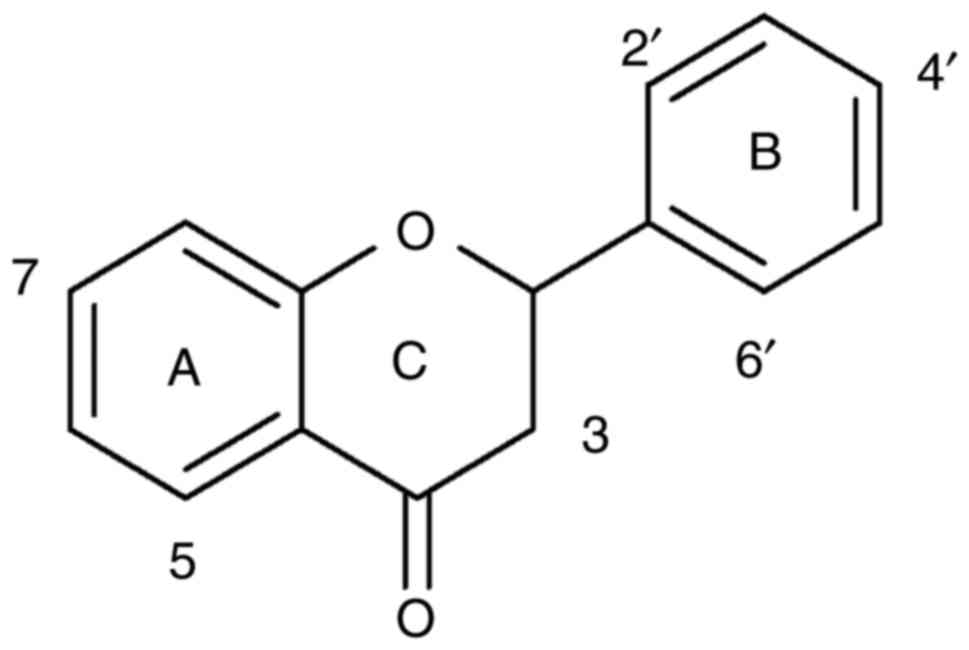
Introduction
Flavanone, an important natural compound, has great
potential to reduce the risk of mortality in patients with
cardiovascular diseases (1–3).
Flavanone is widely distributed in plants, fruits and vegetables,
and consists of a large group of naturally existing polyphenolic
compounds (4). Farrerol, a major
bioactive component in the traditional Chinese herb ‘Man-shan-hong’
derived from the dried leaves of Rhododendron dauricum L.,
has numerous biological effects, including antithrombotic,
anti-inflammatory, antioxidant, antiangiogenesis, vasorelaxant and
antihypertensive effects (5–8).
There has been great interest in the cardiovascular effects of
flavonoids in humans. It has previously been reported that the
greatest levels of vasorelaxant and antioxidant activity were
detected in flavonols and flavones (9,10).
Previous research revealed that farrerol derivatives exhibited
significant anti-atherosclerosis activity in rat vascular smooth
muscle cells (VSMCs) and also exhibited strong cytoprotective
activity against hydrogen peroxide-induced injury in human
umbilical vein endothelial cells. Notably, they had preliminary
structure-activity associations, indicating that farrerol
derivatives could be used to treat and/or prevent cardiovascular
disease (11,12).
Farrerol has been demonstrated to induce a relaxing
effect in the rat aorta (13);
however, there is a lack of research regarding the
structure-activity associations of farrerol derivatives with
vasorelaxant properties. The present study aimed to use farrerol as
the lead compound, and modify the structure to identify more potent
vasorelaxant agents. In the present study, the vasorelaxant actions
of farrerol derivatives in isolated rat thoracic aorta rings were
examined, and the inhibitory effects of farrerol derivatives in rat
aortic VSMCs contraction were investigated.
As we know, a greater understanding of the
structural characteristics that optimize vasorelaxant activity is
of critical importance to develop flavanone derivatives as
potential therapeutic agents. Thus, the present study evaluated the
structure-activity associations of farrerol derivatives in order to
obtain highly active vasodilator compounds. The results may be
useful for the treatment and/or prevention of vascular disease
resulting from abnormal vascular contractility.
Materials and methods
Animals
A total of 120 Male Sprague-Dawley rats aged 12
weeks (weight, 210±20 g) were obtained from the Animal Center of
Shanxi Medical University (Taiyuan, China), with free access to
food and water. Rats were housed five per cage. All of the rats
were maintained at 22±3°C and 45±15% humidity with a normal 12-h
light/dark cycle. All protocols were approved by the Animal Care
and Use Committee of the Shanxi Medical University (Taiyuan,
China), and all animal procedures were performed in accordance with
the Ethical Guidelines for Animal Research in Shanxi Medical
University.
