Introduction
Hypoxia is an important cause of pathological
alterations in various diseases, including diabetic retinopathy
(1). Since normal retinal
metabolism requires the consumption of a large amount of oxygen,
hypoxia results in damage, or even death, of retinal neuronal
cells, and other tissues and cells (2). Photoreceptor cells are the primary
neurons of the visual pathway and damage to these cells severely
affects visual functions.
Toll-like receptor 4 (TLR4) is a member of Toll-like
receptors (TLRs) family, which are germline-encoded innate immune
receptors that detect invading microbials and induce immune
responses (3). TLR4 expression is
upregulated via hypoxia-inducible factor-1α (HIF-1α) in response to
hypoxia condition (4). Previous
studies have shown that TLR4 is involved in retinal ischemic injury
(5,6). However, the role of TLR4 and its
downstream NADPH oxidase 4 (NOX4) under hypoxic condition is not
fully understood.
Progranulin is a multi-functional growth factor
composed of 593 amino acids, which is expressed in various tissues
in the body (7). Progranulin gene
mutations are not only associated with numerous neurodegenerative
diseases (8), but also serve
regulatory roles in inflammatory diseases. It has been reported
that progranulin suppressed TLR4-driven inflammatory response in
osteoarthritic tissues (9).
However, few studies have investigated the functions of progranulin
in retinopathy. In order to determine the function of progranulin
on the retina under hypoxic conditions, a hypoxia model was
established using the classic method of intravitreal injection with
cobalt chloride (CoCl2). Exogenous progranulin was
applied, after which, alterations in the function and morphology of
mouse photoreceptors, and in retinal vascular leukostasis, were
detected.
Materials and methods
Reagents and instruments
Progranulin, CoCl2, ketamine, xylazine
and resatorvid were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Peanut agglutinin (PNA; cat. no. FL-1071),
goat anti-rabbit fluorescent secondary antibody (cat. no. FI-1000)
and DAPI were purchased from Vector Laboratories, Inc. (Burlingame,
CA, USA). The rabbit anti-mouse caspase-3 (cat. no. MAB835-SP),
goat anti-mouse p53 (cat. no. AF1355-SP) and goat anti-mouse
β-catenin (cat. no. AF1329-SP) primary antibodies were purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). The rabbit
anti-mouse tumor necrosis factor (TNF)-α (cat. no. sc-52746),
nuclear factor (NF)-κB/p65 (cat. no. sc-8008), vascular endothelial
growth factor (VEGF; cat. no. sc-7269), HIF-1α (cat. no. sc-13515)
and β-actin (cat. no. sc-517582) primary antibodies were purchased
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The rabbit
anti-mouse s-opsin (cat. no. ABN1660) and NOX4 (cat. no. ABC271)
primary antibodies were purchased from EMD Millipore (Billerica,
MA, USA). The rabbit anti-mouse glycogen synthase kinase (GSK)-3β
(cat. no. ab32391), TLR4 (cat. no. ab13867) primary antibodies, HRP
conjugated goat anti-rabbit (cat. no. ab6721) and rabbit anti-goat
(cat. no. ab6741) secondary antibodies were purchased from Abcam
(Cambridge, UK). PBS, citric acid buffer and goat serum were
purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The
western blotting electrophoresis system and the protein
concentration determination reagent kit were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). Sodium hyaluronate gel was
purchased from Shandong Bausch & Lomb Freda Pharmaceutical Co.,
Ltd. (Shandong, China). Compound tropicamide eye drops were
purchased from Santen Pharmaceutical Co., Ltd. (Osaka, Japan). The
micro-injectors were purchased from the Hamilton Company (Reno, NV,
USA). The Espion electroretinography system was purchased from
Diagnosys Systems, Inc. (Westford, MA, USA). The inverted
fluorescence microscope was purchased from Olympus Corporation
(Tokyo, Japan), and the spectrophotometer was purchased from
SPECTRO Analytical Instruments GmbH (Kleve, Germany).
Experimental animals and groups
The study complied with the Animal Research:
Reporting of In Vivo Experiments guidelines (10) and was conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(Bethesda, MD, USA). The present study was approved by the
Committee on the Ethics of Animal Experiments, Nanchang University
(Nanchang, China). A total of 48 healthy, inbred, male C57BL/6 mice
(age, 8 weeks; weight, 20–30 g), without eye diseases, were
purchased from the Department of Animal Sciences, Nanchang
University. They were housed at a temperature of 18–23°C, humidity
of 40–60% with a 14-h light/10-h dark cycle and food and water
accessible at all times All experimental manipulations followed the
regulations of the Institutional Animal Care and Use Committee,
Nanchang University. The mice were numbered using ear tags and were
randomly divided into two groups: Normal condition and hypoxic
condition groups (n=24 mice/group).
Intravitreal injection with
CoCl2 to establish a hypoxic model
The mice in the two groups were anesthetized with a
mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) via
intraperitoneal injection, and iodophor was used to disinfect the
area around the eyes. A Hamilton micro-injector needle was inserted
1 mm outside the limbus in the optic nerve direction under a
microscope. The drugs were slowly dispensed when the needle tip was
observed to be in the pupillary area. In the hypoxic condition
group, 2 µl 9 mM CoCl2 was intravitreally injected into
both eyes. In the normal condition group, 2 µl PBS was
intravitreally injected into both eyes. After 6 h, 18 mice from
each group received intraperitoneal anesthesia as above. The
aforementioned intravitreal injection steps were repeated through
the previous puncture sites. The right eyes of the mice in the two
groups were injected with 0.5 µl 10 mM progranulin, and the left
eyes were injected with an equal amount of PBS. The remaining 6
mice in each group were injected with 0.5 µl 8 mM resatorvid in
both eyes; after 1 h, the right eyes of these mice were injected
with 0.5 µl 10 mM progranulin, and the left eyes with an equal
amount of PBS.
