Introduction
Acute lung injury (ALI) and its severe form, acute
respiratory distress syndrome (ARDS), are serious clinical issues
with no effective treatment (1).
Despite tremendous improvements in treatment modalities and
understanding of the associated respiratory physiology, the
mortality rate for patients with ALI/ARDS is 40% (1). The characteristic features of
ALI/ARDS are refractory hypoxemia, alveolar-capillary barrier
disruption, pulmonary alveoli edema and impaired gas exchange
(2). Alveolar epithelial cells
(AECs) are essential to maintain homeostatic pulmonary functions
and are the primary injury site during ALI/ARDS (3,4). AEC
damage causes fluid clearance dysfunction and decreases surfactant
production (4). Numerous
mechanisms are involved in diffuse AEC damage. However, excessive
inflammatory reactions and apoptosis are the two primary factors
(5). Therefore, preservation and
repair of AECs by controlling inflammation and apoptosis are
critical in ALL/ARDS treatment (5).
Mesenchymal stem cells (MSCs) are multipotent cells
that exhibit a high immunoregulatory capacity (6). Accumulating evidence indicates that
MSCs are an effective modality for cell-based therapies in various
ALL/ARDS models, including endotoxin-induced, live bacteria-induced
and sepsis-associated models, and pancreatitis-associated lung
injury (7). Studies involving MSCs
have focused on the effect of MSCs on the integrity and alveolar
fluid clearance function of AECs (8). At present, increasing evidence
suggests that the protective effect of MSCs is largely attributed
to paracrine mechanisms (9). MSCs
provide beneficial paracrine effects by secreting a broad range of
cytokines, chemokines and growth factors, which protects endogenous
cells, inhibits apoptosis and attenuates the inflammatory response
(9). However, which paracrine
factors and signaling pathways are involved in the immunoregulatory
effects of MSCs on inflammation and apoptosis remain unclear.
Toll-like receptor 4 (TLR4) is a sensing receptor
for lipopolysaccharide (LPS) (10). TLR4 signaling serves a key role in
host defense, innate immunity and inflammation, and is involved in
several acute and chronic diseases (11). However, unchecked or inappropriate
TLR4 activation serves as an amplifier of the inflammatory
response, which causes inflammation and immunity-associated tissue
damage (1). Additionally,
TLR4/nuclear factor k-light-chain-enhancer of activated B cells
(NF-κB) signal activation triggers apoptotic cascades (12). Control of aberrant TLR4 activation
contributes to improving the prognosis of inflammation and
apoptosis-associated diseases including ALI/ARDS.
We hypothesized that MSCs may secrete several
soluble factors to attenuate LPS-induced inflammation and apoptosis
through the inhibition of TLR4 signals. Therefore, the present
study was performed to investigate the effects of bone
marrow-derived MSCs (BM-MSCs) on the inflammatory reaction and
apoptosis of LPS-stimulated AECs in the A549 cell line, and to
explore the involved mechanisms using a coculture system.
Materials and methods
Cell lines and culture
The human AEC line A549 and human BM-MSCs were
purchased from Procell Life Science & Technology Co., Ltd
(Wuhan, China) and Cyagen Biosciences Inc. (Guangzhou, China),
respectively. The cells were maintained in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotics (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2. Characterization of surface markers [endoglin
(CD105+), CD44 antigen (CD44+), hematopoietic
progenitor cell antigen CD34 (CD34−) and receptor-type
tyrosine-protein phosphatase C (CD45−)] and verification
of the differentiation potential of MSCs (osteogenesis,
adipogenesis and chondrogenesis) was performed by the supplier
(Cyagen Biosciences Inc. Guangzhou, China).
Cell Counting Kit-8 (CCK-8) assay of
A549 cells
A549 cells were seeded in 96-well plates (Corning
Incorporated, Corning, NY, USA) at a density of 2×103
cells/well and treated with 0, 10, 20, 40, 80, 100, 150 and 200
µg/ml LPS (Escherichia coli 0127: B8; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 6 h. The cell viability assay was
performed using a CCK-8 kit (Beyotime Institute of Biotechnology,
Haimen, China), following the manufacturer's protocol. CCK-8
solution (10 µl) was added to each well, followed by incubation for
2 h at 37°C. The optical density at 450 nm was then measured with a
microplate reader (BioTek Instruments, Inc., Vermont, USA).
Coculture system and experimental
design
MSCs and A549 cells were cocultured using an
indirect Transwell system (Corning Incorporated). For the Cell
Counting Kit-8 (CCK-8) assay, 96-well plates were used (Corning
Incorporated). Briefly, 2×103 A549 cells/well were
seeded in 96-well plates. Then, 0, 1×103,
1×104 or 1×105 MSCs/well were plated in the
upper chamber of Transwell plates and cocultured with A549 cells
overnight. LPS (100 µg/ml) was added to the lower chamber for 6 h.
A cell viability assay was then performed using a CCK-8 kit
(Beyotime Institute of Biotechnology). For the other experiments,
6-well plates were used (Corning Incorporated). Briefly, A549 cells
(2×103/well) were seeded in the bottom chamber of the
Transwell plate, and MSCs (1×105/well) were seeded in
the upper chamber. The coculture groups were A549 + LPS, A549/MSC +
LPS, A549 alone and A549/MSC + PBS. Following overnight coculture
at 37°C in a humidified chamber with 5% CO2, 100 µg/ml
LPS was added for 6 h. Then, A549 cells were collected for
apoptosis analysis by using flow cytometry, and total/nucleus
protein of A549 cell was extracted for protein expression, NF-κB
and coimmunoprecipitation analyses as described subsequently.
Furthermore, the culture supernatants were collected for ELISA
assays as described subsequently.
A549 cell apoptosis evaluation by flow
cytometry
Following LPS stimulation as aforementioned, A549
cells were collected, washed with ice-cold PBS and stained with
final concentration of 0.2 µg/ml Annexin V-fluorescein
isothiocyanate (FITC) and final concentration of 2 µg/ml propidium
iodide (PI; Beyotime Institute of Biotechnology) at 4°C in the
dark, following the manufacturer's protocol. Annexin V-FITC and PI
fluorescence emissions were detected in FL1 and FL2 channels using
a flow cytometer (BD Biosciences, San Jose, CA, USA). The apoptotic
ratio was determined by FlowJo 7.6.4 software (Tree Star, Inc.,
Ashland, OR, USA).
Western blot analysis of caspase-3,
B-cell lymphoma 2 (Bcl-2), myeloid differentiation factor 88
(MyD88), TLR4 and toll-interleukin-1 receptor domain-containing
adaptor inducing interferon (TRIF) in A549 cells
Following LPS stimulation as aforementioned, A549
cells were lysed with radioimmunoprecipitation assay (RIPA) lysis
buffer containing a protease inhibitor cocktail (Beyotime Institute
of Biotechnology). Protein concentrations were measured using a
bicinchoninic acid (BCA) assay (Beyotime Institute of
Biotechnology). Protein samples were analyzed by western blot
analysis, as described previously (10). Equal amounts of protein (30 µg)
were separated by 10% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred onto a polyvinylidene fluoride
filter membrane (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and blocked with 5% dry non-fat milk (Beyotime Institute of
Biotechnology) at 37°C for 2 h. The membrane was incubated with
anti-cleaved Caspase-3 (1:1,000, cat. no. AC033),
anti-phosphorylated (phosphor)-Bcl-2 (1:1,000, cat. no. AB116)
(both Beyotime Institute of Biotechnology), anti-MyD88 (1:1,000,
cat. no. sc-136970), anti-TRIF (1:500, cat. no. sc-514384),
anti-TLR4 (1:500, cat. no. sc-293072) and anti-β-actin (1:1,000,
cat. no. sc-58673) (all Santa Cruz Biotechnology, Inc.) primary
antibodies overnight at 4°C, and then with a horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (1:1,000,
cat. no. A0216; Beyotime Institute of Biotechnology) at 37°C for 2
h. The membrane was washed, and the signals were detected by
enhanced chemiluminescence (Beyotime Institute of Biotechnology).
Protein expression levels were normalized by Quantity One (version
4.62; Bio-Rad Laboratories Inc., Hercules, CA, USA).
ELISA analysis of cytokine levels and
NF-κB activity in A549 cells
Following LPS stimulation as aforementioned, culture
supernatants were collected and centrifuged at 1,000 × g for 20 min
at 4°C. Subsequently, tumor necrosis factor-α (TNF-α) (cat. no.
CSB-E04740h), interleukin-8 (IL-8) (cat. no. CSB-E04641h), and
IL-1β (cat. no. CSB-E08053h) levels were measured by ELISA kits
(Cusabio Technology, LLC., Wuhan, China), following the
manufacturer's protocols. In addition, nuclear extracts of A549
cells were prepared using a Nuclear Extract kit (Active Motif,
Carlsbad, CA, USA), following the manufacturer's instructions.
Protein concentrations of nuclear extracts were quantified as
aforementioned using a BCA assay. DNA-binding activities of NF-κB
p65 were assessed by performing an ELISA using the TransAM™ NF-κB
Transcription Factor Assay kit (cat. no. 40596; Active Motif),
following the manufacturer's protocol.
Coimmunoprecipitation in A549
cells
A549 cells were pooled and lysed with RIPA lysis
buffer containing the protease inhibitor cocktail following LPS
stimulation as aforementioned. Cell lysates were centrifuged at
14,000 × g for 15 min at 4°C. Then, protein concentrations were
quantified using a BCA kit as aforementioned. The supernatant was
incubated overnight with anti-TLR4 (1:100; cat. no. sc-293072;
Santa Cruz Biotechnology, Inc.) or normal goat IgG (1:100; cat. no.
