Introduction
As the primary barrier to the body, the skin
protects against dehydration, mechanical injury and microbial
infection (1). Skin is composed of
an outer epidermis and the underlying dermis separated by a
basement membrane. Hair follicles are highly sensitive mini-organs
comprising epidermal keratinocytes and mesenchymal compartments
(2–4). Hair growth is a cyclic regeneration
phenomenon regulated by connections between epithelial and dermal
compartments (5–8). Hair follicles go through repeated
cycles of anagen (growth), catagen (regression) and telogen
(quiescence) throughout the life of mammals (9–12).
Prior to the start of each cycle there is a phase of hair follicle
morphogenesis. Hair follicle morphogenesis and the subsequent hair
phases follow a precise timescale (13). The period beginning with hair
morphogenesis until the first hair cycle is considered to be the
maturation of the hair follicle.
Nestin, originally discovered in neuroepithelial
stem cells, is an intermediate filament protein expressed during
the early stages of development (1,14).
Hair follicles contain a distinct population of follicular stem
cells that express Nestin (15).
Using Nestin-green fluorescent protein (GFP) transgenic mice,
researchers demonstrated that during telogen and in early anagen,
Nestin-GFP+ cells are primarily in the bulge area
(9,16). However, in mid- and late anagen,
the GFP-expressing cells are located in the upper outer-root sheath
in addition to the bulge area (9).
However, Nestin expression between morphogenesis and the postnatal
regular hair cycle is not well studied. In the present study, it
was demonstrated that during morphogenesis, Nestin-GFP expression
was detected rarely, and gradually increased during maturation (0–4
weeks) in hair follicle dermal cells. In mature hair follicle
dermal cells, Nestin and Ki67 were highly expressed in anagen,
while during telogen, they were markedly decreased. Additionally,
the lineage tracing data demonstrated that peri-follicular
Nestin+ cells during morphogenesis differentiated into
vascular cells.
Materials and methods
Animals and treatment
Nestin-GFP mice were provided by Dr Grigori
Enikolopov at Cold Spring Harbor Laboratory (Cold Spring Harbor,
NY, USA). Nestin-CreERT2mice (stock no. 003771) and
B6.129X1-Gt (ROSA) 26Sortm1 [enhanced yellow fluorescent protein
(EYFP)] Cos/J mice (stock no. 006148) were purchased from Jackson
Laboratory (Bar Harbor, ME, USA). Five male mice were used in each
group. The mice were 8-days-old, 2-weeks-old, 4-weeks-old,
8-weeks-old and 12-weeks-old, whose average weight were 5, 7, 12,
19 and 25 g, respectively. For the lineage tracing experiment, mice
were injected with tamoxifen (80 mg/kg) to induce Cre-ER activity 8
days (P8) following birth and tested at 4 weeks old. All animal
experiments were performed under the approval of the Institutional
Animal Care and Use Committee at Southern Medical University
(Guangzhou, China). Mice were housed at the Department of
Laboratory Animal Science, Southern Medical University (Guangzhou,
China), and maintained under a 12-h light/dark cycle, an ambient
temperature of 22±2°C and a constant humility of 60±10%, with food
and water provided ad libitum.
Flow cytometry analysis
For flow cytometry analysis of
Nestin-GFP− and GFP+ cells from the whole
skin, the skin from Nestin-GFP mice was dissected. Following hair
removal, the skin was lightly defatted before 2 ml of Trypsin-EDTA
(0.5%, 10X; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
added, followed by incubation at 4°C overnight. Dermis were
separated and cut into small pieces, followed by digestion in
protease solution [2 mg/ml collagenase I and 2.5 mg/ml trypsin in
phosphate-buffered saline (PBS) at 37°C] for 1 h. Cells within the
supernatant were collected for flow cytometry. Following red blood
cell lysis (in order to remove the hemocytes) with commercial
ammonium chloride-potassium lysis buffer (Quality Biological, Inc.,
Gaithersburg, MD, USA), the cells were analyzed according to CD45
and GFP expression. Flow cytometry analysis was performed using a
FACSCalibur flow cytometer and CellQuest software (version 5.1,
Becton-Dickinson Biosciences). The primary antibodies used were
FITC-conjugated anti-mouse GFP (cat. no. 338008; 1:200; BioLegend,
Inc., San Diego, CA, USA), PerCP-conjugated anti-mouse CD45 (cat.
no. 103130; 1:200; BioLegend, Inc.). Briefly, dermal cells were
blocked with 1% BSA (cat. no. 05470; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 30 min on ice and following washing with
PBS, the primary antibodies were added and incubated on ice for 15
min.
