Introduction
Glioblastoma multiforme (GBM) is the most common and
the most invasive subtype of brain cancer; it is characterized by
symptoms that include personality changes, headaches, nausea and
unconsciousness (1,2). GBM originates from normal brain cells
or low-grade astrocytoma, and may be induced by genetic disorders
and radiation exposure (3,4). Clinical techniques for the treatment
of GBM include surgery combined with radiation therapy or
chemotherapy; however, the survival benefit is limited to ~12-15
months, or even shorter if the disease recurs (4).
Gene therapy is a novel strategy for treating
cancers (5). Transient receptor
potential genes are overexpressed in GBM, which promote the
survival of patients (6,7). It has been previously reported that
low expression of B cell-specific Moloney murine leukemia virus
integration site 1 suppresses proliferation and promotes apoptosis
of U251 GBM cells, and enhances the chemosensitivity of these cells
to cisplatin (8,9). The expression level of epidermal
growth factor-containing fibulin extracellular matrix protein 1
(EFEMP1) was associated with the survival of patients with
GBM treated with temozolomide (TMZ) (10); thus, EFEMP1 is considered a
target for overcoming TMZ-resistance in GBM. Enhancer of zeste
homolog 2 (EZH2) overexpression was associated with tumor
grade and predicts short overall survival in patients with GBM
(11); thus, EZH2 may be a
promising prognostic factor and therapeutic target for patients.
Additionally, HOX transcript antisense RNA (HOTAIR)
overexpression was associated with poor outcome in patients with
GBM, and HOTAIR may be a therapeutic molecular target for
this disease (12,13). O-6-Methylguanine-DNA
methyltransferase (MGMT) methylation status and mutations in
the isocitrate dehydrogenase 1 (IDH1) gene are two known
clinicopathological factors linked to overall survival of patients
with GBM (14). MGMT
methylation is significantly associated with the clinical prognosis
of GBM (15); IDH1 is a
prognostic marker of GBM, and mutations in this gene diminish the
malignant progression of glioma (16). In addition, high expression of
interleukin-13R mRNA is strongly associated with poor prognosis of
GBM (17). POZ/BTB and AT
hook-containing zinc-finger 1 is another prognostic marker of GBM;
it is overexpressed in GBM-derived glioma-initiating stem cells,
and is associated with the characteristic stem cell capacity to
grow as neurospheres in vitro (18). Despite this collective knowledge,
the genes involved in the prognosis of GBM have not been
comprehensively reported.
In 2008, Murat et al (19) established the GSE7696 gene
expression profile, and demonstrated that high expression levels of
epidermal growth factor receptor and stem cell-related
‘self-renewal’ signature are involved in the resistance to
concomitant chemoradiotherapy of GBM. In 2011, Lambiv et al
(20) used the GSE7696 data set to
explore the action mechanism of tumor suppressor gene Wnt
inhibitory factor 1 (WIF1) in GBM models, and concluded that
WIF1 may have a tumor suppressive role in GBM through
senescence.
However, this data set has not been fully explored.
Using the GSE7696 data, additional key genes associated with the
prognosis of GBM were investigated using comprehensive
bioinformatics methods, such as survival analysis, enrichment
analysis and hierarchical clustering. Results from the present
study provided novel insights into the prognosis of GBM and may aid
in the development of novel therapeutic approaches.
Materials and methods
Data source
The GSE7696 microarray data set, based on the GPL570
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array
(Thermo Fisher Scientific, Inc.) platform, along with the
corresponding clinical information was downloaded from the Gene
Expression Omnibus database (www.ncbi.nlm.nih.gov/geo). The data set included 10
recurrent GBM tissue samples (2 females and 8 males; mean age,
51.31 years), 70 primary GBM tissue samples (19 females and 51
males; mean age, 48.07 years) and 4 normal brain tissue samples
(sex and age information not available). All patients participated
in a phase II or randomized phase III trial (21,22),
with informed consent provided. The GSE7696 data set is available
and the study was approved by the local ethics committee (19).
Selection of probes with expression
changes in primary GBM tissue samples
The normalized data of GSE7696 were obtained, and
the primary GBM tissue samples data were selected to use in the
present study. Firstly, the unloaded probes were removed.
Subsequently, the probes with varied expressions among 70 different
patients with primary GBM were identified by the following steps.
Firstly, the variance in probe expression level in each sample was
calculated, and probes with variance <20% of the total probe
variance were excluded. Secondly, the median probe expression in
each sample was calculated and, as in the prior step, probes with a
value <20% of the total median probe expression were removed.
Finally, the probes with expression changes among these primary GBM
tissue samples were selected.
Survival analysis and pathway
enrichment analysis
Using the ‘survival’ package (23) in R (www.r-project.org), univariate survival analysis was
conducted for the above probes with expression changes, and those
with P<0.05 were considered as seed genes. The Kyoto
Encyclopedia of Genes and Genomes (KEGG; www.genome.ad.jp/kegg) is a database used to search
for gene functions, connecting genomic with functional information
(24). Using the DAVID database
(david.abcc.ncifcrf.gov) (25), KEGG pathway enrichment analysis was
performed for the seed genes to explore their functions. The
categories with P<0.05 were considered to indicate a
statistically significant difference.
Screening of prognostic feature genes,
unsupervised hierarchical clustering and analysis of prognostic
characteristics
Using the ‘rbsurv’ package in R (bioconductor.org/packages/release/bioc/html/rbsurv.html)
(26), robust likelihood-based
survival modeling was constructed to identify prognostic feature
genes (27). The samples were
classified based on the expression profiles of the prognostic
feature genes using unsupervised hierarchical clustering (28). Thereafter, the prognostic
differences among the classified samples were analyzed by the
Kaplan-Meier survival analysis (29). The expression differences of the
feature genes between primary GBM and normal samples were analyzed;
and the scatter plot of the gene expression levels were drawn using
the corrplot package (https://cran.r-project.org/web/packages/corrplot/vignettes/corrplot-intro.html)
in R (version 3.4.4).
Functional and pathway enrichment
analysis for the feature genes
The Gene Ontology (GO) database (www.geneontology.org) is used to predict potential
functions of genes and their products (30). The prognostic feature genes were
examined with GO functional and KEGG pathway enrichment analysis by
the ‘clusterProfiler’ package in R (bioconductor.org/packages/release/bioc/html/clusterProfiler.html),
with the threshold q-value <0.05 (31).
Multivariate survival analysis
The prognostic feature genes underwent multivariate
survival analysis to check their overall influences on prognosis.