A7028; Beyotime Institute of Biotechnology) for 3 h at 4°C, and
then with 20 µl protein A/G-agarose (Beyotime Institute of
Biotechnology) at 4°C while rocking overnight. The pellets obtained
following centrifugation at 4°C in 14,000 × g for 5 sec were washed
five times with washing buffer (50 mM Tris, pH 7.5, 7 mM
MgCl2, 2 mM EDTA, and 1 mM PMSF) and resolved by 1X
SDS-PAGE after boiling for 10 min. The immunocomplexes were
analyzed by western blot analysis using anti-MyD88, anti-TRIF or
anti-TLR4 (Santa Cruz Biotechnology) antibodies as described
previously.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) of keratinocyte growth factor
(KGF) and angiopoietin-1 (ANGPT1) mRNAs in MSCs
Following coculture with A549 cells and LPS
treatment for 6 h, total RNA from each group (MSC, MSC + A549 +
PBS, MSC + LPS and MSC + A549 + LPS) was prepared and reverse
transcribed into cDNA, and then RT-qPCR was performed as described
previously (1), using β-actin as
an internal standard. The primer sequences were as follows: KGF
(sense), 5′-GAACAAGGAAGGAAAACTCTATGCAA-3′; KGF (antisense),
5′-AAGTGGGCTGTTTTTTGTTCTTTCT-3′; ANGPT1 (sense),
5′-TGGCTGCAAAAACTTGAGAATTAC-3′; ANGPT1 (antisense)
5′-TTCTGGTCTGCTCTGCAGTCTG-3′. The relative expression of each
target gene was calculated by the 2−ΔΔCq method
(13).
ELISAs of KGF and ANGPT1 levels
Following LPS (100 µg/ml) stimulation as
aforementioned for 6 h, the culture supernatants of each group from
co-culture system were collected as described previously. Then, KGF
(Human KGF ELISA Kit; cat. no. CSB-E08939h) and ANGPT1 (Human
ANGPT1 ELISA Kit; cat. no. CSB-EL001706HU) levels were measured by
ELISA kits (Cusabio Technology, LLC.), following the manufacturer's
protocols.
Preparation of MSC conditioned medium
(MSC-CM)
MSC-CM was prepared as follows: MSCs
(1×105/well) were seeded and cocultured with A549 cells
(2×103/well) in 6-well plates overnight as
aforementioned. LPS (100 µg/ml) was added into the plate for 6 h.
The conditioned medium was harvested, filtrated with a 0.22 µm
filter under sterile conditions and stored at −20°C until use.
KGF/ANGPT1 neutralization
experiment
Various concentrations (0, 0.5, 1.0, 2.0 and 4.0
µg/ml) of KGF (cat. no. AF-251-SP; R&D Systems, Inc.
Minneapolis, MN, USA) and ANGPT1 (cat. no. A0604; Merck KGaA)
neutralizing antibodies were added to MSC-CM to neutralize KGF
and/or ANGPT1 activities in MSC-CM, respectively as described
previously (14–16). Following incubation at 37°C for 1
h, the concentration of KGF (Human KGF ELISA Kit; cat. no.
CSB-E08939h) and ANGPT1 (Human ANGPT1 ELISA Kit; cat. no.
CSB-EL001706HU) in MSC-CM was detected by ELISA kits as
aforementioned. CM treated with unspecific IgG (2 µg/ml) antibodies
(cat. no. sc-52000; Santa Cruz Biotechnology, Inc.) served as
respective negative controls. Following preparation of various
kinds of CM (MSC-CM, MSC-CM + IgG, MSC-CM + KGF-Ab, MSC-CM +
ANGPT1-Ab and MSC-CM + KGF/ANGPT1-Ab), A549 cells were seeded and
cultured in appropriate plates overnight as aforementioned. Then,
LPS (100 µg/ml) or LPS combined with various kinds of CM was added
to the plates for 6 h to stimulate A549 cells. The A549 cell
viability assay was performed and the levels of TNF-α, IL-8 and
IL-1β in cell culture supernatants were calculated by ELISA assays
as aforementioned.
Statistical analysis
Statistical analyses were performed using SPSS
software version 16.0 (SPSS Inc, Chicago, IL, USA). Data are
presented as means ± standard error of the mean and were compared
by one-way analysis of variance. If the variance was homogeneous,
the Least Significant Difference test was adopted for group
comparisons. If the variance was not homogeneous, Dunnett's T3 test
was used for group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
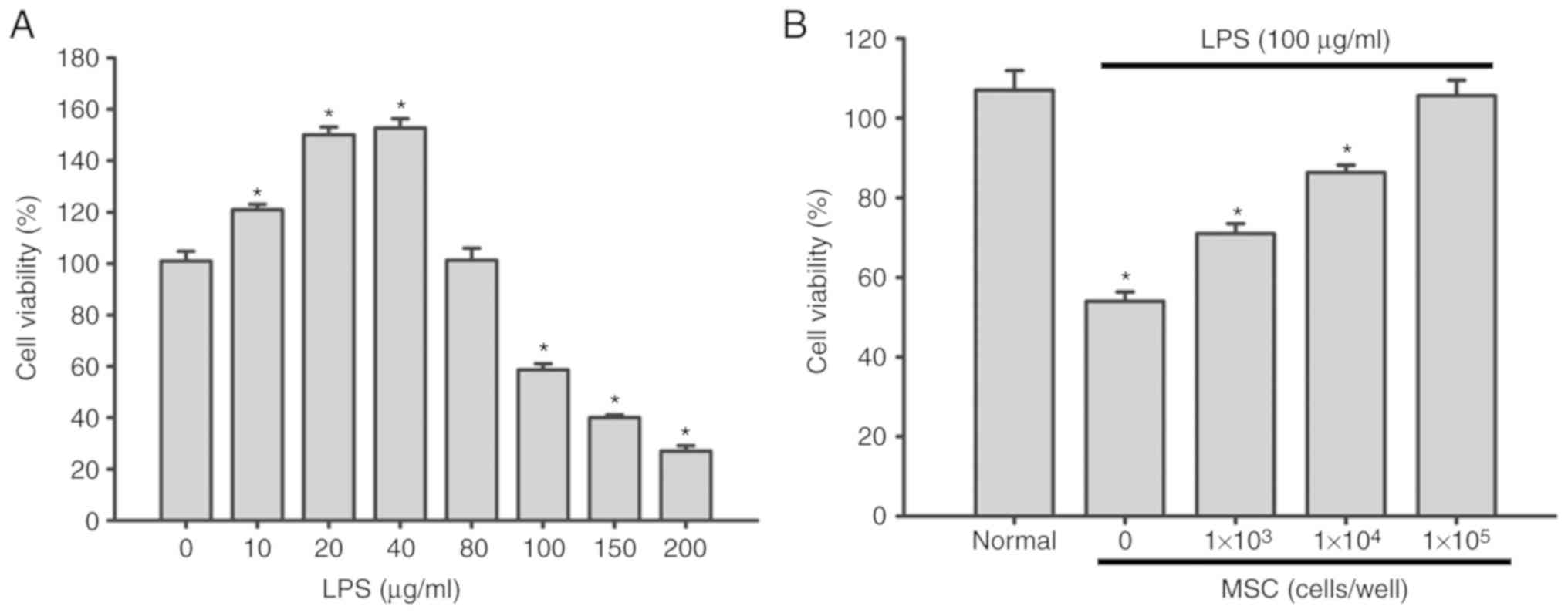
Effect of LPS on the viability of A549
cells
As demonstrated in Fig.
1A, treatment with 10–40 µg/ml LPS for 6 h promoted A549 cell
proliferation in a dose-dependent manner (P<0.05). However, when
the LPS concentration was ≥80 µg/ml, the survival rate of A549
cells was significantly decreased (P<0.05). Specifically, 150
µg/ml LPS exhibited obvious cytotoxicity in A549 cells, decreasing
cell viability to ~40% (Fig. 1A).
Therefore, 100 µg/ml LPS was used for subsequent experiments, which
has also been applied in previous studies (17,18).
MSCs increase the proliferation of
LPS-damaged A549 cells
The CCK-8 assay demonstrated that A549 cell
proliferation was suppressed following exposure to 100 µg/ml LPS
(P<0.05). However, following coculture with MSCs, the viability
of A549 cells was significantly increased in an MSC cell
number-dependent manner (P<0.05; Fig. 1B).
MSCs inhibit LPS-induced apoptosis of
A549 cells
Levels of apoptosis were assessed by flow cytometry
of Annexin-FITC/PI-stained cells. The number of apoptotic cells was
increased by treatment with LPS compared with the control
(P<0.05). However, coculture with MSCs weakened the promoting
effect of LPS on apoptosis (P<0.05). Following pretreatment with
MSCs, the apoptotic rate of A549 cells was decreased under
noninflammatory conditions (P<0.05; Fig. 2).