Immunofluorescence
Following sacrifice, the skin of the mice was
resected and fixed in 4% ice-cold paraformaldehyde solution for 1 h
and decalcified by immersion in 30% sucrose for 24 h. Finally,
tissues were embedded in optimal cutting temperature compound
(Sakura Finetek USA, Inc, Torrance, CA, USA). Sections of skin
(10-µm thick) were harvested for immunofluorescence staining.
For the staining, the sections were incubated with
the following primary antibodies: Mouse Ki67 (cat. no. ab15580;
1:200), GFP (cat. no. ab290; 1:200) and cluster of differentiation
(CD)31 (cat. no. ab28364; 1:50) (all from Abcam, Cambridge, UK).
Slides were rinsed with TBST (cat. no. T5912; Sigma-Aldrich; Merck
KGaA), blocked with 3% BSA (cat. no. 05470; Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature and then incubated with the
primary antibody overnight at 4°C, followed by incubation with FITC
or Cy3-conjugated secondary antibodies (FITC-conjugated secondary
antibodies: cat. no. 711–546-152; 1:1,000; Cy3-conjugated secondary
antibodies: cat. no. 711-167-003; 1:1,000; Jackson ImmunoResearch
Europe, Ltd., Newmarket UK) for 1 h at room temperature in the
dark. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich; Merck KGaA). The sections were mounted with the
ProLong Antifade kit (Molecular Probes; Thermo Fisher Scientific,
Inc.) and were observed under a Zeiss LSM780 confocal microscope
(Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments. One-way analysis of variance followed
by the Bonferroni post hoc test was applied. All data were normally
distributed and had similar variation between groups. Statistical
analysis was performed using SAS version 9.3 software (SAS
Institute, Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Maturation of hair follicle dermal
cells is characterized by gain-of-nestin expression
Nestin is required for the self-renewal,
proliferation and cell cycle progression of hair follicle cells
