Introduction
Diabetic peripheral neuropathy (DPN) is a frequent
complication of diabetes that influences the nervous system
(1) and >50% of patients with
diabetes are affected. The major manifestations of DPN are
paresthesia, hyperalgesia, sensory loss and foot ulceration, which
are associated with considerable morbidity, mortality and impaired
quality of life in patients (2).
Surgery or symptomatic treatments are currently the only options
for treating DPN (3,4). Drugs that are currently used for DPN
treatment have demonstrated variable efficacy and the use of them
are limited due to adverse effects (5,6).
These limitations have led to more extensive research into finding
effective alternative options for treating DPN.
L-carnitine (β-hydroxy-γ-N-trimethylaminobutyric
acid; LC), the biologically active form of carnitine, is an amino
acid-like agent involved in the transit of long chain fatty acids
that is synthesized in various tissues (7) and serves an important role in fatty
acid oxidation (8). Bach et
al (9) reported that the
skeletal muscle, heart, intestines, testis and in particular liver,
can hydroxylate γ-butyrobetaine into carnitine. LC was demonstrated
to have a role in ameliorating diabetic neuropathy by decreasing
insulin resistance and increasing cellular uptake of glucose
(10,11). Stevens et al (12) also demonstrated that diabetic
patients with complications, including hyperlipidemia and
neuropathy exhibited lower concentrations of LC compared with
patients who did not harbour these complications. Additional
studies have revealed the preventive and therapeutic effects of LC
on peripheral nerve function and structural abnormalities (13–16)
as well as on endothelial blood flow (17).
Insulin-like growth factor-1 (IGF-1) is an
insulin-like hormone that participates in maintaining glucose
homeostasis. It is structurally homologous to insulin and shares
similar in vitro metabolic activity (18). Numerous prospective epidemiological
studies have revealed that alterations in serum levels of IGF-1 are
associated with the development of type 2 diabetes mellitus (T2DM)
(19–23). IGF-1 binding proteins (IGFBPs) are
hypothesized to have a role in glucose metabolism, with IGFBP-1
acutely regulating glucose levels through its effects on free IGF-I
(24).
Additionally, studies have indicated that LC
supplementation in the plasma is associated with the levels of
IGF-1 (25) and LC may contribute
to the activation of IGF-1 (26).
However, the role of LC in ameliorating diabetic neuropathy has not
been clarified. In the present study, the effect of exogenous LC on
DPN in streptozotocin (STZ)-induced diabetic mice was investigated
and possible mechanisms of action were examined.
Materials and methods
Animals
A total of 40 male Kunming specific-pathogen-free
mice (weight, 15–17 g; age, 3 weeks), were obtained from the
Institute of Drug Control (Qingdao, China). The mice had free
access to food and water, and were housed in a
temperature-controlled (24±1°C; humidity 50–60%) animal room
(illumination from 7:00 to 19:00). All mice were fed a high-fat
diet (HFD; 59% standard mouse food, 18% lard, 20% sugar and 3% egg
yolk). Only 27 of the 40 mice survived due to the low survival rate
of diabetic mice. The study was approved by the Ethics Committee of
Medical Department of Qingdao University (Qingdao, China). The
protocols used for handling the mice were provided by the Qingdao
University Center for Human Functional Experiments of the Medical
College Animal Care Committee (Qingdao, China), which meets the
guidelines set by the National Institutes of Health Guide for Care
and Use of Laboratory Animals.
LC treatment
LC was obtained from the Northeast Pharmaceutical
Group Co., Ltd. (Shenyang, China) and was prepared in double
distilled water (DDW). To study the preventive effect of LC on
peripheral neuropathy in type 2 diabetic mice, the mice were
randomly divided into five groups (n=8/group): Control, diabetes,
pre-treatment, treatment and post-treatment (27). For the pre-treatment group,
intragastric administration of 125 mg/kg LC was performed once a
day until the mice were 12 weeks old. For the treatment group, mice
were administered 125 mg/kg LC 24 h following diabetes induction (9
weeks + 24 h) until the mice were 12 weeks old. For the
post-treatment group, mice were randomly divided into two equal
groups and administered either LC (125 mg/kg) or DDW when mice were
raised 13 weeks until they were 16 weeks old. A summary of the
experimental design is presented in Table I.
 | Table I.Experimental design. |
Table I.
Experimental design.
|
| Treatment |
|---|
|
|
|
|---|
| Groups | 3 weeks | 6 weeks | 9 weeks | 12 weeks |
|---|
| Control | HFD+DDW | HFD+DDW (CB) | HFD+DDW (CB) | – |
| DM | HFD+DDW | HFD+DDW (STZ) | HFD+DDW (STZ) | – |
| Pre-treatment | HFD+LC | HFD+LC (STZ) | HFD+LC (STZ) | – |
| Treatment | HFD+DDW | HFD+DDW (STZ) | HFD+LC (STZ) | – |
| Post-treatment | HFD+DDW | HFD+DDW (STZ) | HFD+DDW (STZ) | HFD+LC/DDW |
STZ treatment
STZ was obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) and was prepared in 0.1 M citrate buffer (pH
4.5). A total of two low doses of 100 mg/kg STZ were delivered to
mice in the diabetic, pre-treatment, treatment and post-treatment
group by intraperitoneal injection at the age of 6 and 9 weeks.
Age-matched mice in the control group were injected with DDW. Mice
with insulin levels within the average range of the control group
and fasting blood glucose levels >12 mmol/l were considered to
be type 2 diabetic mice (28).
Body weight, food intake and blood
glucose measurements
Body weight and daily food intake were monitored
using an electric balance [PL1501-S; Mettler-Toledo International
Trading (Shanghai) Co., Ltd., Shanghai, China] once a week. Blood
samples were collected from the tail vein at the age of 3 weeks, 6
weeks (24 h following the first STZ injection), 9 weeks (24 h
following the second STZ injection) and 12 weeks. Blood glucose was
determined using a glucometer (B. Braun, Melsungen AG, Hesse,
Germany) for all the samples.
Preparation of plasma, sciatic nerve
and pancreas samples
All mice were sacrificed at the age of 12 weeks
except for mice in the post-treatment group, which were sacrificed
at 16 weeks of age. Blood samples (1 ml) were drawn from the
retro-orbital sinus, followed by centrifugation at 4°C for 15 min
at 3,000 × g and were then stored at −20°C. A total of two segments
of sciatic nerve were removed from the two lateral sides. One
segment of sciatic nerve was used for ultra-structural observation
under an electron microscope. The sciatic nerve was immediately
immersed at room temperature for 1 h, then at 4°C for >3 h in
Sotelo fixing solution, composed of 1% paraformaldehyde and 2.5%
glutaraldehyde in cacodylate buffer 0.1 mol/l, adjusted at pH 7.35.
