Introduction
Colon cancer is the second most lethal malignancy
worldwide, which is associated with >600,000 cases of mortality
per year (1). It is estimated that
there are >1.4 million people living in the United States with
this disease, and an additional 134,490 cases are diagnosed
annually (2). Clinicians are faced
with a great challenge with regards to colon cancer treatment and
targeting the molecular features of this disorder are critical for
therapeutic success (3);
therefore, understanding the molecular pathogenesis of colorectal
cancer is crucial for disease management.
Prolyl 4-hydroxylase, β polypeptide (P4HB) is the
β-subunit of prolyl 4-hydroxylase, which acts as an endoplasmic
reticulum chaperone to suppress aggregation of misfolded proteins
(4). P4HB expression in lung
cancer tissues is increased compared with in adjacent tissues and
normal lung epithelium, and it has been suggested to induce the
growth of lung carcinoma (5). P4HB
is also upregulated in high-grade glioma (6). Downregulation of P4HB decreases
temozolomide resistance in malignant glioma via endoplasmic
reticulum stress response pathways (7). Our previous study revealed that P4HB
is upregulated in human hepatocellular carcinoma (HCC) tissues, and
it promotes HCC cell proliferation and migration (8). These findings indicate that P4HB may
serve an important role in tumorigenesis; however, little is
currently known about the effects of P4HB and its underlying
molecular mechanisms in colon cancer.
It is believed that reactive oxygen species (ROS)
serve a crucial role in cell apoptosis, and increasing evidence has
indicated that ROS regulate the apoptosis of cancer cells (9). ROS function as ‘redox messengers’ in
intracellular signaling and regulation, whereas excessive ROS
accelerate cell death (9,10). ROS result in a cellular redox
imbalance in various cancer cells, which may be associated with
oncogenic stimulation (11,12).
Notably, the regulation of ROS may be a promising therapeutic
approach against colon cancer. Chen et al (13) demonstrated that a novel
benzimidazole acridine derivative induced human colon cancer cell
apoptosis via the upregulation of ROS. ROS has also been revealed
to enhance cisplatin-induced colon cancer cell apoptosis (14). Furthermore, Wang et al
(15) revealed that
dihydrotanshinone induces p53-independent but ROS-dependent
apoptosis of colon cancer cells.
ROS may activate downstream signaling pathways to
regulate the phosphorylation status of transcription factors,
including signal transducer and activator of transcription (STAT)
(16). STAT proteins are recruited
to receptors by binding to phosphotyrosine residues in the Src
homology 2 domain of the STAT protein (17). STAT is subsequently phosphorylated
by Janus kinase 2 and translocated into the nucleus. The STAT
family is a group of latent cytoplasmic proteins that regulate
various metabolic processes (18,19).
The STAT family includes seven structurally and functionally
associated proteins: STAT1-4, STAT5a and b, and STAT6 (18). STAT3 is constitutively aberrantly
activated in ~70% of human solid tumors, and it modulates the
expression of oncogenes controlling the proliferation and
metastasis of tumor cells (20,21).
STAT3 is often a downstream effector of numerous oncogenic
mutations (22). Substantive
evidence has indicated that downregulating STAT3 may mitigate the
malignant behavior of cancer cells (23,24).
The present study aimed to investigate the effects
of P4HB on colon cancer. The results showed that P4HB was
significantly upregulated in colon cancer tissues, whereas P4HB
knockdown significantly increased cell apoptosis. Furthermore, P4HB
knockdown reduced the activation of STAT3 and increased ROS
accumulation. These data indicated that P4HB may inhibit colon
cancer cell apoptosis via the ROS/STAT3 signaling pathway.
Materials and methods
Reagents
N-acetyl cysteine (NAC), an antioxidant, was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
As described previously (15),
colon cancer cells (5×105 cells/cm2) were
pretreated with NAC (10 mM) for 1 h at 37°C and 5%
CO2.
Clinical tissues
The present study was approved by the Medical Ethics
Committee of the Seventh People's Hospital of Shanghai University
of Traditional Chinese Medicine (Shanghai, China). All subjects
provided written informed consent, and none of them received
chemoradiotherapy prior to surgery. From May 2015 to July 2017, 9
patients (5 males and 4 females) with an average age of 52.4 years
were enrolled in the present study. Patients that underwent
chemoradiotherapy prior to surgery were excluded. Colon cancer
tissues and adjacent normal colon tissues were obtained during
surgery.
Immunohistochemistry (IHC)
According to the manufacturer's protocol, P4HB IHC
staining was performed manually using a Bosterbio IHC kit (cat. no.
RC1865; Boster Biological Technology, Pleasanton, CA, USA).
Briefly, the clinical samples were fixed in 10% neutral formalin
for 2 days at room temperature and embedded in paraffin, after
which 3-µm sections were cut and mounted onto slides. Slides were
incubated at 56°C, deparaffinized in xylene and dehydrated in a
graded series of alcohol. Heat-induced (121°C) antigen retrieval
was conducted with sodium citrate (pH 6.7) in a pressure-cooker for
30 min at room temperature. Following washing in wash buffer,
peroxidase-blocking reagent (included in IHC kit) was applied for
15 min at room temperature. Subsequently, sections were incubated
overnight at 4°C with rabbit anti-P4HB monoclonal antibody (1:100;
ab137110; Abcam, Cambridge, UK). Subsequently, a secondary antibody
(included in IHC kit) was applied for 30 min at room temperature.
