Introduction
Lung cancer is one of the most common type of
life-threatening malignancies worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for 80–85% of cases in lung cancer (2). Surgery-oriented multimodality therapy
is the most effective method for the treatment of lung cancer
(3); however, there are no
symptoms in most patients with lung cancer at early-stage of this
disease, and majority of the patients are diagnosed at the moderate
to advanced stage, which has deprived them from the opportunity of
radical surgery (4). Recently,
molecular therapy against the targeting of genes has become a
promising treatment of tumors (5).
In summary, investigating the mechanisms underlying lung cancer and
identifying the potential molecular targets are important in the
treatment of this disease.
MicroRNAs (miRNAs) are a class of endogenous and
highly conserved single-stranded non-coding small RNAs consisting
of 18–22 nucleotides. MiRNAs can bind with the 3′-untranslated
region (UTR) of their downstream target gene, consequently
degrading mRNA or inhibiting translation at the post-transcription
level. Each miRNA regulates numerous target genes; while a specific
target mRNA can also be regulated by several miRNAs at the same
time (6). They function in a
complex regulatory network and act as oncogene or tumor suppressor
during the development of tumors (7,8).
Recent research has revealed that miR-495 functions as tumor
suppressor gene in the majority of solid tumors, including gastric
cancer (9), esophageal cancer
(10), colorectal cancer (11), renal cell carcinoma (12) and bladder cancer (13); however, the underlying mechanism
remains unknown. To investigate the regulatory function of miR-495
in NSCLC, the expression levels of miR-495 in NSCLC and
paracarcinoma tissues, as well as in NSCLC cells and normal
pulmonary bronchial epithelial cells were compared in the present
study. In addition, the association between the expression of
miR-495 and the clinicopathological features of patients with NSCLC
was evaluated. A549 lung cancer cells were transfected with miR-495
mimics; the effects of miR-495 overexpression on the proliferation
of A549 cells were revealed. Additionally, whether high mobility
group A2 (HMGA2) is a potential target gene of miR-495 was
investigated via bioinformatics analysis in the present study.
Materials and methods
Patient samples
A total of 122 pairs of NSCLC and matched
paracarcinoma samples (≥5 cm from tumor margin; 80 males and 42
females; aged 45–77 years old) were obtained via surgery in Weihai
Central Hospital (Weihai, China) from June 2014 to June 2016. The
tissues were snap-frozen in liquid nitrogen within 30 min following
surgery and stored at −80°C until further use. None of the patients
received chemotherapy or radiotherapy prior to surgery. In
addition, no malignancy in other organs was reported. miR-495
expression was categorized into low and high groups according to
the mean value. All pathological sections were examined and
verified by two senior pathologists. The clinical features of
patients were presented in Table
I. TNM staging was carried out according to the 7th edition of
the Union for International Cancer Control (UICC) staging Committee
TNM staging standards (14).
Informed consent was obtained from all patients. The present study
was approved by the Medical Ethics Committee of Weihai Central
Hospital.
 | Table I.Associations between miR-495
expression and the clinicopathological characteristics of patients
with non-small cell lung cancer. |
Table I.
Associations between miR-495
expression and the clinicopathological characteristics of patients
with non-small cell lung cancer.
|
|
| miR-495 [n
(%)] |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | n | Low expression | High
expression | P-value |
|---|
| Gender |
|
|
| 0.316 |
|
Male | 80 | 40 (50.00) | 40 (50.00) |
|
|
Female | 42 | 25 (59.52) | 17 (40.48) |
|
| Age (year) |
|
|
| 0.179 |
|
>60 | 72 | 42 (58.33) | 30 (41.67) |
|
|
≤60 | 50 | 23 (46.00) | 27 (54.00) |
|
| Tumor size |
|
|
| 0.312 |
| >5
cm | 53 | 31 (58.49) | 22 (41.51) |
|
| ≤5
cm | 69 | 34 (49.28) | 35 (50.72) |
|
| Tumor
differentiation |
|
|
| 0.008a |
| Well +
Moderate | 77 | 34 (44.16) | 43 (55.84) |
|
|
Poorly | 45 | 31 (68.89) | 14 (31.11) |
|
| TNM stage |
|
|
| 0.024a |
| I +
II | 84 | 39 (46.43) | 45 (53.57) |
|
|
III | 38 | 26 (68.42) | 12 (31.58) |
|
| Smoking |
|
|
| 0.298 |
|
Yes | 51 | 30 (58.82) | 21 (41.18) |
|
| No | 71 | 35 (49.30) | 36 (50.70) |
|
| Lymphatic
metastasis |
|
|
| 0.005a |
|
Yes | 72 | 46 (63.89) | 26 (36.11) |
|
| No | 50 | 19 (38.00) | 31 (62.00) |
|
| Pathology
classification |
|
|
| 0.081 |
|
Squamous cell carcinoma | 54 | 24 (44.44) | 30 (55.56) |
|
|
Adenocarcinoma | 68 | 41 (60.29) | 27 (39.71) |
|
Cell culture
Human NSCLC cell lines A549, H460, H1650, H520 and
SK-MES-1, as well as the normal human pulmonary bronchial
epithelial cell line 16HBE were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Roswell Park Memorial Institute 1640 medium (RPMI-1640) (HyClone;
GE Healthcare, Chicago, IL, USA) containing 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified atmosphere of 5% CO2. Cell lines
with the highest relative expression of miR-495 were selected for
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from tissues or cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocols.