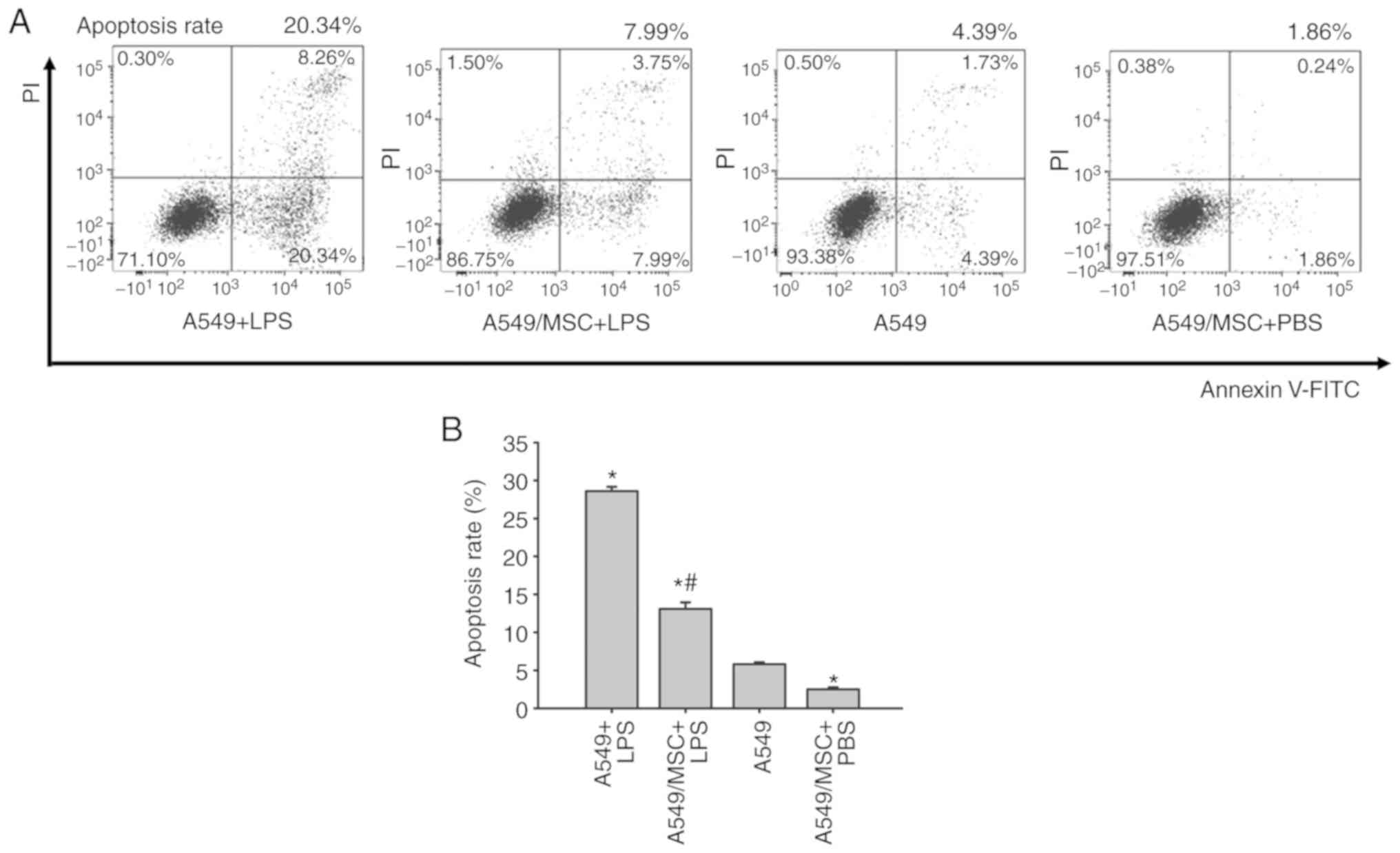 | Figure 2.Coculture with MSCs inhibits A549
cell apoptosis induced by LPS. (A) A549 cells with or without
coculture with MSCs were treated with LPS (100 µg/ml) or PBS for 6
h, and apoptosis of A549 was analyzed by flow cytometry.
Representative examples of flow cytometry analysis for A549 cell
apoptosis rate in each group are presented. (B) The cellular
apoptosis rate analyses of all groups. Data are expressed as means
± standard error of the mean of three independent experiments.
*P<0.05 vs. normal A549 cells, #P<0.05 vs. A549 +
LPS group. LPS, lipopolysaccharide; MSC, mesenchymal stem cell;
FITC, fluorescein isothiocyanate; PI, propidium iodide; A549 + LPS,
LPS-stimulated A549 cells; A549/MSC + LPS, A549 cells cocultured
with MSCs following LPS-stimulation; A549, untreated A549 cells;
A549/MSC + PBS, A549 cells cocultured with MSCs following
PBS-treatment. |
MSCs modulate caspase-3 and Bcl-2
expression levels in A549 cells
The expression levels of caspase-3 and Bcl-2, which
are important effector molecules during apoptosis (19), were analyzed. Cleaved caspase-3
expression was upregulated, whereas phospho-Bcl-2 expression was
downregulated following LPS treatment (P<0.05). However,
coculture with MSCs significantly reversed these changes in protein
expression (P<0.05). MSCs also markedly modulated caspase-3 and
Bcl-2 expression in A549 cells under non-inflammatory conditions
(P<0.05; Fig. 3).
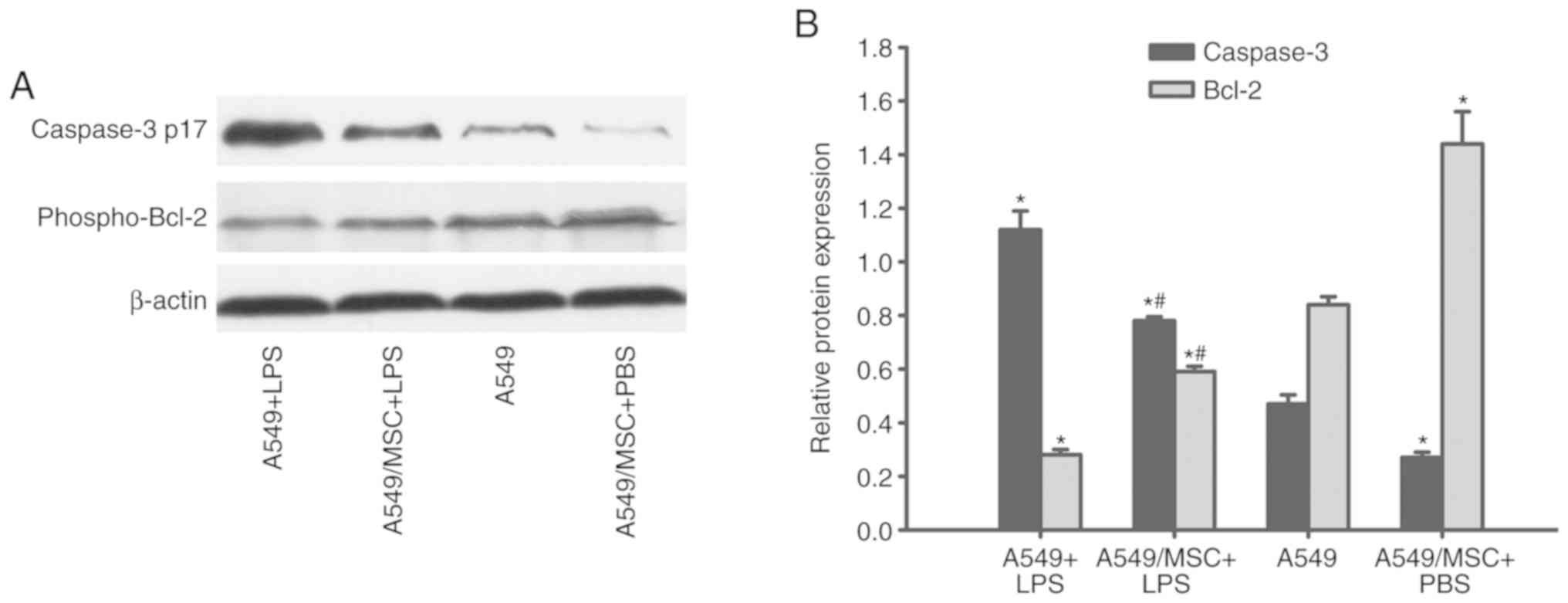 | Figure 3.Coculture with MSCs modulates protein
expression of caspase-3 and Bcl-2 in A549 cells. A549 cells with or
without coculture with MSCs were treated with LPS (100 µg/ml) or
PBS for 6 h. The cleaved-caspase-3 and phosphor-Bcl-2 protein
levels in A549 cells were determined by western blot analysis. (A)
Representative examples of cleaved-caspase-3 and phosphor-Bcl-2
protein bands. (B) Densitometric analyses of MyD88 and TRIF bands.
Data are expressed as means ± standard error of the mean of three
independent experiments. *P<0.05 vs. normal A549 cells,
#P<0.05 vs. A549 + LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; phosphor,
phosphorylated; Bcl-2, B-cell lymphoma 2; caspase-3 p17, cleaved
caspase fragment; A549 + LPS, LPS-stimulated A549 cells; A549/MSC +
LPS, A549 cells cocultured with MSCs following LPS-stimulation;
A549, untreated A549 cells; A549/MSC + PBS, A549 cells cocultured
with MSCs following PBS-treatment. |
MSCs attenuate the production of
LPS-induced inflammatory factors in A549 cells
ELISAs demonstrated that the concentrations of
TNF-α, IL-8 and IL-1β were low in the culture supernatant of
untreated A549 cells. Following LPS stimulation, there was a marked
increase in the levels of TNF-α, IL-8 and IL-1β (P<0.05;
Fig. 4). However, the increases
induced by LPS were inhibited following coculture with MSCs
(P<0.05; Fig. 4). Notably,
coculture with MSCs inhibited the release of TNF-α, IL-8 and IL-1β
in the absence of LPS stimulation compared with the untreated A549
cells (P<0.05; Fig. 4).