In addition, the ‘survivalROC’ package in R (cran.r-project.org/web/packages/survivalROC/index.html)
was used to draw the receiver operating characteristic (ROC) curve
and calculate the area under the ROC curve (AUC) (32). Specifically, the survivalROC
package was used to calculate the ‘true positive rate’ and ‘false
positive rate’ of each sample, and the differences between the
‘true positive rate’ and ‘false positive rate’ for each sample were
subsequently calculated. The sample with the smallest difference
value was set as the cut-off in the multifactorial cox regression
analysis, and samples with a higher value than this cut-off were
deemed as the high-risk group, while those with a lower value were
the low-risk group.
Validation of the prognostic feature
genes using an independent data set
The GBM dataset in The Cancer Genome Atlas (TCGA;
cancergenome.nih.gov) database
(downloaded in January 27, 2015; based on the Illumina HiSeq
platform, and included RNA-sequencing data in level 3 and clinical
follow-up data) was obtained to validate the prognostic feature
genes. The 172 samples in the data set comprised 13 recurrent GBM
samples, 154 primary GBM samples and 5 adjacent normal tissue
samples. Of the 154 primary GBM samples, 2 samples had no survival
information. Finally, 152 primary GBM samples were selected for
analysis. The data were transformed using log2(x+1),
followed by Cox regression analysis using the ‘survival’ package in
R to compare the differences of prognosis and recurrence among the
samples in different groups. Differences in the identified factors
(age and sex distribution) were assessed using R software. The
Mann-Whitney test analysis was performed using the respective
internal function in R, ‘wilcox.test’. The χ2 value was
calculated using the ‘chisq.test’ function in R.
Results
Survival analysis and pathway
enrichment analysis for seed genes
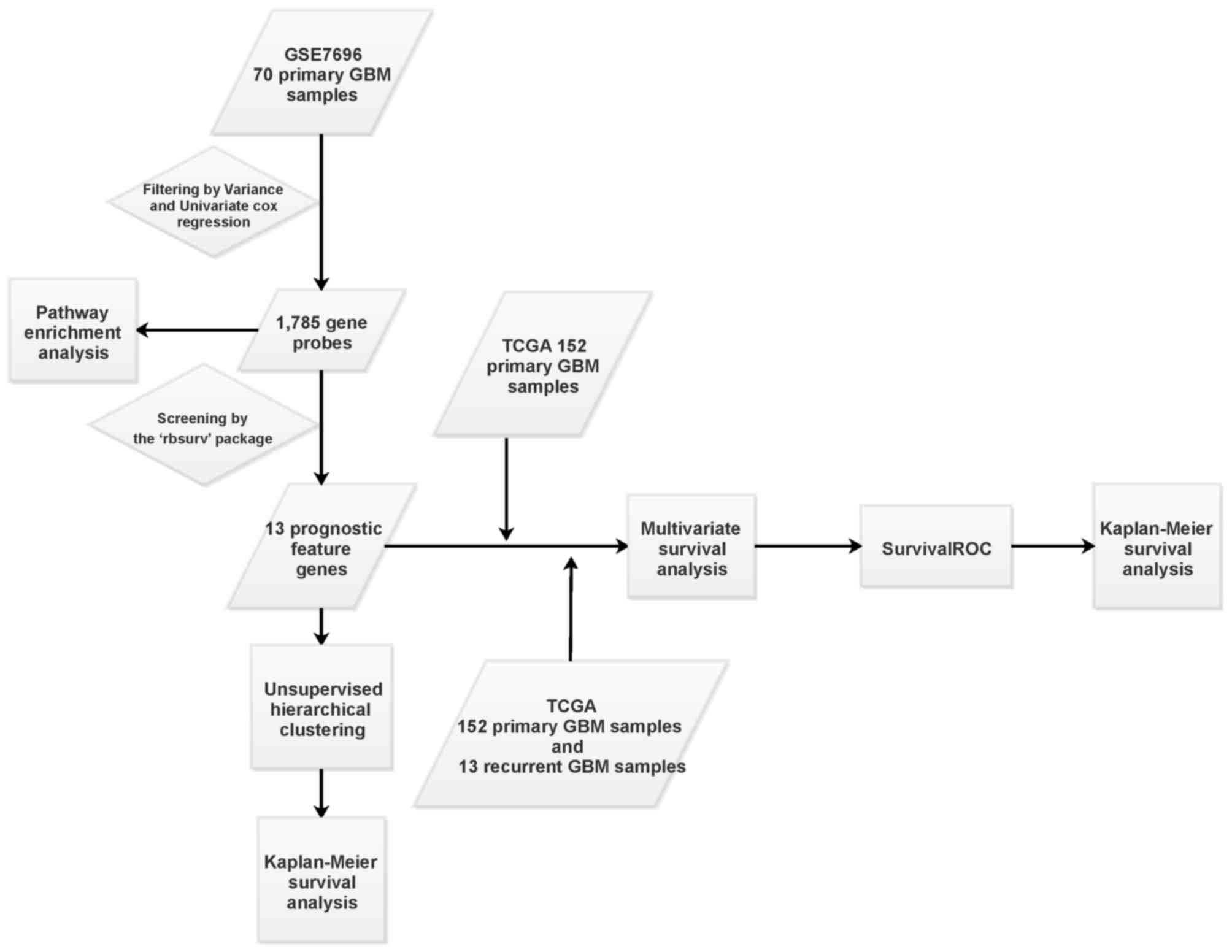
The bioinformatics analysis process used in the
present study is outlined in Fig.
1. Following calculation of the variance and median for the
probes in the 70 primary GBM, a total of 38,370 probes with
expression changes were identified. Based on the univariate
survival analysis using the ‘survival’ package, a total of 1,785
gene probes significantly correlated with prognosis of GBM were
selected as seed genes. KEGG pathway enrichment analysis identified
six pathways that were significantly enriched for these seed genes,
including ‘ribosome’, ‘regulation of actin cytoskeleton’,
‘endometrial cancer’, ‘pathways in cancer’, ‘amino sugar and
nucleotide sugar metabolism’ and ‘non-small cell lung cancer’
(Table I).
 | Table I.Pathways significantly enriched for
the 1,785 seed genes. |
Table I.
