Introduction
Although inflammation has not yet been proven to be
a direct cause of Parkinson's disease (PD), studies have identified
its participation in PD progression and it is an important factor
in the progressive deterioration of PD (1,2). The
main supporting evidence includes microglia and T lymphocyte
proliferation and activation in the substantia nigra-striatum
system in patients with PD and PD animal models (3). In addition, epidemiological surveys
have also demonstrated that the PD incidence in individuals that
have used non-steroidal anti-inflammatory drugs (NSAIDs) for a
period of time is significantly lower than that of the general
population (1,2).
The activation of microglia is the main component of
intracerebral inflammation. Microglia produce a variety of
inflammatory cytokines, including tumor necrosis factor-α (TNF-α),
interleukin-1α (IL-1α), IL-6, interferon-γ (IFN-γ) and nitric
oxide. Additionally, microglia generate cytokines that inhibit
inflammation, including IL-10 and IL-4. If microglial cells
maintain an activated status for a long period and release
inflammatory mediators continuously, they may damage the
surrounding neurons (4–7).
It is widely accepted that the infiltration of
lymphocytes into the brain is one of the key processes in cerebral
inflammation. More cluster of differentiation (CD)8+ and
CD4+ T cells were identified in the brain of patients
with PD compared with healthy individuals. These CD8+
and CD4+ T cells were in close contact with blood
vessels and near the dopaminergic neurons, suggesting that they
migrated from the blood vessels and interacted with the
dopaminergic neurons (8). This
phenomenon was not identified in the red nucleus, which is not an
area injured in PD (8).
In addition, substantial evidence has demonstrated
that systemic inflammatory processes are closely associated with
intracerebral inflammation and exacerbate neurodegeneration in PD
animal models and patients (9).
According to a recent study, under physiological conditions, the
cerebral spinal fluid is drained from the central nervous system
(CNS) into the lymphatic ducts in the meninges, which connect to
the deep cervical venous system (10). Changes in blood-brain barrier
function usually occur in the brain of patients with PD (11). Under abnormal inflammatory
conditions, peripheral inflammation is involved in CNS damage via
the following mechanisms: i) T and B lymphocytes, and dendritic
cells in the venous system respond to the environmental variations
and migrate from the central lymph ducts to attack the central
neurons (12); ii) peripheral
proinflammatory cytokines infiltrate into the brain and transform
microglia from the ‘resting'state into the ‘active’ state (7,9); and
iii) macrophages pathologically diffuse from the blood to the CNS,
transform into microglial cells and contribute to the development
of intracranial degeneration (13).
As dopaminergic neurons are more vulnerable than
other neurons or glial cells, one of the common consequences of
chronic inflammation in the brain is the degeneration of neurons in
the substantia nigra and, finally, PD. Therefore, in addition to
treatment with levodopa, manipulating chronic inflammation may be a
novel therapeutic direction for PD (14–16).
The role of mesenchymal stem cells (MSCs) in
immunoregulation was initially identified during hematopoietic stem
cell transplantation (HSCT). Intravenous transplantation of MSCs
reduces the incidence and attenuates the severity of
graft-versus-host disease significantly following HSCT (17,18).
In recent years, it has been determined that MSCs can inhibit T
cell proliferation, inhibit T cell-produced immune cytokines,
inhibit B cell-synthesized antibodies and suppress natural killer
cell function (19–22). In addition, MSCs express major
histocompatibility complex class I (MHC I) molecules but do not
express MHC II molecules; therefore, MSCs exhibit limited
immunogenicity and can be applied in allogeneic transplantation
without causing severe immunorejection. These properties
potentially enable MSCs to participate in the clinical treatment of
immune-associated diseases. Bone marrow-derived MSCs (BM-MSCs) can
avoid the majority of ethical issues associated with stem cell
transplantation. It has been demonstrated that BM-MSCs inhibit T
lymphocyte proliferation and the production of proinflammatory
cytokines (23).
These immunoregulatory results of MSCs have been
predominantly derived from normal young animal models or healthy
human subjects (23). However, the
peripheral blood mononuclear cells (PBMCs) of patients with PD are
not exactly the same as those of healthy people (24–26).
For example, the studies of PD models and post-mortem PD human
brains have suggested that at least two cellular functions are
damaged; proteostasis and mitochondrial respiration (27). It is uncertain whether the
lymphocytes or PBMCs of older people, particularly patients with
PD, still exhibit the ability to respond to BM-MSC
immunosuppression. The present study attempted to answer this
question. Mouse and human MSCs require stimulation by
proinflammatory cytokines to obtain immunosuppressive ability
(28). IFN-γ is important in
activating MSCs; however, the role of other cytokines remains to be
elucidated (23,29). The current study aimed to determine
which cytokines or cytokine combinations were more effective than
others in stimulating the immunosuppressive potential of
BM-MSCs.
Materials and methods
BM-MSC culture and identification
Spontaneously aborted male fetuses at 16, 17 and 25
weeks of pregnancy were provided by the Obstetrics and Gynecology
Department of Xuanwu Hospital (Beijing, China) during the year 2012
with informed consent of the patients in addition to the approval
of the Xuanwu Hospital Ethics Committee. The bone marrow was
flushed out and plated in T75 culture bottles at a density of
106 cells/ml in medium consisting of α-Dulbecco's
modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 20 ng/ml epidermal growth factor (R&D Systems, Inc.,
Minneapolis, MN, USA), 10% fetal bovine serum (FBS), 100 U/ml
penicillin and streptomycin and 2 mmol/l L-glutamine (all from
Thermo Fisher Scientific, Inc.). The cells were incubated at 37°C
in 5% CO2 for 4 days, then the floating cells were
discarded. When the adherent cells grew to 80% confluence, they
were digested using 0.25% trypsin-EDTA and were passaged at a ratio
of 1:3.
BM-MSCs of passage 3–5 were identified by surface
antigens using FACSCalibur flow cytometry (BD Biosciences, San
Jose, CA, USA), and by differentiation into adipose, bone and
cartilage using a human MSC differentiation kit (Lonza Group, Ltd.,
Basel, Switzerland). The differentiation of human MSC into adipose,
bone and cartilage, and the corresponding Oil Red O staining, von
Kossa staining and Safranin O staining, are all in accordance with
the protocols of the human MSC differentiation kit.
For flow cytometry analysis, the Human MSC Analysis
kit was used (BD Biosciences). In this kit, the MSC-positive
cocktail includes: Fluorescein isothiocyanate (FITC)-CD90,
phycoerythrin (PE)-CD44, peridinin chlorophyll protein complex
(PerCP)-CD105 and allophycocyanin (APC)-CD73. The PE channel is
used in combination with the negative MSC panel [PE-CD45, PE-CD34,
PE-CD11b, PE-CD19 and PE-major histocompatibility complex, class
II, DR (HLA-DR)]. The isotype controls for positive panel were
‘mIgG1, κ FITC (clone X40)’, ‘mIgG1, κ APC (clone X40)’, ‘mIgG1, κ
PerCP-Cy5.5’ and ‘mIgG2a, κ PE’. The isotype control for negative
panel was ‘PE Mouse IgG2b, κ Isotype’. Flow cytometric analysis was
performed on 10,000 events, and data were analyzed with CellQuest
(version 3.2.1; BD Biosciences) and FlowJo software (version 7.6.5;
FlowJo LLC, Ashland, OR, USA).
Recruitment of healthy individuals and
patients with PD
For recruitment, healthy people with no
abnormalities in their blood and major organs were identified by
the Medical Examination Department of Xuanwu Hospital and
recruited. The recruitment period was between January and December
2012. Age 26–30 was regarded as young, and age 56–60 was considered
middle-aged. Study subjects were divided into middle-aged PD,
middle-aged healthy and young healthy. Each group contained 15, all
of them male. Participant consent and ethical approval for the
collection and use of the blood samples were obtained from the
Ethics Committee of Xuanwu Hospital.
Patients with PD were diagnosed with primary PD in
accordance with the UK Parkinson's Disease Society Brain Bank
Clinical Diagnostic Criteria (30). In brief the inclusion/exclusion
criteria were: Hoehn-Yahr score of the ‘open period’ was 2.5–4;
diagnosed with PD for ≥3 years (30); treated with levodopa plus
peripheral decarboxylase enzyme inhibitor or other anti-PD drug
treatment and have reached a stable dose for ≥30 days; had
responses to the drugs, which meant that the Unified Parkinson's
Disease Rating Scale motor scoring of the ‘open period’ increased
≥33% compared with the ‘closed period’. Daily total time of the
‘closed period’ was 2–6 h (morning stiffness was not included);
magnetic resonance imaging examination revealed no significant
brain lesions including atrophy (30); Hamilton Rating Scale for Depression
score of patients was ≤12; without significant cognitive impairment
(31), Mini-Mental State Exam
scores of patients were >24 (for those whose education level was
middle school or higher) or >22 (primary school or higher)
(30). The clinical and
hematological parameters of the three study groups are presented in
Table I.
 | Table I.Clinical and hematological parameters
of the PD and the healthy groups. |
Table I.
