Introduction
Hepatic fibrosis is an inevitable process that
occurs in the progression of various chronic liver diseases,
ultimately leading to cirrhosis. It is a reversible pathological
change caused by acute/chronic liver injury (1). Liver fibrosis manifests as the
excessive deposition of extracellular matrix (ECM) in the liver.
Long-term aggravation of liver fibrosis results in diffuse liver
damage, which may lead to the development of cirrhosis and liver
cancer (1). The activation and
proliferation of hepatic stellate cells (HSCs) is critically
involved in the process of liver fibrosis (2–4). It
is therefore of great importance to identify a reliable,
non-invasive and convenient method for the early diagnosis of liver
fibrosis.
MicroRNAs (miRNAs/miRs) are a class of
evolutionarily conserved small (18–24 nucleotides) single-stranded
non-coding RNAs that mediate post-transcriptional gene suppression,
via the degradation of target mRNAs or the suppression of mRNA
translation following binding (5,6).
Increasing evidence suggests that dysregulated miRNAs serve a
crucial role in a number of diseases, including hepatocellular
carcinoma and liver fibrosis (7,8). For
example, it was reported that the miRNA-15a/plasminogen activator
inhibitor 2 axis promotes the migration of cholangiocarcinoma cells
(9). miR-122 and other miRNAs may
have potential as circulating biomarkers in drug-induced liver
injury (10). miRNA-351 directly
targets the vitamin D receptor to promote schistosomiasis-induced
hepatic fibrosis (11). However,
to the best of our knowledge, there have been no studies focusing
on the miRNA-mRNA network involved in liver fibrosis to date.
In the current study, the expression profiles of
miRNAs and mRNAs in liver fibrosis were investigated and compared
with normal liver samples. The identification of novel
differentially expressed miRNAs may provide novel insights,
allowing for the early diagnosis and treatment of liver fibrosis. A
total of 71 differentially expressed miRNAs (including 56
upregulated and 15 downregulated miRNAs) in were identified
fibrotic tissues compared with normal liver tissues. Integrated
analysis with human liver cirrhosis data from Gene Expression
Omnibus (GEO) datasets revealed 5 miRNAs that were significantly
increased in both human and mouse fibrotic liver tissues. A
functional miRNA-mRNA network was constructed based on their
inverse correlation. Target mRNAs identified in this network were
further confirmed in activated hepatic stellate cells (HSCs). The
results of the present study may provide novel insights to increase
our understanding of the underlying mechanism involved in liver
fibrosis.
Materials and methods
Liver fibrosis model and RNA
isolation
A mouse model of liver fibrosis was constructed and
RNA was isolated as previously reported (12). In brief, 6-week-old male C57BL/6
mice (weight, ~20 g) were purchased from Shanghai Laboratory Animal
Center (Shanghai, China) and maintained under a 12 h light/dark
cycle with 40–60% humidity, at 22–25°C, with free access to food
and water. Following acclimatization for one week, the 12 mice were
randomly divided into control and treatment groups (n=6/each
group). CCl4 (0.5 µl/g body weight; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) diluted nine times in corn oil
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was administered to
mice via intraperitoneal injection by two times per week, for 8
weeks. The control group was injected with an equivalent volume of
corn oil. After 8 weeks, mice were anaesthetized by intraperitoneal
injection of 0.8% pentobarbital (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). and sacrificed and liver tissues were
harvested. Total RNA was isolated from four fibrotic and four
normal liver tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. The study was approved by the Ethical
Committee of Fudan University and all experiments were performed
according to the approved guidelines and regulations.
Histopathology analyses and
immunohistochemistry
Liver specimens were fixed in 4% formaldehyde
(Sigma-Aldrich; Merck KGaA) at room temperature for 12 h,
dehydrated, embedded in paraffin at room temperature for 1 h and
sectioned at 3 um. The sections were processed for hematoxylin and
eosin (H&E) and Masson's trichrome (both Sigma-Aldrich; Merck
KGaA) staining for assessment of the degree of liver fibrosis. For
the H&E staining, the tissue section was incubated at 65°C for
1 h for dewaxing. Samples were stained with hematoxylin for 1 min,
followed by eosin staining for 10 sec at room temperature, and,
then the samples were dried at room temperature and sealed. The
middle part of the visual field was examined by light microscopy.
Masson Trichrome staining was performed following the protocol of
trichrome stain (Masson) kit from Sigma-Aldrich. Deparaffinized the
slides to deionized water, then mordanted in preheated Bouin's
Solution at 56°C for 15 min. Cooled the slides in tap water (18–26
°C) and washed in running tap water to remove yellow color from
sections. Put the slides in Working Weigert's Iron Haematoxylin
Solution for 5 min, Biebrich Scarlet-Acid Fucshin for 5 min,
working Phosphotungstic/Phosphomolybdic Acid Solution for 5 min,
Aniline Blue Solution for 5 min, 1% acetic acid for 2 min both at
room temperature. Finally, rinsed slides, dehydrated through
alcohol and cleared in xylene. A second set of tissue sections
(0.5×0.5 cm) were prepared for immunohistochemical assessment of
the tissues. Briefly, endogenous peroxidase activity was blocked by
immersion of deparaffinized sections in 3% H2O2 in methanol for 30
min at room temperature. Antigen retrieval was performed by
steaming slides in 0.01 M citrate buffer (pH 6.0) and heated them
until the temperature reached 95–100°C for 30 min. The samples were
incubated for 30 min at room temperature in 5% normal blocking
serum, and incubated with a 1:100 dilution of polyclonal rabbit
anti-α-SMA (1:200, cat. no. ab5694, Abcam, Cambridge, UK) overnight
at 4°C. The slides were then incubated with HRP-conjugated goat
anti-rabbit secondary antibody (1:2,000, ab7090; Abcam) for 60 min
at room temperature. Between each incubation, sections were washed
three times with PBS. Sections were developed with
3,3-diaminobenzidine tetrahydrochloride and hydrogen peroxide and
subsequently counterstained with hematoxylin. The sections were
imaged using a Nikon Eclipse 80i microscope (Nikon Corporation,
Tokyo, Japan). Hematoxylin and eosin, α-smooth muscle actin (SMA)
and Masson staining demonstrated that liver fibrosis was
successfully established, which was also verified in our previous
study (12).