Pathways significantly enriched for
the 1,785 seed genes.
| Term | Count | P-value |
|---|
|
hsa03010:Ribosome | 43 |
2.31×10−26 |
| hsa04810:Regulation
of actin cytoskeleton | 27 |
4.09×10−03 |
|
hsa05213:Endometrial cancer | 10 |
9.29×10−03 |
| hsa05200:Pathways
in cancer | 34 |
2.08×10−02 |
| hsa00520:Amino
sugar and nucleotide sugar metabolism | 8 |
3.19×10−02 |
| hsa05223:Non-small
cell lung cancer | 9 |
3.31×10−02 |
Screening of prognostic feature genes,
unsupervised hierarchical clustering and analysis of prognostic
characteristics
Robust likelihood-based survival modeling identified
13 prognostic feature genes from the 1,785 seed genes, including
collagen type XXVIII α1 chain (COL28A1), PDS5
cohesin-associated factor A (PDS5A; also known as sister
chromatid cohesion protein 112), zinc-finger DHHC-type containing 2
(ZDHHC2), zinc-finger protein 24 (ZNF24), myosin VA
(MYO5A) and myeloid/lymphoid or mixed-lineage leukemia
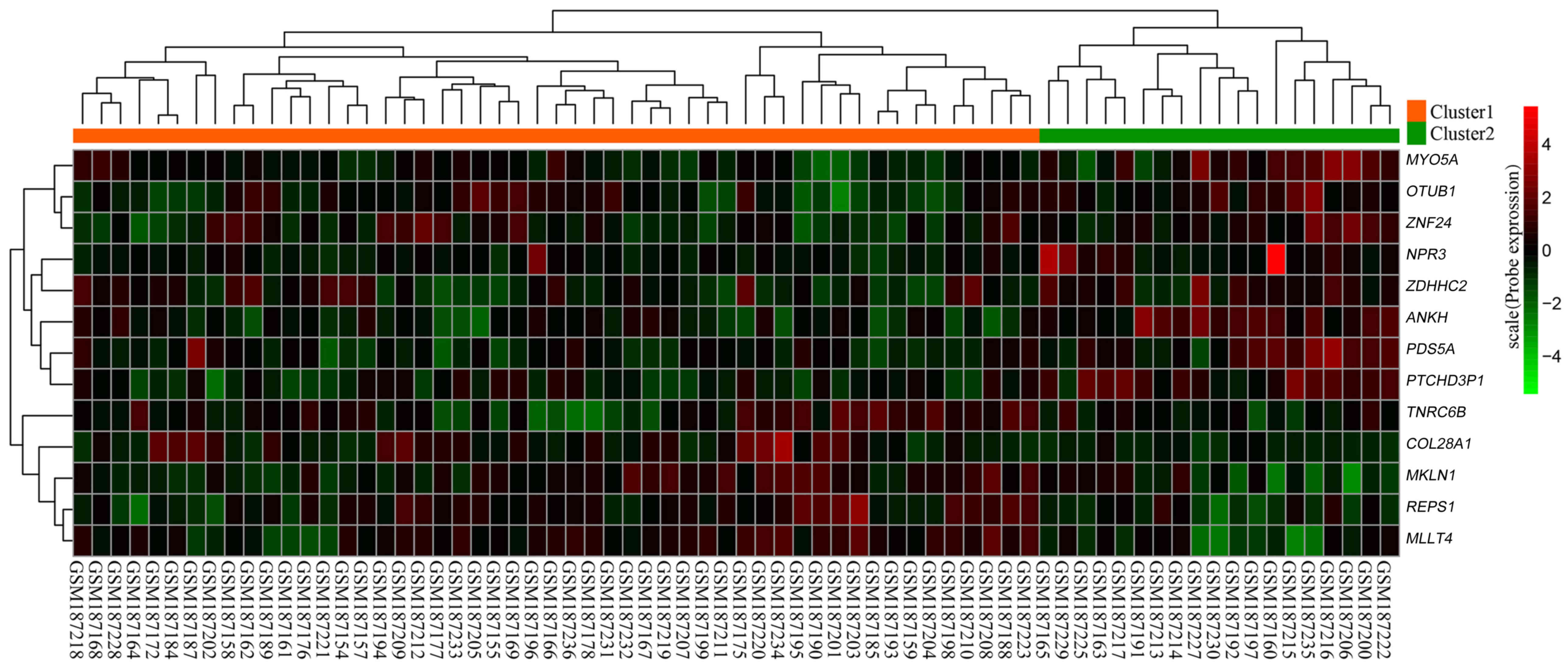
translocated to 4 (MLLT4) (Table II). Unsupervised hierarchical
clustering was conducted for these 13 prognostic feature genes. A
heat map demonstrated that these genes may be used to classify the
70 primary GBM samples into two clusters: Cluster 1 contained 51
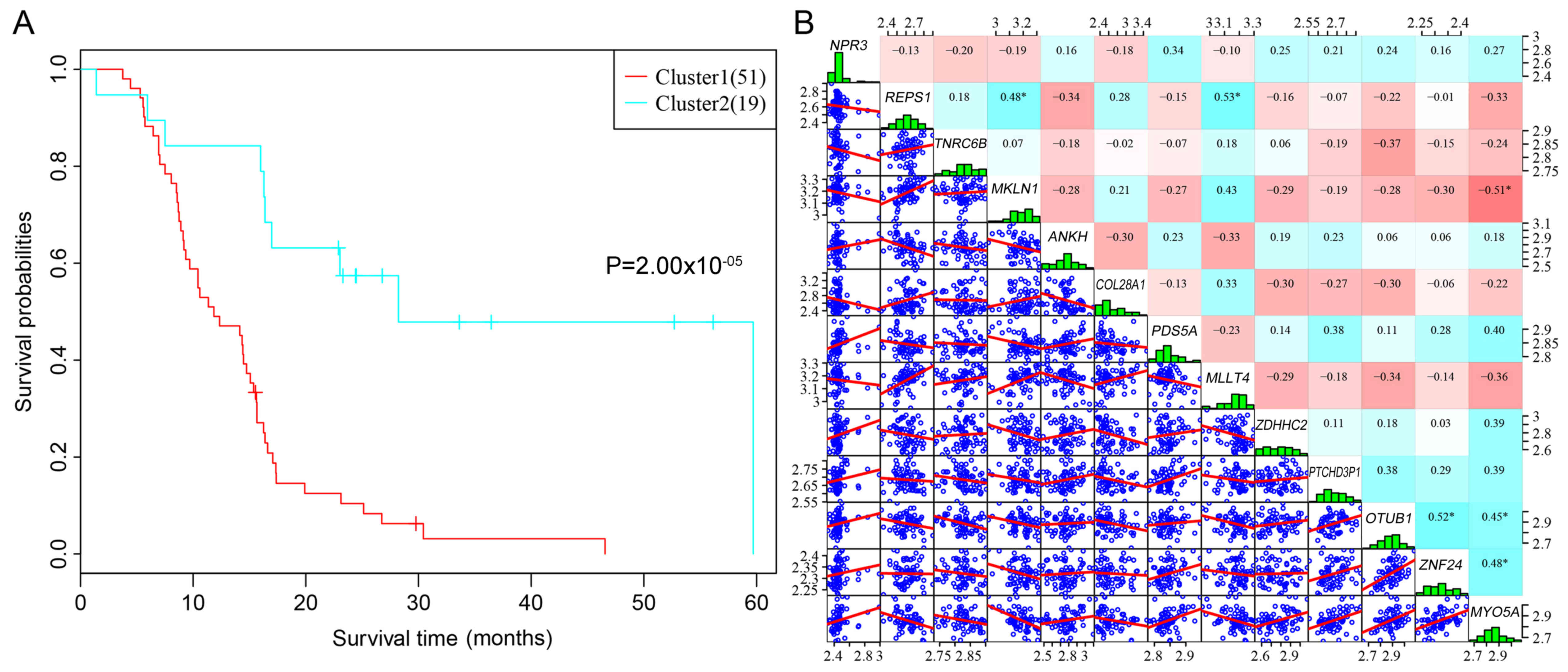
samples and Cluster 2 contained 19 samples (Fig. 2). Subsequently, Kaplan-Meier
survival analysis was used to analyze the prognostic differences
between the two clusters. Patients in the Cluster 1 and in Cluster
2 had significant differences in their prognosis, which indicated
that the 13 prognostic feature genes may effectively differentiate
between high-risk and low-risk patients in a clinical setting
(Fig. 3A). In addition, the
expression levels of the 13 prognostic feature genes were
relatively low, which suggested that they had low redundancy
(Fig. 3B).
