Introduction
Prolonged inflammation can lead to many diseases,
such as inflammatory bowel disease, arthritis, and septic shock
syndrome. Excessive inflammatory responses can induce disease
pathogenesis and may interrupt tissue functions (1). The exposure of immune cells and
macrophages to specific agonists stimulates complex signaling
cascades, which immediately generate the production of
pro-inflammatory cytokines and chemokines, notably macrophage
inflammatory protein-2 (MIP2), interleukin-6 (IL-6), nitric oxide
(NO), tumor necrosis factor-α (TNF-α), inducible nitric oxide
synthase (iNOS), and cyclooxygenase-2 (COX2), which play a vital
part in the initiation of the inflammatory response (2–4).
Heme oxygenase-1 (HO-1) is a member of the heat
shock protein (HSP) family; it also participates in heme catabolism
as a rate-limiting enzyme, and this catabolism releases the three
by-products including carbon monoxide (CO), free iron
(Fe2+), and biliverdin (5). Biliverdin reductase causes the
conversion of biliverdin to bilirubin. The complete protective
effects of HO-1 comprise the antioxidant effects of both biliverdin
and bilirubin (6,7). HO-1 and its by-products play a
significant role in the resolution stage of inflammation, in which
macrophages are targeted (8).
Previous experiments have determined that the expression of HO-1
prevents the production of chemokines and pro-inflammatory
cytokines, such as IL-1β, IL-6, and TNF-α, in stimulated
macrophages (9,10). Moreover, the upregulation of HO-1
expression mitigates the expression of the pro-inflammatory
proteins COX-2 and iNOS, and consequently suppressed the production
of COX-2-derived prostaglandin E2 (PGE2) and
iNOS-derived NO production (11,12).
Through the inactivation of nuclear factor-κB (NF-κB), HO-1 also
prevents iNOS expression and NO production (13,14).
Hence, a group of therapeutic agents have been found to upregulate
the expression of HO-1 and utilize anti-inflammatory responses
mediated by HO-1 initiation (13,15).
NF-κB is an important aspect of the inflammation response. NF-κB is
stimulated by lipopolysaccharides (LPS) through the phosphorylation
of inhibitor of κB-α (IκB-α). Subsequently, NF-κB translocates to
the nucleus after detachment from IκB-α (16,17).
After NF-κB reaches at the nucleus, this molecule attaches to
defined sites on the DNA and coordinates the transcription of its
target genes that are responsible for the production of
pro-inflammatory mediators and cytokines (18).
Extraction is a vital stage in phytochemical
processing for the introduction of new bioactive components from
natural sources. The choice of a compatible extraction method is
also vital for the standardization of natural herbal products, as
it is applied for the isolation of desirable soluble components.
Many types of extraction methods are popular, including
conventional methods such as decoction, percolation, hot continuous
extraction, and maceration. During the last three decades, an
alternative technique known as microwave-assisted solvent
extraction (MASE) has emerged as attractive (19). Microwaves are non-ionizing
electromagnetic waves with a frequency of between 300 MHz and 300
GHz, positioned in the electromagnetic spectrum between infrared
and X-ray radiation (20). Dipolar
rotation and ionic conduction are the two important mechanisms that
occur simultaneously in electric field heating (21). Hence, a significant advantage of
modern MASE systems is solvent-free functionality. MASE also offers
an environmentally friendly green extraction technique for
essential oils and other volatile natural products.
Sappan Lignum, the dried heartwood of Caesalpinia
sappan Linn., has been used extensively in the Asia-Pacific
region as a traditional herbal medicine. It has also been used as a
natural red dye, conventional food and beverage ingredient, and as
a traditional Chinese medicine component for analgesic and
anti-inflammatory remedies or to promote menstruation and blood
circulation (22). Studies of
Sappan Lignum have reported various pharmacological effects,
including anti-atherosclerotic, analgesic (23), antioxidant (24), hypoglycemic (25), anti-complementary (26), vasorelaxation (27), anti-inflammatory (28), spasmolytic activity (29), cytotoxic (30), muscle contractile (31), anticonvulsant (32), antiviral (33), and antibacterial (34) activities. The ethyl acetate (EtOAc)
extract of Sappan Lignum exerted significant inhibitory effects on
mitosis and growth-related signaling (35). In our previous study, we initially
made each microwave-assisted solvent extract from twenty herbal
plants. Later, we checked the anti-inflammatory effect of these
twenty microwave-assisted solvent extracts, the microwave-70% EtOH
extract of Sappan Lignum has good anti-inflammatory actions among
all of the samples. Therefore, we compared the anti-inflammatory
effects of Sappan Lignum extracted by using heat-70% EtOH
extraction (CSE-H-70E) or microwave-70% EtOH extraction
(CSE-MW-70E) in this investigation.
Materials and methods
Plant materials and extraction
The heartwood of C. sappan was purchased from
Omni Herb, Daegu, Korea in March 2012. The voucher specimen
(WK-2012-0301) was deposited at the Herbarium of the College of
Pharmacy, Wonkwang University (Korea). The dried heartwood of C.
sappan was cut and extracted in 70% EtOH by using heat or
microwave extraction. The dried heartwood of C. sappan (30
g) was extracted in 70% EtOH (500 ml in a 1 L round flask) for 15
min at 80°C by using a normal heat-extraction method. The heat-70%
EtOH extraction (CSE-H-70E) was evaporated in vacuo to yield 240,
210, and 258 mg of residue (0.79±0.08% v/v; Table I). In addition, C. sappan
(30 g) was extracted with 70% EtOH (500 ml in a 1 L round flask) by
using microwave-extraction (three extractions at 2450 Hz and 700 W
for 5 min). During the microwave extraction, 500 ml water was
reacted as the coolant and the water was changed each time.
Finally, the microwave-70% EtOH extraction (CSE-MW-70E) was
evaporated in vacuo to yield 345, 297, and 375 mg of residue
(1.13±0.13% v/v; Table I). Values
are presented as mean ± standard deviation (mean ± SD).
 | Table I.Comparison between the extracted mass
and percentage yield of CSE-H-70E and CSE-MW-70E. |
Table I.
Comparison between the extracted mass
and percentage yield of CSE-H-70E and CSE-MW-70E.
|
| Yield, mg |
|
|---|
|
|
|
|
|---|
| Sample | Exp. 1 | Exp. 2 | Exp. 3 | Yield, % |
|---|
| CSE-H-70E | 240 | 210 | 258 | 0.79±0.08 |
| CSE-MW-70E | 345 | 297 | 375 | 1.13±0.13 |
Chemical and reagents
RPMI-1640, fetal bovine serum (FBS), and other cell
culture reagents were purchased from Gibco-BRL Co. (Grand Island,
NY, USA). All chemicals were obtained from Sigma-Aldrich; Merck
KGaA (Darmstadt, German). Antibodies to anti-iNOS, anti-COX-2,
anti-HO-1, and β-actin were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
Cell culture and viability assay
RAW264.7 cells were maintained at a density of
1×107 cells/ml in RPMI-1640 medium supplemented with 10%
FBS and 1% antibiotics and were incubated at 37°C in a humidified
atmosphere containing 5% CO2. To determine the cell
viability by using an MTT assay, the cells were maintained at
1×104 cells/well and then treated with the extract.
After incubation, the medium was removed from each well, 200 µl
fresh medium was placed in each well, and the cells were incubated
with 0.5 mg/ml MTT for 3 h (36).
