Introduction
Nucleotide-binding oligomerization domain-like
receptors (NLRs) are a class of cytoplasmic pattern-recognition
receptors involved in identifying harmful substances in the
cytoplasm and forming inflammasomes to transform these antigens
into immune-initiating signals (1,2).
There are four subfamilies in NLRs, including NLRA, NLRB, NLRC and
NLRP (3). The NLRP family (NLRPs)
is distinguished by a pyrin domain in the N-terminal. Among the 22
known NLRs, the NLRP family contains 14 members, NLRP1-14 (4). The current understanding of NLRPs is
concentrated on NLRP1, NLRP3 and NLRP6. When stimulated by a
cytoplasmic antigen signal, these proteins recruit the adaptor
protein ASC by the pyrin domain to form a multi-protein complex,
which further activates caspase-1 and cleaves effector
pro-inflammatory cytokines interleukin (IL)-1b and IL-18 (5–7).
However, the studies of NLRP2 are limited and the majority of them
focus on its functions in reproduction and embryonic development.
For example, Tilburgs et al (8) identified that NLRP2 was involved in
preventing unwanted antifetal responses by suppressing nuclear
factor κB signaling and major histocompatibility complex, class I,
C expression in human trophoblasts. Mahadevan et al
(9) identified that maternally
expressed NLRP2 links the subcortical maternal complex to
fertility, embryogenesis and epigenetic reprogramming. Peng et
al (10) identified that NLRP2
served an important role in early embryonic development in mice.
However, NLRP2, as a systemic protein associated with biological
reproduction and embryonic development, must serve an important
role in a variety of biological processes, although relevant
studies are lacking.
Vascular endothelial cells, located between the
bloodstream and tissues, are involved in numerous physiological and
pathological processes, including (tumor) angiogenesis,
inflammation and wound healing (11,12).
A recent study revealed that NLRP2 was significantly upregulated in
a mouse model of ischemic stroke and served important roles in the
pathophysiological processes (13). Therefore, the present study
hypothesized that NLRP2 may exhibit protective effects on vascular
endothelial cells.
In the present study, the expression of NLRP2 in
human umbilical vein endothelial cells (HUVECs) was knocked-down to
investigate its functions in HUVEC proliferation, apoptosis, cell
cycle and motility. By this, it was hoped to identify the function
of NLRP2 in HUVECs and elucidate the underlying signaling
pathway.
Materials and methods
Cell culture and transfection
HUVECs were purchased from the Chinese Academy of
Sciences cell bank (Shanghai, China) and cultured in RPMI-1640
culture medium (Hyclone, GE Healthcare Life Sciences, Logan, UT,
USA) at 37°C in 5% CO2. The medium additions were fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 10% penicillin and streptomycin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) at 100 units
and 0.1 mg/ml, respectively. When the cells entered into the
logarithmic growth phase, cells were digested to form a single-cell
suspension and plated in 6-well plates. When the cell density
reached ~80%, 20 nM short interfering siNLRP2 or a scrambled siRNA
(siNC) was transfected into the cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. The sequences of siRNAs
were as follows: siNLRP2, 5′-CGUACAGAAGCUGCUUUCCGGAGU-3′ and siNC,
5′-UUCUCCGAACGUGUCACGUTT-3′. Following transfection for 24 h, the
mRNA expression of NLRP2 was determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
Following transfection with siNLARP2 or siNC for 48
h, total RNA from HUVECs was extracted using TRIzol reagent (Cwbio,
Beijing, China). Then, 1 µg total RNA was reverse transcribed to
cDNA using a reverse transcription system (Promega Corporation,
Madison, WI, USA). Subsequently, PCR was performed using GoTaq qPCR
master mix (Promega Corporation) on a 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). In the 25 µl
reaction system, 300 nmol/l primers were used. Thermal cycling
conditions were: 2 min at 50°C and 10 min at 95°C, followed by 40
cycles of 15 sec at 95°C and 1 min at 60°C. The primers were as
follows: glycogen synthase kinase-3β left,
5′-GAATTGCTGCGATGCGACAT-3′ and right, 5′-TCGAAGAGCTAGGCAGAGGT-3′;
GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′, reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The fold-change in the expression of
each gene was calculated using the 2−ΔΔCq method
(14).
Cell Counting kit-8 (CCK-8)
proliferation assay
HUVECs were digested to prepare a single-cell
suspension and plated into a 96-well plate at a density of
1–5×103/well. siNLRP2 or siNC was transfected into
HUVECs using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. Cell
viability was measured every 24 h. A total of 10 µl of CCK-8
reagent was added to each well prior to the assay and incubated for
1.5 h in a 37°C incubator, and the optical density value at 450 nm
was measured using a microplate reader to plot the proliferation
curve.
Flow cytometry for apoptosis
detection
Cell apoptosis was analyzed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit I (4A
Biotech Co., Ltd., Beijing, China). Briefly, following transfection
with siNLRP2 or siNC for 48 h, HUVECs were collected and
centrifuged at 300 × g, 25°C for 5 min. Then, cells were
resuspended in 4°C precooled PBS and centrifuged at 300 × g, 25°C
for 5 min. The supernatant was carefully removed. Cells were
resuspended by adding 1X binding buffer and adjusting the cell
density to 1–5×106/ml. To 100 µl of cell suspension in a
5 ml flow tube, 5 µl of Annexin V/FITC was added, mixed and
incubated for 5 min. Then, 10 µl of propidium iodide (PI) and 400
µl of PBS were added prior to analyzing by a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). FlowJo software (version
7.6.1; FlowJo LLC, Ashland, OR, USA) was used for statistical
analysis.
