Introduction
Allogeneic hematopoietic stem cell transplantation
(HSCT) is an effective therapy for a number of hematological
disorders. Despite good progress in the prevention and treatment of
complications that are often associated with transplantation, acute
graft-vs.-host disease (aGVHD) occurs in 30–70% of patients
undergoing allogeneic HSCT and remains a leading cause of
nonrelapse mortality (1,2). This disease occurs when immune cells
transplanted from a genetically non-identical donor recognize and
are activated by alloantigens in HSCT recipients, resulting in
organ damage, predominantly to the skin, gastrointestinal (GI)
tract, and liver. Corticosteroids, which elicit a response rate of
50–80%, are considered the first-line treatment for aGVHD (2,3).
However, patients who are unresponsive to this initial therapy
exhibit only a 10–30% likelihood of long-term survival (1,4).
Although available, second-line pharmacological strategies are
limited by their substantial impairment of the recipient's immune
system and subsequent increases in opportunistic infections
(5,6). Therefore, the development of novel
treatment strategies to improve the overall survival of HSCT
recipients is of significant clinical relevance.
Mesenchymal stem cells (MSCs) are a type of
multipotent adult stem cell that can be isolated from several
tissues, including the bone marrow (BM), adipose tissue, and
palatine tonsils. MSCs possess the capacity to suppress
immunological responses, support hematopoiesis, and stimulate
tissue repair (7,8). Clinical applications of human MSCs
for the prevention and treatment of GVHD are evolving rapidly. To
this end, clinical studies have demonstrated the efficacy of
systemic infusions of culture-expanded allogeneic human BM-MSCs for
the treatment of patients with steroid-refractory aGVHD (9). In fact, allogeneic BM-MSC products
have been used clinically in some countries as off-the-shelf
treatments for steroid-resistant aGVHD (10). Previous evidence has suggested that
MSCs inhibit immune cell functions primarily through the local
secretion of soluble immune modulators and partially through
cell-to-cell contact-dependent mechanisms. However, although MSCs
are thought to be a less important source of immunogenic cells for
transplantation, the half-life of infused MSCs and the risk of
immune rejection following either their repeated administration or
their use at high doses have not clearly been defined. Further,
GVHD-affected organs may require more ‘remote’ and ‘efficient’
immunomodulatory effects following systemic infusion to minimize
the loss of cells that directly adhere to tissues.
Thus, the use of conditioned medium (CM) derived
from MSCs (MSC-CM) could be a viable cellular approach to
overcoming the limitations of the use of MSCs directly as a
clinical treatment. Previously, we demonstrated that human palatine
tonsil-derived MSCs (T-MSCs) abundantly secrete immunomodulatory
proteins and that T-MSC-CM effectively attenuates inflammation both
in vitro and in vivo (11–13).
In the current study, we examined the effects of
T-MSC-CM on the prevention of GVHD in a mouse model of the disease.
Survival, weight loss, pathological changes, and lymphocyte gene
expression were evaluated to address the efficacy of T-MSC-CM as an
alternative treatment for GVHD in patients undergoing HSCT.
Materials and methods
Animals
Female BALB/c and male C57BL/6 mice were purchased
from OrientBio (Eumsung, Korea). All animals were maintained at
21–23°C with 51–54% humidity under pathogen-free conditions on a
12-h light/dark cycle with free access to food and water. All
procedures were approved by the Animal Care and Use Committee of
College of Medicine, Ewha Womans University (Seoul, Korea; approval
no. ESM18-0403).
Preparation of CM
To generate MSC-CM, BM-MSCs, adipose tissue-derived
MSCs (AT-MSCs), and T-MSCs (at passages 7–8) were cultured in low
glucose Dulbecco's modified Eagle's medium (DMEM; Welgene, Daegu,
Korea) in 100-mm tissue culture plates. The T-MSCs were obtained
and maintained as previously reported (14). The T-MSCs were obtained and
maintained as previously reported (14). The AT-MSCs were generously provided
by RNL Bio (Seoul, Korea), and the BM-MSCs were purchased from the
Severance Hospital Cell Therapy Center (Seoul, Korea). At 80%
confluence, the cells were washed four times with PBS, and the
medium was replaced with serum-free DMEM to generate CM. The medium
was collected after 48 h of culture as previously reported
(15,16), centrifuged at 1,300 rpm for 5 min,
and passed through a 0.2-µm filter. The CM was concentrated 20-fold
by centrifugal filtration by using centricone (molecular weight
cut-off value of 3K; Amicon Ultra-15; EMD Millipore, Billerica, MA,
USA) that provide highest yield in protein recovery. The
concentrated CM was then frozen and stored at −80°C for future use.
As a negative control, the serum-free culture medium was processed
in the same manner.
Western blotting
Equal amounts of CM from each MSC type (BM-MSCs,
AT-MSCs, and T-MSCs) were loaded onto a polyacrylamide gel,
separated by electrophoresis, transferred to polyvinylidene
difluoride membranes, blocked, and incubated with primary
antibodies overnight at 4°C. The following primary antibodies were
used: TSG-6 (1:200, diluted in 3% BSA (Bovogen Biologicals, Pty,
Ltd., East Keilor, Victoria, Australia) containing TBST; cat. no.
sc-398307); and β-actin [1:3,000; diluted in 3% BSA containing
TBST; cat. no. sc-47778; both Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA]. The membranes were washed 3 times for 10 min in
TBST and incubated with anti-mouse (cat. no. BR170-6516; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) horseradish
peroxidase-conjugated secondary antibodies (1:3,000; diluted in
TBST) for 1 h at room temperature. Following incubation, membranes
were washed 3 times for 10 min in TBST and developed using
SuperSignal West Femto Maximum Sensitivity Substrate (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Images were
obtained using ImageQuant LAS 4000 (GE Healthcare Life Sciences,
Little Chalfont, UK). The pixel densities of the TSG-6 bands were
divided by the pixel densities of the corresponding β-actin bands
for the quantitation of protein levels using UN-SCAN-IT-gel 6.1
software (Silk Scientific, Inc., Orem, UT, USA).
ELISA
To quantify the amounts of TSG-6 secreted from
BM-MSCs, AT-MSCs, and T-MSCs, CM was collected, and the levels of
secreted TSG-6 were determined using a human TSG-6 ELISA kit, in
accordance with the manufacturer's recommended protocol (cat. no.