The purity of the extracted RNA was determined using an ultraviolet
spectrophotometer. cDNA was synthesized using a PrimeScript RT
reagent kit (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocols. The primers of miR-495, HMGA2 and the
internal reference U6, GAPDH were obtained from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). The primer sequences used were as
follows: miR-495 forward, 5′-GGAGCTTGAGCGGATGGCGA-3′ and reverse,
5′-TTAGCGGAGCGGGAGGGCGA-3′; U6 forward, 5′-CATCACCATCAGGAGAGTCG-3′
and reverse, 5′-TGACGCTTGCCCACAGCCTT3′; HMGA2 forward,
5′-CCAACCGGTGAGCCCTCT-3′ and reverse, 5′-TTGAGCTGCTTTAGAGGGAC-3′;
GAPDH forward, 5′-CGCTGAGTACGTCGTGGAGT-3′ and reverse,
5′-GTCGCTGTTGAAGTCAGAGGAG-3′. qPCR was performed using a
GoTaq® RT-qPCR system (Promega Corporation) according to
the manufacturer's protocols. The reaction conditions were as
follows: 2 min at 94°C, 20 sec at 94°C and 30 sec at 60°C for 40
cycles. The relative expression of miR-495 and HMGA2 mRNA were
calculated using the 2−ΔΔCq method (15). All assays were performed in
triplicate.
Cell transfection
A549 cells at the logarithmic phase were seeded on
the 6-well plate (1.5×106 cells/well) and cultured
overnight in RPMI-1640 without antibiotics.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for transfection according to the
manufacturer's protocols. Following transfection for 24 h, the
cells were collected for subsequent experiments. The cells were
divided into 3 groups: i) Negative control (NC) group, transfection
with mimics-NC plasmid (50 nM, sense: 5′-UUCUCCGAACGUGUCACGUTT-3′;
anti-sense: 5′-ACGUGACACGUUCGGAATT-3′); ii) miR-495 mimics group,
transfection with miR-495 mimics plasmid (50 nM, sense:
5′-AAACAAACAUGGUGCACUUCUU-3′; anti-sense:
5′-GAAGUGCACCAUGUUUGUUUUU-3′) and iii) miR-495 mimics + HMGA2
group, co-transfection with miR-495 mimics and HMGA2-expressing
plasmids. The miR-495 mimics, mimics-NC and HMGA2 expression
plasmids were synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China).
Cell counting kit-8 (CCK-8) assay
Cells were harvested following transfection and
inoculated with 10 µl CCK-8 solution (Blue Skies Biotech.
Inc.; LakePharma., Inc., Worcester, MA, USA) for 24, 48, 72 and 96
h according to the manufacturer's protocols. Following an
incubation at 37°C for an additional 2 h in the dark, the
absorbance at 450 nm was measured using a plate reader.
Bioinformatic prediction and
dual-luciferase reporter assay
HMGA2 was identified as the potential direct target
of miR-495 via the bioinformatics database miRanda version August
2010 Release (available at: www.microrna.org). The wild-type (wt)
pmirGLO-HMGA2-wtUTR and mutant (mut) pmirGLO-HMGA2-mtUTR plasmids
were synthesized by Shanghai GenePharma Co., Ltd. A549 cells
(5×105 cells/well) were plated onto 6-well plates. Then,
100 ng pmirGLO-HMGA2-wtUTR or pmirGLO-HMGA2-mtUTR were used to
co-transfect A549 cells with 50 nM miR-495 mimics or mimics-NC
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were harvested 24 h post-transfection.
Luciferase activity was evaluated 48 h post-transfection using a
Dual-Luciferase® Reporter Assay (Promega Corporation),
and the levels of firefly luciferase activity were normalized to
that of Renilla luciferase.