Then they were post-fixed by immersion in 1% osmium tetroxide for
30 min at room temperature and dehydrated in graded alcohol
solutions and propylene oxide. Following embedding in Epon 812,
microsections of 1–2 µm were cut and stained at room temperature
for 15–30 min on the copper grid with 4% uranyl acetate and then
with Reynold's lead citrate for 5–10 min. Pancreas tissues were
homogenized in 0.01 M PBS and the supernatants obtained by
centrifugation at 4°C for 15 min at 12,000 × g were stored at
−20°C.
Determination of plasma and pancreas
LC levels using high-performance liquid chromatography (HPLC)
Concentrations of LC in the plasma and pancreas were
detected by HPLC using an excitation and emission wavelengths of
248 and 418 nm, respectively. This method was first established in
the authors' lab (29). HPLC was
performed using a 474 fluorescence detector (Waters 2690
Separations Module; Waters Corporation, Milford, MA, USA). A
reversed-phase Hypersil BDS-C18 column (200×4.6 mm, 5 mm particle
size, Thermo Fisher Scientific, Inc., Waltham, MA, USA) equipped
with a precolumn Hypersil BDSC18 (10×4 mm, 5 mm particle size) was
used. The mobile phase was composed of methanol- [ammoniun formate
20 mM, formic acid 0.5%, triethylamine 0.2% (pH 3)] 67:33 v/v, and
its flow rate was 1 ml/min. The samples were refrigerated in an
auto sampler for storage, which was a part of the Waters 2690
Alliance system. The complete procedure was run for 60 min. The
injection volumes for test samples and standards varied between 50
and 100 µl depending on the sample concentration. The column was
equilibrated with a minimum of 5 injections (50 µl) of sample
diluent buffer until a stable baseline was obtained. Experiments
were performed at ambient temperature.
Measurement of plasma insulin
levels
The plasma insulin levels were detected by ELISA
using a mouse insulin ELISA kit (Catalog # EMINS; Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Absorbance at 450 nm was measured using a microplate
reader (M5; MD-SpectraMax; Molecular Devices, LLC., San Jose, CA,
USA).
Tail-flick test
The tail-flick latency test was used to measure the
reaction latency of the peripheral nerve to thermal stimulus. The
tails of mice were immersed in 49°C water and when a withdrawal
response occurred, the response latency was recorded. The cut-off
time for maximum latency was 10 sec to avoid tail tissue damage.
The tests were carried out once a week starting at 9 weeks of age
(following the second STZ injection).
Peripheral neuropathy measurement
A sample of the sciatic nerve was processed
according to standard procedures for the preparation of samples for
electron microscope visualization (30). The ultrastructure of the sciatic
nerve was observed by JEOL 1200 EX transmission electron microscope
(JEOL USA, Inc., Peabody, MA, USA) and ultra-structural
morphometric analysis was performed on electron micrographs of
nerves using ImageJ (v1.8.0; National Institutes of Health,
Bethesda, MD, USA). The area of the axon (A) and myelin sheath (MS)
of sciatic nerves was used to calculate the ratio of axon to myelin
sheath area (A/MS).
Measurement of IGF-1 concentration in
sciatic nerves and plasma
The concentration of IGF-1 in the sciatic nerve and
plasma was determined using a mouse IGF-1 Quantikine ELISA kit
(cat. no. MG100; R&D; Bio-Techne, Minneapolis, MN, USA). The
total protein was quantified using the bicinchoninic acid Protein
Assay kit (cat. no. 80815–500; Tiandz, Inc., Beijing, China).
Tissue IGF-1 content is expressed as IGF-1 µg/g total protein.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of 2 repeated experiments. Statistical differences between
dependent variables were examined with one-way analysis of variance
followed by Student-Newman-Keuls post hoc test. All data were
analyzed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
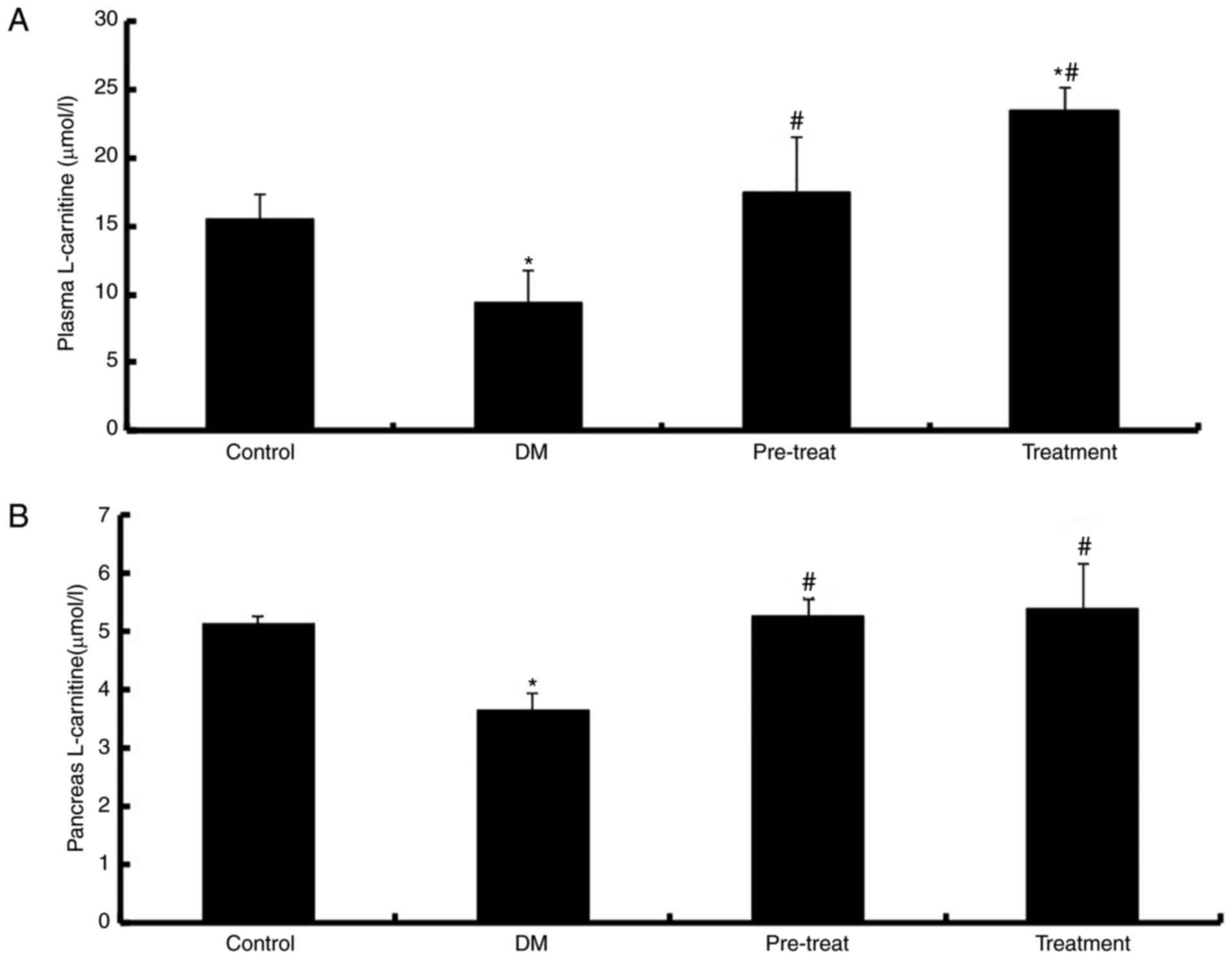
Concentration of L-carnitine in the
plasma and pancreas
De Palo et al (31) previously reported that diabetes
mellitus causes a significant decrease in LC levels. To investigate
the effect of LC in diabetic mice, intragastric administration of
LC was performed. The results demonstrated that there were
significantly decreased levels of LC in the plasma and pancreas of
diabetic mice compared with the control (P<0.01). LC levels were
significantly elevated in the plasma (P<0.01; Fig. 1A) and pancreas (P<0.01; Fig. 1B) of mice treated with LC compared
with diabetic mice. In addition, the plasma LC levels of mice in
the treatment group were increased compared with in the control
group.