ELH-TSG-6; RayBiotech, Norcross, GA, USA).
In vitro chemotaxis assay
Spleen and draining lymph node (dLN) cells isolated
from normal healthy male C57BL/6 mice were suspended in chemotaxis
medium composed of RPMI-1640 (Welgene), 1% fatty acid-free bovine
serum albumin, 2 mM glutamine, and 20 mM HEPES. The cells
(2×106 in 100 µl chemotaxis medium) were placed in the
upper chamber of 24-well Transwell plates (5 µM pore size; Costar,
Corning, NY, USA); 600 µl chemotaxis medium lacking chemokines
(control) or containing recombinant CCL2 (Peprotech, Rocky Hill,
NJ, USA) at various concentrations (50, 100, and 200 ng/ml) were
placed in the lower chamber. T-MSC-CM generated from 106
cells or rhTSG-6 (200 ng/ml; Peprotech) was added to the lower
chamber separately. The number of SP or dLN cells that migrated
into the lower chamber after 4 h was determined by trypan blue
staining.
Induction of GVHD
Female BALB/c recipient mice received busulfan (BU;
20 mg/kg/day) daily for 4 days, followed by cyclophosphamide (CY;
100 mg/kg/day) daily for 2 days via intraperitoneal injection.
After 1 day of rest, a BM transfer (BMT) was performed on day 0, as
previously described (17,18). For BM cells (BMCs) isolation, male
C57BL/6 donor mice were killed by cervical dislocation and their
limbs were removed. The BM was flushed from the medullary cavities
of both the femurs and tibias and a single cell suspension was
prepared. For the spleen cells (SPC), the spleen of male C57BL/6
donor mice was minced and dispersed into a single-cell suspension.
After pelleting those cells, the erythrocytes were lysed using
hypotonic buffer containing 0.75% NH4Cl.
Female BALB/c recipient mice were injected with
1×107 BMCs combined with 1.5×107 SPCs in a
total volume of 200 µl via lateral tail vein injection (GVHD
group). Mice transplanted with BMCs alone that did not induce GVHD
served as the healthy control group as we previously established
(18–20). Mice transplanted with BMCs and SPCs
suspended in T-MSC-CM generated from 106 cells were
defined as the GVHD-T-MSC-CM group. The GVHD-T-MSC-CM group was
injected with T-MSC-CM (from 106 cells, 200 µl) once
more after 3 days via the tail veil. The expected concentration of
TSG-6 in the T-MSC-CM injection was 300 ng per mouse. Mice
transplanted with BMCs, SPCs, and rhTSG-6 (1 ug/mouse)
simultaneously via the tail vein were defined as the GVHD-rhTSG-6
group. We used eighteen female recipient mice and eighteen male
donor mice per group. Thus, total number of animals used in the
study is one hundred forty four.
During the experimental periods, humane endpoints
was determined as the time when the animal lose weight over 20%
from the starting weight. In that case, mice showed poor mobility
and we immediately sacrificed.
Assessment of GVHD
Recipient mice were examined daily for 3 weeks;
survival and weight loss were recorded. For clinical scoring, mice
were sacrificed by cervical dislocation on days 7 and 21 following
GVHD induction. Tissue samples from the liver, small intestine,
large intestine, and skin were collected and fixed in 4%
paraformaldehyde, and embedded in paraffin. After sectioning, the
tissue sections were stained with hematoxylin and eosin and
analyzed to confirm the presence of GVHD. To assess the severity of
GVHD in mouse organs, seven parameters for skin (necrotic
keratinocytes, lymphoid infiltration of the dermis, lymphocyte
exocytosis, vascular degeneration of the epidermal-dermal junction,
intraepithelial lymphoid infiltration, deficient Langerhans cells,
and edema of the intercellular space), small intestine (villous
blunting, crypt regeneration, crypt epithelial cell apoptosis,
crypt loss, intraintestinal obstruction by cell debris,
inflammatory cell infiltration of the lamina propria, and mucosal
ulceration), and large intestine (crypt regeneration, crypt
epithelial cell apoptosis, crypt loss, liquefaction of superficial
epithelial cells, degeneration of superficial epithelial cells,
inflammatory cell infiltration of the lamina propria, and mucosal
ulceration) were scored, whereas 10 parameters (portal tract
expansion by inflammatory cell infiltrates, lymphocyte infiltration
of bile ducts, bile duct epithelial cell apoptosis, bile duct
epithelial cell sloughing, vascular endothelialitis, parenchymal
apoptosis, parenchymal microabscesses, parenchymal mitotic figures,
hepatocellular cholestasis, and hepatocellular steatosis) were
scored for the liver. The scoring of each parameter was as follows:
0, normal; 0.5, focal and rare; 1, focal and mild; 2, diffuse and
mild; 3, diffuse and moderate; and 4, diffuse and severe. The
scores were added to achieve a total score for each organ;
therefore, the maximum score was 28 for the skin, small intestine,
and large intestine, and 40 for the liver.
Reverse transcription-quantitative
polymerase chain reaction
To confirm the expression of CD4 and CD19 in the
skin, liver, small intestine, and large intestine, total RNA was
extracted from organs harvested on day 21 after GVHD induction.
Total RNA (1 µg) was transcribed into complementary DNA using a
reverse transcription reagent (ELPIS-Biotech Inc., Daejeon, Korea),
according to the manufacturer's instructions. Amplification was
performed in duplicate by 40 cycles of 15 sec denaturation step at
95°C and a 1 min amplification and signal acquisition step at 60°C
using StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using SYBR-Green (Kapa Biosystems, Inc.,
Wilmington, MA, USA). All gene expression values were normalized to
the expression of the GAPDH reference gene using the following
primers: mouse CD4 (115 bp) forward, 5′-TCCTAGCTGTCACTCAAGGGA-3′
and reverse, 5′-TCAGAGAACTTCCAGGTGAAGA-3′; mouse CD19 (164 bp)
forward, 5′-GGAGGCAATGTTGTGCTGC-3′ and reverse,
5′-ACAATCACTAGCAAGATGCCC-3′; and mouse GAPDH (173 bp) forward,
5′-GGTAAAGTGGATATTGTTGCCATCAATG-3′ and reverse,
5′-GGAGGGATCTCGCTCCTGGAAGATGGTG-3′. The relative fold expression
and changes were calculated 2−ΔΔCt method (21).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical significance was determined by two-way
analysis of variance (ANOVA) in conjunction with Dunnett's post hoc
test over non-treated group for chemotaxis assay. Two-way ANOVA in
conjunction with Dunnett's post hoc test over GVHD group were used
for weight loss and total clinical scoring. One-way ANOVA in
conjunction with Sidak's post-hoc test were used in quantitative
real-time PCR. Survival curves were plotted using Kaplan-Meier
estimates. All analysis was performed using GraphPad Prism, version
7 software (GraphPad Software, Inc., La Jolla, CA, USA). For all
analyses, P<0.05 was considered to indicate a statistically
significant difference..
