Introduction
Aneurysm is caused by injured normal structure of
the aortic wall due to congenital or acquired disorders, especially
by an injured elastic fiber layer of arterial walls, which leads to
the gradual dilation or enlargement of the aorta in local or
multiple places (1). In general,
aneurysm refers to an artery with a diameter exceeding 50% of the
normal size. Abdominal aortic aneurysm (AAA) refers to a high-risk
disease caused by the rupture of blood vessel walls under the
impact of blood flow, due to abnormal dilation or limited expansion
of abdominal aorta (1). The
incidence rate of AAA in China has reached 2%, and increases year
by year (2). Due to the lack of
understanding towards the incidence and underlying molecular
mechanisms of AAA, in addition to surgery, there is no drug which
can effectively treat or slow down the development of AAA (3).
According to previous studies, AAA may be associated
with smoking, sex, oxidative stress, matrix protein, blood lipid
levels and other factors (4). The
activities of reactive oxygen species, O2, nicotinamide
adenine dinucleotide phosphate (NADPH) oxidase, and the expression
of NADPH oxidase p47phox ligand, were demonstrated to be increased
in human AAA tissue samples (5).
In addition, matrix metalloproteinase (MMP) was also detected in
human AAA tissue samples; increased activity of MMPs promotes the
degradation of elastin and collagen in artery walls, leading to the
expansion of arterial walls (6).
Moreover, AAA destroys the extracellular matrix, all of which
results in AAA (7). Studies also
demonstrated that triglycerides and cholesterol are also associated
with the incidence of AAA (6,7).
Phosphatidylinositol 3-kinase (PI3K), a member of
the kinase family, is widely distributed in the human body. In the
1980s, the PI3K family attracted the attention of the medical field
(8). PI3K, an important catalytic
enzyme system regulating metabolic pathways of phospholipids, is
produced by phospholipid messenger molecules. It specifically binds
to the phosphatidylinositol base and transfers the phosphate group
of ATP to phosphorylate PI into inositol lipids (8). Lymphokine-activated killer
T-cell-originated protein kinase (PBK) forms an important signaling
pathway, termed the PI3K-protein kinase B (Akt) signaling pathway,
which together with Akt, located in its downstream, serves an
important role in the survival, differentiation, proliferation and
apoptosis of cells (9).
Nuclear factor (NF)-κB, a transcription factor, has
been confirmed to be widely distributed in eukaryotic cells, and
can quickly transfer into the nucleus and specifically bind to
special sites of cell gene promoters or enhancer sequences, to
promote transcription and expression (10). Studies have confirmed that NF-κB is
closely associated with many major pathophysiological processes,
such as the immune response and inflammation as well as the
proliferation, differentiation, metastasis and apoptosis of tissue
cells, serving an important role in the incidence and development
of many diseases (11,12).
Gamboge is the dry resin secreted by Garcinia
hanbaryi Hook.f. Gamboge mainly consists of 70–80% resin and
15–25% gum (13). The major
constituents of gamboge include gambogic acid, neogambogic acid,
allogambogic acid, morellin, isomorellin, morellic acid and
isomorellic acid, among which gambogic acid is the main effective
constituent (14). In recent
years, many studies have further investigated the antineoplastic
mechanism of gambogic acid, proving that gambogic acid can induce
cell differentiation and tumor cell apoptosis, as well as
inhibiting angiogenesis and lowering the activity of telomerase to
block the cell cycle and reverse drug-resistance, so as to kill
tumor cells (15). The present
study aimed to test the effect of gambogic acid on the prevention
of angiotensin (Ang) II-induced AAA, and to explore its underlying
mechanism.
Materials and methods
Animals
Animal experiments were performed according to
protocols approved by the institutional animal care and use
committee of The First Hospital of Qiqihar City. The present study
was approved by the Animal Ethical and Welfare Committee of The
First Hospital of Qiqihar City (Qiqihar, China). Male C57BL/6 mice
(ApoE−/− mice, 20–22 g, 6 weeks old; n=46) were
purchased from Animal Laboratory of Harbin Medical Sciences
University (Harbin, China) and were raised in specific
pathogen-free conditions with a 12-h light/dark cycle at 24±2°C and
50–60% humidity. An AAA model was induced by chronic infusion of
1,000 ng/kg/min Ang II using mini-osmotic pumps. All mice were
randomly distributed into five groups: Sham (n=6), AAA model
(n=10), 5 mg/kg Gambogic acid (n=10), 10 mg/kg Gambogic acid (n=10)
and 20 mg/kg Gambogic acid (n=10). Sham and AAA model groups were
gavaged with normal saline. The 5, 10 and 20 mg/kg Gambogic acid
groups were gavaged with 5, 10 or 20 mg/kg every 3 days Gambogic
acid, respectively, for 4 weeks.
Staining of toluidine blue
All mice were anesthetized with sodium pentobarbital
(50 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
sacrificed. The aortas of all mice were immediately separated,
washed in PBS, and perfused with 4% paraformaldehyde for 30 min.
Tissue samples were embedded into paraffin and cut into 5–6 µM
sections. All tissue sections were deparaffinized and hydrated in
several changes of ethanol and Tissue-Clear® (Sakura
Finetek Europe B.V., Flemingweg, The Netherlands). All tissue
sections were stained with toluidine blue working solution at room
temperature for 30 min and dehydrated with ethanol. Samples were
normalized to aortic vessel wall area (mm2) and total
numbers per aorta since were calculated in numbers.
ELISA kits
All mice were anesthetized with sodium pentobarbital
(50 mg/kg) and sacrificed. Aortic tissues samples (50 mg) were
homogenized with radioimmunoprecipitation assay (RIPA) lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) on ice for 15
min and centrifuged at 14,000 × g at 4°C for 10 min. Protein
content was measured by Bicinchoninic Acid (BCA) assay (Beyotime
Institute of Biotechnology), and equal protein (10 µg) was
incubated with corresponding ELISA kits. Tumor necrosis factor-α
(TNF-α; cat. no. H052), interleukin (IL)-1β (cat. no. H052), IL-6
(cat. no. H002), IL-18 (cat. no. H015), glutathione peroxidase
(GSH-PX; cat. no. A005), GSH (cat. no. A006-2), malondialdehyde
(MDA; cat. no. A003-1) and superoxide dismutase (SOD; cat. no.
