Introduction
Liver cancer is the third leading cause of
cancer-associated morbidity and mortality worldwide (1), and primarily results from chronic
hepatitis, nonalcoholic fatty liver disease and other hereditary
conditions (2). In recent years,
the morbidity and mortality rates associated with liver cancer have
shown an increasing tendency (3).
Furthermore, due to the difficulties associated with establishing a
correct diagnosis during the early stages of the disease, the
scarcity of targeted drugs and the accompanying rapid progression
of the illness, the survival rate of liver cancer is low (4). At present, surgical methods,
including liver transplantation and resection, are the first-choice
therapies for the treatment of patients with liver cancer in the
early stages of the disease (5,6).
However, the majority of patients with liver cancer have already
progressed to an advanced stage at the time of diagnosis, having
therefore lost the best opportunity for treatment, and must depend
on radiotherapy, chemotherapy and other non-surgical treatment
methods (7). Therefore, the
development of novel and effective preventative and treatment drugs
for liver cancer with minimal side effects is an urgent
requirement.
Natural products isolated from traditional Chinese
medicinal plants are one of the most important sources of
anti-cancer drugs that are used for the effective treatment of
cancer in China. These may assist patients through enhancing their
anticancer ability and working as remedial agents against adverse
reactions caused by radiotherapy or chemotherapy (8). The flowering plant species
Vladimiria (V.) souliei, classified in the
Compositae family, is mainly distributed in the western and eastern
Sichuan province and eastern Tibet in China (9). Costunolide (cos) is one of the major
sesquiterpenes extracted from V. souliei and is considered
to be the active constituent of this plant. It has been
demonstrated to have various pharmacological properties, including
serving as an anti-lung injury, anti-hepatitis B virus and
anti-liver injury agent. Cos has also been reported to inhibit
ethanol-induced gastric ulcers in mice and to exert anti-cancer
effects in vitro (10–14).
Thus far, cos has been extensively studied with
respect to its potential anti-cancer activity in vitro. It
exhibited strong inhibitory effects on the proliferation of human
osteosarcoma U2OS (15), breast
cancer MCF-7 (16), bladder cancer
T24 (17), leukemia HL-60
(18), and cervical cancer Hela
(19) cell lines, indicating that
cos is a potential therapeutic candidate for the treatment of
cancer. It was previously reported that cos treatment could inhibit
cell proliferation of HepG2 cells (IC50 27.5 µM) (20); however, to the best our knowledge
the protective mechanisms of cos against liver cancer have not been
elucidated.
Therefore, the present study aimed to investigate
the effects of cos against liver cancer and to determine whether
apoptotic pathways contribute to the anticancer effects of cos in
HepG2 cells. The growth inhibitory effects of cos on HepG2 cells
were examined using MTT assay, while cell morphology was examined
in order to determine the extent of cellular apoptosis. Cell cycle
distribution and apoptosis analyses were also conducted using flow
cytometry. The protein expression levels of B-cell lymphoma 2
(Bcl-2), Bcl-2-associated X protein (Bax), and caspases-3, −8 and
−9 were further detected by western blotting. It was demonstrated
that cos effectively inhibited the proliferation and induced the
apoptosis of HepG2 cells. Taken together, the results of the
present study suggested that cos may be a promising therapeutic
agent for the treatment of liver cancer, and the potential
mechanism underlying its effects was, in part, elucidated.
Materials and methods
Plant material
The roots of V. souliei were identified by
Professor Min Chen, the corresponding author of the present study,
of the College of Pharmaceutical Sciences, Southwest University
(Chongqing, China). The plants were collected at Luding County
(Sichuan, China) in October 2015, and a voucher specimen (no.
2015-14) was deposited at the College of Pharmaceutical Sciences,
Southwest University.
Extraction and isolation of cos
Powder derived from the air-dried roots (11.0 kg) of
V. souliei was extracted three times with 95% ethanol
overnight at room temperature. The ethanol extract was evaporated
in vacuo to yield a semisolid (1.12 kg), which was then
suspended in water and partitioned successively with petroleum
ether, ethyl acetate and n-butanol. The ethyl acetate solution was
concentrated to yield 296 g of residue, which was subjected to
silica gel column chromatography elution with petroleum ether/ethyl
acetate, using mixtures of increasing polarity (99:1 to 10:1) to
obtain a total of 16 fractions. Cos (4.23 g) was purified from
fraction 7 (purity, >98%) by crystallization and
recrystallization, and its structure was confirmed by spectroscopic
methods, including 1H-nuclear magnetic resonance and mass
spectrometric analyses, by comparing with data in the literature
(21).
Reagents and materials
Human liver cancer HepG2 cells were purchased from
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). Dimethyl sulfoxide (DMSO), the Annexin
V/fluorescein isothiocyanate (FITC) apoptosis detection kit were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), streptomycin and penicillin were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). The MTT assay kit, cell
cycle analysis kit, rabbit monoclonal primary antibodies against
β-actin (cat. no. AF0003), Bax (cat. no. AF1270), Bcl-2 (cat. no.
AF1915), caspase-3 (cat. no. AF1213), caspase-8 (cat. no. AF1243)
and caspase-9 (cat. no. AF1264), and horseradish
peroxidase-conjugated secondary antibodies (cat. no. A0208) were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Cos (purified via recrystallization to a purity >98%, as
described above) was dissolved in DMSO and stored at 4°C.
Cell culture and treatment
HepG2 cells were cultured in DMEM supplemented with
10% FBS, 100 µg/ml streptomycin and 100 µg/ml penicillin at 37°C in
a humidified atmosphere containing 5% CO2. Cells in the control
group were treated with DMSO, whereas HepG2 cells in the
experimental groups were exposed to 2.5, 5, 10, 20 and 40 µmol/l
cos for 3, 6, 12, 24, 36 and 48 h. In the cos treatment groups, the
final concentration of DMSO added to the cells was <0.1%.