Results
T-MSCs secrete TSG-6 and effectively
inhibit chemotaxis diverse cell populations including immune
cells
The recruitment of donor immune cells, including T
and B cells, into recipient target organs is critical for the
maximal induction of GVHD. Based on our previous findings that
T-MSCs abundantly secrete immunomodulatory cytokines, we sought to
determine whether T-MSCs secrete proteins that regulate immune cell
migration. TSG-6 was originally considered a potent inhibitor of
neutrophil extravasation via the disruption of CXCL8 activity
(22), but TSG-6 also diminishes
the activity of various chemokines, including CXCL4, CXCL12, CCL2,
CCL5, CCL7, CCL19, CCL21, and CCL27, by direct binding (23). Thus, we tested whether T-MSCs
produce TSG-6 endogenously and whether either T-MSC-CM or TSG-6
inhibits the migration of a heterogeneous population of immune
cells under chemotactic conditions. As shown in Fig. 1A, the secreted form of TSG-6 was
abundantly generated by both T-MSCs and AT-MSCs without any
preconditioning, but not by BM-MSCs, according to the results of
the western blot analysis. Secreted TSG-6 was detected at high
levels in T-MSCs, according to the ELISA results (Fig. 1B). Next, we investigated the
effects of TSG-6 on CCL2, a potent chemokine commonly produced by
target organs in GVHD, using a Transwell assay. The concentration
of TSG-6 in T-MSC-CM (106 cell derived) is 136 ng, and
the final concentration of TSG-6 in T-MSC-CM is expected as 217
ng/ml in the Transwell assay. We choose 106
cells-derived T-MSC-CM as the concentration of TSG-6 in T-MSC-CM is
most similar when we use 200 ng/ml of rhTSG-6. In this system, CCL2
upregulated the migration of SPCs and dLN cells in a dose-dependent
manner. Under these conditions, we showed that T-MSC-CM and rhTSG-6
independently ablated the CCL2-induced migration of immune cells,
which was significantly more pronounced in SPCs than in dLN cells
(Fig. 1C and D). Given that the T
cells used in this assay were not purified, the inhibitory effects
of TSG-6 or TSG-6-containing T-MSC-CM may extend beyond specific
cell types.
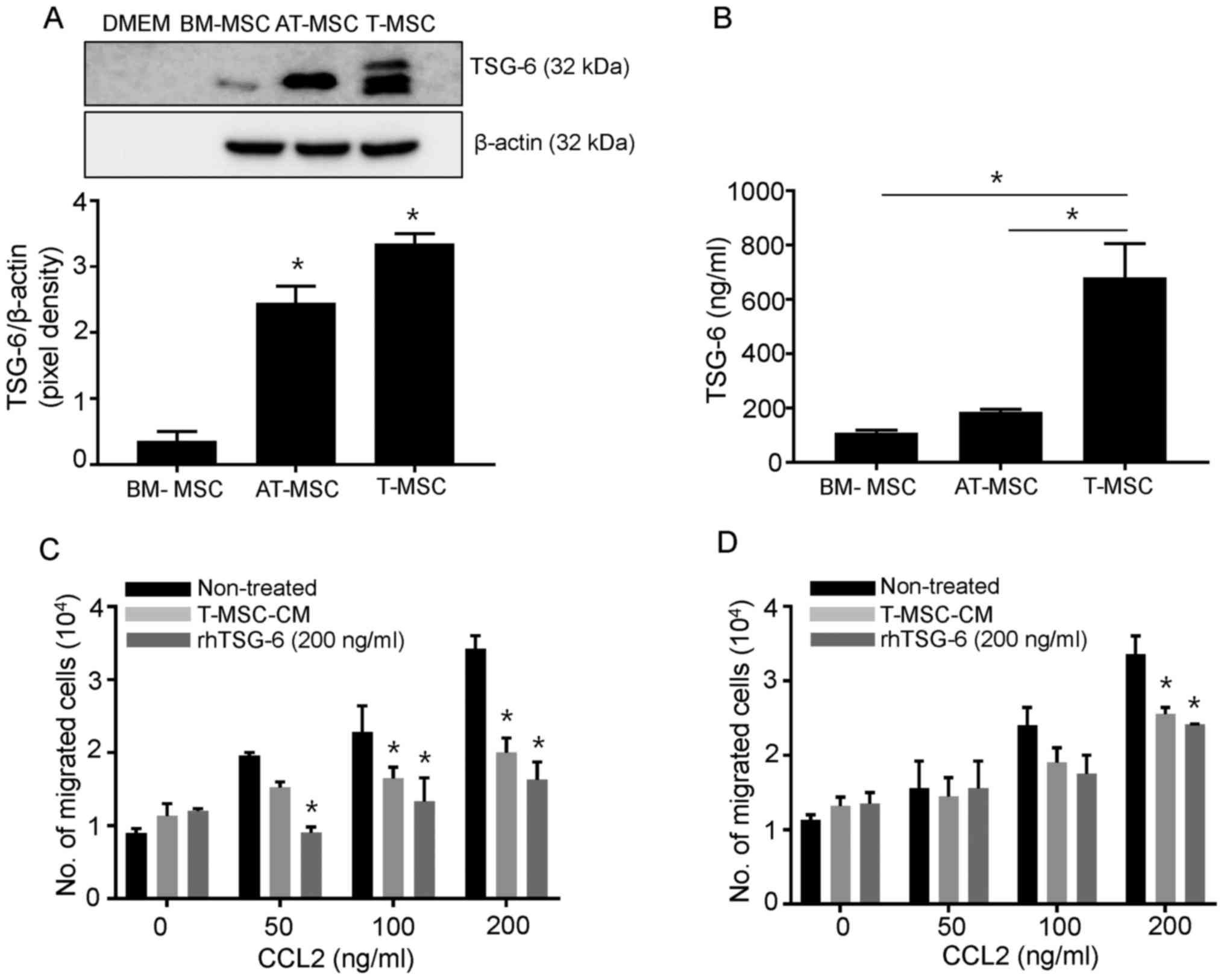 | Figure 1.T-MSCs constitutively secrete TSG-6
and inhibit SPC and dLN cell chemotaxis in vitro. (A) Cell
culture supernatants were collected and subjected to western blot
analysis to detect the secreted form of TSG-6 in BM-MSCs, AT-MSCs
and T-MSCs. DMEM media alone was loaded as the negative control.