Clinical and hematological parameters
of the PD and the healthy groups.
| Parameter | Middle-aged PD | Middle-aged
healthy | Young healthy |
|---|
| Number | 15 | 15 | 15 |
| Sex | Male | Male | Male |
| Age (mean ±
SD) | 57.67±1.40 | 58.13±1.41 | 27.8±1.42 |
| Age at symptoms
onset (mean ± SD) | 52.87±1.41 | NA | NA |
| Symptom duration
(mean ± SD) | 4.80±1.33 | NA | NA |
| Unilateral symptoms
(number) | 9 | NA | NA |
| Bilateral symptoms
(number) | 7 | NA | NA |
| Tremor
(number) | 3 | NA | NA |
|
Bradykinesia/rigidity (number) | 4 | NA | NA |
| Hoehn and Yahr
(mean ± SD) | 2.83±0.55 | NA | NA |
| UPDRS motor score
(mean ± SD) | 15.31±0.59 | NA | NA |
| MMSE score (mean ±
SD) | 25.07±1.84 | NA | NA |
| White blood cells
(109/l) | 5.63±0.43 | 5.39±0.58 | 5.45±0.62 |
| Red blood cells
(109/l) | 4.76±0.34 | 4.63±0.39 | 4.58±0.29 |
| Neutrophils
(%) | 60.05±3.78 | 59.88±5.37 | 57.52±5.76 |
| Lymphocytes
(%) | 32.03±4.78 | 30.97±4.71 | 28.61±4.78 |
| Monocytes (%) | 8.82±1.57 | 7.78±1.22 | 8.13±1.46 |
| Hemoglobin
(g/l) | 143.25±2.56 | 140.35±1.47 | 142.38±2.49 |
Culture of PBMCs
To isolate PBMCs, 700 µl hydroxyethyl starch (B.
Braun Medical, Inc., Bethlehem, PA, USA) was added to 2 ml
peripheral blood and allowed to incubate for 30 min with slight
agitation. Subsequently, the transparent supernatant (~1.5 ml) was
transferred to a new 15 ml centrifuge tube and mixed with 4.5 ml of
erythrocyte lysis buffer (eBioscience; Thermo Fisher Scientific,
Inc.). The mixture was centrifuged at 300 × g for 5 min. Following
removal of the supernatant, the cells were washed twice in 5 ml PBS
(Thermo Fisher Scientific, Inc.). The cells were counted and
resuspended in complete medium [RPMI-1640 (Thermo Fisher
Scientific, Inc.) + 10% FBS (Thermo Fisher Scientific, Inc.) + 200
pg/ml IL-2]. To induce PBMC proliferation in vitro, the
cells (1×106/ml) were activated by 10 µg/ml
phytohemagglutinin (PHA; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 24 h (19). The PHA
was then removed and the cells were cultured with complete medium
again.
Determination of cytokines effective
in inducing immunosuppression in BM-MSCs
Cytokines that are effective for the activation of
MSCs was determined by adding the recombinant proteins IFN-γ,
TNF-α, IL-1α, and IL-1β, one by one or in combination, to the
BM-MSC culture medium at a concentration of 20 ng/ml.
The cytokines were removed, and the BM-MSCs at
5×104/cm2 were incubated in 96-well plates
with PBMCs at a density of 104 cells/well. The PBMCs
were isolated from the healthy young, healthy middle-aged and the
patients with PD groups. These PBMCs were incubated in complete
medium (RPMI-1640+10% FBS+200 pg/ml IL-2) for 24–48 h and were
pretreated with PHA for 24 h.
Prior to co-culture, PHA was washed
off
The cytokine-treated BM-MSCs and PHA-treated PBMCs
were co-cultured in the 96-well plate for 4 days in the PBMC medium
(RPMI-1640+10% FBS+200 pg/ml IL-2).
Responsiveness of PBMCs to BM-MSC
immunosuppression
The experimental protocol is schematically
represented in Fig. 1A. The
responsiveness of PBMCs toward the inhibitory effects of BM-MSC was
compared among the three groups. The PBMCs from the three groups
were isolated and cultured for 24–48 h in a 37°C incubator with 5%
CO2 and saturated humidity. In the experiments in which
PHA-treated PBMCs were used, BM-MSCs were plated into 96-well
culture plates at the density of 1×104 cells per well.
Subsequently, 2×105 PBMCs were added to the BM-MSC
culture. The co-cultures of BM-MSCs and PBMCs with or without PHA
were incubated for 4 days. The relative proliferation rate, which
was used as the basis for comparing the extent of suppression among
the three groups, was the Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) absorbance ratios of
the co-cultures with or without PHA. The proliferation was assayed
by the CCK-8 method according to the manufacturer's protocol.
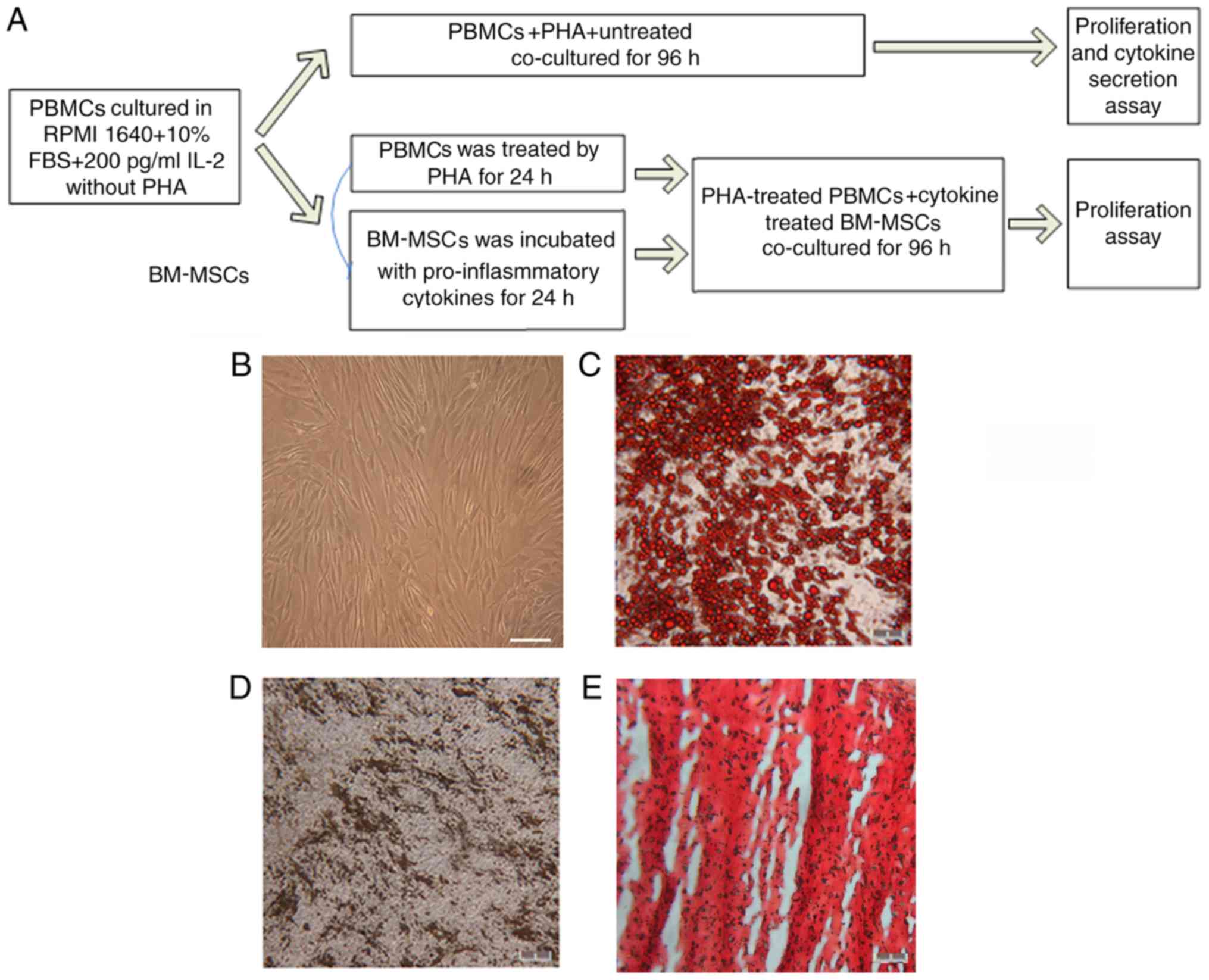 | Figure 1.Differentiation and cytometry
characterization of cultured human BM-MSCs. (A) Schematic paradigm
of the co-culture experiments. (B) Following 3–5 passages, the
BM-MSCs exhibited spindle-like shape. (C) Formation of lipid
vacuoles was detected by Oil Red O staining. (D) Osteogenesis was
tested by von Kossa staining of the mineralized matrix. (E)
Chondrogenesis was indicated by Safranin O staining. Scale bar, 100
µm. (F) The cells gated for analysis. With isotype control,
cytometry tests identified that BM-MSCs at passage 3–5 were
positive for (G) CD105 (H) CD44, (I) CD90 and (J) CD73, and
negative for (K) CD34, CD11b, CD19, CD45 and HLA-DR. (L) The
summary of quantified flow cytometry results as percentages. n=3.
PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin;
BM-MSCs, bone marrow-derived mesenchymal stem cells; CD, cluster of
differentiation. |
In the experiments in which IFN-γ, TNF-α, IL-1α, and
IL-1β were used to activate MSCs, the BM-MSCs were treated with
these cytokines one by one or in combinations for 24 h. The
cytokines were then washed off. The MSCs were digested and plated
into 96-well culture plates at a density of 1×104 cells
per well. The PBMCs were co-cultured with PHA for 24 h to be
induced to proliferate. Then, PHA was removed. The PHA-treated
PBMCs were seeded at a density of 2×105cells/well into
the 96-well plate containing 104 BM-MSCs and incubated
for 4 days.
To compare the immunosuppression among the three
groups, the relative proliferation rate was determined as the CCK-8
absorbance ratios of the co-culture with cytokine-treated BM-MSCs
and with untreated BM-MSCs.
Assessment of the BM-MSC suppression
of PBMC proliferation
The proliferation was assayed by the CCK-8 method
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according
to the manufacturer's protocol. To elucidate which type of cells in
this co-culture, BM-MSCs or PBMCs, decreased their proliferative
activity, the proliferation of BM-MSCs was inhibited by mitomycin C
(10 µg/ml) treatment for 2 h. Following the treatment, the
mitomycin C was removed. The MSCs were cultured for another 24 h
and then were co-cultured with PBMCs. The effects of mitomycin C on
BM-MSC proliferation were confirmed by Ki67 staining. In brief,
2×104 treated BM-MSCs were fixed by 4% paraformaldehyde
prior to the co-culture with PBMCs. The fixed cells were incubated
in 0.3% Triton X-100 PBS for 1 h at room temperature, blocked with
2% donkey serum (Jackson Immuno-Research Laboratories, Inc., West
Grove, PA, USA) at room temperature for 45 min, and incubated
overnight with monoclonal antibodies for Ki67 (cat. no. sc-23900,
1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C. The
next day, the cells were incubated with FITC-conjugated secondary
antibodies (cat. no. 715-095-151,1:300; Jackson Immuno-Research
Laboratories, Inc., West Grove, PA, USA) for 2 h at room
temperature, followed by DAPI nucleus staining at 1 µg/ml at room
temperature for 20 min, and observed under an inverted fluorescence
microscope (magnification, ×200; Leica DMI 4000B; Leica
Microsystems GmbH, Wetzlar, Germany).
Detection of cytokines and chemokines
in the co-culture
The suppression of PBMCs by BM-MSCs includes the
suppression of cytokine production. A multiplex human cytokine
assay was performed, which included IL-2, IL-4, IL-6, IL-10, IFN-γ
and TNF-α. The BM-MSCs were seeded at 1×104 density per
well in a 96-well culture plate. The concentration of the cytokines
in the BM-MCS culture medium was used as a control. Since the
BM-MSCs and PBMCs secrete certain cytokines physiologically, the
BM-MSCs cultured in the PBMC medium with PHA and the PBMCs cultured
in the presence or absence of PHA, were measured for the secretion
of cytokines. For the co-cultures, 2×105 PBMCs were
cultured together with BM-MSCs for 96 h in the presence of PHA. The
co-culture supernatant of the PBMCs was assayed for six different
cytokines and chemokines with a Th1/Th2/Th17 bead array kit (cat.
no. BD 560484, BD Biosciences, San Jose, CA, USA) using cytometric
bead array (CBA) technology.
Statistical analysis
The data were analyzed with SPSS version 10.0 (SPSS,
Inc., Chicago, IL, USA). Quantitative data are expressed as the
means ± standard deviation. Data were analyzed by one-way analysis
of variance and least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Culture and identification of human
BM-MSCs
The primary human BM-MSCs were cultured for 3–5 days
and the cells were spindle-shaped and polygonal. Following the
removal of non-adherent cells, the adherent cells further grew into
a relatively uniform morphology of spindles. At days 10–14, the
cultured cells reached 80% confluence and were digested by 0.25%
trypsin-EDTA and passaged at a ratio of 1:3. Then, the cells were
passaged every 3–5 days (Fig. 1B).
To identify the BM-MSCs, differentiation and cytometry tests were
applied. The BM-MSCs possessed the ability to differentiate into
adipocytes, osteocytes and chondrocytes (Fig. 1C-E). The cytometry examination
identified that the positive percentages of BM-MSCs at passages 3–5
for CD105, CD44, CD90 and CD73 were 99.98±0.02, 99.79±0.26,
99.94±0.06 and 99.99±0.01%, respectively, and negative for CD34,
CD19, CD45 or CD11b. Furthermore, BM-MSCs did not express the MHC
II molecule HLA-DR (Fig. 1F-K).
The quantified flow cytometry results are summarized in Fig. 1L.
BM-MSCs suppress mitogen-induced PBMC
proliferation
Initially, the co-culture system was used to examine
whether PBMCs were required to be induced by mitogen to obtain the
ability to stimulate BM-MSCs to exert their inhibitory effects. The
PBMCs from the healthy young people were incubated in a 96-well
plate at a density of 2×105 cells/well with
104 cells/well BM-MSCs for 4 days (with or without PHA).
Then, the effects of BM-MSCs on the proliferation of PBMCs were
tested by the CCK-8 method. The morphology of PBMCs from the young
healthy group, cultured alone or with BM-MSCs, is shown in Fig. 2A-C.
