Introduction
Foetal growth restriction (FGR) is one of main
causes of perinatal morbidity and mortality (1), but currently there is no clinically
effective treatment. Placental development is well known to
influence foetal growth, but the pathophysiology of failure of
placental development remains completely unelucidated. Recently E2F
transcription factor (E2F) 7 and E2F8 have been reported to be
essential for murine placental development and to regulate gene
expressions as repressors of the classic activator E2F3a (2). Members of the E2F family are
transcriptional factors controlling cell cycle, although E2F7 and
E2F8 have recently been identified as atypical E2F family members
(3). The functions of E2F7 and
E2F8 have also been investigated, and they are reported to control
angiogenesis (3–5).
E2f8 expression peaks on embryonic day (E)
10.5 and E15.5 in the murine placenta; it is expressed in three
major trophoblast lineages-labyrinth trophoblasts,
spongiotrophoblasts, and trophoblast giant cells (TGCs)-in the
murine placenta.
E2f7−/−;E2f8−/− murine
placentas are reported to be smaller, showing failed invasion into
the maternal decidua and a poor vascular network in comparison with
wild-type placentas (2). In
addition, ablation of E2f7 and E2f8 in all
trophoblasts results in FGR along with the collapse of placental
architecture. The human placenta, as well as the murine placenta,
is classified as chorioallantoic placenta. However, there are
structural differences between the human and murine placentas,
including the cell types (6).
Therefore, experiments using human placental samples and human cell
lines will require translation of the findings from a mouse mutant
model into human placental pathology. Behaviour of TGCs is similar
to that of human extravillous trophoblasts (EVTs), both of which
invade maternal decidua and become polyploid (7). The function of spongiotrophoblasts
remains unknown, but some spongiotrophoblast cells differentiate
into TGCs and are thought to be analogous to the cytotrophoblasts
of cell columns that anchor villi in the human placenta (7). Labyrinth trophoblasts are analogous
in function to syncytiotrophoblasts (7). In
E2f7−/−;E2f8−/− murine
placentas, significant defects were observed in spongiotrophoblasts
and TGCs, which suggests that E2F7 and E2F8 play important roles in
these cell types.
From these findings, we hypothesised that atypical
E2Fs also play a critical role in the development of the human
placenta, which requires successful invasion of EVTs into the
maternal uterus. Insufficient EVT invasion leads to human placental
pathologies, including FGR. TGCs in the mouse placenta are
analogous to EVTs, and E2F8 is involved in polyploidy in TGCs
(2). Therefore, in this study we
focused on the role of E2F8 in human EVTs. To demonstrate the
involvement of E2F8 on human placental development could lead to
establish the new therapy for underdevelopment of placenta
related-pathologies including hypertensive disorders of pregnancy
or FGR. For this end, we investigated the localisation of E2F8 in
the human placenta and its function in human EVTs.
Materials and methods
Immunohistochemistry and
immunofluorescence
A first trimester pregnant uterus was previously
obtained from a patient who underwent a hysterectomy during
pregnancy (8,9). Third trimester placental samples were
previously obtained from three pregnant women without any obstetric
complications. After formalin fixation, paraffin-embedded tissue
sections were cut at a thickness of 4 µm. In the present study, for
heat-induced epitope retrieval, deparaffinised sections in 10 mM
EDTA buffer (pH 9.0) were heated at 95°C for 20 min using a
microwave oven. Immunohistochemical staining was performed using
the Histofine SAB-PO(R) kit (Nichirei Bioscience Inc., Tokyo,
Japan) by the avidin-biotin immunoperoxidase technique. Briefly,
endogenous peroxidase activity was blocked by incubation with 0.3%
H2O2 in methanol for 20 min, and nonspecific
immunoglobulin binding was blocked by treatment with 10% normal
goat serum for 10 min. The sections were incubated at 4°C overnight
with 4 µg/mL of anti-human E2F8 antibody (bs-4265R; Bioss Inc.,
Woburn, MA, USA). For the negative control, primary antibody was
replaced by goat serum (Nichirei Bioscience Inc.). The sections
were then rinsed and incubated for 10 min with biotinylated
secondary antibody (Nichirei Bioscience Inc.). After washing, the
sections were incubated for 5 min with horseradish
peroxidase-conjugated streptavidin and treated with
diaminobenzidine (DAB; Dako Agilent Technologies, Inc., Santa
Clara, CA, USA) in 0.01% H2O2 for 5 min. The sections were
counterstained with Meyer's haematoxylin (Wako Pure Chemical
Industries, Ltd., Osaka, Japan).
For immunofluorescence, the sections were incubated
at 4°C overnight with 4 µg/mL of anti-human E2F8 antibody and
anti-human Cytokeratin AE1/AE3 antibody (ready to use, PMD072;
Diagnostic BioSystems Inc., Pleasanton, CA, USA) or with an
anti-human E2F8 antibody and 4 µg/mL of anti-Vimentin antibody
(sc-6260; Santa Cruz Biotechnology Inc., Dallas, TX, USA). The
sections were then rinsed and incubated at room temperature for 30
min with 2 µg/mL Alexa Fluor 488-conjugated anti-mouse IgG
(A-11029; Thermo Fisher Scientific Inc., Waltham, MA, USA) and
Alexa Fluor 568-conjugated anti-rabbit IgG (A-11036; Thermo Fisher
Scientific Inc.). For the negative controls, primary antibodies
were replaced by 4 µg/mL mouse nonspecific IgG control antibody
(555746; BD Biosciences, San Jose, CA, USA) and 4 µg/mL rabbit
nonspecific IgG control antibody (I-1,000; Vector Laboratories
Inc., Burlingame, CA, USA), respectively. The slides were mounted
with Fluorescence Mounting Medium (S3023; Dako Agilent Technologies
Inc.). The prepared slides for immunohistochemistry and
immunofluorescence were photographed with AXIO Imager A1 (Carl
Zeiss Microscopy Co., Ltd., Tokyo, Japan) and a BZ9000 (Keyence
Corporation, Osaka, Japan), respectively.
Human chorionic villous explant
culture
Placental tissue specimens were obtained from
healthy women at 6 to 9 weeks of gestation undergoing legal
abortions (n=6). Villous explant cultures were established as
previously reported (8), with
slight modifications. Briefly, placental tissues were placed in
ice-cold PBS, washed several times, and aseptically dissected to
remove decidual tissues and foetal membranes. After the placental
tissues were minced, the villous fragments were placed in 10-cm
collagen 1-coated dishes (AGC Techno Glass Co., Ltd., Shizuoka,
Japan). The explants were cultured in Placental Epithelial Cell
Growth Medium (ready-to-use) (PromoCell, Heidelberg, Germany) with
100 U/mL penicillin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan),
100 µg/mL streptomycin (Meiji Seika Pharma Co., Ltd.), and 25 µg/mL
amphotericin B (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
tissue specimens were incubated at 37°C in a 5% CO2 atmosphere. The
samples of primary cultured EVTs, the cell outgrowth from the
explants, were collected with trypsin and filtered with a 100-µm
mesh (Greiner Bio-One Co., Ltd., Frickenhausen, Germany) to extract
total RNA. The present study was approved by the Ethics Committee
of Nagoya University Hospital (Nagoya, Japan; approval no. 648).