Immunoblotting
Anesthetized mice were sacrificed by an experienced
technician, 24 eyeballs from 12 mice were removed, and the retinal
tissues were collected and placed in an Eppendorf tube containing
200 µl RIPA lysis buffer (1% Nonidet P-40 (NP-40), 1% sodium
deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M sodium phosphate, pH
7.2, 2 mM EDTA, 50 mM sodium fluoride, 0.2 mM fresh sodium
orthovanadate and 100 U/ml protease inhibitor aprotinin). The
samples were sonicated (100–200 W) for 10 sec three times and were
then centrifuged (10,000 × g) for 20 min at 4°C. The supernatant
was collected, and the protein concentrations of the samples were
determined. Equal amounts (50 µg/lane) of protein samples were
boiled in loading buffer and subjected to SDS-PAGE (5% stacking gel
and 10% separating gel). The proteins were transferred onto
polyvinylidene difluoride (PVDF) membrane for 120 min. The membrane
was blocked with 5% non-fat dried milk in TBST at 37°C for 2 h then
incubated with the primary antibodies (1:1,000) at 4°C overnight.
The following day, the membrane was washed in TBST (Tris-buffered
saline with 0.1% Tween-20) three times and incubated with a
horseradish peroxidase-conjugated secondary antibody (1:2,000) at
room temperature for 1 h. Pierce ECL Western Blotting Substrate
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added in a
dark room for gel image analysis and measurement of the gray
density values of protein bands using UVP GelDoc-It Imager (UVP,
LLC, Phoenix, AZ, USA) and VisionWorks® Image
Acquisition & Analysis Software (version 7.0; UVP, LLC).
Electroretinogram (ERG)
Following intravitreal injection of progranulin or
PBS, mydriasis was induced in 12 mice from each group using
compound tropicamide eye drops; subsequently, the mice were placed
in a dark room overnight. The next day, following administration of
intraperitoneal anesthesia with mixture of ketamine (100 mg/kg) and
xylazine (10 mg/kg), the mice were placed onto a heating pad.
Reference and grounding electrodes were inserted into the palate
and tail. A platinum corneal electrode was placed at the corneas of
both eyes and lubricated using sodium hyaluronate gel. These
manipulations were all performed in a dark room with dim red-light
illumination. The light intensities (0.0004, 0.04, 4, 400 and 2,000
cd.s/m2) were established in the ERG to record the
scotopic ERG reaction. The light was turned on for adaption for 10
min and the photopic ERG reaction was then recorded under 2,000
cd.s/m2 light intensity.
Immunofluorescence
co-localization
Following completion of the ERG, six anesthetized
mice from each group were sacrificed under anesthetic by a
well-trained technician. The eyeballs were removed and directly
placed in 4% paraformaldehyde for 1 h of fixation at room
temperature. Following the removal of the cornea and lens, the
eyeballs were placed in 4% paraformaldehyde again for fixation for
15 min at room temperature. After washing in PBS and dehydrating in
gradient alcohol, the eyeballs were cleared in xylene I and xylene
II, immersed in paraffin, embedded, sectioned (5-µm) and the slides
dried for 30 min on a slide warmer at 37°C. The paraffin-embedded
sections were deparaffinized in xylene I and xylene II, rinsed in
anhydrous alcohol and placed in gradient alcohol. Antigen retrieval
was performed using 0.01 mol/l citric acid buffer at 98°C for 20
min. The sections were allowed to cool, rinsed three times with
PBS, incubated with goat serum in a moisture box for 1 h and were
then incubated with primary antibodies as above (1:1,000) at 4°C
overnight. The primary antibody was replaced with PBS in the
negative control group. Subsequently, the sections were rinsed
three times with PBS and were incubated with a secondary antibody
as above (1:2,000) and PNA at room temperature for 1 h. Finally,
the sections were mounted in DAPI containing an anti-quenching
agent. The results were observed under a fluorescence
microscope.
Retinal vascular leukostasis
assay
A total of 6 mice from each group were randomly
selected following completion of the ERG. The mice were
intraperitoneally anesthetized with a mixture of ketamine (100
mg/kg) and xylazine (10 mg/kg) (Sigma-Aldrich; Merck KGaA), after
which, the thoracic skin and ribs of the mice were cut to expose
the thoracic cavity. The descending aorta was clamped, the right
atrial appendage was cut, and a 27 G needle was inserted into the
left ventricle. Non-adherent leukocytes were washed away by
perfusion with a 10-ml mixture of PBS and heparin (0.1 mg/ml).
Subsequently, the adherent leukocytes were labeled with a mixed
solution containing 20 µg/ml PBS and fluorescein isothiocyanate
(FITC)-labeled concanavalin A (ConA; 5 mg/kg; Sigma-Aldrich; Merck
KGaA). FITC-ConA that did not interact with the leukocytes was
washed away using 10 ml PBS. Following sacrifice of the
anesthetized mice via spinal dislocation by a well-trained
technician, six pairs of eyeballs from each group were removed and
directly placed into 4% paraformaldehyde for fixation for 1 h at
room temperature. Finally, the retinal tissues were flat mounted,
and leukocytes were counted under a fluorescence microscope.
Statistical analysis
In vivo experiments were repeated ≥3 times.