A001-1-1) levels were measured using ELISA kits (Nanjing Jiancheng
Biological Engineering Institute, Nanjing, China). The absorbance
was measured in a spectrophotometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 450 nm.
Western blot analysis
All mice were anesthetized with sodium pentobarbital
(50 mg/kg) and sacrificed. Aortic tissues samples (50 mg) were
homogenized with RIPA lysis buffer on ice for 15 min and
centrifuged at 14,000 × g at 4°C for 10 min. Protein content was
measured using BCA assay. Equal protein (50 µg) was separated on
10% SDS-PAGE gels and blotted onto a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
non-fat powdered milk in TBS with Tween-20 for 1 h at 37°C, and
incubated with the following primary antibodies: transforming
growth factor (TGF)-β (cat. no. sc-7892; 1:500), MMP-2 (cat. no.
sc-10736; 1:500), MMP-9 (cat. no. sc-10737; 1:500), PI3K (cat. no.
sc-7174; 1:500), phosphorylated (p)-Akt (cat. no. sc-7985-R;
1:500), p-mechanistic target of rapamycin (mTOR; cat. no.
sc-101738; 1:500; all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), p-p70-S6 kinase 1 (cat. no. p-p70S6K1; 9204; 1:2,000; Cell
Signaling Technology, Inc.), NF-κB (cat. no. sc-109; 1:500) and
GAPDH (cat. no. sc-25778; 1:500; both Santa Cruz Biotechnology,
Inc.) overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (cat. no. sc-2030;
1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Membrane
was developed using Enhanced Chemiluminescence Prime Western
Blotting reagent (GE Healthcare Life Sciences, Little Chalfont, UK)
and calculated using GeneTools software using a Syngene gel
documentation system.
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS 19.0 (IBM, Corp. Armonk, NY, USA). Data were analyzed
using one-way analysis of variance followed by Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
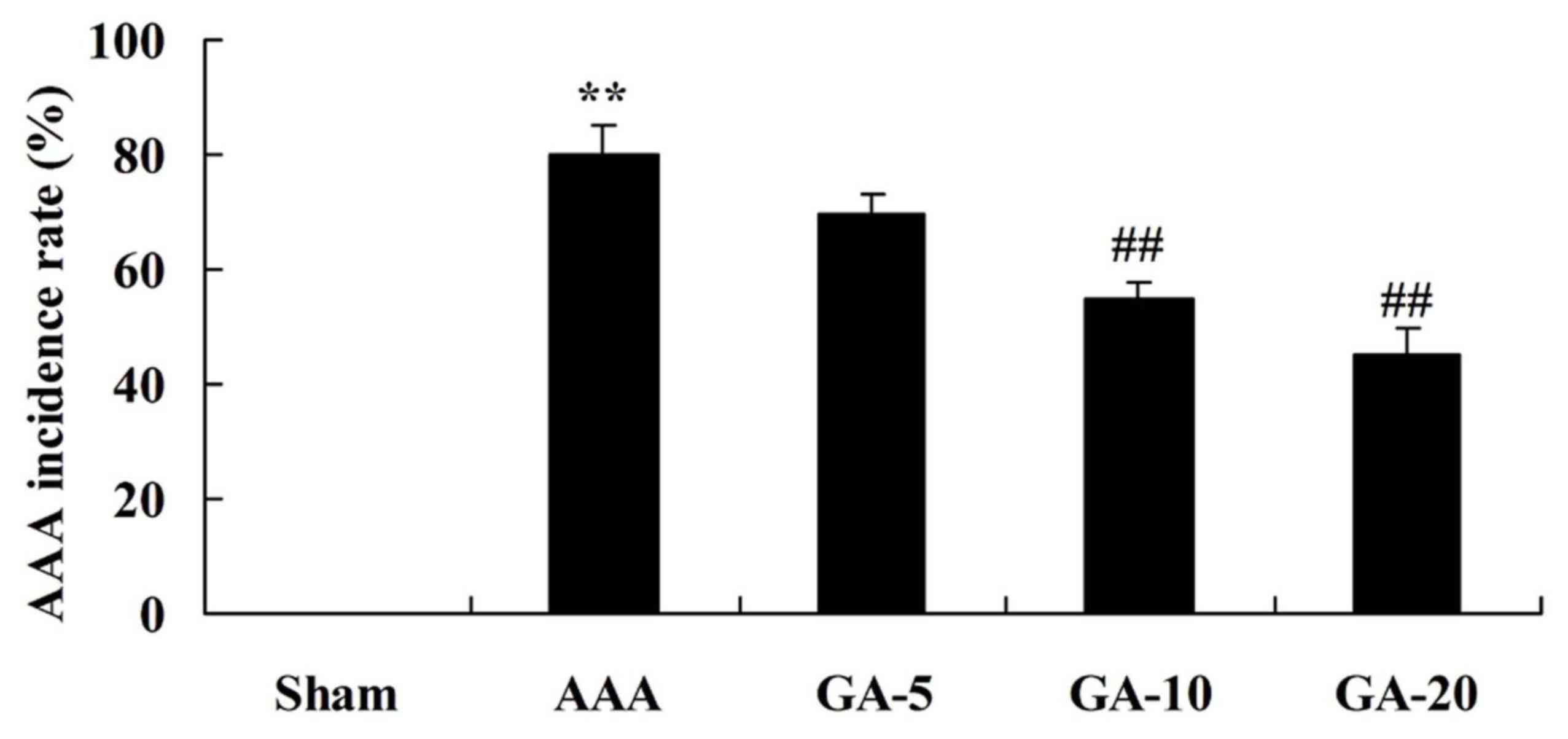
Effect of Gambogic acid on AAA
incidence rate in AAA mice
The chemical structure of Gambogic acid is presented
in Fig. 1. As demonstrated in
Fig. 2, the AAA incidence rate of
AAA model group was significantly higher than that of sham group.
Treatment with 5 and 10 mg/kg Gambogic acid significantly inhibited
AAA incidence rate of AAA mice, compared with the AAA model group
(Fig. 2).