Flow cytometry for cell cycle
analysis
Following transfection with siNLRP2 or siNC for 48
h, HUVECs were collected and washed with PBS three times. Following
fixation with 70% ethanol at −20°C overnight, cells were incubated
with RNAase (0.1 mg/ml) and PI (0.02 mg/ml) at 37°C for 30 min.
Flow cytometry was used to analyze cell proportion in
G1, S and G2/M phases. Flowjo software
(version 7.6.1) was used for statistical analysis.
Wound healing assay
HUVECs were plated in 6-well plates and cultured to
100% confluence. A single-line scratch of ~600–700 µm was created
using a p200 pipette tip and cell debris was removed by gentle
washing with PBS. Then, the medium was replaced with fresh medium
containing either siNLRP2 or siNC. Cells were imaged at 0 and 48 h
by using an inverted microscope (magnification, ×40; Olympus
Corporation, Tokyo, Japan), and the wound closure was evaluated
using ImageJ software (version 1.46, National Institutes of Health,
Bethesda, MD, USA).
Transwell invasion and migration
assay
For the invasion assay, Matrigel (BD Biosciences)
was added to Transwell inserts (Corning Incorporated, Corning, NY,
USA) and solidified for 4–6 h in a 37°C incubator. Then, 500 µl of
serum-free medium was added to the bottom chamber to hydrate the
polyethylene terephthalate (PET) membrane for 2 h. HUVECs
(5×104) transfected with siNLRP2 or siNC were plated in
the top chamber in 200 µl of serum-free medium and 500 µl of medium
containing 10% FBS was added to the bottom chamber. Following
incubation for 48 h, cells remaining on the top surface of the PET
membrane were removed and invading cells were fixed with 4%
paraformaldehyde for 30 min, stained with 0.1% crystal violet for
20 min, and washed with PBS at room temperature. Cell numbers were
counted in 5 random fields under an inverted microscope
(magnification, ×100). The migration assay procedure was similar to
the invasion experiment except that no Matrigel was used.
Western blot analysis
HUVECs having been transfected for 48 h were lysed
in radio immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) supplemented with 1% protease
cocktail inhibitor I (Calbiochem; Merck KGaA, Darmstadt, Germany).
The protein concentration was measured using a BCA Protein Assay
kit (Pierce; Thermo Fisher Scientific, Inc.); 20 µg of proteins
were analyzed by 12% SDS-PAGE and transferred onto PVDF membranes.
Subsequently, the membrane underwent blocking in nonfat milk for
1.5 h at room temperature, was probed with primary antibodies at
4°C overnight and incubated with anti-rabbit or anti-mouse
secondary antibodies for 1 h. Finally, the protein bands were
visualized by using a Pierce ECL Western Blotting Substrate
(Pierce; Thermo Fisher Scientific, Inc.) and the density was
quantified using ImageJ software (version 1.46). The primary
antibodies used were as follows: anti-NLRP2 (cat. no. SAB3500325,
1:1,000), anti-GAPDH (cat. no. G9545, 1:10,000; both Sigma-Aldrich;
Merck KGaA), anti-caspase-3 (cat. no. ab13847, 1:1,000), anti-p53
(cat. no. ab26; 1:1,000), anti-Bcl-2-like protein 4 (Bax; cat. no.
ab32503; 1:1,000), anti-B-cell lymphoma 2 (Bcl-2; cat. no. ab32124;
1:1,000), anti-p70S6 kinase (p70S6K; cat. no. ab176651, 1:1,000),
anti-cyclin-dependent kinase 4 (CDK4; cat. no. ab108357, 1:1,000),
anti-cyclinD1 (cat. no. ab134175. 1:1,000), anti-p-extracellular
signal-regulated kinase (ERK; cat. no. ab192591, 1:1,000), anti-ERK
(cat. no. ab224313, 1:1,000), anti-Raf (cat. no. ab137435; 1:1,000;
all Abcam, Cambridge, UK). Horseradish peroxidase-conjugated goat
anti-mouse or goat anti-rabbit IgG antibody (cat. no. A0192 and
A0208, 1:1,000, Beyotime Institute of Biotechnology) was used as
secondary antibodies.
Statistical analysis
Each experiment was performed at least three times.