(17–19). The mouse hair cycle follows a
precise time scale. A gradual induction of Nestin-GFP signaling was
detected in male Nestin-GFP+ mice in hair follicle
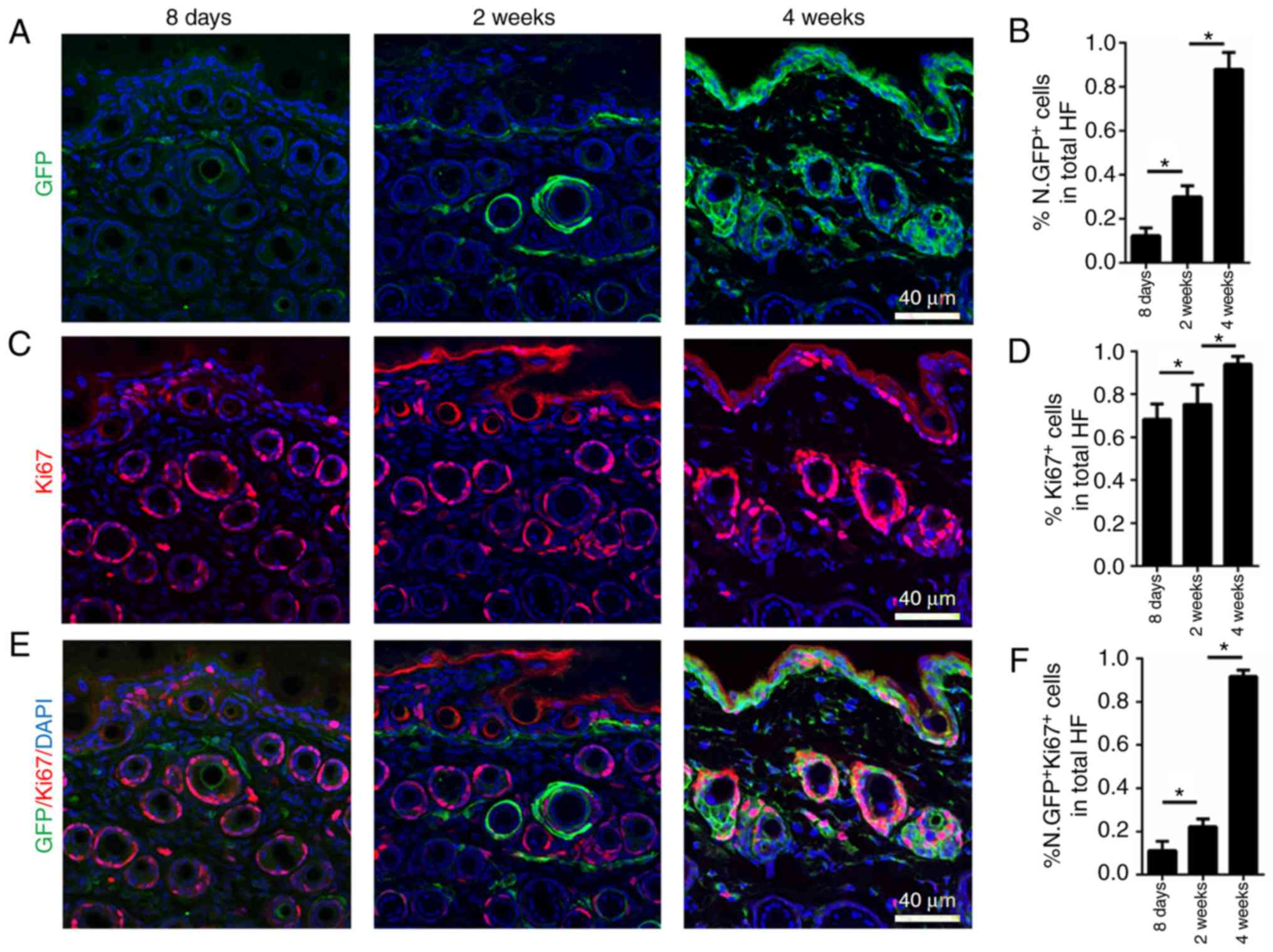
dermal cells at P8, and in 2- and 4-week-old mice (Fig. 1A and B). According to the
immunofluorescence staining results, Nestin-GFP was expressed at
low levels at P8 (morphogenesis) in hair follicle dermal cells and
progressively increased at 2 and 4 weeks of age (first catagen and
anagen; Fig. 1A and B). Therefore,
it appeared that the period spanning morphogenesis until the first
anagen represented the maturation of hair follicle and, following
this, hair follicles follow a regular hair cycle.
Mature hair follicle dermal cells
co-express nestin and Ki67 during anagen
Given the induction of Nestin-GFP signaling in male
Nestin-GFP mice during maturation, the proliferation capacity of
hair follicle dermal cells during this period was investigated by
immunofluorescence staining with the Ki67 proliferation marker. The
high amount of Ki67+ dermal cells observed at P8
(morphogenesis) indicated that the cells have high proliferative
activity during morphogenesis. Ki67 expression was substantially
maintained at 2 weeks of age, when the first catagen begins, and
increased at 4 weeks, when first anagen begins (Fig. 1C and D). Notably,
co-immunofluorescence staining of Ki67 and Nestin-GFP at different
time points indicated that at P8, few Ki67+ hair
follicle dermal cells also expressed Nestin-GFP. At 2 weeks, nearly
20% of Ki67+ hair follicle dermal cells expressed
Nestin-GFP (Fig. 1E and F). In
4-week-old mice, nearly all Ki67+ hair follicle dermal
cells expressed Nestin-GFP, indicating that mature
Nestin+ hair follicle dermal cells are of high
proliferative capacity during the first anagen (Fig. 1E and F). During the maturation of
hair follicles, hair follicle dermal cells begin expressing Nestin,
and mature cells are of high proliferative capacity.