miRNA sequencing
Total RNA was used to prepare miRNA libraries and
the purified libraries were sequenced on an Illumina HiSeq 2000
platform (Illumina, Inc., San Diego, CA, USA). In brief, the raw
data were processed with R software (version 3.3.2; www.r-project.org) to ensure its quality. Clean data
were mapped to the Ensemble database (GRCh37) (13) and compared with miRBase (release
21; www.mirbase.org) to identify mature
miRNAs. The count and reads per million total read values of the
miRNAs were collected for each sample. Individual miRNAs were
analyzed to identify significant differences using the DESeq2
package (version 1.4.0) (14) in
R. The parameters for differentially expressed miRNAs were set with
a false discovery rate of <0.05 and |log2 fold change
(FC)|>=1. The differentially expressed miRNAs are shown by
heatmap and volcano plot using R software The miRNA expression
profiles (GSE49012 and GSE40744) in human liver cirrhosis tissues
were downloaded from publicly available Gene Expression Omnibus
(GEO) datasets (www.ncbi.nlm.nih.gov/geo) (15,16).
miRNA target prediction
miRWalk (version 2.0) (17) collects 13 predicted data sets from
existing miRNA target databases to predict all possible miRNA-mRNA
interactions. Putative interactions between the sequenced miRNAs
and mRNAs were evaluated using miRWalk. The search was restricted
to miRNAs with a minimum seed length of seven nucleotides and
binding sites in the 3′untranslated region (UTR) of target genes.
P<0.05 was considered to indicate a statistically significant
difference.
Functional analysis
To explore the functional roles of target genes, the
Database for Annotation, Visualization and Integrated Discovery
(version 6.8; www.david.ncifcrf.gov) software (18,19)
was used, which integrates the Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) databases to analyze
biological functions. Fisher's exact test was performed to evaluate
the enrichment values of GO terms and KEGG pathways. P<0.05 was
considered to indicate a statistically significant difference. The
miRNA-mRNA interaction regulatory network was constructed using
Cytoscape software (20) (version
3.3.0; www.cytoscape.org).
Activation of LX-2 cells
LX-2 HSC cells were cultured in DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS, Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere containing 5% CO2 at 37°C for 24
h. LX-2 cells were starved for 12 h in serum-free DMEM, following
which they were treated with 10 ng/ml TGF-β (R&D Systems China
Co., Ltd., Shanghai, China). After 24 h of TGF-β treatment, the
cells were harvested for RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
SYBR Green-based qPCR (Thermo Fisher Scientific,
Inc.) was used to measure mRNA expression. Total RNA (2 µg) was
reverse transcribed into cDNA using the reverse transcription kit
(Takara Biotechnology Co., Ltd. Dalian, China). The protocol for
reverse transcription was as follows: 25°C for 10 min; 37°C for 120
min and 85°C for 5 min. The thermocycling conditions for RT-qPCR
were as follows: Initial denaturation at 95°C for 10 min; 40 cycles
of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec
and extension at 72°C for 30 sec; followed by melting curve
analysis. All experiments were performed in triplicate. The
comparative ∆Cq method (21) was
used to analyze the data, with GAPDH as an endogenous reference
gene. The RT-PCR primers are listed in Table I.
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| Gene (human) | Forward primer
(5′to 3′) | Reverse primer
(5′to 3′) |
|---|
| RELB |
CAGCCTCGTGGGGAAAGAC |
GCCCAGGTTGTTAAAACTGTGC |
|
RAP1A |
CGTGAGTACAAGCTAGTGGTCC |
CCAGGATTTCGAGCATACACTG |
|
PPP3CB |
CCCCAACACATCGCTTGACAT |
GGCAGCACCCTCATTGATAATTC |
|
ARRB1 |
AAAGGGACCCGAGTGTTCAAG |
CGTCACATAGACTCTCCGCT |
|
MAP2K4 |
TGCAGGGTAAACGCAAAGCA |
CTCCTGTAGGATTGGGATTCAGA |
|
FGF1 |
CTCCCGAAGGATTAAACGACG |
GTCAGTGCTGCCTGAATGCT |
|
MAP3K4 |
CTCGACAGATGAAACGCATGT |
CCAGTGTCTTTATGTGGAGGC |
|
PPKCB |
AGCCCCACGTTTTGTGACC |
GCTGGGAACATTCATCACGC |
|
GAPDH |
ACAACTTTGGTATCGTGGAAGG |
GCCATCACGCCACAGTTTC |
| Gene (mouse) |
|
Relb |
CACCGGGTACACCCACATAG |
ATGCCCAGGTTGTTAAAGCTG |
|
Rap1a |
ATGCGTGAGTACAAGCTAGTAGT |
AATCTACCTCGACTTGCTTTCTG |
|
Ppp3cb |
AAAGCGTGCTGACACTCAAG |
TGGAGAGAATCCTCGTATTGCT |
|
Arrb1 |
AGGCAAGCCCCAATGGAAAG |
AGTGTCACGTAGACTCGCCTT |
|
Map2k4 |
AATCGACAGCACGGTTTACTC |
GCAGTGAAATCCCAGTGTTGTT |
|
Fgf1 |
GGGGAGATCACAACCTTCGC |
GTCCCTTGTCCCATCCACG |
|
Map3k4 |
GAGTCGGCTCGCAAAAGTATG |
GTGAGGTGCCGTAGAGAGTC |
|
Ppkcb |
ATGAGTTCGTCACGTTCTCCT |
CCATACAGCAGCGATCCACAG |
|
Gapdh |
AGGTCGGTGTGAACGGATTTG |
GGGGTCGTTGATGGCAACA |
Western blotting
Protein samples were extracted from cells using
radioimmunoprecipitation lysis buffer (Beyotime Biotechnology,
Inc., Haimen, China). Total protein concentration was quantified by
Bicinchoninic acid Protein Assay kit (Beyotime Biotechnology).
Equal amounts of protein (30 µg/lane) were separated on 8–12%
polyacrylamide gels and transferred onto polyvinylidene difluoride
membranes. Subsequently, the membranes were blocked with 5%
fat-free dry milk at room temperature for 1 h. Membranes were
incubated with mouse anti-α-SMA (cat. no. A5228, 1:1,000;
Sigma-Aldrich; Merck KGaA), anti-collagen α-1(I) chain (COL1α1;
cat. no. ABT123, 1:1,000, EMD Millipore, Billerica, MA, USA) or
anti-GAPDH (cat. no. ab8245, 1:4,000; Abcam, Cambridge, UK)
antibodies in Tris buffered saline with 0.1% Tween-20 (TBST)
overnight on a shaker at 4°C. Membranes were subsequently washed
three times in TBST and incubated with horseradish peroxidase
(HRP)-conjugated rabbit anti-mouse IgG secondary antibodies
(1:5,000, cat. no. ab6728, Abcam) at room temperature for 1 h.