High-performance liquid chromatography
(HPLC)
Chromatography was performed by using a YL-9100
series HPLC instrument (YoungLin, Korea). In all experiments, C-18
column (SHISEIDO CAPCELL PAK, 4.6×150 mm; 5 µm) was used as the
stationary phase and the injection volume was 20 µl. Samples were
prepared that contained 2 mg/ml CSE-H-70E and CSE-MW-70E. The
mobile phase consisted of water plus 0.1% formic acid (A) and MeOH
(B) eluted as a linear gradient of 0–50 min from 10% B to 100% B.
The detection wavelength used was 254 nm and the flow rate was 0.7
ml/min.
Western blotting
The cells were harvested and pelleted by
centrifugation at 14,000 × g for 3 min at 4°C. The cells
were washed with PBS and lysed in 20 mM Tris-HCl buffer (pH 7.4)
containing a protease inhibitor mixture (0.1 mM
phenylmethanesulfonyl fluoride, 5 mg/ml aprotinin, 5 mg/ml
pepstatin A, and 1 mg/ml chymostatin). The protein concentration of
the lysed samples was determined by using a Lowry protein assay kit
(Sigma-Aldrich; Merck KGaA), and 30 µg of protein from each sample
was resolved by 7.5 or 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), and then electrophoretically
transferred onto a Hybond enhanced chemiluminescence (ECL)
nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Non-specific
binding to the membrane was blocked by incubation in 5% skimmed
milk and the membrane was sequentially processed by incubation with
primary antibodies (Santa Cruz Biotechnology and Cell Signaling
Technology), horseradish peroxidase-conjugated secondary
antibodies, and detected by the application of ECL (Amersham
Pharmacia Biotech, Piscataway, NJ, USA).
Determination of nitrite as an
indicator of NO production
The production of nitrite, a stable end-product of
NO oxidation, was used for the measurement of iNOS activity. The
concentration of nitrite in the conditioned medium was determined
by a method based on the Griess reaction (37). The NO levels were determined from
the concentration of nitrite as assessed by the Griess reaction.
The supernatant (100 µl) was mixed with Griess reagent (100 µl),
and then the absorbance at 525 nm was determined by using an ELISA
plate reader from Bio-Rad.
PGE2, IL-1β, IL-6, IL-12
and TNF-α assays and DNA binding activity of NF-κB
The culture medium was collected and the level of
PGE2, IL-1β, IL-6, IL-12 and TNF-α present in each
sample was determined by using a commercially available kit from
R&D Systems, Inc. The DNA-binding activity of NF-κB in nuclear
extracts was measured by using the TransAM kit (Active Motif,
Carlsbad, CA, USA). The assays, including PGE2, IL-1β,
IL-6, IL-12 and TNF-α production and NF-κB DNA-binding activity,
were performed in accordance with the manufacturer's
instructions.
Reverse transcription-polymerase chain
reaction (PCR)
Total RNA was isolated from the cells by using
TRIzol (Invitrogen, Carlsbad, CA, USA) in accordance with the
manufacturer's recommendations, and quantified
spectrophotometrically at 260 nm. The following primer sequences
were designed by using PrimerQuest (Integrated DNA Technologies,
Cambridge, MA, USA): HO-1, forward, 5′-CTCTTGGCTGGCTTCCTT-3′ and
reverse, 5′-GGCTCCTTCCTCCTTTCC-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward, 5′-ACTTTGGTATCGTGGAAGGACT-3′ and
reverse, 5′-GTAGAGGCAGGGATGATGTTCT-3′. The optimal conditions for
PCR amplification of the cDNA were established by in accordance
with the manufacturer's instructions. The data were analyzed by
using StepOne software (Applied Biosystems, Carlsbad, CA, USA), and
the cycle number at the linear amplification threshold (Ct) values
for the endogenous control gene (GAPDH) and the target gene were
recorded. The relative gene expression (target gene expression
normalized to the expression of the endogenous control gene) was
calculated by using the comparative Ct method
(2−ΔΔCt).
Statistical analysis
The data are expressed as the mean ± standard
deviation of three independent experiments. To compare between
three groups, one-way analysis of variance followed by the
Newman-Keuls post hoc test was used. Statistical analysis was
computed using GraphPad Prism software, version 3.03 (GraphPad
Software Inc., San Diego, CA, USA).
Results
HPLC analysis of CSE-H-70E and
CSE-MW-70E
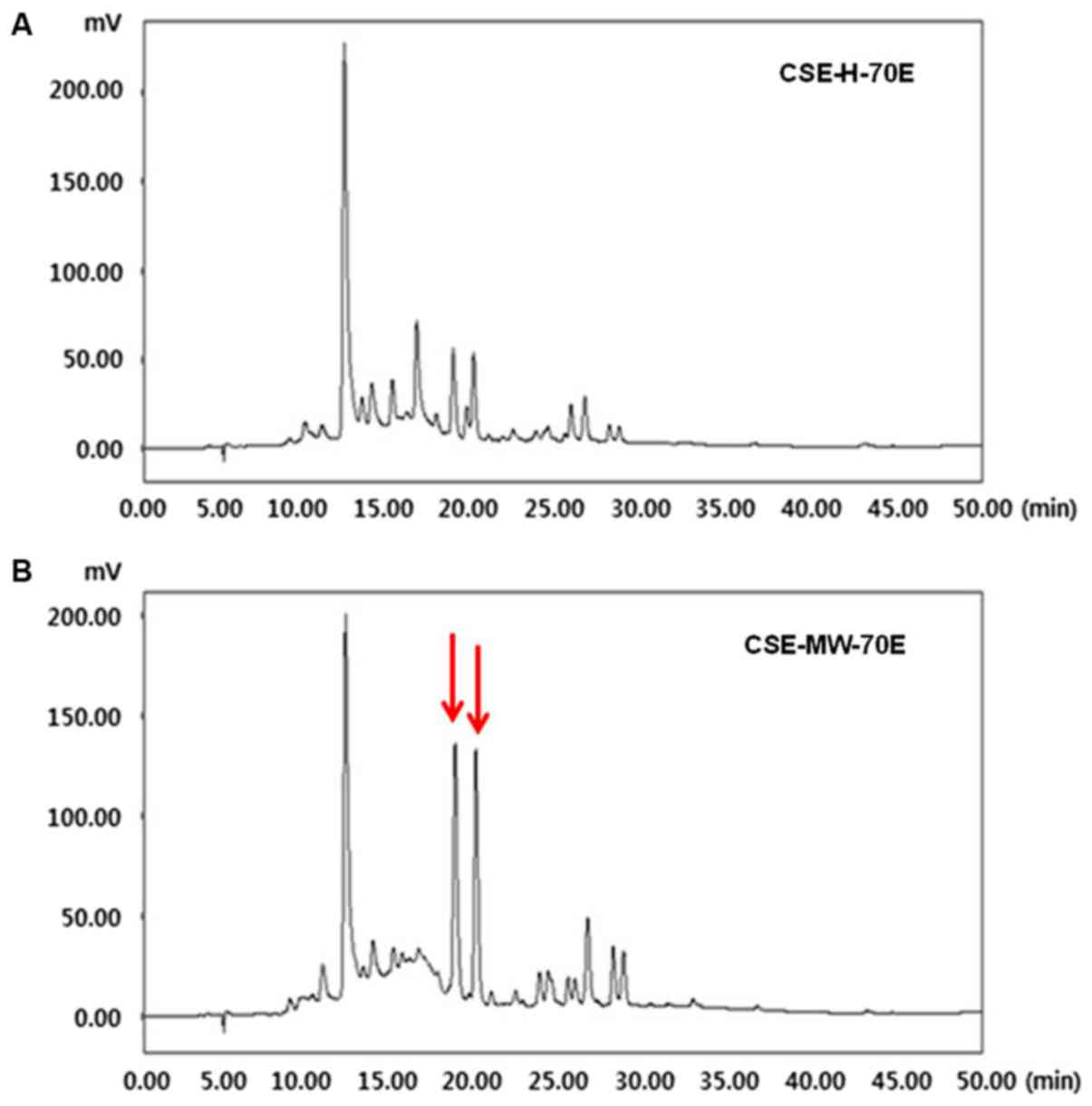
The HPLC analysis of CSE-H-70E and CSE-MW-70E was
presented in the form of chromatograms of the detected reaction at
254 nm. As shown in Fig. 1A and B,
the retention time of the main peak was 12.00 min for both
fractions. However, in Fig. 1B,
two identical peaks were observed at ~19-20 min for CSE-MW-70E.