Reagents
Phenylephrine (PE; purity ≥98%), Angiotensin II (Ang
II; purity ≥98%) and Quercetin (purity ≥98%) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Potassium chloride
(KCl), sodium chloride (NaCl), magnesium chloride
(MgCl2), glucose, calcium chloride (CaCl2)
and the other reagents were of analytical purity and were purchased
from Sangon Biotech Co., Ltd., (Shanghai, China). The purity of all
of the farrerol derivatives was >98%, as determined by high
performance liquid chromatography analysis; all of the compounds
were synthesized according to the previously reported methods
(11,12) and the codes for all the compounds
were presented in Table I. All of
the farrerol derivatives used in these experiments were racemates.
The basic structures of flavanone is illustrated in Fig. 1. Quercetin was used as a positive
control drug. All of the farrerol derivatives and quercetin were
dissolved in dimethyl sulfoxide (DMSO) and then diluted in
distilled water in order to yield a final concentration of DMSO
<0.5% (v/v).
 | Table I.Vasorelaxant effects of all of the
farrerol derivatives on phenylephrine-induced contractions in rat
aortic rings. |
Measurements of aortic ring
tension
Male Sprague-Dawley rats were sacrificed by cervical
dislocation in the presence of anesthesia with 5% chloral hydrate
(200 mg/kg), then the blood was drained. The chests were opened,
and the aortas were isolated then immediately transferred into 4°C
Krebs-bicarbonate solution (pH=7.4), composed of NaCl 118.0 mM, KCl
4.7 mM, KH2PO4 1.2 mM, NaHCO3 25.0
mM, CaCl2 2.5 mM, MgCl2, 1.18 mM and
D-glucose 11.0 mM. The aortas were cleaned of adherent connective
tissues, then were cut into 4 mm length rings.
Isolated rat aortic rings were suspended on two
stainless steel hooks in baths containing 5 ml of the
Krebs-bicarbonate solution (maintained at 37°C and supplemented
with 100% O2). The upper hook was connected to a force
transducer and changes in isometric force were recorded using Chart
5.4 (PowerLab; ADInstruments Inc., Colorado Springs, CO, USA).
According to a previous study, passive tension was adjusted to 2 g
and all subsequent measurements representing the force were
generated above this baseline (14). Integrity of the endothelium was
assumed when 10−6 M acetylcholine induced >70%
relaxation of the aortic rings precontracted with 60 mM KCl. A 2 h
equilibration period was applied prior to any experimental
relaxation effects of different farrerol derivatives to the aortic
rings precontracted with 1 µM PE (15). When the contraction induced by 1 µM
PE was sustained, one of the drugs (different farrerol derivatives,
positive control quercetin or vehicle DMSO) was added cumulatively
to the chamber (13). Drugs at
each concentration were added for ~10 min at 37°C. The end
concentrations of farrerol derivatives or quercetin in the chamber
were increased stepwise (1, 3, 10, 30 and 100 µM). The relaxation
response to a concentration of the farrerol derivatives was allowed
to develop to a relatively stable plateau (~10 min), then the next
concentration of farrerol derivative was added. The vasorelaxant
activity of each farrerol derivative was detected in 6 aortic rings
from 6 different rats (1 aortic ring/rat).
Cell culture and identification
Rats were anesthetized by intraperitoneal
administration of 5% chloral hydrate (0.7 ml/100 g), sacrificed by
cervical dislocation, and then disinfected with 75% ethanol. The
thoracic aorta was immediately removed under aseptic conditions,
and was then rinsed thrice with PBS (4°C) for 5 min each. The
thoracic aorta was opened longitudinally. Rat aortic VSMCs were
obtained from the medial layer as previously described (16). Briefly, the endothelium was gently
scrapped off with a scalpel, then the vascular adventitia was
carefully stripped with ophthalmic tweezers. The medial layer of
the thoracic aorta was then cut into small pieces (1
mm2), which were plated into a tissue culture flask and
were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12
nutrient solution (Sigma Aldrich; Merck KGaA) supplemented with 20%
fetal bovine serum (Sigma-Aldrich; Merck KGaA), L-glutamine
(Sigma-Aldrich; Merck KGaA), streptomycin (100 U/ml; Sigma-Aldrich;
Merck KGaA) and penicillin (100 U/ml, Sigma-Aldrich; Merck KGaA).