Immunoblots were visualized using Enhanced Chemiluminescent Plus
western blotting substrate (Thermo Fisher Scientific, Inc.).
Statistical analysis
Student's t-test was performed to compare two
variables using SPSS v20 software (IBM Corp., Armonk, NY, USA). All
data are expressed as the mean ± standard deviation. FC≥2 and
P<0.05 were considered to indicate a statistically significant
difference. All experiments were repeated in triplicate.
Results
Identification of differentially
expressed miRNAs in liver fibrosis
To identify miRNAs that may be significant in liver
fibrogenesis, miRNA sequencing was performed to compare their
expression between fibrotic liver and normal liver tissues. As
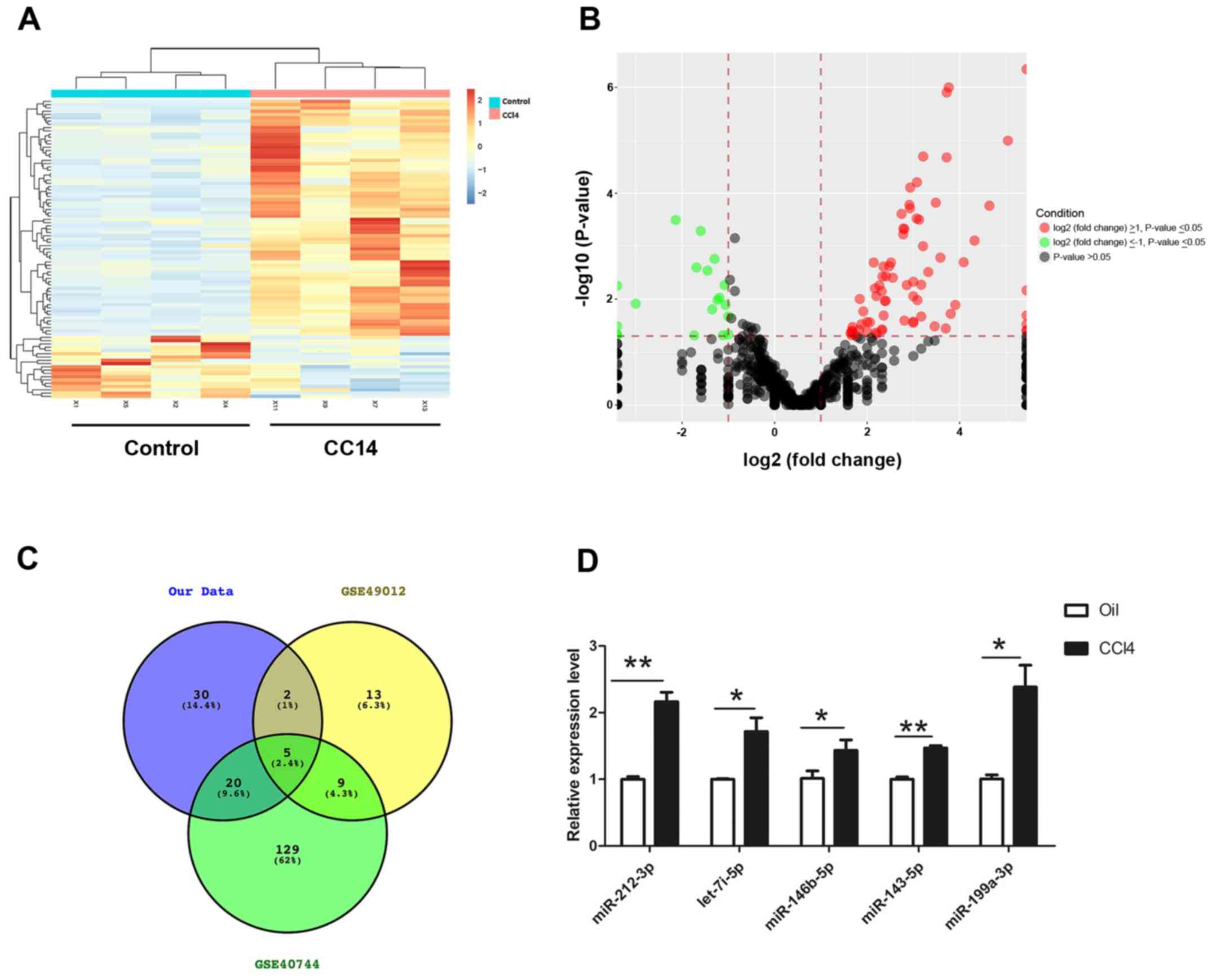
shown in Fig. 1, 71 miRNAs that
were significantly differentially expressed between fibrotic liver
and normal liver tissues were identified. Among these, 56 were
upregulated and 15 were downregulated in fibrotic samples. A
heatmap and volcano plot were produced to show the differentially
expressed miRNAs using R software, of which the top 20 dysregulated
miRNAs were summarized and listed in Table II. To identify conserved miRNAs in
human clinical samples and animal experiments, two miRNA profiles
(GSE49012 and GSE40744) were downloaded from publicly available GEO
datasets (www.ncbi.nlm.nih.gov/geo). The data suggested that
five miRNAs (miR-212-3p, miR-146b-5p, let-7i-5p, miR-143-5p and
miR-199a-3p) were also upregulated in human liver fibrotic samples,
which was consistent with the results of the present study
(Fig. 1C). RT-qPCR was performed
to verify the expression of these five upregulated miRNAs in
CCl4-induced fibrotic and control normal liver tissues.
It was confirmed that miR-212-3p, miR-146b-5p, let-7i-5p,
miR-143-5p and miR-199a-3p were significantly increased in
CCl4-treated liver tissues compared with control samples
(Fig. 1D).
 | Table II.Dysregulated microRNAs in
CCl4-induced liver fibrosis (>2-fold; P<0.05). |
Table II.