Cell lysates were harvested and endogenous levels of β-actin were
detected for normalization. The pixel densities of the TSG-6 bands
were divided by the pixel densities of the corresponding β-actin
bands. AT-MSC and T-MSC produce significantly higher extent of
TSG-6 in comparison with those of BM-MSC. Data are presented as the
mean ± SEM. *P<0.05 vs. BM-MSC group). (B) TSG-6 levels in cell
supernatants from BM-MSCs, AT-MSCs, and T-MSCs were measured by
ELISA. Data are presented as the mean ± SEM (*P<0.05). The
migration of mouse SPCs (C) and dLN cells (D) in response to CCL2
is shown using the Transwell migration assay. Data are presented as
the mean ± SEM. *P<0.05 vs. Non-treated group. SEM, standard
error of the mean; DMEM, Dulbecco's modified Eagle's medium;
BM-MSC, bone marrow-derived mesenchymal stem cell; AT-MSC, adipose
tissue-derived mesenchymal stem cell; T-MSC, tonsil-derived
mesenchymal stem cell; TSG-6, tumor necrosis factor stimulated
gene-6; T-MSC-CM, tonsil-derived mesenchymal stem cell conditioned
medium; rhTSG-6, recombinant human tumor necrosis factor stimulated
gene-6; SPC, spleen cell; dLN, draining lymph node; CCL2, chemokine
(C-C motif) ligand 2. |
T-MSC-CM attenuates the clinical
manifestations of GVHD
A mouse model of GVHD was generated, as shown in
Fig. 2A. GVHD was induced in mice
conditioned with BU and CY prior to the injection of allogenic BMCs
and SPCs. Despite the severe mortality of GVHD mice observed 7 days
after cell transplantation, the addition of either T-MSC-CM or
rhTSG-6 prolonged survival (Fig.
2B). Rapid and severe weight loss was also observed in these
mice within 7 days of BMC/SPC transplantation, which correlated
with the mortality rates observed during that period. However, the
administration of either T-MSC-CM or rhTSG-6 resulted in
significantly lesser weight loss and faster recovery rates in
comparison with GVHD mice (Fig.
2C).
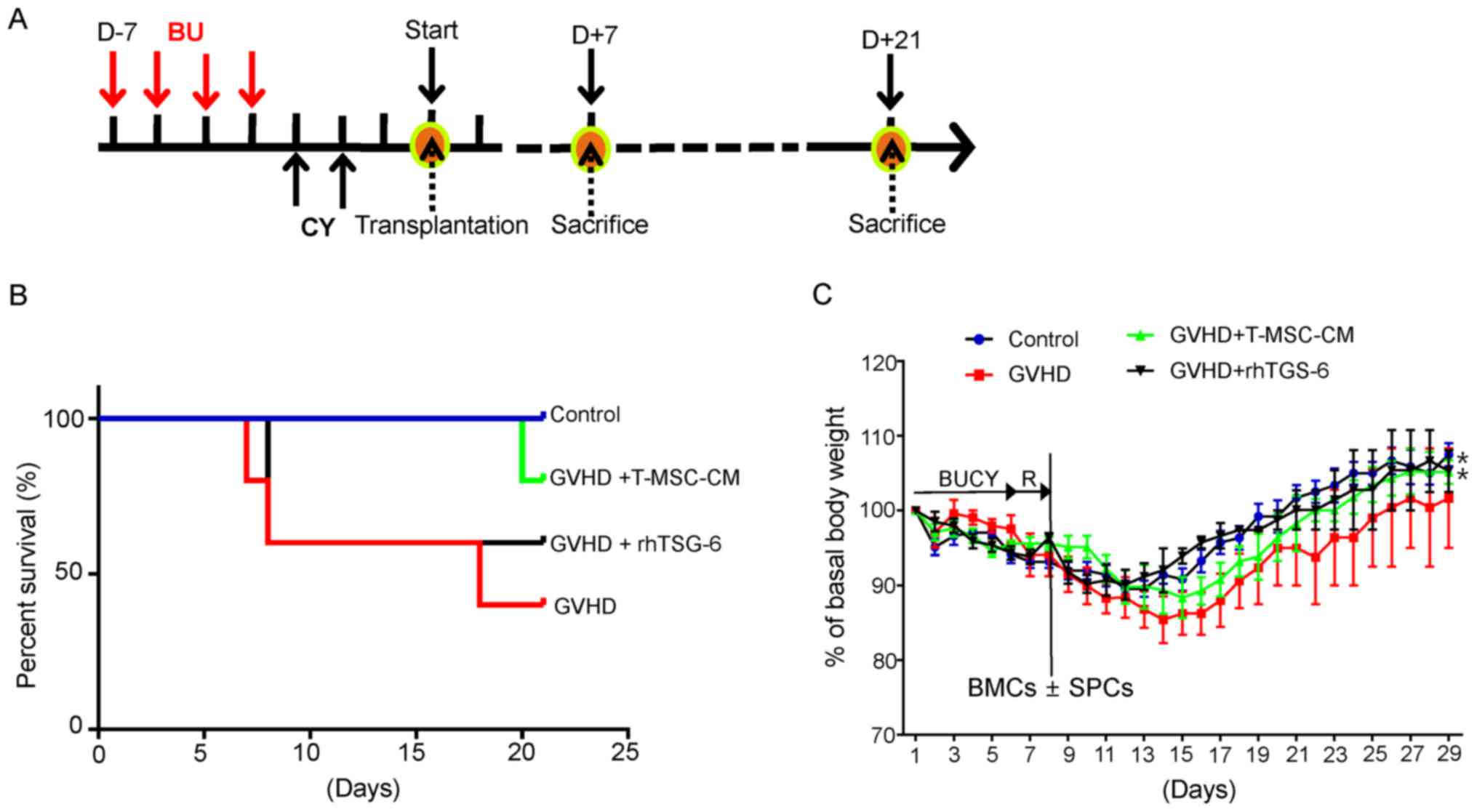 | Figure 2.T-MSC-CM or rhTSG-6 reduces GVHD
severity in mice. (A) Female BALB/c recipient mice received BU (20
mg/kg/day) for 4 days, followed by CY (100 mg/kg/day) for 2 days.