Microscopic observation
Morphological changes associated with apoptosis were
assessed using light microscopy. Following the corresponding
treatments, the morphology of HepG2 cells was observed under an
inverted light microscope (Olympus CKX53; Olympus Corporation,
Tokyo, Japan), and images were captured at a magnification of
×100.
Cell viability assay
MTT assay was used to measure the viability of the
HepG2 cells. Briefly, cells were plated in 96-well culture plates
(1×104 cells/well) and incubated at 37°C with cos at various
concentrations (2.5, 5, 10, 20, and 40 µmol/l) for 48 h. In
addition, cells were treated with 10 µmol/l cos for 3, 6, 12, 24,
36 and 48 h. Subsequently, MTT solution (5 mg/ml) was added to each
well. After 3 h of incubation, the formazan precipitate was
dissolved in 100 ml DMSO, and the absorbance was measured at a
wavelength of 450 nm using a microplate reader (Bio-Rad 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The half-maximal
inhibitory concentration (IC50) values were calculated by nonlinear
regression analysis using Origin version 8.0 (OriginLab Software,
Inc., Northampton, MA, USA) and GraphPad Prism version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) software packages.
Each sample was analyzed in triplicate.
Cell cycle analysis
Cell cycle distribution was analyzed using flow
cytometric analysis. Briefly, cells were plated in 10-cm plates,
and treated with DMSO or with the various concentrations (2.5, 5,
10, 20, and 40 µmol/l) of cos for 48 h. Subsequently, the treated
cells were collected, washed twice with cold phosphate-buffered
saline (PBS), fixed with 70% ethanol and stained with propidium
iodide (PI). The cells were then analyzed using a flow cytometer
(Accuri C6; Bio-Rad Laboratories, Inc.), and the data obtained were
analyzed with FlowJo software (version X; FlowJo LLC, Ashland, OR,
USA).
Apoptosis assay
Cells were treated with DMSO or various
concentrations (2.5, 5, 10, 20, and 40 µmol/l) of cos. After 48 h
of incubation, the cells were harvested and washed twice in cold
PBS for cell apoptotic analysis. The cells were subsequently
treated with 5 µl Annexin V/FITC and 5 µl PI solution, and then
incubated at room temperature for 15 min in the dark. Finally, the
cells were analyzed for apoptosis using an Accuri C6 flow
cytometer. According to the protocol provided with the apoptosis
kit, the number of cells in each cell cycle phase was calculated
using Cell ModFit software (BD Biosciences, Franklin Lakes, NJ,
USA). The experiments were performed in triplicate. The apoptotic
rate was the sum of the proportion of cells in the early (Annexin
V/FITC+ PI-) and late (Annexin V/FITC+ PI+) stages of
apoptosis.
Western blot assay
Approximately 5×105 HepG2 cells plated in 6-well
culture plates were used for western blotting. Total protein
fractions were isolated, the medium was removed, and cells were
washed twice with ice-cold PBS prior to lysis using cell lysis
buffer (containing 20 mM Tris, 150 mM NaCl and 1% Triton X-100).
The lysates were collected by scraping from the plates and
subsequently centrifuged at 12,000 × g at 4°C for 5 min. Protein
concentrations were determined using the BCA protein assay method
(Beyotime Institute of Biotechnology). Samples (50 µg) were
separated by SDS-PAGE (10% gels) and subsequently transferred onto
nitrocellulose membranes for 1.5 h. Following blocking with 10%
dried fat-free milk in Tris-buffered saline buffer with 0.05%
Tween-20 for 1 h at 37°C, the membranes were incubated with the
primary antibodies overnight at 4°C. The primary antibodies used
were as follows: Anti-Bax (1:1,000 dilution), anti-Bcl-2 (1:1,000),
anti-caspases-3, −8 and −9 (1:1,000), and anti-β-actin (1:1,500).
Subsequently, the membranes were incubated with secondary goat
anti-rabbit antibodies (1:1,000) at 37°C for 1 h. Proteins were
detected using an EasyBlot ECL kit (cat. no. C506668-0100; Sangon
Biotech Co., Ltd., Shanghai, China). Using ImageJ software version
1.50 (National Institutes of Health, Bethesda, MD, USA) for
grayscale analysis, the relative expression levels of the target
proteins were expressed as the ratio of the gray value of Bax,
caspase-3, caspase-8, caspase-9 and Bcl-2 protein to the gray value
of β-actin.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA) was used
to perform statistical analyses. One-way analysis of variance
followed by least significant difference post hoc test was used to
compare data containing multiple groups. Student's t-test was
performed to determine statistically significant differences
between two groups. P<0.05 was considered to indicate
statistically significant values.