Dysregulated microRNAs in
CCl4-induced liver fibrosis (>2-fold; P<0.05).
| A, Upregulated |
|---|
|
|---|
|
|
CCl4/Corn Oil |
CCl4/Corn Oil |
|
|---|
|
|
|
|
|
|---|
| Probe ID | Log2
change | Fold-change | P-value |
|---|
| mmu-miR-212-3p | 5.04 | 33.0 | 0.04778 |
|
mmu-miR-3081-3p | 4.64 | 25.0 | 0.03257 |
| mmu-miR-1983 | 4.32 | 20.0 | 0.02042 |
| mmu-miR-411-3p | 4.09 | 17.0 | 0.03905 |
| mmu-miR-147-5p | 3.91 | 15.0 | 0.03943 |
| mmu-miR-543-3p | 3.81 | 14.0 | 0.02985 |
| mmu-miR-582-3p | 3.77 | 13.6 | 0.00001 |
| mmu-miR-708-3p | 3.72 | 13.2 | 0.00017 |
| mmu-miR-134-5p | 3.72 | 13.2 | 0.00078 |
|
mmu-miR-376b-3p | 3.70 | 13.0 | 0.00202 |
|
| B,
Downregulated |
|
|
|
CCl4/Corn Oil |
CCl4/Corn Oil |
|
|
|
|
|
|
| Probe
ID | Log2
change |
Fold-change | P-value |
|
|
mmu-miR-193a-5p | −1 | 0.50 | 0.02130 |
| mmu-miR-26b-3p | −1 | 0.50 | 0.04648 |
| mmu-miR-137-3p | −1.12 | 0.46 | 0.04818 |
| mmu-miR-871-3 | −1.19 | 0.44 | 0.00943 |
|
mmu-miR-1948-5p | −1.23 | 0.43 | 0.01038 |
| mmu-miR-365-3p | −1.30 | 0.41 | 0.00174 |
| mmu-miR-192-3p | −1.35 | 0.39 | 0.01571 |
| mmu-miR-741-3p | −1.45 | 0.37 | 0.00290 |
| mmu-miR-6390 | −1.60 | 0.33 | 0.00052 |
| mmu-miR-122 | −1.69 | 0.31 | 0.00252 |
GO analysis and pathway analysis
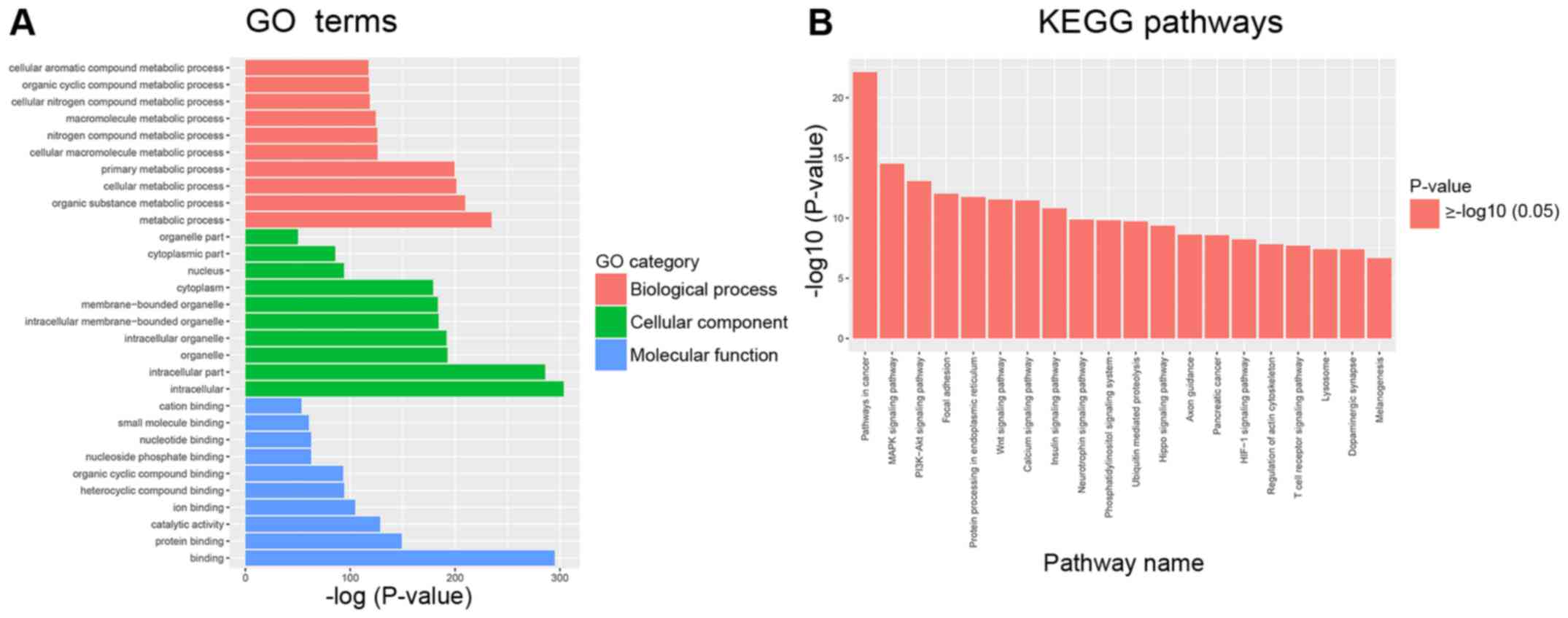
Bioinformatics analyses were used to analyze miRNA
function based on their target mRNAs. GO analysis was performed to
evaluate mRNA enrichments in terms of biological process, cellular
component and molecular function. The top 10 enriched GO terms were
involved in the regulation of cellular and metabolic processes
(Fig. 2A). KEGG analysis revealed
the top 10 pathways, which included the mitogen-activated protein
kinase (MAPK), phosphoinositide 3-kinase (PI3K)-protein kinase B
(Akt), focal adhesion and Wnt signaling pathways. All of these
pathways are associated with HSC activation and extracellular
matrix ECM remodeling (22–24).
These results suggested that some differentially expressed miRNAs
may be involved in the progression of liver fibrosis (Fig. 2B).
