Introduction
Polycystic ovary syndrome (PCOS) is an endocrine
disorder characterized by ovulatory dysfunction, hyperandrogenism
and polycystic ovarian morphology; this disorder occurs in 5–20% of
women of reproductive age worldwide (1). PCOS is one of the leading causes of
anovulatory infertility (2) and is
an important risk factor for type 2 diabetes mellitus (3). However, at present, the pathogenesis
of PCOS remains unclear. It is known that abnormal gonadotropin
levels may cause anovulation and hyperandrogenism (4). Alterations in ovarian
folliculogenesis, which are common in PCOS, are induced by the
imbalance between testosterone and follicle-stimulating hormone,
caused by excessive production of luteinizing hormone (5). In spite of efforts made regarding the
prevention and treatment of PCOS, long-term outcomes remain
generally unsatisfactory. Therefore, novel targets are required to
improve the clinical treatment outcomes of PCOS.
PCOS is caused by elevated androgens and is
characterized by altered cell proliferation, differentiation,
steroidogenesis, follicle maturation and apoptosis (6). Numerous intra- and extra-ovarian
factors, including tumor necrosis factor α, vascular endothelial
growth factor, epidermal growth factors and interleukins have been
reported to be involved in the pathogenesis of PCOS (6). Alongside mRNAs that encode protein
products, the human genome transcribes a larger set of non-coding
RNAs (ncRNAs) (7). Long ncRNAs
(lncRNAs) are a subgroup of ncRNAs composed of >200 nucleotides,
which serve critical roles in the pathogenesis of various human
diseases (8). It has been reported
that the development of PCOS is accompanied by alterations in the
expression levels of certain lncRNAs (9), thus indicating the involvement of
lncRNAs in this disease. LncRNA BANCR is a recently identified
lncRNA that has pivotal roles in various malignancies, including
endometrial cancer (10) and
melanoma (11), mainly by
promoting the proliferation of cancer cells through interactions
with numerous pathways. Our preliminary experiments detected
altered expression of BANCR in PCOS tissues compared with in
healthy controls (data not shown). The present study aimed to
examine the potential involvement of lncRNA BANCR in PCOS. It was
demonstrated that lncRNA BANCR may participate in PCOS by promoting
cell apoptosis through the upregulation of B-cell lymphoma
(Bcl)-2-associated X protein (Bax).
Patients and methods
Subjects and granulosa cells
(GCs)
The present study recruited 44 patients with PCOS;
these patients were diagnosed and treated for the first time at The
Second Affiliated Hospital of Guangzhou University of Chinese
Medicine, Guangdong Provincial Hospital of Chinese Medicine
(Guangdong, China) between January 2016 and January 2017. The
patients were aged between 22 and 39 years old, with a mean age of
31.2±3.4 years. GCs were collected from each patient. Briefly, GCs
were separated from whole blood samples via centrifugation at 1,875
× g and room temperature for 10 min in tubes containing 80%
Percoll; GCs formed a layer on the Percoll solution. GCs were also
collected from non-PCOS patients (n=34; age range, 21–39; mean,
30.9±3.1) undergoing in vitro fertilization, which served as
a control. GCs were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 (1:1; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum (FBS, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 3 days prior to
experimentation. There was no significant difference in age between
the patient and control groups. The present study was approved by
the ethics committee of The Second Affiliated Hospital of Guangzhou
University of Chinese Medicine, Guangdong Provincial Hospital of
Chinese Medicine. All patients provided written informed
consent.