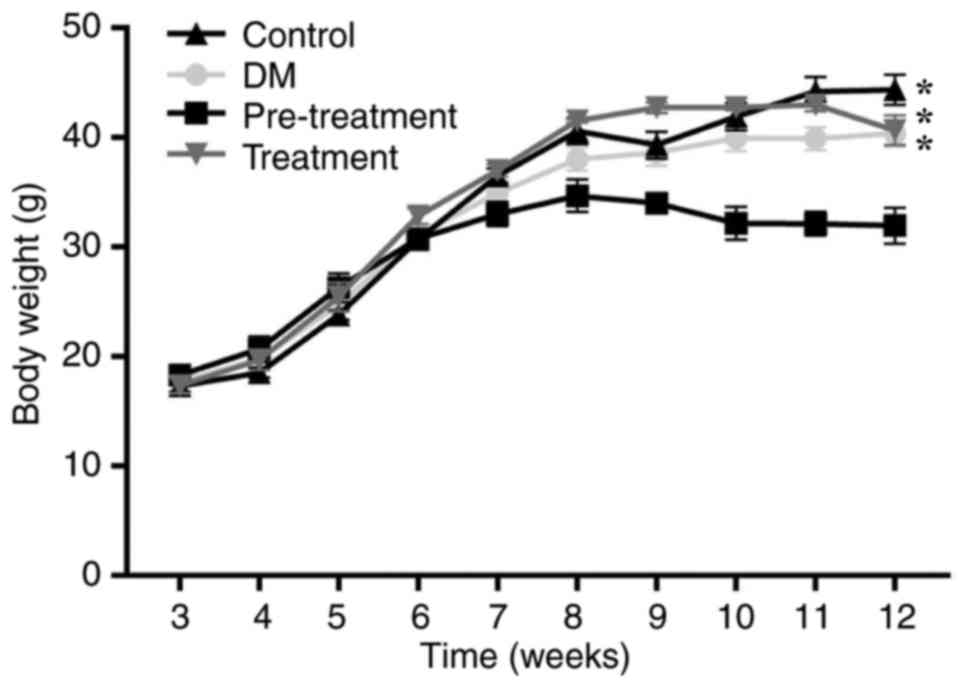
Changes in blood glucose levels,
plasma insulin levels, body weight and food intake
Following intragastric administration of LC, blood
glucose levels, plasma insulin levels, body weight and food intake
were monitored. Blood glucose levels of mice treated with LC were
significantly increased compared the control group (P<0.01), but
significantly decreased compared with the diabetes group
(P<0.05; Table II). The blood
glucose levels of mice in the pre-treatment group, where mice where
treated with LC prior to the appearance of diabetes, were lower
compared with mice in the treatment group, where mice were treated
with LC following the appearance of diabetes. Higher plasma insulin
levels were observed in mice treated with LC compared with in
diabetic mice. Overall, LC appeared to have beneficial effects on
diabetes. When mice were aged 12 weeks, the average body weight of
mice treated with LC prior to the appearance of diabetes was lower
compared with other mice. From week 9 onwards, there was a
significant difference in body weight between the pre-treatment and
control group (P<0.05; Fig. 2).
The food intake of diabetic mice was increased compared with the
mice in the control group, which was consistent with clinical
characteristics of T2DM (32). The
food intake of mice treated with LC was significantly decreased
compared with mice in the control group and the diabetes group
(P<0.05; Table II). These
results indicated that LC ameliorated certain symptoms of
diabetes.
 | Table II.Blood glucose, plasma insulin levels
and food intake in diabetic mice with or without L-carnitine
treatment. |
Table II.
Blood glucose, plasma insulin levels
and food intake in diabetic mice with or without L-carnitine
treatment.