 | Table II.A total of 13 prognostic feature
genes were identified from the 1,785 seed genes. |
Table II.
A total of 13 prognostic feature
genes were identified from the 1,785 seed genes.
| Gene ID | nloglik | AIC | Gene |
|---|
| 219789_at | 198.94 | 399.87 | NPR3 |
| 215201_at | 193.8 | 391.6 | REPS1 |
| 213254_at | 190.47 | 386.95 | TNRC6B |
| 225526_at | 186.37 | 380.73 | MKLN1 |
| 223093_at | 183.88 | 377.77 | ANKH |
| 239921_at | 180.76 | 373.52 | COL28A1 |
| 217331_at | 177.81 | 369.62 | PDS5A |
| 224685_at | 177.78 | 371.55 | MLLT4 |
| 244779_at | 176.76 | 371.52 | ZDHHC2 |
| 228786_at | 176.17 | 372.34 |
PTCHD3P1 |
| 201245_s_at | 175.81 | 373.63 | OTUB1 |
| 1554045_at | 173.56 | 371.12 | ZNF24 |
| 204527_at | 170.83 | 367.67 | MYO5A |
Functional annotation of prognostic
feature genes
Using the ‘clusterProfiler’ package, functional
annotation of the prognostic feature genes were conducted. The
results identified three genes, including, natriuretic peptide
receptor 3 (NPR3), ANKH inorganic pyrophosphate transport
regulator and OTU deubiquitinase, ubiquitin aldehyde binding 1
(OTUB1) were significantly enriched in functions, including
‘natriuretic peptide receptor activity’, ‘inorganic phosphate
transmembrane transporter activity’ and ‘NEDD8-specific protease
activity’, respectively. In addition, myeloid/lymphoid or
mixed-lineage leukemia translocated to 4 (MLLT4; also known
as afadin, adherens junction formation factor) was indicated to be
involved in six KEGG pathways, including ‘adherens junction’,
‘leukocyte transendothelial migration’, ‘tight junction’, ‘cAMP
signaling pathway’, ‘Rap1 signaling pathway’ and ‘Ras signaling
pathway’. COL28A1, PDS5A, ZDHHC2 and MYO5A were
enriched in ‘collagen biosynthesis and modifying enzymes’,
‘separation of sister chromatids’, ‘surfactant metabolism’ and
‘regulation of actin dynamics for phagocytic cup formation’
pathways, respectively.
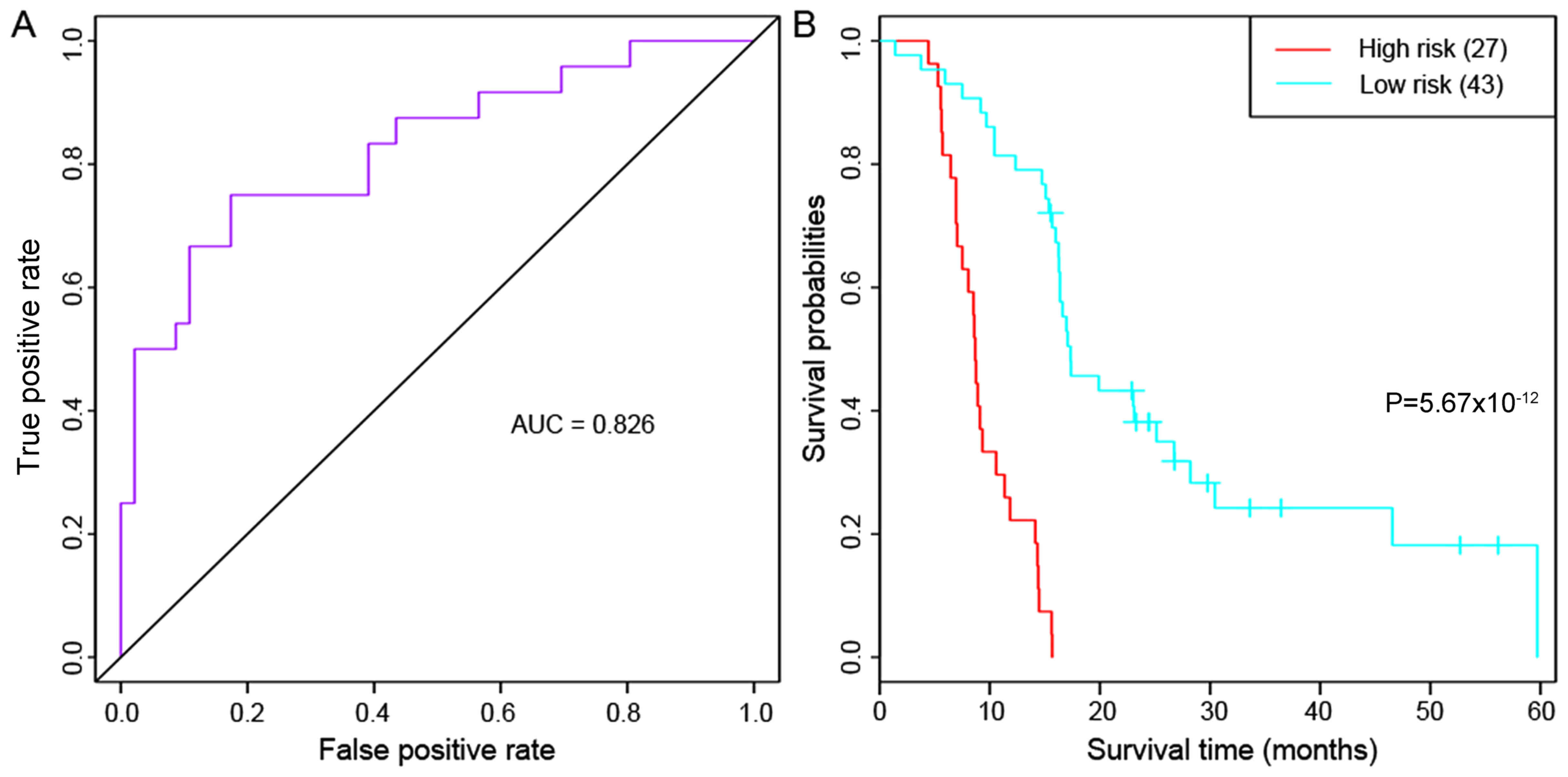
Multivariate survival analysis
To check the overall influence of the prognostic
feature genes on prognosis, multivariate survival analysis was
carried out. These genes were classified as having a positive
effect on prognosis (area under the ROC curve=0.826) for the 70
primary GBM samples (Fig. 4A).