Horseradish peroxidase-streptavidin (included in IHC kit) was used
to detect immunoactivity, followed by counterstaining with
hematoxylin for 1 min at room temperature. Under a light microscope
(magnifications, ×100 and 200; Olympus Corporation, Tokyo, Japan),
each section was imaged and semi-quantitatively analyzed according
to a previously published method (25).
Cell culture
The HT29 human colon cancer cell line was purchased
from the Cell Bank of Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum (FBS; HyClone; GE Healthcare, Chicago, IL,
USA) at 37°C and 5% CO2.
Lentiviral infection
The HT29 human colon cancer cell line was used for
lentiviral infection. A lentiviral short hairpin RNA (shRNA)
construct targeting P4HB (SHCLNG-NM_000918) was obtained from
Sigma-Aldrich (Merck KGaA). Three shRNA sequences targeting P4HB
were designed (Table I). The
oligonucleotides were phosphorylated, annealed and cloned into the
pLKO.1 vector (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocol. Briefly, the cells were seeded at
1×105 cells/well in a 12-well plate prior to lentiviral
particle infection and incubated with 1 ml DMEM supplemented with
10% FBS for 6 h. Subsequently, cells were infected with lentiviral
particles (1×109). This lentiviral transgenic system
possessed >90% gene transfer effectiveness and a multiplicity of
infection of 100 for the inhibition of P4HB expression. After 24 h,
the virus-containing medium of infected cells was substituted with
DMEM with 10% FBS, and infected cells were incubated with 1 µg/ml
puromycin for 72 h at 37°C and 5% CO2. Empty lentiviral
vectors (1×109) were used as a negative control. After
the screening for 72 h, the infected cells the subsequent
experiments.
 | Table I.Sequences interfering with P4HB. |
Table I.
Sequences interfering with P4HB.
| shRNA | Sequence
(5′-3′) |
|---|
| shRNA1 |
CCGGGCTCCCATTTGGGATAAACTGCTCGAGCAGTTTATCCCAAATGGGAGCTTTTTG |
| shRNA2 |
CCGGAGGTGAAATCAAGACTCACATCTCGAGATGTGAGTCTTGATTTCACCTTTTTTG |
| shRNA3 |
CCGGGTGTGGTCACTGCAAACAGTTCTCGAGAACTGTTTGCAGTGACCACACTTTTTG |
| ShCtrl |
CCTTCTCCGAACGTGTCACGT |
Cell proliferation
To evaluate the proliferative ability of colon
cancer cells, the cells were seeded into 96-well plates
(2×103 cells/well). Following 24 h, the medium was
removed, and the cells were treated with 10% Cell Counting kit 8
(CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) in
100 µl DMEM without FBS for 2 h at room temperature. Absorbance at
450 nm, which is directly proportional to the rate of cell
proliferation, was measured using a microplate reader.
Assessment of apoptosis
Apoptosis was evaluated using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) dual
staining kit (C1052; Beyotime Institute of Biotechnology).
Following treatment (shRNA transfection or NAC pretreatment), the
HT29 cells (1×106 cells) were trypsinized and
centrifuged at 400 × g for 5 min at 4°C. Cells were then dissolved
in 100 µl Annexin V-FITC binding buffer, and were incubated with 5
µl Annexin V-FITC and 5 µl PI for 15 min at room temperature in the
dark. Harvested cells were analyzed by fluorescence-activated cell
sorting using a flow cytometer.
Detection of ROS
According to a previous study (26), intracellular ROS can be detected
using the peroxide-sensitive fluorophore 2′,7′-dichlorofluorescein
diacetate (DCF-DA; Beyotime Institute of Biotechnology). Briefly,
the cells (1×105 cells) were plated in six-well plates,
washed with DMEM without FBS, and incubated with 10 µM DCF-DA at
37°C for 20–30 min. Fluorescence distribution was detected using a
fluorescence spectrophotometer at an excitation wavelength of 488
nm.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer supplemented with a protease inhibitor (Beyotime
Institute of Biotechnology). The concentration of total protein was
detected by the BCA method. Whole cell extracts containing equal
quantities of proteins (50 µg) were separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and were then
transferred onto a polyvinylidene fluoride membrane. Following
blocking in 5% bovine serum albumin (cat. no. BA7019, Boster
Biological Technology) for 1 h at room temperature, the membranes
were incubated overnight at 4°C with antibodies specific to β-actin
(1:8,000; cat. no. 4970), phosphorylated (p)-STAT3 (1:1,000; cat.
no. 4074), total (t)-STAT3 (1:1,000; cat. no. 12640), B-cell
lymphoma (Bcl)-2 (1:1,000; cat. no. 3498S; all Cell Signaling
Technology, Inc., Danvers, MA, USA), cleaved caspase-3 (1:1,000;
cat. no. 9661; Abcam), c-Myc (1:1,000; cat. no. 13987) and p53
(1:1,000; cat. no. 2527; both Cell Signaling Technology, Inc.).
Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000;
cat. no. BA1099; Boster Biological Technology) was applied as a
secondary antibody for 1 h at 37°C. For all western blots, β-actin
served as an internal control. Protein expression was
semi-quantified using Bio-Rad Quantity One software 3.76 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). All experiments were
performed at least in triplicate. Data are presented as the means ±
standard deviation. Statistical significance was determined using a
two-tailed Student's t-test. Comparisons among multiple groups were
analyzed by a one-way analysis of variance, followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
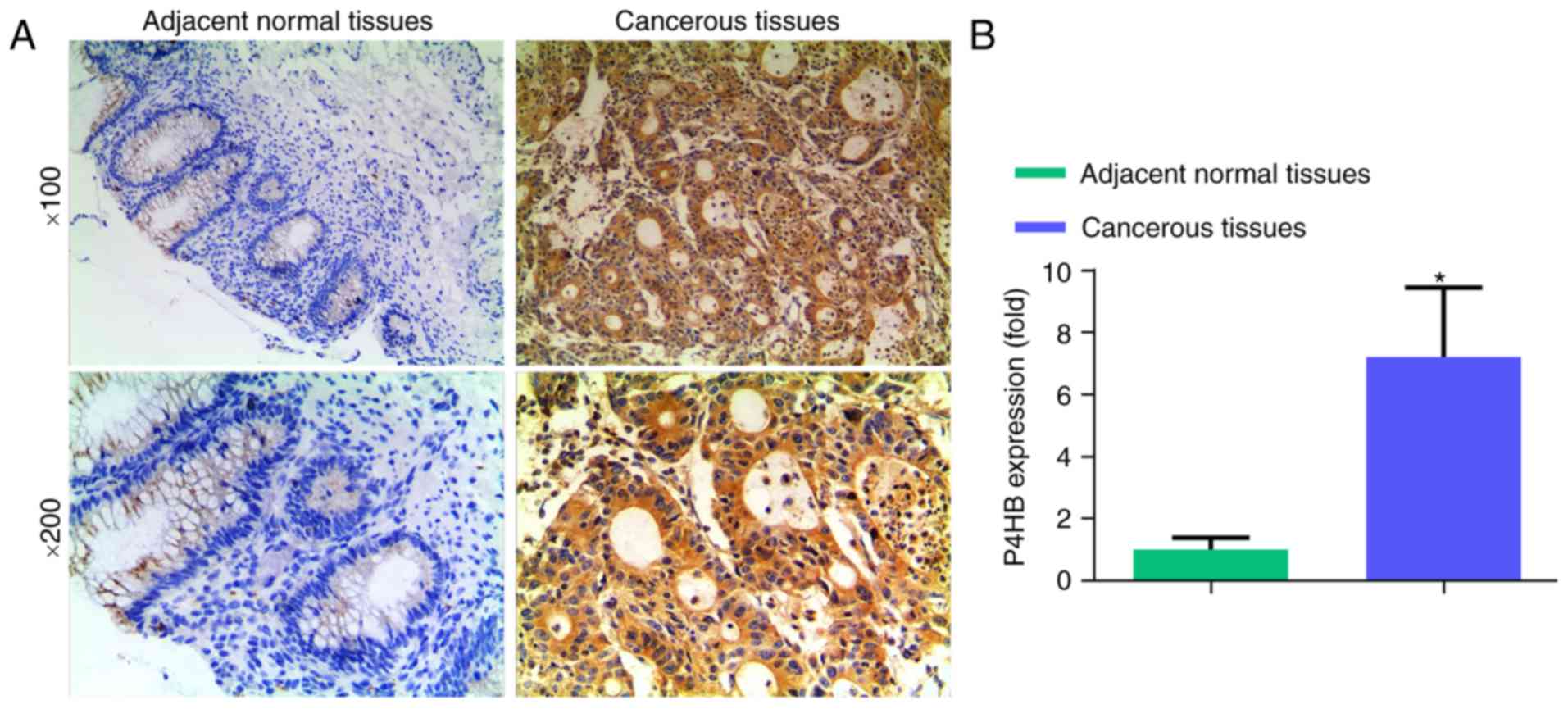
P4HB levels are lower in normal colon
tissues than in colon cancer tissues
To assess the expression levels of P4HB in normal
and cancerous colon tissues, IHC was performed on nine clinical
specimens. Significantly higher P4HB levels were identified in
colon cancer tissues compared with in normal colon tissues
(Fig. 1; P<0.05).
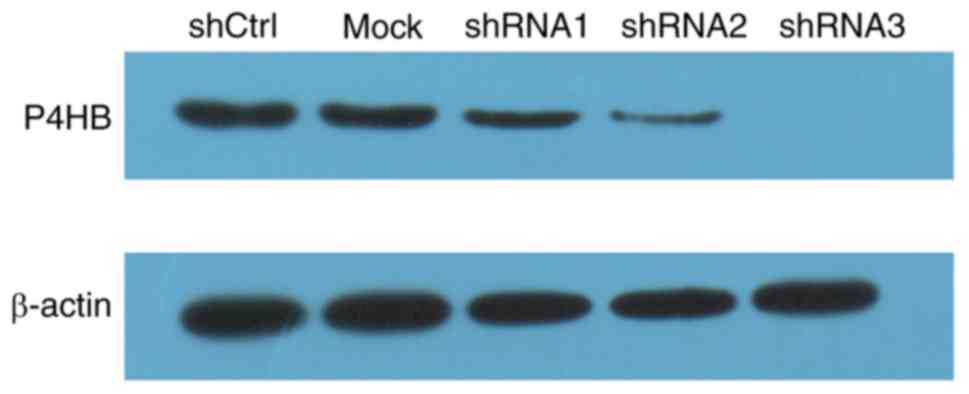
Knockdown of P4HB in colon cancer
cells by lentiviral infection
To investigate the effects of P4HB, lentiviral
vectors were used to downregulate P4HB expression in colon cancer
HT29 cells. As shown in Fig. 2,
P4HB levels were knocked down using three shRNAs. shRNA3 was chosen
for subsequent experiments, as it most effectively downregulated
P4HB.