Construction of the miRNA-mRNA
interaction network
To further investigate the association between
distinct miRNAs and target mRNAs in fibrotic liver tissues, the
mRNA sequencing profile of the CCl4-induced liver
fibrosis mouse model was extracted from our previous study
(12). A miRNA-mRNA regulatory
network associated with liver fibrosis was constructed based on the
inverse expression relationships between the five miRNAs
upregulated in both mouse and human fibrotic liver tissues and
mRNAs significantly enriched in the MAPK, PI3K-Akt, focal adhesion
and Wnt signaling pathways. As shown in Fig. 3A, the network contained five
miRNAs, 22 mRNAs and 23 miRNA-mRNA connections. Target mRNAs in the
network, such as RELB proto-oncogene (RELB), RAS-related protein 1a
(RAP1A), protein phosphatase 3 catalytic subunit β (PPP3CB),
arrestin β 1 (ARRB1), fibroblast growth factor 1 (FGF1), protein
kinase C β (PRKCB) and members of the MAPK family (MAP3K4, MAPK8,
MAPKAPK5, MAP2K5, MAP2K4, MAP3K1), were summarized in Table III. The RT-qPCR results revealed
that the expression of RELB, RAP1A, PPP3CB, MAP2K4, ARRB1, MAP3K4,
FGF1 and PRKCB was decreased in CCl4-induced fibrotic
liver tissues (Fig. 3B). The
integrated analysis revealed their regulatory interactions in liver
fibrosis.
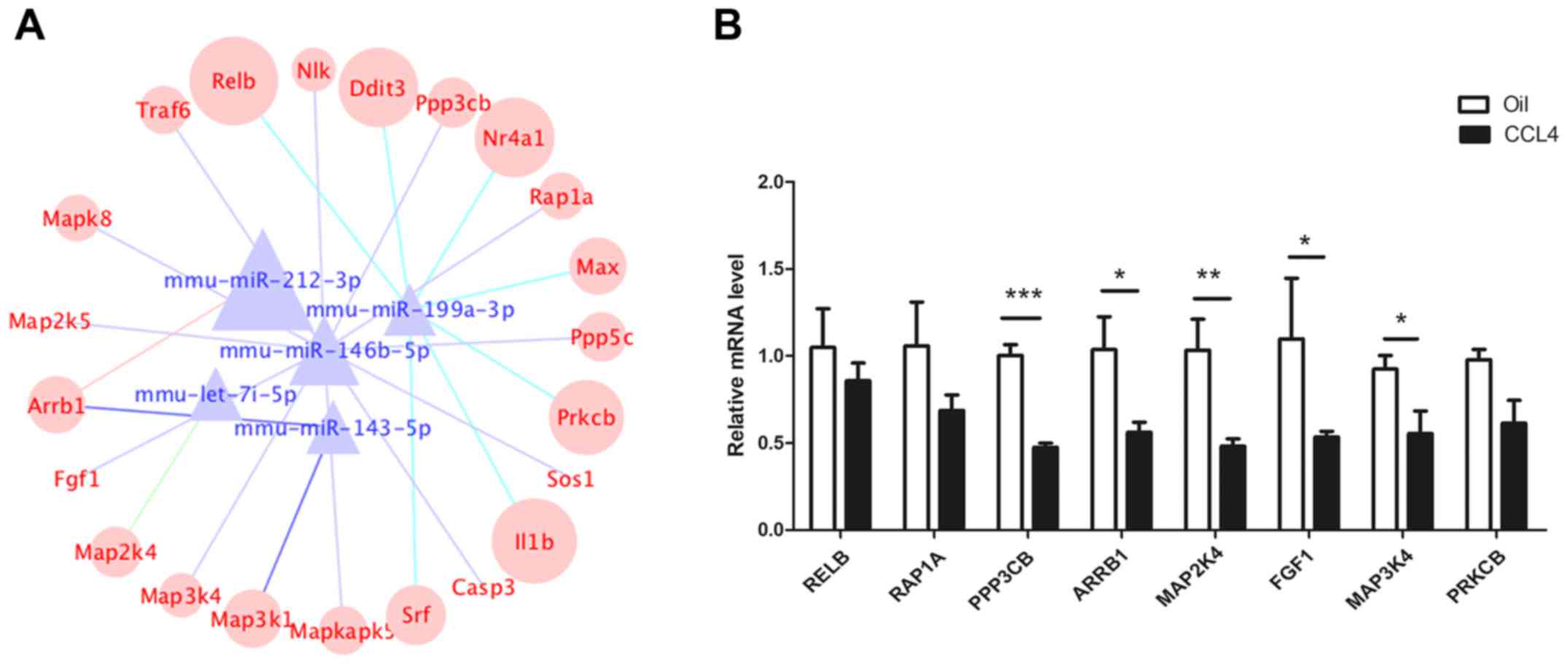 | Figure 3.Construction of a miRNA-mRNA
co-expression network. (A) The co-expression network was comprised
of five highly conserved miRNAs and 22 mRNAs. The different color
lines represent different miRNAs. (B) mRNA expression of RELB,
RAP1A, PPP3CB, ARRB1, MAP2K4, FGF1, MAP3K4 and PRKCB in
CCl4-induced fibrotic and normal liver tissues.
*P<0.05, **P<0.01, ***P<0.001. Relb, RELB proto-oncogene;
RAP1A, RAS-related protein 1a; PPP3CB, protein phosphatase 3
catalytic subunit β; ARRB1, arrestin β 1; MAP2/3K,
mitogen-activated protein kinase kinase; FGF1, fibroblast growth
factor 1; PRKCB, protein kinase C β. |
 | Table III.Putative target genes involved in the
microRNA-mRNA network. |
Table III.
Putative target genes involved in the
microRNA-mRNA network.