Furthermore, Kaplan-Meier survival analysis demonstrated that the
samples in the high-risk and low-risk groups differed significantly
in their prognosis (P=5.67×10−12; Fig. 4B), which indicated that the 13
prognostic feature genes may effectively distinguish samples with a
different prognostic risk.
Validation of the prognostic feature
genes using another independent data set
The GBM data set was downloaded from the TCGA
database and used to validate the prognostic feature genes.
Survival analysis demonstrated that the 13 prognostic feature genes
had good classification effects on samples in the validation data
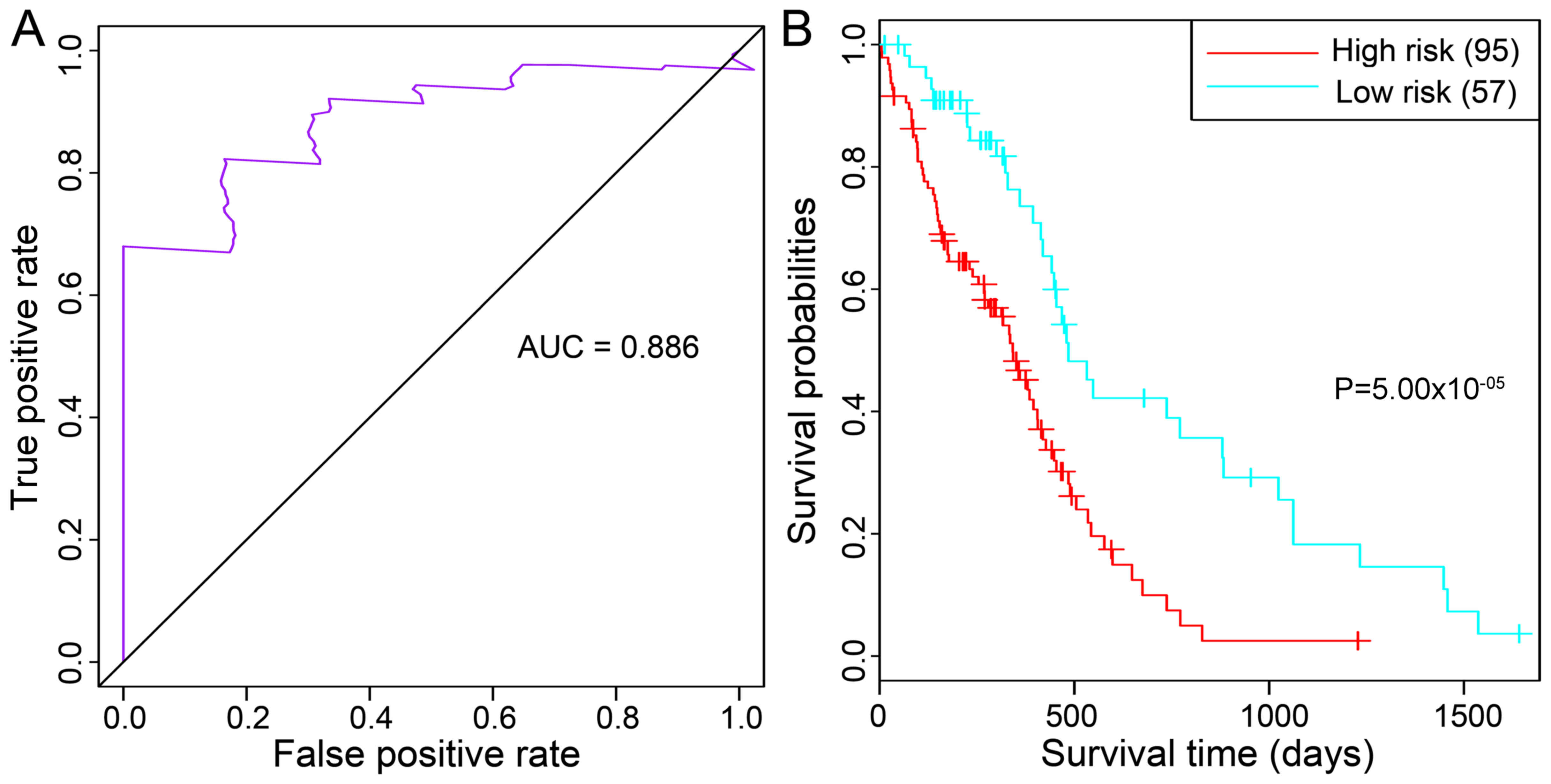
set (area under the ROC curve =0.886) (Fig. 5A). The high-risk and low-risk
groups differed significantly in their prognosis
(P=5.00×10−5; Fig. 5B).
Furthermore, age distribution analysis of the classified primary
GBM samples demonstrated that samples in the high-risk and low-risk
groups differed significantly in age, with the high-risk group
being older than the low-risk group (61.54 vs. 56.96; P=0.04396;
Table III). There was no
significant difference concerning sex distribution of the
classified samples.
 | Table III.Age distribution of the samples in
the high-risk and low-risk groups. |
Table III.
Age distribution of the samples in
the high-risk and low-risk groups.
| Sample group | Min age | Mean age | Max age | Mann-Whitney
test | Female | Male | P-value |
|---|
| High risk
(n=95) | 21 | 61.54 | 89 | P=0.04396 | 32 | 64 | P=0.51 |
| Low risk
(n=57) | 21 | 56.96 | 85 |
| 22 | 35 |
|
Discussion
In the present study, a total of 1,785 gene probes
with significant prognostic differences were selected as seed
genes. From these, 13 prognostic feature genes, including
COL28A1, PDS5A, ZDHHC2, ZNF24, MYO5A, MLLT4, NPR3, ANKH and
OTUB1, were further screened. The prognostic feature genes
performed well for the classification of samples into different
prognostic risk categories. Additionally, the GBM data set
downloaded from the TCGA database further confirmed the association
of the 13 prognostic feature genes with the prognosis of GBM.