All recipients received one day of rest before experimental
transplantation. The mice were categorized into four groups:
Transplantation of BMCs only (control); transplantation of BMCs
plus SPCs (GVHD); BMCs plus SPCs with T-MSC-CM (GVHD-T-MSC-CM); and
BMCs plus SPCs with rhTSG-6 (GVHD-rhTSG-6). After transplantation,
the mice were monitored daily for 3 weeks. (B) A survival rate
analysis of the different treatment groups (n=8 for each group) was
performed using Kaplan-Meier estimates. (C) The total body weight
of experimental BALB/C recipients was monitored over the study
duration. Basal body weight was determined as weight at start.
T-MSC-CM or rhTSG-6 treated mice showed significant recovery in
comparison with GVHD group. Data are presented as the mean ±
standard error of the mean. *P<0.05 vs. GVHD group. D, day; BU,
busulfan; CY, cyclophosphamide; GVHD, graft-vs.-host disease;
T-MSC-CM, tonsil-derived mesenchymal stem cell conditioned medium;
rhTSG-6, recombinant human tumor necrosis factor stimulated gene-
6; BUCY, busulfan cyclophosphamide; R, recovery; BMCs, bone marrow
cells; SPCs, spleen cells. |
T-MSC-CM or rhTSG-6 attenuates GVHD
response
Major target organs of GVHD, including the skin,
liver, small intestine, and large intestine, showed clear
histopathological evidence of GVHD on day 7 (Fig. 3A) that was more severe than on day
21 (Fig. 3B). Skin samples
exhibited a clear disruption of the epidermis and thinning of the
dermis on day 7 post-transplantation, and liver samples showed
extramedullary hematopoiesis and inflammatory infiltrates in the
portal triad. Mucosal crypts in the small and large intestines were
severely disrupted, exhibiting hyperplasia and hyperchromatic
nuclei, with numerous cells displaying apoptotic characteristics.
The total scores for GVHD severity are shown in Fig. 3C. This score was highest on day 21
in the GVHD group, but T-MSC-CM and rhTSG-6 improved this score by
over 20 points.
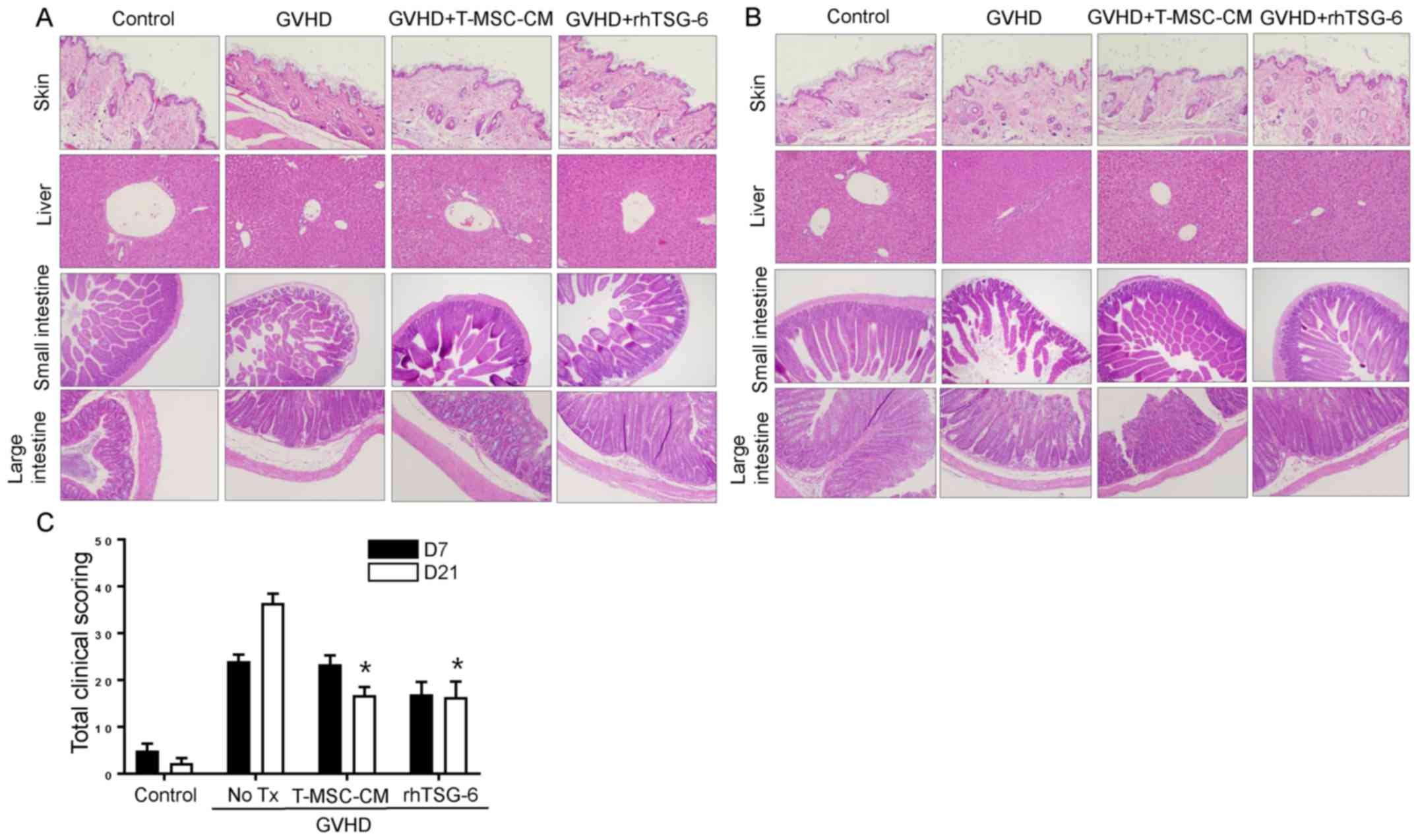 | Figure 3.T-MSC-CM or rhTSG-6 attenuate the GVHD