| Group | Control | DM | Pre-treatment | Treatment |
|---|
| Blood glucose
(mmol/l) | 6.23±0.36 |
19.81±2.03c |
10.65±0.92a,c |
13.61±1.93c |
| Insulin
(mIU/l) | 12.55±0.42 | 12.02±0.64 |
17.50±2.69a |
21.80±2.56a |
| Food intake
(g/day) | 9.00±0.80 | 9.40±0.40 |
5.40±0.50a |
7.80±0.23a,b |
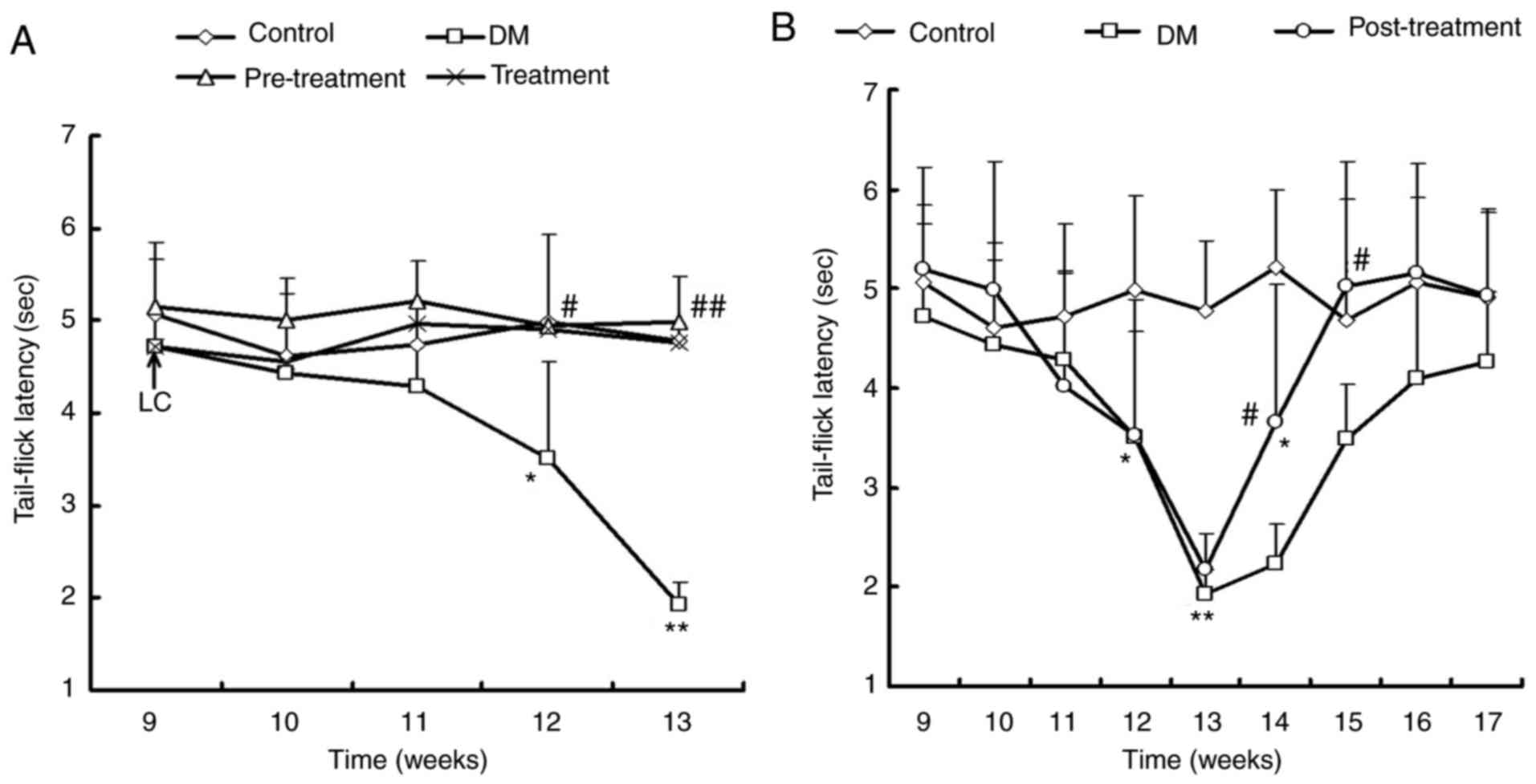
Effect of LC on the tail-flick latency
test
To determine the effect of LC on hyperalgesia in
diabetes, the tail-flick latency test was performed. Non-diabetic
control mice had a stable tail-flick latency of ~4.9±0.2 sec
throughout the entire procedure. However, diabetic mice exhibited
enhanced thermal hyperalgesia from the age of 12–13 weeks (3–4
weeks following diabetes induction). The tail-flick latencies in
diabetic mice were significantly shortened to 1.9 sec at 13 weeks
of age (P<0.01; Fig. 3A). In
the pre-treatment group, neither hyperalgesia nor hypoalgesia were
observed in tail-flick latencies test compared with the control
group (Fig. 3A). The treatment
group also demonstrated significantly improved tail-flick latencies
compared with diabetic mice (P<0.05).
Furthermore, the effects of LC on tail-flick
responses in mice with advanced stage diabetes were investigated.
In the post-treatment group, the latency of tail-flick responses
were significantly decreased to the shortest time (~1.9 sec) at 13
weeks old and then gradually increased to the same value as the
control mice at 16 weeks old (Fig.
3B), which demonstrated that LC ameliorated hyperalgesia. At 17
weeks old, there were no significant differences between the
control and post-treatment group (P>0.05; Fig. 3B).
Effect of LC on peripheral
neuropathy
Hur et al (33) reported that nerve conduction
velocity was decreased in patients with T2DM. Ding et al
(34) demonstrated that sciatic
nerves exhibited signs of damage in diabetic control rats,
including swelling and dilatation of mitochondria and endoplasmic
reticulum in Schwann cells as well as deranged myelin sheaths with
lighter electron density. To investigate the influence of LC on
peripheral neuropathy, examination of the ultrastructure and
morphology of the sciatic nerve was performed. As presented in
Fig. 4, most myelinated nerve
fibers in the control group mice exhibited complete and regular
structures and clear mitochondrial cristae. The nerves of diabetic
mice revealed delamination of myelin lamellae, atrophy and deletion
of axons as well as deformed nerve fibers, with swelling and
rupture of mitochondria in Schwann cells. Following administration
of LC, these effects were ameliorated. The morphometry of nerves in
the pre-treatment group was similar compared to that observed for
the control group. The A/MS reflects the degree of axonal atrophy
and myelin sheath swelling, which was different between diabetic
nerves and nerves from other groups (Fig. 4). The A/MS of the diabetic group
was 0.49, which was decreased compared with 0.73 in the control
group, 0.71 in the pre-treatment group and 0.77 in the treatment
group.