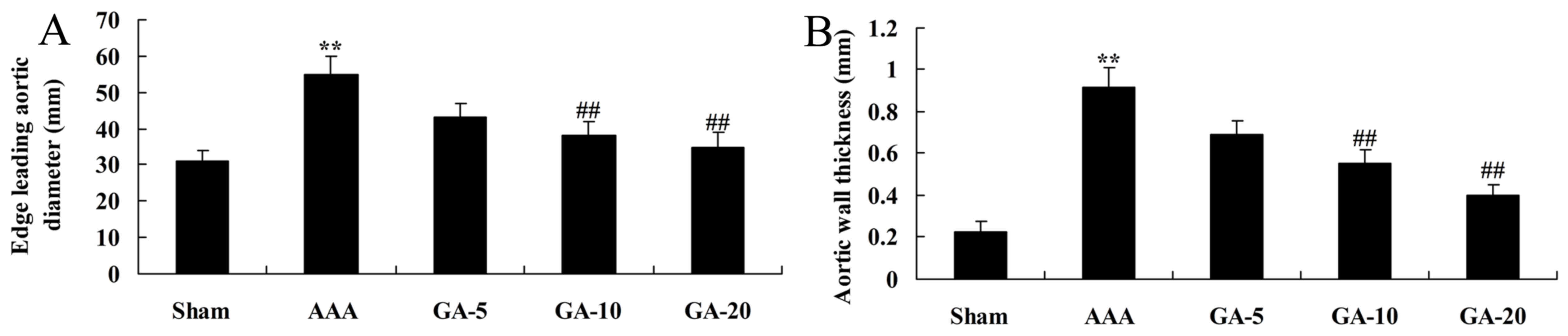
Effect of Gambogic acid on vascular
remodeling in AAA mice
As presented in Fig.
3, there was a significant increase of edge leading aortic
diameter and aortic wall thickness in AAA model mice, compared with
the sham control group. Furthermore, 5 and 10 mg/kg Gambogic acid
significantly reduced edge leading aortic diameter and aortic wall
thickness in AAA mice, compared with the AAA model group (Fig. 3).
Effect of Gambogic acid on
inflammation reactions in AAA mice
As demonstrated in Fig.
4, TNF-α, IL-1β, IL-6 and IL-18 contents of AAA model mice were
markedly higher than those of the sham control group. However, 5
and 10 mg/kg Gambogic acid treatment significantly decreased TNF-α,
IL-1β, IL-6 and IL-18 contents in AAA mice, compared with the AAA
model group (Fig. 4).
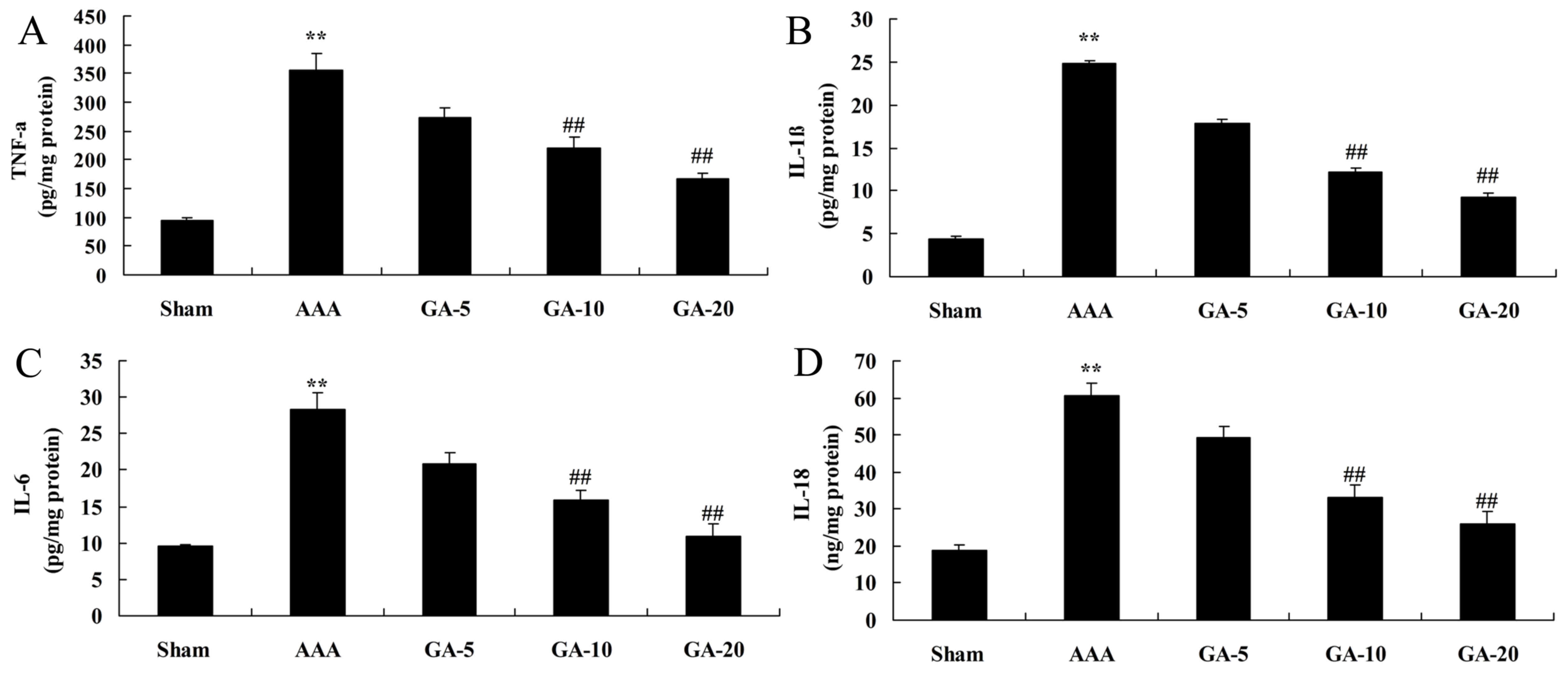 | Figure 4.Effect of Gambogic acid on
inflammation reactions in AAA mice. The effect of Gambogic acid on
(A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-18 levels in AAA mice.