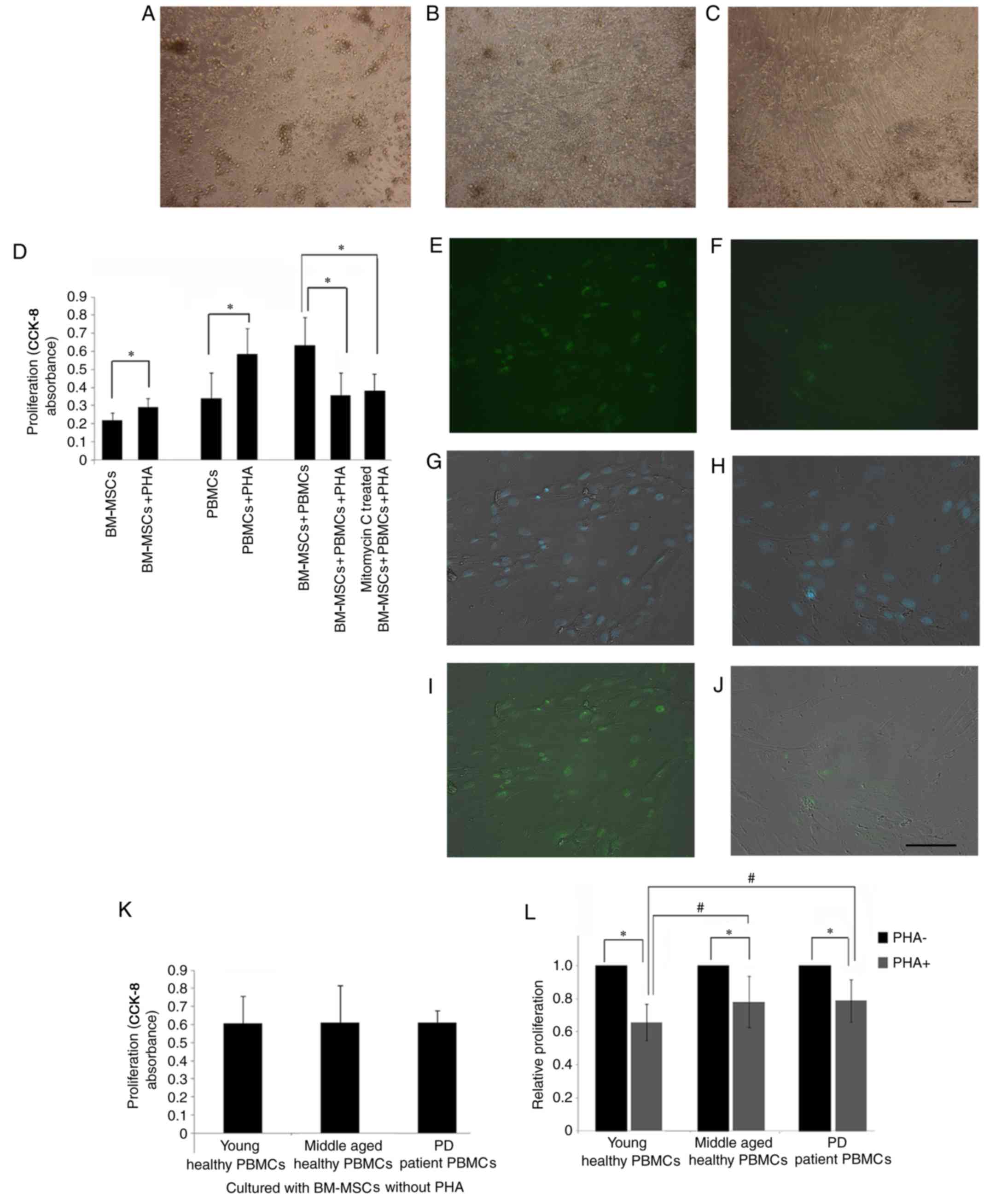 | Figure 2.Proliferation suppression of PBMCs by
PHA-treated BM-MSCs. (A) PBMCs isolated from young healthy
individuals. (B) Co-culture of BM-MSCs and PBMCs without PHA at day
3. (C) Co-culture of PHA-activated PBMCs and BM-MSCs at day 3. (D)
In the presence of PHA, the proliferation of PBMCs and BM-MSCs
increased significantly (*P<0.05). When incubated with BM-MSCs
and mitomycin C-treated BM-MSCs, PHA-activated PBMC proliferation
decreased significantly. Ki67 staining of the MSCs prior to (E, G
and I) and following mitomycin C treatment (F, H and J) were
demonstrated. (E and F) Ki67 staining. (G and H) Merged image of
DAPI nucleus staining and phase contrast. (I and J) Merged image of
Ki67 and phase contrast. (K) Without PHA, the PBMC proliferation in
the young healthy, the middle-aged healthy, and the middle-aged PD
individuals was not significantly different. (L) Following
treatment with PHA, the PBMC proliferation in the young healthy,
the middle-aged healthy and the middle-aged PD groups decreased
significantly (*P<0.05). The proliferation of PBMCs suppressed
by BM-MSCs of the young healthy subjects was significantly lower
than that of the middle-aged healthy and the PD individuals
(#P<0.05). Scale bar, 50 µm, n=6. PBMCs, peripheral
blood mononuclear cells; PHA, phytohemagglutinin; BM-MSCs, bone
marrow-derived mesenchymal stem cells; PD, Parkinson's disease. |
The direct effects of PHA on the proliferation of
PBMCs and BM-MSCs were determined. In the presence of PHA, the
proliferation of PBMCs and BM-MSCs increased significantly
(Fig. 2D). The effect of BM-MSCs
on the proliferation of PHA-activated PBMCs was then examined.
BM-MSCs were co-cultured with fresh PBMCs in the presence of PHA
and it was identified that, when PBMCs were activated by PHA, their
proliferation was markedly suppressed by BM-MSCs (Fig. 2D). These results indicated that
BM-MSCs exert an inhibitory effect when co-cultured with activated
PBMCs.
Following the mitomycin C treatment, the percentage
of Ki67 positive MSCs was 7.56±4.35%, significantly lower than
prior to the treatment (64.8±7.57%; Fig. 2E-I). These mitomycin C treated
BM-MSCs were used in co-culture with PBMC and identified that even
these MSCs still decreased the proliferation of PBMC to the same
level as that of untreated BM-MSCs (Fig. 2D).
When the PBMCs were not stimulated by PHA, the
influence of BM-MSCs on the proliferation of PBMCs was not
suppressive. In fact, the measured proliferation value of
co-cultured BM-MSCs and PBMCs increased compared with that of PBMCs
cultured with PHA, but did not reach statistical significance
(Fig. 2D). These results confirmed
that the immunosuppressive ability of BM-MSCs is not innate but is
induced by the PHA-treated PBMCs.
Responsiveness of PHA-treated PBMCs
from the middle-aged healthy and PD groups toward BM-MSC
suppression of proliferation
The PBMCs from the young healthy, the middle-aged
healthy and the PD patient groups were seeded in a 96-well plate at
a density of 2×105 cells/well and were co-cultured with
104 cells/well BM-MSCs (with or without PHA-treatment).
Without PHA treatment, no significant change was observed in the
CCK-8 absorbance values among the three groups (Fig. 2K), demonstrating that there was no
difference in the proliferation of PBMCs from the three groups
co-cultured with BM-MSCs.
When PHA was added, compared with that of the
PHA-untreated PBMCs, the proliferation of PBMCs co-cultured with
BM-MSC was significantly decreased (Fig. 2L). This result was observed in the
young healthy group, healthy middle-aged group and the PD group
(P<0.05). The proliferation of PHA-treated PBMCs in the young
group was lower compared with the middle-aged group and PD group
(P<0.05). The PBMC proliferation of the middle-aged and PD group
demonstrated no significant difference following the suppression by
BM-MSCs (Fig. 2L).
Effect of cytokines on the activation
of BM-MSCs
To detect which proinflammatory cytokines are
required for BM-MSCs to exert inhibitory effects, the relative
proliferation rates of PBMCs from young healthy males were compared
following co-culture with BM-MSCs pre-treated with several
cytokines (20 ng/ml).
All the proinflammatory cytokines tested, IL-1α,
IL-1β, TNF-α and IFN-γ-treated BM-MSCs, demonstrated a suppressive
effect on the proliferation of PBMCs, but none of them reached
statistical significance, and no significant difference was
observed among the inhibitory abilities of these proinflammatory
cytokines (Fig. 3A).