Western blotting
Transfected A549 cells were collected and washed
with PBS for three times. The total protein of cells was extracted
with the protein lysis buffer (Cell Signaling Technology Inc.,
Danvers, MA, USA), and the concentration of protein was determined
using a bicinchoninic acid assay. Protein samples (40 µg)
were separated on a 10% SDS-PAGE gel for 30 min and transferred to
polyvinylidene difluoride membranes. Then, the membranes were
blocked in tris-buffered-saline-Tween-20 (TBST; containing 0.1%
Tween-20) with 5% skimmed milk for 2 h at room temperature and
incubated with primary antibodies against HMGA2 (1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-130024) and
β-actin (1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA;
cat. no. sc-130065) at 4°C overnight with agitation. Following
washing, the membranes were incubated with goat anti-mouse
horseradish peroxidase-conjugated secondary antibody (1:2,000;
Santa Cruz Biotechnology, Inc.; sc-516102) at room temperature for
2 h. Signals were visualized using an enhanced chemiluminescence
luminescent reagent (cat. no. sw2030; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and were developed
using a gel imaging system. β-actin was used as the internal
reference. Bands were analyzed with Image J software (version
1.51j8; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
employed to perform statistical analysis. Data were presented as
the mean ± standard deviation and analyzed using a Student's t-test
or analysis of variance (ANOVA). A Student-Newman-Keuls test was
used as a post-hoc test following ANOVA. Enumeration data were
compared using a χ2 test. All experiments were repeated
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
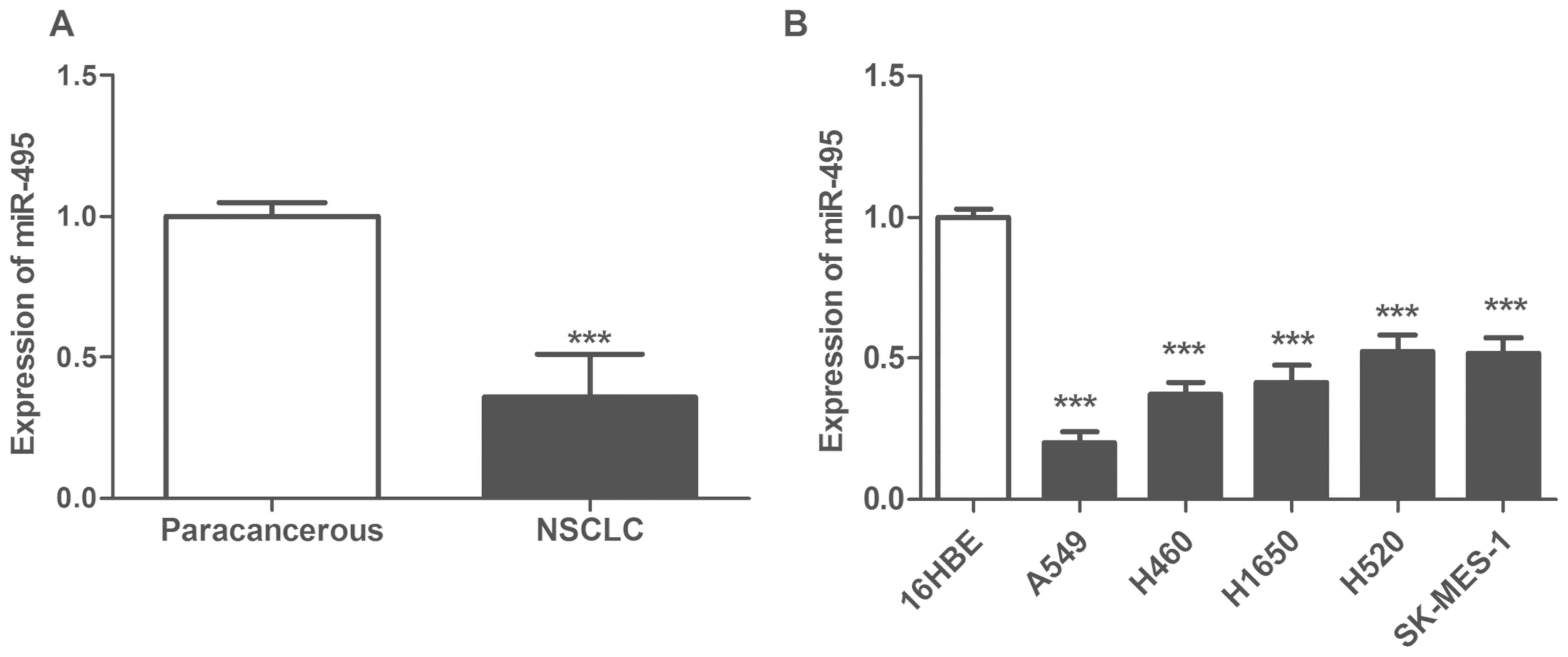
Expression of miR-495 is downregulated
in NSCLC tissues and cells
The expression levels of miR-495 were significantly
downregulated in NSCLC tissues compared with in matched
paracarcinoma tissues (P<0.05; Fig.
1A). Furthermore, the expression of miR-495 was also
significantly reduced in NSCLC cell lines A549, H460, H1650, H520
and SK-MES-1 compared with in the normal pulmonary bronchial
epithelial cell line 16HBE (P<0.05; Fig. 1B). In addition, the relative
expression levels of miR-495 in A549 cells were the lowest.
Therefore, A549 was selected for further cytological study to
ectopically overexpress miR-495.
Expression of miR-495 is associated
with the clinicopathological parameters of patients with NSCLC
The mean miR-495 expression levels in all patients
were used as the threshold to divide the patients into miR-495 high
and low expression groups. Downregulated expression of miR-495 was
associated with tumor differentiation, lymph node metastasis and
clinical stage (P<0.05; Table
I), but was not associated with age, sex, tumor size, smoking
and pathological classification (Table
I).