Collagen XVI may promote the invasion of glioma
cells by damaging cell-cell interactions or mediating the
β1-integrin activation pattern, which provides new approaches for
treating cancer in neuro-oncology (33). Fibrillar collagens and the collagen
internalization receptor, endocytic receptor 180 (Endo180), are
overexpressed in GBM (34).
Additionally, Endo180 affects the invasion and progression of
tumors (34). Collagen type I α1
(CO1A1) was reported to serve a suppressive biological role
in the progression of glioma, suggesting that it may be applied to
treating the disease (35). The
COL28A1 protein is a filament-forming collagen and is detected in
the adult sciatic nerve (36). In
our study, COL28A1 was enriched in ‘collagen biosynthesis
and modifying enzymes’ pathway. Therefore, COL28A1 may serve
a role in the progression of GBM.
PDS5A is tissue-specific and has two-fold
effects in tumorigenesis, acting as a tumor suppressor or an
oncogenic factor to promote tumor proliferation (37). PDS5A was previously reported
to be overexpressed in high-grade gliomas and is positively
associated with the World Health Organization grade of gliomas
(37). Based on our enrichment
result, PDS5A was involved in ‘separation of sister
chromatids’ pathway, indicating it might influence the tumor's DNA
synthesis phase in GBM development.
ZDHHC2 on chromosome 8p21.3–22 belongs to the
DHHC-domain protein family of protein acyltransferases (38). As the largest transcription factor
family in humans, zinc-finger proteins serve roles in multiple
biological processes, including autophagy, metabolism, development
and differentiation, and in cancer progression (39). In addition, ZDHHC2 is
associated with prognosis in a number of types of cancer. In
gastric cancer, decreased ZDHHC2 expression is associated
with lymph node metastasis and indicates a poor prognosis (40). In the present study, ZDHHC2
was highly associated with the ‘surfactant metabolism’ pathway and
identified as a prognostic gene in GBM, suggesting that it might
have an important role in GBM prognosis.
ZNF24 plays a critical role in brain
development and various cancer types. Furthermore, ZNF24 may
contribute to cell cycle promotion and maintenance of the
progenitor stage of neural cells (41). Platelet-derived growth factor
receptor (PDGFR) signaling is a crucial mechanism for the
initiation and development of GBM (42). In GBM cell lines, ZNF24
negatively regulates two transcription factors, vascular
endothelial growth factor (VEGF) and PDGFR-β (43).
In the present study, the above three genes were
among the 13 crucial prognostic genes of GBM. The collective
findings implicate PDS5A, ZDHHC2 and ZNF24 are
involved in the pathogenesis of GBM. Although no studies have
reported the potential roles of ZDHHC2 in GBM, this gene may
also be associated with the prognosis of GBM, based on our
results.
Myosin II is required for the invasion of glioma
cells and is a promising target for the anti-invasive treatment of
malignant brain tumors (44,45).
In 1321N1 GBM cells, MYO1C is crucial for lamellipodia
formation to produce a protein complex promoting cell migration
(46). MYO6 was implicated
in human glioma and its inhibition may be a promising therapeutic
method for the disease (47). The
activation of myosin-associated contractility sensitizes GBM
tumor-initiating cells, which subsequently weakens the invasive
ability of the cells (48). In the
present study, MYO5A was enriched in ‘regulation of actin
dynamics for phagocytic cup formation’. Thus, MYO5A may
serve a role in the progression of GBM.
MLLT4 is one of the aliases of the gene,
afadin, adherens junction formation factor, which belongs to an
adhesion system and encodes a protein participating in cell
junctions during embryogenesis. Neurofibromin 1 (NF1)
mutations have been identified in several cancer types, including
GBM (49). Furthermore, in a mouse
model of NF1-associated optic pathway gliomas (OPG), cyclic
adenosine 3′,5′-monophosphate (cAMP) inhibits the growth of OPG
(50). Notably, MLLT4 is
one of the NF1-regulated effectors downstream of RAS
(51). Results from the present
studies support the possible importance of MLLT4 in GBM
pathogenesis through the participation in the adherens junction,
cAMP signaling and Ras signaling pathways. In addition, a number of
pathways, such as leukocyte transendothelial migration, are
enriched in GBM-derived extracellular vesicles (EVs), which
suggested that GBM cells may exhibit mechanisms to selectively
combine these proteins in EVs (52). In the present study, leukocyte
transendothelial migration was one of the six enriched pathways
identified for MLLT3; therefore, this pathway may also be an
important regulation target in GBM development. With regard to the
tight junction pathway, junctional adhesion molecule (JAM) is one
family of the immunoglobulin-like superfamily expressed in tight
junctions, and abnormal JAM-A expression was reported to contribute
to the progression of GBM (53).
This may be evidence of the gene expression alterations in the
tight junction pathway in the development of GBM.
NPR3 encodes a natriuretic peptide receptor,
which regulates metabolic processes. A long noncoding RNA (lncRNA),
BCYRN1, has an oncogenic role in colorectal cancer cells by
upregulating NPR3 expression (54). In clear cell renal cell carcinoma
(ccRCC), the lncRNA MRCCAT1 has been observed to promote ccRCC
metastasis by inhibiting NPR3 (55). ANKH is identified as a novel
putative oncogene in small cell lung cancer cell lines (56). In cervical cancer, ANKH is
also suggested as an oncogene and the upregulation is validated by
reverse transcription-quantitative polymerase chain reaction
(57). To the best of our
knowledge, there have been no studies reporting an association
between these two genes, NPR3 and ANKH, and glioma.
However, in the present study, these were important prognostic
genes of GBM and were enriched in crucial pathways, suggesting that
they may be novel markers in GBM and correlate with prognosis.
The OTUB1 protein is a deubiquitinating enzyme. In
glioma tissues, expression of OTUB1 is increased and the
expression level is associated with the glioma grade; on the other
hand, knockdown of OTUB1 suppresses the tumor cell migration
(58). This finding suggests that
OTUB1 serves an important role in the etiology of glioma
(58).
However, all these predictive results need to be
further validated by in vitro and in vivo
experiments. Notably, patients in the high-risk group were slightly
older compared with patients in the low-risk group; although the
difference was minor, it was significant. A previous study
identified age as a risk factor for the process of
dexamethasone-induced leukocytosis, which is associated with poor
survival in the newly diagnosed GBM (59). Therefore, the present study
suggested that age may be a risk factor of GBM, but this needs to
be validated using larger data sets.