Written informed consent was obtained from each patient for use of
the chorionic villous explant culture samples collected between
October 2014 to January 2015.
Cell culture
The establishment of the human EVT cell line
HTR-8/SVneo has been reported previously (10). The cells were grown in RPMI 1640
medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
heat-inactivated foetal calf serum (FCS; Biological Industries,
Kibbutz Beit Haemek, Israel), penicillin (100 U/mL), and
streptomycin (100 µg/mL) at 37°C in a humidified atmosphere
containing 5% CO2.
Total RNA isolation and reverse
transcription (RT)
Total RNA isolation was performed using the RNeasy
mini kit followed by treatment with RNase-Free DNase I (Qiagen
GmbH, Hilden, Germany), as suggested by the manufacturer. Total RNA
from primary cultured EVTs was reverse-transcribed in a 20 µl
reaction volume using ReverTra Ace (Toyobo Life Science, Osaka,
Japan). Total RNA from HTR-8/SVneo was reverse-transcribed using
High-Capacity cDNA Reverse Transcription kits and RNase Inhibitor
(Thermo Fisher Scientific Inc.).
RT-quantitative polymerase chain
reaction (RT-qPCR) and semi-quantitative (sq)-PCR
RT-qPCR was carried out with an Applied
Biosystems® StepOne plus (Thermo Fisher Scientific Inc.)
to measure mRNA expression of E2F8, E2F7, E2F1, E2F3, TIMP-1,
TIMP-2, PAI-1, and GAPDH using Fast SYBR®
Green Master Mix (Thermo Fisher Scientific Inc.). The cycling
parameters were as follows: Holding stage of 95°C for 20 sec, 40
cycles at 95°C for 3 sec, 60°C for 30 sec, one melting curve stage
of 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. The
amplification specificity was confirmed by melting curve analysis.
Using GAPDH as an endogenous reference gene, relative
expression was estimated using the comparative Cq
(2−ΔΔCq) method (11).
Data were automatically processed by StepOne plus software (Thermo
Fisher Scientific Inc.). All of the primer sequences are listed in
Table I.
 | Table I.List of primers. |
Table I.
List of primers.
| Genes | Direction | Primer sequences
(5′-3′) | Length (bp) |
|---|
| E2F1 | Forward |
CTCCTGAGACCCAGCTCCAA | 114 |
|
| Reverse |
ATCCCACCTACGGTCTCCTCA |
|
| E2F3 | Forward |
GTATGATACGTCTCTTGGTCTGC | 78 |
|
| Reverse |
CAAATCCAATACCCCATCGGG |
|
| E2F7 | Forward |
AAAGGGACTATTCCGACCCAT | 168 |
|
| Reverse |
ACTTGGATAGCGAGCTAGAAACT |
|
| E2F8 | Forward |
AAGTACGCCGAGCAGATTATG | 127 |
|
| Reverse |
ATGTCTGGGTGTCCATTTGGG |
|
| TIMP-1 | Forward |
CGGCCTTCTGCAATTCCGACC | 146 |
|
| Reverse |
GGATGTCAGCGGCATCCCCTA |
|
| TIMP-2 | Forward |
CTCGGCAGTGTGTGGGGTC | 137 |
|
| Reverse |
TGGGTGGTGCTCAGGGTGTC |
|
| PAI-1 | Forward |
CAGACCAAGAGCCTCTCCAC | 181 |
|
| Reverse |
GACTGTTCCTGTGGGGTTGT |
|
| GAPDH | Forward |
CATCCATGACAACTTTGGTATCGT | 107 |
|
| Reverse |
CCATCACGCCACAGTTTCC |
|
To estimate the amount of cDNA for sqPCR, the Cq
value of GAPDH was obtained by RT-qPCR, because it is
inversely correlated with the amount of template cDNA present in
the reaction. Equal amounts of cDNA were used as templates for PCR.
sqPCR was performed on the cDNA of primary cultured EVTs by using a
Veriti Thermal Cycler (Thermo Fisher Scientific Inc.) with Blend
taq (Toyobo Life Science), as previously reported (12).
The sqPCR conditions were as follows:
Pre-denaturation at 94°C for 5 min, 35 cycles of denaturation at
94°C for 30 sec, annealing at 55°C for 30 sec, and extension at
72°C for 1 min. The amplification products were electrophoresed on
15% polyacrylamide gels.
Knockdown of E2F8 expression
To knockdown E2F8 expression, HTR-8/SVneo
cells were infected with retrovirus expressing shRNA against E2F8
or non-target control shRNA. Oligonucleotides encoding shRNA
specific to human E2F8 (5′-GCAGCCAATGATACCTCAAAG-3′)
(shE2F8) and luciferase for control
(5′-CTTACGCTGAGTACTTCGA-3′) (shControl) were cloned into a
retroviral expression vector pSIREN-RetroQ (Takara Bio, Inc., Otsu,
Japan). Insertion of expression constructs was confirmed by DNA
sequence analysis. 293T cells (RCB220; RIKEN BioResource Center,
Tsukuba, Japan) were co-transfected with the pSIREN-RetroQ encoding
either shE2F8 or shControl in combination with the
pVPack-GP and pVPack-Ampho vectors (Agilent Technologies, Inc.,
Santa Clara, CA, USA) using Lipofectamine 3000 (Thermo Fisher
Scientific Inc.). After 6 h, the culture medium of 293T cells was
replaced with fresh RPMI 1640 supplemented with 10% FCS, penicillin
(100 U/mL), and streptomycin (100 µg/mL). The 293T cell supernatant
was collected after 48 h. The supernatant and 8 µg/mL Polybrene
(Nacalai Tesque Inc., Kyoto, Japan) were added to HTR-8/SVneo cells
when the cell density reached about 50%. After 20 h of incubation,
the infected cells were selected in RPMI 1640 with 10% FCS, 100
U/mL penicillin, 100 µg/mL streptomycin, and 1 µg/mL puromycin
(Nacalai Tesque Inc.). Subsequent experiments were performed using
the pooled populations of puromycin-resistant cells after drug
selection.