Effects of CSE-H-70E and CSE-MW-70E on
the expression of nitrite, PGE2 production, and iNOS and
COX-2 proteins in LPS-stimulated RAW264.7 cells
The primary indication of the anti-inflammatory
effects of CSE-H-70E and CSE-MW-70E in LPS-induced RAW264.7
macrophages was assessed through the estimation of the
concentrations of NO and PGE2 and the expression of the
iNOS and COX-2 proteins. We found that CSE-MW-70E was meaningfully
more competent in terms of its anti-inflammatory effects than
CSE-H-70E. RAW264.7 cells pretreated with CSE-MW-70E for 24 h
exhibited lower iNOS expression (Fig.
2) and decreased PGE2 production derived from COX-2
(Fig. 2). Similarly, CSE-MW-70E
mitigated COX-2 expression and dose-dependently suppressed the
production of NO (Fig. 2).
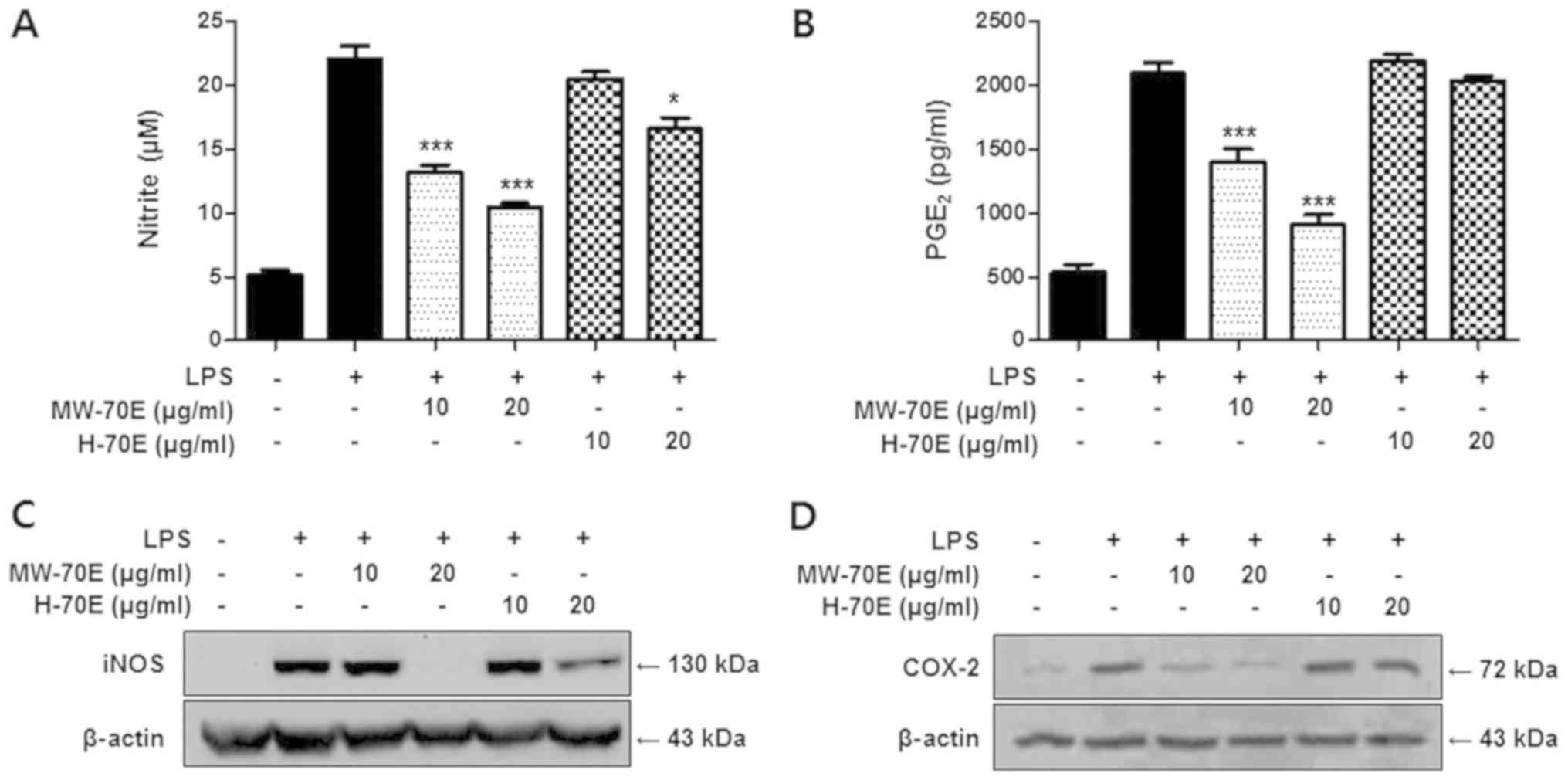 | Figure 2.CSE-MW-70E suppresses the production
of NO and PGE2, and decreases iNOS and COX-2 protein
expression. Effects of CSE-H-70E or CSE-MW-70E on (A) nitrite, (B)
PGE2, (C) iNOS and (D) COX-2 levels in RAW264.7
macrophages. Cells were pretreated for 3 h with indicated
concentrations of Sappan Lignum extracts and stimulated 24 h with
LPS (1 µg/m). Western blotting was performed; representative blots
from three independent experiments are shown. The data are
presented as the mean ± standard deviation of three experiments.
*P<0.05, ***P<0.001 vs. the LPS-treated group. CSE-H-70E,
heat-70% EtOH extraction; CSE-MW-70E, microwave-70% EtOH
extraction; PGE2, prostaglandin E2; iNOS,
inducible nitric oxide synthase; COX-2, cyclooxygenase-2; LPS,
lipopolysaccharide. |
Effects of CSE-H-70E and CSE-MW-70E on
pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and NF-κB
binding activity in LPS-stimulated RAW264.7 cells
Further anti-inflammatory effects of CSE-H-70E and
CSE-MW-70E were evaluated through the assessment of the TNF-α,
IL-1β and IL-6 levels in LPS-stimulated RAW264.7 macrophages. Cells
were pre-incubated with CSE-H-70E and CSE-MW-70E for 3 h and then
treated with LPS for 24 h; CSE-MW-70E caused
concentration-dependent decreases in IL-1β, IL-6 and TNF-α relative
to CSE-H-70E (Fig. 3A-C). In
addition, to confirm whether the NF-κB pathway was engaged in the
suppression of inflammatory responses induced by CSE-H-70E and
CSE-MW-70E, we measured NF-κB DNA binding activity in nuclear
extracts from RAW264.7 cells that were stimulated by LPS for 1 h.