In conclusion, the 13-gene set was tested and
verified, and very efficiently predicted the prognosis of GBM in
independent data sets. In addition, COL28A1, PDS5A, ZDHHC2,
ZNF24, MYO5A and MLLT4 were implicated as key genes
involved in the prognosis of GBM. The adherens junction, cAMP and
Ras signaling pathways may be important in the progression of GBM,
and age may be a risk factor for prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY performed data analyses and wrote the manuscript.
LJ contributed significantly to the data analysis and manuscript
revision. XS conceived and designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young RM, Jamshidi A, Davis G and Sherman
JH: Current trends in the surgical management and treatment of
adult glioblastoma. Ann Transl Med. 3:1212015.PubMed/NCBI
|
|
3
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallego O: Nonsurgical treatment of
recurrent glioblastoma. Curr Oncol. 22:e273–e281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fulci G and Chiocca EA: The status of gene
therapy for brain tumors. Expert Opin Biol Ther. 7:197–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alptekin M, Eroglu S, Tutar E, Sencan S,
Geyik MA, Ulasli M, Demiryurek AT and Camci C: Gene expressions of
TRP channels in glioblastoma multiforme and relation with survival.
Tumor Biology. 36:9209–9213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Luan Y, Yu R, Zhang Z, Zhang J and
Wang W: Transient receptor potential (TRP) channels, promising
potential diagnostic and therapeutic tools for cancer. Biosci
Trends. 8:1–10. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong Y, Shang C, Xue YX and Liu YH:
Silencing of Bmi-1 gene enhances chemotherapy sensitivity in human
glioblastoma cells. Med Sci Monit. 21:1002–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye L, Wang C, Yu G, Jiang Y, Sun D, Zhang
Z, Yu X, Li X, Wei W, Liu P, et al: Bmi-1 induces radioresistance
by suppressing senescence in human U87 glioma cells. Oncol Lett.
8:2601–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiddingh L, Tannous BA, Teng J, Tops B,
Jeuken J, Hulleman E, Boots-Sprenger SH, Vandertop WP, Noske DP,
Kaspers GJ, et al: EFEMP1 induces γ-secretase/Notch-mediated
temozolomide resistance in glioblastoma. Oncotarget. 5:363–374.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Chen L, Han L, Shi Z, Zhang J, Pu
P and Kang C: EZH2 is a negative prognostic factor and exhibits
pro-oncogenic activity in glioblastoma. Cancer Lett. 356:929–936.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Ren Y, Zhang J, Zhang C, Zhang K,
Han L, Kong L, Wei J, Chen L, Yang J, et al: HOTAIR is a
therapeutic target in glioblastoma. Oncotarget. 6:8353–8365.
2015.PubMed/NCBI
|
|
13
|
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi
Z, Zhang A, Ye M, Wang Q, Liu C, et al: Long non-coding RNA HOTAIR
promotes glioblastoma cell cycle progression in an EZH2 dependent
manner. Oncotarget. 6:537–546. 2015.PubMed/NCBI
|
|
14
|
Insin P and Prueksaritanond N: Evaluation
of four risk of malignancy indices (RMI) in the preoperative
diagnosis of ovarian malignancy at Rajavithi hospital. Thai J
Obstet Gynaecol. 21:2013.
|
|
15
|
Sim J, Nam DH, Kim Y, Lee IH, Choi JW, Sa
JK and Suh YL: Comparison of 1p and 19q status of glioblastoma by
whole exome sequencing, array-comparative genomic hybridization,
and fluorescence in situ hybridization. Med Oncol. 35:602018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Q, Cai G, Yu Q, Shen J, Gu Z, Chen J,
Shi W and Shi J: IDH1 mutation diminishes aggressive phenotype in
glioma stem cells. Int J Oncol. 52:270–278. 2017.PubMed/NCBI
|
|
17
|
Han J and Puri RK: Analysis of the cancer
genome atlas (TCGA) database identifies an inverse relationship
between interleukin-13 receptor α1 and α2 gene expression and poor
prognosis and drug resistance in subjects with glioblastoma
multiforme. J Neurooncol. 136:463–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guadagno E, Vitiello M, Francesca P, Calì
G, Caponnetto F, Cesselli D, Camorani S, Borrelli G, Califano M,
Cappabianca P, et al: PATZ1 is a new prognostic marker of
glioblastoma associated with the stem-like phenotype and enriched
in the proneural subtype. Oncotarget. 8:59282–59300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murat A, Migliavacca E, Gorlia T, Lambiv
WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven
MC, et al: Stem cell-related ‘self-renewal’ signature and high
epidermal growth factor receptor expression associated with
resistance to concomitant chemoradiotherapy in glioblastoma. J Clin
Oncol. 26:3015–3024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lambiv WL, Vassallo I, Delorenzi M, Shay
T, Diserens AC, Misra A, Feuerstein B, Murat A, Migliavacca E,
Hamou MF, et al: The Wnt inhibitory factor 1 (WIF1) is targeted in
glioblastoma and has a tumor suppressing function potentially by
induction of senescence. Neuro Oncol. 13:736–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stupp R, Dietrich PY, Ostermann Kraljevic
S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G,
Miralbell R, et al: Promising survival for patients with newly
diagnosed glioblastoma multiforme treated with concomitant
radiation plus temozolomide followed by adjuvant temozolomide. J
Clin Oncol. 20:1375–1382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Therneau TM: Survival analysis (R package
survival version 2.39–5). 2015.
|
|
24
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Boil. 8:R1832007. View Article : Google Scholar
|
|
26
|
Cho HJ, Kim S, Kang J and Lee JW: How to
use the rbsurv Package. 2010.
|
|
27
|
Renaud G, Stenzel U, Maricic T, Wiebe V
and Kelso J: deML: Robust demultiplexing of Illumina sequences
using a likelihood-based approach. Bioinformatics. 31:770–772.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang SJ: Unsupervised hierarchical
clustering based on sequential partitioning and merging.
International Symposium on Vlsi Design, Automation and Test.
2016.
|
|
29
|
Porcher R: CORR Insights(®):
Kaplan-meier survival analysis overestimates the risk of revision
arthroplasty: A meta-analysis. Clin Orthop Relat Res.
473:3431–3442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith B, Williams J and Schulze-Kremer S:
The ontology of the gene ontology. AMIA Annu Symp Proc. 609–613.
2003.PubMed/NCBI
|
|
31
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heagerty PJ, Thomas L and Pepe MS:
Time-dependent ROC curves for censored survival data and a
diagnostic marker. Biometrics. 56:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bauer R, Ratzinger S, Wales L, Bosserhoff
A, Senner V, Grifka J and Grässel S: Inhibition of collagen XVI
expression reduces glioma cell invasiveness. Cell Physiol Biochem.