Proliferation assay
The cells were plated in 96-well plates in
triplicate at a density of 1,650 cells in a 100 µl volume and
cultured with RPMI 1640 supplemented with 10% FCS. After 1, 2, and
3 days in culture, cell proliferation was examined using a Cell
Counting Kit-8 (Dojindo Molecular Technologies Inc., Kumamoto,
Japan) according to the manufacturer's instruction. Absorbance was
measured at 450 nm using a microplate reader (Thermo Scientific™
Multiskan™ FC; Thermo Fisher Scientific Inc.). The experiments were
repeated three times.
Cell cycle analysis
Cells were seeded at 5×105 cells per
10-cm culture dish, cultured for 72 h, trypsinised, and fixed with
70% ethanol in PBS. RNase (0.25 mg/ml; Thermo Fisher Scientific
Inc.) and propidium iodide (50 µg/mL; Sigma-Aldrich; Merck KGaA)
were added to the fixed cells, and incubated for 30 min on ice.
Then they were analysed with an Attune Acoustic Focusing Cytometer
(Thermo Fisher Scientific Inc.). The experiment was repeated three
times.
Invasion assay
Cell invasion was determined by the ability of the
cells to cross the 8-µm pores of polycarbonate membranes (6.5 mm
filter; 8 µm pore size; Corning Costar Inc., Corning, NY, USA)
coated with 5 µg/well Matrigel (Becton Dickinson; BD Biosciences).
In brief, the shControl and shE2F8 cells
(6×104 cells/well) were placed in the upper chamber, and
700 µl of RPMI 1640 medium containing 10% FCS was added to the
lower chamber. Samples were then incubated at 37°C under a 5%
CO2 atmosphere for 16 h, and the cells on the upper
surface of the membrane were removed with a sterile cotton swab.
The cells which had moved through the membrane to the lower
surface, were stained with Giemsa Stain Solution (Wako Pure
Chemical Industries, Ltd.). The surfaces of the membrane were
photographed using a BX43 microscope with a DP21 camera and
cellSens software (Olympus Corp., Tokyo, Japan) at magnification,
×4 and the number of invading cells were counted in a
2.3-mm2 area. The experiment was performed in triplicate
and repeated three times.
Gelatin zymography
To determine the activity of the secreted proteases
involved in cell invasion and migration, supernatant from
shE2F8 and shControl cells was assayed by zymography.
These cells (2×105 cells) were seeded onto 3.5-cm culture dishes
and cultured as described above. Twenty-four h after seeding, the
cells were washed with serum-free medium and replaced with another
500 µl of serum-free RPMI 1640. After 24 h incubation, the
supernatant was collected and centrifuged to remove cells.
Tris-Glycine SDS Sample Buffer without reducing agent was added to
the supernatant, and 20 µl of the sample was electrophoresed on 10%
SDS-polyacrylamide gels containing 0.03% gelatin. After
electrophoresis, the gels were washed three times with wash buffer
[50 mM Tris-HCl (pH 7.4), 2.5% Triton X-100] and incubated at 37°C
in reaction buffer [50 mM Tris-HCl (pH 7.4), containing 10 mM
CaCl2] for 24 h. The gels were fixed with 50% methanol and 10%
acetic acid, stained with a solution of 0.2% Coomassie Brilliant
Blue in 50% methanol and 10% acetic acid for 30 min and then washed
with 20% methanol and 10% acetic acid. The gelatinases were then
detected as unstained bands. Matrix metalloproteinase (MMP) marker
(Primary Cell, Ishikari, Japan) containing pro-MMP-9, pro-MMP-2,
and MMP-2 was loaded into the gels. The gels were digitised with
ImageQuant LAS 4010 (GE Healthcare UK Ltd., Little Chalfont, UK)
using a white transilluminator, and the band intensity was measured
using ImageQuant TL (GE Healthcare UK Ltd.). The experiment was
repeated three times.
Assay of adhesion to extracellular
matrix-coated dish
Experiments were performed as previously reported
(13). Briefly, 4×104
cells were plated in 100 µl of serum-free RPMI 1640 in 96-well
microtiter plates coated with fibronectin, laminin, collagen 1, or
collagen 4 (Corning Inc.). Then the culture plates were centrifuged
at 500 rpm for 30 sec and incubated at 37°C in a humidified
atmosphere with 5% CO2 for 2 h. After incubation, the
plates were washed three times with assay buffer to remove
non-adherent cells. Then, the adherent cells were evaluated for
cell viability using the modified
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay using a CellTiter 96® AQueous One Solution Cell
Proliferation Assay kit (Promega, Madison, WI, USA). Absorbance was
measured at 490 nm using a microplate reader, Viento 808 IU (BioTek
Instruments, Inc., Winooski, VT, USA). Three individual experiments
were performed in triplicate.
Mass spectrometry analysis
shE2F8 and shControl cells were lysed
in lysis buffer [50 mM Tris-HCl (pH 7.6), 150 mM NaCl, cOmplete™
EDTA-free Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA)]
and sonicated for 5 sec on ice. The lysates were then centrifuged
for 30 min at 13,000 rpm and the supernatant fraction collected.
Their total protein concentrations were assessed by the BCA assay
(Pierce™ BCA Protein Assay kit-Reducing Agent Compatible; Thermo
Fisher Scientific, Inc.), using bovine serum albumin for generating
the standard curve. The procedure was performed in accordance with
a previous report (14).
The protein samples were digested with trypsin and
subjected to mass spectrometry analysis using the Q Exactive Hybrid
Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific
Inc.) in combination with an Advance LC System (Bruker Corporation,
Billerica, MA, USA) Then, the samples were injected into the
Advance LC System equipped with a MonoCap C18 0.1 mm in diameter
and 150 mm in length (GL Sciences Inc., Tokyo, Japan). Finally,
multiple MS/MS spectra were submitted to the Mascot program,
version 2.5.1 (Matrix Science Inc., Boston, MA, USA) for the MS/MS
ion search. The MS data were analysed using the Mascot software
(Matrix Science, Wyndham Place, UK) with a threshold of a 1.2-fold
change to identify differentially expressed proteins in
shE2F8 cells compared to those in shControl cells, in
accordance with a previous report (15,16).
The proteins thus identified were categorised using the Database
for Annotation, Visualization and Integrated Discovery (DAVID,
david.abcc.ncifcrf.gov, and version
6.8). Proteomap was generated to visualise the differential
contribution of biological pathways (bionic-vis.biologie.uni-greifswald.de/, v2.0).