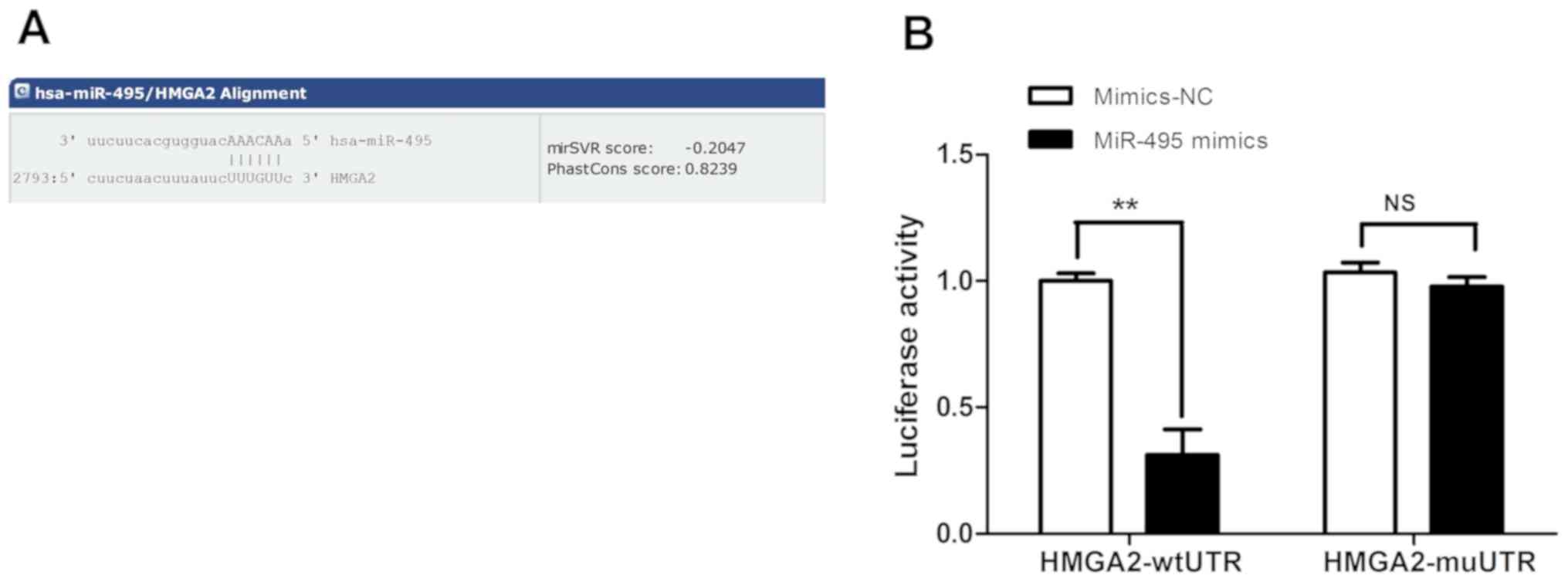
HMGA2 is a direct target gene of
miR-495
HMGA2 was predicted to be a potential direct target
gene of miR-495 using the target gene prediction database miRanda
and gene functional analysis. In addition, there were complementary
binding sites to the seed sequence of miR-495 in the 3′-UTR of
HMGA2 (Fig. 2A). To further
investigate whether HMGA2 was the direct regulatory target gene of
miR-495, A549 cells were co-transfected with miR-495 mimics or
mimics-NC, and pmirGLO-HMGA2-wtUTR or pmirGLO-HMGA2-mtUTR. The
results of the dual-luciferase reporter assay revealed that the
luciferase activity of cells in the miR-495mimics +
pmirGLO-HMGA2-wtUTR group was significantly reduced compared with
that of the mimics-NC + pmirGLO-HMGA2-wtUTR group (Fig. 2B). Additionally, no significant
difference was observed between cells in miR-495mimics +
pmirGLO-HMGA2-mt group and the mimics-NC + pmirGLO-HMGA2-mtUTR
group (P>0.05; Fig. 2B). These
results indicated that miR-495 could specifically bind to the
3′-UTR of HMGA2; however, this effect was not detected in the
presence of 3′-UTR-mutated HMGA2, suggesting that HMGA2 may be the
direct target of miR-495.