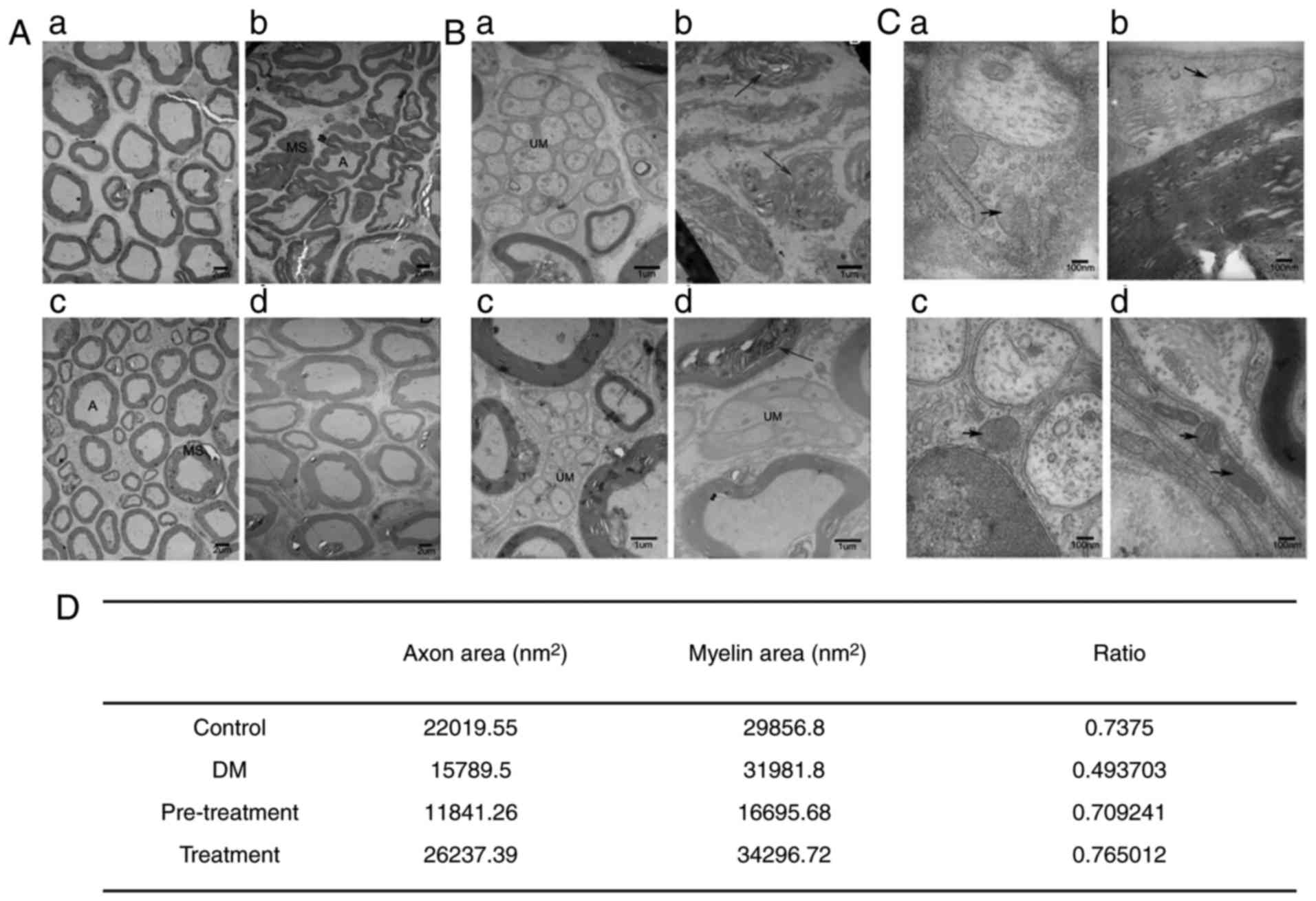 | Figure 4.Ultrastructural morphologic analysis
of sciatic nerves isolated from type 2 diabetic mice with or
without L-carnitine treatment, using transmission electron
microscopy. (A) Scale bar, 2 µm. (B) Scale bar, 1 µm. (C) Scale
bar, 100 nm. (a) Control group, (b) DM group, (c) pre-treatment
group and (d) treatment group. The arrows indicate delamination of
myelin lamellae, atrophy and deletion of axon, and deformed nerve
fibers. (D) The ratio of axon to myelin sheath area was determined.
A, axon; DM, diabetes mellitus; MS, myelin sheath; UM,
ultrastructural morphologic. |
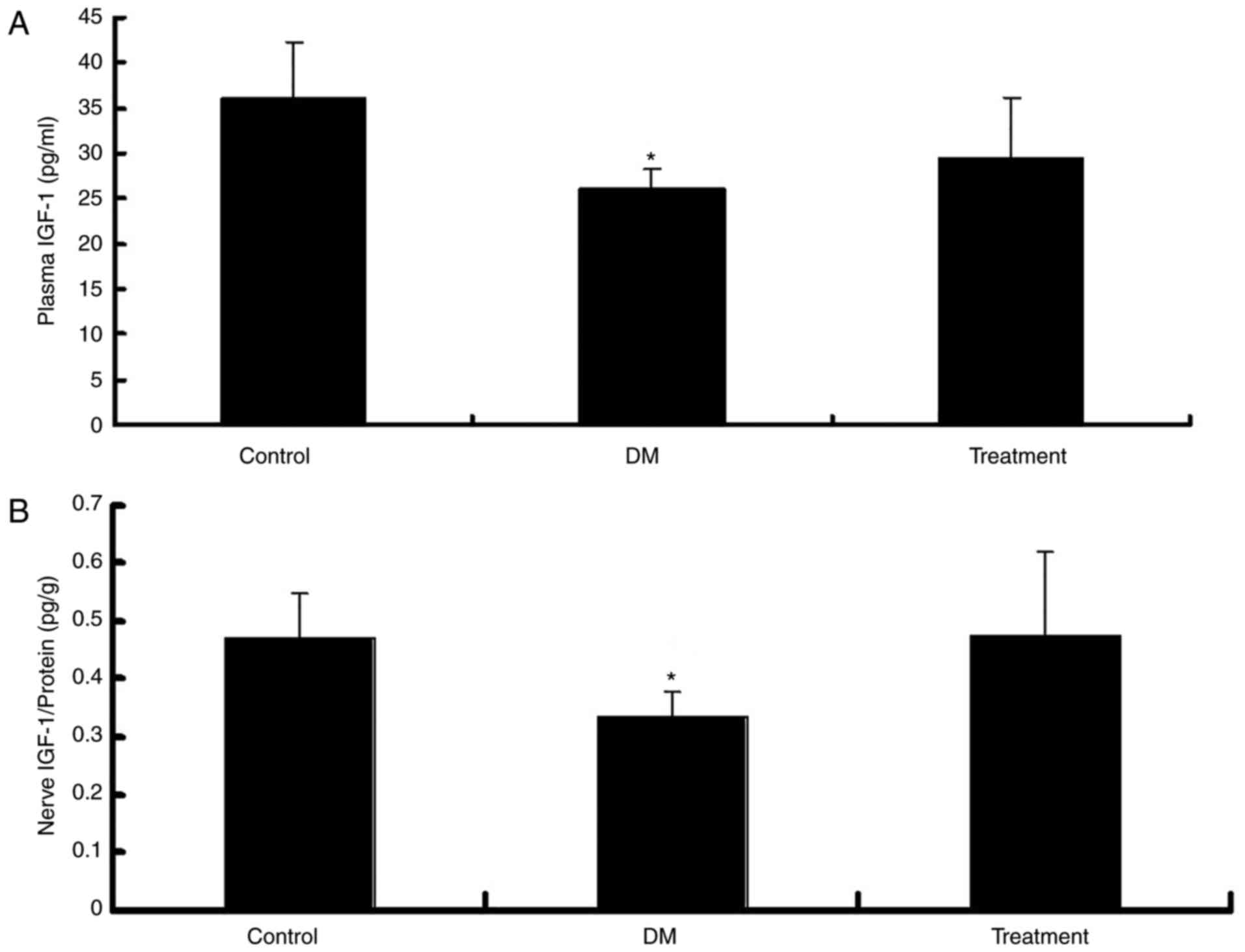
IGF-1 content in the plasma and
sciatic nerve
To confirm that LC ameliorates peripheral neuropathy
in diabetic mice via an increase in IGF-I levels, the IGF-I levels
in LC-treated mice were measured. As expected, the IGF-1 levels in
the plasma (Fig. 5A) and nerve
fibers (Fig. 5B) of diabetic mice
were significantly decreased (P<0.05). However, the exogenous
administration of LC in the treatment group normalized the levels
of IGF-1 in both the plasma and the sciatic nerve (Fig. 5).