This process resulted a relative 20-fold rise in the DNA binding
activity of NF-κB, which was inhibited by CSE-MW-70E in a
concentration-dependent manner (Fig.
3D).
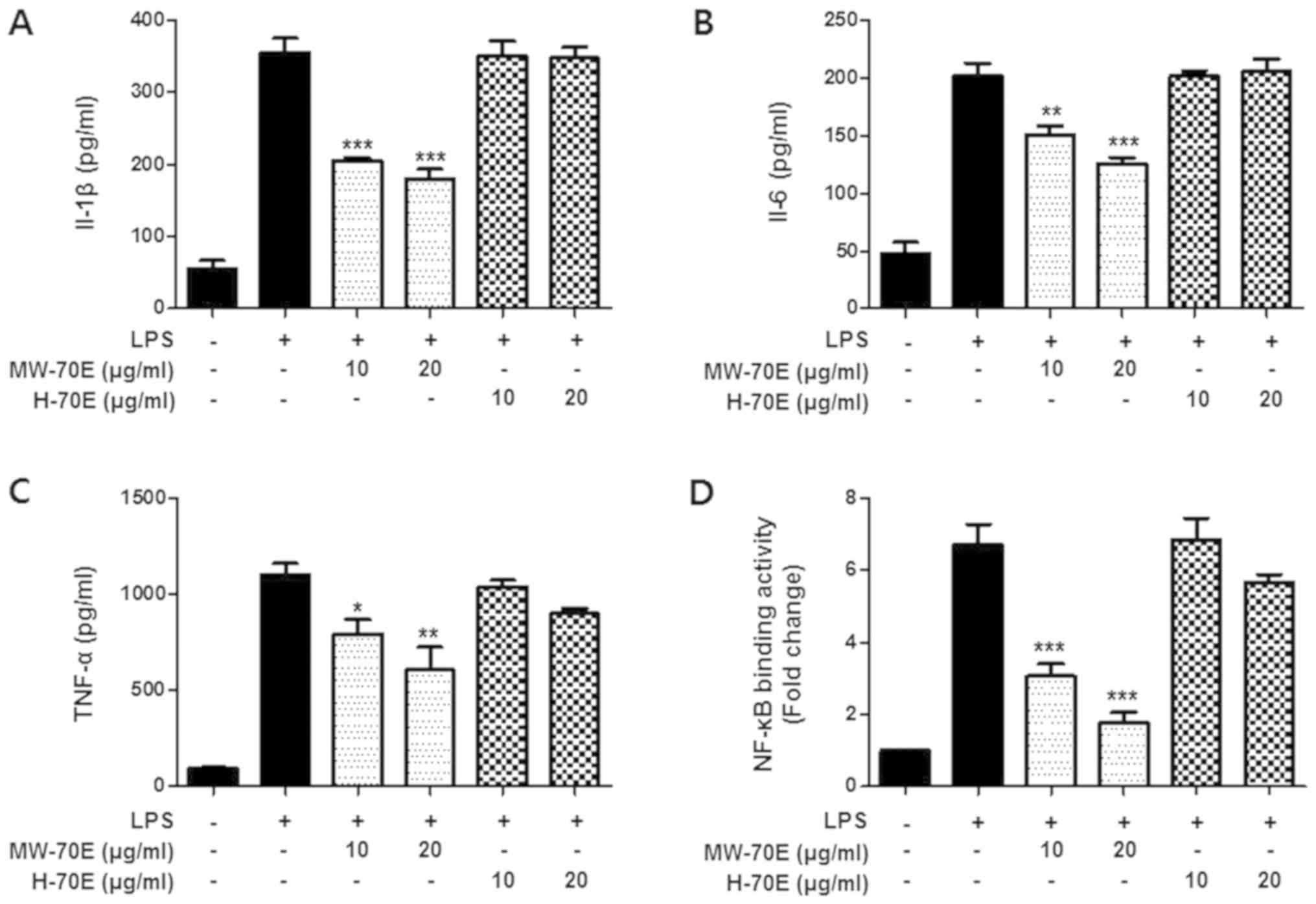 | Figure 3.CSE-MW-70E suppresses LPS-induced
production of IL-1 β, IL-6, TNF-α and NF-κB binding activity.
Effects of CSE-H-70E or CSE-MW-70E on (A) IL-1β, (B) IL-6 and (C)
TNF-α concentrations and (D) NF-κB binding activity in RAW264.7
macrophages. (A-C) The cells were pretreated for 3 h with the
indicated concentrations of Sappan Lignum extracts and stimulated
for 24 h with LPS (1 µg/ml). (D) The cells were pretreated for 3 h
with the indicated concentrations of Sappan Lignum extracts and
stimulated for 1 h with LPS (1 µg/ml). A commercially available
NF-κB ELISA (Active Motif) kit was used to test the nuclear
extracts and determine the degree of NF-κB binding. The data are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05, **P<0.01, ***P<0.001 vs. the
LPS-treated group. CSE-H-70E, heat-70% EtOH extraction; CSE-MW-70E,
microwave-70% EtOH extraction; IL, interleukin; TNF-α, tumor
necrosis factor-α; NF-κB, nuclear factor-κB; LPS,
lipopolysaccharide. |
Effects of CSE-H-70E and CSE-MW-70E on
heme oxygenase-1 expression in RAW264.7 macrophages
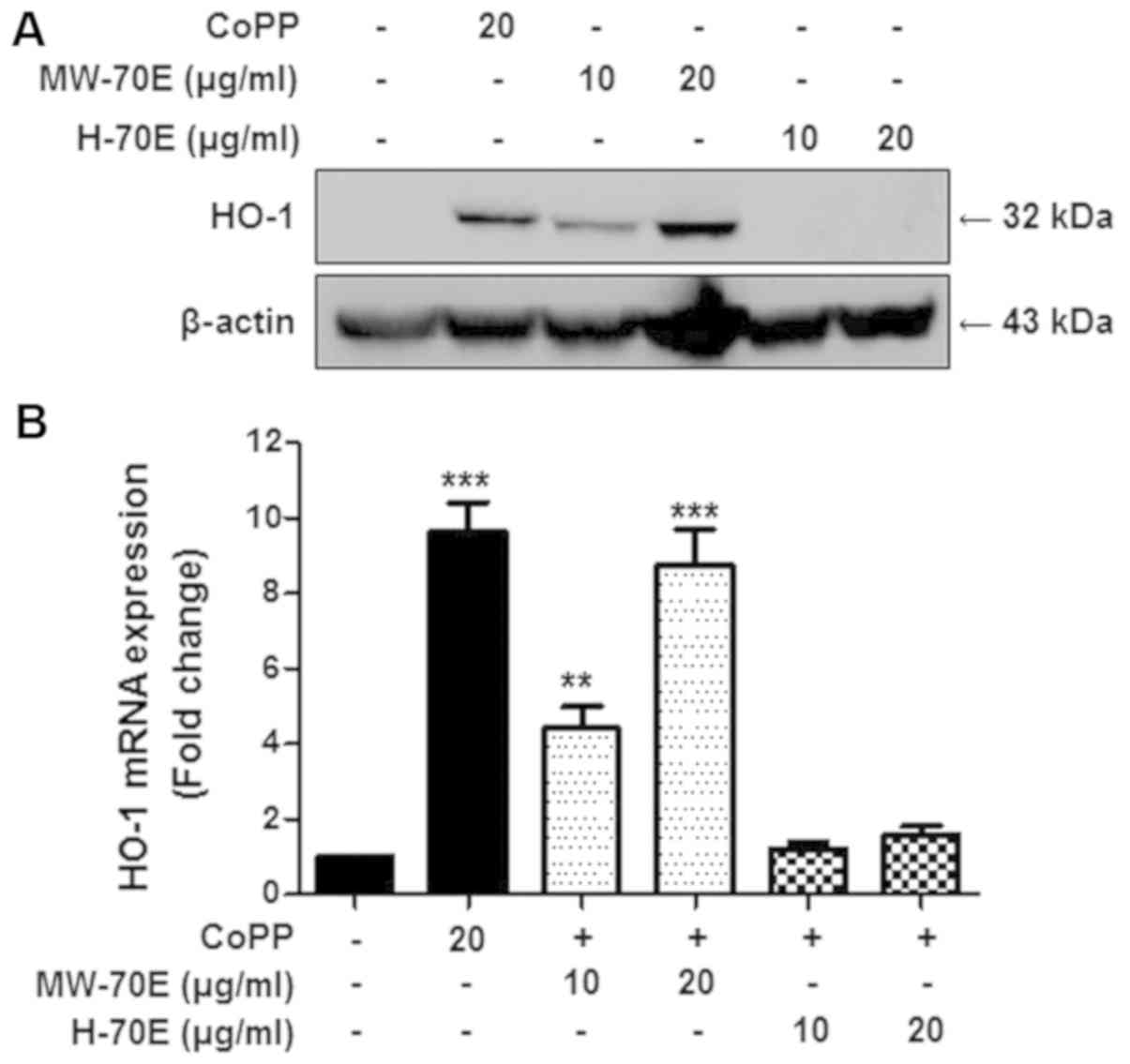
We subsequently examined the effects of CSE-H-70E
and CSE-MW-70E on HO-1 expression in RAW264.7 macrophages. The
cells were treated with different concentrations of CSE-H-70E and