All data analyses were conducted by using SPSS 17.0 software (SPSS
Inc., Chicago, IL, USA). The results were manifested as mean ±
standard deviation. All the comparisons were performed using
unpaired student t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of HUVEC proliferation by
knocking down NLRP2 expression
To investigate the action of NLRP2 in HUVECs, NLRP2
expression was knocked-down using RNA interference technology
(siNLRP2), along with a non-targeting scrambled siRNA (siNC) that
was constructed to use as a negative control in all assays. The
interference efficiency of siNLRP2 on NLRP2 expression was examined
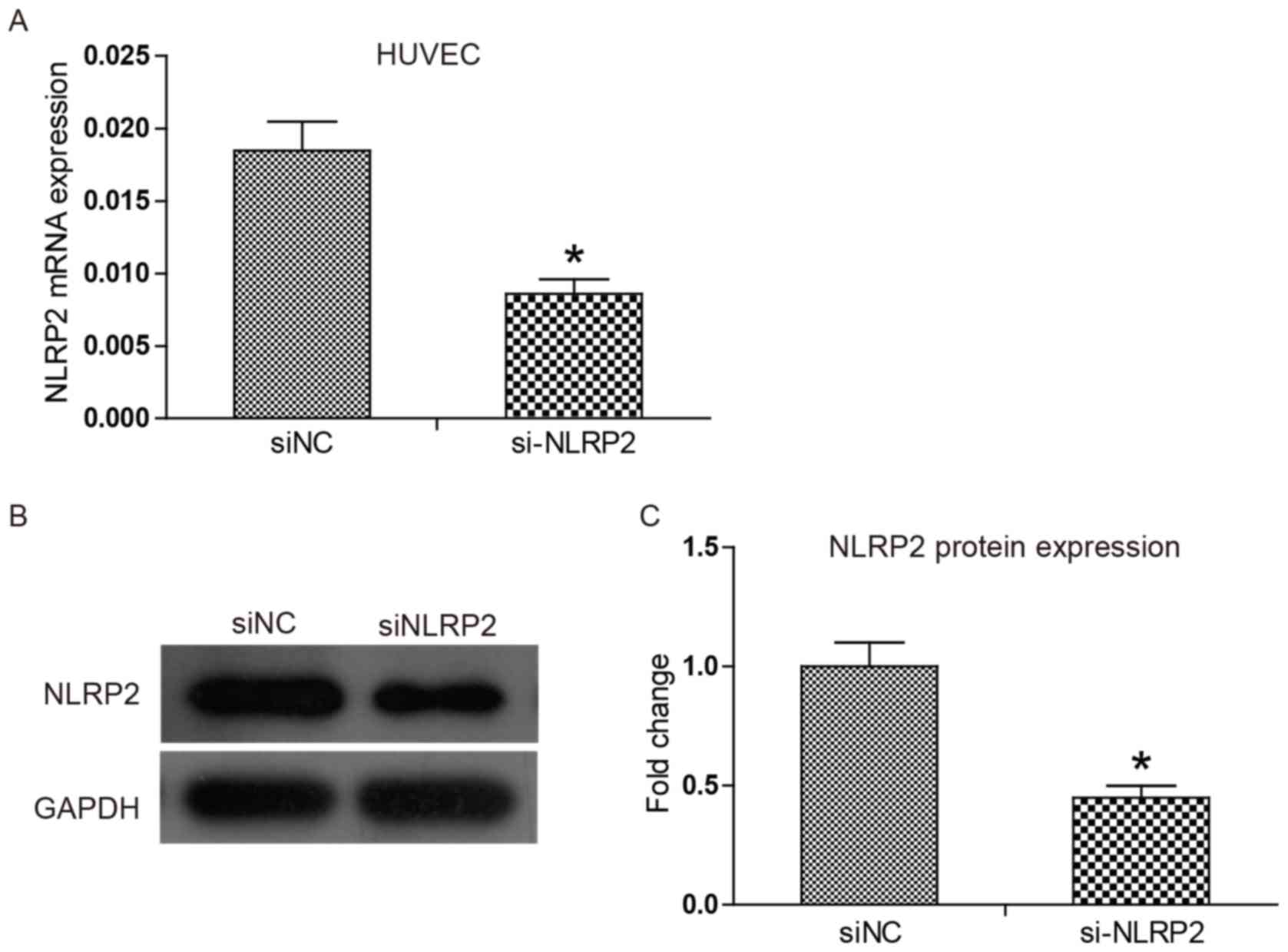
via mRNA levels using RT-qPCR. Fig.
1A demonstrates that siNLRP2 exerted high efficiency in
knocking down NLRP2 mRNA expression compared with the siNC, with an
inhibition rate reaching 54%. Western blotting results in Fig. 1B and C indicate that protein
expression of NLRP2 was also significantly reduced in the siNLRP2
group compared with the siNC group (P<0.05). The results of the
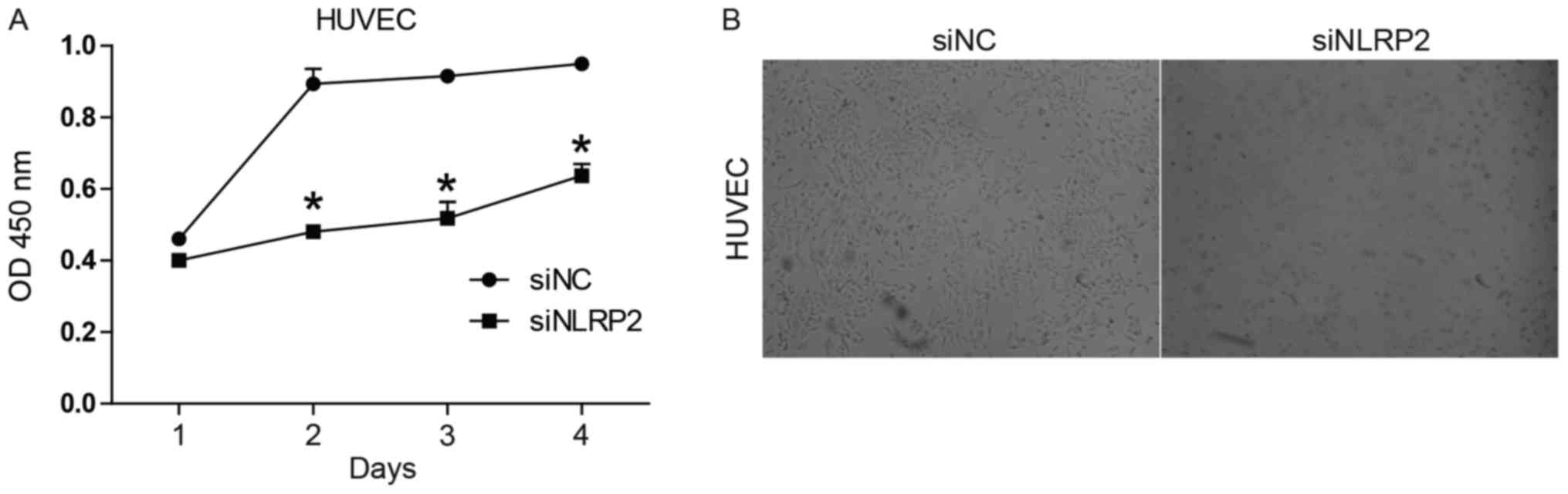
in vitro CCK-8 proliferation assay indicated that the cell
viability of siNLRP2-transfected HUVECs was notably decreased in
comparison with siNC-transfected cells (P<0.05; Fig. 2A). Observation of HUVEC morphology
(Fig. 2B) suggested that
transfection of siNLRP2 led to apoptosis-like changes including
cell shrinkage and the formation of vacuoles.