The rat aortic VSMCs were verified through immunohistochemical
staining of α-actin. The VSMCs were cultured until they reached 80%
confluency and were trypsinized (0.05%) every 3 to 5 days. Cells
between passages 4–8 were used for subsequent experiments.
Cell contraction assay
Collagen gels were prepared as described previously
(17,18), then added to 24-well culture
dishes. Rat aortic VSMCs (1×105 cells per well) were
seeded into wells containing 100 µl neutralization solution and 400
µl collagen, with a final collagen concentration of 1.5 mg/ml. The
VSMCs were then allowed to embed into the collagen gel. The VSMCs
were cultured at 37°C for 24 h, and were then used for the
following treatments. Firstly, the original area of a collagen gel
was measured. Ang II (10 ng/l) was added to cells at 37°C for 1 h,
then farrerol derivatives, quercetin or DMEM were added to cells at
37°C for 4 h, successively. The collagen gel area was then
measured. Gel images were captured using a camera following the
administration of derivatives, and the areas (cm2) of
the gels were measured using ImageJ software version 1.50 (National
Institutes of Health, Bethesda, MD, USA). Enhanced contractions
were characterized by a decreased gel area, and reduced
contractions were characterized by increased gel area. Changes to
the surface area of collagen gels were expressed as a percentage
relative to the untreated control. Three different fields were
analyzed for each experiment that was performed.
Statistical analysis
All data are presented as mean ± standard deviation.
The RC50 was defined as the concentration of flavanone
derivatives that induced a 50% reduction in the maximum relaxation
from the contraction elicited by 1 µM PE and was calculated from
the concentration-response curve resulting from a nonlinear
regression (curve fit) performed using GraphPad Prism 7.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis
was performed using the Student's paired t-test and one-way
repeated measures analysis of variance followed by Bonferroni's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Vasorelaxant activity of all compounds
on the aorta pre-contracted by PE
Treatment with 1 µM PE induced steady contractions
in endothelium-intact rat aortic rings, and all of the farrerol
derivatives examined at concentrations ranging from 1–100 µM,
produced concentration-dependent relaxation (Fig. 2), which could be reversed if the
farrerol derivative was replaced with Krebs solution. The relative