CSE-MW-70E (10–20 µg/ml) for 12 h. Our data showed that CSE-MW-70E
and the positive control, CoPP, induced a dose-dependent increase
in HO-1 protein (Fig. 4A) and HO-1
mRNA (Fig. 4B) expression.
Effects of SnPP on nitrite,
PGE2, IL-1β, IL-6, TNF-α and NF-κB DNA-binding activity
in CSE-MW-70E pretreated LPS-stimulated RAW264.7 macrophages
To confirm the interaction between the induction of
HO-1 and the anti-inflammatory activity of CSE-MW-70E, we studied
the effects of tin protoporphyrin (SnPP). The cells were pretreated
for 3 h with CSE-MW-70E (20 µg/ml) in the presence or absence of
SnPP (50 µM) and activated with LPS (1 µg/ml) for 24 h (Fig. 5A-E) or 1 h (Fig. 5F). As shown in Fig. 6, pretreatment with SnPP partly
prevented the suppressive effects of CSE-MW-70E on the production
of pro-inflammatory mediators and cytokines in LPS-activated
cells.
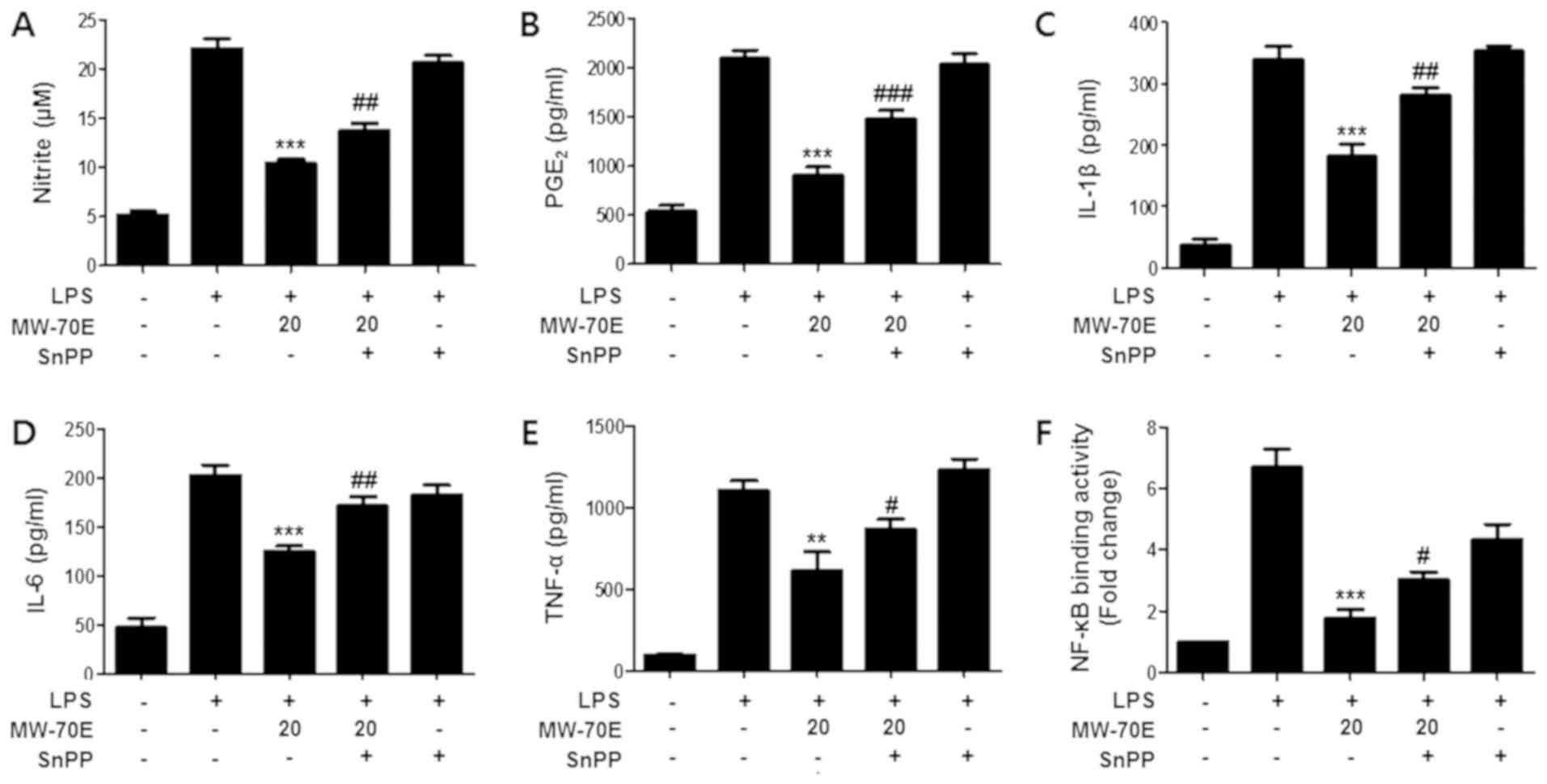 | Figure 5.Pretreatment with SnPP partly
reverses the suppressive effects of CSE-MW-70E against the
production of pro-inflammatory mediators, cytokines and NF-κB DNA
binding activity. Effects of SnPP on (A) nitrite, (B)
PGE2, (C) IL-1β, (D) IL-6, (E) TNF-α and (F) NF-κB
DNA-binding activity in CSE-MW-70E pretreated LPS-stimulated
RAW264.7 macrophages. The cells were pretreated for 3 h with Sappan
Lignum extracts (20 µg/ml) in the presence or absence of SnPP (50
µM) and stimulated with LPS (1 µg/ml) for (A-E) 24 h or 1 h (F).