Dynamic nestin expression during the
normal hair cycle in hair follicle dermal cells
Whether Nestin is highly expressed during the normal
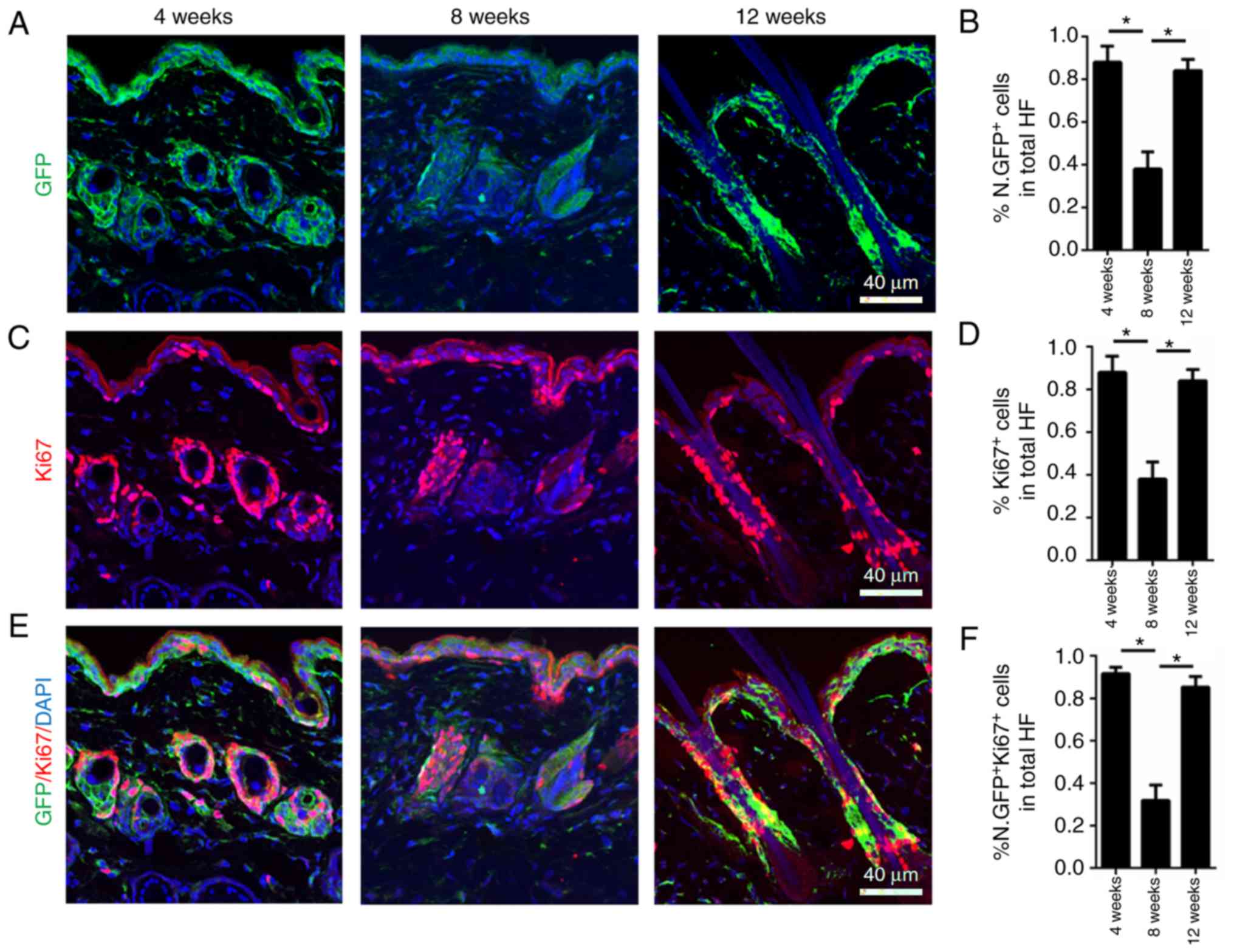
hair cycle was also investigated. To determine this, skin from 4-,
8- and 12-week-old male Nestin-GFP+ mice was collected,
corresponding to the first anagen, second telogen and second anagen
of the murine hair cycle, respectively. Nestin-GFP was highly
expressed in anagen (growing phase; 4 and 12 weeks) and
significantly decreased in telogen (quiescent phase; 8 weeks;
Fig. 2A and B).