Enhancement of apoptosis by knocking
down NLRP2 expression
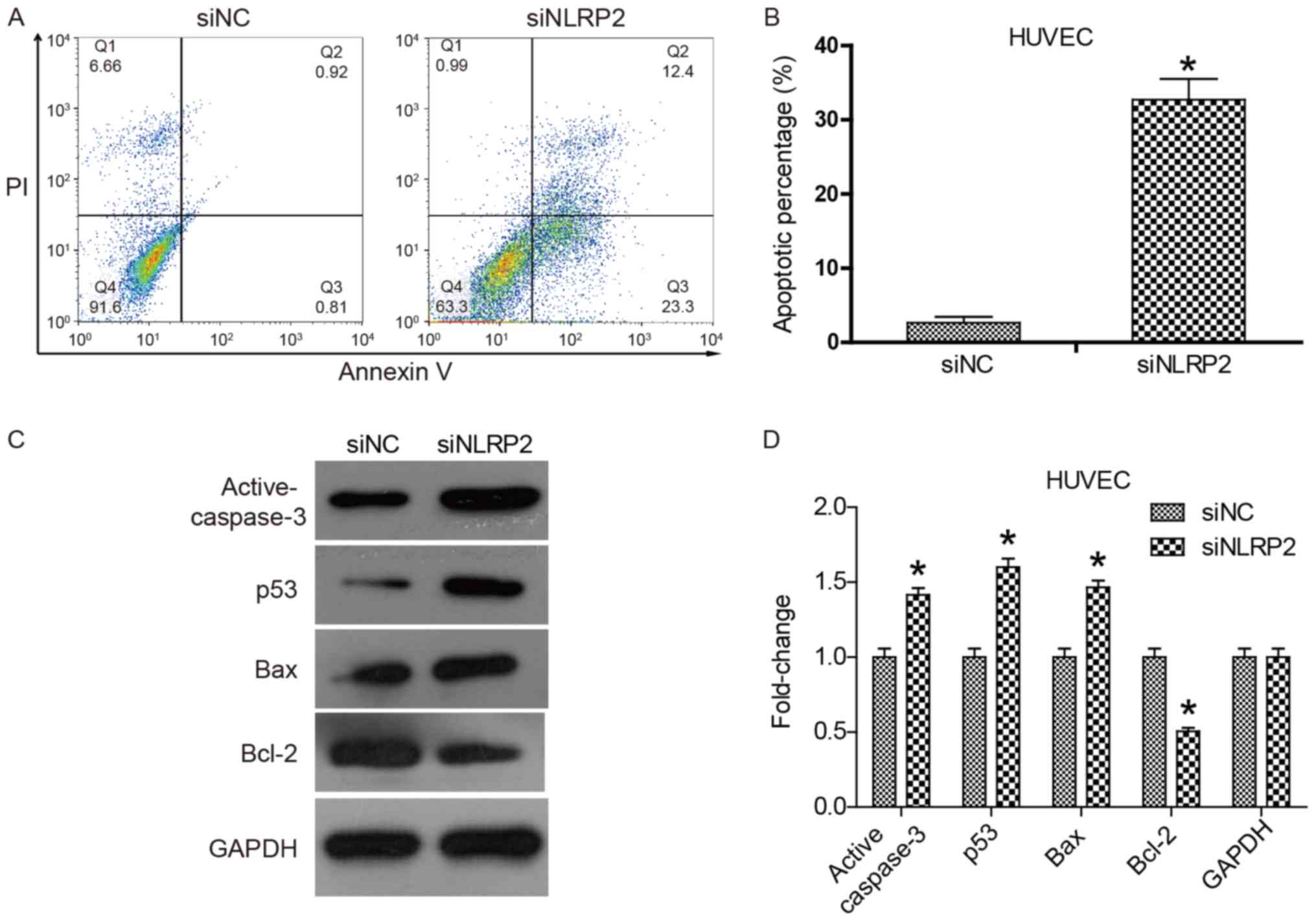
Flow cytometry was performed to assess apoptosis of
HUVECs. Measurement was based on staining with Annexin V-FITC and
PI. The resulting plot, presented in Fig. 3A, is divided into 4 quadrants.
Early apoptotic cells populating the lower right quadrant are
stained only by AnnexinV-FITC. Late apoptotic cells populating the
upper right quadrant are stained by Annexin V-FITC and PI. This
suggested that siNLRP2 significantly promoted early and late
apoptosis of HUVECs compared with siNC (P<0.05). The total
apoptotic proportion increased from 1.73 to 35.7% (P<0.05;
Fig. 3A and B). To clarify the
pro-apoptosis mechanism of siNLRP2, the expression of
apoptosis-associated genes was investigated. The results suggested
that the expression of the pro-apoptotic genes p53, Bax and
active-caspase 3 was significantly increased and the expression of
the anti-apoptotic gene Bcl-2 was significantly decreased in the
siNLRP2 group (P<0.05; Fig. 3C and
D). These gene expression changes would cause cytochrome
c release and mitochondria-dependent cell apoptosis. These
results suggested that knockdown of NLRP2 with siNLRP2 promoted
HUVEC cell apoptosis by regulating apoptosis-associated gene
expression.