response. (A) Histological tissue sections of the skin, liver,
small intestine, and large intestine were collected from
experimental mice on day 7 post-transplantation (original
magnification, ×100 for liver and ×200 for skin, small intestine
and large intestine). (B) Histological tissue sections of the skin,
liver, small intestine, and large intestine were obtained from
experimental mice on day 21 (original magnification, ×100 for liver
and ×200 for skin, small intestine, and large intestine). (C)
Slides of the skin, liver, small intestine, and large intestine
obtained from experimental mice on days 7 and 21
post-transplantation were stained with hematoxylin and eosin and
scored for GVHD severity according to standard criteria and added
to determine the total score. Either T-MSC-CM and rhTSG-6
significantly reduce the scoring on post 21 day from GVHD
induction. Data are presented as the mean ± standard error of the
mean. *P<0.05 vs. GVHD group (n=8). GVHD, graft-vs.-host
disease; T-MSC-CM, tonsil-derived mesenchymal stem cell conditioned
medium; rhTSG-6, recombinant human tumor necrosis factor stimulated
gene-6; No Tx, no treatment. |
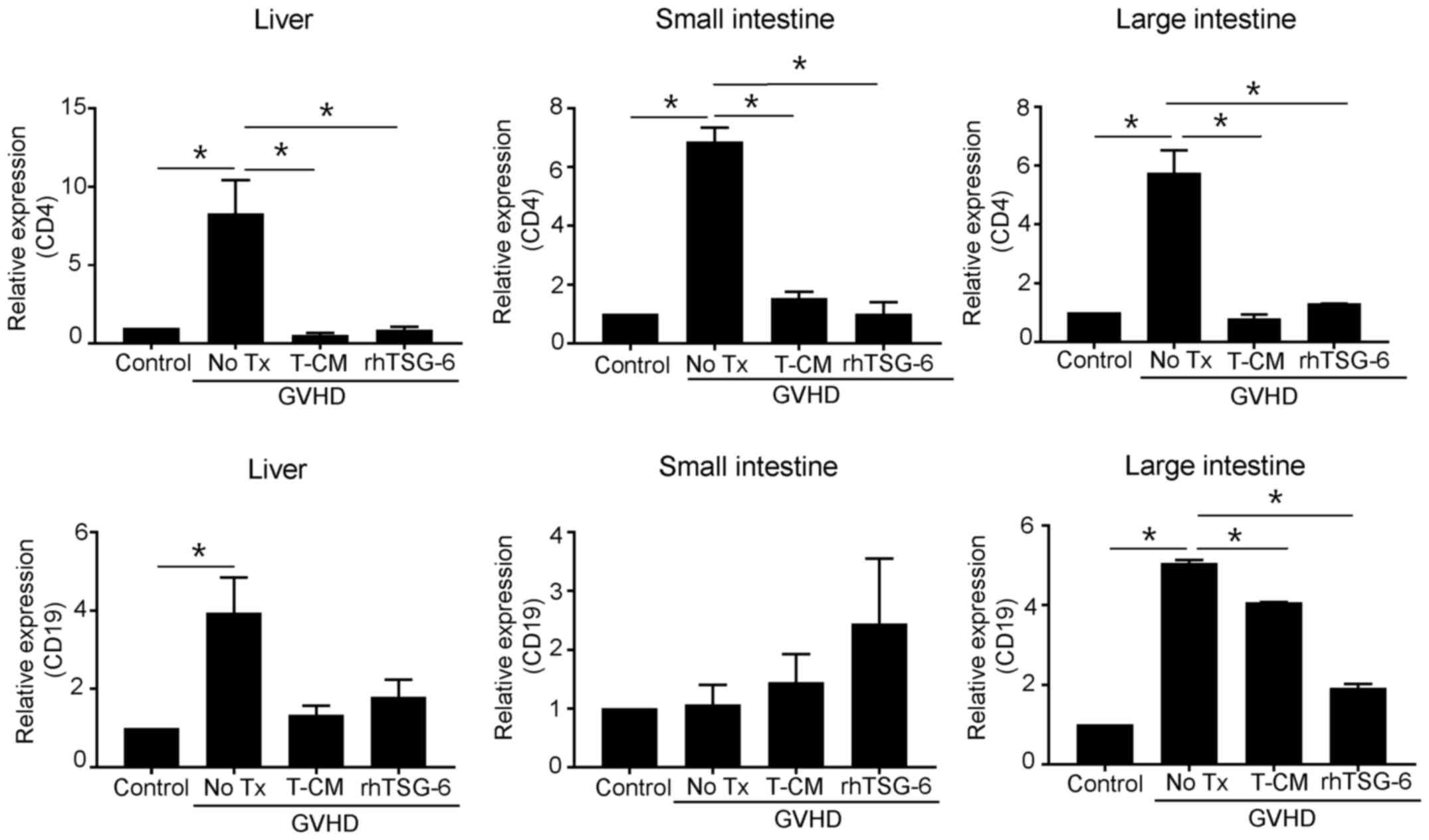
T-MSC-CM and rhTSG-6 downregulate
lymphocyte gene expression in GVHD-targeted organs
Because we observed significant inhibition of immune
cell chemotaxis in the presence of T-MSC-CM, we speculated that the
differential recruitment and expansion of lymphocytes, such as T or
B cells, occurred in each experimental mouse group. To test our
theory, we compared the expression of CD4 and CD19 in the liver,
small intestine, and large intestine (Fig. 4). Our results revealed that GVHD
greatly induced the expression of CD4 in all organs, whereas
similar to the effects of rhTSG-6, T-MSC-CM significantly
downregulated CD4 expression. The highest levels of CD19 expression
were observed in the large intestine in GVHD mice, but these levels
were reduced by T-MSC-CM. The liver also exhibited increased CD19
expression in GVHD mice that was inhibited by T-MSC-CM.