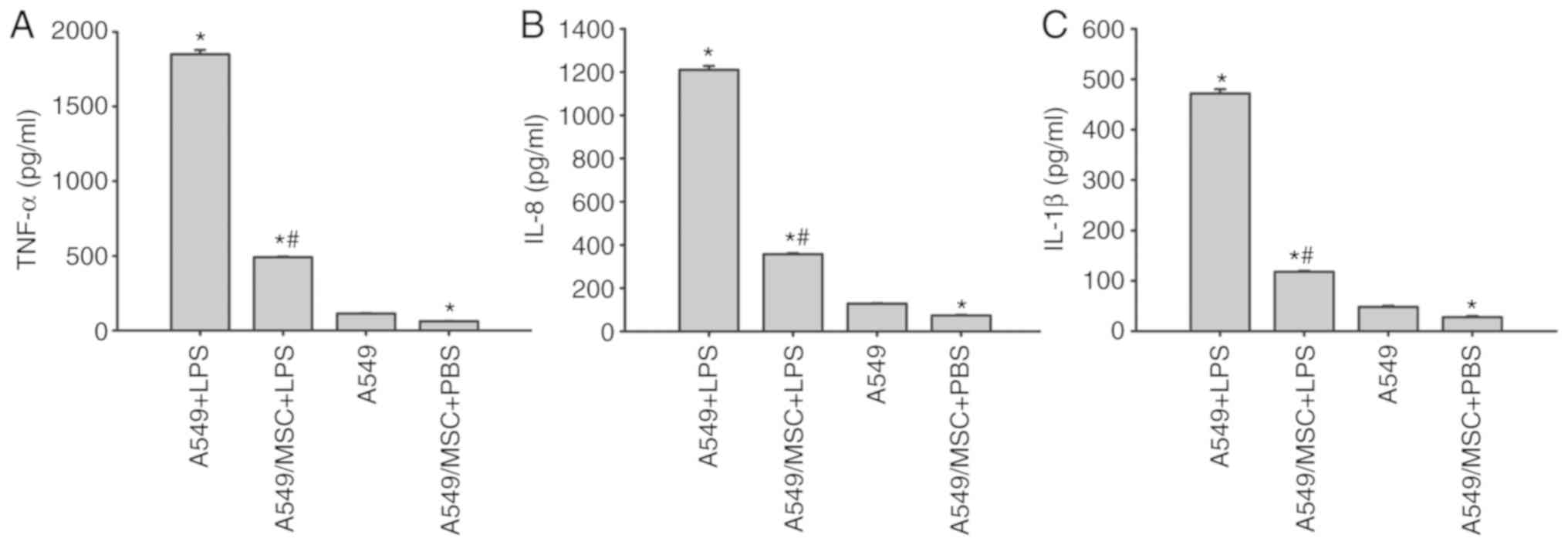 | Figure 4.Coculture with MSCs inhibits the
production of (A) TNF-α (A), (B) IL-8 and (C) IL-1β derived from
A549 cells induced by LPS. A549 cells with or without coculture
with MSCs were treated with LPS (100 µg/ml) or PBS for 6 h.
Coculture supernatants were collected, and the levels of TNF-α,
IL-8 and IL-1β were then measured by ELISAs. Data are expressed as
means ± standard error of the mean of three independent
experiments. *P<0.05 vs. normal A549 cells,
#P<0.05 vs. A549 + LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; TNF-α, tumor
necrosis factor α; Il, interleukin; A549 + LPS, LPS-stimulated A549
cells; A549/MSC + LPS, A549 cells cocultured with MSCs following
LPS-stimulation; A549, untreated A549 cells; A549/MSC + PBS, A549
cells cocultured with MSCs following PBS-treatment. |
MSCs attenuate the LPS-induced NF-κB
activation in A549 cells
NF-κB is a critical transcription factor required
for the maximal expression of a number of cytokines (10). As presented in Fig. 5, all LPS-stimulated A549 cells
exhibited a marked increase in NF-κB DNA binding in comparison with
the A549 cells not treated with LPS. The increases in NF-κB
activity induced by LPS were decreased following coculture with
MSCs (P<0.05; Fig. 5).
Concomitantly, coculture with MSCs inhibited the NF-κB activation
in the absence of LPS stimulation compared with the untreated A549
cells (P<0.05; Fig. 5).
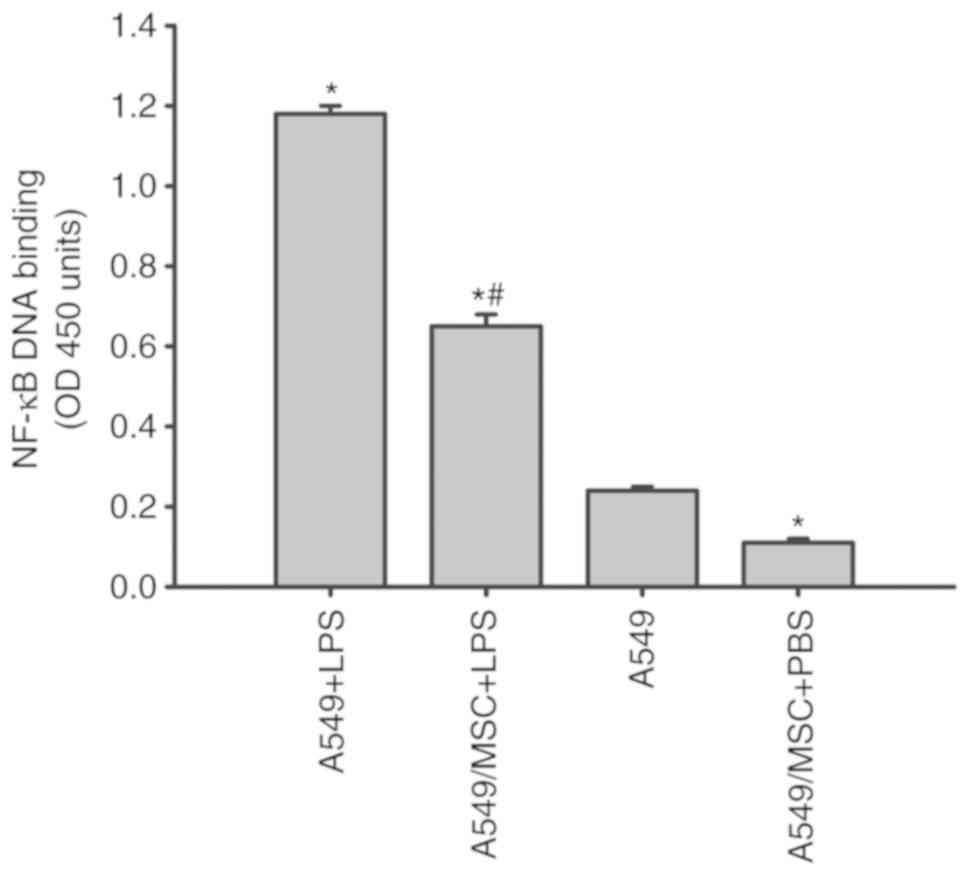 | Figure 5.Coculture with MSCs inhibits NF-κB
DNA binding activity in A549 cells induced by LPS. A549 cells with
or without coculture with MSCs were treated with LPS (100 µg/ml) or
PBS for 6 h. Nuclear extracts of A549 cells were collected, and the
activity of NF-κB was then measured by ELISA. Data are expressed as
means ± standard error of the mean of three independent
experiments. *P<0.05 vs. normal A549 cells,
#P<0.05 vs. A549 + LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; NF-κB, nuclear
factor κ-light-chain-enhancer of activated B cells; OD, optical
density; A549 + LPS, LPS-stimulated A549 cells; A549/MSC + LPS,
A549 cells cocultured with MSCs following LPS-stimulation; A549,
untreated A549 cells; A549/MSC + PBS, A549 cells cocultured with
MSCs following PBS-treatment. |
MSCs decrease the expression of MyD88,
TLR4 and TRIF in A549 cells
In response to TLR4 activation, the adaptors MyD88,
TLR4 and TRIF are overexpressed (11). Therefore, the expression of MyD88,
TLR4 and TRIF were measured by western blot analysis. LPS
significantly upregulated the expression of MyD88, TLR4 and TRIF in
A549 cells (P<0.05; Fig. 6).
However, coculture with MSCs decreased MyD88, TLR4, and TRIF
expression in LPS-stimulated A549 cells (P<0.05; Fig. 6). Under non-inflammatory
conditions, coculture with MSCs downregulated MyD88, TLR4 and TRIF
expression in A549 cells (P<0.05; Fig. 6).