Discussion
A previous study demonstrated that HFD-fed,
STZ-induced, insulin-resistant diabetic mice are good models for
patients with T2DM that have risk factors for obesity and distinct
metabolic characteristics (35).
This model is considered more cost-effective compared with genetic
models. In the present study, diabetic mice induced by a
combination of STZ and HFD revealed similar characteristics to
patients with T2DM patients, including hyperglycemia and obesity.
The weight loss caused by LC pre-treatment was expected; however,
to the best of our knowledge this has not been reported previously.
This could be due to the protective effect of LC and this effect
could be used to avoid the harm caused by HFD. Blood glucose levels
in the diabetes group were significantly increased, whilst the
plasma insulin levels were not increased compared with the control
group, which indicated successful induction of T2DM in the
mice.
LC is a conditionally essential nutrient that serves
a critical role in energy production and fatty acid metabolism
(6). Li et al (36) reported that LC could improve
clinical symptoms and neurophysiological parameters in patients
with DPN. Preclinical trials have revealed the preventive and
therapeutic effects of LC on peripheral nerve function and
structural abnormalities as well as on endometrial blood flow
(37,38). However, little is known about the
effect of LC on diabetes mellitus in humans. In the present study,
LC levels in the plasma and pancreas were elevated following LC
supplementation by intragastric administration.
A previous study indicated that there was no
statistical difference in body weight between the diabetes and
control group, but food intake of the diabetes group was
significantly increased compared with the control group (39). In the present study, the body
weight of the pre-treatment group mice was lower compared with the
three other experimental groups, which may have prevented the
appearance of diabetes. However, the food intake of the
pre-treatment and treatment group was lower compared with the
diabetes group, which suggested the effect of LC on ameliorating
diabetes mellitus. In addition, blood glucose levels of mice
treated with LC were lower compared with the diabetic group, which
demonstrated the protective effect of LC on hyperglycemia in
diabetes mellitus. The results demonstrated that pre-treatment with
LC inhibited the development of diabetes.
DPN occurs with hyperalgesia (40). There were several reports on the
changes of nociceptive threshold in experimental diabetic animal
models, whereby newly diagnosed diabetes revealed thermal and
mechanical hyperalgesia, and long term-treated diabetes
demonstrated hypoalgesia (41–43).
The alterations to the nociceptive threshold were examined 3 weeks
following T2DM induction and hyperalgesia was recorded. The
progression of thermal hyperalgesia was attenuated by treatment
with LC at both the early and advanced stage of T2DM, which
indicated a role for LC in alleviating peripheral neuropathy.
Previous studies indicated that hyperalgesia in
diabetic mice is simultaneously followed by a series of
neuropathological presentations, including axonal degeneration and
segmental demyelination (44,45).
Therefore, morphological damage of the sciatic nerve was examined,
including axonal atrophy, axonal loss, Schwann cell swelling,
demyelination and mitochondrial swelling in neurons. The TEM images
revealed stabilized swollen myelin sheaths, regenerated atrophic
axons and normalized mitochondria of Schwann cell in LC treated
groups, suggesting that LC improved DPN in the present study.
Under experimental conditions, it has been
demonstrated that DPN is provoked by multiple interactive
pathogenic mechanisms, including oxidative stress, protein kinase C
and microvascular disease (3).
Numerous attempts have been made to address the underlying
mechanisms therapeutically, but the quantifiable benefits remain
limited. Previous studies considered carnitine as an active
antioxidant, as carnitine ameliorated DPN by improving cellular
energy metabolism by promoting long chain fatty acid β-oxidation,
stimulating glucose disposal and improving insulin resistance
further by restoring Na+/K+-adenosine
triphosphatase activity (26). One
of these studies also revealed that supplementation with carnitine
leads to an increase in IGF-1 levels and activation of the
IGF-1/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3
K)/protein kinase B (Akt) signaling pathway in the skeletal muscle
of rats (26). Whilst insulin
promotes the absorption of glucose from the blood into liver and
skeletal muscle cells (46), IGF-1
can directly suppress renal gluconeogenesis in mice, reduce hepatic
glucose production and stimulate peripheral glucose uptake
(47). In the present study, the
levels of IGF-1 in the plasma and sciatic nerves were decreased in
type 2 diabetic mice, which was consistent with a previous study
(26). The results also
demonstrated that LC increased the levels of plasma insulin and
IGF-1, and prevented the onset of diabetes. Therefore, the
preventive and therapeutic effects of LC on DPN may be mediated
through the endogenous IGF-1 system.
The beneficial effects of insulin, C-peptide and
IGF-1 on neuropathy have been described, particularly for the
treatment of diabetic polyneuropathy (48–50).
Insulin is regarded as a nerve promotion factor that upregulates
and stabilizes neuron filaments as well as tubulin in a
dose-dependent manner. In STZ-induced rats, local unilateral
administration of insulin to the sciatic nerve resulted in an
increased number of small myelinated fibers and prevented slow
nerve conduction in the treated nerve (26). Similarly, IGF-1, which is
homologous to insulin and has partial common receptor affinity, can
activate post-receptor signaling in neurons to ameliorate diabetic
neuropathy (50). Binding of
insulin/IGF-I to insulin receptor or IGF-I receptor activates
autophosphorylation of the receptor and causes phosphorylation of
several intracellular substrate proteins. This in turn leads to
activation of the PI3 K/Akt and mitogen-activated protein kinase
pathway, which alter neuronal apoptosis-inducing factors (26). Insulin and IGF-1 share certain
common effects in diabetes, including increasing glucose uptake,
promoting protein synthesis and regulating cell proliferation and
differentiation. As a neural growth factor, IGF-1 promotes the
regeneration of sensory nerves (51), motor nerves (52) and Schwann cells (53). In addition, IGF-1 can prevent
apoptosis of dorsal root ganglion neurons in vitro under
high glucose conditions. Therefore, it is reasonable to suggest
that IGF-1 mediates the therapeutic effects of LC on DPN.
In conclusion, LC ameliorated peripheral neuropathy
in diabetic mice, which may be due to an increase in IGF-I levels.
The results indicated that LC was an effective choice for the
relief of symptoms associated with progressive DPN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31371168 and
31872791).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
RW, LW, JD and RM designed the experiments. RW and
LW performed the experiments. CZ, YL, LS and YZ analyzed the data.