Human granulosa-like tumor cell line
(KGN) and transfection
The KGN human granulosa-like tumor cell line was
recently established in our laboratory using tissue samples from a
patient with invasive ovarian granulosa cell carcinoma, according
to methods described by Nishi et al (12). KGN cells were cultured in DMEM/F12
(1:1) containing 10% FBS. BANCR cDNA was amplified from cDNA
derived from the total RNA of GCs using primers carrying the
EcoRI restriction enzyme sites at the flanking ends. The
EcoRI-EcoRI fragment containing BANCR cDNA was
inserted into the GV299 lentiviral vector (Shanghai GeneChem Co.,
Ltd., Shanghai, China) to establish a BANCR expression vector. KGN
cells were cultured overnight to reach 80–90% confluence and 10 nM
vectors were transfected into cells. Empty vectors without BANCR
cDNA were also transfected into KGN cells to serve as negative
controls.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol® Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from GCs and
KGN cells. To detect the effects of insulin on BANCR, GCs were
first treated with insulin (5, 10 and 50 ng/ml; Sigma-Aldrich;
Merck KGaA) for 24 h at 37°C in a 5% CO2 prior to RNA
extraction. Total RNA was quantified using a NanoDrop™
2000 Spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA), and RNA samples with an A260/A280 ratio of
1.8–2.0 were used to synthesize cDNA through RT using SuperScript
III Reverse Transcriptase kit (Thermo Fisher Scientific, lnc.)
according to manufacturer's instructions. PCR reaction systems were
prepared using SYBR® Green Quantitative RT-qPCR kit
(Sigma-Aldrich; Merck KGaA). PCR reactions were performed on an ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Primers used in PCR reactions were as follows:
BANCR forward, 5′-ACAGGACTCCATGGCAAACG-3′ and reverse
5′-ATGAAGAAAGCCTGGTGCAGT-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
qPCR was carried out using the following thermocycling conditions:
95°C for 50 sec, followed by 40 cycles at 95°C for 12 sec and 60°C
for 45 sec. All data were processed using the 2−ΔΔCq
method (13). Relative expression
levels of BANCR were normalized to the endogenous control
β-actin.
Cell proliferation assay
Cell Counting kit-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was performed to measure the proliferation
of KGN cells with or without BANCR overexpression, according to the
manufacturer's protocol. Briefly, 3,000 cells/100 µl were added to
each well of a 96-well plate. Cells were cultured at 37°C in a 5%
CO2 incubator, and 10 µl CCK-8 was added to each well
after 24, 48, 72 and 96 h later. Cells were incubated with CCK-8
for 4 h at 37°C. Finally, an Epoch Microplate Spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA) was used to measure
optical density values at 450 nm. Cell proliferation was normalized
to control cells.
Cell apoptosis assay
Cells were suspended in serum-free medium
(6×104 cells/ml) and were transferred to a 6-well plate
(10 ml cell suspension/well). After cell culture for 48 h, cells
were subjected to 0.25% trypsin digestion. Subsequently, cells were
stained with Annexin V-fluorescein isothiocyanate (FITC; 1:50;
Dojindo Molecular Technologies, Inc.) and propidium iodide (PI,
1:50). Flow cytometry was performed to detect apoptotic cells. Data
were analyzed using BD FACSuite™ software version 1.0
(BD Biosciences, San Jose, CA, USA). Cell apoptotic rates were
calculated based on the sum of % Annexin V-FITC+/PI- and
% Annexin V-FITC+/PI+ cells.
Western blotting
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total
proteins from KGN cells. Bicinchoninic acid assay was used to
quantify protein samples. Subsequently, proteins (30 µg/lane) were
separated by 10% SDS-PAGE and were transferred to polyvinylidene
fluoride membranes, which were blocked with 5% skimmed milk at room
temperature for 1 h. Tris-buffered saline-3% Tween-20 (TBST) was
used to wash the membranes three times (5 min/wash), after which,
membranes were incubated with the following primary antibodies:
Rabbit anti-Bax antibody (1:2,000, cat. no. ab32503), anti-p53
(1:2,000, cat. no. ab31333) and anti-GAPDH (1:1,000, cat. no.