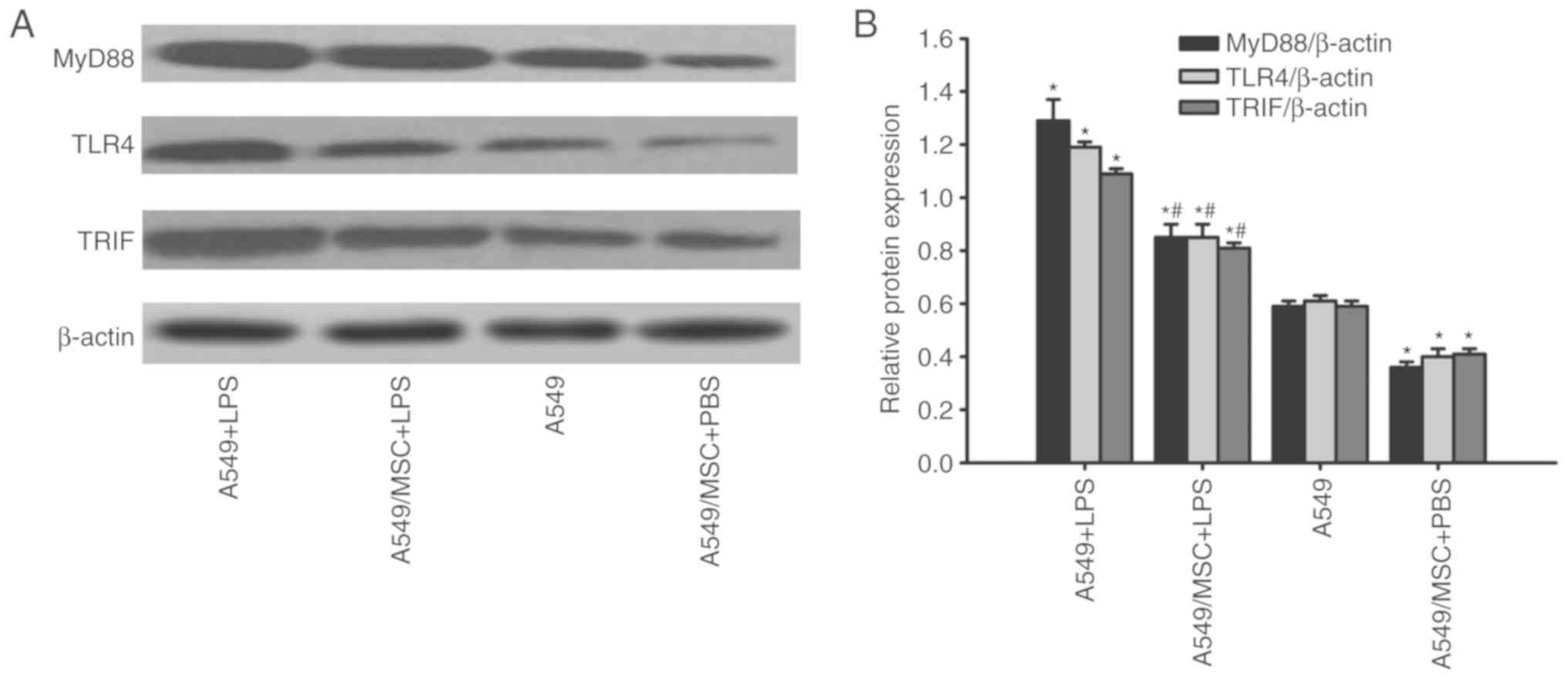 | Figure 6.Coculture with MSCs suppresses TLR4,
MyD88 and TRIF protein expression in A549 cells. A549 cells with or
without coculture with MSCs were treated with LPS (100 µg/ml) or
PBS for 6 h. TLR4, MyD88 and TRIF protein expression in A549 cells
was detected by western blot analysis. (A) Representative examples
of TLR4, MyD88, and TRIF bands. (B) Densitometric analyses of TLR4,
MyD88 and TRIF bands. Data are expressed as means ± standard error
of the mean of three independent experiments. *P<0.05 vs. normal
A549 cells, #P<0.05 vs. A549 + LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; TLR4, Toll-like
receptor-4; MyD88, myeloid differentiation factor 88; TRIF,
toll-interleukin-1 receptor domain-containing adaptor inducing
interferon; A549 + LPS, LPS-stimulated A549 cells; A549/MSC + LPS,
A549 cells cocultured with MSCs following LPS-stimulation; A549,
untreated A549 cells; A549/MSC + PBS, A549 cells cocultured with
MSCs following PBS-treatment. |
MSCs suppress TLR4/MyD88 and TLR4/TRIF
complex formation in A549 cells
TLR4/MyD88 and TLR4/TRIF complex formation is a
prerequisite for TLR signal transduction (1). Therefore, the effects of MSCs on
these complexes were examined by coimmunoprecipitation. As
demonstrated in Fig. 7, enhanced
formation of these complexes was observed following LPS stimulation
(P<0.05). However, the interactions of TLR4/MyD88 and TLR4/TRIF
were attenuated by coculture with MSCs with or without LPS
stimulation (P<0.05; Fig. 7).
MyD88 and TRIF expression was not detected after coprecipitation
with normal nonspecific IgG (Fig.
7).
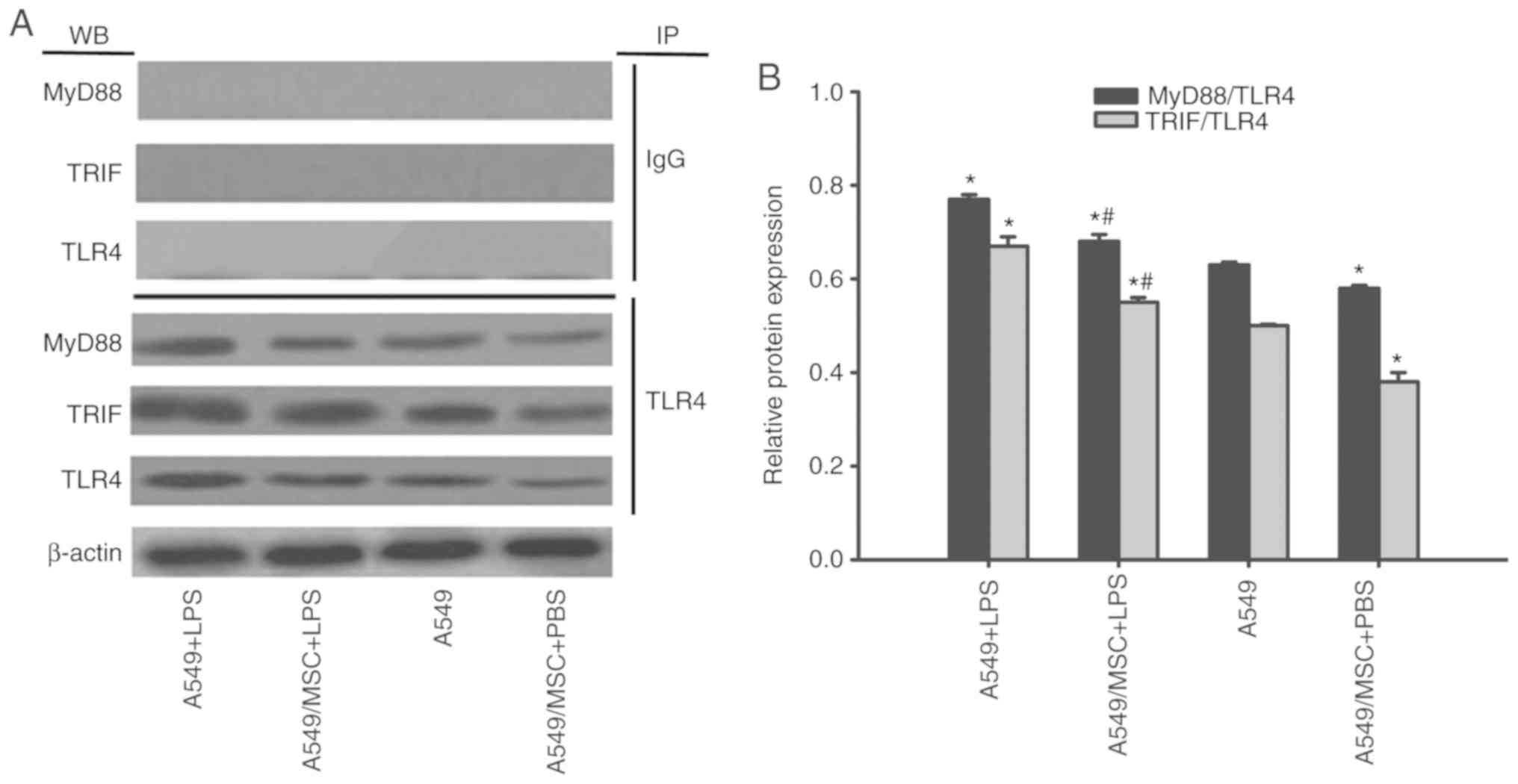 | Figure 7.Coculture with MSCs suppresses
TLR4/MyD88 and TLR4/TRIF complex formation in A549 cells. A549
cells with or without coculture with MSCs were treated with LPS
(100 µg/ml) or PBS for 6 h. The interaction of TLR with MyD88 or
TRIF was evaluated by coimmunoprecipitation. (A) Representative
coimmunoprecipitated blots of MyD88 and TRIF. (B) Densitometric
analyses of MyD88 and TRIF bands. Data are expressed as means ±
standard error of the mean of three independent experiments.