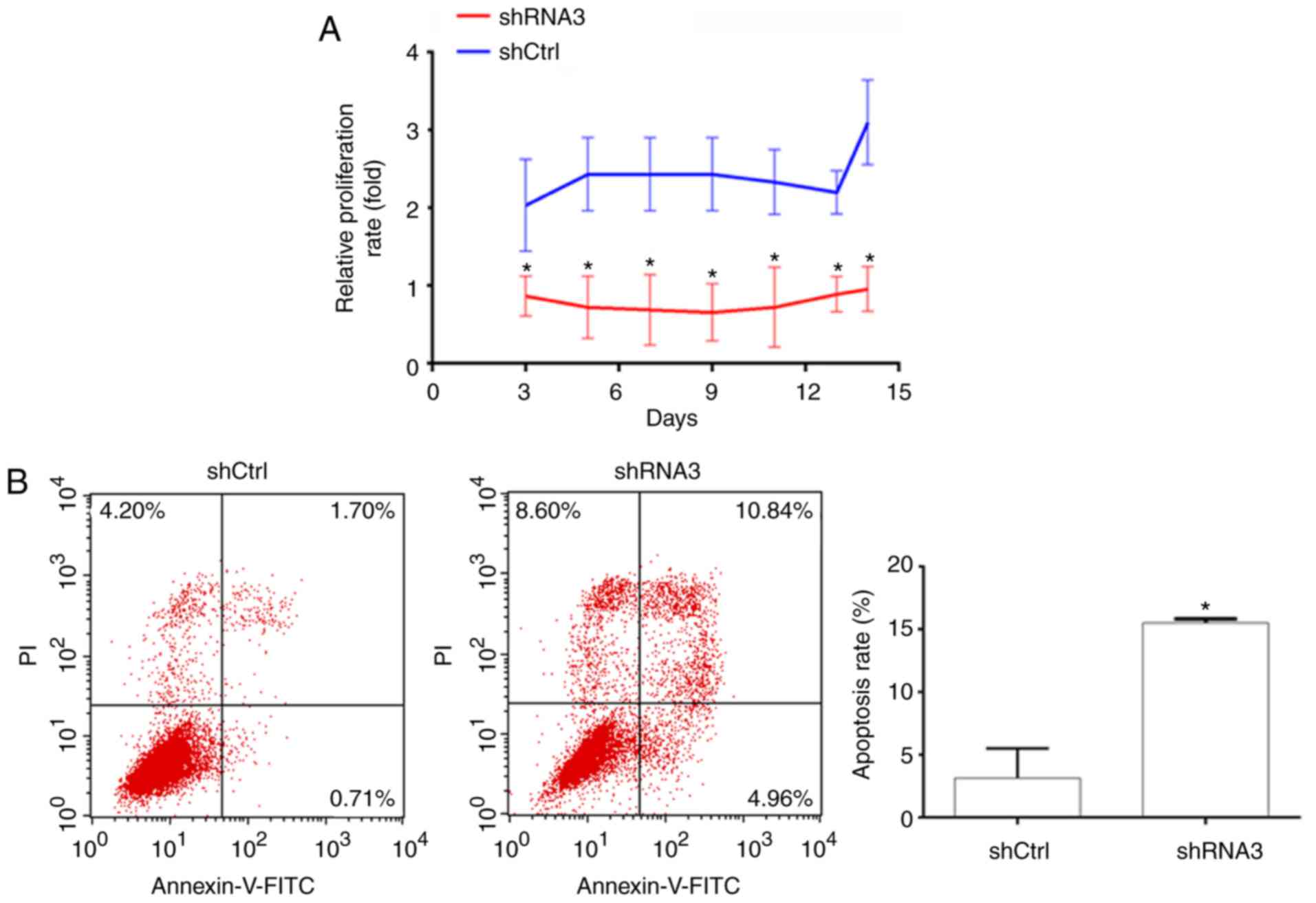
Knockdown of PH4B inhibits
proliferation and promotes apoptosis of human HT29 cells
The effects of P4HB on cell proliferation were
identified using the CCK8 assay, which revealed that proliferation
was significantly reduced in the P4HB-knockdown group compared
within the control group (Fig.
3A).
Cell apoptosis was evaluated by Annexin V/PI
staining. The percentage of cells undergoing early or late
apoptosis was demonstrated in Fig.
3B. Compared with in the control group, apoptosis was
significantly increased by P4HB knockdown (P<0.05). As
illustrated in Fig. 4A, the
P4HB-knockdown group exhibited markedly higher protein expression
levels of p53 and cleaved caspase-3 compared with in the control
group. Furthermore, Bcl-2 levels were lower in the P4HB-knockdown
group than in the control group.