Results
Effect of cos on the proliferation and
morphology of HepG2 cells
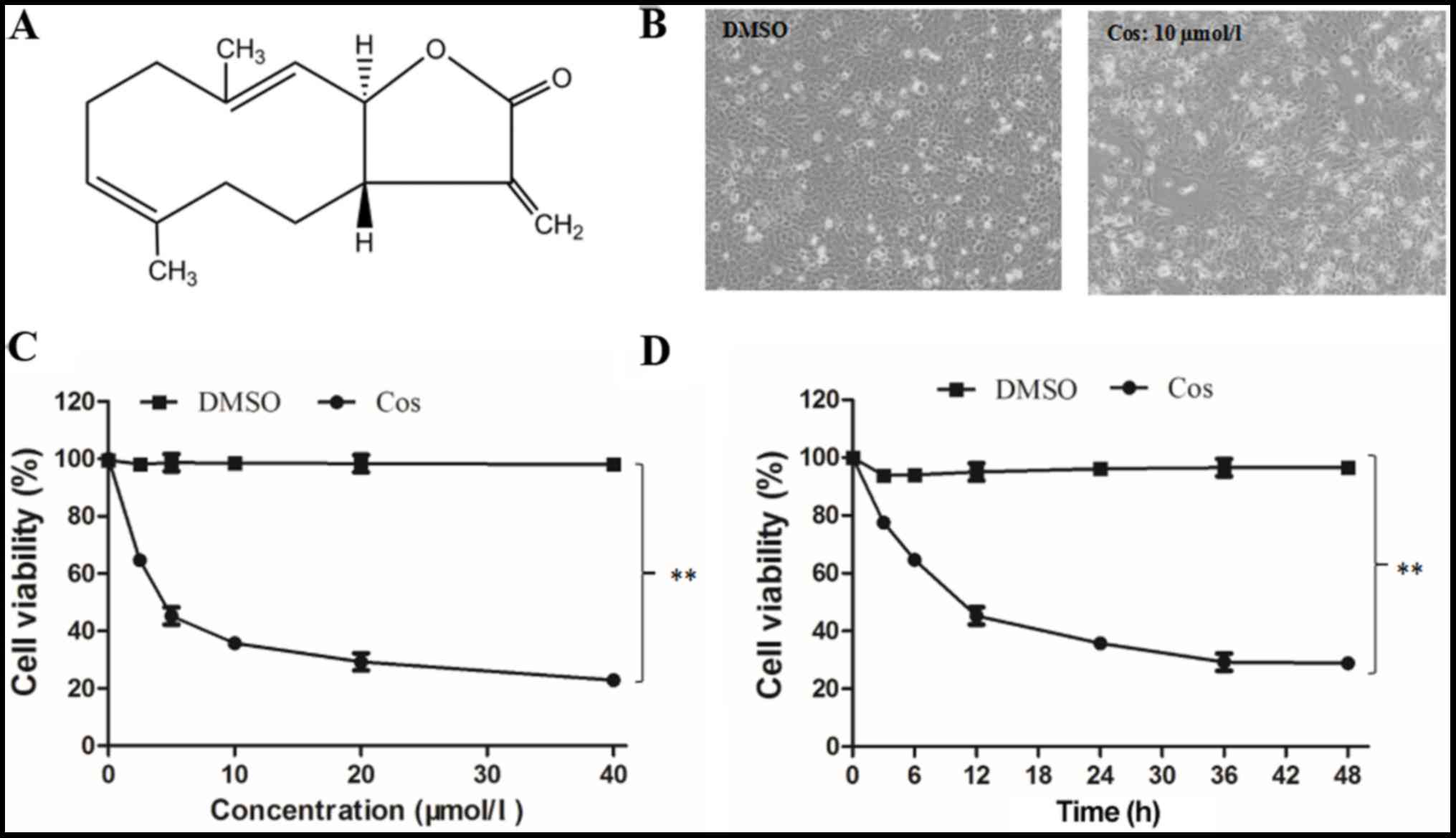
The chemical structure of cos, which is a
sesquiterpene lactone with an α-methylene-γ-lactone ring, is shown
in Fig. 1A. HepG2 cells in the
DMSO group appeared to have a regular phenotype and to adhere to
the wall of the plate, as observed in images captured under an
inverted microscope (Fig. 1B). By
contrast, the majority of the cells pretreated with 10 µmol/l cos
for 48 h appeared to have a smaller volume, irregular shape and
smaller size, while they were separated from adjacent cells and
lost their adhesive property, revealing a clear reduction in their
proliferative capacity. At the end of the treatment period, the
adhesion of these cells gradually declined, and they eventually
separated and floated freely (Fig.
1B). Furthermore, the results revealed that cos effectively
inhibited the proliferation of HepG2 cells in a dose-dependent
manner, as compared with the DMSO-treated cell group (Fig. 1C). After 48 h of treatment with
cos, the IC50 was determined to be 18.09±1.74 µM. The inhibitory
effect of cos was also induced in a time-dependent manner, with the
proliferation of HepG2 cells progressively decreasing over the time
course of the experiment (3, 6, 12, 24, 36 and 48 h), as shown in
Fig. 1D. Taken together, these
results suggested that cos effectively inhibited HepG2 cell
proliferation in a dose- and time-dependent manner.
Effect of cos on cell cycle
distribution of HepG2 cells
HepG2 cells in the control group were treated with
DMSO, whereas cells in the experimental groups were exposed to 2.5,
5, 10, 20 and 40 µmol/l cos for 48 h. PI staining and flow
cytometric analysis were used to detect the effect of cos on the
cell cycle distribution of HepG2 cells, and the results are shown
in Fig. 2. Compared with the
DMSO-treated group, the percentage of cells in G1/G0 phase
following treatment with the various concentrations of cos did not
change significantly, whereas the percentage of cells in the G2/M
phase was significantly increased and that in S phase was
significantly decreased. Following treatment with 0, 2.5, 5, 10, 20
and 40 µmol/l cos for 48 h, the percentage of HepG2 cells in the
G2/M phase was 18.52±2.58, 20.90±4.21, 26.54±3.67, 29.08±6.32,
32.17±3.14 and 33.42±8.97%, respectively. These results suggested
that cos treatment led to cell cycle arrest at G2/M phase in a
dose-dependent manner.