ab9485; all Abcam, Cambridge, UK) overnight at 4°C. Subsequently,
TBST was used to wash the membranes three times (5 min/wash),
followed by incubation with an anti-rabbit immunoglobulin
G-horseradish peroxidase secondary antibody (1:1,000; cat. no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 1 h. TBST was used to wash the membranes a further
three times (5 min/wash). Enhanced chemiluminescence
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was then used to
develop the signal. Relative expression levels were normalized to
the endogenous control GAPDH using ImageJ version 1.47 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
analyze results. Experiments were performed in triplicate. Data are
expressed as the means ± standard deviation; comparisons among
multiple groups were determined using one-way analysis of variance
followed by the least significant difference post hoc test, whereas
differences between two groups were analyzed by Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
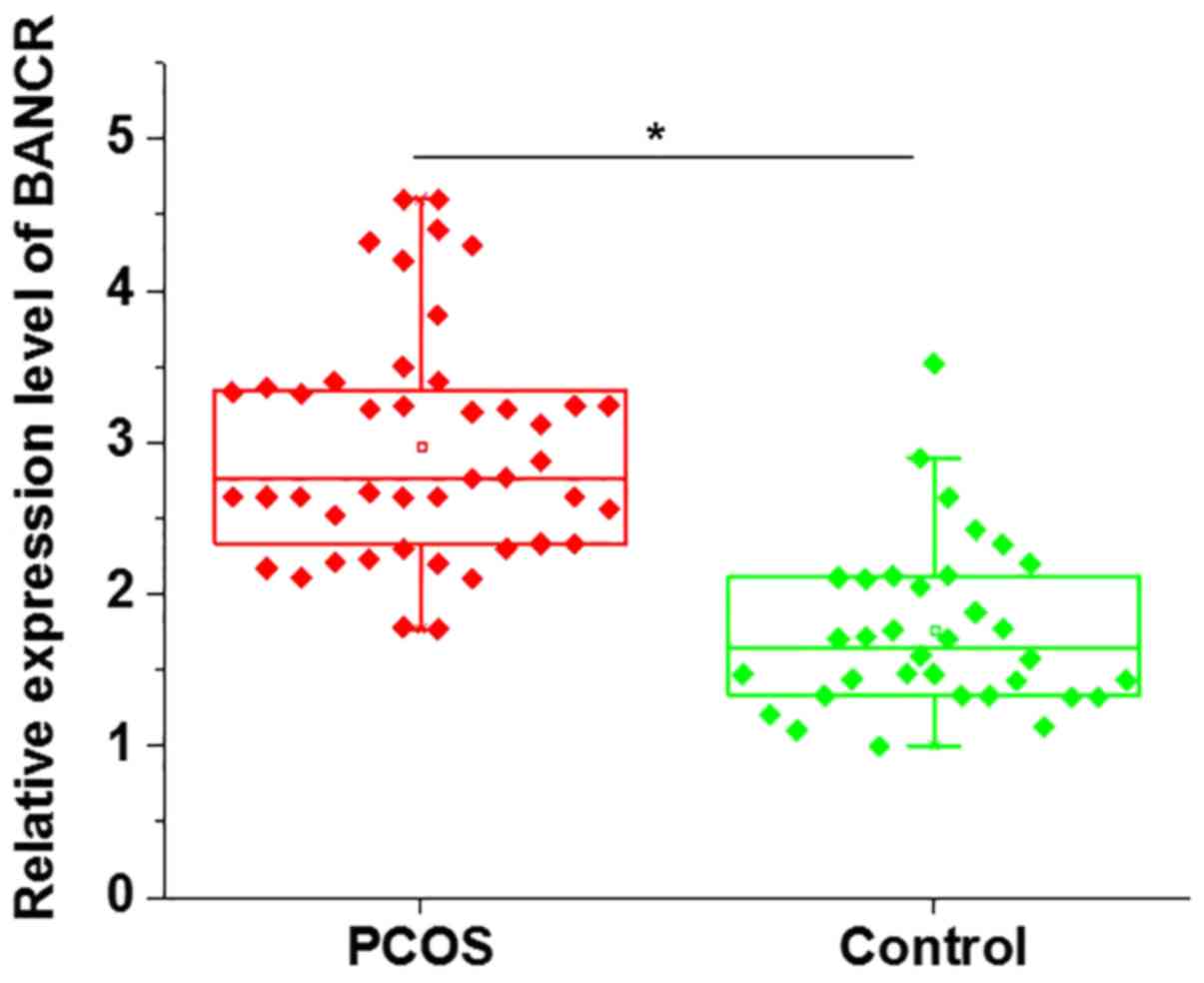
Comparison of BANCR expression in GCs
between patients with PCOS and controls
The mRNA expression levels of BANCR in GCs derived
from patients with PCOS and healthy controls were detected by
RT-qPCR. As shown in Fig. 1, the
expression levels of BANCR were significantly higher in GCs from
patients with PCOS than in controls. These data suggested that
BANCR may be involved in the pathogenesis of PCOS.