Nestin may serve as marker of high
proliferation during the normal hair cycle
To investigate Ki67 expression during the normal
hair cycle in hair follicle dermal cells, Ki67 immunofluorescence
staining was performed on 4-, 8- and 12-week-old male
Nestin-GFP+ mice. High Ki67 expression levels were
observed at 4 and 12 weeks, representing the first and second
anagen of the murine hair cycle, respectively, indicating that the
cells were in a highly proliferative state. At 8 weeks, when mouse
hair follicles were in telogen (quiescent phase), Ki67 expression
levels were significantly decreased (Fig. 2C and D). Co-immunofluorescence
staining of Ki67 and Nestin-GFP indicated that nearly all
Ki67+ cells were also Nestin-GFP+, and the
expression of the two markers was increased in anagen and decreased
in telogen (Fig. 2E and F). The
fact that Nestin had the same expression pattern as Ki67 during the
normal hair cycle in hair follicle dermal cells indicated that
Nestin may serve as a marker of high proliferation during the
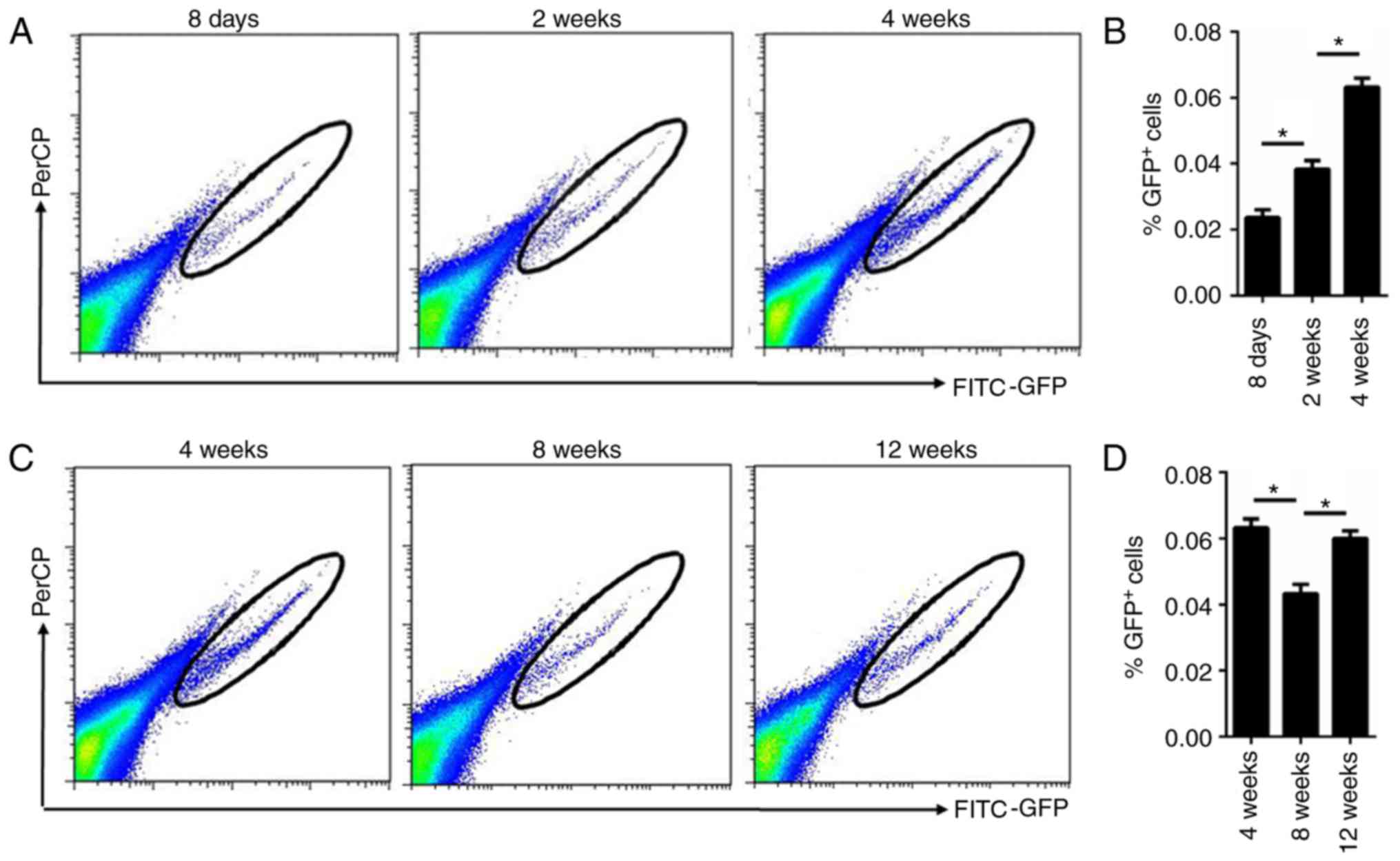
normal hair cycle. Furthermore, flow cytometry analysis of the
isolated cells demonstrated that the percentage of GFP+
cells was gradually increased in mice of 2 and 4 weeks of age
compared with those at 8 days of age, which was in accordance with
the immunofluorescence staining results (Fig. 3A and B). Additionally, flow
cytometry analysis of the isolated cells demonstrated that the
percentage of GFP+ cells was increased during anagen (12
weeks) and decreased during telogen (8 weeks; Fig. 3C and D).