order of diastolic activity of the different farrerol derivatives
examined in the present study is listed in Table I. In these experiments, the
vasodilator amplitude of quercetin (positive control drug) in the
isolated rat aortic rings was 55.74±4.19%, which was less than that
observed with farrerol (65.13±2.12%). In addition, only three
farrerol derivatives (a2, a3 and b2) had a weaker relaxation effect
when compared with that observed following quercetin application to
isolated rat aortas.
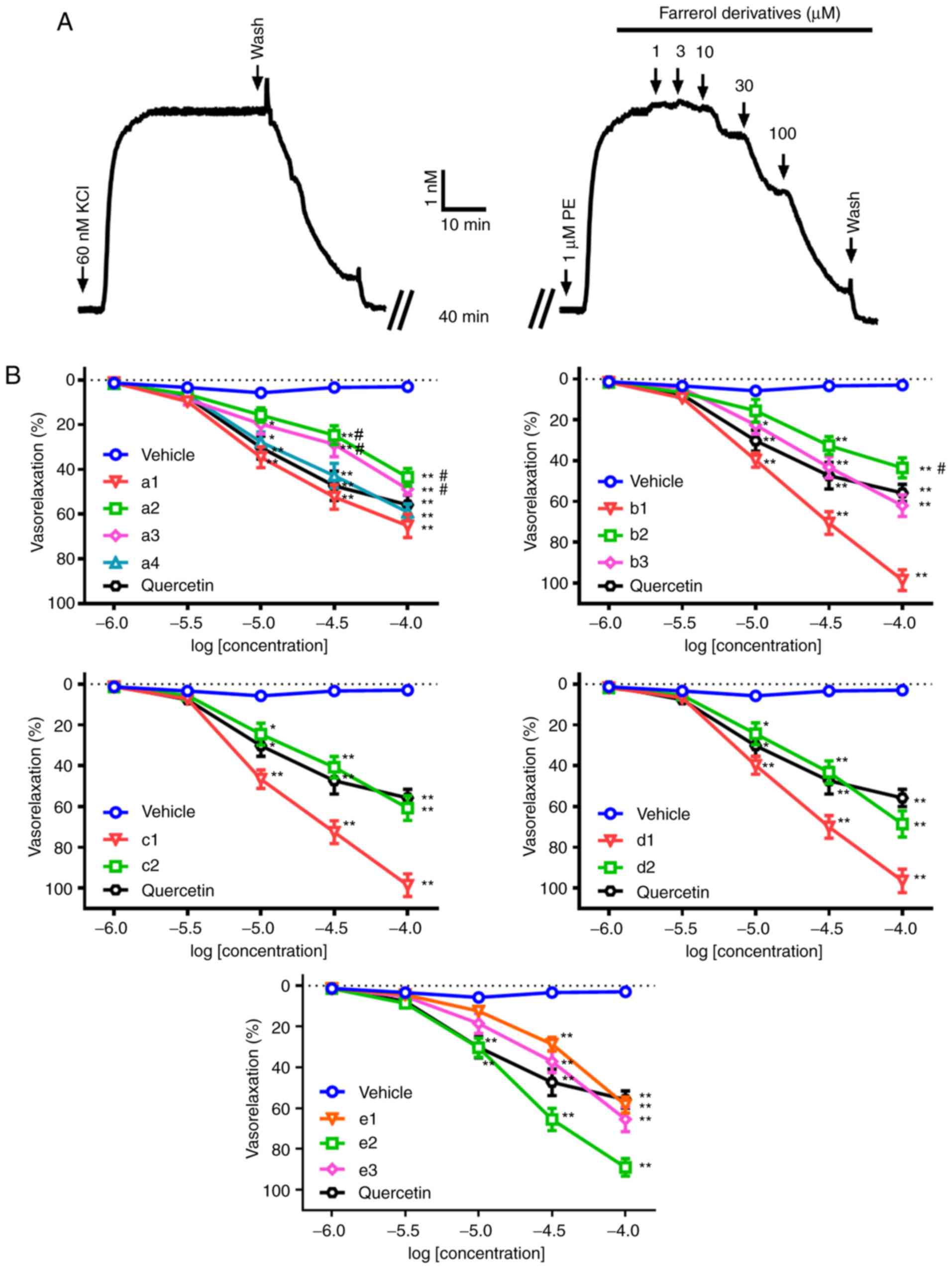 | Figure 2.Concentration-response curves for the
relaxation induced by different farrerol derivatives in isolated
rat aortic rings pre-contracted with PE. (A) Original tension
recording of the vasorelaxation induced by cumulative addition of
farrerol derivatives on rat aortic rings pre-contracted with 1 µM
PE. When the precontraction was sustained, different concentrations
(1, 3, 10, 30 and 100 µM) of farrerol derivatives, positive control
quercetin or vehicle were added to achieve the appropriate
concentrations. (B) Pooled data (mean ± standard deviation; n=6).
Vasorelaxations were expressed as percentages of the
pre-contraction induced by 1 µM PE. The farrerol derivatives were
presented in Table I (a1-a4,
b1-b3, c1-c2, d1-d2, e1-e3). *P<0.05 and **P<0.01 vs. vehicle
group; #P<0.05 vs. quercetin group. PE,
phenylephrine. |
Results of cell contraction
analysis
Collagen gels embedded with rat aortic VSMCs were
prepared using primary cultured cells with very stable
characteristics of the contractile phenotype. Collagen gel
contraction experiments revealed a significant effect on gel
surface area (enhanced contraction was characterized by decreased
gel area and reduced contraction was characterized by increased gel
area). The surface area of the collagen gels was significantly
decreased upon the addition of Ang II (10 ng/l), indicating
increased contractility. Collagen gel contractions were attenuated
when incubated with farrerol derivatives in a dose-dependent manner
and were relatively unaffected by DMEM (Fig. 3). The results of the cell
contraction assay were consistent with of those of the aortic ring
tension measurements. The inhibitory percentage of quercetin on the
initial collagen area was 37.25±4.76%, which was greater than that
of farrerol (20.48±3.52%). It was also revealed that only three
farrerol derivatives (a2, a3 and b2) had greater inhibitory
percentages than that of quercetin in regard to the initial
collagen area.