Data are expressed as the mean ± standard deviation. **P<0.01
vs. sham group; ##P<0.01 vs. AAA model group. Sham,
sham group; AAA, AAA model group; GA-5, Gambogic acid treatment
group (5 mg/kg); GA-10, Gambogic acid treatment group (10 mg/kg);
GA-20, Gambogic acid treatment group (20 mg/kg); AAA, abdominal
aortic aneurysm; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
Effect of Gambogic acid on oxidative
stress in AAA mice
As presented in Fig.
5, GSH-PX, GSH and SOD levels were significantly inhibited, and
MDA levels were significantly promoted, in the AAA model group,
compared with the sham control group. Gambogic acid (5 and 10
mg/kg) significantly increased GSH-PX, GSH and SOD levels, and
reduced MDA levels in AAA mice, compared with the AAA model group
(Fig. 5).
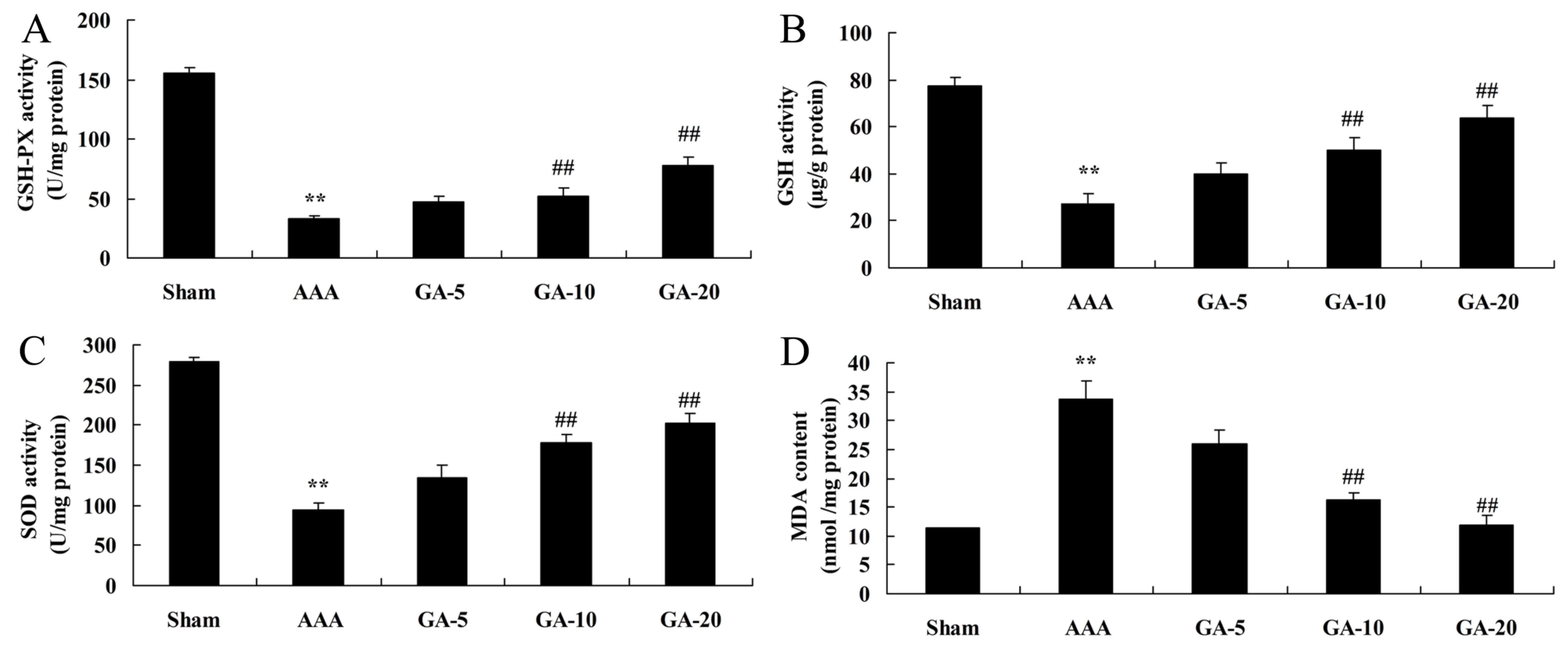 | Figure 5.Effect of Gambogic acid on oxidative
stress in AAA mice. The effect of Gambogic acid on (A) GSH-PX, (B)
GSH, (C) SOD and (D) MDA in AAA mice. Data are expressed as the
mean ± standard deviation. **P<0.01 vs. sham group;
##P<0.01 vs. AAA model group. Sham, sham group; AAA,
AAA model group; GA-5, Gambogic acid treatment group (5 mg/kg);
GA-10, Gambogic acid treatment group (10 mg/kg); GA-20, Gambogic
acid treatment group (20 mg/kg); AAA, abdominal aortic aneurysm;
GSH, glutathione; GSH-PX, glutathione peroxidase; SOD, superoxide
dismutase; MDA, malondialdehyde. |
Effect of Gambogic acid on TGF-β,
MMP-2, MMP-9 and NF-κB protein expression in AAA mice
Western blotting demonstrated that TGF-β, MMP-2,
MMP-9 and NF-κB protein expression in AAA model mice was
significantly increased, compared with the sham control group
(Fig. 6). However, compared with
the AAA model groups, 5 and 10 mg/kg Gambogic acid treatment
significantly suppressed TGF-β, MMP-2, MMP-9 and NF-κB protein
expression in AAA mice (Fig.
6).