The data are presented as the mean ± standard deviation of three
experiments. **P<0.01, ***P<0.001 vs. with the group treated
with LPS alone; #P<0.05, ##P<0.01,
###P<0.001 vs. the group treated with CSE-MW-70E and
LPS. SnPP, tin protoporphyrin; PGE2, prostaglandin
E2; IL, interleukin; TNF-α, tumor necrosis factor-α;
NF-κB, nuclear factor-κB; CSE-MW-70E, microwave-70% EtOH
extraction; LPS, lipopolysaccharide. |
Discussion
Different extracts of C. sappan have been
shown to have anti-inflammatory and anti-arthritic activities
(38,39). The EtOAc extract of Sappan Lignum
was found to exert significant inhibitory effects on mitosis and
growth-related signaling (35).
The anti-inflammatory effects of various compounds from the EtOAc
extract of Sappan Lignum were recently investigated (40). 70% EtOH is generally one of the
efficient extraction solvent conditions. Both polar and nonpolar
compounds can be effectively extracted, especially for extracting
major active ingredients such as glycoside, flavonoid, and
polyphenols, etc. However, the anti-inflammatory effect of 70% EtOH
Sappan Lignum extract prepared by heat extraction or microwave
extraction was not previously elucidated.
MASE has become increasingly popular over the last
few decades (19), as it was shown
to be more efficient for most applications compared with
traditional extraction methods (20). Microwaves increase decoction
temperature as hot continuous extraction and maceration, it is also
similar to normal heat extraction method. However, microwave
extraction has many advantages such as shorter extraction time,
less solvent consumption, higher extraction rate, and better yield
with lower energy consumption (41).
One of the complex biological responses of the body
is inflammation, which causes adverse stimuli, such as pathogens or
irritants that may disturb the function of cells (42). The response to inflammation is a
complicated reaction of the immune system that is monitored by
different inflammatory mediators and cytokines. Three isoforms of
NOS are well-known: nNOS, eNOS, and iNOS (43). The production of NO in
immune-activated macrophages is stimulated by iNOS, a crucial
pro-inflammatory enzyme (44). In
the current study, we observed that CSE-MW-70E dose-dependently
suppressed the production of NO and PGE2 and decreased
iNOS and COX-2 protein expression (Fig. 2). The pro-inflammatory activities
of cytokines, such as TNF-α, IL-1β, and IL-6, exert significant
roles in the control of tumor progression and inflammation
(45). In this study, we showed
that the pretreatment of murine macrophages (RAW 264.7 cells) with
CSE-MW-70E suppressed LPS-induced TNF-α, IL-6, and IL-1β production
(Fig. 3). NF-κB is a fundamental
molecule in a crucial signaling pathway associated with
inflammatory diseases, and coordinates various pro-inflammatory
genes and cytokines (46). We
measured the NF-κB DNA-binding activity and found that CSE-MW-70E
inhibited the increase in NF-κB DNA-binding activity in a
dose-dependent manner in RAW264.7 cells stimulated with LPS to a
greater extent than CSE-H-70E (Fig.
3). These findings suggested that the NF-κB pathway was a
crucial target for the action of CSE-MW-70E in the suppression of
the induction of pro-inflammatory enzymes, mediators, and
cytokines. The anti-inflammatory effects of the reaction of HO-1
were also recently demonstrated in a number of inflammatory models,
along with its antioxidant effects (47,48).
HO-1 exerts cytoprotective effects through its anti-inflammatory,
anti-proliferative, and antiapoptotic activities, which are
mediated by relatively few heme products containing CO, biliverdin,
and free iron (Fe2+). CO restrains pro-inflammatory
responses and boosts anti-inflammatory responses in macrophages. By
minimizing the expression of iNOS and COX-2, HO-1 and CO can also
suppress the production of NO and PGE2 (49). To evaluate whether the initiation
of HO-1 plays a significant role in the CSE-H-70E and CSE-MW-70E,
derived suppression of the pro-inflammatory responses, RAW264.7
cells were pretreated with CSE-H-70E and CSE-MW-70E, and the
expression of HO-1 mRNA and protein was evaluated. We observed that
pretreatment with CSE-MW-70E for 12 h significantly increased the
expression of HO-1 mRNA and protein (Fig. 4).
As SnPP is a competitive inhibitor of the HO-1
response, we tested its influence on the suppressive effects of
CSE-MW-70E on the production of pro-inflammatory mediators and
cytokines in LPS-stimulated cells to confirm the interaction
between HO-1 induction and the anti-inflammatory activity of
CSE-MW-70E. Macrophages were pretreated with CSE-MW-70E (20 µg/ml)
for 3 h in the presence or absence of SnPP (50 µM) and then
activated by LPS. As shown in Fig.
5, pretreatment with SnPP partly reversed the suppressive
effects of CSE-MW-70E against the production of proinflammatory
mediators, cytokines, and NF-κB DNA binding activity in the cells.
These findings provided further evidence that the anti-inflammatory
activity of CSE-MW-70E was linked to the effects on the HO-1
expression pathway. The present study is not enough to validate the
anti-inflammatory effects of Caesalpinia sappan L., because
major compounds have not yet identified and not conducted in
vivo study. However, this study was focused and designed to
investigate the difference between extraction efficiency and the
active ingredient according to the extraction method.