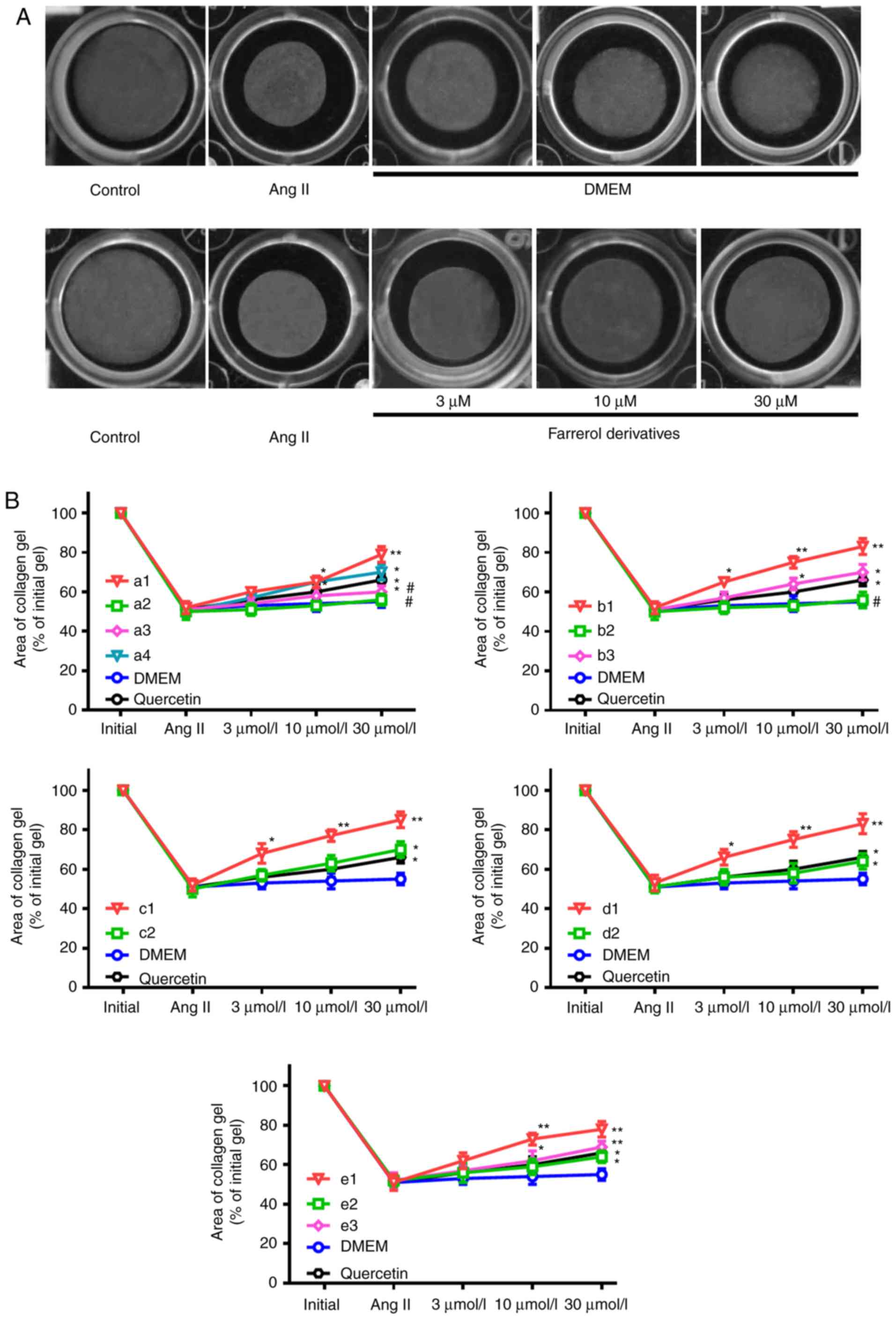 | Figure 3.Effects of different farrerol
derivatives on collagen gel contraction. (A) A representative
photograph of collagen gel contraction. Ang II (10 ng/l) caused
collagen gel/VSMCs contractions, which were attenuated by farrerol
derivatives or positive control quercetin in a
concentration-dependent manner (3, 10 and 30 µM) and were
relatively unaffected by DMEM (magnification, ×100). (B)
Comparisons of the collagen gel surface area following the
application of different farrerol derivatives, positive control
quercetin or DMEM. Changes in collagen gel surface area were
expressed as the ratio of the experimental gel area to that of the
untreated control. Three different fields were analyzed for each
experiment that was performed (n=3). The farrerol derivatives were
presented in Table I (a1-a4,
b1-b3, c1-c2, d1-d2, e1-e3). *P<0.05 and **P<0.01 vs. DMEM
group; #P<0.05 vs. quercetin group. Ang II,
Angiotensin II; VSMCs, vascular smooth muscle cells; DMEM,
Dulbecco's modified Eagle's medium. |
Discussion
Farrerol, isolated from Rhododendron dauricum
L., is a traditional Chinese medicine that has been reported to
have beneficial effects for multiple conditions, including
bronchitis and asthma (13). Our
preliminary experiment determined the cytotoxicity of all of the
farrerol derivatives in rat aortic VSMCs using MTT assays, the
results revealed that there was no cytotoxicity up to 60 µM (data
not shown). Our previous study demonstrated that farrerol could
induce relaxation in rat aortic rings precontracted by PE in a
dose-dependent manner (13). Owing
to the structural similarity of farrerol derivatives to that of
farrerol, farrerol was also used to explore the possible mechanisms
involved as the lead compound. It was hypothesized that the
relaxation effect of farrerol derivatives may be associated with
the molecular structure, which was confirmed by two different
experimental methods (the rat aortic tension test and collagen gel
contraction assay) in the present study.