RW, LW and YZ wrote the manuscript. RM and JD revised the
manuscript. All authors reviewed, read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Medical Department of Qingdao University (Qingdao,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DPN
|
Diabetic peripheral neuropathy
|
|
LC
|
L-carnitine
|
|
IGF-1
|
insulin-like growth factor-1
|
|
T2DM
|
type 2 diabetes mellitus
|
|
HFD
|
high-fat diet
|
|
STZ
|
streptozotocin
|
|
DDW
|
double distilled water
|
References
|
1
|
Wooten K: Clinical features and
electrodiagnosis of diabetic peripheral neuropathy in the
dysvascular patient. Phys Med Rehabil Clin N Am. 20:657–676. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tesfaye S and Selvarajah D: Advances in
the epidemiology, pathogenesis and management of diabetic
peripheral neuropathy. Diabetes Metab Res Rev. 28 Suppl 1:S8–S14.
2012. View Article : Google Scholar
|
|
3
|
Sima AA: Diabetic neuropathy: Pathogenetic
background, current and future therapies. Expert Rev Neurother.
1:225–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sima AA, Bril V, Nathaniel V, McEwen TA,
Brown MB, Lattimer SA and Greene DA: Regeneration and repair of
myelinated fibers in sural-nerve biopsy specimens from patients
with diabetic neuropathy treated with sorbinil. N Engl J Med.
319:548–555. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziegler D, Reljanovic M, Mehnert H and
Gries FA: Alpha-lipoic acid in the treatment of diabetic
polyneuropathy in Germany: Current evidence from clinical trials.
Exp Clin Endocrinol Diabetes. 107:421–430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flanagan JL, Simmons PA, Vehige J, Willcox
MD and Garrett Q: Role of carnitine in disease. Nutr Metab (Lond).
7:302010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salmanoglu DS, Gurpinar T, Vural K,
Ekerbicer N, Dariverenli E and Var A: Melatonin and L-carnitin
improves endothelial disfunction and oxidative stress in Type 2
diabetic rats. Redox Biol. 8:199–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ido Y, McHowat J, Chang KC,
Arrigoni-Martelli E, Orfalian Z, Kilo C, Corr PB and Williamson JR:
Neural dysfunction and metabolic imbalances in diabetic rats.
Prevention by acetyl-L-carnitine. Diabetes. 43:1469–1477. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bach AC: Carnitine in human nutrition. Z
Ernahrungswiss. 21:257–265. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evans JD, Jacobs TF and Evans EW: Role of
acetyl-L-carnitine in the treatment of diabetic peripheral
neuropathy. Ann Pharmacother. 42:1686–1691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sima AA: Acetyl-L-carnitine in diabetic
polyneuropathy: Experimental and clinical data. CNS Drugs. 21 Suppl
1:13–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stevens MJ, Lattimer SA, Feldman EL,
Helton ED, Millington DS, Sima AA and Greene DA: Acetyl-L-carnitine
deficiency as a cause of altered nerve myo-inositol content, Na,
K-ATPase activity and motor conduction velocity in the
streptozotocin-diabetic rat. Metabolism. 45:865–872. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lowitt S, Malone JI, Salem AF, Korthals J
and Benford S: Acetyl-L-carnitine corrects the altered peripheral
nerve function of experimental diabetes. Metabolism. 44:677–680.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onofrj M, Fulgente T, Melchionda D,
Marchionni A, Tomasello F, Salpietro FM, Alafaci C, De Sanctis E,
Pennisi G, Bella R, et al: L-acetylcarnitine as a new therapeutic
approach for peripheral neuropathies with pain. Int J Clin
Pharmacol Res. 15:9–15. 1995.PubMed/NCBI
|
|
15
|
Quatraro A, Roca P, Donzella C, Acampora
R, Marfella R and Giugliano D: Acetyl-L-carnitine for symptomatic
diabetic neuropathy. Diabetologia. 38:1231995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scarpini E, Sacilotto G, Baron P, Cusini M
and Scarlato G: Effect of acetyl-L-carnitine in the treatment of
painful peripheral neuropathies in HIV+ patients. J Peripher Nerv
Syst. 2:250–252. 1997.PubMed/NCBI
|
|
17
|
Sima AA, Calvani M, Mehra M and Amato A;
Acetyl-L-Carnitine Study Group: Acetyl-L-carnitine improves pain,
nerve regeneration, and vibratory perception in patients with
chronic diabetic neuropathy: An analysis of two randomized
placebo-controlled trials. Diabetes Care. 28:89–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajpathak SN, He M, Sun Q, Kaplan RC,
Muzumdar R, Rohan TE, Gunter MJ, Pollak M, Kim M, Pessin JE, et al:
Insulin-like growth factor axis and risk of type 2 diabetes in
women. Diabetes. 61:2248–2254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pires KM, Buffolo M, Schaaf C, David
Symons J, Cox J, Abel ED, Selzman CH and Boudina S: Activation of
IGF-1 receptors and Akt signaling by systemic hyperinsulinemia
contributes to cardiac hypertrophy but does not regulate cardiac
autophagy in obese diabetic mice. J Mol Cell Cardiol. 113:39–50.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanazawa I, Notsu M, Miyake H, Tanaka K
and Sugimoto T: Assessment using serum insulin-like growth factor-I
and bone mineral density is useful for detecting prevalent
vertebral fractures in patients with type 2 diabetes mellitus.
Osteoporosis Int. 29:2527–2535. 2018. View Article : Google Scholar
|
|
21
|
Cao LH, Lu FM, Lu XJ and Zhu LY: Study on
the relationship between insulin growth factor 1 and liver fibrosis
in patients with chronic hepatitis C with type 2 diabetes mellitus.
J Cell Biochem. 119:9513–9518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai C, Li N, Song G, Yang Y and Ning X:
Insulin-like growth factor 1 regulates growth of endometrial
carcinoma through PI3k signaling pathway in insulin-resistant type
2 diabetes. Am J Transl Res. 8:3329–3336. 2016.PubMed/NCBI
|
|
23
|
Hjortebjerg R, Laugesen E, Høyem P, Oxvig
C, Stausbøl-Grøn B, Knudsen ST, Kim WY, Poulsen PL, Hansen TK,
Bjerre M and Frystyk J: The IGF system in patients with type 2
diabetes: Associations with markers of cardiovascular target organ
damage. Eur J Endocrinol. 176:521–531. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katz LE, DeLeón DD, Zhao H and Jawad AF:
Free and total insulin-like growth factor (IGF)-I levels decline
during fasting: Relationships with insulin and IGF-binding
protein-1. J Clin Endocrinol Metab. 87:2978–2983. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge P, Cui Y, Liu F, Luan J, Zhou X and Han
J: L-carnitine affects osteoblast differentiation in NIH3T3
fibroblasts by the IGF-1/PI3K/Akt signalling pathway. Biosci
Trends. 9:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keller J, Couturier A, Haferkamp M, Most E
and Eder K: Supplementation of carnitine leads to an activation of
the IGF-1/PI3 K/Akt signalling pathway and down regulates the E3
ligase MuRF1 in skeletal muscle of rats. Nutr Metab (Lond).