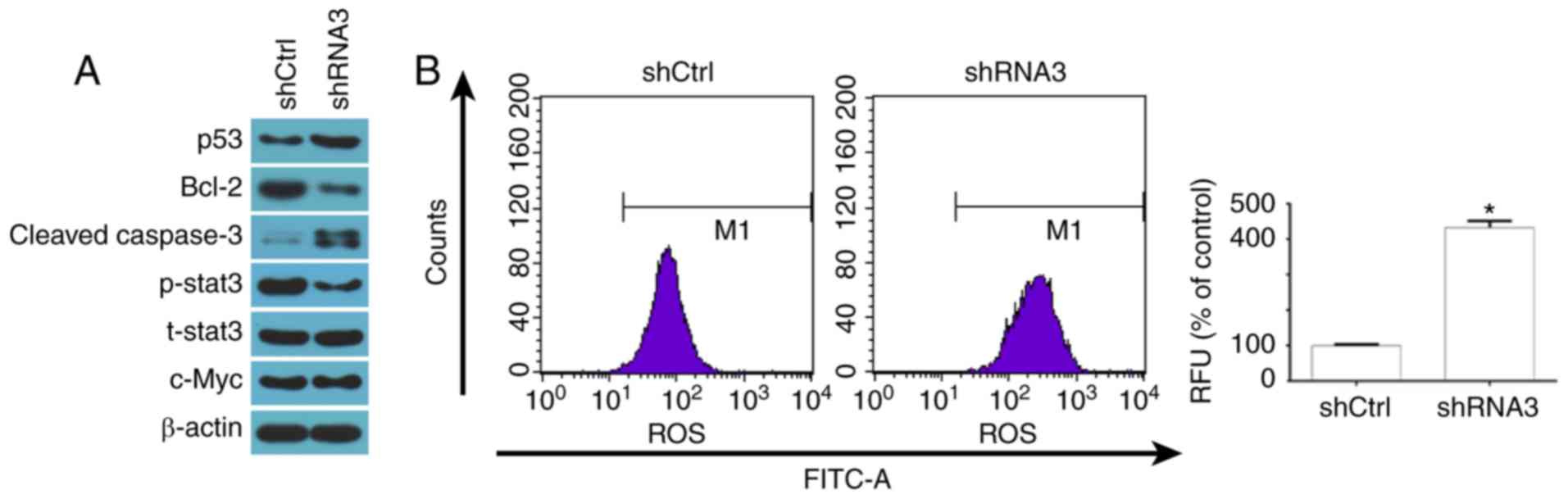 | Figure 4.Effects of P4HB knockdown on markers
of apoptosis. (A) Representative images of the protein levels of
p53, Bcl-2, cleaved caspase-3, p-STAT3, t-STAT3 and c-Myc as
assessed by western blot analysis. (B) P4HB knockdown increased the
generation of ROS. Images are representative of three independent
experiments. Data are presented as the means ± standard deviation.
*P<0.05 vs. the shCtrl group. Bcl-2, B-cell lymphoma 2; Ctrl,
control; FITC, fluorescein isothiocyanate; p-, phosphorylated;
P4HB, prolyl 4-hydroxylase, β polypeptide; RFU, relative
fluorescence unit; shRNA, short hairpin RNA; STAT3, signal
transducer and activator of transcription 3; t, total. |
P4HB knockdown regulates p-STAT3
expression and promotes the accumulation of ROS
To explore the molecular mechanism underlying the
effects of P4HB on colon cancer cells, ROS generation and STAT3
levels, which are key regulators of colon cancer cell proliferation
and apoptosis, were evaluated. P4HB knockdown markedly inhibited
activation of p-STAT3 (Fig. 4A)
and significantly increased the accumulation of ROS (Fig. 4B). Furthermore, a previous study
demonstrated that ROS downregulates p-STAT3 by downregulating c-Myc
(27); therefore, the expression
of c-Myc was also examined. However, no significant difference was
detected in the levels of c-Myc (Fig.
4A), which indicated that ROS may downregulate p-STAT3 levels
via other signaling pathways.
Inhibiting ROS accumulation rescues
the increased cell apoptosis induced by P4HB knockdown
NAC was used to scavenge intracellular ROS.
According to a previous study (15), the cells were pretreated with NAC
(10 mM) for 1 h. As demonstrated in Fig. 5A, intracellular ROS levels were
decreased by NAC. The levels of apoptosis and apoptosis-associated
proteins (Bcl-2, cleaved-caspase-3) were markedly decreased,
whereas proliferation significantly increased in response to NAC
compared with in cells without NAC (Fig. 5B-D). The increased apoptosis
induced by P4HB knockdown was also reduced by NAC. Finally, NAC
effectively reversed the effects of P4HB knockdown on the
suppression of p-STAT3 (Fig.
5B).