Western blot analysis
Cells were lysed in sample buffer [50 mM Tris-HCl
(pH 6.8), 5% glycerol, 2% SDS] and boiled at 95°C for 3 min. The
whole-cell lysates were resolved by 10% SDS-PAGE and transferred
onto a Polyvinylidene Difluoride membrane (Immobilon-P; EMD
Millipore, Billerica, MA, USA). MMP-1 was detected using 0.092
µg/mL rabbit anti-human MMP-1 polyclonal antibody (ab137332; Abcam,
Cambridge, UK). Horseradish peroxidase-conjugated antibody was used
as a secondary antibody (0.019 µg/mL, NA934; GE Healthcare UK
Ltd.). The 0.2 µg/mL mouse anti-beta actin monoclonal antibody
(017-24573; WAKO Pure Chemical Industries, Ltd.) was used for
standardising the amount of sample loaded. The chemiluminescent
signals were detected using ECL Plus (GE Healthcare UK Ltd.) and
scanned using ImageQuant LAS 4010 (GE Healthcare UK Ltd.). The band
intensity was measured using ImageQuant TL (GE Healthcare UK Ltd.)
and the experiment was performed seven times.
Statistical analysis
R2.15.2 software was used for statistical analysis
(cran.r-project.org/bin/windows/base/old/2.15.2/). The
data were expressed as the mean ± standard deviation and
statistical analyses were performed with a Student's t-test or
Welch's t-test, according to whether equal variance was assumed or
not, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
E2F8 expression in human placenta
In the first trimester, the localisation of E2F8 was
investigated, and double staining of cytokeratin (CK), as an
epithelial marker, and vimentin, as a stromal marker, was
performed. E2F8 was expressed in EVTs and decidual cells,
co-stained with CK and vimentin, respectively (Fig. 1A). E2F8 expression was strong in
cytotrophoblasts in the cell columns and villi, but weak in
syncytiotrophoblasts (Fig. 1B).
Then, we also confirmed the expression of E2F8 in EVTs in the third
trimester (Fig. 1B). It was found
that E2F8 mRNA was expressed in primary cultured EVTs from 6
to 9 weeks of gestation (n=6) and HTR-8/SVneo cells, an EVT cell
line (Fig. 1C).
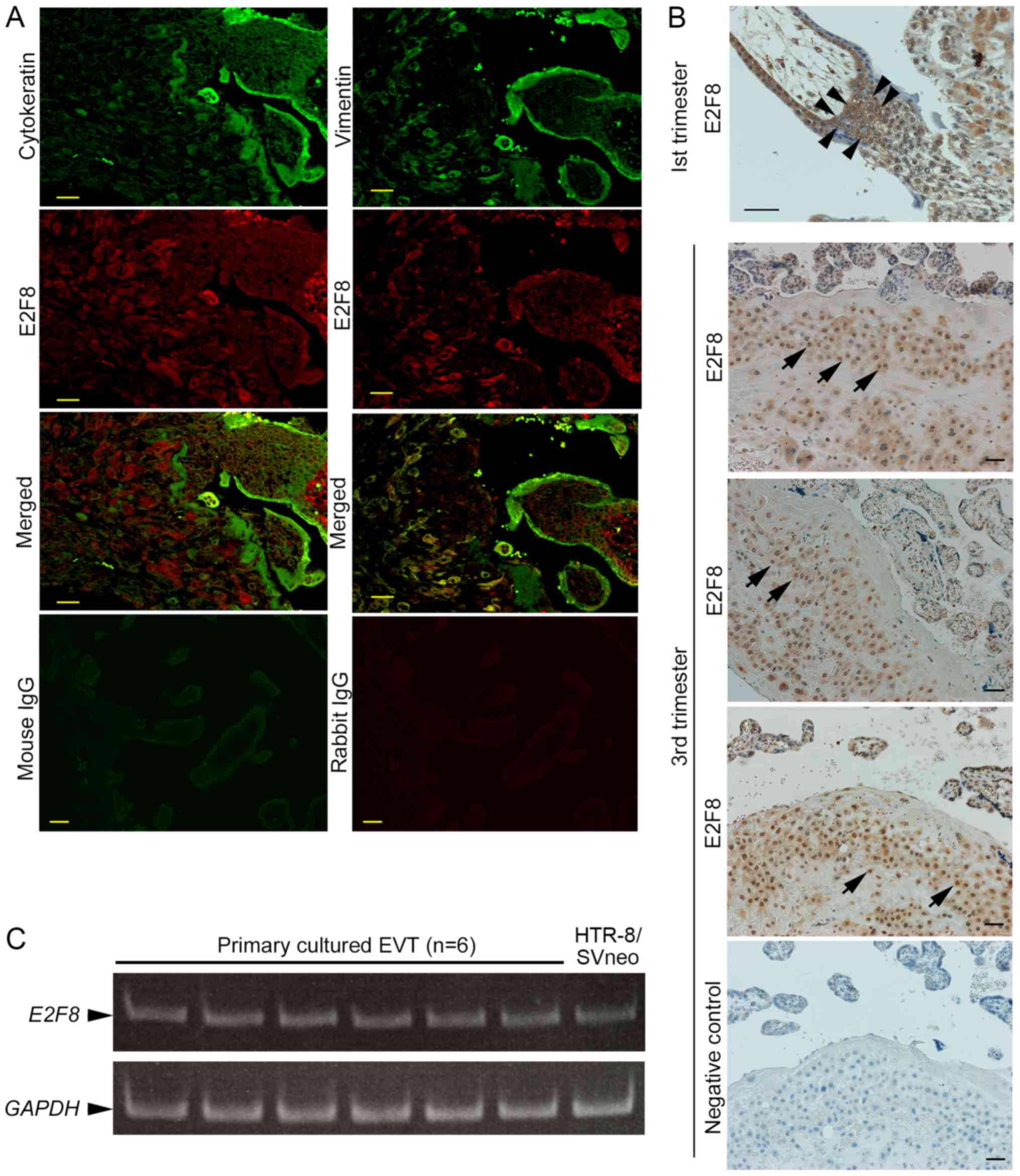 | Figure 1.Localisation of E2F8 in the human
placenta. (A) Double immunofluorescence staining was performed on
samples of a first trimester human placenta. Staining was conducted
for Cytokeratin (green), an epithelial cell marker, and E2F8 (red),
or Vimentin (green), a marker of stromal cells, and E2F8 (red). The
specimen exhibiting no staining with nonspecific rabbit or mouse
IgG served as the negative controls. Magnification, ×200; Scale
bars, 40 µm. (B) Immunohistochemical staining of E2F8 in first and
third trimester human placentas. E2F8 was detected in
cytotrophoblasts (arrowheads) from the first trimester cell column
and third trimester EVTs (arrows). The negative control is
presented in the bottom panel (Magnification, ×200; scale bars, 40
µm). (C) E2F8 mRNA expression in cultured primary EVTs (6 to
9 weeks of gestation; n=6) was determined by semi-quantitative
polymerase chain reaction. HTR-8/SVneo is a human extravillous
trophoblast cell line derived from first trimester human
trophoblasts. E2F, E2F transcription factor; EVTs, extravillous
trophoblasts; IgG, immunoglobulin G. |
E2F8 silencing in HTR-8/SVneo
To elucidate the role of E2F8 in EVTs, HTR-8/SVneo
cells were stably infected with a retrovirus expressing shRNA
targeting E2F8. E2F8 mRNA was clearly reduced in
shE2F8 cells compared with shConrtol cells (Fig. 2A, P<0.01). In shE2F8
cells, the expression of other E2F family proteins, including E2F7,
E2F1, and E2F3, was also examined to exclude the effects of the
changed expression levels of these factors. E2F8 silencing
did not significantly affect expression of E2F7, E2F1, or
E2F3 (Fig. 2A, P=0.461,
P=0.550, and P=0.386, respectively). Then, the role of E2F8 in the
cell cycle, proliferation, and invasion in HTR-8/SVneo cells was
examined. E2F8 silencing did not significantly affect the
cell cycle (Fig. 2B) or
proliferation (Fig. 2C). However,
the number of invaded cells in the shE2F8 cells was
significantly larger than that in shControl cells (Fig. 2D, P<0.01).