The statistical analyses were performed using SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). The gray density values of the
western blots and the numbers of adherent leukocytes were expressed
as means ± standard deviation. The ERG results were expressed as
means ± standard error of the mean. The comparisons between the
parameters in the differently treated eyes were performed using
one-way analysis of variance followed by a post hoc
Student-Newman-Keul test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of the hypoxic mouse
model
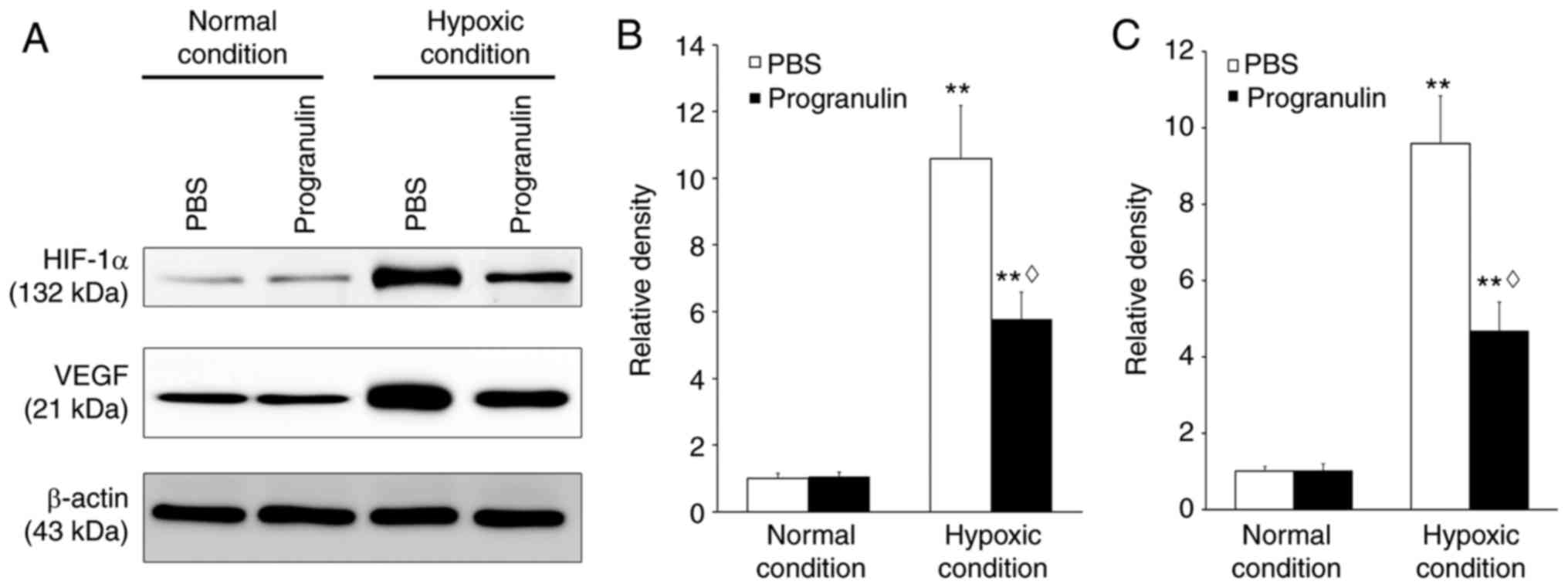
Retinal HIF-1α and VEGF expression were detected to
verify whether hypoxia was successfully induced. Immunoblotting
revealed that the expression levels of HIF-1α were significantly
upregulated under hypoxic conditions compared with under normal
conditions (P<0.01). However, under hypoxic conditions, the
expression of HIF-1α in the progranulin-treated eyes was ~54.58%
that in the PBS-treated eyes (P<0.05; Fig. 1A and B). Similar results were
obtained regarding the expression levels of VEGF, which were also
significantly upregulated under hypoxic conditions compared with
under normal conditions (P<0.01). However, under hypoxic
conditions, the expression of retinal VEGF in the
progranulin-treated eyes was ~48.91% that in the PBS-treated eyes
(P<0.05; Fig. 1A and C).
Progranulin attenuates the functional
damage of photoreceptor cells during hypoxia
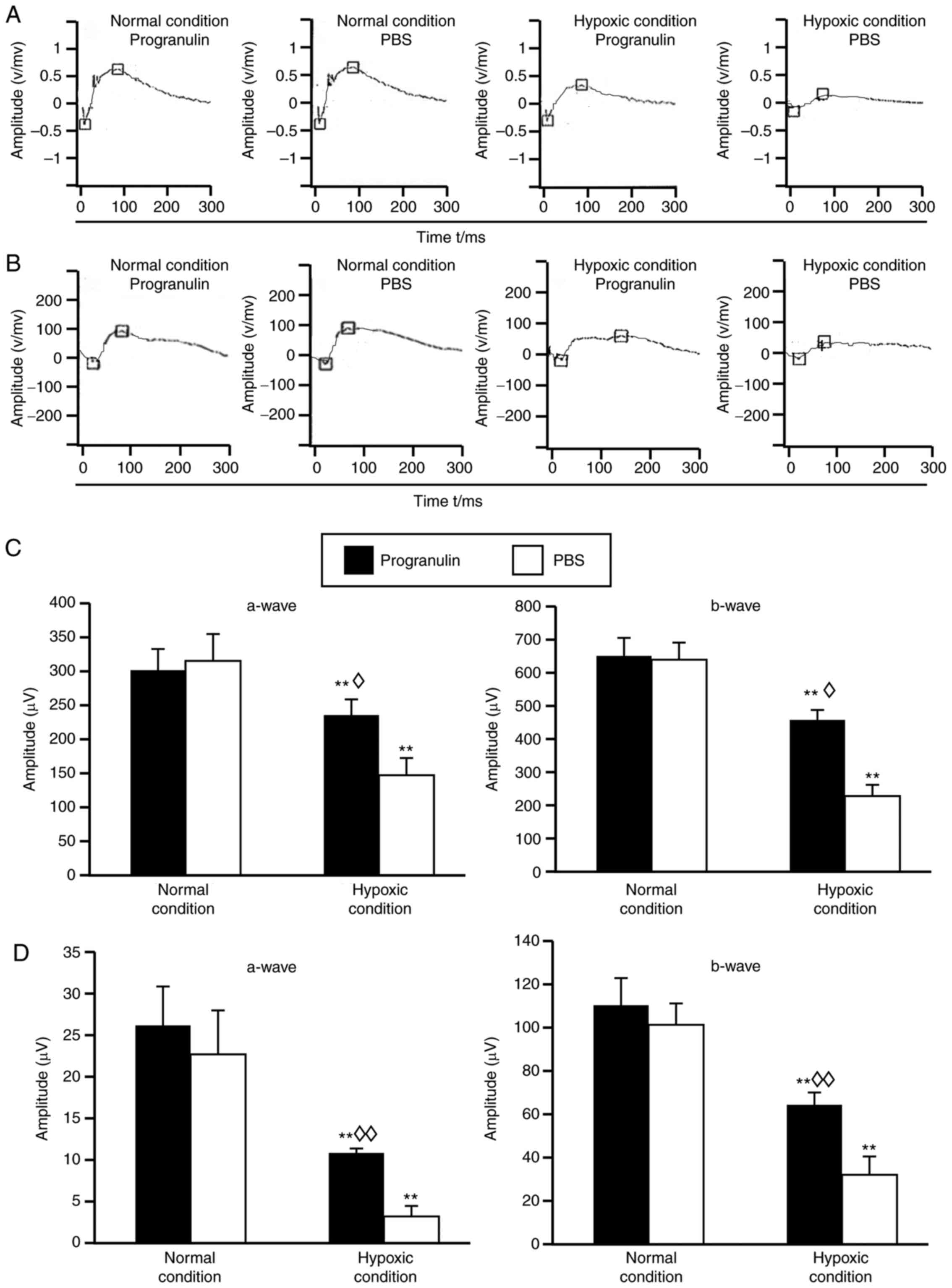
Under hypoxic conditions, the amplitudes of a-waves
and b-waves in the mouse scotopic ERG (Fig. 2A) and photopic ERG (Fig. 2B) were reduced compared with in the
normal control group, and statistical analysis demonstrated that
the differences were significant (Fig.