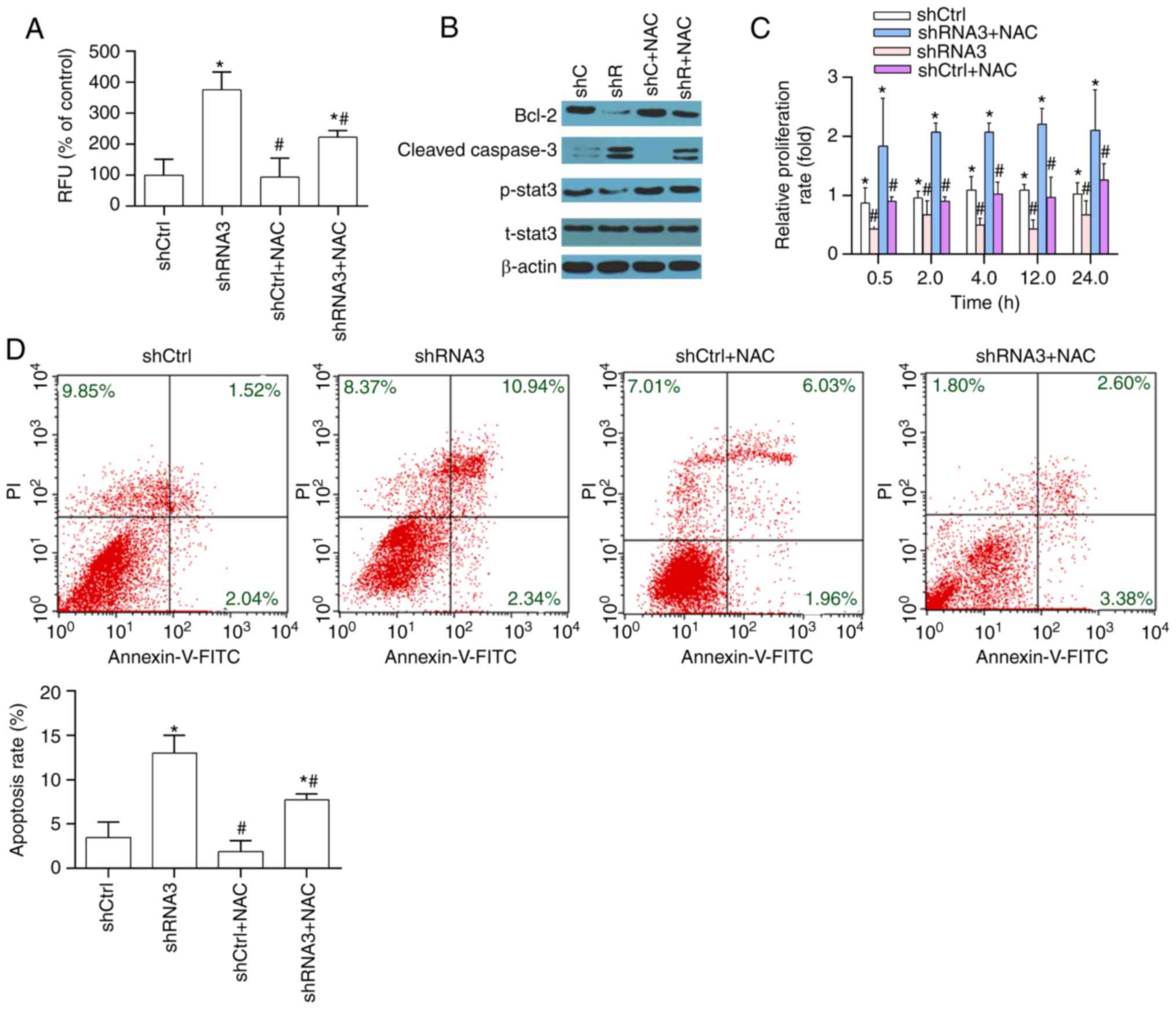 | Figure 5.Inhibiting accumulation of ROS
reduces apoptosis induced by P4HB knockdown. (A) ROS generation, as
measured by flow cytometry. (B and C) Protein levels of Bcl-2,
cleaved caspase-3, p-STAT3 and t-STAT3 were assessed by western
blot analyses. (D) Annexin V/PI staining was performed to assess
cell apoptosis. Images are representative of three independent
experiments. All data are presented as the means ± standard
deviation. *P<0.05 vs. the shCtrl group. #P<0.05
vs. the shRNA3 +NAC group. Bcl, B-cell lymphoma; Ctrl, control;
FITC, fluorescein isothiocyanate; p-, phosphorylated; P4HB, prolyl
4-hydroxylase, β polypeptide; RFU, relative fluorescence unit; PI,
propidium iodide; shRNA, short hairpin RNA; STAT3, signal
transducer and activator of transcription 3; t, total. |
Discussion
Colon cancer is one of most lethal malignancies, and
there are still great challenges concerning its treatment.
According to previous studies, P4HB is associated with
tumorigenesis (5,6). The present study aimed to investigate
the effects of P4HB on human colon cancer. In the present study,
compared with in normal colon tissues, P4HB levels were
significantly upregulated in colon cancer tissues. P4HB knockdown
significantly decreased cell proliferation and increased cell
apoptosis in a human cancer cell line. Additionally, downregulation
of P4HB suppressed p-STAT3 expression and accelerated the
accumulation of ROS. Notably, the increased apoptosis observed
after P4HB knockdown was rescued by inhibition of ROS. Notably, the
downregulation of ROS could also upregulate p-STAT3 levels. Taken
together, these findings suggested that P4HB knockdown may decrease
cell proliferation and increase cell apoptosis in a human cancer
cell line.