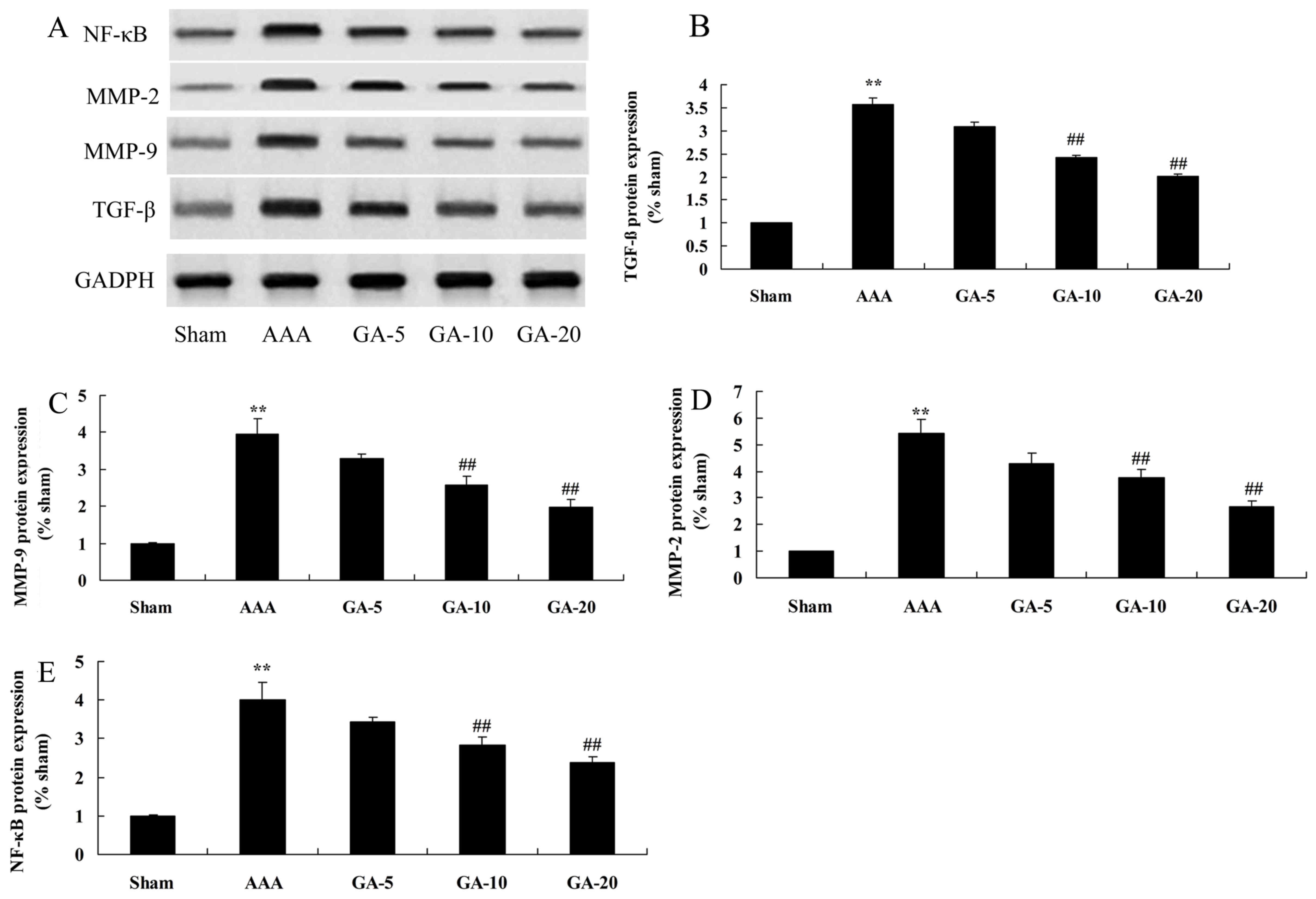 | Figure 6.Effect of Gambogic acid on TGF-β,
MMP-2, MMP-9 and NF-κB protein expression in AAA mice. Data are
expressed as the mean ± standard deviation. **P<0.01 vs. sham
group; ##P<0.01 vs. AAA model group. (A)
Representative western blot images. Quantification of (B) TGF-β,
(C) MMP-9, (D) MMP-2 and (E) NF-κB protein expression levels. Sham,
sham group; AAA, AAA model group; GA-5, Gambogic acid treatment
group (5 mg/kg); GA-10, Gambogic acid treatment group (10 mg/kg);
GA-20, Gambogic acid treatment group (20 mg/kg); AAA, abdominal
aortic aneurysm; TGF-β, transforming growth factor-β; MMP, matrix
metalloproteinase; NF-κB, nuclear factor-κB. |
Effect of Gambogic acid on PI3K,
p-Akt, p-mTOR and p-p70S6K1 protein expression in AAA mice
Western blotting was performed to elucidate the
potential role of PI3K, p-Akt, p-mTOR and p-p70S6K1 in AAA mice
treated by Gambogic acid. As presented in Fig. 7, PI3K, p-Akt, p-mTOR and p-p70S6K1
protein expression were significantly increased in AAA mice
compared with the sham control group. Gambogic acid treatment
significantly induced PI3K, p-Akt, p-mTOR and p-p70S6K1 protein
expression in AAA mice, compared with the AAA model group (Fig. 7).