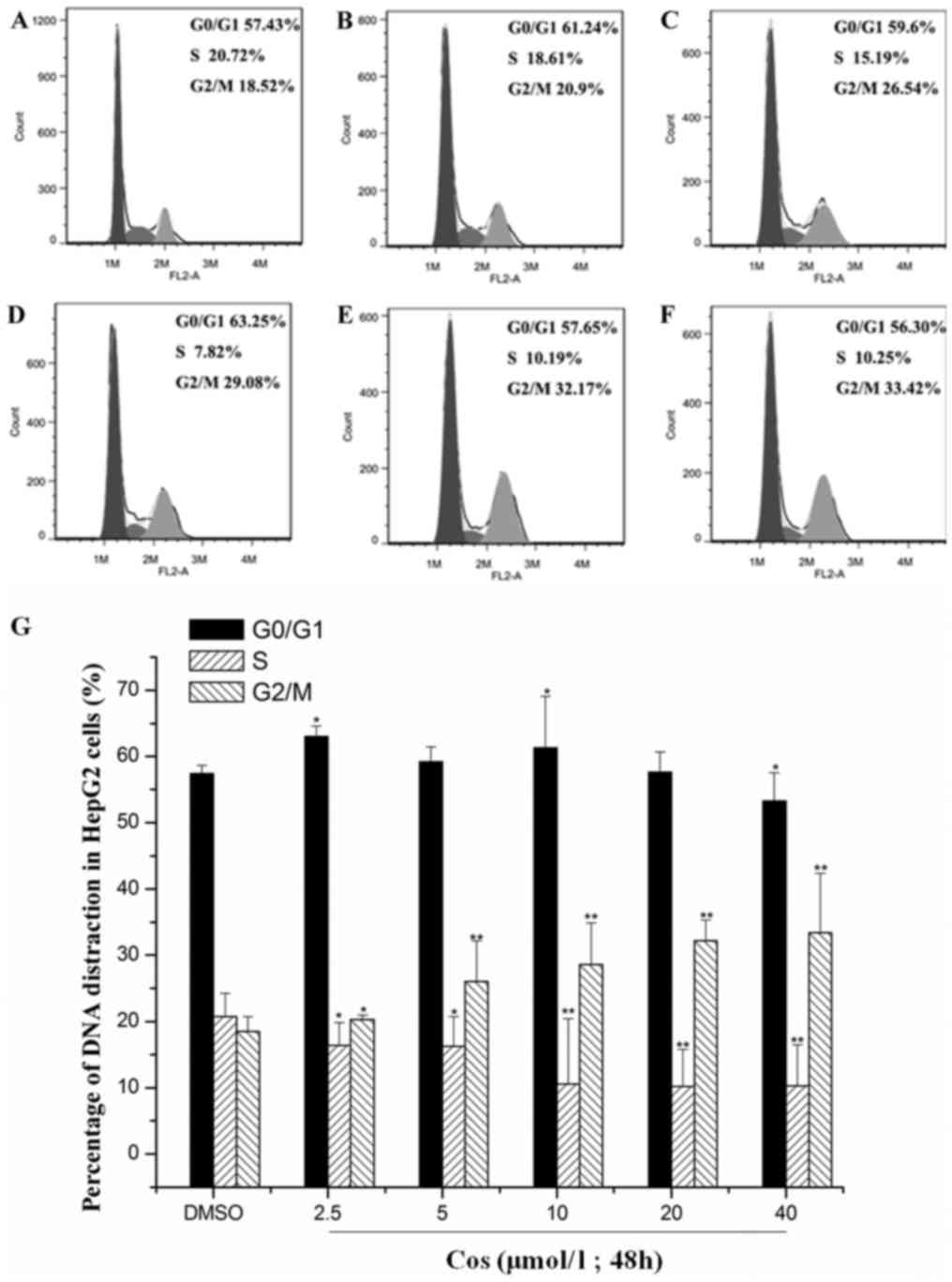 | Figure 2.Effect of cos on cell cycle
distribution of HepG2 cells. Following stimulation with cos or DMSO
for 48 h, HepG2 cells were harvested, fixed with ethanol and
stained with propidium iodide. The number of cells at each cell
cycle phase was subsequently determined using flow cytometry in
cells treated with (A) DMSO, or with (B) 2.5 µmol/l, (C) 5 µmol/l,
(D) 10 µmol/l, (E) 20 µmol/l and (F) 40 µmol/l cos, respectively.
(G) Percentages of HepG2 cells at each phase of the cell cycle,
analyzed using Origin version 8.0 software. Data are expressed as
the mean ± standard deviation from triplicate determinations.
*P<0.05 and **P<0.01, vs. control (DMSO) group. Cos,
costunolide; DMSO, dimethyl sulfoxide. |
Effect of cos on the apoptosis of
HepG2 cells
Translocation of phosphatidylserine (PS) to the
outer leaflet of the cellular membrane is the key step in the early
stages of apoptosis. Annexin V selectively binds to PS, and this
process enables the identification of cells undergoing apoptosis.
Different populations of cells may be observed when cells are
double-stained with Annexin V and PI (22). HepG2 cells in the control group
were treated with DMSO, whereas the treatment groups were exposed
to 2.5, 5, 10, 20 and 40 µmol/l cos for 48 h. As revealed in
Fig. 3, the apoptotic rate of the
HepG2 cells increased significantly with an increasing
concentration of cos. The apoptotic rates of HepG2 cells upon
treatment with 2.5, 5, 10, 20 and 40 µmol/l cos were 6.04±1.24,
8.04±1.57, 17.03±2.31, 37.19±3.19 and 55.33±2.12%,
respectively.