| Gene | Official full
name | Reference
sequence | Targeting
miRNA |
|---|
| ARRB1 | Arrestin β 1 | NM_178220.3 | mmu-miR-212-3p |
|
|
|
| mmu-miR-143-5p |
| NLK | Nemo like
kinase | NM_008702.3 |
mmu-miR-146b-5p |
| PPP3CB | Protein phosphatase
3 catalytic subunit β | NM_008914.3 |
mmu-miR-146b-5p |
| MAPK8 | Mitogen-activated
protein kinase 8 | NM_001310454.1 |
mmu-miR-146b-5p |
| MAP3K4 | Mitogen-activated
protein kinase kinase kinase 4 | NM_011948.2 |
mmu-miR-146b-5p |
| SOS1 | SOS Ras/Rac guanine
nucleotide exchange factor 1 | NM_009231.2 |
mmu-miR-146b-5p |
| FGF1 | Fibroblast growth
factor 1 | NM_001033789.2 |
mmu-miR-146b-5p |
| TRAF6 | TNF
receptor-associated factor 6 | NM_009424.3 |
mmu-miR-146b-5p |
| RAP1A | RAS-related protein
1a | NM_145541.5 |
mmu-miR-146b-5p |
| MAPKAPK5 | MAP
kinase-activated protein kinase 5 | NM_010765.2 |
mmu-miR-146b-5p |
| PPP5 | Protein phosphatase
5, catalytic subunit | NM_011155.2 |
mmu-miR-146b-5p |
| CASP3 | Caspase 3 | NM_001284409.1 |
mmu-miR-146b-5p |
| MAP2K | mitogen-activated
protein kinase kinase 5 | NM_011840.2 |
mmu-miR-146b-5p |
| MAP2K4 | Mitogen-activated
protein kinase kinase 4 | NM_001316368.1 | mmu-let-7i-5p |
| DDIT | DNA-damage
inducible transcript 3 | NM_001003913.2 |
mmu-miR-199a-3p |
| MAX | Max protein | NM_008558.2 |
mmu-miR-199a-3p |
| NR4A1 | Nuclear receptor
subfamily 4 | NM_010444.2 |
mmu-miR-199a-3p |
| SRF | Serum response
factor | NM_020493.2 |
mmu-miR-199a-3p |
| IL1B | Interleukin 1
β | NM_008361.4 |
mmu-miR-199a-3p |
| PRKCB | Protein kinase C
β | NM_008855.2 |
mmu-miR-199a-3p |
| RELB |
Reticuloendotheliosis viral (v-rel)
oncogene related B | NM_009046.2 |
mmu-miR-199a-3p |
| MAP3K1 | Mitogen-activated
protein kinase kinase kinase 1 | NM_011945.2 | mmu-miR-143-5p |
Target mRNAs in the network are
associated with HSC activation
HSCs have an essential role in liver fibrosis, in
which they undergo activation or transdifferentiation from
quiescent to myofibroblast-like cells as a result of injury
(25). LX-2 is a widely used human
HSC line that can be activated by TGF-β1. To further target mRNAs
in HSC activation, the expression of mRNAs in LX-2 cells was
examined after 24 h of TGF-β1 (10 ng/ml) stimulation. Following
stimulation, the expression of COL1α1 and α-SMA was significantly
upregulated (Fig. 4A), suggesting
that LX-2 cells were activated. In addition, miR-212-3p,
miR-146b-5p, let-7i-5p, miR-143-5p and miR-199a-3p expression was
significantly upregulated in LX-2 cells following TGF-β1 treatment
(Fig. 4B). Of the 22 mRNAs
assessed, the expression of RELB, RAP1A, PPP3CB, MAP2K4, ARRB1,
MAP3K4, FGF1 and PRKCB was also significantly decreased (Fig. 4C). The expression pattern of these
mRNAs was inverse to that of their regulatory miRNAs (miR-199a-3p
for RELB and PRKCB; miR-146b-5p for RAP1A, FGF1 and PPP3CB;
miR-143a-3p for MAP3K4; and let-7i-5p for MAP2K4) in the activated
HSCs. These results suggested that these miRNAs have an inhibitory
effect on their target genes and may serve important roles in liver
fibrosis.
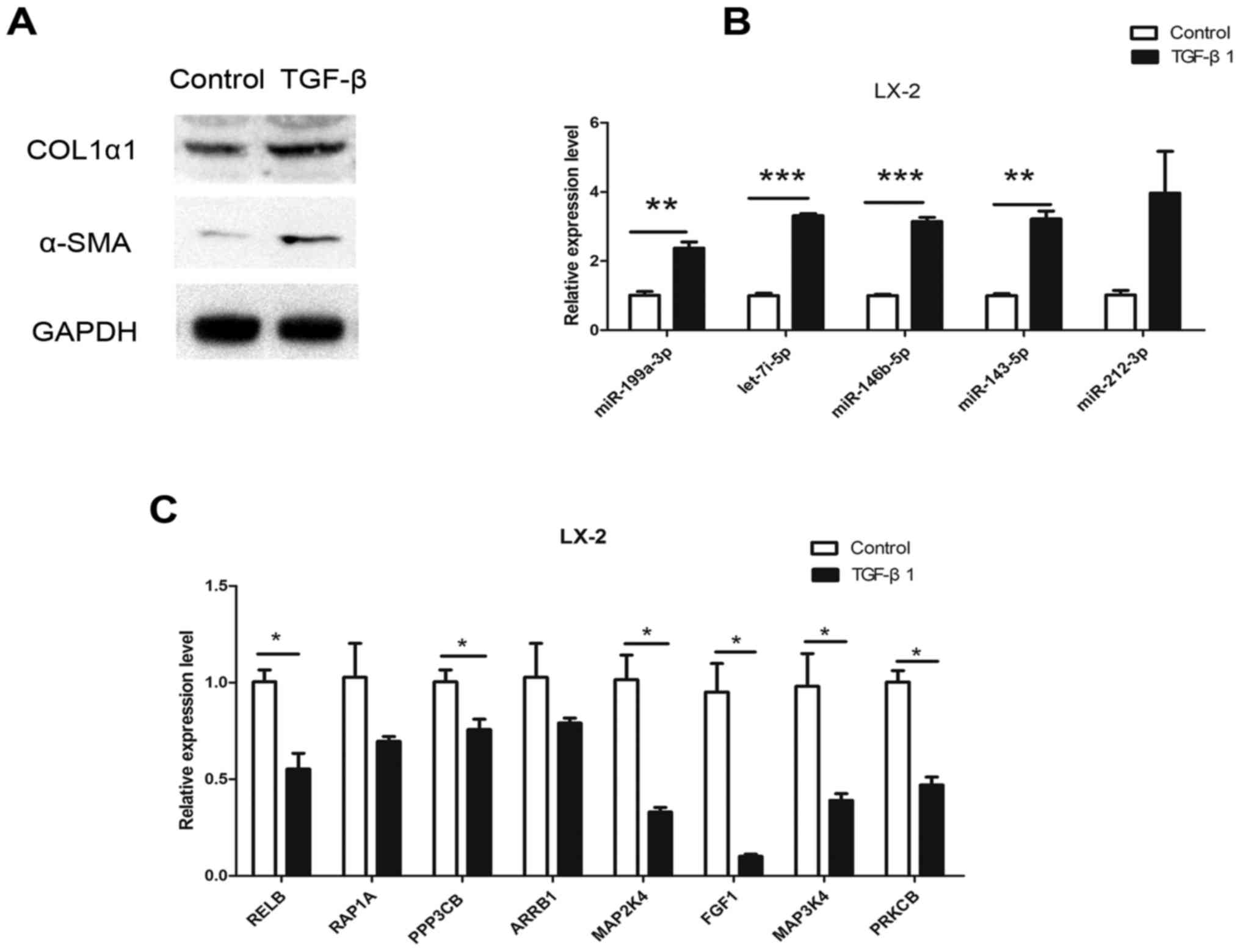 | Figure 4.Expression of dysregulated miRNAs and
target mRNAs in LX-2 cells following TGF-β stimulation for 24 h.