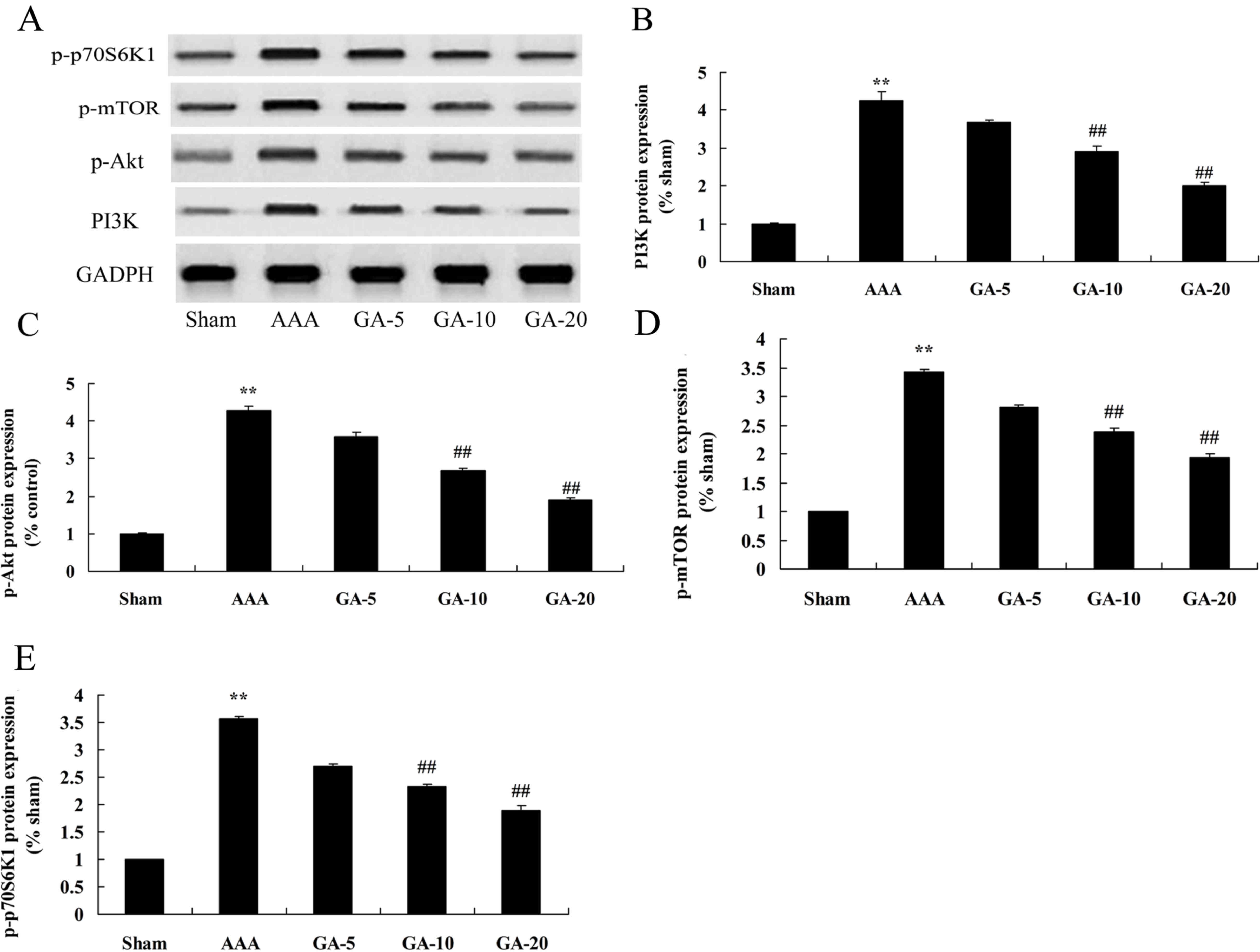 | Figure 7.Effect of Gambogic acid on PI3K,
p-Akt, p-mTOR, and p-p70S6K1 protein expression in AAA mice. (A)
Representative western blot images. Quantification of (B) PI3K, (C)
p-Akt, (D) p-mTOR and (E) p-p70S6K1 protein expression levels.
**P<0.01 vs. sham group; ##P<0.01 vs. AAA model
group. Sham, sham group; AAA, AAA model group; GA-5, Gambogic acid
treatment group (5 mg/kg); GA-10, Gambogic acid treatment group (10
mg/kg); GA-20, Gambogic acid treatment group (20 mg/kg); AAA,
abdominal aortic aneurysm; p-, phosphorylated; mTOR, mechanistic
target of rapamycin; Akt, protein kinase B, PI3K,
phosphatidylinositol 3-kinase. |
Discussion
AAA is one of the most dangerous vascular
degenerative diseases in vascular surgery. In the most serious
cases, as the weak artery walls cannot withstand the impact of
blood flow, AAA will lead to the rupture of walls of aneurysm,
causing sudden mortality (16).
With the aging population of China, the incidence of AAA is
increasing year by year, and has become one of the diseases that
threatens the life and health of many people (17). With the deepening of the
understanding towards AAA and the development of imaging
examination approaches, the diagnosis rate of the disease has been
greatly improved, but its specific pathogenesis remains unknown
(18). At present, people have
demonstrated that the incidence of AAA is associated smoking, sex,
oxidative stress, matrix proteins and blood lipids (19). The latest research has revealed
that the inflammatory reaction serves an important role in the
incidence and development of AAA (20). In this study, it was demonstrated
that gambogic acid significantly inhibited the rate of AAA
incidence, and reduced edge leading aortic diameter and aortic wall
thickness in AAA mice. The results demonstrated that gambogic acid
had an obvious improving effect on AAA.
In addition to the above factors, the incidence of
AAA is also closely associated with the inflammatory reaction
(21). According to current
research, AAA is a chronic inflammatory disease, which is
characterized by continuous arterial dilatation mainly caused by
the invasion of inflammatory cells and the destruction of
intermediate elastic protein matrix (22). Numerous inflammation-associated
factors are closely associated with the pathogenesis of AAA
(22). In the present study, it
was demonstrated that Gambogic acid treatment significantly
decreased TNF-α, IL-1β, IL-6 and IL-18 contents, increased GSH-PX,
GSH and SOD levels, and reduced MDA levels in AAA mice. Wen et
al (23) suggested that
Gambogic acid exhibits anti-psoriatic efficacy through inhibition
inflammation.
In the case of the TGF-β neutralizing antibody, the
T lymphocyte deletion signal transducer as well as signal
transducer and activator of transcription 3 also significantly
promote the incidence of AAA induced by AngII (24). In a previous study, when AngII was
used to induce AAA in C57 or low density lipoprotein
receptor-deficient mice, the activity of TGF-β was inhibited,
leading to the necrosis of smooth muscular cells, degradation of
elastin, exacerbation of intravascular inflammation in mice, thus
eventually worsening AAA (24).
Similarly, as a calcineurin immune-suppressing drug, cyclosporin-A
can promote the transcription of TGF-β1 and activate latent TGF-β1,
thereby alleviating AAA induced by elastase or calcium chloride
infusion; on the contrary, the TGF-β antibody offsets the effect of
cyclosporin-A in the treatment of AAA, suggesting that TGF-β serves
an important role in the incidence and development of AAA (25). In the present study, the results
suggested that Gambogic acid treatment significantly suppressed
TGF-β protein expression in AAA mice. Qu et al (26) observed that Gambogic acid prevented
pulmonary fibrosis by suppressing the TGF-β1/Smad3 signaling
pathway.