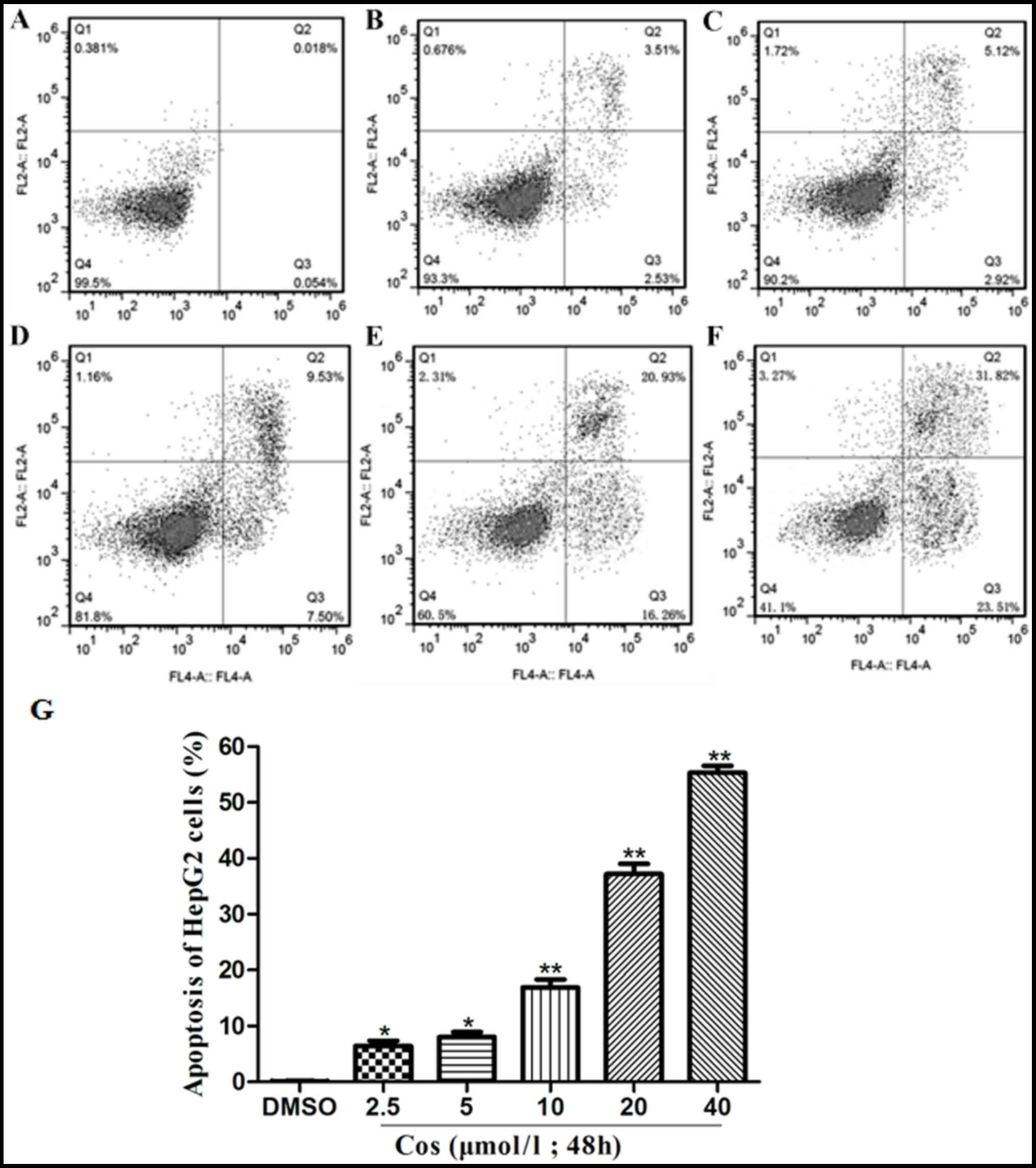 | Figure 3.Effect of cos on apoptosis of HepG2
cells. HepG2 cells were treated with cos or DMSO for 48 h, and then
stained with fluorescein isothiocyanate-conjugated Annexin V in a
buffer containing propidium iodide, followed by flow cytometric
analysis. The flow cytometry results are shown for cells treated
with (A) DMSO, or with (B) 2.5 µmol/l, (C) 5 µmol/l, (D) 10 µmol/l,
(E) 20 µmol/l and (F) 40 µmol/l cos. (G) Percentages of apoptotic
HepG2 cells, analyzed using GraphPad Prism version 5.0 software.
Data are expressed as the mean ± standard deviation from triplicate
determinations. *P<0.05 and **P<0.01, vs. control (DMSO)
group. Cos, costunolide; DMSO, dimethyl sulfoxide. |
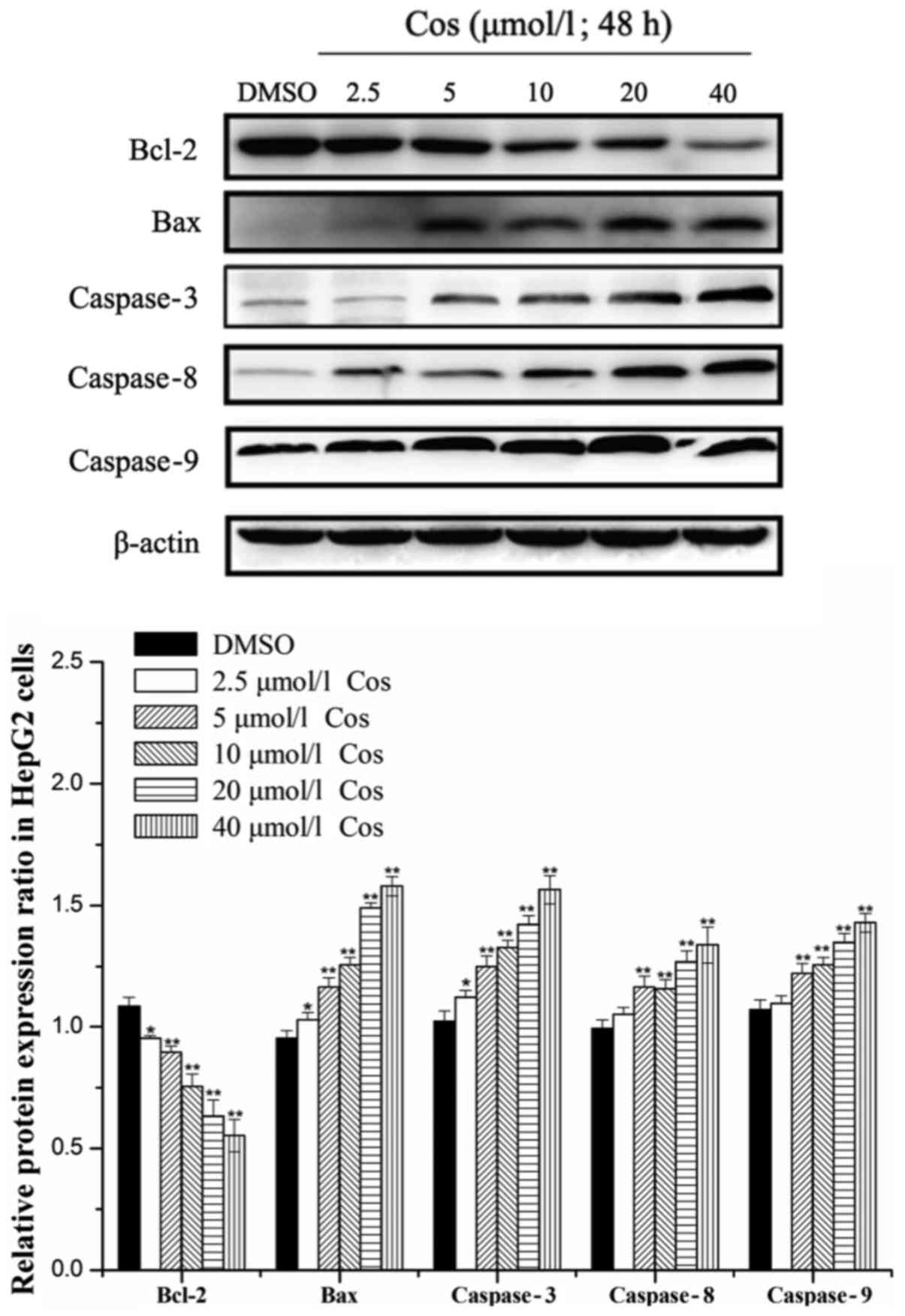
Effect of the cos on the expression
levels of caspases-3, −8 and −9, Bax and Bcl-2 in HepG2 cells
Bax and Bcl-2 proteins exert pivotal roles in
caspase activation and the regulation of apoptosis (23). In addition, caspase family
proteins, the central components of the apoptotic response, are a
conserved family of enzymes that induce irreversible cell death
(24). Caspases-3, −8 and −9 stand
at the nexus of critical regulatory networks controlling cell
apoptosis, and are components of the pathway that ultimately
mediates the activation and execution of apoptosis. Therefore, the
levels of caspases-3, −8 and −9, as well as Bax and Bcl-2 proteins,
were investigated in the present study. Cos treatment was observed
to induce the apoptosis of HepG2 cells by upregulating the protein
expression levels of Bax, and caspases-3, −8 and −9, and by
downregulating the expression of Bcl-2 protein in a dose-dependent
manner after 48 h of treatment (Fig.
4). Taken together, these results suggested that cos exerted an
inhibitory effect on the growth of HepG2 cells.
Discussion
Cos, a sesquiterpene lactone that contains an
α-methylene-γ-lactone ring structure, belongs to a class of
compounds that react with enzymes containing thiol groups and their
functional proteases, and interfere with the key biological
processes of cells, exhibiting a variety of pharmacological
activities (25). It has been
reported that cos exerts a strong inhibitory effect on the
proliferation and apoptosis of a variety of tumor cells at
concentrations ranging between 2.5 and 40 µmol/l (15–19).
Therefore, in the present study, the concentrations of 2.5, 5, 10,