Nestin positive cells also express
CD31
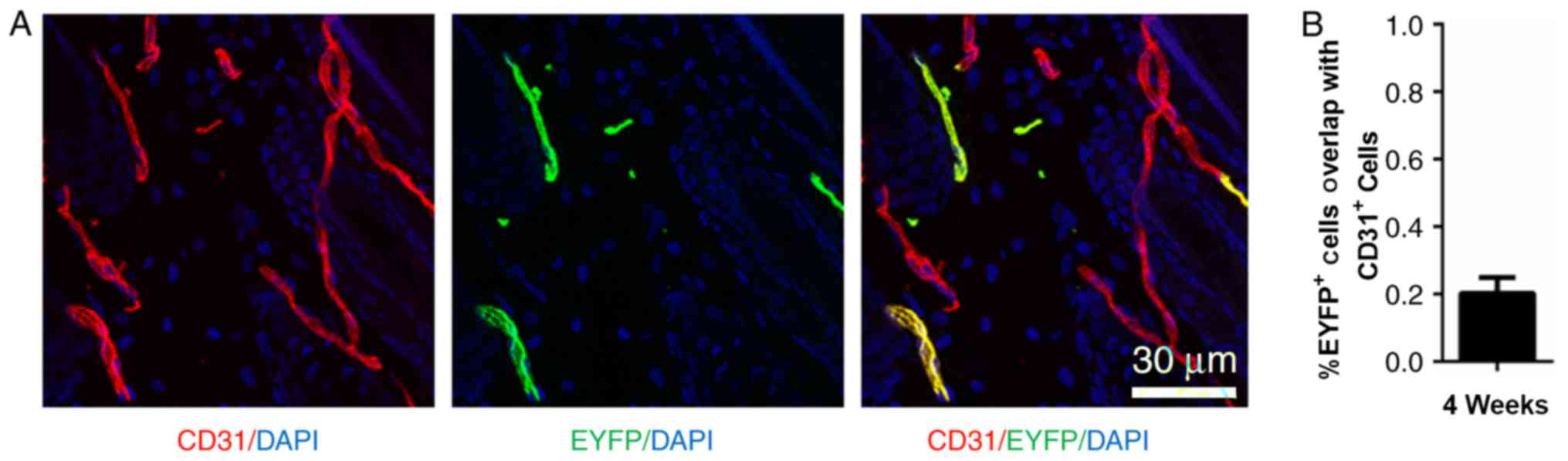
At P8, when morphogenesis occurs, Nestin-GFP was
rarely expressed in hair follicle dermal cells. However,
Nestin-GFP+ cells were detected in the peri-follicular
area (Fig. 1A). The lineage fate
of the Nestin+ cells was traced at P8 using
Nestin-Cre::ROSA26-EYFP mice. Examination of the fate of
Nestin-EYFP+ cells 3 weeks post-tamoxifen injection
revealed that ~20% of the EYFP-labeled cells were also
CD31+ (Fig. 4).