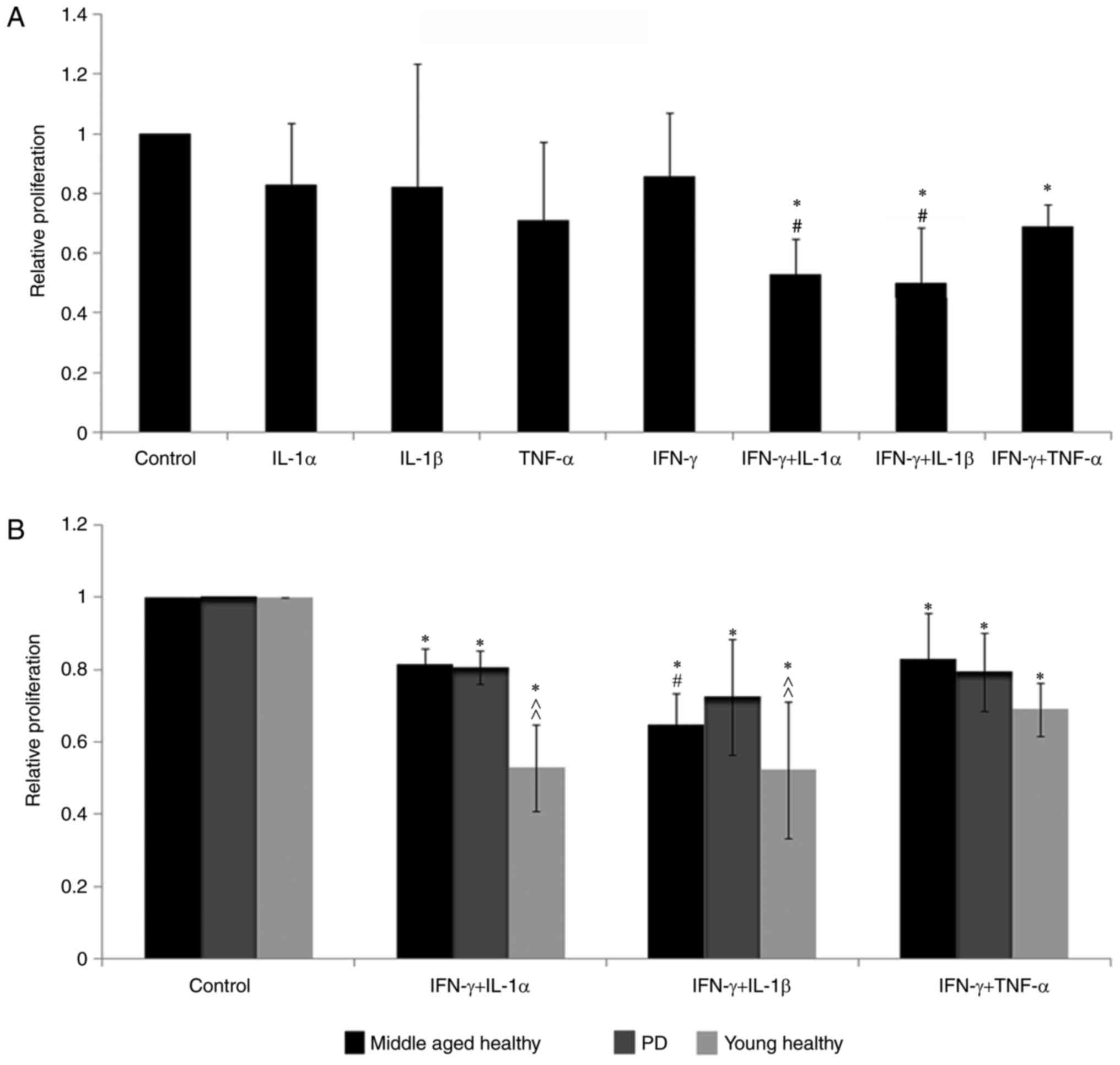 | Figure 3.Proinflammatory cytokines are
required by BM-MSCs to suppress the proliferation of PBMCs. (A)
IL-1α, IL-1β, TNF-α or IFN-γ alone did not induce BM-MSCs to become
immunosuppressive. Compared with the cytokine-untreated cells,
BM-MSCs treated with the combinations of IFN-γ + IL-1α, IFN-γ +
IL-1β and IFN-γ + TNF-α decreased the PBMC proliferation in young
individuals significantly (*P<0.05). IFN-γ + IL-1α and IFN-γ +
IL-1β were more powerful than IFN-γ + TNF-α in inducing BM-MSCs to
suppress the PBMC proliferation in young individuals to lower
levels (#P<0.05). (B) Proliferation of PBMCs treated
with all three combinations in the young healthy, the middle-aged
healthy and the PD groups decreased significantly compared with
those in the control group (untreated BM-MSCs with PBMCs;
*P<0.05). In the middle-aged healthy group, IFN-γ + IL-1β was
more effective than IFN-γ + TNF-α in inducing BM-MSCs to be
immunosuppressive (#P<0.05), while IFN-γ + IL-1α and
IFN-γ + TNF-α did not show any significant difference. Following
the treatment with IFN-γ + IL-1β or IFN-γ + IL-1α, the PBMC
proliferation in young healthy subjects was significantly lower
than that in the middle-aged healthy and the PD individuals
(^^P<0.01). n=6. BM-MSCs, bone marrow-derived mesenchymal stem
cells; PBMCs, peripheral blood mononuclear cells; IL, interleukin;
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ, PD,
Parkinson's disease. |
The combination of proinflammatory cytokines was
more effective than any single one in inhibiting PBMC
proliferation. BM-MSCs pretreated with IFN-γ combined with IL-1α,
IL-1β or TNF-α significantly inhibited the proliferation of PBMCs
(P<0.05). The combinations of IFN-γ + IL-1α and IFN-γ +
IL-1β-treated BM-MSCs had greater inhibitory effects on PBMCs than
the combination of IFN-γ + TNF-α-treated BM-MSCs (P<0.05), and
there was no significant difference between IFN-γ + IL-1α and IFN-γ
+ IL-1β-treated BM-MSCs (Fig.
3A).
Responsiveness of PBMCs from the
middle-aged healthy and PD groups toward cytokine-pretreated BM-MSC
suppression of proliferation
The responses of the PBMCs isolated from middle-aged
healthy individuals and patients with PD to BM-MSCs that were
activated by proinhibitory cytokine combinations were then
tested.
Similar to the suppression experiment in the young
healthy group, the combinations of IFN-γ + IL-1α, IFN-γ + IL-1β and
IFN-γ + TNF-α-treated BM-MSCs significantly decreased PBMC
proliferation (P<0.05) in middle-aged individuals and patients
with PD compared with the co-culture of PBMCs and BM-MSCs without
adding proinhibitory cytokines (control group in Fig. 3B).
The next question was which response was most
prominent among the young healthy, middle-aged healthy and PD
patient groups. The relative proliferation rate of PBMCs inhibited
by IFN-γ + IL-1α- or IFN-γ + IL-1β-treated BM-MSCs in the healthy
young people was significantly lower compared with middle-aged
healthy people and PD patients (P<0.01; Fig. 3B). No significant difference was
observed in the decrease in PBMC proliferation between the healthy
middle-aged people and the patients with PD group.
The final question was which proinflammatory
cytokine combination was most effective in inducing BM-MSCs to be
inhibitory in the healthy middle-aged people and the patients with
PD. Among the combinations of the three inflammatory cytokines,
similar to the results in the healthy young people, BM-MSCs treated
with IFN-γ + IL-1β had the most significant inhibitory effect on
PBMCs compared with those treated with IFN-γ + TNF-α in the healthy
middle-aged people (P<0.05), while no significant difference was
observed between the effect of BM-MSCs treated with IFN-γ + IL-1α
and those treated with IFN-γ + TNF-α (Fig. 3B).
BM-MSCs reduce the PBMC production of
proinflammatory cytokines in young healthy males
To identify the immunosuppressive effects mediated
by BM-MSCs on cytokine production in the young, middle-age and PD
groups, the generation of cytokines during the incubation of the
BM-MSCs and PHA-stimulated PBMCs (Fig.
4) was investigated.
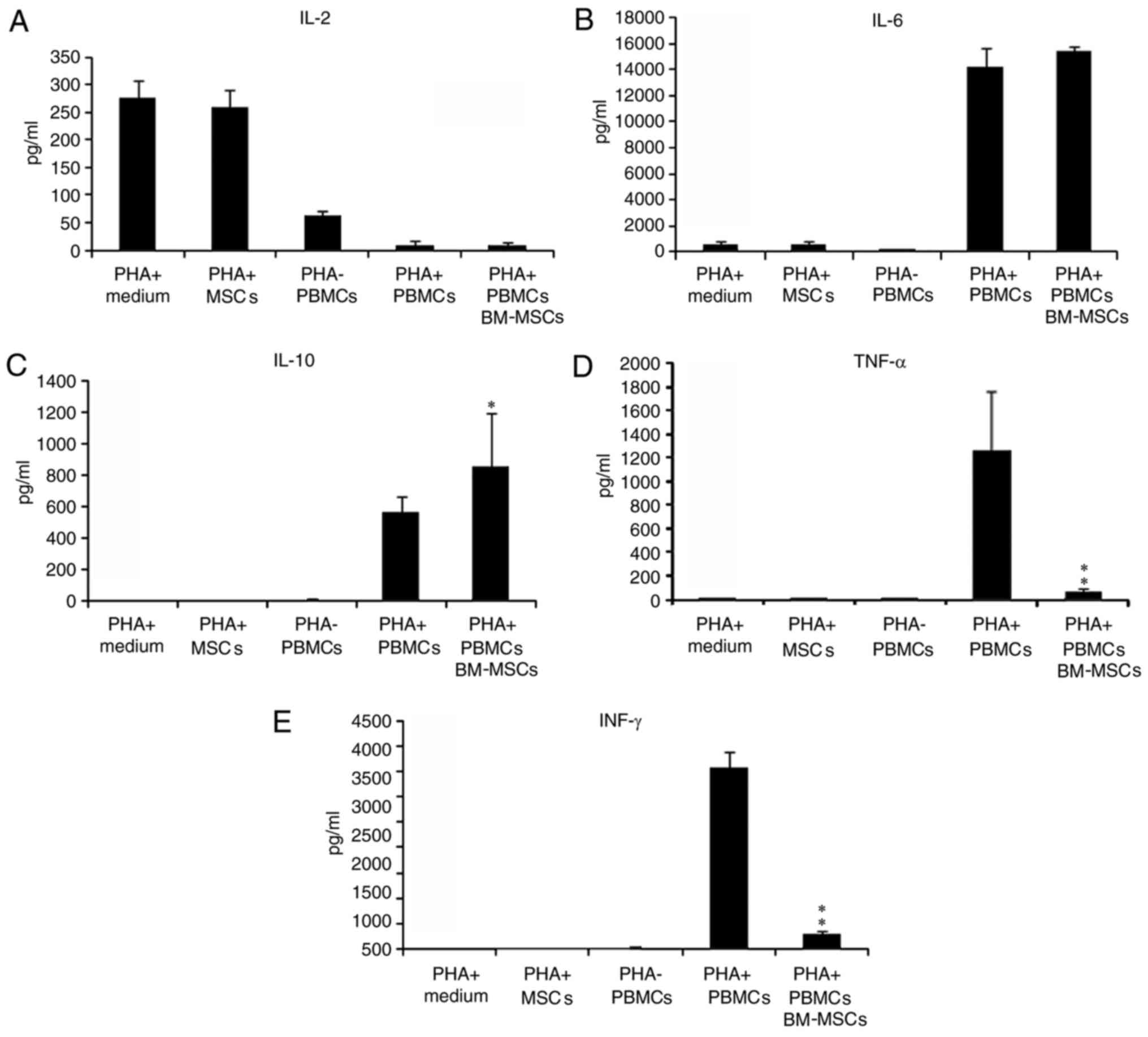 | Figure 4.Influence of BM-MSCs on cytokine
production in the co-culture of BM-MSCs and PBMCs. (A) BM-MSCs did
not produce IL-2 even with PHA. The co-culture with PBMCs consumed
IL-2, and the activated PBMCs lowered IL-2 levels to 9.8±6.6 pg/ml.