(A) α-SMA and COL1α1 protein expression in LX-2 cells following 10
ng/ml TGF-β stimulation. (B) RT-qPCR results for miR-212-3p,
miR-146b-5p, let-7i-5p, miR-143-5p and miR-199a-3p. (C) RT-qPCR
results for RELB, RAP1A, PPP3CB, ARRB1, MAP2K4, FGF1, MAP3K4 and
PRKCB. *P<0.05, **P<0.01, ***P<0.001. α-SMA, α-smooth
muscle actin; COL1α1, collagen α-1(I) chain; TGF-β, transforming
growth factor-β; miR, microRNA; RELB, RELB proto-oncogene; RAP1A,
RAS-related protein 1a; PPP3CB, protein phosphatase 3 catalytic
subunit β; ARRB1, arrestin β 1; MAP2/3K, mitogen-activated protein
kinase kinase; FGF1, fibroblast growth factor 1; PRKCB, protein
kinase C β. |
Discussion
An increasing number of studies have reported that
miRNAs serve a role in regulating the progression and metastasis of
various cancers (26–30). However, the role of miRNAs and
their specific mechanism of action in liver fibrogenesis remain to
be elucidated (31). In the
present study, miRNA sequencing was performed in
CCl4-induced liver fibrosis and integrated with miRNA
array data from clinical liver fibrosis samples to compare miRNA
expression profiles between fibrotic and normal liver tissues. It
was demonstrated that five miRNAs (miR-212-3p, miR-146b-5p,
let-7i-5p, miR-199a-3p and miR-143-5p) were significantly
upregulated in CCl4-treated livers and human liver
cirrhosis samples compared with normal liver tissues. Furthermore,
GO and KEGG pathway enrichment analysis was performed for miRNA
target genes to identify the signaling pathways involved in hepatic
fibrosis. GO enrichment analysis indicated that the majority of the
miRNA target genes were involved in cellular metabolic processes
closely associated with the onset and development of hepatic
fibrosis
The results of KEGG pathway enrichment analysis
indicated a total of 159 signaling pathways that may be essential
for the development of liver fibrosis. Several of these pathways
were already known to be involved in the progression of liver
fibrosis, such as the MAPK, focal adhesion, Wnt, p53, mTOR and
PI3K-Akt signaling pathways (22–24).
Various metabolic pathways were also identified in the present
study's analysis such as glycan metabolism, cysteine and methionine
metabolism and tyrosine metabolism pathway.
HSCs are an important cell type in the process of
liver fibrosis, as they can be activated by a large number of
cytokines secreted by damaged hepatocytes in chronic liver injury.
Activated HSCs promote the deposition of ECM, which in turn leads
to the development of liver fibrosis. (32). Therefore, several molecules
involved in the activation of HSCs may have potential as
therapeutic targets in liver fibrosis (33–35).
Previous studies have demonstrated that MAPK signaling plays a key
role in HSC activation and liver fibrogenesis: MAPK signaling
promotes the activation and proliferation of HSCs, increases
collagen mRNA stability and leads to the accumulation of ECM
(36,37). These findings suggest that
dysregulated miRNAs that affect MAPK signaling may affect HSC
activation in hepatic fibrosis. In the current study, MAPK-related
genes, including RELB, RAP1A, PPP3CB, ARRB1, MAP2K4, FGF1, MAP3K4
and PRKCB, were dysregulated in TGF-β-induced activation of HSCs,
suggesting that they may be essential for HSC activation.
A number of studies have reported that miRNAs are
involved in the regulation of liver fibrosis (38–40).
let-7i, miR-342-3p, miR-188-5p and miR-34a are upregulated in
CCl4-treated fibrotic livers compared with control
samples, while miR-202, miR-378b and miR-378d are downregulated
(41). It was previously reported
that a module containing five downregulated miRNAs (miR-130b-3p,
miR-148a-3p, miR-345-5p, miR-378a-3p and miR-422a) was associated
with HSC activation (42). The
miR-199 family is closely associated with the progression of liver
fibrosis in humans and mice (43).
In our previous study, it was demonstrated that the miR-212
promotes HSC activation by targeting Smad family member 7 (44). Similarly, the results of the
present study revealed that miR-342-3p, let-7i, miR-212 and miR-199
were significantly upregulated in cirrhotic liver tissues, whereas
miR-378a-3p was significantly downregulated. These miRNAs may
therefore be promising biomarkers for the early diagnosis of liver
cirrhosis.
In conclusion, the miRNA expression profile
generated in the present study revealed that the expression of
several miRNAs (miR-212-3p, miR-146b-5p, let-7i-5p, miR-143-5p,
miR-199a-3p) was associated with liver fibrosis. A miRNA-mRNA
network was identified, comprised of 5 miRNAs and 22 mRNAs that
were associated with liver fibrosis. This study may provide novel
insights and potential biomarkers for the early diagnosis and
treatment of liver fibrosis. Future studies should focus on the
biological functions of these miRNAs.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81420108005 and
81300327) and the Ministry of Science and Technology of China
(grant no. 2013CB945401).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JinL performed the cell experiments, analyzed
the data and wrote the paper; YM, JW and JieZ conceived the mouse
experimental design and performed the animal experiments. JieL and
JunZ designed the study and revised the paper.
Ethics approval and consent to
participate
The animal study was approved by the Animal Care and
Use Committee at the Fudan University. Animals were anesthetized
and sacrificed using acceptable methods.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Liver fibrosis-from bench to
bedside. J Hepatol. 1 Suppl 38:S38–S53. 2003. View Article : Google Scholar
|
|
3
|
Moreira RK: Hepatic stellate cells and
liver fibrosis. Arch Pathol Lab Med. 131:1728–1734. 2007.PubMed/NCBI
|
|
4
|
Ellis EL and Mann DA: Clinical evidence
for the regression of liver fibrosis. J Hepatol. 56:1171–1180.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W and Qin C: General hallmarks of
microRNAs in brain evolution and development. RNA Biol. 12:701–708.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XW, Heegaard NH and Orum H: MicroRNAs
in liver disease. Gastroenterology. 142:1431–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szabo G and Bala S: MicroRNAs in liver
disease. Nat Rev Gastroenterol Hepatol. 10:542–552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Utaijaratrasmi P, Vaeteewoottacharn K,
Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C, Khuntikeo N,
Ishimaru N, Sirivatanauksorn Y, Pongpaibul A, et al: The
microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated
fibroblasts promotes migration of cancer cells. Mol Cancer.