MMP is the major protease that causes the
degradation of extracellular matrix of arterial walls. Matrix
metalloproteinases are a series of homologous zinc- and
calcium-dependent proteases, which exist in the form of inactive
zymogen (27). The extracellular
matrix, including elastic fibers, collagen, laminin and
fibronectin, can be degraded through the cutting and activation of
N2 end by enzyme, and has been recognized to serve a very important
role in the incidence and development of aortic aneurysm (28). Most members of the MMP family serve
important role in this process, in which MMP-2 and MMP-9 are
especially important (29).
Studies on arterial medial smooth muscle cells have demonstrated
that normal arterial smooth muscle cells are the main components of
artery intima media, which are not only associated with the
diastolic and systolic function of arterial walls, but also
regulate the synthesis and repair of extracellular matrix
components, such as elastins and collagens (27,29).
They also demonstrated that Gambogic acid significantly inhibits
MMP-2 and MMP-9 protein expression in AAA mice. Qi et al
(30) indicated that Gambogic acid
induced suppression of MDA-MB-435 human breast carcinoma cell lung
metastasis through mediation of MMP-2/9 expression inhibition.
Regarding the association between PI3K and tumors,
disorder of the PI3K-Akt signaling pathway has been demonstrated to
lead to a variety of human cancers, including lung cancer,
nasopharyngeal cancer, liver cancer, gastrointestinal cancer,
breast cancer, ovarian cancer, renal cancer, prostate cancer,
lymphoma, malignant glioma and medulloblastoma (31). The association between PI3K and
non-tumor diseases, such as liver fibrosis, Alzheimer's disease,
diabetes and cardiovascular disease has also attracted significant
attention. It was demonstrated that selectively inhibiting the
PI3K-Akt signaling pathway can promote the autophagy of
macrophages, reduce the infiltration of plaque macrophages, and
significantly alleviate the inflammatory response, thus improving
atherosclerotic plaque (32). PI3K
also contributes to the progression of atherosclerosis through
affecting vascular endothelial cells, using a variety of signals
can be transduced and regulated through the PI3K-Akt signaling
pathway. Regulation of this pathway can directly or indirectly
promote the pathological progression of atherosclerosis (9). In the present study, it was observed
that Gambogic acid treatment significantly induced PI3K, p-Akt,
p-mTOR and p-p70S6K1 protein expression in AAA mice. Wang et
al identified that Gambogic acid suppresses the activity of
multiple myeloma cells through the PI3K-Akt signaling pathway
(33).
Normally, NF-κB exists in the cytoplasm and binds to
inhibitive protein IκB to stay in an inactive state (34). When cells are stimulated, IκB
kinase complex (IKK) will be activated and phosphorylate IκB to
dissociate IκB with NF-κB, then free NF-κB will quickly transfer
into nucleus and bind to the target gene sequence specifically,
thus regulating gene expression associated with various major
pathophysiological reactions such as proliferation,
differentiation, metastasis and apoptosis of cells, including the
secretion of extracellular matrix degrading enzymes such as MMP and
urokinase (35). In our
experiments, Gambogic acid significantly suppressed NF-κB protein
expression in AAA mice. Liu et al (36) reported that GA induced apoptosis
via suppression of NF-κB pathway of esophageal squamous cell
carcinoma cells.
In conclusion, the results of the present study
indicated that Gambogic acid prevents AngII-induced AAA incidence
rate, edge leading aortic diameter and aortic wall thickness in AAA
mice. These data support that Gambogic acid decreased the levels of
proinflammatory cytokines, oxidative stress, and TGF-β, MMP-2,
MMP-9 protein expression in AAA mice through the PI3K/Akt/mTOR and
NF-κB signaling pathways. Furthermore, Gambogic acid treatment may
provide a promising approach for the prevention of AAA in the
future.
Acknowledgements
The authors would like to thank Dr Wang Qingshan for
his help writing the manuscript.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
PS designed the experiment; QL, PS and HL performed
the experiment. PS analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethical
and Welfare Committee of The First Hospital of Qiqihar City
(Qiqihar, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Zhang Y, Zhang D, Wang F, Xu D, Guo Y and
Cui W: Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497)
as novel non-invasive biomarkers for detection of cervical cancer.
Sci Rep. 5:179422015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng S, Gao D, Gao C, Wei P, Niu M and
Shuai C: MicroRNAs regulate signaling pathways in osteogenic
differentiation of mesenchymal stem cells (review). Mol Med Rep.
14:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang M, Liang L, Li L, Han K, Li Q, Peng
Y, Peng X and Zeng K: Increased miR-424-5p expression in peripheral
blood mononuclear cells from patients with pemphigus. Mol Med Rep.
15:3479–3484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svoboda M, Riha J, Wlcek K, Jaeger W and
Thalhammer T: Organic anion transporting polypeptides (OATPs):
Regulation of expression and function. Curr Drug Metab. 12:139–153.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer Zu, Schwabedissen HE, Böttcher K,
Chaudhry A, Kroemer HK, Schuetz EG and Kim RB: Liver X receptor α
and farnesoid X receptor are major transcriptional regulators of
OATP1B1. Hepatology. 52:1797–1807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao R, Yan F, Zeng Z, Farhan M, Little P,
Quirion R, Srivastava LK and Zheng W: Amiodarone-induced retinal
neuronal cell apoptosis attenuated by IGF-1 via counter regulation
of the PI3k/Akt/FoxO3a pathway. Mol Neurobiol. 54:6931–6943. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu W, Bijur GN, Styles NA and Li X:
Regulation of FOXO3a by brain-derived neurotrophic factor in
differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol
Brain Res. 126:45–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol protects PC12
cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoo HI, Kim BK and Yoon SK:
MicroRNA-330-5p negatively regulates ITGA5 expression in human
colorectal cancer. Oncol Rep. 36:3023–3029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian Y, Guo S, Wu X, Ma L and Zhao X:
Minocycline alleviates sevoflurane-induced cognitive impairment in
aged rats. Cell Mol Neurobiol. 35:585–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang ZJ, Wang YW, Li CL, Ma LQ and Zhao X:
Pre-treatment with a Xingnaojing preparation ameliorates
sevoflurane-induced neuroapoptosis in the infant rat striatum. Mol
Med Rep. 11:1615–1622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chmielarz P, Konovalova J, Najam SS, Alter
H, Piepponen TP, Erfle H, Sonntag KC, Schütz G, Vinnikov IA and
Domanskyi A: Dicer and microRNAs protect adult dopamine neurons.