10:282013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia Y, Li Q, Zhong W, Dong J, Wang Z and
Wang C: L-carnitine ameliorated fatty liver in high-calorie
diet/STZ-induced type 2 diabetic mice by improving mitochondrial
function. Diabetol Metab Syndr. 3:312011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reed MJ, Meszaros K, Entes LJ, Claypool
MD, Pinkett JG, Gadbois TM and Reaven GM: A new rat model of type 2
diabetes: the fat-fed, streptozotocin-treated rat. Metabolism.
49:1390–1394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Y, Wang YX, Liu CJ, Wang LX, Han ZW
and Wang CB: Comparison of pharmacokinetics of L-carnitine,
acetyl-L-carnitine and propionyl-L-carnitine after single oral
administration of L-carnitine in healthy volunteers. Clin Invest
Med. 32:E13–E19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tisi A, Federico R, Moreno S, Lucretti S,
Moschou PN, Roubelakis-Angelakis KA, Angelini R and Cona A:
Perturbation of polyamine catabolism can strongly affect root
development and xylem differentiation. Plant Physiol. 157:200–215.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Palo E, Gatti R, Sicolo N, Padovan D,
Vettor R and Federspil G: Plasma and urine free L-carnitine in
human diabetes mellitus. Acta Diabetol Lat. 18:91–95. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McGeoch SC, Holtrop G, Fyfe C, Lobley GE,
Pearson DW, Abraham P, Megson IL, Macrury SM and Johnstone AM: Food
intake and dietary glycaemic index in free-living adults with and
without type 2 diabetes mellitus. Nutrients. 3:683–693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hur J, Dauch JR, Hinder LM, Hayes JM,
Backus C, Pennathur S, Kretzler M, Brosius FC III and Feldman EL:
The metabolic syndrome and microvascular complications in a murine
model of type 2 diabetes. Diabetes. 64:3294–3304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding Y, Dai X, Zhang Z, Jiang Y, Ma X, Cai
X and Li Y: Proanthocyanidins protect against early diabetic
peripheral neuropathy by modulating endoplasmic reticulum stress. J
Nutr Biochem. 25:765–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Srinivasan K and Ramarao P: Animal models
in type 2 diabetes research: An overview. Indian J Med Res.
125:451–472. 2007.PubMed/NCBI
|
|
36
|
Li S, Chen X, Li Q, Du J, Liu Z, Peng Y,
Xu M, Li Q, Lei M, Wang C, et al: Effects of acetyl-L-carnitine and
methylcobalamin for diabetic peripheral neuropathy: A multicenter,
randomized, double-blind, controlled trial. J Diabetes Investig.
7:777–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fagher B, Cederblad G, Eriksson M, Monti
M, Moritz U, Nilsson-Ehle P and Thysell H: L-carnitine and
haemodialysis: Double blind study on muscle function and metabolism
and peripheral nerve function. Scand J Clin Lab Invest. 45:169–178.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arioz DT, Kanat-Pektas M, Tuncer N, Koken
T, Unlu BS, Koken G and Yilmazer M: L-Carnitine: A new insight into
the pathogenesis of endometrial cancer. Arch Gynecol Obstet.
291:1147–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, Lv XY, Li J, Xu ZG and Chen L:
The characterization of high-fat diet and multiple low-dose
streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res.
2008:7040452008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jolivalt CG, Frizzi KE, Guernsey L,
Marquez A, Ochoa J, Rodriguez M and Calcutt NA: Peripheral
neuropathy in mouse models of diabetes. Curr Protoc Mouse Biol.
6:223–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drel VR, Mashtalir N, Ilnytska O, Shin J,
Li F, Lyzogubov VV and Obrosova IG: The leptin-deficient (ob/ob)
mouse: A new animal model of peripheral neuropathy of type 2
diabetes and obesity. Diabetes. 55:3335–3343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohsawa M, Miyata S, Carlsson A and Kamei
J: Preventive effect of acetyl-L-carnitine on the thermal
hypoalgesia in streptozotocin-induced diabetic mice. Eur J
Pharmacol. 588:213–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Calcutt NA, Freshwater JD and Mizisin AP:
Prevention of sensory disorders in diabetic Sprague-Dawley rats by
aldose reductase inhibition or treatment with ciliary neurotrophic
factor. Diabetologia. 47:718–724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Malik RA: Pathology of human diabetic
neuropathy. Handb Clin Neurol. 126:249–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dyck PJ and Giannini C: Pathologic
alterations in the diabetic neuropathies of humans: A review. J
Neuropathol Exp Neurol. 55:1181–1193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dimitriadis G, Mitrou P, Lambadiari V,
Maratou E and Raptis SA: Insulin effects in muscle and adipose
tissue. Diabetes Res Clin Pract. 93 Suppl 1:S52–S59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Simpson HL, Jackson NC, Shojaee-Moradie F,
Jones RH, Russell-Jones DL, Sönksen PH, Dunger DB and Umpleby AM:
Insulin-like growth factor I has a direct effect on glucose and
protein metabolism, but no effect on lipid metabolism in type 1
diabetes. J Clin Endocrinol Metab. 89:425–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de la Hoz CL, Cheng C, Fernyhough P and
Zochodne DW: A model of chronic diabetic polyneuropathy: Benefits
from intranasal insulin are modified by sex and RAGE deletion. Am J
Physiol Endocrinol Metab. 312:E407–E419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kamiya H, Zhang W and Sima AA: The
beneficial effects of C-Peptide on diabetic polyneuropathy. Rev
Diabet Stud. 6:187–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao T, Bogdanova N, Ghauri S, Zhang G, Lin
J and Sheikh K: Systemic IGF-1 gene delivery by rAAV9 improves
spontaneous autoimmune peripheral polyneuropathy (SAPP). Sci Rep.
8:54082018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fernyhough P, Willars GB, Lindsay RM and
Tomlinson DR: Insulin and insulin-like growth factor I enhance
regeneration in cultured adult rat sensory neurones. Brain Res.
607:117–124. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Near SL, Whalen LR, Miller JA and Ishii
DN: Insulin-like growth factor II stimulates motor nerve
regeneration. Proc Natl Acad Sci USA. 89:11716–11720. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang YM, Kuo WH, Lai TY, Shih YT, Tsai
FJ, Tsai CH, Shu WT, Chen YY, Chen YS, Kuo WW and Huang CY: RSC96
schwann cell proliferation and survival induced by dilong through
PI3K/Akt signaling mediated by IGF-I. Evid Based Complement
Alternat Med. 2011:2161482011. View Article : Google Scholar : PubMed/NCBI
|