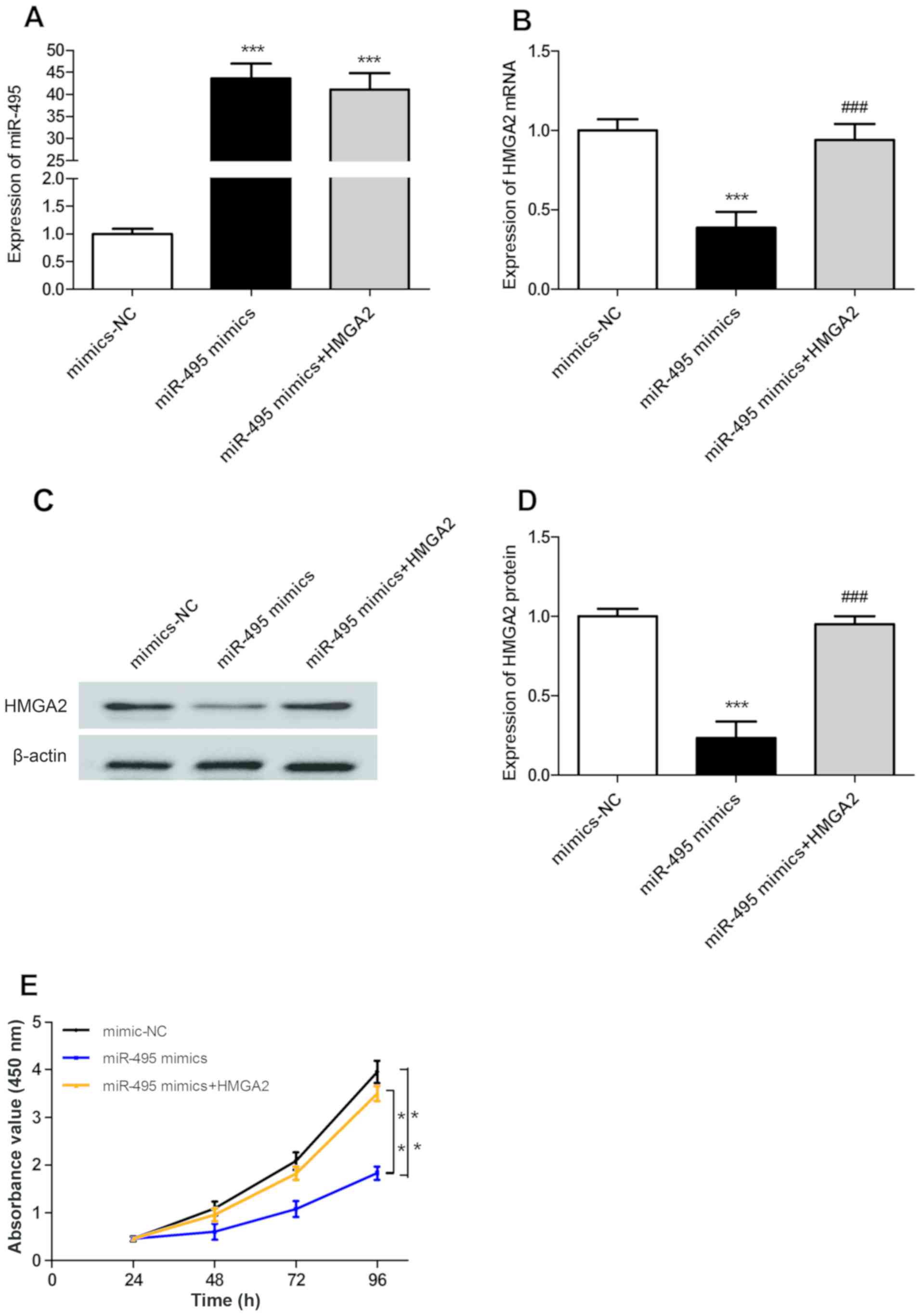
MiR-495 inhibits the proliferation of
A549 cells in a HMGA2-dependant manner
The expression levels of miR-495 in A549 cells in
the miR-495 mimics and miR-495 mimics + HMGA2 groups were
significantly upregulated compared with in the mimic-NC group
(P<0.001; Fig. 3A); however,
there was no significant difference in miR-495 expression between
miR-495 mimics and miR-495 mimics + HMGA2 groups (P>0.05;
Fig. 3A). The mRNA and protein
expression levels of HMGA2 in transfected A549 cells were evaluated
using RT-qPCR and western blotting. There were no significant
differences in the mRNA and protein expression levels of HMGA2
between mimic-NC and miR-495 mimics + HMGA2 groups (P>0.05;
Fig. 3B-D). However, the mRNA and
protein expression levels of HMGA2 in the miR-495 mimics group were
significantly downregulated compared with those in the mimic-NC
group (P<0.001; Fig. 3B-D).
Furthermore, the expression of HMGA2 in the miR-495 mimics + HMGA2
group was significantly upregulated compared with in the miR-495
mimics group (P<0.001; Fig.
3B-D). In addition, the results of the CCK-8 assay revealed
that upregulated expression of miR-495 could significantly inhibit
the proliferation of A549 cells compared with the control
(P<0.01), while HMGA2 overexpression could significantly
reversed this inhibition (P<0.01; Fig. 3E). The above results show that
miR-495 inhibits the proliferation of A549 cells in a
HMGA2-dependant manner.
Discussion
Lung cancer is a frequently-occurring malignancy,
and its initiation and development are complex processes involving
numerous genetic alterations (16); however, the molecular mechanisms
underlying this disease remain unclear. Recently, the roles of
miRNA in numerous diseases have attracted increasing attention with
advances in of epigenetic research (17). Aberrant expression of miRNA in the
majority of malignancies has been revealed; miRNAs serve an
indispensable role in the initiation and development of
malignancies (18,19).