*P<0.05 vs. normal A549 cells, #P<0.05 vs. A549 +
LPS group. LPS, lipopolysaccharide; MSC, mesenchymal stem cell;
TLR4, Toll-like receptor-4; MyD88, myeloid differentiation factor
88; TRIF, toll-interleukin-1 receptor domain-containing adaptor
inducing interferon; WB, western blot analysis; IP,
immunoprecipitation; A549 + LPS, LPS-stimulated A549 cells;
A549/MSC + LPS, A549 cells cocultured with MSCs following
LPS-stimulation; A549, untreated A549 cells; A549/MSC + PBS, A549
cells cocultured with MSCs following PBS-treatment. |
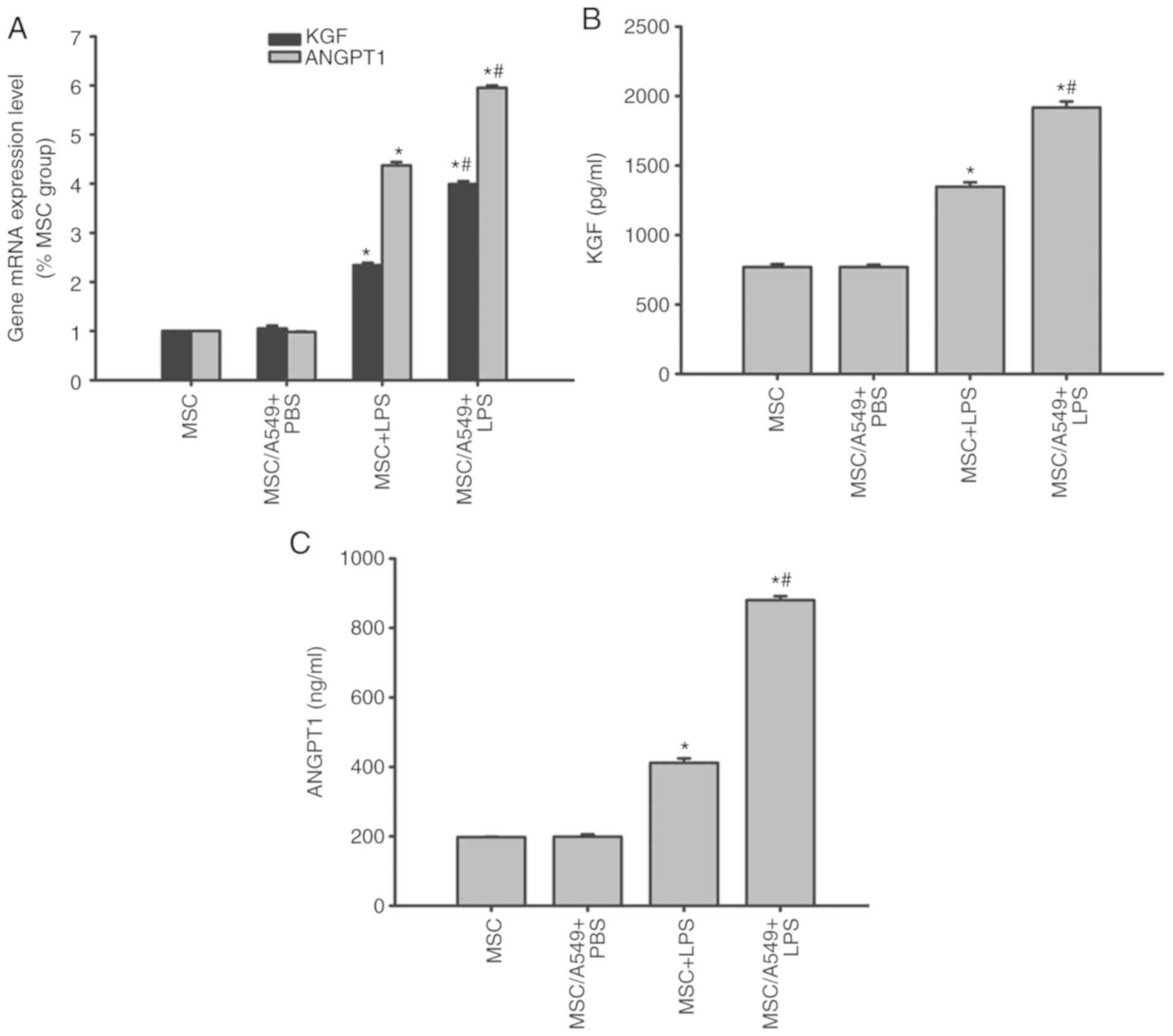
KGF and ANGPT1 expression in MSCs
KGF and ANGPT1 are MSC-derived paracrine factors
that target the alveolar epithelium (8). Therefore, the expression levels of
KGF and ANGPT1 in MSCs prior to and following treatments were
compared by RT-qPCR and ELISAs. KGF and ANGPT1 mRNA and protein
expression of MSCs cocultured with A549 cells was similar to that
of normal MSCs prior to LPS stimulation (P>0.05; Fig. 8). Following LPS stimulation, KGF
and ANGPT1 mRNA and protein levels were upregulated in
non-cocultured MSCs, which were increased in the MSC/A549 coculture
system (P<0.05; Fig. 8).
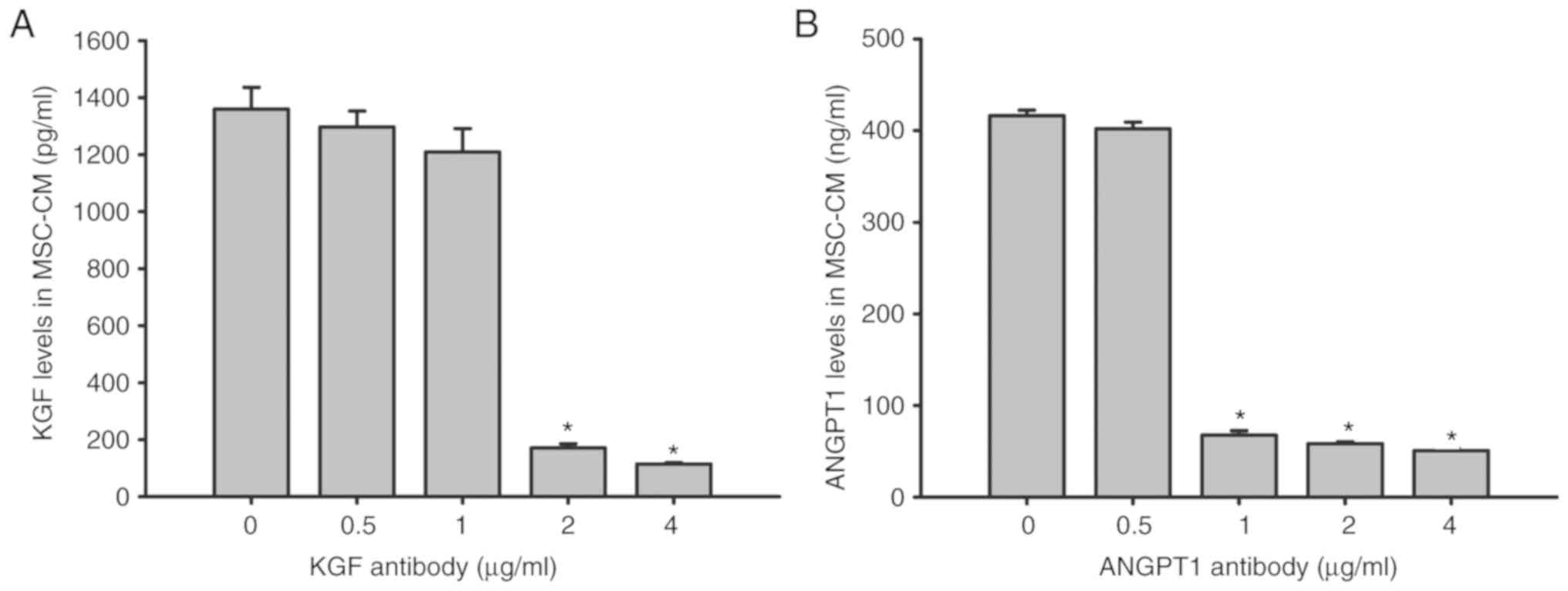
Effect of KGF and ANGPT1 neutralizing
antibody on KGF and ANGPT1 levels in MSC-CM
KGF and ANGPT1 concentration levels were
additionally examined by ELISAs following the addition of anti-KGF
(0.5, 1.0, 2.0 and 4.0 µg/ml) and anti-ANGPT1 (0.5, 1.0, 2.0 and
4.0 µg/ml) antibodies to MSC-CM. The results indicated that the
levels of KGF and ANGPT1 were ~1,360 pg/ml and 416 ng/ml,
respectively, in MSC-CM after 6 h of LPS stimulation. However, KGF
in MSC-CM was significantly neutralized with ≥2 µg/ml anti-KGF
antibody (P<0.05), while ≥1 µg/ml anti-ANGPT1 antibody
significantly neutralized ANGPT1 in MSC-CM (P<0.05; Fig. 9).
Effect of KGF and ANGPT1 neutralizing
antibody on the viability of A549 cells
To analyze the specific effect of KGF and ANGPT1 on
the viability of A549 cells, the effect of MSC-CM on cell viability
in the presence of neutralizing antibodies against KGF and ANGPT1
was evaluated. The results demonstrated that MSC-CM reversed the
inhibitory effect of LPS on cell viability of A549 cells
(P<0.05; Fig. 10). However,
the beneficial effect of MSC-CM on cell viability was abrogated by
the administration of KGF or ANGPT1 neutralizing antibody
(P<0.05; Fig. 10). Compared
with monotherapy, the combination of anti-KGF and anti-ANGPT1
antibodies additionally impaired the protective effect of MSC-CM on
cell viability of A549 cells, but the difference did not reach
significance (P>0.05; Fig.
10). Unspecific IgG antibodies had no effect on the
cytoprotective effect of MSC-CM (P>0.05; Fig. 10).