20 and 40 µmol/l cos were selected for investigation. The results
demonstrated that cos was able to inhibit the proliferation of
HepG2 cells in a concentration-dependent manner, according to
cytotoxicity analysis.
Carcinogenesis is a multistep process that may be
activated by alterations in the activity of oncogenes and
transcription, and such changes affect cell proliferation, cell
cycle regulation and cell apoptosis (26). Since abnormal proliferation is
observed in cancer cells, inhibition of cell cycle progression is
an important means of intervention in the treatment of cancer.
Multiple checkpoints regulate cell proliferation during cell cycle
progression (27). In the present
study, it was identified that cos arrested the cell cycle at the
G2/M phase, induced apoptosis in vitro.
It has been reported that the volatile oil and cos
obtained from the roots of Saussurea lappa markedly
inhibited the proliferation of SMMC-7721 and Hep3B cells in
vitro (28). The anti-cancer
mechanism of volatile oil on HepG2 cells may be attributed to
increases in the proportion of cells in the G2/M and S phases,
accompanied by a decrease in the G0/G1 phase. In a previous study
examining the effect of cos on SGC-7901, apoptosis of the SGC-7901
cells was induced by cos in a time-dependent manner, and the cell
cycle was blocked in the G2/M phase (29). These previous findings are in
agreement with the results of the present study.
Apoptosis is the physiological process of programmed
cell death that results in tissue damage. For the majority of
commonly used anticancer drugs, activation of apoptotic pathways in
order to kill cancer cells remains the predominant anticancer
mechanism (30). A previous study
indicated that there are two apoptotic pathways that may be
activated: The mitochondria-dependent ‘intrinsic’ cytochrome
c/caspase-9 pathway, and the death receptor-mediated
‘extrinsic’ caspase-8 pathway (31). In particular, the mitochondrial
pathway is considered to be involved in the apoptosis of cancer
cells induced by phytochemicals (32). It is known that suppression of
anti-apoptotic members or activation of pro-apoptotic members of
the Bcl-2 family usually leads to an altered mitochondrial membrane
permeability, which subsequently induces apoptosis (33). The Bcl-2 family of proteins
contains the anti-apoptotic protein Bcl-2 and the pro-apoptotic
protein Bax, which inhibit or promote apoptosis, respectively.