Cell cycle arrest by knocking down
NLRP2 expression
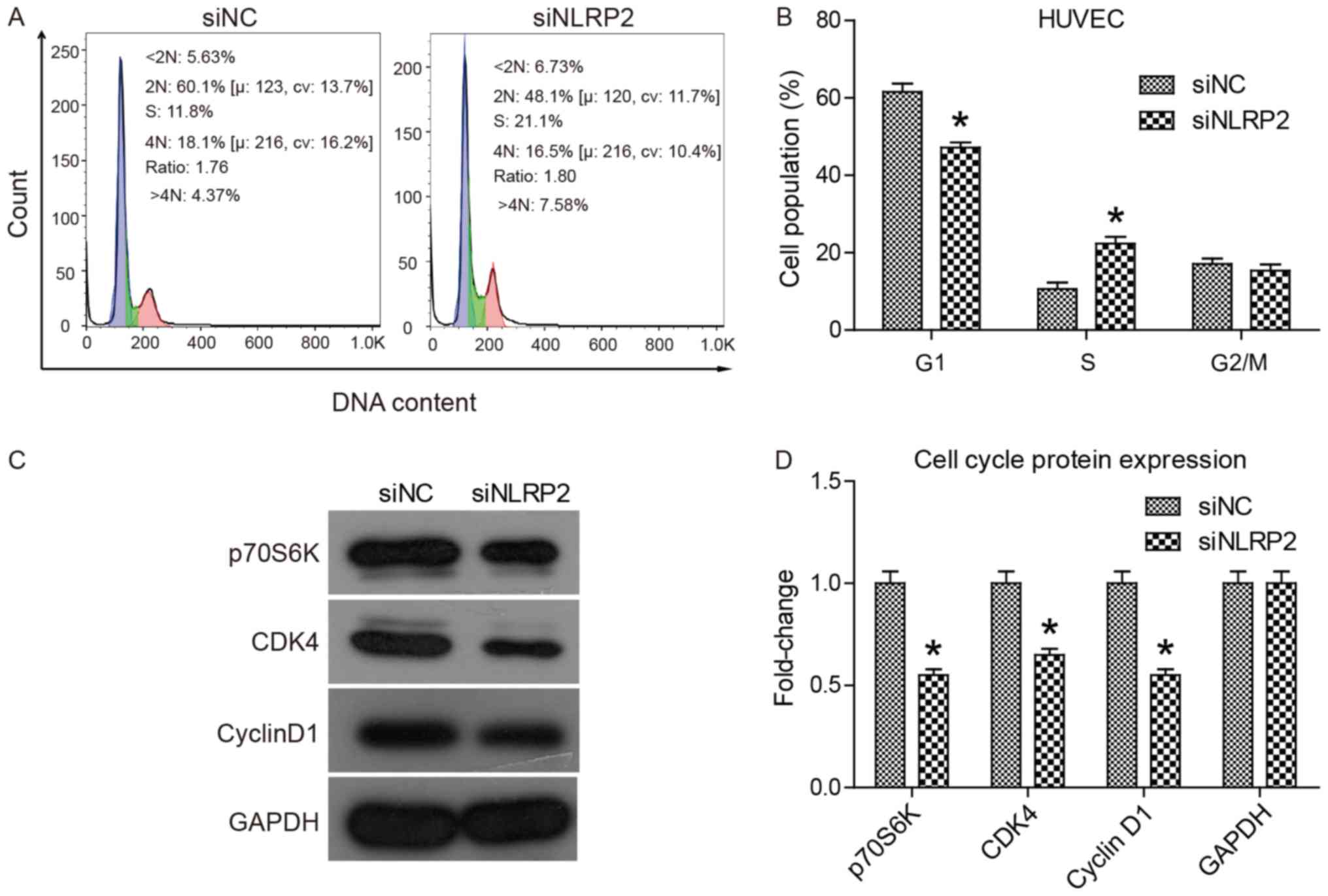
To determine if knockdown of NLRP2 expression
interfered with cell cycle progression, a cell cycle analysis was
performed. The principle of this assay is based on alterations in
DNA content throughout the cell cycle progression. DNA content was
detected by PI staining and flow cytometry. The data in Fig. 4A and B demonstrated that siNLRP2
transfection significantly increased the proportion of S-phase
cells (from 11.8 to 21.1%; P<0.05), but the proportion of
G1-phase cells dropped from 60.1–48.1%. This suggested
that knockdown of NLRP2 could arrest cell cycle of HUVECs at S
phase. To explain the underlying mechanism, a western blot analysis
was performed to determine alterations in the expression of cell
cycle-associated genes including p70S6K, CDK4, cyclin D1 and p53.
The results, presented in Fig. 4C and
D, indicated that the expression of cell cycle driving genes
including p70S6K, CDK4 and cyclin D1 was significantly decreased
(P<0.05). Overall, the results suggested that knockdown of NLRP2
halted cell cycle progression at the S phase by regulating cell
cycle-associated gene expression.