MiR-495 is a novel miRNA that is located in
chromosome 14q32.31 (20). It
serves an important role in cell proliferation, apoptosis, and the
immune and inflammatory responses (21). It has been revealed that aberrant
expression of miR-495 was associated with tumor cell proliferation,
apoptosis and chemoresistance (22). In addition, miR-495 functioned as
tumor suppressor in the majority of solid tumors (9–13);
however, the expression of miR-495 was upregulated in some solid
tumors, including hepatocellular carcinoma (23) and breast cancer (24), and thus may be considered as an
oncogene. In the present study, the expression levels of miR-495 in
NSCLC and matched paracarcinoma tissues, as well as in human lung
cancer and normal human pulmonary bronchial epithelial cells were
compared. The results revealed that miR-495 expression in NSCLC
tissues and cells was significantly downregulated. In addition, to
investigate the effects of miR-495 on the proliferation of lung
cancer cells, miR-495 mimics were successfully transfected into
A549 cells. Thus, A549 cells overexpressing miR-495 were
established; overexpression of miR-495 significantly inhibited the
proliferation of A549 cells, indicating that miR-495 could function
as a tumor suppressor gene in NSCLC.
MiRNAs may regulate numerous target genes; however,
each target gene can also be regulated by several miRNAs. The role
of miRNA in tumors depends on its regulatory functions on
downstream target genes (25). Li
et al (9) reported that
miR-495 could inhibit the invasion and metastasis of gastric cancer
cells in a phosphatase of regenerating liver 3-dependent manner.
Mao et al (10) revealed
that miR-495 could suppress the invasion of esophageal cancer by
targeting protein kinase. Chu et al (26) reported that miR-495 could inhibit
the proliferation and metastasis of NSCLC cells in a metastasis
associated 1 family member 3-dependent manner. In the present
study, the potential target gene of miR-495 was predicted to be
HMGA2 using target gene prediction database miRanda and gene
functional analysis; there were complementary binding sites to the
seed sequence of miR-495 in the 3′-UTR of HMGA2. Additionally, a
dual-luciferase reporter gene assay revealed that miR-495 could
specifically bind to the 3′-UTR of HMGA2. Furthermore, the
expression levels of HMGA2 were further determined using RT-qPCR
and western blotting; upregulation of miR-495 significantly
downregulated HMGA2 expression. The findings of the present study
demonstrated that miR-495 inhibited the expression of HMGA2, thus
HMGA2 may be one of the direct regulatory target genes of
miR-495.
HMGA2 is a non-histone chromosomal protein and
member of the high mobility group (27). The role of HMGA2 in tumors has been
widely studied, and HMGA2 was reported to be a potential oncogene
(28). HMGA2 is abundantly
expressed in solid tumor tissues, including thyroid cancer
(29), ovarian cancer (30), prostate cancer (31), gallbladder adenocarcinoma (32), esophageal squamous carcinoma
(33), bladder cancer (34), NSCLC (35) and gastric cancer (36). In addition, HMGA2 binds to the
promoter of the cyclin gene, upregulating the expression of cyclin
and accelerating the G2/M stage of the cell cycle, and consequently
promoting tumorigenesis (37). E2F
transcription factor 1 (E2F1) is a member of the transcription
factor E2F family and regulates the progression of the cell cycle
from G1 to S phase (38). Fedele
et al (39) revealed that
overexpressed HMGA2 could bind to phosphorylated retinoblastoma
protein, consequently enhancing the activity of E2F1 and resulting
in the impaired proliferation of tumor cells. Cyclin A serves an
important role in promoting the mitosis and proliferation of cells;
it is an important regulatory protein of the cell cycle (40). HMGA2 activates the promoter of the
cyclin A gene to induce its expression, thereby disrupting the cell
cycle and promoting the occurrence of tumors (41). A recent study also demonstrated
that in human pituitary adenoma, HMGA2 directly associated with the
promoter of the cyclin B2 gene and upregulate its expression,
consequently accelerating the transition of G2 to M phase (42). Additionally, Morishita et al
(43) revealed that HMGA2 promoted
epithelial mesenchymal transition, activated transcription growth
factor β (TGF-β) receptor II, and activated the TGF-β signaling
pathway, which facilitated the invasion and metastasis of tumor
cells. It was reported that downregulated expression level of HMGA2
in NSCLC cells notably suppressed the proliferation of lung cancer
cells (44). In the present study,
A549 cells were co-transfected with miR-495 mimics and
HMGA2-overexpressing plasmids. The results revealed that the
expression levels of HMGA2 in the miR-495 mimics + HMGA2 group were
significantly upregulated compared with the control, indicating
that the inhibitory effects of miR-495 on HMGA2 expression was
successfully reversed. Furthermore, the results of CCK-8 assay
suggested that HMGA2 overexpression significantly reversed the
inhibition of miR-495 mimics on the proliferation of A549 cells,
indicating that miR-495 suppressed the proliferation of A549 cells
in a HMGA2-dependent manner.