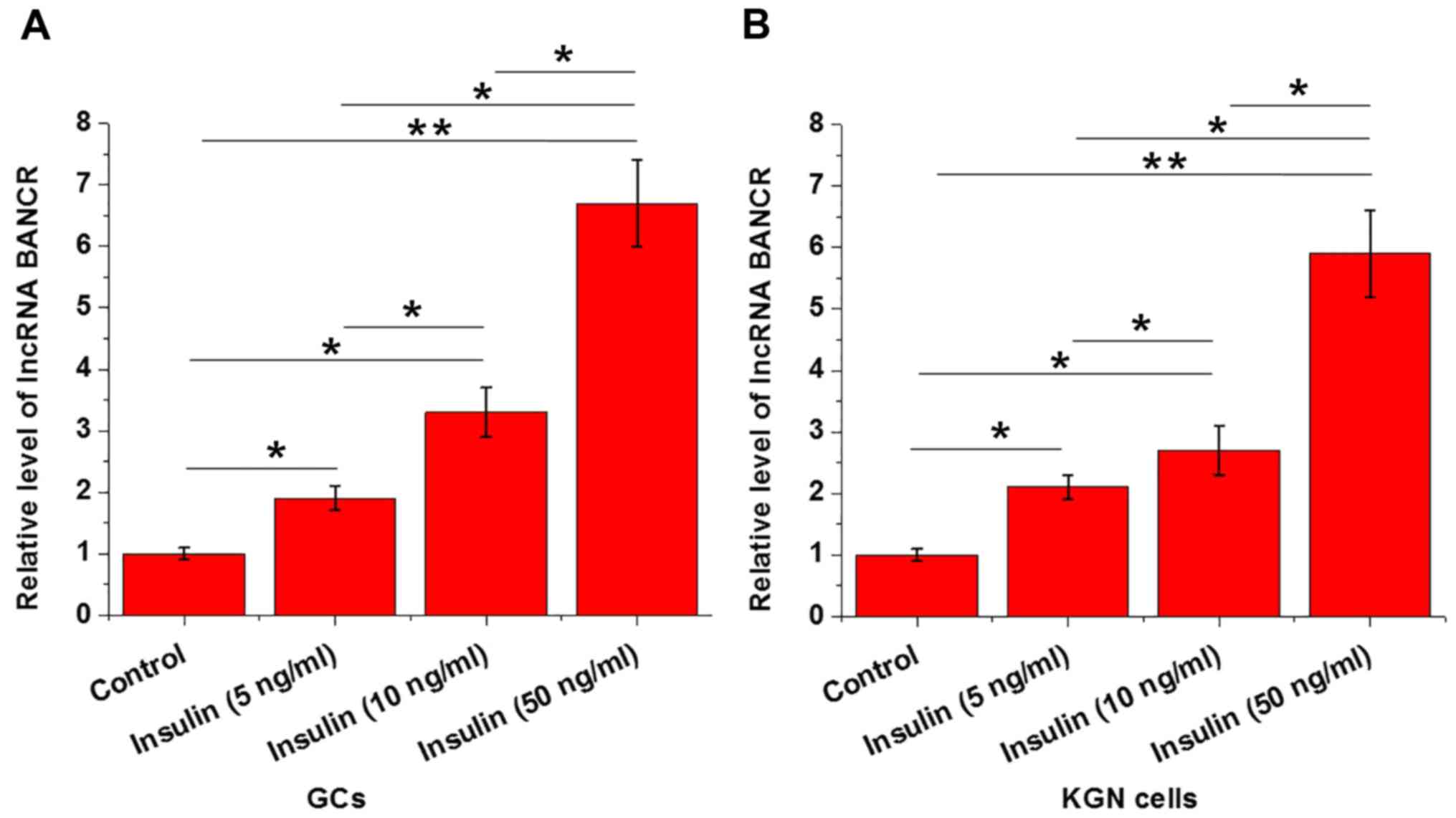
Effects of insulin treatment on BANCR
expression in GCs and KGN cells
Insulin treatment was performed by adding various
concentrations of insulin (5, 10 and 50 ng/ml; Sigma-Aldrich; Merck
KGaA) into the culture media. As presented in Fig. 2, insulin treatment significantly
upregulated the expression levels of BANCR in GCs (Fig. 2A) and KGN cells (Fig. 2B) in a dose-dependent manner.