2C and D). In addition, the ERG amplitudes were reduced in the
PBS-injected eyes compared with in the progranulin-injected eyes
(Fig. 2A and B), and statistical
analysis demonstrated that the differences were significant
(Fig. 2C and D).
Progranulin attenuates morphological
damage of photoreceptor cells during hypoxia
Immunofluorescence co-localization was performed to
detect the morphology of retinal photoreceptor cells. S-opsin was
used to label the outer segments of the cone cells, PNA was used to
label the whole cone cells and DAPI was used to label the nuclei.
S-opsin and PNA reflected morphological alterations to the rod
cells, as did the thickness of the outer nuclear layer. The results
demonstrated that the thickness of the retinal outer nuclear layer
was significantly decreased in mice under hypoxic conditions
compared with in the normal control group. Furthermore, the
thickness of the retinal outer nuclear layer was smaller in the
PBS-injected eyes compared with in the progranulin-injected eyes.
The morphology of the cone cells also exhibited significant
alterations compared with the normal control group, with the
morphology of the outer segments becoming shorter, smaller and
disordered. The changes were more evident in the cone cells in the
PBS-injected eyes than in the progranulin-injected eyes, and the
cone cells were sparser in the former group (Fig. 3).
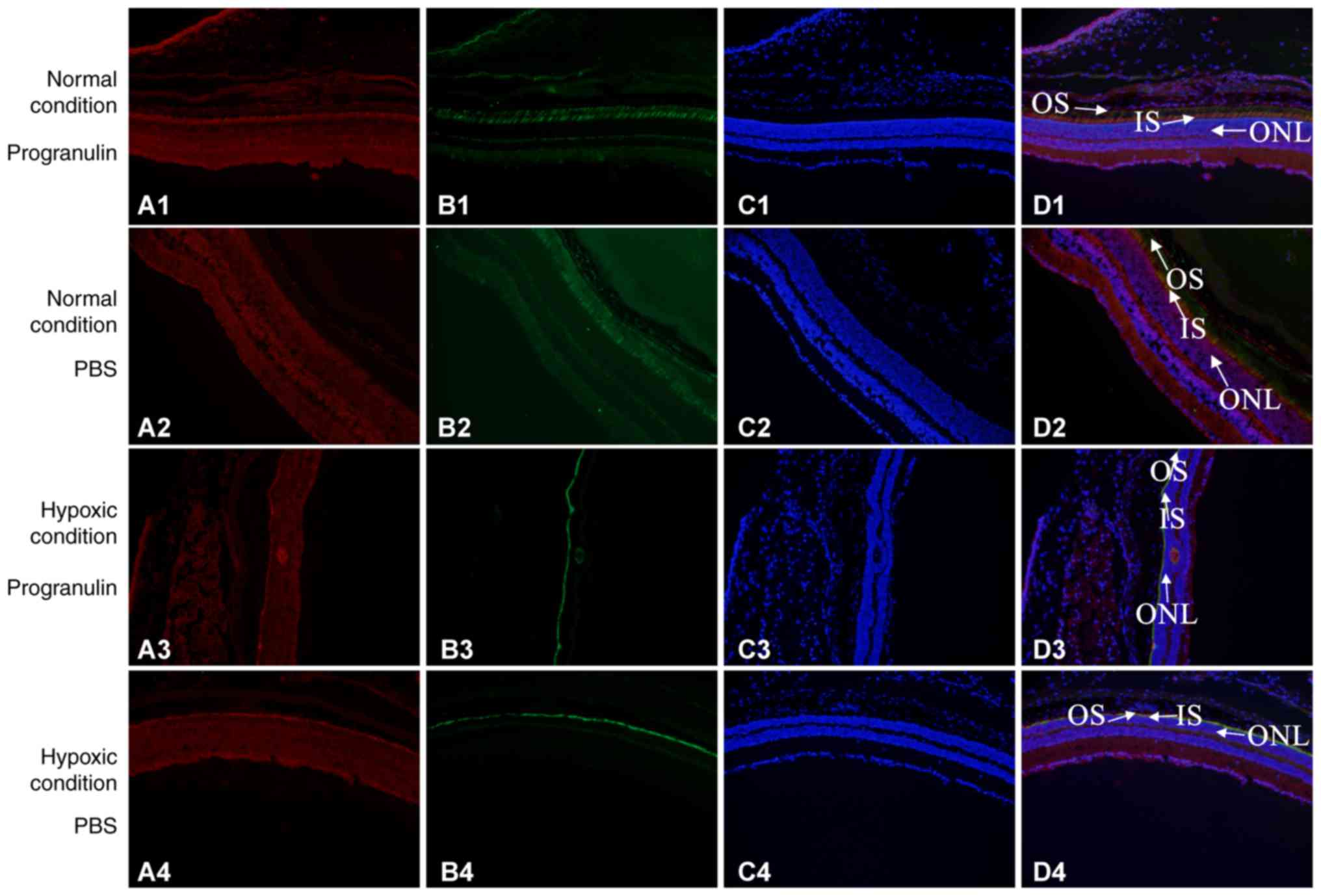 | Figure 3.Detection of morphological alterations
in mouse retinal photoreceptors under the hypoxic condition using
immunofluorescence co-localization. Group 1, normal condition +
progranulin injection; Group 2, normal condition + PBS injection;