Discussion
In mice, the first two postnatal hair cycles are
synchronized (13). Hair follicles
are an ideal system for studying how stem cells interact with
progeny in the niche between quiescence and regeneration (5). Murine hair follicles undergo a
precise hair cycle, which follows a specific timescale following
hair morphogenesis (13). From
birth to 2 weeks of age, murine hair follicles are in a morphogenic
phase, followed by 2-, 3- and 4-weeks of age when hair follicles
undergo the first postnatal catagen, telogen and anagen,
respectively. The second hair cycle occurs at 6-, 7- and 12-weeks
of age, indicating the second catagen, telogen and anagen,
respectively (13). Using this
guide to classify hair phases, a previous study demonstrated that
Nestin is expressed in different hair follicle locations in
different developmental phases (9). However, Nestin expression between
morphogenesis and the postnatal regular hair cycle is not well
studied.
Nestin, a type-VI intermediate filament protein,
serves as a marker for neural stem cells and is also known to be
expressed in follicle stem cells (14). Previous studies have demonstrated
that Nestin is required for the self-renewal, proliferation and
cell cycle progression of the cells, particularly in neural
progenitor cells (17–19). Consistent with all these findings,
the present study demonstrated that during the hair follicle cycle,
there was a high percentage of Ki67+ cells among the
Nestin-expressing cells in the dermis layer at 4 and 12 weeks
following birth, which represent the first and second anagen of the
murine hair cycle, respectively. Notably, at 8 weeks of age, which
is the first telogen (quiescent phase),
Ki67+/Nestin+ hair follicle cell numbers
decreased. These results indicated the high proliferative capacity
of these cells, likely due to a high demand for hair follicle
replacement during anagen and not during telogen. All these results
suggested that Nestin+ cells in the dermis layer
proliferate more rapidly compared with Nestin− cells in
the same region. A notable phenomenon from the present study is
that during early hair follicle morphogenesis,
Nestin-GFP+ cells gradually appeared in the dermis layer
until the first anagen, which indicated that Nestin expression may
be a sign of hair follicle maturation.
Previous findings revealed that
Nestin-Cre+ and Nestin-GFP+ cells are able to
label endothelial, mesenchymal lineage and Schwann precursor cells
(20–24). At P8, when morphogenesis occurs,
Nestin-GFP was rarely expressed in hair follicle dermal cells.
However, Nestin-GFP+ cells were detected in the
peri-follicular area. In the present study, a single dose of
tamoxifen was administered to P8 Nestin-CreERT2;
ROSA26-EYFP in order to preform lineage tracing, and skin samples
were harvested after 3 weeks. It was illustrated that during hair
follicle morphogenesis, peri-follicular Nestin+ cells
also expressed CD31, which is an endothelial cell marker. This
finding may divide Nestin+ cells in the dermis layer
into two different populations: Peri-follicular Nestin+
cells during morphogenesis that are of endothelial lineage, and
hair follicle Nestin+ cells that are hair follicle
precursor cells.
In conclusion, the expression of Nestin in hair
follicles during morphogenesis and maturation was investigated.
Additionally, Nestin may serve as a marker of high proliferation
during the normal hair cycle, and was highly expressed during
anagen and decreased during telogen in the murine hair cycle.
Furthermore, certain Nestin+ cells may serve a role in
other processes, including angiogenesis during morphogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81471900, 81701929 and
81772104) and the Natural Science Foundation of Guangdong Province
(grant nos. 2015A030311001 and 2017A030310120) and the Science and
Technology Planning Project of Guangzhou City (grant no.
201508020262).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RC performed the experiments and wrote the
manuscript. YM analyzed the data. ZH designed the study and
critically revised the manuscript for important intellectual
content.
Ethics approval and consent to
participate
All animal experiments were performed under the
approval of the Institutional Animal Care and Use Committee at
Southern Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Falodah FA and Al-Karim S: Immuno- and
gene expression analysis of EGFR and Nestin during mice skin
development. Tissue Cell. 48:274–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mistriotis P and Andreadis ST: Hair
follicle: A novel source of multipotent stem cells for tissue
engineering and regenerative medicine. Tissue Eng Part B Rev.
19:265–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeo M, Lee W and Ito M: Wound healing
and skin regeneration. Cold Spring Harb Perspect Med.
5:a0232672015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Myung PS, Takeo M, Ito M and Atit RP:
Epithelial Wnt ligand secretion is required for adult hair follicle
growth and regeneration. J Invest Dermatol. 133:31–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu YC, Li L and Fuchs E: Emerging
interactions between skin stem cells and their niches. Nat Med.