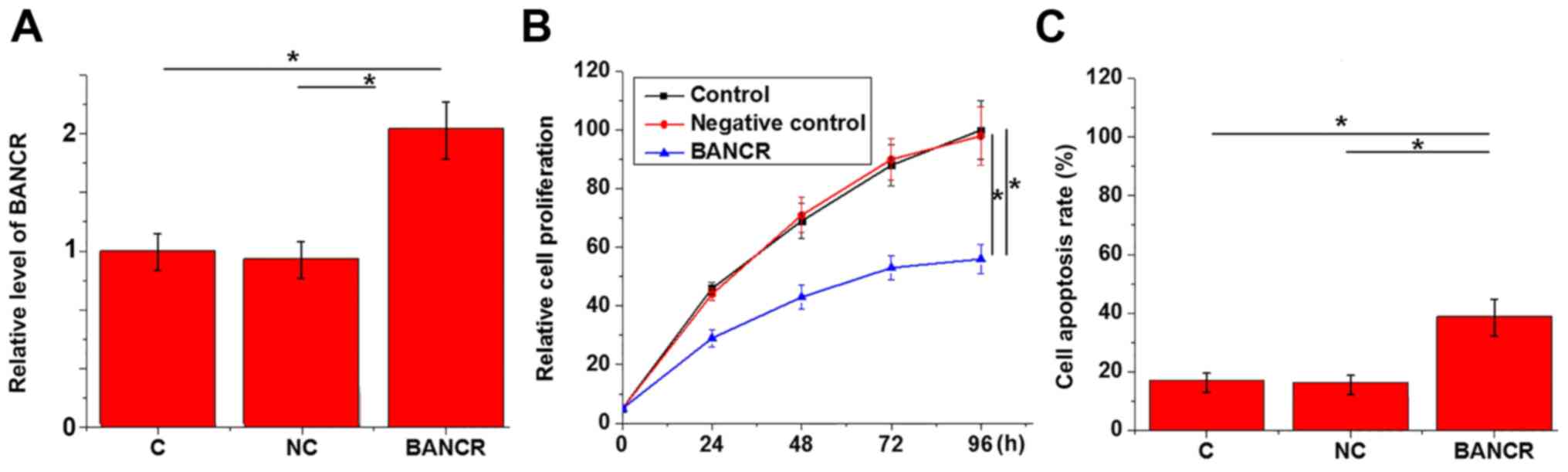
Effects of BANCR overexpression on
proliferation and apoptosis of KGN cells
CCK-8 assay and MTT assay were performed to
investigate the effects of BANCR overexpression on the
proliferation and apoptosis of KGN cells. BANCR vector
significantly increased BANCR expression, compared with the
controls (Fig. 3A). As shown in
Fig. 3B, compared with control
cells and negative control cells, BANCR overexpression
significantly inhibited the proliferation of KGN cells (P<0.05).
In addition, BANCR overexpression significantly promoted apoptosis
of KGN cells (Fig. 3C;
P<0.05).