Group 3, hypoxic condition + progranulin injection; Group 4,
hypoxic condition + PBS injection. (A) Red, s-opsin. (B) Green,
PNA. (C) Blue, DAPI. (D) Merge. Original magnification, ×100; IS,
inner segment of cone cells; ONL, outer nuclear layer; OS, outer
segment of cone cell. |
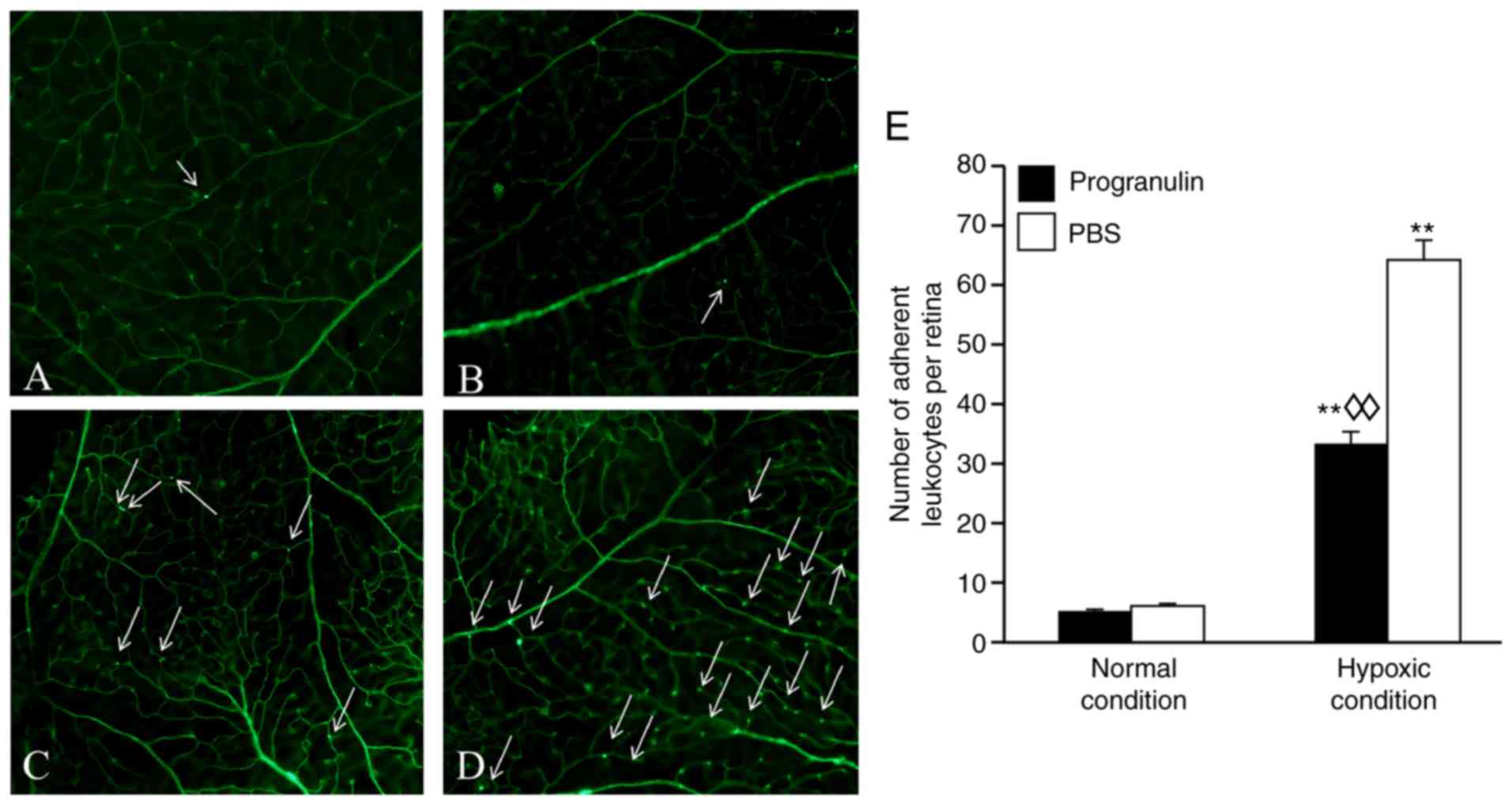
Progranulin reduces the number of
adherent leukocytes in the retinal blood vessels under hypoxic
conditions
Adherent leukocytes in mouse retinal blood vessels
are an indicator used to measure retinal inflammatory responses
(11). The number of adherent
leukocytes under hypoxic conditions was significantly increased
compared with in the normal control group (Fig. 4). In addition, the number of
adherent leukocytes in the progranulin-injected eyes (Fig. 4A and C) was ~52.31% that in the
PBS-injected eyes (Fig. 4B and D);
these differences were significant (Fig. 4E).
Progranulin exerts retinal protective
effects through regulation and inhibition of the TLR4-NOX4
signaling pathway
The expression levels of caspase signaling
pathway-associated proteins, caspase-3 and p53, and Wnt/β-catenin
signaling pathway-associated proteins, GSK-3β and β-catenin, were
evaluated in the retina. The expression levels of caspase-3, p53,
GSK-3β and β-catenin were significantly upregulated under hypoxic
conditions compared with in the normal control group; however, the
differences in expression between the progranulin- and PBS-injected
eyes were not significant (Fig.
5A-D). Therefore, it was concluded that progranulin did not
exert its function through the regulation of these two signaling
pathways.