In summary, the present study revealed that miR-495
expression was downregulated in NSCLC tissues and cells. Therefore,
HMGA2 may be one of the direct target genes of miR-495. In
addition, miR-495 could inhibit the expression of HMGA2,
consequently suppressing the proliferation of lung cancer cells.
The results of the present study may provide insight into the
mechanisms underlying the inhibition of lung cancer cell
proliferation. In conclusion, miR-495 may be considered as a
potential target in the treatment of lung cancer via gene therapy
in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the experiments. JS, YQ and TS performed
the experiments. JS and HW analyzed the data. JS wrote the
manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Weihai Central Hospital (Weihai, China). Informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ridge CA, Mcerlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakelee H, Kelly K and Edelman MJ: 50
Years of progress in the systemic therapy of non-small cell lung
cancer. Am Soc Clin Oncol Educ Book. 177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhan P, Qian Q and Yu LK: Prognostic value
of COX-2 expression in patients with non-small cell lung cancer: A
systematic review and meta-analysis. J Thorac Dis. 5:40–47.
2013.PubMed/NCBI
|
|
5
|
Sheng M, Zhao Y, Wang F, Li S, Wang X,
Shou T, Luo Y and Tang W: Targeted drugs for unselected patients
with advanced non-small-cell lung cancer: A network meta-analysis.
J Thorac Dis. 8:98–115. 2016.PubMed/NCBI
|
|
6
|
Acunzo M and Croce CM: MicroRNA in cancer
and cachexia-a mini-review. J Infect Dis. 212 Suppl 1:S74–S77.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorel O and Dewals BG: MicroRNAs in large
herpesvirus DNA genomes: Recent advances. Biomol Concepts.
7:229–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Zhang G, Li D, Jie Z, Chen H, Xiong
J, Liu Y, Cao Y, Jiang M, Le Z and Tan S: Methylation-associated
silencing of miR-495 inhibit the migration and invasion of human
gastric cancer cells by directly targeting PRL-3. Biochem Biophys
Res Commun. 456:344–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao Y, Li L, Liu J, Wang L and Zhou Y:
MiR-495 inhibits esophageal squamous cell carcinoma progression by
targeting Akt1. Oncotarget. 7:51223–51236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan L, Yao J and Qiu J: miRNA-495
suppresses proliferation and migration of colorectal cancer cells
by targeting FAM83D. Biomed Pharmacother. 96:974–981. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Liu WG, Lu JH, Liu ZJ, Li HB, Liu
GJ, She HY, Li GY and Shi XH: LncRNA UCA1 promotes renal cell
carcinoma proliferation through epigenetically repressing p21
expression and negatively regulating miR-495. Tumour Biol.
39:10104283177016322017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan M, Mu X, Liu Z, Tao L, Wang J, Ge J
and Qiu J: microRNA-495 promotes bladder cancer cell growth and
invasion by targeting phosphatase and tensin homolog. Biochem
Biophys Res Commun. 483:867–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Wu N, Zheng Q, Feng Y, Yan S, Lv
C, Li S, Wang Y and Yang Y: Evaluation of the 7th edition of the
TNM classification for lung cancer at a single institution. J
Cancer Res Clin Oncol. 140:1189–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schimittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehta A, Dobersch S, Romero-Olmedo AJ and
Barreto G: Epigenetics in lung cancer diagnosis and therapy. Cancer
Metastasis Rev. 34:229–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hesse M and Arenz C: MicroRNA maturation
and human disease. Methods Mol Biol 1095. 11–25. 2014. View Article : Google Scholar
|
|
18
|
Salido-Guadarrama I, Romero-Cordoba S,
Peralta-Zaragoza O, Hidalgo-Miranda A and Rodríguez-Dorantes M:
MicroRNAs transported by exosomes in body fluids as mediators of
intercelluar communication in cancer. Onco Targets Ther.
7:1327–1338. 2014.PubMed/NCBI
|
|
19
|
Wang Z, Yao H, Lin S, Zhu X, Shen Z, Lu G,
Poon WS, Xie D, Lin MC and Kung HF: Transcriptional and epigenetic
regulation of human microRNAs. Cancer Lett. 331:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Song Y, Liu D, Zhao J, Xu J, Ren J,
Hu Y, Wang Z, Hou Y and Zhao G: MiR-495 promotes senescence of
mesenchymal stem cells by targeting Bmi-1. Cell Physiol Biochem.