These proteins have been described as critical regulators of the
mitochondrial apoptosis pathway, regulating mitochondrial membrane
permeability to control mitochondrial apoptosis (34). Caspase family proteins also serve a
key role in inducing apoptosis, and are involved in the ultimate
pathway for the execution of apoptosis. Caspase-3 is the key
protease of apoptosis; when it is activated, the cascade pathway of
downstream apoptosis is inevitably triggered (35). Caspase-9, an essential initiator
caspase required for apoptosis signaling through the mitochondrial
pathway, is activated on the apoptosome complex (36). Caspase-8 is considered to be
predominantly a pro-apoptotic protease that is mainly involved in
signal transduction via death receptors of the tumor necrosis
factor receptor family, such as Fas (37). Therefore, in the present study, the
effects of cos on the expression levels of critical regulators of
the mitochondrial apoptotic pathway, including Bax, Bcl-2, and
caspases-3, −8 and −9, were examined in HepG2 cells. Western
blotting revealed that cos induced apoptosis of HepG2 cells via the
upregulation of Bax and caspases-3, −8 and −9, and the
downregulation of Bcl-2 protein expression. A previous study
indicated that cos may induce apoptosis in the breast cancer cell
line MDA-MB-231 via the activation of Fas in the exogenous pathway,
upregulation of caspases-8 and −3, and downregulation of poly
(ADP-ribose) polymerase (PARP) expression (38). In addition, the apoptosis of
SGC-790 cells has been reported to be induced by cos via
downregulation of the expression levels of Bcl-2 and upregulation
of caspase-3 (29). It was also
reported that, in the human platinum-resistant ovarian cancer cell
line SKOV3PT, cos exerted a clear inhibitory effect by
downregulating the activation of Bcl-2 protein and upregulating
caspases involved in the apoptosis signaling pathway (39). Furthermore, apoptosis of esophageal
cancer cells induced by cos was revealed to be mediated by
upregulation of Bax protein expression, downregulation of Bcl-2
protein expression, and activation of caspase-3 and PARP (40). The results of these previous
studies on the anti-cancer effects of cos are consistent with those
obtained in the present study. Taken together, these results
confirm that cos treatment induced the activation of apoptosis.
In conclusion, the present study demonstrated that
cos, a natural sesquiterpene lactone isolated from V.
souliei, markedly inhibited the proliferation of HepG2 cells
in vitro. The survival rate of tumor cells was gradually
decreased as the concentration of cos was increased. Furthermore,
the apoptosis rates of HepG2 cells increased with an increasing
concentration of cos in the range of 2.5–40 µmol/l. Cos was also
demonstrated to induce cell cycle arrest in the G2/M phase, thereby
affecting the proliferation of HepG2 cells. In terms of the
underlying mechanism, cos was able to induce apoptosis in the HepG2
cells by upregulating the protein expression levels of Bax and
caspases-3, −8 and −9, and downregulating the expression of Bcl-2
protein, thereby inhibiting the growth of the HepG2 cells. Taken
together, these results suggest that cos may be a promising
candidate or leading compound for drug development, targeting liver
cancer. This study has verified previous results on the inhibitory
effects of cos on HepG2 cells and has also provided preliminary
data in support of further animal experiments and clinical
trials.
Acknowledgements
The authors would like to thank Dr Selvaraj
Subramaniyam (College of Pharmaceutical Sciences, Southwest
University) for his assistance with useful discussions and a
critical reading of the manuscript.
Funding
The present study was supported by the Chongqing
Social Undertaking and Livelihood Security Project (grant no.
cstc2017shmsA130079) and the National Natural Science Foundation of
China (grant no. 81774005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC conceived and designed the study. JM performed
most of the experiments and wrote the paper. MY and YT performed
part of the experiments. YH analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Firtina Karagonlar Z, Koc D, Iscan E,
Erdal E and Atabey N: Elevated hepatocyte growth factor expression
as an autocrine c-Met activation mechanism in acquired resistance
to sorafenib in hepatocellular carcinoma cells. Cancer Sci.
107:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 (5 Suppl 1):S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu J and Wang HY: Precision diagnosis and
treatment of liver cancer in China. Cancer Lett. 412:283–288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laursen L: A preventable cancer. Nature.
516:S2–S3. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarnagin WR: Management of small
hepatocellular carcinoma: A review of transplantation, resection,
and ablation. Ann Surg Oncol. 17:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bitzer M, Horger M, Giannini EG, Ganten
TM, Wörns MA, Siveke JT, Dollinger MM, Gerken G, Scheulen ME, Wege
H, et al: Resminostat plus sorafenib as second-line therapy of
advanced hepatocellular carcinoma-The SHELTER study. J Hepatol.
65:280–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kojiro M: Histopathology of liver cancers.
Best Pract Res Clin Gastroenterol. 19:39–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin WF, Lu JY, Cheng BB and Ling CQ:
Progress in research on the effects of traditional Chinese medicine
on the tumor microenvironment. J Integr Med. 15:282–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. 1. China Medical
Science Press; Shanghai: pp. 35–36. 2015
|
|
10
|
Butturini E, Di Paola R, Suzuki H,
Paterniti I, Ahmad A, Mariotto S and Cuzzocrea S: Costunolide and
dehydrocostuslactone, two natural sesquiterpene lactones,
ameliorate the inflammatory process associated to experimental
pleurisy in mice. Eur J Pharmacol. 730:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HC, Chou CK, Lee SD, Wang JC and Yeh
SF: Active compounds from Saussurea lappa Clarks that suppress
hepatitis B virus surface antigen gene expression in human hepatoma
cells. Antiviral Res. 27:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang X, Zhao L, Shi M, Wei Z,
Yang Z, Guo C and Fu Y: Costunolide protects
lipopolysaccharide/d-galactosamine-induced acute liver injury in
mice by inhibiting NF-κB signaling pathway. J Surg Res. 220:40–45.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng H, Chen Y, Zhang J, Wang L, Jin Z,
Huang H, Man S and Gao W: Evaluation of protective effects of
costunolide and dehydrocostuslactone on ethanol-induced gastric
ulcer in mice based on multi-pathway regulation. Chem Biol
Interact. 250:68–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava SK, Abraham A, Bhat B, Jaggi M,
Singh AT, Sanna VK, Singh G, Agarwal SK, Mukherjee R and Burman AC:
Synthesis of 13-amino Costunolide derivatives as anticancer agents.