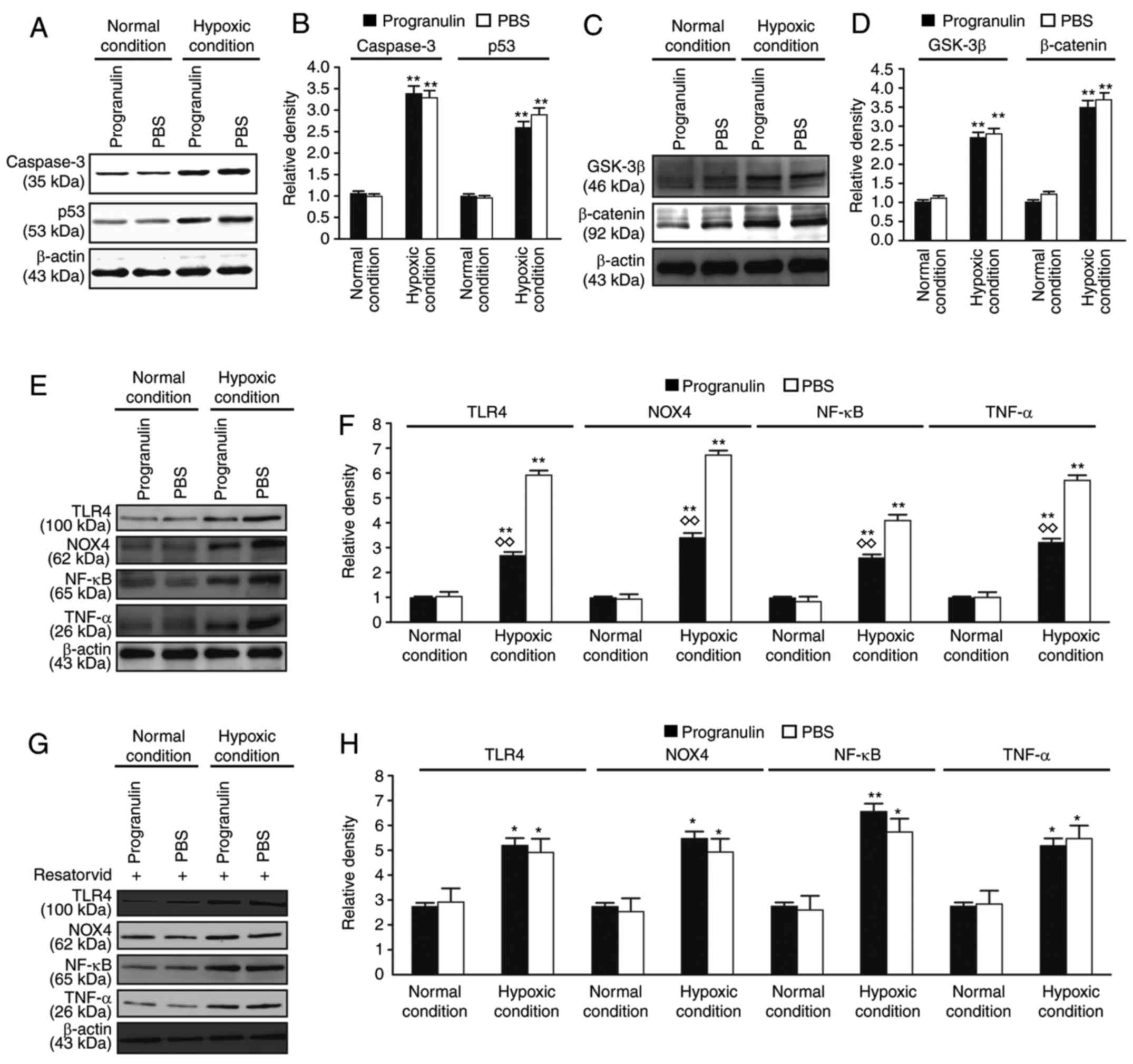 | Figure 5.Detection of the expression levels of
caspase, Wnt/β-catenin and TLR4-NOX4 signaling pathway-associated
proteins by western blotting. (A) Detection of the expression
levels of the caspase signaling pathway-associated proteins,
caspase-3 and p53, using western blotting. (B) Statistical
analysis. (C) Detection of the expression levels of the
Wnt/β-catenin signaling pathway-associated proteins, GSK-3β and
β-catenin, using western blotting. (D) Statistical analysis. (E)
Detection of the expression levels of TLR4, NOX4, TNF-α and
NF-κB/p65 using western blotting. (F) Statistical analysis. (G)
Detection of the expression levels of TLR4, NOX4, TNF-α and
NF-κB/p65 following treatment with the inhibitor resatorvid. (H)
Statistical analysis. Data are presented as the means ± standard
error of the mean, n=6. *P<0.05, **P<0.01 vs. the normal
condition; ◊◊P<0.01 vs. the PBS group. GSK-3β,
glycogen synthase kinase-3β; NF-κB, nuclear factor-κB; NOX4, NADPH
oxidase 4; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis
factor-α. |
The expression levels of TLR4-NOX4 signaling
pathway-associated proteins, TLR4 and NOX4, and downstream
inflammatory factors, TNF-α and NF-κB, were also examined in the
retina. The expression levels of TLR4, NOX4, TNF-α and NF-κB were
significantly upregulated under hypoxic conditions compared with
under normal conditions. These results suggested that the TLR4-NOX4
signaling pathway was activated in the retina under hypoxic
conditions. In addition, the expression levels of the
aforementioned proteins were significantly lower in the
progranulin-injected eyes compared with in the PBS-injected eyes
(Fig. 5E and F). Subsequently,
resatorvid, a TLR4 inhibitor originally developed to stop the
progression of sepsis (12), was
intravitreally injected to observe its effects on the mouse retina.
Western blotting results demonstrated that the expression levels of
TLR4 and NOX4, and the downstream proteins, TNF-α and NF-κB, were
not significantly different between PBS- and progranulin-injected
eyes (Fig. 4G and H). These
results preliminarily suggested that progranulin may exert its
function through regulation and inhibition of the TLR4-NOX4
signaling pathway.