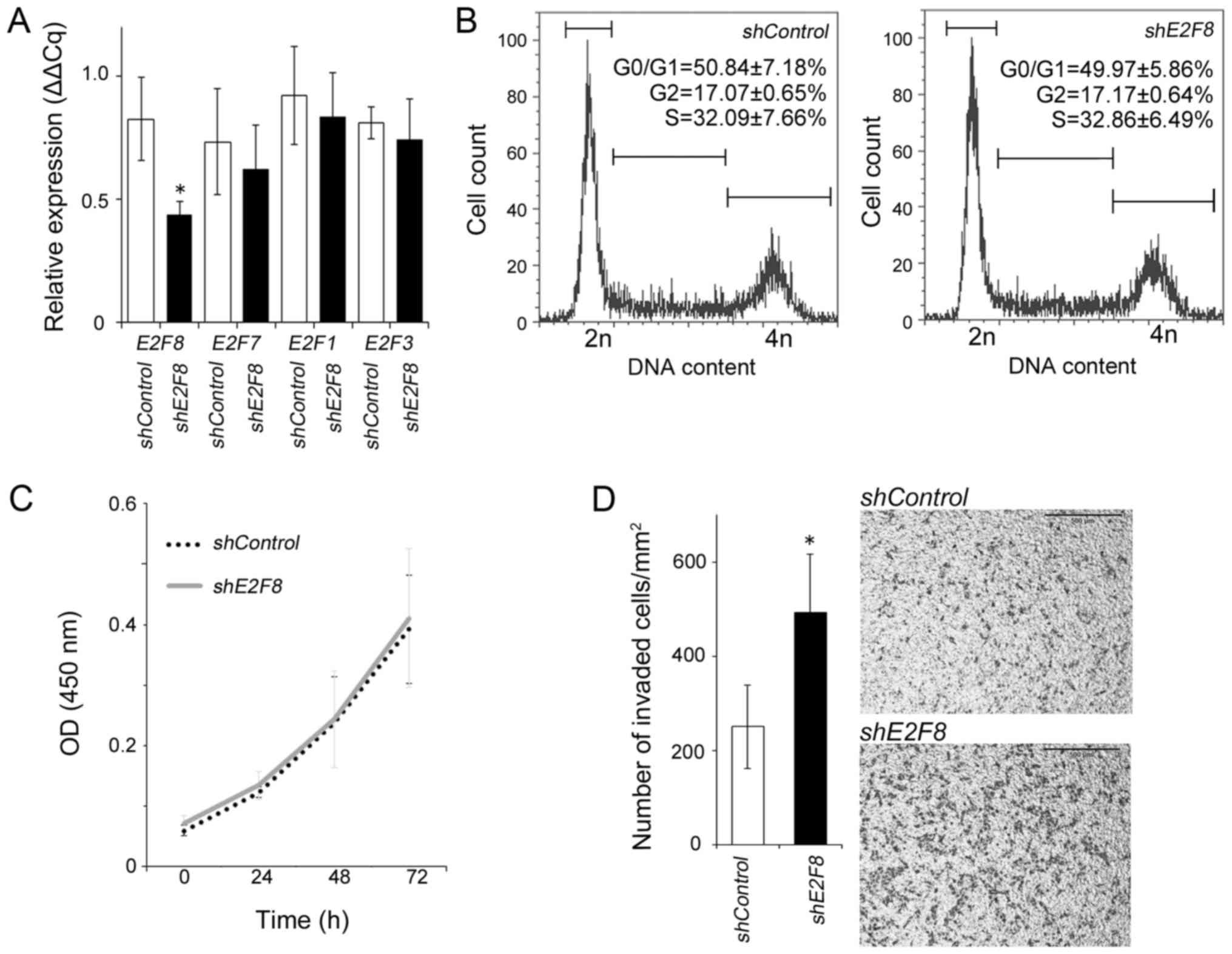 | Figure 2.Effects of E2F8 knockdown on
the mRNA expression of E2F family members, and cell proliferation
and invasion in HTR-8/SVneo. (A) mRNA levels of E2F8, E2F7,
E2F1, and E2F3 as determined by reverse
transcription-quantitative polymerase chain reaction analysis of
shE2F8 and shControl cells. The E2F8 mRNA
levels significantly decreased in shE2F8 cells when compared
with those of shControl cells; however, no significant
difference in E2F7, E2F1 or E2F3 mRNA levels was
observed between the two groups. The results are expressed as the
mean ± standard deviation (n=4/group). (B) Cell cycle progression
was analysed by flow cytometry 72 h following plating. Data are
shown as the percentage of the corresponding phases (n=3). (C) Time
course of cell proliferation was measured by Cell Counting Kit-8.
Cell growth curves were not significantly different between the
shControl and shE2F8 cells. shControl and
shE2F8 cells are represented as dotted and solid lines,
respectively. (D) The number of invaded cells (per mm2)
was significantly increased in shE2F8 cells when compared
with shControl cells. Representative images of
transwell invasion assays with shControl (upper image) and
shE2F8 (lower image) cells are presented. Magnification,
×100; Scale bar, 500 µm. The results are expressed as the mean ±
standard deviation. *P<0.01 vs. shControl. E2F, E2F
transcription factor; sh-, short hairpin RNA; OD, optical
density. |
The effect of E2F8 silencing on molecules involved
in HTR-8/SVneo invasion and adhesion. We examined whether MMP-2,
MMP-9, TIMP-1, TIMP-2 and PAI-1 expression was
altered by E2F8 silencing. These molecules are well known as
factors related to the invasive potential of EVTs. The expression
of pro-MMP-2 and pro-MMP-9 in shE2F8 cells were found to be
slightly higher than that in shControl cells, but not
significantly changed (Fig. 3A,
P=0.546 and P=0.556, respectively). E2F8 silencing did not
significantly affect the expression of TIMP-1, TIMP-2, or
PAI-1 (Fig. 3B, P=0.097,
P=0.962, and P=0.765, respectively).