17:102018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howell LS, Ireland L, Park BK and Goldring
CE: MiR-122 and other microRNAs as potential circulating biomarkers
of drug-induced liver injury. Expert Rev Mol Diagn. 18:47–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He X, Sun Y, Lei N, Fan X, Zhang C, Wang
Y, Zheng K, Zhang D and Pan W: MicroRNA-351 promotes
schistosomiasis-induced hepatic fibrosis by targeting the vitamin D
receptor. Proc Natl Acad Sci USA. 115:180–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu J, Ma Y, Wang J, Zhu J, Liu J
and Zhang J: Integrated profiling of long non-coding RNAs and mRNAs
identifies novel regulators associated with liver fibrosis. Pathol
Res Pract. 214:1794–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aken BL, Achuthan P, Akanni W, Amode MR,
Bernsdorff F, Bhai J, Billis K, Carvalho-Silva D, Cummins C,
Clapham P, et al: Ensembl 2017. Nucleic Acids Res. 45:D635–d642.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vuppalanchi R, Liang T, Goswami CP,
Nalamasu R, Li L, Jones D, Wei R, Liu W, Sarasani V, Janga SC and
Chalasani N: Relationship between differential hepatic microRNA
expression and decreased hepatic cytochrome P450 3A activity in
cirrhosis. Plos One. 8:e744712013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diaz G, Melis M, Tice A, Kleiner DE,
Mishra L, Zamboni F and Farci P: Identification of microRNAs
specifically expressed in hepatitis C virus-associated
hepatocellular carcinoma. Int J Cancer. 133:816–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Britton RS and Bacon BR: Intracellular
signaling pathways in stellate cell activation. Alcohol Clin Exp
Res. 23:922–925. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao XK, Yu L, Cheng ML, Che P, Lu YY,
Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, et al: Focal adhesion kinase
regulates hepatic stellate cell activation and liver fibrosis. Sci
Rep. 7:40322017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Ma J, Chen L, Xie XL and Jiang H:
Inhibition of focal adhesion kinase on hepatic stellate-cell
adhesion and migration. Am J Med Sci. 353:41–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gantier MP, McCoy CE, Rusinova I, Saulep
D, Wang D, Xu D, Irving AT, Behlke MA, Hertzog PJ, Mackay F and
Williams BR: Analysis of microRNA turnover in mammalian cells
following Dicer1 ablation. Nucleic Acids Res. 39:5692–5703. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber JA, Baxter DH, Zhang S, Huang DY,
Huang KH, Lee MJ, Galas DJ and Wang K: The microRNA spectrum in 12
body fluids. Clin Chem. 56:1733–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HM, Nguyen DT and Lu LF: Progress and
challenge of microRNA research in immunity. Front Genet. 5:1782014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anthony PP, Ishak KG, Nayak NC, Poulsen
HE, Scheuer PJ and Sobin LH: The morphology of cirrhosis.
Recommendations on definition, nomenclature, and classification by
a working group sponsored by the World Health Organization. J Clin
Pathol. 31:395–414. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li D, He L, Guo H, Chen H and Shan H:
Targeting activated hepatic stellate cells (aHSCs) for liver
fibrosis imaging. EJNMMI Res. 5:712015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trautwein C, Friedman SL, Schuppan D and
Pinzani M: Hepatic fibrosis: Concept to treatment. J Hepatol. 62
Suppl 1:S15–S24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mallat A and Lotersztajn S: Reversion of
hepatic stellate cell to a quiescent phenotype: From myth to
reality? J Hepatol. 59:383–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hattori S, Dhar DK, Hara N, Tonomoto Y,
Onoda T, Ono T, Yamanoi A, Tachibana M, Tsuchiya M and Nagasue N:
FR-167653, a selective p38 MAPK inhibitor, exerts salutary effect
on liver cirrhosis through downregulation of Runx2. Lab Invest.
87:591–601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Furukawa F, Matsuzaki K, Mori S, Tahashi
Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki
Y, et al: p38 MAPK mediates fibrogenic signal through Smad3
phosphorylation in rat myofibroblasts. Hepatology. 38:879–889.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fernandez-Ramos D, Fernandez-Tussy P,
Lopitz-Otsoa F, Gutiérrez-de-Juan V, Navasa N, Barbier-Torres L,
Zubiete-Franco I, Simón J, Fernández AF, Arbelaiz A, et al:
MiR-873-5p acts as an epigenetic regulator in early stages of liver
fibrosis and cirrhosis. Cell Death Dis. 9:9582018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou L, Liu S, Han M, Ma Y, Feng S, Zhao
J, Lu H, Yuan X and Cheng J: miR-185 inhibits fibrogenic activation
of hepatic stellate cells and prevents liver fibrosis. Mol Ther
Nucleic Acids. 10:91–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao L, Xue D, Shen D, Ma W, Zhang J, Wang
X, Zhang W, Wu L, Pan K, Yang Y, et al: MicroRNA-942 mediates
hepatic stellate cell activation by regulating BAMBI expression in
human liver fibrosis. Arch Toxicol. 92:2935–2946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hyun J, Park J, Wang S, Kim J, Lee HH, Seo
YS and Jung Y: MicroRNA expression profiling in CCl(4)-induced
liver fibrosis of Mus musculus. Int J Mol Sci. 17:E9612016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen W, Zhao W, Yang A, Xu A, Wang H, Cong
M, Liu T, Wang P and You H: Integrated analysis of microRNA and
gene expression profiles reveals a functional regulatory module
associated with liver fibrosis. Gene. 636:87–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murakami Y, Toyoda H, Tanaka M, Kuroda M,
Harada Y, Matsuda F, Tajima A, Kosaka N, Ochiya T and Shimotohno K:
The progression of liver fibrosis is related with overexpression of
the miR-199 and 200 families. PloS One. 6:e160812011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu J, Zhang Z, Zhang Y, Li W, Zheng W, Yu
J, Wang B, Chen L, Zhuo Q, Chen L, et al: MicroRNA-212 activates
hepatic stellate cells and promotes liver fibrosis via targeting
SMAD7. Biochem Biophys Res Commun. 496:176–183. 2018. View Article : Google Scholar : PubMed/NCBI
|