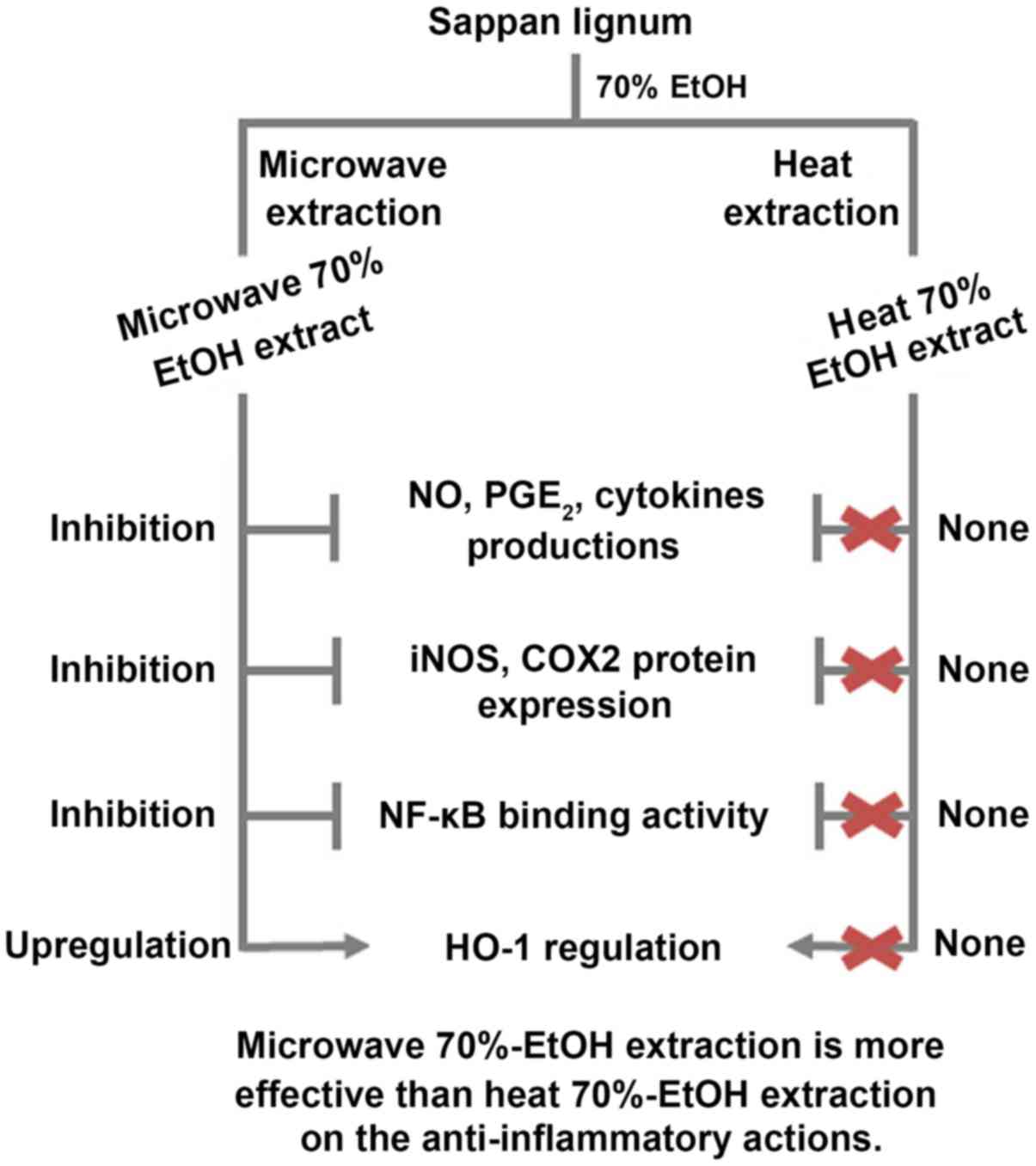
In conclusion, it was found that the extraction
method influenced the biological activity of Sappan Lignum. Our
results showed that two types of Sappan Lignum 70% ethanol extract
(CSE-H-70E and CSE-MW-70E) induced anti-inflammatory responses that
were associated with their inhibitory effects against NF-κB pathway
activation in LPS-stimulated cells. CSE-MW-70E has a greater
potential for therapeutic applications than the CSE-H-70E. In
addition, it was proven that the anti-inflammatory activity of
CSE-MW-70E was correlated with its ability to HO-1 mRNA and protein
expression (Fig. 6). In this
manuscript we tried to compare the extraction efficiency and
evaluation of biological activities of these two extracts of Sappan
Lignum and. Thus, HPLC analysis showed there is a significant
difference between these two extracts pattern at ~20 min (Fig. 1). Our bioassay results also showed
that the anti-inflammatory effects are excellent due to the
difference of these patterns, that might be active components.
These results suggested that 70% ethanol-microwave extraction of
Sappan Lignum (CSE-MW-70E) may be a favorable therapeutic agent and
should be investigated further to establish its potential as a
remedy for a range of inflammatory diseases. Further detailed
investigation into the anti-inflammatory effects of CSE-MW-70E in
in vitro and in vivo models of inflammatory diseases
would assist in the development of its therapeutic potential.
Safety of Caesalpinia sappan L. should be discussed in order
to develop as a medicine. In our experiments, cell viability assay
was assessed, and subsequently in vitro assay model was
evaluated within a non-toxic concentration range. However, in order
to evaluate the toxicity of Caesalpinia sappan L., it is
necessary to conduct the clinical test through in vivo model
assay. Therefore, in further study, we will conduct an
anti-inflammatory mechanism experiment on in vivo model to
confirm the possibility of development as a medicine.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
nos. 2015R1C1A1A02036465 and NRF-2018R1C1B6001913).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MDAC, MC and WK participated in the acquisition,
analysis and interpretation of the data, and contributed to the
writing of the manuscript. HL participated in the acquisition,
analysis and interpretation of the data. SCK, HO and ERW designed
the study and contributed to the writing of the manuscript. YCK and
DSL designed the study, participated in the acquisition, analysis,
and interpretation of the data, and contributed to the writing of
the manuscript. All authors read approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medzhitov R: Inflammation 2010: New
adventures of an old flame. Cell. 140:771–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Widowati W, Darsono L, Suherman J, Fauziah
N, Maesaroh M and Erawijantari PP: Anti-inflammatory effect of
mangosteen (Garcinia mangostana L.) peel extract and its
compounds in LPS-induced RAW264.7 cells. Nat Prod Sci. 22:147–153.
2016. View Article : Google Scholar
|
|
3
|
Kim NH, Yang MH, Heo JD, Sung SH and Jeong
EJ: Dihydrobenzofuran neolignans isolated from Euonymus
alatus leaves and twigs attenuated inflammatory responses in
the activated RAW264.7 macrophage cells. Nat Prod Sci. 22:53–59.
2016. View Article : Google Scholar
|
|
4
|
Kim KJ, Yoon KY, Yoon HS, Oh SR and Lee
BY: Brazilein suppresses inflammation through inactivation of
IRAK4-NF-κB pathway in LPS-induced Raw264.7 macrophage cells. Int J
Mol Sci. 16:27589–27598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee DS and Jeong GS: Butein provides
neuroprotective and anti-neuroinflammatory effects through
Nrf2/ARE-dependent haem oxygenase 1 expression by activating the
PI3K/Akt pathway. Br J Pharmacol. 173:2894–2909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baranano DE, Rao M, Ferris CD and Snyder
SH: Biliverdin reductase: A major physiologic cytoprotectant. Proc
Natl Acad Sci USA. 99:16093–16098. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee TS, Tsai HL and Chau LY: Induction of
heme oxygenase-1 expression in murine macrophages is essential for
the anti-inflammatory effect of low dose 15-deoxy-Delta
12,14-prostaglandin J2. J Biol Chem. 278:19325–19330. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morse D, Pischke SE, Zhou Z, Davis RJ,
Flavell RA, Loop T, Otterbein SL, Otterbein LE and Choi AM:
Suppression of inflammatory cytokine production by carbon monoxide
involves the JNK pathway and AP-1. J Biol Chem. 278:36993–36998.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim KS, Lee DS, Kim DC, Yoon CS, Ko W, Oh
H and Kim YC: Anti-Inflammatory effects and mechanisms of action of
coussaric and betulinic acids isolated from Diospyros kaki in
lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules.