A considerable amount of evidence has indicated that
P4HB is associated with tumorigenesis (7,8). Our
previous study reported that P4HB overexpression promotes HCC cell
growth, migration, invasion and epithelial-to-mesenchymal
transition, and the knockdown of P4HB expression by small molecules
or small interfering RNA may be used as a therapeutic target in HCC
(8). Apoptosis is a gene-directed
program that eliminates excess, abnormal cells in vivo. A
hallmark of cancer is the capability of cancer cells to resist cell
death (28). Therefore, disturbed
regulation of apoptosis is a vital factor in tumorigenesis
(29). Evasion of apoptosis may
have an important role in tumor initiation and therapy resistance
(30). p53 is a tumor suppressor,
which serves a key role in apoptosis and is frequently inactivated
in colon cancer cells (31). In
addition, Bcl-2 functions as a suppressor and is a key regulator of
apoptosis. Alterations in the Bcl-2/Bax ratio may have an anti- or
proapoptotic effect and may result in caspase activation, thereby
inducing apoptosis (27). In the
present study, depletion of P4HB upregulated p53 and cleaved
caspase-3 expression, and inactivated the expression of Bcl-2.
These data indicated that P4HB knockdown increased the apoptosis of
colon cancer cells in vitro.
Elevated ROS levels may promote tumor onset and
progression by increasing DNA damage and genomic instability
(32). ROS-induced oxidative
stress can also directly provoke programmed cell death, including
apoptosis and autophagy (33).
Ding et al (34) revealed
that ROS overload prompts apoptosis of colon cancer cells;
therefore, targeting ROS in colon cancer cells may be exploited as
an anticancer strategy. ROS may activate downstream signaling
pathways to regulate the phosphorylation status of transcription
factors, including STAT3 (16).
Inappropriate activation of STAT3 serves a crucial role in the
apoptosis of tumor cells. Chae et al (35) demonstrated that CAY10598 induced
apoptosis of colon cancer cells by activating ROS/STAT3 signaling.
Kasiappan et al (27) also
revealed that the ROS/STAT3 signaling pathway serves an important
role in regulating the behaviors of tumor cells. Considering the
critical role of ROS/STAT3 in cancer cells, the expression levels
of ROS/STAT3 signaling pathway members were investigated in a colon
cancer cell line. The present results were consistent with previous
reports (27,35). The results demonstrated that P4HB
knockdown induced colon cancer cell apoptosis by increasing ROS
generation and downregulating STAT3, which may be associated with
subsequent activation of the intrinsic apoptosis pathway.
Furthermore, blocking the generation of ROS by NAC rescued the
increased cell apoptosis induced by P4HB knockdown. Notably, the
decreased ROS levels effectively antagonized the effects of P4HB on
the activation of p-STAT3. Levels of ROS are controlled at both the
level of production and by degradation. The predominant
transcriptional response that increases the production of
antioxidant proteins in cancer cells is through the activation of
nuclear factor (erythroid-derived 2)-like 2 (NRF2). The levels of
ROS have been reported to be regulated by antioxidant proteins,
such as NRF2 (36). Notably, P4HB
may be associated with the expression of NRF2 (36).
There are several limitations in the present study.
Firstly, these in vitro results need to be verified in other
colon cancer cell lines and in animal models. In addition, it is
well known that P4HB and its downstream targets may induce ROS
accumulation; however, the mechanisms by which P4HB regulates ROS
levels remain unclear. Therefore, further studies are required.
In conclusion, the present data suggested that P4HB
knockdown may induce apoptosis of human colon cancer HT29 cells
through the generation of ROS and inactivation of the STAT3
signaling pathway; however, these results require further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Shanghai Pudong Commission of Health and Family Planning (grant no.
PWRd2016-12), Shanghai Pudong Science and Technology Committee
Foundation (grant no. PKJ2016-Y50), Talents Training Program of
Seventh People's Hospital of Shanghai University of TCM (grant nos.
BDX2016-01 and QMX2016-04).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author'scontributions
WX and JW designed the experiments. YZ, JY, QZ, QX
and LL conducted the experiments. YZ and JY analyzed the data. WX
wrote and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Seventh People's Hospital of Shanghai University
of Traditional Chinese Medicine (reference no. 2013876). All
subjects provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahnen DJ, Wade SW, Jones WF, Sifri R,
Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A
and You YN: The increasing incidence of young-onset colorectal
cancer: A call to action. Mayo Clin Proc. 89:216–224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hagan S, Orr MC and Doyle B: Targeted
therapies in colorectal cancer-an integrative view by PPPM. EPMA J.
4:32013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noiva R: Protein disulfide isomerase: The
multifunctional redox chaperone of the endoplasmic reticulum. Semin
Cell Dev Biol. 10:481–493. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SM, Lin LZ, Zhou DH, Zhou JX and
Xiong SQ: Expression of prolyl 4-hydroxylase beta-polypeptide in
non-small cell lung cancer treated with Chinese medicines. Chin J
Integr Med. 21:689–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun S, Wong TS, Zhang XQ, Pu JK, Lee NP,
Day PJ, Ng GK, Lui WM and Leung GK: Protein alterations associated
with temozolomide resistance in subclones of human glioblastoma
cell lines. J Neurooncol. 107:89–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee
NP, Day PJ, Lui WM, Fung CF and Leung GK: Inhibition of prolyl
4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide
resistance in malignant glioma via the endoplasmic reticulum stress
response (ERSR) pathways. Neuro Oncol. 15:562–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia W, Zhuang J, Wang G, Ni J, Wang J and
Ye Y: P4HB promotes HCC tumorigenesis through downregulation of
GRP78 and subsequent upregulation of epithelial-to-mesenchymal
transition. Oncotarget. 8:8512–8521. 2017.PubMed/NCBI
|
|
9
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JE, Park JH, Shin IC and Koh HC:
Reactive oxygen species regulated mitochondria-mediated apoptosis
in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol.