27:217–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huijbers IJ, Iravani M, Popov S, Robertson
D, Alsarraj S, Jones C and Isacke CM: A role for fibrillar collagen
deposition and the collagen internalization receptor endo180 in
glioma invasion. PLoS One. 5:e98082010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Honma K, Miyata T and Ochiya T: Type I
collagen gene suppresses tumor growth and invasion of malignant
human glioma cells. Cancer Cell Int. 7:122007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Veit G, Kobbe B, Keene DR, Paulsson M,
Koch M and Wagener R: Collagen XXVIII, a novel von Willebrand
factor A domain-containing protein with many imperfections in the
collagenous domain. J Biol Chem. 281:3494–3504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hagemann C, Weigelin B, Schommer S,
Schulze M, Al-Jomah N, Anacker J, Gerngras S, Kühnel S, Kessler AF,
Polat B, et al: The cohesin-interacting protein, precocious
dissociation of sisters 5A/sister chromatid cohesion protein 112,
is up-regulated in human astrocytic tumors. Int J Mol Med.
27:39–51. 2011.PubMed/NCBI
|
|
38
|
Oyama T, Miyoshi Y, Koyama K, Nakagawa H,
Yamori T, Ito T, Matsuda H, Arakawa H and Nakamura Y: Isolation of
a novel gene on 8p21.3-22 whose expression is reduced significantly
in human colorectal cancers with liver metastasis. Genes
Chromosomes Cancer. 29:9–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jen J and Wang YC: Zinc finger proteins in
cancer progression. J Biomed Sci. 23:532016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan SM, Tang JJ, Huang CY, Xi SY, Huang
MY, Liang JZ, Jiang YX, Li YH, Zhou ZW, Ernberg I, et al: Reduced
expression of ZDHHC2 is associated with lymph node metastasis and
poor prognosis in gastric adenocarcinoma. PLoS One. 8:e563662013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khalfallah O, Ravassard P, Che SL, Fligny
C, Serre A, Bayard E, Faucon-Biguet N, Mallet J, Meloni R and
Nardelli J: Zinc finger protein 191 (ZNF191/Zfp191) is necessary to
maintain neural cells as cycling progenitor. Stem Cells.
27:1643–1653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carrasco-Garcia E, Martinez-Lacaci I,
Mayor-López L, Tristante E, Carballo-Santana M, García-Morales P,
Ventero Martin MP, Fuentes-Baile M, Rodriguez-Lescure Á and Saceda
M: PDGFR and IGF-1R inhibitors induce a G2/M arrest and subsequent
cell death in human glioblastoma cell lines. Cells. 7:E1312018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li JZ, Chen X, Liu Y, Ding L, Qiu L, Hu ZL
and Zhang J: The transcriptional repression of platelet-derived
growth factor receptor-beta by the zinc finger transcription factor
ZNF24. Biochem Biophys Res Commun. 397:318–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beadle C, Assanah MC, Monzo P, Vallee R,
Rosenfeld SS and Canoll P: The role of myosin II in glioma invasion
of the brain. Mol Biol Cell. 19:3357–3368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ivkovic S, Beadle C, Noticewala S, Massey
SC, Swanson KR, Toro LN, Bresnick AR, Canoll P and Rosenfeld SS:
Direct inhibition of myosin II effectively blocks glioma invasion
in the presence of multiple motogens. Mol Biol Cell. 23:533–542.
2011. View Article : Google Scholar
|
|
46
|
Edimo WE, Ramos AR, Ghosh S, Vanderwinden
JM and Erneux C: The SHIP2 interactor Myo1c is required for cell
migration in 1321 N1 glioblastoma cells. Biochem Biophys Res
Commun. 476:508–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu R, Fang XH and Zhong P: Myosin VI
contributes to malignant proliferation of human glioma cells.
Korean J Physiol Pharmacol. 20:139–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong SY, Ulrich TA, Deleyrolle LP, MacKay
JL, Lin JM, Martuscello RT, Jundi MA, Reynolds BA and Kumar S:
Constitutive activation of myosin-dependent contractility
sensitizes glioma tumor-initiating cells to mechanical inputs and
reduces tissue invasion. Cancer Res. 75:1113–1122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Warrington NM, Sun T, Luo J, Mckinstry RC,
Parkin PC, Ganzhorn S, Spoljaric D, Albers AC, Merkelson A, Stewart
DR, et al: The cyclic AMP pathway is a sex-specific modifier of
glioma risk in type I neurofibromatosis patients. Cancer Res.
75:16–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kiuru M and Busam KJ: The NF1 gene in
tumor syndromes and melanoma. Lab Invest. 97:146–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Vrij J, Maas SL, Kwappenberg KM,
Schnoor R, Kleijn A, Dekker L, Luider TM, de Witte LD, Litjens M,
van Strien ME, et al: Glioblastoma-derived extracellular vesicles
modify the phenotype of monocytic cells. Int J Cancer.
137:1630–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Leech AO, Cruz RG, Hill AD and Hopkins AM:
Paradigms lost-an emerging role for over-expression of tight
junction adhesion proteins in cancer pathogenesis. Ann Transl Med.
3:1842015.PubMed/NCBI
|
|
54
|
Gu L, Lu LS, Zhou DL and Liu ZC: Long
noncoding RNA BCYRN1 promotes the proliferation of colorectal
cancer cells via Up-regulating NPR3 expression. Cell Physiol
Biochem. 48:2337–2349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li JK, Chen C, Liu JY, Shi JZ, Liu SP, Liu
B, Wu DS, Fang ZY, Bao Y, Jiang MM, et al: Long noncoding RNA
MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via
inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer.
16:1112017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Coe BP, Henderson LJ, Garnis C, Tsao MS,
Gazdar AF, Minna J, Lam S, Macaulay C and Lam WL: High-resolution
chromosome arm 5p array CGH analysis of small cell lung carcinoma
cell lines. Genes Chromosomes Cancer. 42:308–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kloth JN, Oosting J, van Wezel T, Szuhai
K, Knijnenburg J, Gorter A, Kenter GG, Fleuren GJ and Jordanova ES:
Combined array-comparative genomic hybridization and
single-nucleotide polymorphism-loss of heterozygosity analysis
reveals complex genetic alterations in cervical cancer. BMC
Genomics. 8:532007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu L, Li J, Bao Z, Xu P, Chang H, Wu J,
Bei Y, Xia L, Wu P, Yan K, et al: Silencing of OTUB1 inhibits
migration of human glioma cells in vitro. Neuropathology.
37:217–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dubinski D, Won SY, Gessler F,
Quick-Weller J, Behmanesh B, Bernatz S, Forster MT, Franz K, Plate
KH, Seifert V, et al: Dexamethasone-induced leukocytosis is
associated with poor survival in newly diagnosed glioblastoma. J
Neurooncol. 137:503–510. 2018. View Article : Google Scholar : PubMed/NCBI
|