(B) BM-MSCs and PBMCs are capable of secreting IL-6. With PHA, the
IL-6 concentration increased significantly. The synergistic effect
of BM-MSCs and PBMCs in producing IL-6 was not marked. (C) With
PHA, the IL-10 concentration increased significantly to
562.34±186.78 pg/ml and was improved to 852.64±214.32 by co-culture
with BM-MSCs (*P<0.05 vs. PHA-activated PBMCs alone). When PBMCs
were activated by PHA, the secretion of (D) TNF-α and (E) IFN-γ was
significantly inhibited by BM-MSCs (*P<0.05 and **P<0.01 vs.
PHA-activated PBMCs alone;). BM-MSCs, bone marrow-derived
mesenchymal stem cells; PBMCs, peripheral blood mononuclear cells;
IL, interleukin; PHA, phytohemagglutinin; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ. |
The concentration of the cytokines in the culture
medium was measured as a control. IL-2, IL-6, IL-10, IFN-γ, and
TNF-α were analyzed (Fig. 4).
Since BM-MSCs and PBMCs physiologically secrete certain cytokines,
the BM-MSCs cultured in the PBMC medium with PHA and the PBMCs
cultured in the presence or absence of PHA were measured for the
secretion of cytokines.
IL-2 is an essential factor for T cell proliferation
in vitro and was added to the medium at a concentration of
200 pg/ml. The IL-2 concentration of the medium measured by the CBA
method was 227.51±30.1 pg/ml. The PHA-treated BM-MSCs alone did not
change the IL-2 concentration, which indicated that BM-MSCs did not
produce IL-2 even with PHA stimulation. The culture of PBMCs
consumed IL-2, and the activated PBMCs lowered the IL-2
concentration to 9.8±6.6 pg/ml. When the PBMCs were co-cultured
with the BM-MSCs, the IL-2 concentration did not change
significantly. This finding suggested that the BM-MSCs do not
affect the production of IL-2 by PBMCs (Fig. 4A).
Regarding IL-6, BM-MSC together with PHA produced
624.87±138.06 pg/ml. PBMCs at the resting state secreted
322.82±100.27 pg/ml IL-6; when activated by PHA, PBMCs produced a
significantly higher level of IL-6, which was 14,242.73±1,452.48
pg/ml. When cultured together, the IL-6 concentration did not
increase significantly. This result suggested that the BM-MSCs and
PBMCs are capable of secreting IL-6 and that the synergistic effect
of BM-MSCs and PBMCs in producing IL-6 was not prominent (Fig. 4B).
The concentration of IL-10 in the BM-MSCs cultured
with PHA demonstrated no difference compared with that in the
medium with added PHA (without MSCs). This result suggested the
BM-MSCs did not produce, or produced a very low level, of IL-10.
PBMCs only produced 9.16±2.47 pg/ml of IL-10 without PHA. With PHA,
however, the IL-10 concentration increased significantly to
562.34±186.78 pg/ml. The concentration of IL-10 was increased to
852.64±214.33 by co-culture with BM-MSCs. These results were
evidence that BM-MSCs stimulate the secretion of IL-10 (Fig. 4C).
As demonstrated in Fig.
4D and E, IFN-γ and TNF-α were not present in the PBMC medium
and not secreted by PHA-treated BM-MSCs. TNF-α was not produced by
PHA-treated PBMCs (Fig. 4D),
whereas the concentration of IFN-γ was as low as 31.91±7.8 pg/ml in
the medium of PHA-treated PBMCs (Fig.
4E). When PBMCs were activated by PHA, the secretion of TNF-α
and IFN-γ was significantly increased to 1,266.9±408.3 and
3,584.1±287.5 pg/ml, respectively. When co-cultured with BM-MSCs,
the concentrations of TNF-α and IFN-γ decreased to 110.7±14.4 and
368.9±105.7 pg/ml, respectively. These results demonstrated that
BM-MSCs downregulated the production of inflammatory cytokines by
PHA-activated PBMCs.
Responsiveness of PBMCs from the
middle-aged healthy and PD groups toward BM-MSC suppression of
cytokine production
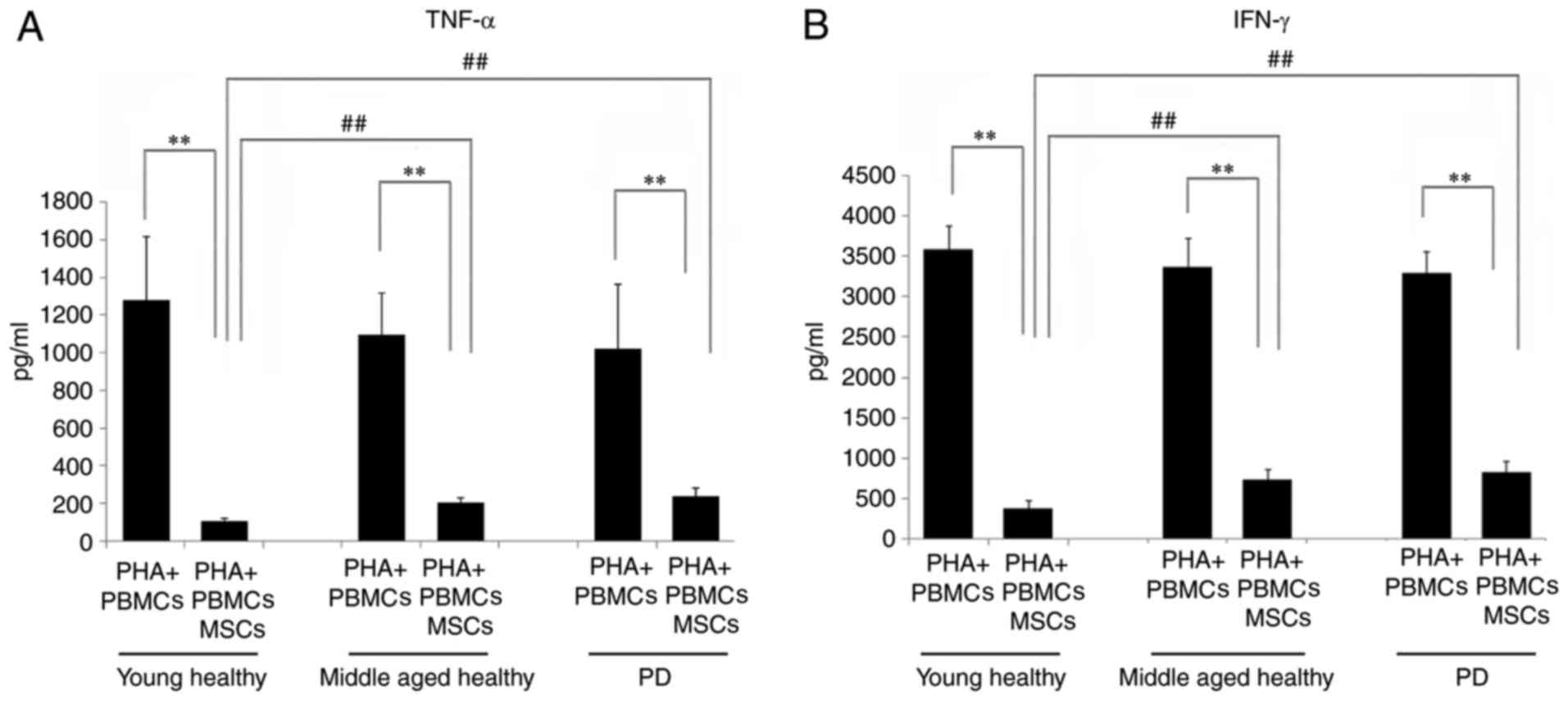
TNF-α and IFN-γ were selected to examine the
responsiveness of the PBMCs towards the BM-MSC suppression of
cytokine production. The levels of TNF-α secreted by PBMCs in the
young healthy group (3,584.1±287.5 pg/ml), the middle-aged healthy
group (3,361.2±363.3 pg/ml) and the PD patient group (3,291.2±270.2
pg/ml) were of the same level and were all decreased markedly by
BM-MSCs (P<0.01). The TNF-α production in the co-cultured PBMCs
of the young healthy group (368.9±105.7 pg/ml) was significantly
lower compared with the middle-aged healthy (733.8±126.9 pg/ml) and
PD patient groups (812.4±145.5 pg/ml; P<0.01; Fig. 5A).