Bioorg Med Chem Lett. 16:4195–4199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Lu T, Wang GD, Ma C and Zhou YF:
Costunolide, an active sesquiterpene lactone, induced apoptosis via
ROS-mediated ER stress and JNK pathway in human U2OS cells. Biomed
Pharmacother. 80:253–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang GM, Shi DD, Peng ZX, Lu YF, Gu X,
Wang Y and Yan C: Study on mechanism of costunolide-induced
apoptosis in breast cancer MCF-7 cells. Chin J Anal Chem.
43:682–688. 2015.
|
|
17
|
Rasul A, Bao R, Malhi M, Zhao B, Tsuji I,
Li J and Li X: Induction of apoptosis by costunolide in bladder
cancer cells is mediated through ROS generation and mitochondrial
dysfunction. Molecules. 18:1418–1433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh GS, Pae HO, Chung HT, Kwon JW, Lee JH,
Kwon TO, Kwon SY, Chon BH and Yun YG: Dehydrocostus lactone
enhances tumor necrosis factor-alpha-induced apoptosis of human
leukemia HL-60 cells. Immunopharmacol Immunotoxicol. 26:163–175.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semsarha F, Taheri MA and Najafi MH:
Interfering with consciousness at cellular (HeLa cell) and
molecular (microtubule protein) levels. Presented at The Science of
Consciousness TSC 2016. Abstract. 299:2016.
|
|
20
|
Wang Z, Zhao Z and Gong XG: Costunolide
induces lung adenocarcinoma cell line A549 cells apoptosis through
ROS (reactive oxygen species)-mediated endoplasmic reticulum
stress. Cell Biol Int. 40:289–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao K, Qian W, Xu Y, Zhou Z, Zhang Q and
Zhang XF: A new sesquiterpenoid from Saussurea lappa roots. Nat
Prod Res. 30:2160–2163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Utsugi T, Schroit AJ, Connor J, Bucana CD
and Fidler IJ: Elevated expression of phosphatidylserine in the
outer membrane leaflet of human tumor cells and recognition by
activated human blood monocytes. Cancer Res. 51:3062–3066.
1991.PubMed/NCBI
|
|
23
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alves JCF: A review on the chemistry of
eremanthine: A sesquiterpene lactone with relevant biological
activity. Org Chem Int. 2011:Article ID 170196. 2011. View Article : Google Scholar
|
|
26
|
Ramasamy K and Agarwal R: Multitargeted
therapy of cancer by silymarin. Cancer Lett. 269:352–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
López-Sáez JF, de la Torre C, Pincheira J
and Giménez-Martín G: Cell proliferation and cancer. Histol
Histopathol. 13:1197–1214. 1998.PubMed/NCBI
|
|
28
|
Lin X, Peng Z, Fu X, Liu C, Xu Y, Ji W,
Fan J, Chen L, Fang L, Huang Y and Su C: Volatile oil from
Saussurea lappa exerts antitumor efficacy by inhibiting epithelial
growth factor receptor tyrosine kinase-mediated signaling pathway
in hepatocellular carcinoma. Oncotarget. 7:79761–79773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rasul A, Yu B, Yang LF, Arshad M, Khan M,
Ma T and Yang H: Costunolide, a sesquiterpene lactone induces G2/M
phase arrest and mitochondria-mediated apoptosis in human gastric
adenocarcinoma SGC-7901 cells. J Med Plants Res. 6:1191–1200.
2012.
|
|
30
|
Lau A, Wang Y and Chiu JF: Reactive oxygen
species: Current knowledge and applications in cancer research and
therapeutic. J Cell Biochem. 104:657–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lang L, Zhu S, Zhang H, Yang P, Fan H, Li
S, Liao Z, Lan X, Cui H and Chen M: A natural phenylpropionate
derivative from Mirabilis himalaica inhibits cell proliferation and
induces apoptosis in HepG2 cells. Bioorg Med Chem Lett.
24:5484–5488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gross A: Bcl-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857:1243–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ko HM, Joo SH, Jo JH, Park WS, Jung WY,
Shin JH and Ahn HJ: Liver-wrapping, nitric oxide-releasing
nanofiber downregulates cleaved caspase-3 and bax expression on rat
hepatic ischemia-reperfusion injury. Transplant Proc. 49:1170–1174.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wurstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salvesen GS: Caspase 8: Igniting the death
machine. Structure. 7:R225–R229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi YK, Seo HS, Choi HS, Choi HS, Kim SR,
Shin YC and Ko SG: Induction of Fas-mediated extrinsic apoptosis,
p21WAF1-related G2/M cell cycle arrest and ROS generation by
costunolide in estrogen receptor-negative breast cancer cells,
MDA-MB-231. Mol Cell Biochem. 363:119–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi YI, Kim JH, Lee KT and Choi JH:
Costunolide induces apoptosis in platinum-resistant human ovarian
cancer cells by generating reactive oxygen species. Gynecol Oncol.
123:588–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua PY, Sun M, Zhang G, Zhang Y, Song G,
Liu Z, Li X, Zhang X and Li B: Costunolide induces apoptosis
through generation of ROS and activation of P53 in human esophageal
cancer Eca-109 cells. J Biochem Mol Toxicol. 30:462–469. 2016.
View Article : Google Scholar : PubMed/NCBI
|