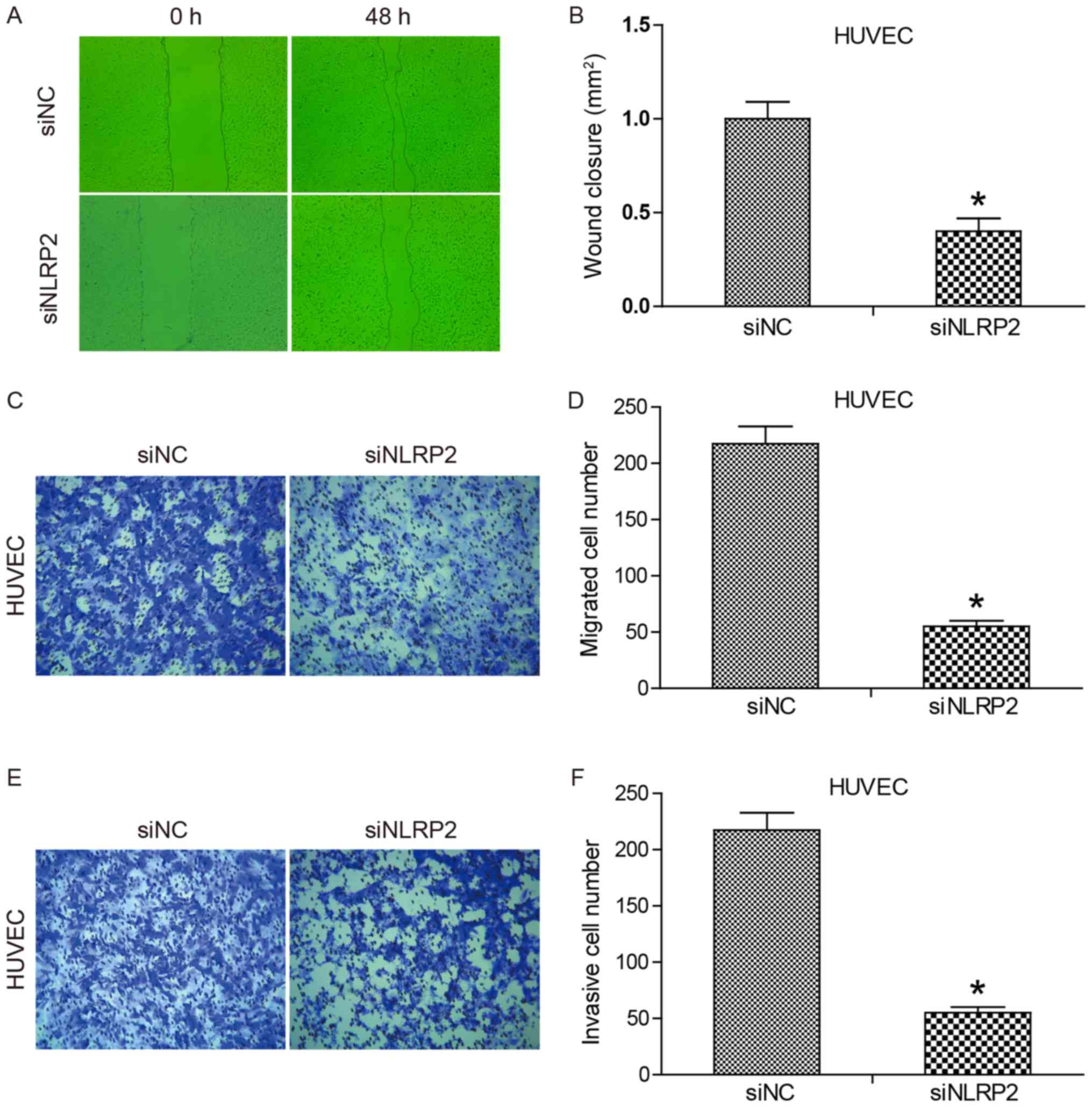
Inhibition of cell migration and
invasion by knocking down NLRP2 expression
To determine if siNLRP2 transfection had an effect
on cell migration, a scratch assay was performed. Wound closure was
examined at 0 and 48 h following wounding (Fig. 5A and B). No significant difference
was observed between siNC and siNLRP2 at 0 h. Following
transfection for 48 h, siNLRP2 led to significantly decreased wound
closure compared with the siNC group (P<0.05). Since migration
and proliferation affect wound closure in the wound healing assay,
a Transwell migration assay was performed to specifically evaluate
cell migration. The results are summarized in Fig. 5C and D. Regarding the migrated cell
numbers, cell migration was reduced 4-fold as a result of
transfection with siNLRP2 compared with siNC. The effect of siNLRP2
transfection on HUVEC cell invasion was tested by using a Transwell
invasion assay. The data (Fig. 5E and
F) demonstrated that the number of HUVECs that invaded the
Matrigel was significantly reduced in the siNLRP2 group compared
with siNC group (P<0.05). Statistical analysis indicated that
the inhibitory effect of siNLRP2 on cell invasion was 76% of that
of siNC. Together, these results demonstrated that knockdown of
NLRP2 expression markedly inhibited HUVEC migration and
invasion.