Discussion
In this study, we demonstrate that T-MSC-CM
attenuates aGVHD responses in a mouse model of the disease. BALB/C
recipient mice that were preconditioned by the administration of BU
and CY were subsequently transplanted with BMCs and SPCs to induce
GVHD. In this model, the administration of T-MSC-CM effectively
prolonged survival, promoted a rapid recovery from weight loss and
improved histological pathogenesis. Furthermore, lymphocyte
expression levels of CD4 and CD19 were downregulated in GVHD mice
injected with T-MSC-CM. Furthermore, the observed regulation of
lymphocyte gene expression was supported by in vitro data,
indicating that T-MSC-CM significantly inhibits the migration of
diverse cell populations including immune cells.
The use of MSCs is a promising strategy for the
treatment of aGVHD. A key advantage of MSCs is that
histocompatibility matching is not required to achieve therapeutic
effects. MSCs do not express human leukocyte antigen class II
histocompatibility antigens or the CD40, CD80, or CD86 accessory
molecules that are required for immune cell activation. An
important biological property of MSCs is that their chemotactic
responses to inflammatory factors are generally restricted to the
migration of neutrophils and other immune-responsive cells. Once at
the site of injury or inflammation, it is thought that MSCs
modulate immune and inflammatory reactions at the
microenvironmental level and stimulate tissue repair of affected
organs (7). Several organs are
targets in GVHD (skin, liver, and GI tract); thus, MSC therapy
might depend on the number of infused cells that successfully
traffic to various sites of tissue damage. However, the optimal
number of cells and the number of administrations might be
difficult to determine. Because more than one tissue is often
damaged, the migration of cells to various tissues might not be
uniform, leading to inefficient tissue repair. In fact, the use of
an increased number of cells did not augment the therapeutic
effects of BM-MSCs in GVHD (24).
Regarding the route of cellular therapy, the direct, regional
administration of cells to target organs in steroid-refractory GVHD
was not as effective as their systemic injection (25). Thus, we assume that the therapeutic
effects of MSCs in GVHD might be primarily attributed to the
secretion of immunomodulatory factors following systemic
infusion.
Previously, we reported that T-MSCs could serve as a
cellular treatment for inflammatory conditions and the regeneration
of damaged tissue via the abundant secretion of immunomodulatory
proteins. Specifically, in this study, we found that T-MSCs produce
high levels of TSG-6, a protein that inhibits immune cell
chemotaxis, by directly binding to several chemokines. Of noted, we
had difficulties in finding secretory loading protein that produced
with similar extent from AT-MSCs, BM-MSCs, T-MSCs. Thus, we used
β-actin as loading control protein in performing western blot. We
believe β-actin can be alternative loading control protein because
we cultured same numbers of cells for harvesting CM (AT-MSC,
BM-MSC, and T-MSC) and used same concentration of protein for
β-actin western blot. Due to its ability to inactivate chemokines,
TSG-6 is assumed to play an inhibitory role in the development of
GVHD by blocking donor cell migration into target organs that
secrete a number of chemokines. When we tested whether rhTSG-6 or
T-MSC-CM could affect the migration of an immune cell population
primarily composed of lymphocytes, both rhTSG-6 and T-MSC-CM
significantly inhibited the chemotactic migration of responder
cells. This finding supports the use of T-MSC-CM to treat GVHD,
considering that the trafficking of donor T cells is critical for
GVHD development. In fact, blocking the migration of donor T cells
effectively attenuated aGVHD and preserved graft-vs.-leukemia
activity in a tumor-bearing GVHD mouse model (18). In this prior study, blocking the
chemotactic migration of donor T cells to GVHD-damaged organs
efficiently enhanced their anti-tumor effects in a leukemic mouse
model. In the current study, T-MSC-CM appeared to cause a similar
inhibition of chemotaxis, possibly via the involvement of
TSG-6.
Donor T cells were shown to partition to lymphoid
tissues within h of a BMT. In the 2–3 days following transfer,
allogeneic T cells expanded within lymphoid tissues. Between days 3
and 21, the number of allogeneic T cells increased in GVHD-targeted
organs, including the GI tract, liver, lung, and skin (26). This finding is consistent with our
in vivo data, showing increased mortality 7 days after cell
transplantation and more severe GVHD responses on day 21
post-transplantation compared with responses on day 7. Both rhTSG-6
and T-MSC-CM significantly recovered weight loss in GVHD mice, but
T-MSC-CM showed higher therapeutic effect on survival. We assume
survival might be affected by more complicated factors that are not
restricted to weight recovery. For example, a mouse in mild weight
loss with severely damaged target organ died earlier rather than
mouse showed rapid weight loss with lesser extent of damages on
target organs.
Further, our qPCR results support the critical
pathophysiological sequence of events, as the expression levels of
CD4 and CD19, surface antigens on T and B cells, respectively, were
significantly increased on day 21 post-transplantation. Likewise,
the downregulation of CD4 and CD19 expression by T-MSC-CM and
rhTSG-6 suggests that immune cell migration and expansion were
abrogated in GVHD organs. When considering that T-MSC-CM contains
various immunomodulatory factors in addition to TSG-6, the effects
of T-MSC-CM shown in this study might not be exclusive to TSG-6.
For example, PD-L1 (27), IL-35
(12), and IL-1ra (13), which were previously reported to be
anti-inflammatory proteins acting on effector T cell, B cells, or
fibrosis, may also exert anti-inflammatory functions in GVHD.
However, we believe that TSG-6 in T-MSC-CM may critically inhibit
donor cell migration into GVHD-targeted organs rather than other
factors in T-MSC-CM. The specific outcome of TSG- inhibition or the
dual treatment of T-MSC-CM with rhTSG-6 or other molecules (e.g.,
PD-L1, IL-35 and IL-1ra) should be handled within our next expanded
GVHD research.
In summary, we demonstrate that T-MSCs effectively
ameliorate GVHD via secretory factors containing TSG-6. These
findings suggest that T-MSC-CM could be a promising cellular agent
for the treatment of transplant rejection.
Acknowledgements
Not applicable.
Funding
The present study was supported by a National
Research Foundation of Korea (NRF) grant funded by the Korean
government (grant no. NRF-2017R1E1A1A01073021). In addition, the
present study was supported by the RP-grant 2018 of Ewha Womans
University.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contribution
K-AC performed experiments and wrote the manuscript.
Y-HK, MP and HJK also performed experiments and analyzed data. S-YW
and J-WP also analyzed data and assisted in writing the manuscript.
K-HR designed the experiments and assisted in writing the
manuscript.