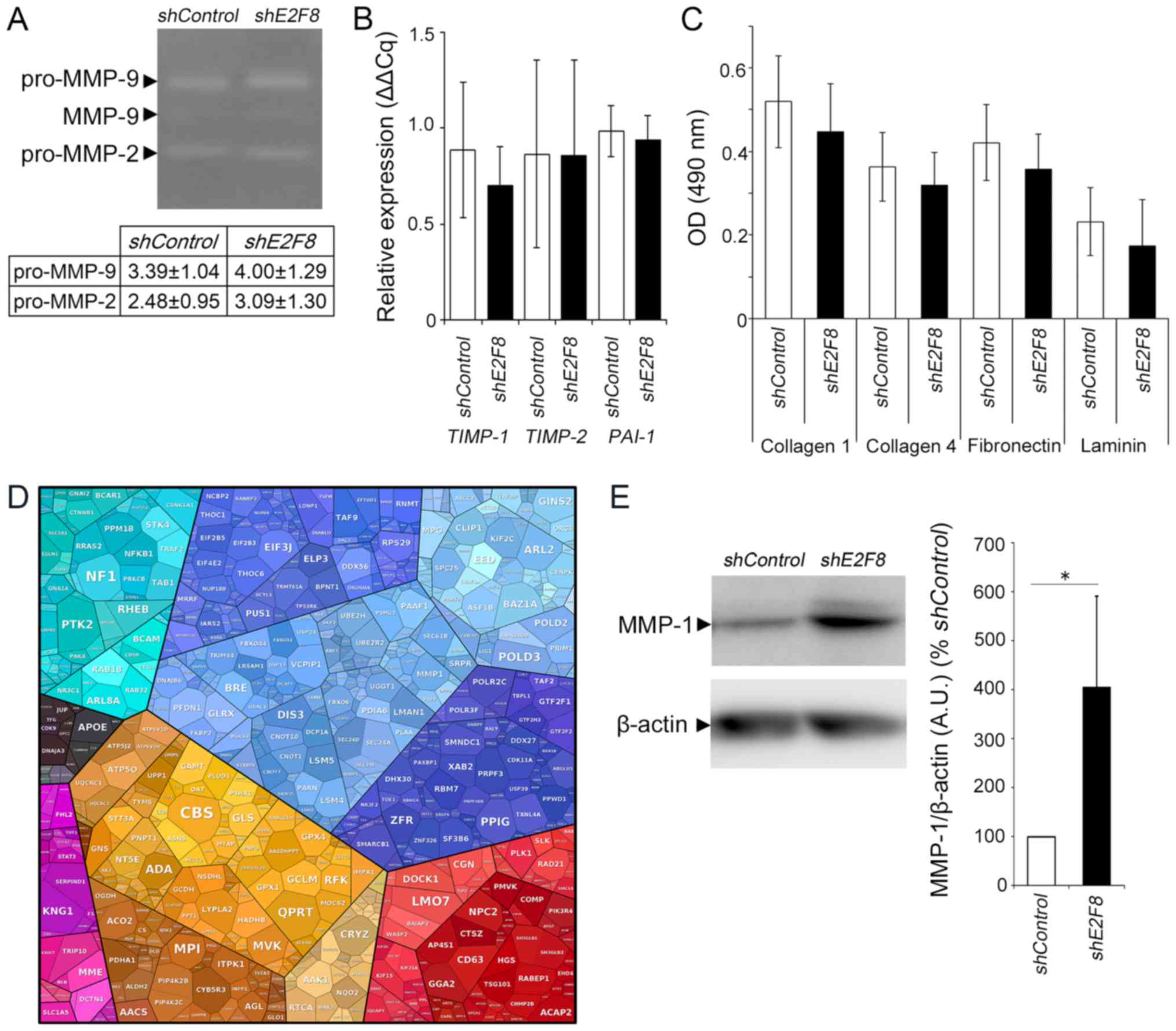 | Figure 3.Effects of E2F8 knockdown on
the mRNA and protein expressions involved in matrix degradation.
(A) MMP-2 and MMP-9 activity by gelatin zymography comparing
shE2F8 and shControl cells. Pro-MMP-2 and pro-MMP-9
activity were quantified by band intensity. Values are expressed as
the mean ± standard deviation of three independent experiments. (B)
mRNA expression levels of TIMP-1, TIMP-2 and PAI-1
were not changed in shE2F8 cells, as determined by reverse
transcription-quantitative polymerase chain reaction analysis.
Statistical bars in the graph are expressed as the mean ± standard
deviation of four independent experiments. (C) shControl or
shE2F8 cells were seeded on collagen 1, collagen 4,
fibronectin and laminin-coated plates. The extent of cell adhesion
is presented as absorbance at 490 nm. Values are expressed as the
mean ± standard deviation of independent experiments performed in
triplicate. (D) Proteomic analysis of shE2F8 and
shControl cells. Proteomap of gene ontology annotation,
where the areas for each protein reflect the magnitude of the fold
change in shE2F8 cells in comparison with shControl
cells. (E) Western blot analysis of MMP-1 in shE2F8 and
shControl cells. The bar graph presents MMP-1 band intensity
normalised to that of b-actin. Values are expressed as the mean ±
standard deviation of independent experiments performed in
septuplicate. *P<0.05, as indicated. E2F, E2F transcription
factor; sh-, short hairpin RNA; OD, optical density; MMP, matrix
metalloproteinase; TIMP, tissue inhibitor of metallopeptidase; PAI,
plasminogen activator inhibitor-1. |
We also investigated the effect of E2F8
silencing on the adhesive ability to collagen 1, collagen 4,
fibronectin, and laminin, but found no significant changes
(Fig. 3C, P=0.475, P=0.544,
P=0.425, and P=0.509, respectively).
The proteomic profile of shE2F8 cells was
analysed and compared with that of shControl cells. The
1,293 proteins were identified as differentially expressed (data
not shown). MMP-1 was listed as one of upregulated proteins in
shE2F8 cells (Fig. 3D),
which was validated by western blot analysis. The MMP-1 expression
in shE2F8 cells was significantly higher than that in
shControl cells (Fig. 3E,
P<0.05).
Discussion
The present study demonstrates E2F8 expression and
function in human EVT cells. The localisation of E2F8 was also
shown in cytotrophoblasts from the cell columns and EVTs in the
human placenta in the first and third trimesters. Thus, E2F8 also
exists in an invasive type of trophoblast in both the human
placenta and murine placenta. Additionally, E2F8 was detected in
decidual cells, as previously reported (17).