Discussion
Progranulin is a multi-functional growth factor
(13), which serves an important
role in various pathophysiological processes, including tumor
development, inflammatory responses, nerve cell survival and axonal
growth regulation. In addition, the progranulin gene affects the
axonal growth of neurons through regulation of GSK3β, which is an
important component of the Wnt/β-catenin signaling pathway.
Progranulin gene knockout in nervous system cells has been reported
to increase the expression of Wnt/β-catenin signaling
pathway-associated proteins (14).
Progranulin also inhibits the caspase pathway; activation of
caspase signaling pathway-associated proteins in the cortex is
intensified in progranulin gene knockout mice, and cortical
neuronal apoptosis is increased (15). Progranulin is also an immune
regulatory factor that serves a regulatory role in inflammatory
diseases. Local injection of progranulin into mice in a multiple
arthritis model inhibits the neutrophil-induced inflammatory
response (16). Tang et al
(17) demonstrated that
progranulin is a novel ligand for the TNF receptor that interacts
with its extracellular domain; progranulin blocks TNF-α-mediated
signaling pathways, including NF-κB, by competing with TNF-α for
its receptors and thus serves important anti-inflammatory roles in
rheumatoid arthritis. Therefore, progranulin is considered a
protein with extensive functionality. However, to date, few reports
have studied its function in retinopathy. Tsuruma et al
(18) reported that progranulin
serves a protective role in a light-induced retina degeneration
model, which is used to mimic age-related macular degeneration
(19). To the best of our
knowledge, the role of progranulin under hypoxic conditions has not
yet been reported, and requires further investigation.
Hypoxia is involved in various retinopathies,
including diabetic retinopathy (20), central retinal vein occlusion and
macular edema (21).
Investigations into the pathological alterations of retinal tissues
under hypoxic conditions may contribute to understanding the
pathogenic mechanisms underlying these diseases. The chemical drug
most commonly used to simulate the hypoxic environment is
CoCl2. The principle underlying this method is to block
intracellular respiratory chain transfer and cause chemical hypoxia
in cells (22,23). Intravitreal injection of
CoCl2, a chemical hypoxia-mimicking agent, is an
emerging in vivo method to simulate retinal tissue hypoxia
in rodents (23). This process has
a short cycle for the establishment of a hypoxia model and is
convenient to use. A previous study performed intravitreal
injection of various CoCl2 concentrations in rats, in
order to investigate the optimal concentration required to induce
hypoxia, and the results have demonstrated an optimal effect at 9
mM CoCl2 (23).
Therefore, this concentration was adopted in the present study.
ERG is mainly used to detect the function of retinal
photoreceptor cells. This method uses low light and a white
standard flash to stimulate photoreceptors to produce electric
potential, which can be recorded non-invasively using a platinum
electrode on the corneal surface (24). Scotopic ERG reflects the functional
status of rod cells, and photopic ERG is typically used to reflect
the functional status of cone cells. The present study demonstrated
that although the amplitudes of a-waves and b-waves in the ERGs of
mice in the hypoxic condition group were lower compared with the
amplitudes in the normal condition group, the reduction in the
amplitude was lower in progranulin-injected eyes compared with in
PBS-injected eyes. These results suggested that progranulin may
exert protective effects on the function of photoreceptor cells.
Following the completion of the functional evaluation,
immunofluorescence co-localization was performed using markers for
whole cone cells (PNA) and the outer segment of cone cells
(s-opsin) (25), in order to
observe alterations in cone cell structure. Furthermore, DAPI
staining was used to observe alterations in the structure of the
outer nuclear layer formed by the rod cells. The number of rod
cells was decreased in PBS-injected eyes compared with in
progranulin-injected eyes, as revealed by a decrease in the
thickness of the outer nuclear layer; in addition, the arrangement
of cone cells was sparse, and the morphology of the outer segment
was short and small. These results suggested that the occurrence of
photoreceptor cell damage and death in the progranulin-injected
eyes was relatively low, even though the photoreceptor cells
experienced a considerable number of deaths under hypoxic
conditions in the present study. These findings suggested that
progranulin may exert protective effects on photoreceptor
cells.
The inflammatory response is an important step in
microvascular diseases. Numerous studies have reported that, in
diabetic animal models, the number of retinal leukocytes and their
adhesive ability is increased, whereas deformability is decreased
(11,26). The passive deformability of
leukocytes decreases when passing through capillaries with smaller
diameters; therefore, the leukocyte adhesion rate increases.
Additionally, the increase in leukocyte adhesion rate is more
evident with the progression of disease; therefore, the retinal
vascular leukostasis assay can be used to analyze the levels of the
inflammatory response. The present study demonstrated that the
number of adherent leukocytes was higher in progranulin-injected
eyes under hypoxic conditions compared with in the normal condition
group, but was only 52.31% that in PBS-injected eyes. As
aforementioned, progranulin is associated with the Wnt/β-catenin,
caspase and TLR4 signaling pathways. Therefore, western blotting
was performed to screen relevant signaling pathways. The results
demonstrated that the protective effects of progranulin on the
retina under hypoxic conditions did not occur through regulation of
the caspase and Wnt/β-catenin signaling pathways; instead, the
regulation involved inhibition of the TLR4-NOX4 signaling
pathway.
In conclusion, the present study reported that
progranulin may exert protective effects on mouse photoreceptor
functions and morphology under CoCl2-induced hypoxia.
Additionally, progranulin reduced the number of adherent leukocytes
in retinal blood vessels. These functions occurred through
inhibition of the TLR4-NOX4 signaling pathway. The specific
mechanism and function underlying regulation of the TLR4-NOX4
signaling pathway by progranulin are currently under investigation.
These results suggested that inhibition of TLR4-NOX4 signaling by
progranulin may become a new direction for future therapy of
retinal hypoxia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81460088 and
81760176), the Jiangxi Provincial Training Program for
Distinguished Young Scholars (grant no. 20171BCB23092), the Jiangxi
Provincial Key R&D Program (grant no. 20171BBG70099), the
Jiangxi Provincial Natural Science Foundation for Youth Scientific
Research (grant no. 20171BAB215032) and the Youth Scientific
Research Foundation of the Second Affiliated Hospital of Nanchang
University (grant no. 2014YNQN12011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZPY and KS designed the study and performed the
experiments. MJY performed the experiments. YLZ analyzed the data,
and KS wrote the manuscript.