Our previous research confirmed that farrerol could
relax precontracted rat aortic rings and inhibit contractions
produced by a vasoconstrictor (13). Furthermore, it was demonstrated
that farrerol could reduce [Ca2+]in in
cultured VSMCs by laser scanning confocal microscopy and suppress
Ca2+ influx via L-type voltage gated Ca2+
channel via the patch clamp technique (13). These results suggested that the
antihypertensive effect of farrerol could be explained by the
direct vasodilatory effect of farrerol on VSMCs. VSMCs serve an
important role in maintaining blood pressure and facilitating the
flow of nutrients in the body (19). PE could cause aortic contraction by
Ca2+ influx through reactive oxygen cluster (ROC) and by
the release of Ca2+ from the sarcoplasmic reticulum
(20,21). In our previous experiments,
farrerol relaxed the precontraction in the rat aortic rings induced
by PE, implying that farrerol may decrease Ca2+ influx
by blocking ROC and inhibiting Ca2+ release from the
sarcoplasmic reticulum (13). In
the present study, farrerol derivatives were evaluated for their
vasodilatory effect on rat aortic rings precontracted by PE. The
results demonstrated that all of the examined farrerol derivatives
exhibited a vasorelaxation effect in rat aortic rings; however, the
vasorelaxation activity varied between the derivatives. Compounds
c1, d1 and b1 exhibited high vasorelaxation activity, the maximum
relaxation percentages were 98.44±5.41, 96.38±3.65 and 98.34±5.01%,
respectively (RC50=12.85, 15.12 and 14.68 µM,
respectively). The results indicated that compounds are more likely
to have positive vasorelaxation activity if they have an
electron-withdrawing substituent in the ortho position of the
phenyl group (ring B). This is due to compounds c1 and d1, which
have an ortho nitro group on the B ring, having the strongest
vasorelaxation activity; whereas, compounds c2 and d2, with a para
electron withdrawing group, displayed weak vasorelaxation activity.
Notably, when there was no substituent on the B ring (b1), the
compound could also exert good vasodilatation activity.
In the present study, it was also revealed that
compounds a1-a4 and e1-e3 exhibited a vasorelaxation effect on
precontracted rat aortic rings. The results indicated that an ortho
electron-withdrawing substituent is crucial for the vasorelaxation
activity, whereas, a hydroxyl or methoxy group is unfavorable. A
number of studies have reported that the extra hydroxyl group
present in the flavanone at the C3 position is important for
vascular activity as they have greater vasorelaxation effects. In
addition, the pattern of substitution of hydroxyl groups on the A
and B rings of compounds also influences activity (22,23),
particularly substitution on the B ring, as flavonols with a
C3′, 4′ diOH or C3′, 4′,5′ triOH orientation
exhibited weak vasodilation activity (24). A para electron-withdrawing group or
an electron-donating group could also decrease the anti-tumor
activities of farrerol derivatives in vitro (11). As demonstrated in the present
study, the results indicated that a para electron-donating group
increased the vasorelaxation effect, as compounds a1 and e2
exhibited high vasodilation activity. In addition, when the
heterocycle is a B ring instead of a phenyl group, the
vasorelaxation ability of compounds b2 and b3 were weaker than b1.
The results indicated that a heterocycle was unfavorable for
relaxation activity. Furthermore, the relaxation effect was greatly
influenced by the molecular structure of the different farrerol
derivatives.
Unimpaired VSMC function is essential for the
maintenance of life. In the present study, using a collagen gel
contraction assay, the inhibitory effect of farrerol derivatives in
decreased collagen gel area induced by Ang II was examined. Ang II
has been reported to be associated with cardiac growth and
hypertensive disease (25,26). The farrerol derivatives examined in
the present study were able to inhibit collagen gel/VSMC
contraction in a concentration-dependent manner, and the inhibitory
strength of different farrerol derivatives was also consistent with
the results of the myogenic experiments. Furthermore, the
inhibitory activity of farrerol derivatives on collagen gel/VSMC
contraction were enhanced by an ortho electron-withdrawing
substituent or a para electron-donating group on the B ring, but
were weakened by a para electron withdrawing group or a heterocycle
on the B ring as opposed to a phenyl group.