However, the function of E2F8 in human EVTs does not
seem to be consistent with that in murine TGCs. In the mouse model,
E2F8 plays a critical role in placental development. Based on these
findings, we speculated that decrease of E2F8 in human EVTs might
have a negative effect on invasive ability, as successful EVT
invasion into maternal decidua is essential for the development of
human placenta. Therefore, we investigated whether E2F8
knockdown might decrease the invasive ability of EVTs, but
unexpectedly E2F8 suppression significantly increased their
invasion. These results suggest that E2F8 suppression would
promote placental growth with increased EVT invasion. From these
findings, E2F8 does not seem to be critical for successful invasion
of human EVTs. A previous study reported that E2F3 expression in
FGR placentas was lower than that in the placentas of normal
control (18). Another research
demonstrated that E2F1 was significantly downregulated in placenta
with severe early-onset preeclampsia (19), which is known to be caused by
failed EVT invasion. Thus, E2F1 and E2F3 could promote placental
growth in human, although their function in human EVTs is not fully
known. On the other hand, in murine placenta E2F3 is thought to
have a negative role in placental growth (20). E2F1 and E2F3 are known to be
essential for cellular proliferation as classical E2F proteins
(21), but E2F8 functionally
antagonises the classical E2F proteins. These findings, together
with the results of the present study, suggest that E2F8 could play
a negative role in human placental development, which is consistent
with the finding that E2F1 and E2F3 could be important in the human
placenta. We speculated that upregulated invasion in shE2F8
cells might be related to E2F1 and E2F3 expression,
but E2F1 and E2F3 expression was unchanged in
shE2F8 cells. A previous research also suggested that the
function of E2F8 in human decidualisation was opposite of that in
mice. Decidualisation is an important change during conception, the
first step of pregnancy. Though E2F8 plays a role in the
development of the human placenta, this may not align with the role
of E2F8 in the murine placenta.
The increased invasiveness caused by E2F8
suppression would be related with increased MMP-1 expression in
shE2F8 cells. A previous study provided evidence to support
that impaired invasion of EVTs in preeclampsia and FGR could result
from reduced production of MMP-1 (22). Other reports demonstrated that
MMP-1 is involved in invasion of EVTs or HTR-8/SVneo (23,24).
The E2F transcription family proteins have the consensus winged
helix DNA-binding motif sequence (25). It was reported that E2F
transcription factors commonly bind to MMP promoter regions and
regulate MMP gene expression (26,27).
These findings suggest that E2F8 might regulate the MMP-1 promoter
negatively and suppress its expression, which might lead to
inhibition of EVT invasion.
E2F8 expression in EVTs and suppression of the
invasive ability of EVTs seem paradoxical, but E2F8 might act in
coordination with E2F1 and E2F3 to control EVT invasion. Moreover,
a part of the findings in murine placenta is consistent with our
results. In murine placenta, E2f7 and E2f8 ablation
in TGCs, spongiotrophoblasts, or both caused no significant change
in placental architecture (2),
which might mean that E2F8 could not either be correlated with
trophoblast invasion in murine placenta. The ablation of
E2f7 and E2f8 in all trophoblasts including
trophoblast progenitor cells in mice showed placental abnormalities
including FGR. In the present study, E2F8 knockdown could
not be performed in other trophoblasts, including cytotrophoblasts
and syncytiotrophoblasts, because their cell lines remain
unestablished. E2F8 might play some role in those trophoblasts and
concomitant human placental function. The present study
demonstrates that E2F8 expression was high in cytotrophoblasts in
the cell columns during the first trimester. Cytotrophoblasts are
thought to have a stem cell-like feature corresponding to that of
murine spongiotrophoblasts. Based on these findings, we speculate
that reduction in E2F8 expression in cytotrophoblasts might promote
cell differentiation into invasive EVTs, although further
investigation is required.
Furthermore, there is another potential limitation
of this study. The role of E2F8 was investigated in HTR-8/SVneo
cells. It is an EVT cell line that is used worldwide, but it is
also known to have a signature different from that of primary EVT
cells (28). Additionally, in this
study, the role of E2F7 was not investigated, but E2F7 has been
reported to be have a synergistic function with E2F8 (29,30).
Thus, the effect of E2F8 suppression on EVT functions in the
present study might be minimal because of E2F7 functions. It is
important to understand the involvement of both E2F7 and E2F8 on
human placental development in vivo, and further
investigation is required for this end. We intend to investigate
the role of E2F7 and correlation between E2F7 and E2F8 by
suppressing E2F7 or both E2F7 and E2F8 in our
future studies.
In conclusion, E2F8 is expressed in EVTs and
cytotrophoblasts in the cell columns of the human placenta, and may
be involved in invasion of EVTs, which is important for human
placental development. Further research is required to reveal the
role of E2F8, as well as other E2Fs, in human placental
development.
Acknowledgements
The human EVT cell line HTR-8/SVneo was a kind gift
from Dr Charles H. Graham (Queen's University, Ontario, Canada).
The authors would like to thank Mr. Kentaro Taki (Division for
Medical Research Engineering, Nagoya University Graduate School of
Medicine, Aichi, Japan) for the technical support.
Funding
The present study was supported by a research grant
from the Yamaguchi Endocrine Research Foundation (2013 grant).
Availability of data and materials
The analysed datasets generated during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TK designed the experiments, analysed the data and
wrote the paper. MM and RM performed the experiments, analysed the
data and wrote the paper. YM performed the experiments, and
analysed and interpreted the data. TU, KI, KN, TN, HT and SS
analysed and interpreted the data. EY, AI, TS and FK reviewed and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nagoya University Hospital (Aichi, Japan; approval
nos. 648 and 2017-0302). Written informed consent was obtained from
each patient for use of the chorionic villous explant culture
samples collected between October 2014 to January 2015. The
requirement for written informed consent for the use of first
trimester uterus and third trimester placental samples was waived
due to the retrospective nature of these experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CK
|
cytokeratin
|
|
E2F
|
E2F transcription factor
|
|
E
|
embryonic day
|
|
EVT
|
extravillous trophoblast
|
|
FGR
|
foetal growth restriction
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
MMP
|
matrix metalloproteinase
|
|
PAI
|
plasminogen activator inhibitor
|
|
PCR
|
polymerase chain reaction
|
|
RT
|
reverse transcription
|
|
TGC
|
trophoblast giant cell
|
|
TIMP
|
tissue inhibitor of
metalloproteinases
|
References
|
1
|
Nardozza LM, Caetano AC, Zamarian AC,
Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB and Araujo
Júnior E: Fetal growth restriction: Current knowledge. Arch Gynecol
Obstet. 295:1061–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ouseph MM, Li J, Chen HZ, Pécot T, Wenzel
P, Thompson JC, Comstock G, Chokshi V, Byrne M, Forde B, et al:
Atypical E2F repressors and activators coordinate placental
development. Dev Cell. 22:849–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weijts BG, Bakker WJ, Cornelissen PW,
Liang KH, Schaftenaar FH, Westendorp B, de Wolf CA, Paciejewska M,
Scheele CL, Kent L, et al: E2F7 and E2F8 promote angiogenesis
through transcriptional activation of VEGFA in cooperation with
HIF1. EMBO J. 31:3871–3884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bakker WJ, Weijts BG, Westendorp B and de
Bruin A: HIF proteins connect the RB-E2F factors to angiogenesis.