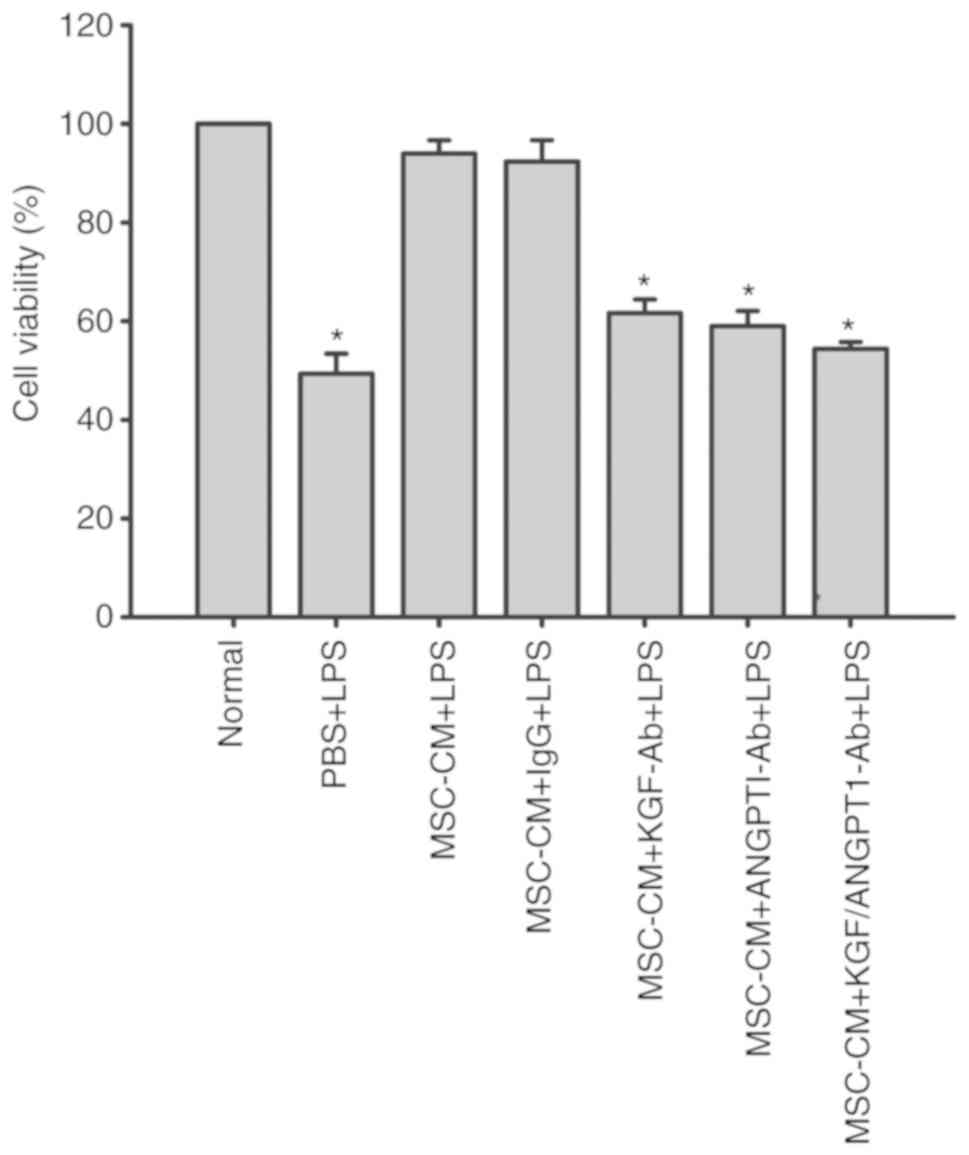 | Figure 10.Coculture with MSCs improves A549
cell viability by secreting paracrine KGF and ANGPT1. A549 cells
were treated with LPS (100 µg/ml) or LPS combined with various
types of CM for 6 h. Then, A549 cell viability was determined by
CCK-8 assays. Data are expressed as means ± standard error of the
mean of three independent experiments. *P<0.05 vs. normal A549
cells. KGF-Ab, keratinocyte growth factor-neutralizing antibody;
ANGPT1-Ab, angiopoietin-1-neutralizing antibody; LPS,
lipopolysaccharide; IgG, Immunoglobulin G; MSC, mesenchymal stem
cell; MSC-CM, MSC conditioned medium; Normal, normal and untreated
A549 cells; PBS + LPS, A549 cells treated with PBS and LPS; MSC-CM
+ LPS, A549 cells treated with MSC-CM and LPS; MSC-CM + IgG + LPS,
A549 cells treated with IgG-pretreated MSC-CM and LPS; MSC-CM +
KGF-Ab + LPS, A549 cells treated with KGF-Ab-pretreated MSC-CM and
LPS; MSC-CM + ANGPT1-Ab + LPS, A549 cells treated with
ANGPT1-Ab-pretreated MSC-CM and LPS; MSC-CM + KGF/ANGPT1-Ab + LPS,
A549 cells treated with KGF/ANGPT1-Ab-pretreated MSC-CM and
LPS. |
Effect of KGF and ANGPT1 neutralizing
antibodies on the release of TNF-α, IL-8 and IL-1β in A549
cells
As indicated in Fig.
11, treatment with MSC-CM significantly inhibited the
production of TNF-α, IL-8 and IL-1β induced by LPS in A549 cells
(P<0.05; Fig. 11). In
addition, unspecific IgG antibodies had no effect on the inhibitory
effect of MSC-CM on the production of inflammatory cytokines
(P>0.05; Fig. 11). However,
the anti-KGF and anti-ANGPT1 antibodies diminished this inhibitory
effect of MSC-CM on the production of TNF-α, IL-8 and IL-1β induced
by LPS in A549 cells (P<0.05 vs. MSC-CM + LPS and MSC-CM + IgG +
LPS; Fig. 11). Concurrently,
treatment with anti-KGF antibodies combined with anti-ANGPT1
antibodies additionally reversed the beneficial effect of MSC-CM
and promoted the release of A549 cell-derived inflammatory
cytokines under LPS simulation (P<0.05; Fig. 11).
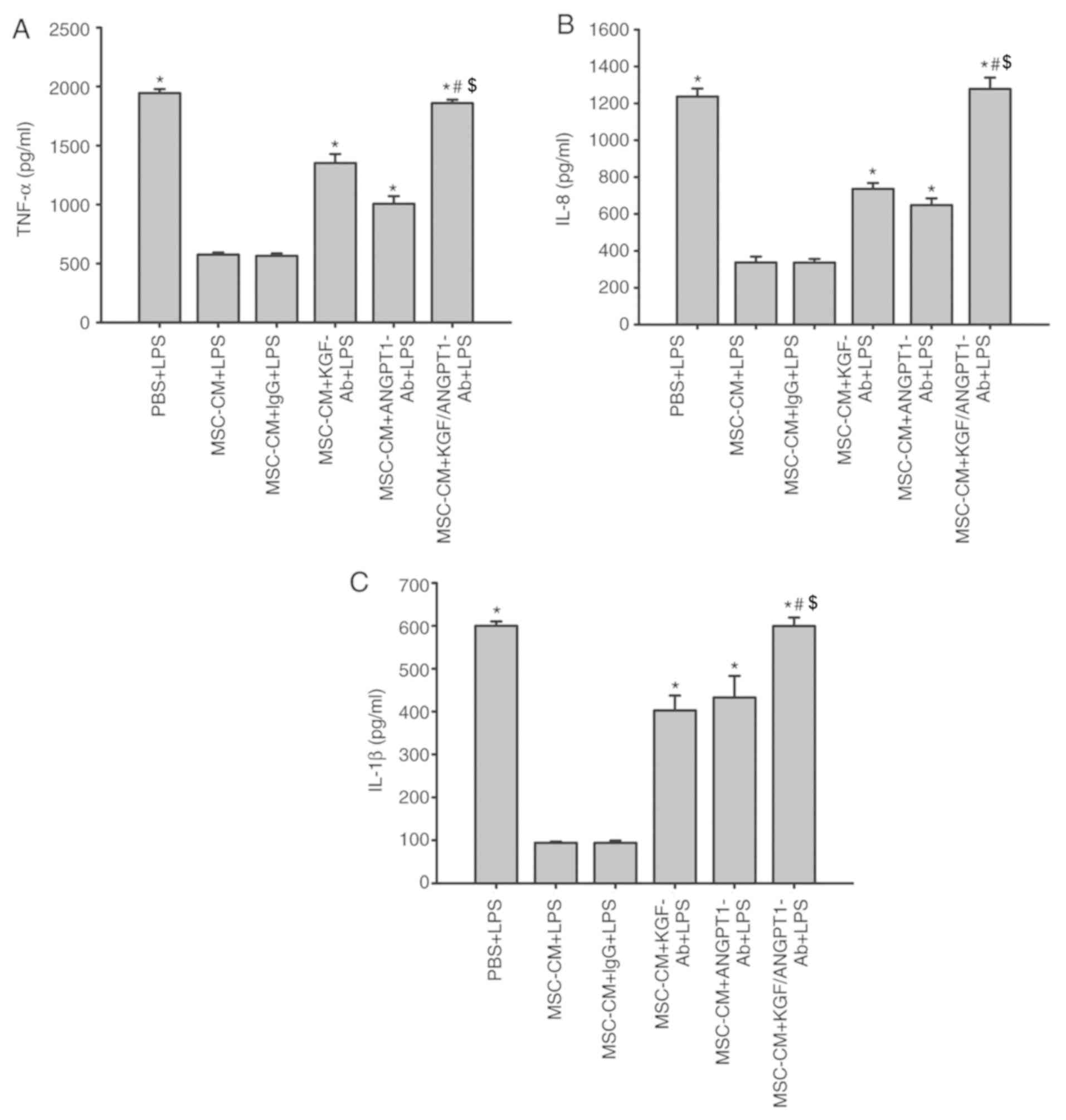 | Figure 11.Coculture with MSCs inhibits the
production of (A) TNF-α, (B) IL-8 and (C) IL-1β derived from A549
cells by secreting paracrine KGF and ANGPT1. A549 cells were
treated with LPS (100 µg/ml) or LPS combined with various types of
CM for 6 h. The levels of TNF-α, IL-8 and IL-1β were then measured
by ELISAs. Data are expressed as means ± standard error of the mean
of three independent experiments. *P<0.05 vs. MSC-CM + LPS,
#P<0.05 vs. MSC-CM + KGF-Ab + LPS and
$P<0.05 vs. MSC-CM + ANGPT1-Ab + LPS). KGF-Ab,
keratinocyte growth factor-neutralizing antibody; ANGPT1-Ab,
angiopoietin-1-neutralizing antibody; LPS, lipopolysaccharide; IgG,
Immunoglobulin G; MSC, mesenchymal stem cell; MSC-CM, MSC
conditioned medium; TNF-α, tumor necrosis factor α; Il,
interleukin; PBS + LPS, A549 cells treated with PBS and LPS; MSC-CM
+ LPS, A549 cells treated with MSC-CM and LPS; MSC-CM + IgG + LPS,
A549 cells treated with IgG-pretreated MSC-CM and LPS; MSC-CM +
KGF-Ab + LPS, A549 cells treated with KGF-Ab-pretreated MSC-CM and
LPS; MSC-CM + ANGPT1-Ab + LPS, A549 cells treated with
ANGPT1-Ab-pretreated MSC-CM and LPS; MSC-CM + KGF/ANGPT1-Ab + LPS,
A549 cells treated with KGF/ANGPT1-Ab-pretreated MSC-CM and
LPS. |
Discussion
In the present study, it was demonstrated that high
concentrations of LPS (>80 µg/ml) were cytotoxic in AECs,
suppressed AEC proliferation, induced apoptosis and provoked severe
inflammatory reactions through activation of TLR4 signaling in
AECs. Coculture with BM-MSCs reversed all of these detrimental
effects of LPS and inhibited TLR4 signal transduction in AECs. The
protective effect of MSCs may be partly associated with their
paracrine secretion of KGF and ANGPT1. To the best of our
knowledge, the present study is the first to demonstrate that
coculture with MSCs protects AECs from LPS-induced injury via
enhanced secretion of KGF and ANGPT1 and consequent inhibition of
TLR4 signaling.