Based on the structure-activity associations of
farrerol derivatives established in the present study, it was
hypothesized that an ortho electron-withdrawing substituent may be
crucial for the vascular activity of the farrerol derivatives,
whereas, a hydroxyl or methoxy group may be unfavorable. A para
electron-donating group increased the activity of the examined
compounds. Meanwhile, it was unfavorable for compound activity when
the heterocycle was on the B ring, as opposed to a phenyl group.
Further in vivo and in vitro tests of different
farrerol derivatives are currently underway. These will provide
important information for further structure modifications and the
results of the present study will hopefully provide a basis to
develop novel anti-cardiovascular drug candidates. The molecular
structure of farrerol contains an S configuration; however, the S
configuration and its determination have yet to be investigated. In
the future research, we will confirm the enantiopurity of farrerol
derivatives by typical polarimetric techniques in order to
elucidate whether they interact with raceme or enantiomers. In the
present study, the main focus was on the structure-vasodilatation
activity associations of farrerol and its derivatives on the
isolated aortic rings and rat aorta VSMCs. Our ongoing research
will further explore the mechanism of farrerol derivatives relaxing
the aorta in rats.
In conclusion, the type and position of the
substituents on the B ring serve an important role in the
relaxation activity of farrerol derivatives. An ortho
electron-withdrawing substituent was crucial for the activity of
the farrerol derivatives, whereas a hydroxyl or methoxy group was
unfavorable. A para electron-donating group could increase the
activity of the compounds. In the future, the group will continue
to synthesize the corresponding derivatives with -NH2 or
-SO3H in the ortho or para positions. In addition, the
heterocyclic structure was unfavorable for compound activity when
the heterocycle was on the B ring instead of a phenyl group. The
effects of compounds with different hydroxyl and vinyl groups in
the C ring should also be considered; thus, the group will continue
to modify the C ring to acquire highly active compounds. The
present study only investigated the structure-activity associations
of a racemate mixture of farrerol derivatives on the rat aorta. In
future experiments, enantiomers from farrerol derivatives will be
separated and studied to elucidate their vasorelaxant activity in
rat aortas.
The results of the present study have facilitated
the identification of the structural characteristics that promote
the vasorelaxation activity of farrerol derivatives, which may lead
to the development of agents useful in the treatment of
cardiovascular disease.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Youth Science and Technology Research Fund of Shanxi Province
(grant nos. 201701D221247 and 201701D221259), the Science and
Technology Innovation Project of Shanxi Higher School (grant nos.
2017146 and 2017147), the Youth Fund of Shanxi Medical University
(grant nos. 02201604 and 02201613), the Startup Foundation for
Doctors of Shanxi Medical University (grant nos. 03201510 and
03201521) and the Fund for Shanxi ‘1331 Project’ Key Subjects
Construction.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XH and XQ performed the experiments and were the
major contributors in writing the manuscript. QL conceived and
designed the study, and was involved in drafting the manuscript and
critically revising the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Animal Care and Use Committee of the Shanxi Medical University
(Taiyuan, China), and were performed in accordance with the Ethical
Guidelines for Animal Research in Shanxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Da Pozzo E, Costa B, Cavallini C, Testai
L, Martelli A, Calderone V and Martini C: The citrus flavanone
naringenin protects myocardial cells against age-associated damage.
Oxid Med Cell Longev. 2017:95361482017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goetz ME, Judd SE, Hartman TJ, Mcclellan
W, Anderson A and Vaccarino V: Flavanone intake is inversely
associated with risk of incident ischemic stroke in the REasons for
geographic and racial differences in stroke (REGARDS) study. J
Nutr. 146:2233–2243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chin KY, Silva LS, Darby IA, Ng DCH and
Woodman OL: Protection against reperfusion injury by
3′,4′-dihydroxyflavonol in rat isolated hearts involves inhibition
of phospholamban and JNK2. Int J Cardiol. 254:265–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ajay M, Gilani AU and Mustafa MR: Effects
of flavonoids on vascular smooth muscle of the isolated rat
thoracic aorta. Life Sci. 74:603–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai F, Gao L, Zhao Y, Wang C and Xie S:
Farrerol inhibited angiogenesis through Akt/mTOR, Erk and
Jak2/Stat3 signal pathway. Phytomedicine. 23:686–693. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JK, Ge R, Tang L and Li QS: Protective
effects of farrerol against hydrogen-peroxide-induced apoptosis in
human endothelium-derived EA.hy926 cells. Can J Physiol Pharmacol.