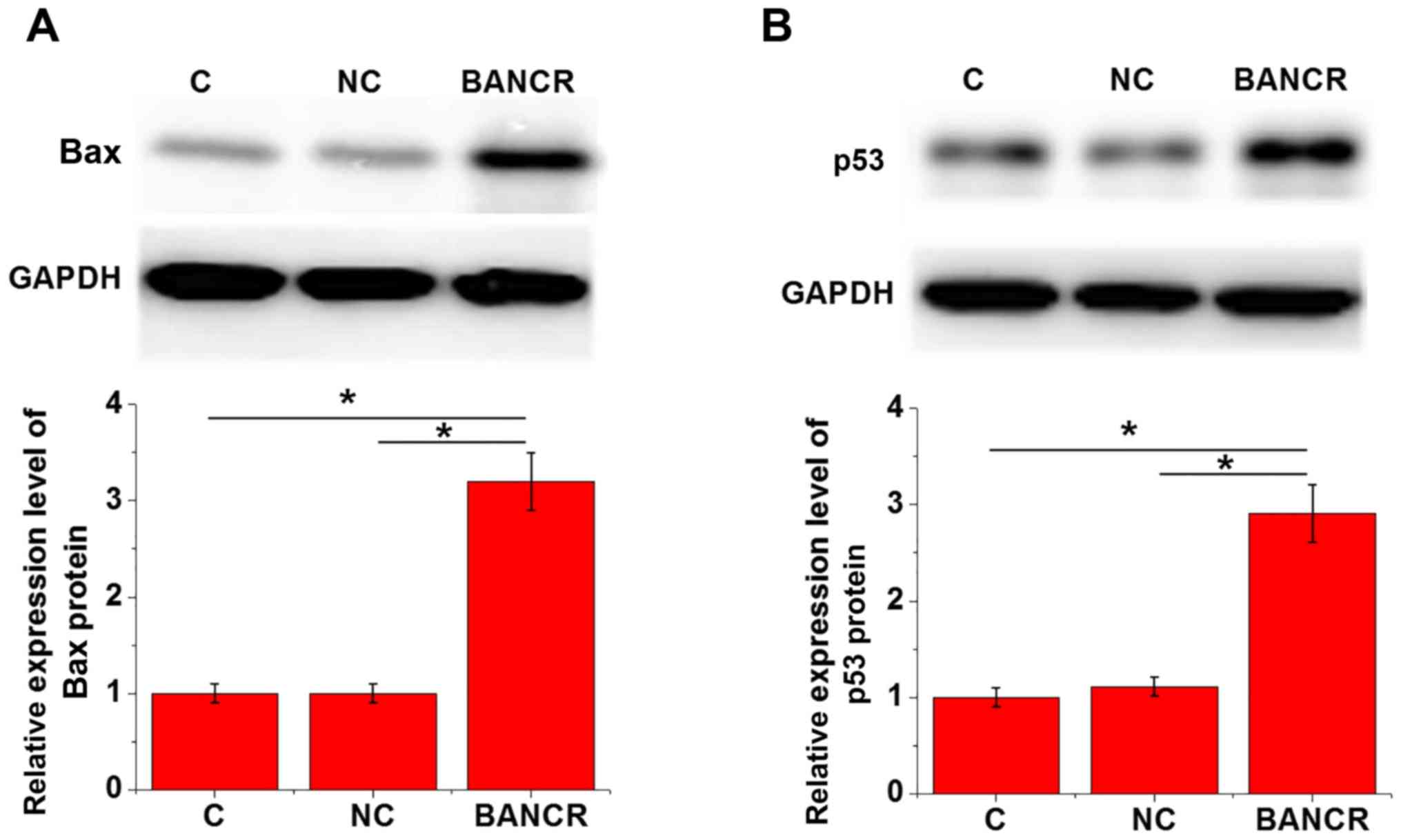
Effects of BANCR overexpression on Bax
and p53 expression in KGN cells
Bax and p53 expression in KGN cells with or without
BANCR overexpression was detected by western blotting. As shown in
Fig. 4, compared with in the
control and negative control cells, BANCR overexpression
significantly upregulated Bax (Fig.
4A) and p53 (Fig. 4B)
expression in KGN cells (P<0.05).
Discussion
The present study reported a potential novel
function of lncRNA BANCR, which has well-characterized functions in
human malignancies. The results demonstrated that lncRNA BANCR may
be upregulated by pathological insulin signaling, and it may
promote the development of PCOS by promoting apoptosis and
inhibiting proliferation of GCs by activating the Bax pro-apoptotic
pathway.