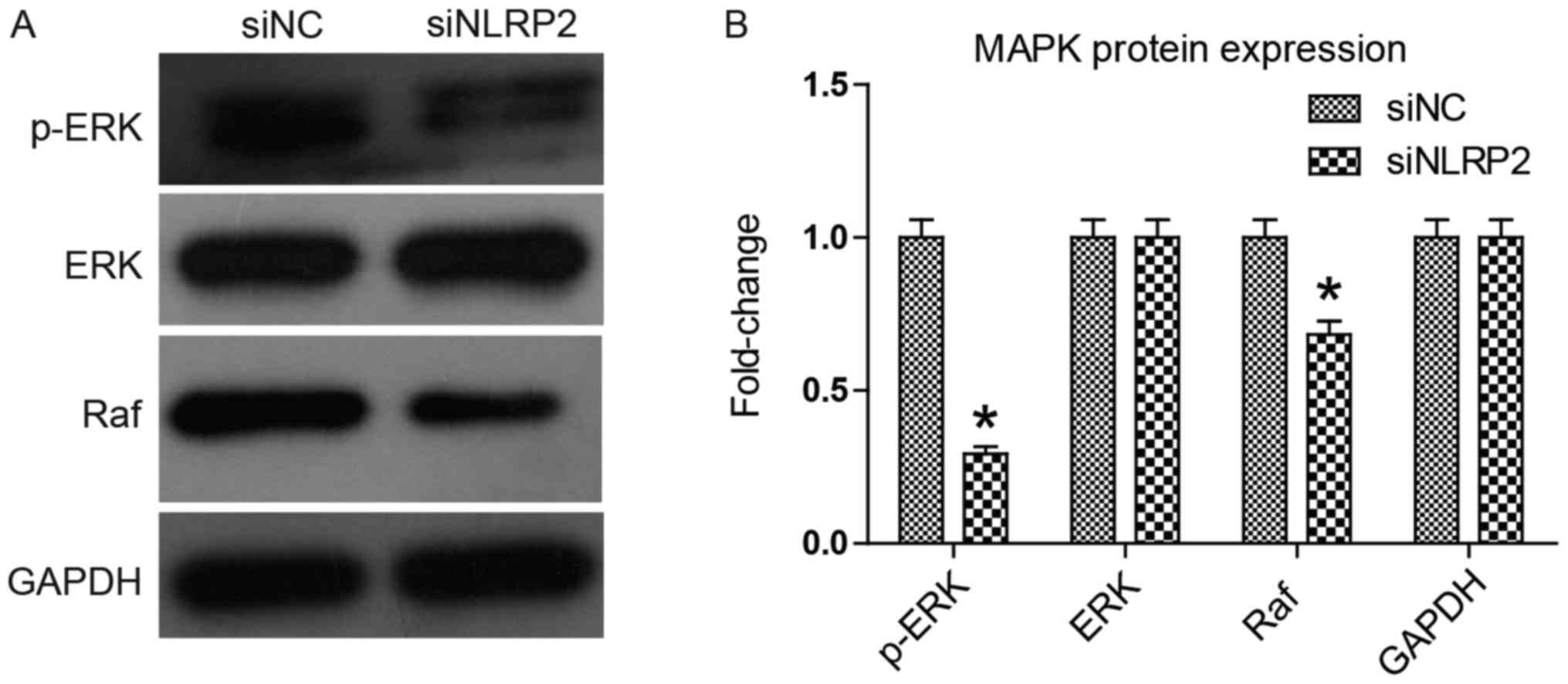
Knocking down NLRP2 inhibits the MAPK
signaling pathway
The MAPK signaling pathway is an important signaling
pathway that regulates cell proliferation, apoptosis and migration
(15). Among the signaling
components, MAPK, ERK and Raf serve a central regulating role, and
their abnormal activation, expression or upregulation frequently
causes an uncontrolled proliferation as in cancer cells (16,17).
The present study investigated the effect of siNLRP2 transfection
on the MAPK signaling pathway by using western blot analysis. The
results in Fig. 6 indicated that
siNLRP2 transfection significantly downregulated Raf expression and
ERK phosphorylation (P<0.05), while no evident change was
identified in ERK expression. This suggests that knocking down
NLRP2 inhibited the MAPK signaling pathway, which explains the
above inhibitory activity of siNLRP2 on HUVECs.
Discussion
NLRs, hosted by the innate immune system, are mainly
involved in recognizing harmful endogenous and exogenous molecular
patterns and forming inflammasomes (5,18).
NLRP2 belongs to a group of NLRPs, which is a subfamily of NLRs. In
addition to mediating the activation of inflammasomes in innate
immune responses (19), NLRP2 has
been mainly described as participating in reproduction and
embryonic development (8,10,20,21).
Other studies have revealed a correlation with ischemic stroke,
bipolar disorder and sibling allogeneic stem cell transplantation
(13,19,22).
However, NLRP2 expression and whether it serves an important
physiological function in vascular endothelial cells has yet to be
elucidated.
The present study used HUVECs as a model to
investigate NLRP2 expression and function in vascular endothelial
cells. siNLRP2 was constructed to knock down the expression of
NLRP2 and a scrambled siRNA was used as a negative control. qPCR
and western blot analysis indicated that NLRP2 was basally
expressed in HUVECs and that siNLRP2 exerted effective
interference. Using a CCK-8 assay, it was identified that siNLRP2
significantly inhibited the proliferation of HUVECs and led to
apoptosis-like morphological changes, including cell shrinkage and
blebbing. The motility of cells, including migration and invasion,
was also identified to be suppressed by siNLRP2 in a wound healing
and in a Transwell assay. An apoptosis and cell cycle detection
assay revealed that siNLRP2 induced apoptosis and inhibited HUVEC
cell cycle progression. In addition, apoptosis-associated gene
expression was also altered in a manner consistent with apoptosis
activation, including increased p53 and active-caspase 3
expression, and a decreased Bcl-2/Bax ratio. With respect to cell
cycle-associated genes, p70S6K, CDK4 and cyclin D1 were
downregulated, and conversely, p53 was upregulated. p53 was
responsible for preventing cell cycle progression and inducing
apoptosis when cells were in abnormal states. Additionally, members
of the MAPK signaling pathway, ERK and Raf, were also investigated.
The results suggested that ERK phosphorylation and Raf expression
were elevated, while ERK expression was unchanged. The results
indicated that downregulation of NLPR2 led to the inhibition of the
MAPK signaling pathway. MAPK cascade activation is the center of a
variety of signaling pathways and serves a key role in cell
proliferation and differentiation (23–26).
In conclusion, for the first time, to the best of
the authors' knowledge, NLRP2 function in HUVECs has been
investigated. The results suggested that NLRP2 serves an important
role in maintaining HUVEC viability and motility via the MAPK
signaling pathway. In combination with a previous study
demonstrating that NLRP2 expression is significantly enhanced in a
mouse model of ischemic stroke (13), it was assumed that NLRP2 may exert
a vessel protection function in ischemic stroke. These results also
provide a novel therapeutic strategy for tumor angiogenesis. Gene
therapy or monoclonal antibodies may be used to selectively inhibit
NLRP2 function in tumors and suppress tumor angiogenesis.