Ethics approval and consent to
participate
All procedures were approved by the College of
Medicine, Ewha Womans University Animal Care and Use Committee
(approval no. ESM18-0403).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferrara JL, Levine JE, Reddy P and Holler
E: Graft-versus-host disease. Lancet. 373:1550–1561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacigalupo A: Management of acute
graft-versus-host disease. Br J Haematol. 137:87–98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacMillan ML, Weisdorf DJ, Wagner JE,
DeFor TE, Burns LJ, Ramsay NK, Davies SM and Blazar BR: Response of
443 patients to steroids as primary therapy for acute
graft-versus-host disease: Comparison of grading systems. Biol
Blood Marrow Transplant. 8:387–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deeg HJ: How I treat refractory acute
GVHD. Blood. 109:4119–4126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez L, Anasetti C and Pidala J: Have we
improved in preventing and treating acute graft-versus-host
disease? Curr Opin Hematol. 18:408–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnett C, Apperley JF and Pavlů J:
Treatment and management of graft-versus-host disease: Improving
response and survival. Ther Adv Hematol. 4:366–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fajardo-Orduña GR, Mayani H and Montesinos
JJ: Hematopoietic support capacity of mesenchymal stem cells:
Biology and clinical potential. Arch Med Res. 46:589–596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dotoli GM, De Santis GC, Orellana MD, de
Lima Prata K, Caruso SR, Fernandes TR, Rensi Colturato VA, Kondo
AT, Hamerschlak N, Simões BP and Covas DT: Mesenchymal stromal cell
infusion to treat steroid-refractory acute GvHD III/IV after
hematopoietic stem cell transplantation. Bone Marrow Transplant.
52:859–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muroi K, Miyamura K, Okada M, Yamashita T,
Murata M, Ishikawa T, Uike N, Hidaka M, Kobayashi R, Imamura M, et
al: Bone marrow-derived mesenchymal stem cells (JR-031) for
steroid-refractory grade III or IV acute graft-versus-host disease:
A phase II/III study. Int J Hematol. 103:243–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho KA, Park M, Kim YH, Ryu KH and Woo SY:
Mesenchymal stem cells inhibit RANK-RANKL interactions between
osteoclasts and Th17 cells via osteoprotegerin activity.
Oncotarget. 8:83419–83431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho KA, Lee JK, Kim YH, Park M, Woo SY and
Ryu KH: Mesenchymal stem cells ameliorate B-cell-mediated immune
responses and increase IL-10-expressing regulatory B cells in an
EBI3-dependent manner. Cell Mol Immunol. Jan 2–2017;(Epub ahead of
print).
|
|
13
|
Cho KA, Park M, Kim YH, Woo SY and Ryu KH:
Conditioned media from human palatine tonsil mesenchymal stem cells
regulates the interaction between myotubes and fibroblasts by
IL-1Ra activity. J Cell Mol Med. 21:130–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC, Chung SM, et al: Tonsil-derived
mesenchymal stromal cells: Evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cantinieaux D, Quertainmont R, Blacher S,
Rossi L, Wanet T, Noël A, Brook G, Schoenen J and Franzen R:
Conditioned medium from bone marrow-derived mesenchymal stem cells
improves recovery after spinal cord injury in rats: An original
strategy to avoid cell transplantation. PLoS One. 8:e695152013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gehmert S, Wenzel C, Loibl M, Brockhoff G,
Huber M, Krutsch W, Nerlich M, Gosau M, Klein S, Schreml S, et al:
Adipose tissue-derived stem cell secreted IGF-1 protects myoblasts
from the negative effect of myostatin. Biomed Res Int.
2014:1290482014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadeghi B, Aghdami N, Hassan Z,
Forouzanfar M, Rozell B, Abedi-Valugerdi M and Hassan M: GVHD after
chemotherapy conditioning in allogeneic transplanted mice. Bone
Marrow Transplant. 42:807–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho KA, Woo SY, Park YS, Park MH and Ryu
KH: Macrophage inflammatory protein-2 (MIP-2)/CXCR2 blockade
attenuates acute graft-versus-host disease while preserving
graft-versus-leukemia activity. Biochem Biophys Res Commun.
426:558–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY,
Choi YB, Hong KM, Woo SY, Seoh JY and Ryu KH: Bioimaging for the
monitoring of the in vivo distribution of infused mesenchymal stem
cells in a mouse model of the graft-versus-host reaction. Cell Biol
Int. 35:417–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY,
Choi YB, Hong KM, Woo SY, Seoh JY, Cho SJ and Ryu KH: Mesenchymal
stromal cells inhibit graft-versus-host disease of mice in a
dose-dependent manner. Cytotherapy. 12:361–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dyer DP, Thomson JM, Hermant A, Jowitt TA,
Handel TM, Proudfoot AE, Day AJ and Milner CM: TSG-6 inhibits
neutrophil migration via direct interaction with the chemokine
CXCL8. J Immunol. 192:2177–2185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dyer DP, Salanga CL, Johns SC, Valdambrini
E, Fuster MM, Milner CM, Day AJ and Handel TM: The
anti-inflammatory protein TSG-6 regulates chemokine function by
inhibiting chemokine/glycosaminoglycan interactions. J Biol Chem.
291:12627–12640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kebriaei P, Isola L, Bahceci E, Holland K,
Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, et
al: Adult human mesenchymal stem cells added to corticosteroid
therapy for the treatment of acute graft-versus-host disease. Biol
Blood Marrow Transplant. 15:804–811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arima N, Nakamura F, Fukunaga A, Hirata H,
Machida H, Kouno S and Ohgushi H: Single intra-arterial injection
of mesenchymal stromal cells for treatment of steroid-refractory
acute graft-versus-host disease: A pilot study. Cytotherapy.
12:265–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wysocki CA, Panoskaltsis-Mortari A, Blazar
BR and Serody JS: Leukocyte migration and graft-versus-host
disease. Blood. 105:4191–4199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JY, Park M, Kim YH, Ryu KH, Lee KH,
Cho KA and Woo SY: Tonsil-derived mesenchymal stem cells (T-MSCs)
prevent Th17-mediated autoimmune response via regulation of the
programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway.
J Tissue Eng Regen Med. 12:e1022–e1033. 2018. View Article : Google Scholar : PubMed/NCBI
|