Similar phenomena were observed for IFN-γ. With the
stimulation by PHA, no significant difference was observed in the
IFN-γ production among the PBMCs isolated from the young healthy
(1,266.9±408.3 pg/ml), middle-aged healthy (1,096.8±225.0 pg/ml)
and patients with PD groups (1,022.5±344.7 pg/ml). When BM-MSCs
were added into the co-culture system, the IFN-γ secretion was
markedly decreased in all three groups compared with that of the
PBMC culture (P<0.01). Following BM-MSC suppression, the IFN-γ
production in the young healthy group (110.7±14.4 pg/ml) was
significantly lower than the middle-aged healthy (208.2±23.1 pg/ml)
and patients with PD groups (243.5±43.2 pg/ml; P<0.01; Fig. 5B).
Discussion
The role of inflammation in the pathogenesis and
progression of PD has gradually been recognized (14–16).
The activation of glial cells, particularly microglia, is one of
the typical signs of intracerebral inflammation (14–16).
Activated microglia can secrete a large amount of inflammatory
cytokines, which can trigger a sustained inflammatory reaction
(14–16). McGeer et al (3) identified that an inflammatory
reaction exists in the substantia nigra of patients with PD with
localized microglial proliferation. Inhibiting microglia-mediated
inflammatory reaction has become a new research direction in PD
treatment (14–16).
Recent studies have demonstrated that untreated
mouse MSCs derived from bone marrow did not possess the ability to
inhibit the immune responses, but that once they were stimulated by
proinflammatory cytokines, MSCs obtained a strong immunosuppressive
ability. These cytokines include TNF-α, IL-1α, IL-1β and IFN-γ. It
is now clear that human BM-MSCs also require similar stimulation by
inflammatory factors to serve an immunosuppressive role (28,32).
The serum levels of IL-2, IL-4, IL-6, IL-10 and
TNF-α are significantly increased in patients with PD (9). It is known that peripheral
inflammation is closely associated with neurodegenerative processes
in the brain by triggering strong responses of microglia (9). Therefore, it is possible to delay the
progression of PD by inhibiting the systemic inflammatory response.
In recent years, numerous experiments have been performed in this
research field. NSAIDs have been commonly used in clinical trials.
Previous studies have confirmed that NSAIDs can inhibit and keep
the inflammatory response in check to a certain extent and thereby
can protect the dopaminergic neurons, but other studies have shown
that the protective effect of NSAIDs is mild and cannot reduce the
risk of Parkinson's disease. Furthermore, the long-term clinical
application of NSAIDs has been proven to exhibit certain side
effects (33,34).
MSCs exhibit immunosuppressive capacity and possess
very limited immunogenicity. Using MSCs to regulate inflammation
and the immune response will potentially become routine in clinical
treatment (33,34). According to the results of mouse
experiments, proinflammatory cytokines including IL-1α, IL-1β,
TNF-α, and IFN-γ, whether released by mitogen-treated PBMCs in
vitro or added to the MSC culture, activate MSCs, and then MSCs
significantly inhibit PBMC proliferation and production of
proinflammatory cytokines (28).
As the immunosuppressive capacity of MSCs depends on
the preactivation of the proinflammatory cytokines, when the
inflammation in the body gradually subsides, the immunosuppressive
capacity of MSCs gradually diminishes. When the inflammatory
reaction recurs, the MSCs will be activated again and then possess
an inhibitory effect. Therefore, the immune regulation by MSCs is a
dynamic process, and it does not inhibit the regular immune
function of patients. Compared with the effects of
immunosuppressive drugs, the merits of MSCs include fewer or no
side effects, long duration of action and regulation by
feedback.
The main purpose of the present study was to verify
the suppressive effects of human BM-MSCs on allogeneic PBMCs from
patients with PD. In the experiments, the surface markers of the
BM-MSCs were identical to those that are well accepted (21–23).
The BM-MSCs possessed the ability to differentiate into adipose
tissue, bone and cartilage. All these results confirmed that the
isolated cells are indeed MSCs. The BM-MSCs isolated from young and
middle-aged healthy participants, and patients with PD markedly
inhibited PBMC proliferation and the proinflammatory cytokine
production of PBMCs in vitro.
The proliferation and proinflammatory cytokine
production of PBMCs from healthy young people were more markedly
suppressed by BM-MSCs than the PBMCs from middle-aged people and
the patients with PD, and no significant difference was observed in
the suppression effect between the PBMCs from patients with PD and
the age-matched middle-aged healthy participants. These results
suggested that the weaker responses of PBMCs in the PD group toward
the suppression of BM-MSCs are not necessarily due to PD but are
more likely to be age-related.
In the present study, the PBMC proliferation and the
extent of MSC suppression were not as marked as previously reported
(28). This may be due to a
difference in experimental design. Firstly, the cells that are
suppressed by MSCs in PBMCs are not lymphocytes alone. Lymphocytes
comprise ~20–50% of the PBMCs. That the present study used PBMCs
marks a difference between this study and those studies that used
CD4- or CD8-positive lymphocytes. Secondly, the PHA-treated PBMCs
and BM-MSCs were only co-cultured for 4 days; more time is required
for the cells to reach the exponential growth phase. Thirdly, the
suppressive capacity of MSCs was usually examined by adding the
cells to lymphocytes at a ratio of 1:5, 1:10 and 1:20. Fewer MSCs
mean a lower degree of suppression. In the present study, the 1:20
ratio was used.
In addition, the current study identified that the
ability of BM-MSCs to inhibit PBMCs requires proinflammatory
cytokines. BM-MSCs stimulated by IFN-γ combined with any one of
IL-1α, IL-1β or TNF-α exerted an inhibitory effect on PBMCs
isolated from the young healthy group, the middle-aged healthy
group and the patients with PD. Among the combination of
proinflammatory cytokines, in young healthy and middle-aged healthy
people, BM-MSCs pretreated with IFN-γ + IL-1β and IFN-γ + IL-1α had
stronger inhibitory effects on PBMCs compared with those treated
with IFN-γ + TNF-α. The inhibitory effect of BM-MSCs induced by
IFN-γ + IL-1β on PBMC proliferation appears stronger than that of
IFN-γ + IL-1α, although without a significant difference.
The results of the present study suggest that
although aging affects the response of PBMCs toward the suppression
of BM-MSCs, male patients with PD in middle age still maintain
responses toward MSC suppression. This finding is direct evidence
that patients with PD aged 56–60 are still eligible for the
anti-inflammatory cell therapy in which BM-MSCs are used. The
results also suggested that the pretreatment of BM-MSCs with IFN-γ
+ IL-1β and IFN-γ + IL-1α prior to transplantation may result in
more favorable immunosuppressive effects.
Acknowledgements
Not applicable.