Ethics approval and consent to
participate
The present study complied with the Animal Research:
Reporting of in vivo Experiments guidelines and was
conducted in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. This study was approved by the Committee on
the Ethics of Animal Experiments of Nanchang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TLR4
|
Toll-like receptor 4
|
|
NOX4
|
NADPH oxidase 4
|
|
TNF
|
tumor necrosis factor
|
|
GSK
|
glycogen synthase kinase
|
|
PNA
|
peanut agglutinin
|
References
|
1
|
Cheng L, Yu H, Yan N, Lai K and Xiang M:
Hypoxia-inducible factor-1α target genes contribute to retinal
neuroprotection. Front Cell Neurosci. 11:202017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaur C, Foulds WS and Ling EA:
Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol.
2:879–889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS
and Lee JY: Hypoxic stress up-regulates the expression of Toll-like
receptor 4 in macrophages via hypoxia-inducible factor. Immunology.
129:516–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dvoriantchikova G, Barakat DJ, Hernandez
E, Shestopalov VI and Ivanov D: Toll-like receptor 4 contributes to
retinal ischemia/reperfusion injury. Mol Vis. 16:1907–1912.
2010.PubMed/NCBI
|
|
6
|
Liu L, Jiang Y and Steinle JJ: Toll-like
receptor 4 reduces occludin and zonula occludens 1 to increase
retinal permeability both in vitro and in vivo. J Vasc Res.
54:367–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tolkatchev D, Malik S, Vinogradova A, Wang
P, Chen Z, Xu P, Bennett HP, Bateman A and Ni F: Structure
dissection of human progranulin identifies well-folded
granulin/epithelin modules with unique functional activities.
Protein Sci. 17:711–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petkau TL and Leavitt BR: Progranulin in
neurodegenerative disease. Trends Neurosci. 37:388–398. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abella V, Scotece M, Conde J, López V,
Pirozzi C, Pino J, Gómez R, Lago F, González-Gay MÁ and Gualillo O:
The novel adipokine progranulin counteracts IL-1 and TLR4-driven
inflammatory response in human and murine chondrocytes via TNFR1.
Sci Rep. 6:203562016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drummond GB, Paterson DJ and McGrath JC:
ARRIVE: New guidelines for reporting animal research. J Physiol.
588:25172010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schröder S, Palinski W and
Schmid-Schönbein GW: Activated monocytes and granulocytes,
capillary nonperfusion, and neovascularization in diabetic
retinopathy. Am J Pathol. 139:81–100. 1991.PubMed/NCBI
|
|
12
|
Sha T, Sunamoto M, Kitazaki T, Sato J, Ii
M and Iizawa Y: Therapeutic effects of TAK-242, a novel selective
Toll-like receptor 4 signal transduction inhibitor, in mouse
endotoxin shock model. Eur J Pharmacol. 571:231–239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jian J, Li G, Hettinghouse A and Liu C:
Progranulin: A key player in autoimmune diseases. Cytokine.
101:48–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen EY, Wexler EM, Versano R, Coppola G,
Gao F, Winden KD, Oldham MC, Martens LH, Zhou P, Farese RV Jr and
Geschwind DH: Functional genomic analyses identify pathways
dysregulated by progranulin deficiency, implicating Wnt signaling.
Neuron. 71:1030–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar-Singh S: Progranulin and TDP-43:
Mechanistic links and future directions. J Mol Neurosci.
45:561–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jian J, Zhao S, Tian Q, Gonzalez-Gugel E,
Mundra JJ, Uddin SM, Liu B, Richbourgh B, Brunetti R and Liu CJ:
Progranulin directly binds to the CRD2 and CRD3 of TNFR
extracellular domains. FEBS Lett. 587:3428–3436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ,
Liu GY, Syed NM, Lai Y, Lin EA, Kong L, et al: The growth factor
progranulin binds to TNF receptors and is therapeutic against
inflammatory arthritis in mice. Science. 332:478–484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuruma K, Yamauchi M, Sugitani S, Otsuka
T, Ohno Y, Nagahara Y, Ikegame Y, Shimazawa M, Yoshimura S, Iwama T
and Hara H: Progranulin, a major secreted protein of mouse
adipose-derived stem cells, inhibits light-induced retinal
degeneration. Stem Cells Transl Med. 3:42–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enoki M, Shinto S, Matsuoka Y, Otsuka A,
Kaidzu S, Tanito M, Shibata T, Uchida K, Ohira A, Yamato M and
Yamada KI: Lipid radicals cause light-induced retinal degeneration.
Chem Commun (Camb). 53:10922–10925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arden GB and Sivaprasad S: Hypoxia and
oxidative stress in the causation of diabetic retinopathy. Curr
Diabetes Rev. 7:291–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stephen J Ryan: Retina. 1. 4th. Elsevier;
Singapore: 2010
|
|
22
|
Niwa M, Aoki H, Hirata A, Tomita H, Green
PG and Hara A: Retinal cell degeneration in animal models. Int J
Mol Sci. 17:E1102016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hara A, Niwa M, Aoki H, Kumada M, Kunisada
T, Oyama T, Yamamoto T, Kozawa O and Mori H: A new model of retinal
photoreceptor cell degeneration induced by a chemical
hypoxia-mimicking agent, cobalt chloride. Brain Res. 1109:192–200.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abcouwer SF and Gardner TW: Diabetic
retinopathy: Loss of neuroretinal adaptation to the diabetic
metabolic environment. Ann NY Acad Sci. 1311:174–190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaillard F, Kuny S and Sauvé Y:
Topographic arrangement of S-cone photoreceptors in the retina of
the diurnal Nile grass rat (Arvicanthis niloticus). Invest
Ophthalmol Vis Sci. 50:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|