Transcription. 4:62–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weijts BG, van Impel A, Schulte-Merker S
and de Bruin A: Atypical E2fs control lymphangiogenesis through
transcriptional regulation of Ccbe1 and Flt4. PLoS One.
8:e736932013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt A, Morales-Prieto DM, Pastuschek
J, Fröhlich K and Markert UR: Only humans have human placentas:
Molecular differences between mice and humans. J Reprod Immunol.
108:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossant J and Cross JC: Placental
development: Lessons from mouse mutants. Nat Rev Genet. 2:538–548.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mano Y, Kotani T, Shibata K, Matsumura H,
Tsuda H, Sumigama S, Yamamoto E, Iwase A, Senga T and Kikkawa F:
The loss of endoglin promotes the invasion of extravillous
trophoblasts. Endocrinology. 152:4386–4394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto E, Ino K, Miyoshi E, Inamori K,
Abe A, Sumigama S, Iwase A, Kajiyama H, Shibata K, Nawa A and
Kikkawa F: N-acetylglucosaminyltransferase V regulates extravillous
trophoblast invasion through glycosylation of alpha5beta1 integrin.
Endocrinology. 150:990–999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto K, Miki R, Nakayama M, Tatsumi N
and Yokouchi Y: Wnt9a secreted from the walls of hepatic sinusoids
is essential for morphogenesis, proliferation, and glycogen
accumulation of chick hepatic epithelium. Dev Biol. 319:234–247.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kajiyama H, Shibata K, Terauchi M, Ino K,
Nawa A and Kikkawa F: Involvement of SDF-1alpha/CXCR4 axis in the
enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int
J Cancer. 122:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moriyama Y, Kotani T, Ushida T, Imai K,
Nakano T, Tsuda H and Kikkawa F: Altered proteomic profile in
umbilical arterial serum from mothers with schizophrenia. Schizophr
Res. (pii)S0920-9964:30101–30104. 2018.
|
|
15
|
Sun Y, Gao C, Wang X, Yuan Y, Liu Y and
Jia J: Serum quantitative proteomic analysis of patients with
keshan disease based on iTRAQ labeling technique: A first term
study. J Trace Elem Med Biol. 44:331–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Wang J, Gao L, Liu H and Liu C:
iTRAQ-based proteomic analysis of neonatal kidney from offspring of
protein restricted rats reveals abnormalities in intraflagellar
transport proteins. Cell Physiol Biochem. 44:185–199. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi QR, Zhao XY, Zuo RJ, Wang TS, Gu XW,
Liu JL and Yang ZM: Involvement of atypical transcription factor
E2F8 in the polyploidization during mouse and human
decidualization. Cell Cycle. 14:1842–1858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Q, Wu W, Xu X, Huang L, Gao Q, Chen
H, Sun H, Xia Y, Sha J, Wang X, et al: miR-141 contributes to fetal
growth restriction by regulating PLAG1 expression. PLoS One.
8:e587372013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaitu'u-Lino TJ, Hastie R, Cannon P,
Nguyen H, Lee S, Hannan NJ and Tong S: Transcription factors E2F1
and E2F3 are expressed in placenta but do not regulate MMP14.
Placenta. 36:932–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chong JL, Tsai SY, Sharma N, Opavsky R,
Price R, Wu L, Fernandez SA and Leone G: E2f3a and E2f3b contribute
to the control of cell proliferation and mouse development. Mol
Cell Biol. 29:414–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Timmers C, Maiti B, Saavedra HI,
Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, et
al: The E2F1-3 transcription factors are essential for cellular
proliferation. Nature. 414:457–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lian IA, Toft JH, Olsen GD, Langaas M,
Bjørge L, Eide IP, Børdahl PE and Austgulen R: Matrix
metalloproteinase 1 in pre-eclampsia and fetal growth restriction:
reduced gene expression in decidual tissue and protein expression
in extravillous trophoblasts. Placenta. 31:615–620. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nystad M, Sitras V, Larsen M and Acharya
G: Placental expression of aminopeptidase-Q (laeverin) and its role
in the pathophysiology of preeclampsia. Am J Obstet Gynecol.
211:686.e1–31. 2014. View Article : Google Scholar
|
|
24
|
Suman P and Gupta SK: Comparative analysis
of the invasion-associated genes expression pattern in first
trimester trophoblastic (HTR-8/SVneo) and JEG-3 choriocarcinoma
cells. Placenta. 33:874–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgunova E, Yin Y, Jolma A, Dave K,
Schmierer B, Popov A, Eremina N, Nilsson L and Taipale J:
Structural insights into the DNA-binding specificity of E2F family
transcription factors. Nat Commun. 6:100502015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schaal C, Pillai S and Chellappan SP: The
Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and
metastasis. Adv Cancer Res. 121:147–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson JL, Pillai S, Pernazza D, Sebti
SM, Lawrence NJ and Chellappan SP: Regulation of matrix
metalloproteinase genes by E2F transcription factors: Rb-Raf-1
interaction as a novel target for metastatic disease. Cancer Res.
72:516–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bilban M, Tauber S, Haslinger P,
Pollheimer J, Saleh L, Pehamberger H, Wagner O and Knöfler M:
Trophoblast invasion: Assessment of cellular models using gene
expression signatures. Placenta. 31:989–996. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiti B, Li J, de Bruin A, Gordon F,
Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W and Leone G:
Cloning and characterization of mouse E2F8, a novel mammalian E2F
family member capable of blocking cellular proliferation. J Biol
Chem. 280:18211–18220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thurlings I, Martínez-López LM, Westendorp
B, Zijp M, Kuiper R, Tooten P, Kent LN, Leone G, Vos HJ, Burgering
B and de Bruin A: Synergistic functions of E2F7 and E2F8 are
critical to suppress stress-induced skin cancer. Oncogene.
36:829–839. 2017. View Article : Google Scholar : PubMed/NCBI
|

