263:148–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matés JM and Sánchez-Jiménez FM: Role of
reactive oxygen species in apoptosis: Implications for cancer
therapy. Int J Biochem Cell Biol. 32:157–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen K, Chu BZ, Liu F, Li B, Gao CM, Li
LL, Sun QS, Shen ZF and Jiang YY: New benzimidazole acridine
derivative induces human colon cancer cell apoptosis in vitro via
the ROS-JNK signaling pathway. Acta Pharmacol Sin. 36:1074–1084.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He G, He G, Zhou R, Pi Z, Zhu T, Jiang L
and Xie Y: Enhancement of cisplatin-induced colon cancer cells
apoptosis by shikonin, a natural inducer of ROS in vitro and in
vivo. Biochem Biophys Res Commun. 469:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Yeung JH, Hu T, Lee WY, Lu L,
Zhang L, Shen J, Chan RL, Wu WK and Cho CH: Dihydrotanshinone
induces p53-independent but ROS-dependent apoptosis in colon cancer
cells. Life Sci. 93:344–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gào X and Schöttker B: Reduction-oxidation
pathways involved in cancer development: A systematic review of
literature reviews. Oncotarget. 8:51888–51906. 2017.PubMed/NCBI
|
|
17
|
Silver DL, Naora H, Liu J, Cheng W and
Montell DJ: Activated signal transducer and activator of
transcription (STAT) 3: Localization in focal adhesions and
function in ovarian cancer cell motility. Cancer Res. 64:3550–3558.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao X, Liu H, Zhang X, Zhang L, Li X, Wang
C and Sun S: Cell surface GRP78 accelerated breast cancer cell
proliferation and migration by activating STAT3. PLoS One.
10:e01256342015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh JE and Frank DA: STAT3-interacting
proteins as modulators of transcription factor function:
Implications to targeted cancer therapy. ChemMedChem. 11:795–801.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chai EZ, Shanmugam MK, Arfuso F,
Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC,
et al: Targeting transcription factor STAT3 for cancer prevention
and therapy. Pharmacol Ther. 162:86–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furtek SL, Backos DS, Matheson CJ and
Reigan P: Strategies and approaches of targeting STAT3 for cancer
treatment. ACS Chem Biol. 11:308–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MF, Li YJ, Yang TL, Lou B and Xie XM:
Losartan inhibits monocytic adhesion induced by ADMA via
downregulation of chemokine receptors in monocytes. Eur J Clin
Pharmacol. 65:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kasiappan R, Jutooru I, Karki K, Hedrick E
and Safe S: Benzyl isothiocyanate (BITC) induces reactive oxygen
species-dependent repression of STAT3 protein by down-regulation of
specificity proteins in pancreatic cancer. J Biol Chem.
291:27122–27133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Cai Q, Lin J, Fang Y, Zhan Y,
Shen A, Wei L, Wang L and Peng J: Chloroform fraction of
Scutellaria barbata D. Don promotes apoptosis and suppresses
proliferation in human colon cancer cells. Mol Med Rep. 9:701–706.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yaacoub K, Pedeux R, Tarte K and
Guillaudeux T: Role of the tumor microenvironment in regulating
apoptosis and cancer progression. Cancer Lett. 378:150–159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pavlou D and Kirmizis A: Depletion of
histone N-terminal-acetyltransferase Naa40 induces p53-independent
apoptosis in colorectal cancer cells via the mitochondrial pathway.
Apoptosis. 21:298–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teppo HR, Soini Y and Karihtala P:
Reactive oxygen species-mediated mechanisms of action of targeted
cancer therapy. Oxid Med Cell Longev. 2017:14852832017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Du Y, Le W, Wang K, Kieffer N and
Zhang J: Redox control of the survival of healthy and diseased
cells. Antioxid Redox Signal. 15:2867–2908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding Y, Wang H, Niu J, Luo M, Gou Y, Miao
L, Zou Z and Cheng Y: Induction of ROS overload by alantolactone
prompts oxidative DNA damage and apoptosis in colorectal cancer
cells. Int J Mol Sci. 17:5582016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chae IG, Kim DH, Kundu J, Jeong CH, Kundu
JK and Chun KS: Generation of ROS by CAY10598 leads to inactivation
of STAT3 signaling and induction of apoptosis in human colon cancer
HCT116 cells. Free Radic Res. 48:1311–1321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee LC, Weng YT, Wu YR, Soong BW, Tseng
YC, Chen CM and Lee-Chen GJ: Downregulation of proteins involved in
the endoplasmic reticulum stress response and Nrf2-ARE signaling in
lymphoblastoid cells of spinocerebellar ataxia type 17. J Neural
Transm (Vienna). 121:601–610. 2014. View Article : Google Scholar : PubMed/NCBI
|