Altogether, the findings of the present study open a new field of
NLRP2 functional research that could help to find novel treatment
strategies for vascular endothelial-associated diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XZ and XL performed the experiments and wrote the
manuscript; YG and LY made substantial contributions to the
acquisition of data. FQ made substantial contributions to the
conception and design of the present study and has given final
approval of the version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maslanik T, Mahaffey L, Tannura K,
Beninson L, Greenwood BN and Fleshner M: The inflammasome and
danger associated molecular patterns (DAMPs) are implicated in
cytokine and chemokine responses following stressor exposure. Brain
Behav Immun. 28:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bauer RN, Diaz-Sanchez D and Jaspers I:
Effects of air pollutants on innate immunity: The role of Toll-like
receptors and nucleotide-binding oligomerization domain-like
receptors. J Allergy Clin Immunol. 129:14–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YK, Shin JS and Nahm MH: NOD-like
receptors in infection, immunity, and diseases. Yonsei Med J.
57:5–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elinav E, Strowig T, Kau AL, Henao-Mejia
J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon
JI and Flavell RA: NLRP6 inflammasome regulates colonic microbial
ecology and risk for colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brickler T, Gresham K, Meza A,
Coutermarsh-Ott S, Williams TM, Rothschild DE, Allen IC and Theus
MH: Nonessential role for the NLRP1 inflammasome complex in a
murine model of traumatic brain injury. Mediators Inflamm.
2016:63735062016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilburgs T, Meissner TB, Ferreira LMR,
Mulder A, Musunuru K, Ye J and Strominger JL: NLRP2 is a suppressor
of NF-κB signaling and HLA-C expression in human trophoblasts†,‡.
Biol Reprod. 96:831–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahadevan S, Sathappan V, Utama B, Lorenzo
I, Kaskar K and Van den Veyver IB: Maternally expressed NLRP2 links
the subcortical maternal complex (SCMC) to fertility, embryogenesis
and epigenetic reprogramming. Sci Rep. 7:446672017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng H, Chang B, Lu C, Su J, Wu Y, Lv P,
Wang Y, Liu J, Zhang B, Quan F, et al: Nlrp2, a maternal effect
gene required for early embryonic development in the mouse. PLoS
One. 7:e303442012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aird WC: Phenotypic heterogeneity of the
endothelium: I. Structure, function, and mechanisms. Circ Res.
100:158–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kinnunen K, Piippo N, Loukovaara S, Hytti
M, Kaarniranta K and Kauppinen A: Lysosomal destabilization
activates the NLRP3 inflammasome in human umbilical vein
endothelial cells (HUVECs). J Cell Commun Signal. 11:275–279. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun X, Song X, Zhang L, Sun J, Wei X, Meng
L and An J: NLRP2 is highly expressed in a mouse model of ischemic
stroke. Biochem Biophys Res Commun. 479:656–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maurer G, Tarkowski B and Baccarini M: Raf
kinases in cancer-roles and therapeutic opportunities. Oncogene.
30:3477–3488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozaki E, Campbell M and Doyle SL:
Targeting the NLRP3 inflammasome in chronic inflammatory diseases:
Current perspectives. J Inflamm Res. 8:15–27. 2015.PubMed/NCBI
|
|
19
|
Vizlin-Hodzic D, Zhai Q, Illes S,
Södersten K, Truvé K, Parris TZ, Sobhan PK, Salmela S, Kosalai ST,
Kanduri C, et al: Early onset of inflammation during ontogeny of
bipolar disorder: The NLRP2 inflammasome gene distinctly
differentiates between patients and healthy controls in the
transition between iPS cell and neural stem cell stages. Transl
Psychiatry. 7:e10102017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fontalba A, Gutierrez O and Fernandez-Luna
JL: NLRP2, an inhibitor of the NF-kappaB pathway, is
transcriptionally activated by NF-kappaB and exhibits a
nonfunctional allelic variant. J Immunol. 179:8519–8524. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aghajanova L, Mahadevan S, Altmäe S,
Stavreus-Evers A, Regan L, Sebire N, Dixon P, Fisher RA and Van den
Veyver IB: No evidence for mutations in NLRP7, NLRP2 or KHDC3L in
women with unexplained recurrent pregnancy loss or infertility. Hum
Reprod. 30:232–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Granell M, Urbano-Ispizua A, Pons A,
Aróstegui JI, Gel B, Navarro A, Jansa S, Artells R, Gaya A, Talarn
C, et al: Common variants in NLRP2 and NLRP3 genes are strong
prognostic factors for the outcome of HLA-identical sibling
allogeneic stem cell transplantation. Blood. 112:4337–4342. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fragale A, Tartaglia M, Wu J and Gelb BD:
Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent
prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum
Mutat. 23:267–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Apáti A, Jánossy J, Brózik A, Bauer PI and
Magócsi M: Calcium induces cell survival and proliferation through
the activation of the MAPK pathway in a human hormone-dependent
leukemia cell line, TF-1. J Biol Chem. 278:9235–9243. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carter BZ, Mak DH, Schober WD,
Cabreira-Hansen M, Beran M, McQueen T, Chen W and Andreeff M:
Regulation of survivin expression through Bcr-Abl/MAPK cascade:
Targeting survivin overcomes imatinib resistance and increases
imatinib sensitivity in imatinib-responsive CML cells. Blood.
107:1555–1563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmid RS, Graff RD, Schaller MD, Chen S,
Schachner M, Hemperly JJ and Maness PF: NCAM stimulates the
Ras-MAPK pathway and CREB phosphorylation in neuronal cells. J
Neurobiol. 38:542–558. 1999. View Article : Google Scholar : PubMed/NCBI
|