20:847–856. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higgins CA, Chen JC, Cerise JE, Jahoda CA
and Christiano AM: Microenvironmental reprogramming by
three-dimensional culture enables dermal papilla cells to induce de
novo human hair-follicle growth. Proc Natl Acad Sci USA.
110:19679–19688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biernaskie J, Paris M, Morozova O, Fagan
BM, Marra M, Pevny L and Miller FD: SKPs derive from hair follicle
precursors and exhibit properties of adult dermal stem cells. Cell
Stem Cell. 5:610–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sennett R and Rendl M:
Mesenchymal-epithelial interactions during hair follicle
morphogenesis and cycling. Semin Cell Dev Biol. 23:917–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Mignone J, Yang M, Matic M, Penman
S, Enikolopov G and Hoffman RM: Nestin expression in hair follicle
sheath progenitor cells. ProcNatlAcad Sci USA. 100:9958–9961. 2003.
View Article : Google Scholar
|
|
10
|
Matsumura H, Mohri Y, Binh NT, Morinaga H,
Fukuda M, Ito M, Kurata S, Hoeijmakers J and Nishimura EK: Hair
follicle aging is driven by transepidermal elimination of stem
cells via COL17A1 proteolysis. Science. 35:1aad43952016.
|
|
11
|
Kandyba E and Kobielak K: Wnt7b is an
important intrinsic regulator of hair follicle stem cell
homeostasis and hair follicle cycling. Stem Cells. 32:886–901.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoffman RM: Nestin-expressing hair
follicle-accessible pluripotent stem cells for nerve and spinal
cord repair. Cells Tissues Organs. 200:42–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller-Röver S, Handjiski B, van der Veen
C, Eichmüller S, Foitzik K, McKay IA, Stenn KS and Paus R: A
comprehensive guide for the accurate classification of murine hair
follicles in distinct hair cycle stages. J Invest Dermatol.
117:3–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie L, Zeng X, Hu J and Chen Q:
Characterization of Nestin, a Selective marker for bone marrow
derived mesenchymal stem cells. Stem Cells Int. 2015:7620982015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amoh Y, Li L, Yang M, Moossa AR, Katsuoka
K, Penman S and Hoffman RM: Nascent blood vessels in the skin arise
from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA.
101:13291–13295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchugonova A, Cao W, Hoffman RM and Koenig
K: Comparison of label-free and GFP multiphoton imaging of hair
follicle-associated pluripotent (HAP) stem cells in mouse whiskers.
Cell Cycle. 14:3430–3433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu W, Lu H, Wang S, Yin W, Liu X, Dong L,
Chiu R, Shen L, Lu WJ and Lan F: Suppression of Nestin reveals a
critical role for p38-EGFR pathway in neural progenitor cell
proliferation. Oncotarget. 7:87052–87063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park D, Xiang AP, Mao FF, Zhang L, Di CG,
Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, et al: Nestin is required for
the proper self-renewal of neural stem cells. Stem Cells.
28:2162–2171. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahlgren CM, Mikhailov A, Vaittinen S,
Pallari HM, Kalimo H, Pant HC and Eriksson JE: Cdk5 regulates the
organization of Nestin and its association with p35. Mol Cell Biol.
23:5090–5106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isern J, Garcia-Garcia A, Martin AM,
Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F and
Méndez-Ferrer S: The neural crest is a source of mesenchymal stem
cells with specialized hematopoietic stem cell niche function.
Elife. 3:e036962014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kusumbe AP, Ramasamy SK, Itkin T, Mäe MA,
Langen UH, Betsholtz C, Lapidot T and Adams RH: Age-dependent
modulation of vascular niches for haematopoietic stem cells.
Nature. 532:380–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ono N, Ono W, Mizoguchi T, Nagasawa T,
Frenette PS and Kronenberg HM: Vasculature-associated cells
expressing nestin in developing bones encompass early cells in the
osteoblast and endothelial lineage. Dev Cell. 29:330–339. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itkin T, Gur-Cohen S, Spencer JA,
Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I,
Poulos MG, et al: Distinct bone marrow blood vessels differentially
regulate haematopoiesis. Nature. 532:323–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|