It has previously been reported that the development
of PCOS is associated with the abnormal expression of numerous
genetic factors (6). However, to
the best of our knowledge, the involvement of lncRNAs in PCOS has
not yet been well studied. In a recent study, Liu et al
reported that the expression levels of lncRNA steroid receptor RNA
activator RNA activator (SRA) in peripheral blood leukocytes is
closely correlated with the development of PCOS (14), whereas the function of lncRNA SRA
and the mechanism of its action in PCOS are unknown. It has been
well established that GCs have a key role in folliculogenesis, and
are essential for the maintenance of a suitable microenvironment
for the initiation of follicle growth, oocyte maturation and
atresia (15). In the present
study, the expression levels of lncRNA BANCR were significantly
upregulated in patients with PCOS compared with in controls, thus
indicating that upregulation of BANCR may be involved in the
pathogenesis of PCOS.
The majority of patients with PCOS will develop
insulin resistance, causing cardiovascular disease,
hyperinsulinemia and an increased risk for type 2 diabetes
(16). Insulin signaling is a key
player in the regulation of hormone production in GCs, as well as
regulation of ovarian function and GC proliferation (17,18).
In the present study, insulin treatment significantly promoted the
expression of lncRNA BANCR in GCs and KGN cells in a dose-dependent
manner. These data suggested that lncRNA BANCR may be involved in
pathological insulin signaling to promote the progression of PCOS.
Therefore, BANCR may serve as a target for the prevention of type 2
diabetes in patients with PCOS.
Unbalanced proliferation and apoptosis of GCs may
lead to abnormal folliculogenesis (19,20).
The KGN cell line has been widely used to investigate cell growth
and apoptosis of human GCs (12).
In the present study, BANCR overexpression significantly promoted
apoptosis and inhibited proliferation of KGN cells, thus indicating
that BANCR may participate in the pathogenesis of PCOS by
stimulating apoptosis and inhibiting proliferation of GCs.
Bax-mediated cell apoptosis serves a pivotal role in the
development of various types of human disease (21,22).
A recent study also reported that expression levels of Bax are
significantly upregulated in patients with PCOS compared with in
healthy controls (23). In this
study, BANCR overexpression significantly upregulated the protein
expression levels of Bax in KGN cells, indicating that the
enhancing effects of BANCR overexpression on apoptosis of KGN cells
may be achieved by upregulating Bax expression.
Notably, other apoptosis-associated proteins,
including insulin-like growth factors (IGFs), IGF-binding proteins,
Bcl-2 and Bcl-extra large were also detected in this study (data
not shown); however, no significant alterations in the expression
levels of these proteins were detected following BANCR
overexpression. Our future studies aim to focus on other pathways
involved in BANCR-mediated regulation of KGN cell apoptosis.
In conclusion, lncRNA BANCR expression was
upregulated in GCs from patients with PCOS. In addition, insulin
treatment significantly upregulated the expression of BANCR in GCs
and KGN cells. BANCR overexpression significantly inhibited
proliferation and promoted apoptosis of KGN cells, and upregulated
the expression levels of pro-apoptotic Bax and p53 in KGN cells.
These findings indicated that lncRNA BANCR may participate in PCOS
by promoting cell apoptosis through the upregulation of Bax.
Acknowledgements
Not applicable.
Funding
The authors are grateful for the financial support
they received in 2014 from The Scientific Research Project of
Guangdong Provincial Administration of Traditional Chinese
Medicine, for the project ‘The research of acupuncture point buried
line combined with daily lifestyle intervention to treat polycystic
ovary syndrome’ (project no. 20141124, project support).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
RY, JC, LW and AD were responsible for the
conception and design of the study. RY and JC performed the
experiments. RY, JC and LW analyzed and interpreted the data. RY
drafted the article. JC, LW and AD were responsible for the
revision of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of The Second Affiliated Hospital of Guangzhou University
of Chinese Medicine, Guangdong Provincial Hospital of Chinese
Medicine. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Azziz R, Carmina E, Chen ZJ, Dunaif A,
Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ and
Yildiz BO: Polycystic ovary syndrome. Nat Rev Dis Primers.
2:160572016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franks S: Polycystic ovary syndrome.
Medicine. 45:527–531. 2017. View Article : Google Scholar
|
|
3
|
Ollila MM, West S, Keinänen-Kiukaanniemi
S, Jokelainen J, Auvinen J, Puukka K, Ruokonen A, Järvelin MR,
Tapanainen JS, Franks S, et al: Overweight and obese but not normal
weight women with PCOS are at increased risk of Type 2 diabetes
mellitus-a prospective, population-based cohort study. Hum Reprod.