91:733–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nijveldt RJ, van Nood E, van Hoorn DE,
Boelens PG, van Norren K and van Leeuwen PA: Flavonoids: A review
of propable mechanisms of action and potential application. Am J
Clin Nutr. 74:418–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin X, Hou X, Zhang K and Li Q: Farrerol
modulates aorta gene expression profile in spontaneously
hypertensive rats. Planta Med. 84:296–303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin X, Lu Y, Peng Z, Fan S and Yao Y:
Systematic chemical analysis approach reveals superior antioxidant
capacity via the synergistic effect of flavonoid compounds in red
vegetative tissues. Front Chem. 6:92018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng X, Xi Y and Jiang W: Protective roles
of flavonoids and flavonoid-rich plant extracts against
urolithiasis: A review. Crit Rev Food Sci Nutr. 12:1–11. 2018.
View Article : Google Scholar
|
|
11
|
Shi L, Feng XE, Cui JR, Fang LH, Du GH and
Li QS: Synthesis and biological activity of flavanone derivatives.
Bioorg Med Chem Lett. 20:5466–5468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi L, Feng XE, Lin WH, Fang LH, Du GH and
Li QS: Synthesis of new flavanone derivatives of farrerol and
preliminary SAR studies on anti-VSMCs vegetation activity. Chem Res
Chin Univ. 27:237–240. 2011.
|
|
13
|
Qin X, Hou X, Zhang M, Liang T, Zhi J, Han
L and Li Q: Relaxation of rat aorta by farrerol correlates with
potency to reduce intracellular calcium of VSMCs. Int J Mol Sci.
15:6641–6656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong ESW, Man RYK, Ng KFJ, Leung SWS and
Vanhoutte PM: L-arginine and arginase products potentiate
dexmedetomidine-induced contractions in the rat aorta.
Anesthesiology. 128:564–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu LG, Zhang MS, Liu Y, Xue WX, Liu DB,
Zhang J and Liang YQ: Vasorelaxant effect of taurine is diminished
by tetraethylammonium in rat isolated arteries. Eur J Pharmacol.
580:169–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu S, Fu J, Chen J, Xiao P, Lan T, Le K,
Cheng F, He L, Shen X, Huang H and Liu P: Development of an
optimized protocol for primary culture of smooth muscle cells from
rat thoracic aortas. Cytotechnology. 61:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zagai U, Sköld CM, Trulson A, Venge P and
Lundahl J: The effect of eosinophils on collagen gel contraction
and implications for tissue remodelling. Clin Exp Immunol.
135:427–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kohyama T, Liu X, Wen FQ, Zhu YK, Wang H,
Kim HJ, Takizawa H, Cieslinski LB, Barnette MS and Rennard SI: PDE4
inhibitors attenuate fibroblast chemotaxis and contraction of
native collagen gels. Am J Respir Cell Mol Biol. 26:694–701. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thorneloe KS and Nelson MT: Ion channels
in smooth muscle: Regulators of intracellular calcium and
contractility. Can J Physiol Pharmacol. 83:215–242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dellis O, Dedos SG, Tovey SC, Dubel SJ and
Taylor CW: Ca2+entry through plasma membrane IP3receptors. Science.
313:229–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia M, Qian L, Zhou X, Gao Q, Bruce IC and
Xia Q: Endothelium-independent relaxation and contraction of rat
aorta induced by ethyl acetate extract from leaves of Morus alba
(L.). J Ethnopharmacol. 120:442–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dugas AJ, Castañeda AJ, Bonin GC, Price
KL, Fischer NH and Winston GW: Evaluation of the total peroxyl
radical-scavenging capacity of flavonoids: Structure-activity
relationships. J Nat Prod. 63:327–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masek A, Chrzescijanska E, Latos M and
Zaborski M: Influence of hydroxyl substitution on flavanone
antioxidants properties. Food Chem. 215:501–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrera MD, Zarzuelo A, Jiménez J,
Marhuenda E and Duarte J: Effects of flavonoids on rat aortic
smooth muscle contractility: Structure-activity relationships. Gen
Pharmacol. 27:273–277. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simo-Cheyou ER, Ju JT, Grygorczyk R and
Srivastava AK: STIM-1 and ORAI-1 channel mediate
angiotensin-II-induced expression of Egr-1 in vascular smooth
muscle cells. J Cell Physiol. 232:3496–3509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caillon A, Mian MOR, Fraulob-Aquino JC,
Huo KG, Barhoumi T, Ouerd S, Sinnaeve PR, Paradis P and Schiffrin
EL: γδ T cells mediate angiotensin II-induced hypertension and
vascular injury. Circulation. 135:2155–2162. 2017. View Article : Google Scholar : PubMed/NCBI
|