Funding
This study was supported by research grants from:
National Science Foundation of China (grant no. 81371377), Natural
Science Foundation of Beijing Municipality (grant no. 7172055),
Beijing science and technology new star program (grant no.
2009B22), Beijing Municipal Science and Technology Commission
(grant no. Z111107067311033), The Health Project of JiangSu
Province (grant no. H201049).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YQG, CSZ, XBL and YAZ designed the experiments. XBL,
XMY, LLL, JLW, CSZ, HQZ and YQG performed the in vitro and
in vivo experiments. XBL, YQG, HQZ and YAZ contributed to
the data analysis. YQG, XBL and YAZ wrote the manuscript and YAZ
gave final approval to the submitted version.
Ethics approval and consent to
participate
Informed consent of the patients was obtained in
addition to the participant consent and ethical approval obtained
from the Ethics Committee of Xuanwu Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BM-MSC
|
bone marrow mesenchymal stem cell
|
|
CBA
|
cytometric bead array
|
|
HSCT
|
hematopoietic stem cell
transplantation
|
|
MHC
|
major histocompatibility antigen
complexes
|
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PD
|
Parkinson's disease
|
References
|
1
|
Hirsch EC and Hunot S: Neuroinflammation
in Parkinson's disease: A target for neuroprotection? Lancet
Neurol. 8:382–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone DK, Reynolds AD, Mosley RL and
Gendelman HE: Innate and adaptive immunity for the pathobiology of
Parkinson's disease. Antioxid Redox Signal. 11:2151–2166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGeer PL, Itagaki S, Boyes BE and McGeer
EG: Reactive microglia are positive for HLA-DR in the substantia
nigra of Parkinson's and Alzheimer's disease brains. Neurology.
38:1285–1291. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hughes V: Microglia: The constant
gardeners. Nature. 485:570–572. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouchi Y, Yoshikawa E, Sekine Y,
Futatsubashi M, Kanno T, Ogusu T and Torizuka T: Microglial
activation and dopamine terminal loss in early Parkinson's disease.
Ann Neurol. 57:168–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long-Smith CM, Sullivan AM and Nolan YM:
The influence of microglia on the pathogenesis of Parkinson's
disease. Prog Neurobiol. 89:277–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marinova-Mutafchieva L, Sadeghian M, Broom
L, Davis JB, Medhurst AD and Dexter DT: Relationship between
microglial activation and dopaminergic neuronal loss in the
substantia nigra: A time course study in a 6-hydroxydopamine model
of Parkinson's disease. J Neurochem. 110:966–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brochard V, Combadière B, Prigent A,
Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer
D, Callebert J, Launay JM, et al: Infiltration of CD4+
lymphocytes into the brain contributes to neurodegeneration in a
mouse model of Parkinson disease. J Clin Invest. 119:182–192.
2009.PubMed/NCBI
|
|
9
|
Ferrari CC and Tarelli R: Parkinson's
disease and systemic inflammation. Parkinsons Dis.
2011:4368132011.PubMed/NCBI
|
|
10
|
Louveau A, Smirnov I, Keyes TJ, Eccles JD,
Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et
al: Structural and functional features of central nervous system
lymphatic vessels. Nature. 523:337–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farkas E, De Jong GI, de Vos RA, Jansen
Steur EN and Luiten PG: Pathological features of cerebral cortical
capillaries are doubled in Alzheimer's disease and Parkinson's
disease. Acta Neuropathol. 100:395–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Odoardi F, Sie C, Streyl K, Ulaganathan
VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J,
Klinkert WE, et al: T cells become licensed in the lung to enter
the central nervous system. Nature. 488:675–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Zhang J, Hu X, Zhang L, Mao L,
Jiang X, Liou AK, Leak RK, Gao Y and Chen J: Microglia/macrophage
polarization dynamics in white matter after traumatic brain injury.
J Cereb Blood Flow Metab. 33:1864–1874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arimoto T, Choi DY, Lu X, Liu M, Nguyen
XV, Zheng N, Stewart CA, Kim HC and Bing G: Interleukin-10 protects
against inflammation-mediated degeneration of dopaminergic neurons
in substantia nigra. Neurobiol Aging. 28:894–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng ZH, Wang TG, Li DD, Fung P, Wilson
BC, Liu B, Ali SF, Langenbach R and Hong JS:
Cyclooxygenase-2-deficient mice are resistant to
1-methyl-4-phenyl1,2,3,6-tetrahydropyridine-induced damage of
dopaminergic neurons in the substantia nigra. Neurosci Lett.
329:354–358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vijitruth R, Liu M, Choi DY, Nguyen XV,
Hunter RL and Bing G: Cyclooxygenase-2 mediates microglial
activation and secondary dopaminergic cell death in the mouse MPTP
model of Parkinson's disease. J Neuroinflammation. 3:62006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corcione A, Benvenuto F, Ferretti E,
Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi
GL, Pistoia V and Uccelli A: Human mesenchymal stem cells modulate
B-cell functions. Blood. 107:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spaggiari GM, Capobianco A, Abdelrazik H,
Becchetti F, Mingari MC and Moretta L: Mesenchymal stem cells
inhibit natural killer-cell proliferation, cytotoxicity, and
cytokine production: Role of indoleamine 2,3-dioxygenase and
prostaglandin E2. Blood. 111:1327–1333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laranjeira P, Gomes J, Pedreiro S, Pedrosa
M, Martinho A, Antunes B, Ribeiro T, Santos F, Domingues R,
Abecasis M, et al: Human bone marrow-derived mesenchymal stromal
cells differentially inhibit cytokine production by peripheral
blood monocytes subpopulations and myeloid dendritic cells. Stem
Cells Int. 2015:8190842015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS,
Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, et al: Comparison of
immunomodulatory properties of mesenchymal stem cells derived from
adult human tissues. Cell Immunol. 259:150–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grozdanov V, Bliederhaeuser C, Ruf WP,
Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A,
Hengerer B, Kassubek J, et al: Inflammatory dysregulation of blood
monocytes in Parkinson's disease patients. Acta Neuropathol.
128:651–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calligaris R, Banica M, Roncaglia P,
Robotti E, Finaurini S, Vlachouli C, Antonutti L, Iorio F,
Carissimo A, Cattaruzza T, et al: Blood transcriptomics of
drug-naive sporadic Parkinson's disease patients. BMC Genomics.
16:8762015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Mo M, Li G, Cen L, Wei L, Xiao Y,
Chen X, Li S, Yang X, Qu S and Xu P: The biomarkers of immune
dysregulation and inflammation response in Parkinson disease.
Transl Neurodegener. 5:162016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Annesley SJ, Lay ST, De Piazza SW,
Sanislav O, Hammersley E, Allan CY, Francione LM, Bui MQ, Chen ZP,
Ngoei KR, et al: Immortalized Parkinson's disease lymphocytes have
enhanced mitochondrial respiratory activity. Dis Model Mech.
9:1295–1305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren G, Zhang L, Zhao X, Xu G, Zhang Y,
Roberts AI, Zhao RC and Shi Y: Mesenchymal stem cell-mediated
immunosuppression occurs via concerted action of chemokines and
nitric oxide. Cell Stem Cell. 2:141–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Du W, Ma FX, Feng X, Bayard F and
Han ZC: High concentrations of TNF-α induce cell death during
interactions between human umbilical cord mesenchymal stem cells
and peripheral blood mononuclear cells. PLoS One. 10:e01286472015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu T, Liu J, Zhang HJ, Hallett M, Zheng Z
and Chan P: Attention to automatic movements in Parkinson's
disease: Modified automatic mode in the striatum. Cerebral Cortex.
25:3330–3342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bech P, Allerup P, Larsen ER, Csillag C
and Licht RW: The Hamilton Depression Scale (HAM-D) and the
Montgomery-Åsberg Depression Scale (MADRS). A psychometric
re-analysis of the European genome-based therapeutic drugs for
depression study using Rasch analysis. Psychiatry Res. 217:226–232.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Ren G, Huang Y, Su J, Han Y, Li J,
Chen X, Cao K, Chen Q, Shou P, et al: Mesenchymal stem cells: A
double-edged sword in regulating immune responses. Cell Death
Differ. 19:1505–1513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asanuma M and Miyazaki I: Common
anti-inflammatory drugs are potentially therapeutic for Parkinson's
disease? Exp Neurol. 206:172–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Samii A, Etminan M, Wiens MO and Jafari S:
NSAID use and the risk of Parkinson's disease: Systematic review
and meta-analysis of observational studies. Drugs Aging.
26:769–779. 2009. View Article : Google Scholar : PubMed/NCBI
|