32:423–431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diamanti-Kandarakis E and Dunaif A:
Insulin resistance and the polycystic ovary syndrome revisited: An
update on mechanisms and implications. Endocr Rev. 33:981–1030.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franks S, Stark J and Hardy K: Follicle
dynamics and anovulation in polycystic ovary syndrome. Hum Reprod
Update. 14:367–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Leo V, Musacchio MC, Cappelli V,
Massaro MG, Morgante G and Petraglia F: Genetic, hormonal and
metabolic aspects of PCOS: An update. Reprod Biol Endocrinol.
14:382016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet 15 Spec No. 1:R17–R29. 2006. View Article : Google Scholar
|
|
8
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang X, Hao C, Bao H, Wang M and Dai H:
Aberrant expression of long noncoding RNAs in cumulus cells
isolated from PCOS patients. J Assist Reprod Genet. 33:111–121.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D, Wang D, Wang N, Long Z and Ren X:
Long non-coding RNA BANCR promotes endometrial cancer cell
proliferation and invasion by regulating MMP2 and MMP1 via ERK/MAPK
signaling pathway. Cell Physiol Biochem. 40:644–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishi Y, Yanase T, Mu YM, Oba K, Ichino I,
Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al: Establishment
and characterization of a steroidogenic human granulosa-like tumor
cell line, KGN, that expresses functional follicle-stimulating
hormone receptor. Endocrinology. 142:437–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Hao C, Huang X, Zhang N, Bao H and
Qu Q: Peripheral blood leukocyte expression level of lncRNA steroid
receptor RNA activator SRA. and its association with polycystic
ovary syndrome: A case control study. Gynecol Endocrinol.
31:363–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eppig JJ: Oocyte control of ovarian
follicular development and function in mammals. Reproduction.
122:829–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Legro RS, Finegood D and Dunaif A: A
fasting glucose to insulin ratio is a useful measure of insulin
sensitivity in women with polycystic ovary syndrome. J Clin
Endocrinol Metab. 83:2694–2698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Li H, Yang S, Ma W, Liu M, Guo S,
Zhan J, Zhang H, Tsang SY, Zhang Z, et al: Cyanidin-3-o-glucoside
directly binds to ERα36 and inhibits EGFR-positive triple-negative
breast cancer. Oncotarget. 7:68864–68882. 2016.PubMed/NCBI
|
|
18
|
Ni XR, Sun ZJ, Hu GH and Wang RH: High
concentration of insulin promotes apoptosis of primary cultured rat
ovarian granulosa cells via its increase in extracellular HMGB1.
Reprod Sci. 22:271–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Das M, Djahanbakhch O, Hacihanefioglu B,
Saridogan E, Ikram M, Ghali L, Raveendran M and Storey A: Granulosa
cell survival and proliferation are altered in polycystic ovary
syndrome. J Clin Endocrinol Metab. 93:881–887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onalan G, Selam B, Baran Y, Cincik M,
Onalan R, Gündüz U, Ural AU and Pabuccu R: Serum and follicular
fluid levels of soluble Fas, soluble Fas ligand and apoptosis of
luteinized granulosa cells in PCOS patients undergoing IVF. Hum
Reprod. 20:2391–2395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gillissen B, Richter A, Richter A,
Preissner R, Schulze-Osthoff K, Essmann F and Daniel PT:
Bax/Bak-independent mitochondrial depolarization and reactive
oxygen species induction by sorafenib overcome resistance to
apoptosis in renal cell carcinoma. J Biol Chem. 292:6478–6492.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mei S, Li L, Wei Q, Hao J, Su Y, Mei C and
Dong Z: Double knockout of Bax and Bak from kidney proximal tubules
reduces unilateral urethral obstruction associated apoptosis and
renal interstitial fibrosis. Sci Rep. 7:448922017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu XQ, Wang YQ, Xu SM, Liu JF, Bi XY, Wang
ZQ and Zhang JP: The WNT/β-catenin signaling pathway may be
involved in granulosa cell apoptosis from patients with PCOS in
North China. J Gynecol Obstet Hum Reprod. 46:93–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|