42:780–796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Yang Y and Feng Z: Suppression of
microRNA-495 alleviates high-glucose-induced retinal ganglion cell
apoptosis by regulating Notch/PTEN/Akt signaling. Biomed
Pharmacother. 106:923–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai Z, Wang J, Wang T, Li Y, Zhao X, Wu G,
Yang Y, Deng W and Zhang Z: The MiR-495/Annexin A3/P53 axis
inhibits the invasion and EMT of colorectal cancer cells. Cell
Physiol Biochem. 44:1882–1895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Cho ME, Li TW, Peng H, Ko KS, Mato
JM and Lu SC: MicroRNAs regulate methionine adenosyltransferase 1A
expression in hepatocellular carcinoma. J Clin Invest. 123:285–298.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang-Verslues WW, Chang PH, Wei PC, Yang
CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY and Lee WH: miR-495
is upregulated by E12/E47 in breast cancer stem cells, and promotes
oncogenesis and hypoxia resistance via downregulation of E-cadherin
and REDD1. Oncogene. 30:2463–2474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu H, Chen X, Wang H, Du Y, Wang Y, Zang
W, Li P, Li J, Chang J, Zhao G and Zhang G: MiR-495 regulates
proliferation and migration in NSCLC by targeting MTA3. Tumour
Biol. 35:3487–3494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang F, Zhao L, Mei D, Jiang L, Geng C, Li
Q, Yao X, Liu Y, Kong Y and Cao J: HMGA2 plays an important role in
Cr (VI)-induced autophagy. Int J Cancer. 141:986–997. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Busch B, Bley N, Müller S, Glaß M, Misiak
D, Lederer M, Vetter M, Strauß HG, Thomssen C and Hüttelmaier S:
The oncogenic triangle of HMGA2, LIN28B and IGF2BP1 antagonizes
tumor-suppressive actions of the let-7 family. Nucleic Acids Res.
44:3845–3864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin L, Lloyd RV, Henry MR, Erickson LA,
Sebo TJ, Rumilla KM and Zhang J: The diagnostic utility of
combination of HMGA2 and IMP3 qRT-PCR testing in thyroid neoplasms.
Appl Immunohistochem Mol Morphol. 23:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Zhang Q, Dong JQ, Chang XH and He
XJ: Overexpression of high mobility group A2 and its correlation
with microRNA let-7 family in serous ovarian cancers. Beijing Da
Xue Xue Bao Yi Xue Ban. 44:749–754. 2012.(In Chinese). PubMed/NCBI
|
|
31
|
Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai
H, Li P, Cao Q, Ju X, Meng X, et al: miR-154 inhibits EMT by
targeting HMGA2 in prostate cancer cells. Mol Cell Biochem.
379:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou Q, Xiong L, Yang Z, Lv F, Yang L and
Miao X: Expression levels of HMGA2 and CD9 and its
clinicopathological significances in the benign and malignant
lesions of the gallbladder. World J Surg Oncol. 10:922012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Lv GD, Qin X, Gen YH, Zheng ST, Liu
T and Lu XM: Role of microRNA let-7 and effect to HMGA2 in
esophageal squamous cell carcinoma. Mol Biol Rep. 39:1239–1246.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding X, Wang Y, Ma X, Guo H, Yan X, Chi Q,
Li J, Hou Y and Wang C: Expression of HMGA2 in bladder cancer and
its association with epithelial-to-mesenchymal transition. Cell
Prolif. 47:146–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meyer B, Loeschke S, Schultze A, Weigel T,
Sandkamp M, Goldmann T, Vollmer E and Bullerdiek J: HMGA2
overexpression in non-small cell lung cancer. Mol Carcinog.
46:503–511. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kong D, Su G, Zha L, Zhang H, Xiang J, Xu
W, Tang Y and Wang Z: Coexpression of HMGA2 and Oct4 predicts an
unfavorable prognosis in human gastric cancer. Med Oncol.
31:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Denechaud PD, Fajas L and Giralt A: E2F1,
a novel regulator of metabolism. Front Endocrinol (Lausanne).
8:3112017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fedele M, Pierantoni GM, Visone R and
Fusco A: E2F1 activation is responsible for pituitary adenomas
induced by HMGA2 gene overexpression. Cell Div. 1:172006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loukil A, Cheung CT, Bendris N, Lemmers B,
Peter M and Blanchard JM: Cyclin A2: At the crossroads of cell
cycle and cell invasion. World J Biol Chem. 6:346–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tessari MA, Gostissa M, Altamura S, Sgarra
R, Rustighi A, Salvagno C, Caretti G, Imbriano C, Mantovani R, Del
Sal G, et al: Transcriptional activation of the cyclin A gene by
the architectural transcription factor HMGA2. Mol Cell Biol.
23:9104–9116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
D'Angelo D, Esposito F and Fusco A:
Epigenetic mechanisms leading to overexpression of HMGA proteins in
human pituitary adenomas. Front Med (Lausanne). 2:392015.PubMed/NCBI
|
|
43
|
Morishita A, Zaidi MR, Mitoro A,
Sankarasharma D, Szabolcs M, Okada Y, D'Armiento J and Chada K:
HMGA2 is a driver of tumor metastasis. Cancer Res. 73:4289–4299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Cello F, Hillion J, Hristov A, Wood LJ,
Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R
and Resar LM: HMGA2 participates in transformation in human lung
cancer. Mol Cancer Res. 6:743–750. 2008. View Article : Google Scholar : PubMed/NCBI
|