21:E12062016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim
HR, Jeon SB, Jeon WK, Chae HJ and Chung HT: Hydrogen sulfide
inhibits nitric oxide production and nuclear factor-kappaB via heme
oxygenase-1 expression in RAW264.7 macrophages stimulated with
lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee DS, Jeong GS, Li B, Park H and Kim YC:
Anti-inflammatory effects of sulfuretin from Rhus verniciflua
stokes via the induction of heme oxygenase-1 expression in murine
macrophages. Int Immunopharmacol. 10:850–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van-Assche T, Huygelen V, Crabtree MJ and
Antoniades C: Gene therapy targeting inflammation in
atherosclerosis. Curr Pharm Des. 17:4210–4223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DS, Yoon CS, Jung YT, Yoon JH, Kim YC
and Oh H: Marine-derived secondary metabolite, Griseusrazin A,
suppresses inflammation through heme oxygenase-1 induction in
activated RAW264.7 macrophages. J Nat Prod. 79:1105–1111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: The nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lappas M, Permezel M, Georgiou HM and Rice
GE: Nuclear factor kappa B regulation of proinflammatory cytokines
in human gestational tissues in vitro. Biol Reprod. 67:668–673.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar FH, Li Y, Wang Z and Kong D:
NF-kappaB signaling pathway and its therapeutic implications in
human diseases. Int Rev Immunol. 27:293–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Co M, Fagerlund A, Engman L, Sunnerheim K,
Sjöberg PJ and Turner C: Extraction of antioxidants from spruce
(Picea abies) bark using eco-friendly solvents. Phytochem
Anal. 23:1–11. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mandal V, Mohan Y and Hemalatha S:
Microwave assisted extraction - An innovative and promising
extraction tool for medicinal plant research. Phcog Rev. 1:7–18.
2007.
|
|
21
|
Kaufmann B and Christen P: Recent
extraction techniques for natural products: Microwave assisted
extraction and pressurized solvent extraction. Phytochem Anal.
13:105–113. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Pharmacopoeia Committee, People's
Republic of China Pharmacopoeia. Beijing, China: Chin Med Sci and
Technol Press; 153. 2010
|
|
23
|
Oh GT, Choi JH, Hong JJ, Kim DY, Lee SB,
Kim JR, Lee CH, Hyun BH, Oh SR, Bok SH and Jeong TS: Dietary
hematein ameliorates fatty streak lesions in the rabbit by the
possible mechanism of reducing VCAM-1 and MCP-1 expression.
Atherosclerosis. 159:17–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J, Yan X, Wang W, Wu H, Hua L and Du L:
Antioxidant activity in vitro of three constituents from
Caesalpinia sappan L. Tsinghua Sci Technol. 13:474–479.
2008. View Article : Google Scholar
|
|
25
|
Parameshwar S, Srinivasan KK and Rao CM:
Oral antidiabetic activities of different extracts of
Caesalpinia bonducella seed kernels. Pharm Biol. 40:590–595.
2002. View Article : Google Scholar
|
|
26
|
Oh SR, Kim DS, Lee IS, Jung KY, Lee JJ and
Lee HK: Anticomplementary activity of constituents from the
heartwood of Caesalpinia sappan. Planta Med. 64:456–458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu CM, Kang JJ, Lee CC, Li CH, Liao JW and
Cheng YW: Induction of vasorelaxation through activation of nitric
oxide synthase in endothelial cells by brazilin. Eur J Pharmacol.
468:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA and
Lee SK: Evaluation of natural products on inhibition of inducible
cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured
mouse macrophage cells. J Ethnopharmacol. 83:153–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodríguez-López V, Salazar L and Estrada
S: Spasmolytic activity of several extracts obtained from some
Mexican medicinal plants. Fitoterapia. 74:725–728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park KJ, Yang SH, Eun YA, Kim SY, Lee HH
and Kang H: Cytotoxic effects of Korean medicinal herbs determined
with hepatocellular carcinoma cell lines. Pharm Biol. 40:189–195.
2002. View Article : Google Scholar
|
|
31
|
Datté JY, Yapo PA, Kouamé-Koffi GG,
Kati-Coulibaly S, Amoikon KE and Offoumou AM: Leaf extract of
Caesalpinia bonduc Roxb. (Caesalpiniaceae) induces an
increase of contractile force in rat skeletal muscle in situ.
Phytomedicine. 11:235–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baek NI, Jeon SG, Ahn EM, Hahn JT, Bahn
JH, Jang JS, Cho SW, Park JK and Choi SY: Anticonvulsant compounds
from the wood of Caesalpinia sappan L. Arch Pharm Res.
23:344–348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiang LC, Chiang W, Liu MC and Lin CC: In
vitro antiviral activities of Caesalpinia pulcherrima and
its related flavonoids. J Antimicrob Chemother. 52:194–198. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim KJ, Yu HH, Jeong SI, Cha JD, Kim SM
and You YO: Inhibitory effects of Caesalpinia sappan on
growth and invasion of methicillin-resistant Staphylococcus
aureus. J Ethnopharmacol. 91:81–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Liu JL, Qi XM, Qi CT and Yu Q:
Inhibitory activities of Lignum Sappan extractives on growth and
growth-related signaling of tumor cells. Chin J Nat Med.
12:607–612. 2014.PubMed/NCBI
|
|
36
|
Berridge MV and Tan AS: Characterization
of the cellular reduction of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT):
Subcellular localization, substrate dependence, and involvement of
mitochondrial electron transport in MTT reduction. Arch Biochem
Biophys. 303:474–482. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Titheradge MA: The enzymatic measurement
of nitrate and nitrite. Methods Mol Biol. 100:83–91.
1998.PubMed/NCBI
|
|
38
|
Wu SQ, Otero M, Unger FM, Goldring MB,
Phrutivorapongkul A, Chiari C, Kolb A, Viernstein H and Toegel S:
Anti-inflammatory activity of an ethanolic Caesalpinia
sappan extract in human chondrocytes and macrophages. J
Ethnopharmacol. 138:364–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toegel S, Wu SQ, Otero M, Goldring MB,
Leelapornpisid P, Chiari C, Kolb A, Unger FM, Windhager R and
Viernstein H: Caesalpinia sappan extract inhibits
IL1β-mediated overexpression of matrix metalloproteinases in human
chondrocytes. Genes Nutr. 7:307–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mueller M, Weinmann D, Toegel S, Holzer W,
Unger FM and Viernstein H: Compounds from Caesalpinia sappan
with anti-inflammatory properties in macrophages and chondrocytes.
Food Funct. 7:1671–1679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mishra A, Mishra S, Bhargav S, Bhargava C
and Thakur M: Microwave assisted extraction, antioxidant potential
and chromatographic studies of some Rasayana drugs. Chin J Integr
Med. 21:523–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
43
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi Y: The regulatory role of nitric
oxide in proinflammatory cytokine expression during the induction
and resolution of inflammation. J Leukoc Biol. 88:1157–1162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Smith D, Hänsch H, Bancroft G and Ehlers
S: T-cell-independent granuloma formation in response to
Mycobacterium avium Role of tumour necrosis factor-alpha and
interferon-gamma. Immunology. 92:413–421. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee DS, Kim KS, Ko W, Li B, Keo S, Jeong
GS, Oh H and Kim YC: The neoflavonoid latifolin isolated from MeOH
extract of Dalbergia odorifera attenuates inflammatory
responses by inhibiting NF-κB activation via Nrf2-mediated heme
oxygenase-1 expression. Phytother Res. 28:1216–1223. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee DS, Ko W, Quang TH, Kim KS, Sohn JH,
Jang JH, Ahn JS, Kim YC and Oh H: Penicillinolide A: A new
anti-inflmmatory metabolite from the marine fungus
Penicillium sp. SF-5292. Mar Drugs. 11:4510–4526. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Otterbein LE, Bach FH, Alam J, Soares M,
Tao Lu H, Wysk M, Davis RJ, Flavell RA and Choi AM: Carbon monoxide
has anti-inflammatory effects involving the mitogen-activated
protein kinase pathway. Nat Med. 6:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|