Cell Death Dis. 8:e28132017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Z, Wang X, Bhardwaj SK, Zhou X,
Little PJ, Quirion R, Srivastava LK and Zheng W: The atypical
antipsychotic agent, clozapine, protects against
corticosterone-induced death of PC12 cells by regulating the
Akt/FoxO3a signaling pathway. Mol Neurobiol. 54:3395–3406. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HY, Kwon HY, Ha Thi HT, Lee HJ, Kim
GI, Hahm KB and Hong S: MicroRNA-132 and microRNA-223 control
positive feedback circuit by regulating FOXO3a in inflammatory
bowel disease. J Gastroenterol Hepatol. 31:1727–1735. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lian R, Lu B, Jiao L, Li S, Wang H, Miao W
and Yu W: MiR-132 plays an oncogenic role in laryngeal squamous
cell carcinoma by targeting FOXO1 and activating the PI3K/AKT
pathway. Eur J Pharmacol. 792:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Breitkopf K, Nagy LE, Beier JI, Mueller S,
Weng H and Dooley S: Current experimental perspectives on the
clinical progression of alcoholic liver disease. Alcohol Clin Exp
Res. 33:1647–1655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zubillaga-Guerrero MI, Alarcón-Romero Ldel
C, Illades-Aguiar B, Flores-Alfaro E, Bermúdez-Morales VH, Deas J
and Peralta-Zaragoza O: MicroRNA miR-16-1 regulates CCNE1 (cyclin
E1) gene expression in human cervical cancer cells. Int J Clin Exp
Med. 8:15999–16006. 2015.PubMed/NCBI
|
|
18
|
Ziaei S and Halaby R: Immunosuppressive,
anti-inflammatory and anti-cancer properties of triptolide: A mini
review. Avicenna J Phytomed. 6:149–164. 2016.PubMed/NCBI
|
|
19
|
Wang F, Yin J, Lu Z, Zhang G, Li J, Xing
T, Zhuang S and Wang N: Limb ischemic preconditioning protects
against contrast-induced nephropathy via renalase. EBioMedicine.
9:356–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu SJ, Yin CX, Ding MC, Xia SY, Shen QM
and Wu JD: Berberine suppresses in vitro migration of human aortic
smooth muscle cells through the inhibitions of MMP-2/9, u-PA, AP-1,
and NF-κB. BMB Rep. 47:388–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Zhang G, Lu Z, Geurts AM, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Li CJ, Wan Y, Smith P, Shang G and
Cui Q: Antioxidative fullerol promotes osteogenesis of human
adipose-derived stem cells. Int J Nanomedicine. 9:4023–4031. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen J, Pei H, Wang X, et al: Gambogic acid
exhibits anti-psoriatic efficacy through inhibition of angiogenesis
and inflammation. J Dermatol Sci. 74:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian Y, Wu X, Guo S, Ma L, Huang W and
Zhao X: Minocycline attenuates sevoflurane-induced cell injury via
activation of Nrf2. Int J Mol Med. 39:869–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou ZB, Yang XY, Tang Y, Zhou X, Zhou LH
and Feng X: Subclinical concentrations of sevoflurane reduce
oxidative stress but do not prevent hippocampal apoptosis. Mol Med
Rep. 14:721–727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu Y, Zhang G, Ji Y, Zhua H, Lv C and
Jiang W: Protective role of gambogic acid in experimental pulmonary
fibrosis in vitro and in vivo. Phytomedicine. 23:350–358. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu G, Wang X, Wu S and Li Q: Involvement
of activation of PI3K/Akt pathway in the protective effects of
puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell
death. Neurochem Int. 60:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang J, Pan J, Chen F, Zheng L, Chen Y,
Zhang S and Feng W: L-3-n-butylphthalide improves cognitive
impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J
Clin Exp Med. 7:1706–1713. 2014.PubMed/NCBI
|
|
29
|
Hossini AM, Quast AS, Plötz M, Grauel K,
Exner T, Küchler J, Stachelscheid H, Eberle J, Rabien A,
Makrantonaki E and Zouboulis CC: PI3K/AKT signaling pathway is
essential for survival of induced pluripotent stem cells. PLoS One.
11:e01547702016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi Q, Gu H, Yang Y, et al: Involvement of
matrix metalloproteinase 2 and 9 in gambogic acid induced
suppression of MDA-MB-435 human breast carcinoma cell lung
metastasis. J Mol Med (Berl). 86:1367–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng Y, Wang W, Kang J, Wang X and Sun L:
Role of the PI3K/AKT signalling pathway in apoptotic cell death in
the cerebral cortex of streptozotocin-induced diabetic rats. Exp
Ther Med. 13:2417–2422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu
R, Liu P, Wang Y, Guo Q and Chen B: Gambogic acid suppresses
hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial
growth factor expression via inhibiting phosphatidylinositol
3-kinase/Akt/mammalian target protein of rapamycin pathway in
multiple myeloma cells. Cancer Sci. 105:1063–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yufune S, Satoh Y, Akai R, Yoshinaga Y,
Kobayashi Y, Endo S and Kazama T: Suppression of ERK
phosphorylation through oxidative stress is involved in the
mechanism underlying sevoflurane-induced toxicity in the developing
brain. Sci Rep. 6:218592016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo XQ, Cao YL, Hao F, Yan ZR, Wang ML and
Liu XW: Tangeretin alters neuronal apoptosis and ameliorates the
severity of seizures in experimental epilepsy-induced rats by
modulating apoptotic protein expressions, regulating matrix
metalloproteinases, and activating the PI3K/Akt cell survival
pathway. Adv Med Sci. 62:246–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WY, Wu XU, Liao CQ, Shen J and Li J:
Apoptotic effect of gambogic acid in esophageal squamous cell
carcinoma cells via suppression of the NF-kappaB pathway. Oncol
Lett. 11:3681–3685. 2016. View Article : Google Scholar : PubMed/NCBI
|