TLR4 is an innate immune receptor that is expressed
in monocytes/macrophages, neutrophils, and dendritic, epithelial
and endothelial cells (20). LPS
causes conformational changes of TLR4. Toll/interleukin-1
receptor-like (TIR) domains of TLR4 recruit TIR domain-containing
adaptor proteins MyD88 in the MyD88-dependent pathway or TRIF in
the MyD88-independent pathway (11). MyD88-dependent and -independent
pathways serve key roles in the activation of NF-κB (21). In the present study, it was
identified that coculture with MSCs downregulated TLR4, MyD88 and
TRIF expression and hampered TLR4/MyD88 and TLR4/TRIF complex
formation. Inhibition of the LPS-induced ‘cytokine storm’ and NF-κB
activation in A549 cells by coculture with MSCs may be attributed
to decreases in the expression these adaptors and their complex
formations. These data suggested that coculture with MSCs
attenuated the LPS-induced inflammation by inhibiting TLR4 signal
activation.
Excessive apoptosis of AECs is a primary factor in
ALI progression (22). It has been
demonstrated that TLR4 signal activation cross talks with caspase
activation (12). Caspase
activation has been implicated as the final common pathway to the
induction of apoptosis (19). In
the present study, it was identified that 100 µg/ml LPS upregulated
the pro-apoptotic protein caspase-3 and downregulated
anti-apoptotic protein Bcl-2. Coculture with MSCs reversed the
modulation of these apoptosis-associated proteins. The
proliferative effect of MSCs on A549 cells was associated with a
suppression of the decrease in Bcl-2 and increase in caspase-3
levels. Considering the cross talk between TLR4 activation and
apoptotic cascades, and the results of the present study, the
inhibitory effect of MSCs on LPS-induced apoptosis and caspase
activation may be attributed to the inhibition of TLR4 signaling.
Additional investigation is required to reveal the detailed
association between TLR4 signals and apoptosis in LPS-stimulated
AECs. Notably, the data from the present study demonstrated that
high doses of LPS induced A549 cell apoptosis, whereas low doses
promoted A549 cell proliferation, which is in concordance with
previous studies (23,24). Low doses of LPS promote cell
proliferation by activating multiple signaling pathways including
the phosphatidylinositol 3-kinase/protein kinase B pathway
(24). Different doses of LPS may
have distinct modulatory effects on various cell types (25).
Excessive inflammation and pneumocyte apoptosis are
the primary pathologies of ALI (26,27).
MSCs are potent tools for improving these lesions (28). MSCs may exert preventive or
inhibitory effects on the inflammatory response via TLR3-regualted
mitogen-activated protein kinase and TLR2/4-NF-κB signaling pathway
in LPS-induced lung injury (26,29).
MSCs have been suggested to possess additional functions for the
treatment of ALI/ARDS, including homing to inflammatory sites,
differentiating into pneumocytes, secreting multiple soluble
factors that may repair injured pneumocytes and performing
immunomodulatory effects (8,30,31).
However, current data suggest that the therapeutic effects of MSCs
are largely mediated through paracrine factors (8). Among MSC-derived factors, KGF and
ANGPT1 with anti-inflammatory, anti-permeability and epithelial
proliferative effects are targeted to AECs (8,32).
The present study revealed that the mRNA and protein expression
levels of KGF and ANGPT1 were markedly upregulated following LPS
stimulation. In the MSC/A549 coculture system, KGF and ANGPT1
expression levels were additionally increased. The additional
increase in KGF and ANGPT1 expression may correlate with
proinflammatory cytokines that also have a stimulatory effect on
KGF and ANGPT1 expression (33).
In coculture systems, LPS may exert its synergistic effects with
proinflammatory cytokines, including TNF-α, IL-8 and IL-1β,
released by A549 cells to additionally promote paracrine factor
secretion. Otherwise, MSCs attenuated inflammation and inhibited
apoptosis in A549 cells without LPS stimulation. It appeared that
MSCs also secreted a certain amount of KGF and ANGPT1 to protect
AECs under non-inflammatory conditions. Notably, KGF and ANGPT1
neutralizing antibodies exhibited inhibitory effects on KGF and
ANGPT1 in MSC-CM and impaired the protective and anti-inflammatory
effect of MSC-CM. Therefore, we hypothesized that the beneficial
effect of MSCs on inflammation and cell viability in AECs following
LPS-stimulation was partly dependent on enhanced KGF and ANGPT1
expression. This result additionally verified the association
between enhanced KGF/ANGPT1 expression and the beneficial effect of
MSCs on A549 cells. In future studies, cytokine array analysis
would be useful to determine whether other paracrine factors are
involved in the epithelial protective effect of MSCs in addition to
KGF and ANGPT1.
Primary human type II AECs are difficult to isolate
and cultivate (34). Although the
A549 cell line is cancerous, it is an AEC line with type II cell
characteristics, which has been widely used as a suitable surrogate
for a primary human type II AEC line to study ALI/ARDS (2,35).
Therefore, A549 cells were selected for the experiments in the
present study. The fact that beneficial effects of MSCs and TLR4
signal inhibition were observed in A549 cells indicate that the
data are valuable to reveal the protective mechanisms of MSCs in
vitro, despite the subtle differences between A549 cells and
primary human AECs. Additionally, a 2-dimensional culture system
was adopted in the present study, which lacked an extracellular
matrix and inflammatory cells. A 3-dimensional cell culture system,
which is closer to the in vivo cell microenvironment, will
be used to corroborate the results in future studies.
In conclusion, the present study demonstrated that
coculture with BM-MSCs attenuates LPS-induced inflammation and
apoptosis in AECs via the TLR4 signaling pathway. The modulation of
TLR4 signals involves downregulation of adaptor proteins TLR4,
MyD88 and TRIF and suppression of TLR4/MyD88 and TLR4/TRIF complex
formation. These beneficial effects of MSCs may be ascribed to
enhanced secretion of KGF and ANGPT1 under inflammatory conditions.
These data provide a novel insight into MSC-based therapeutic
strategies for treating ALI. Future studies will focus on the
aforementioned issues and detailed mechanisms of MSC-mediated
inhibition of TLR4 signals.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81300050), the Beijing
Natural Science Foundation (grant no. 7182163) and the Innovative
Cultivation Foundation of Navy General Hospital of the People's
Liberation Army (grant no. CXPY201417).
Availability of data and material
All data generated or analyzed during this study are
included in this article.
Authors' contributions
JM designed the present study. XC performed the
analysis of NF-κB DNA binding activity, collected data, made
figures and wrote manuscript. LT performed the cell culture, cell
viability, western blot analysis and reverse transcription
quantitative polymerase chain reaction assay. WW performed the
ELISAs, and coimmunoprecipitation and flow cytometric assays. ZH
made substantial contributions to the analysis of data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
MSC
|
mesenchymal stem cell
|
|
TLR
|
toll-like receptor
|
|
LPS
|
lipopolysaccharide
|
|
LDH
|
lactate dehydrogenase
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
|
MyD88
|
myeloid differentiation factor 88
|
|
TRIF
|
toll-interleukin-1 receptor
domain-containing adaptor inducing interferon
|
|
KGF
|
keratinocyte growth factor
|
|
ANGPT1
|
angiopoietin-1
|
References
|
1
|
Chen X, Tang L, Feng J, Wang Y, Han Z and
Meng J: Downregulation of paralemmin-3 ameliorates
lipopolysaccharide-induced acute lung injury in rats by regulating
inflammatory response and inhibiting formation of TLR4/MyD88 and
TLR4/TRIF complexes. Inflammation. 40:1983–1999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong Y, Yu Z, Gao Y, Deng L, Wang M, Chen
Y, Li J and Cheng B: FABP4 inhibitors suppress inflammation and
oxidative stress in murine and cell models of acute lung injury.
Biochem Biophys Res Commun. 496:1115–1121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyp MF, Mabry SM, Navarro A, Menden H,
Perez RE, Sampath V and Ekekezie II: Lung epithelial-specific
TRIP-1 overexpression maintains epithelial integrity during
hyperoxia exposure. Physiol Rep. 6:2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu BY, Li YL, Luan B, Zhang YL, Jia TM and
Qiao JY: MiR-26a protects type II alveolar epithelial cells against
mitochondrial apoptosis. Eur Rev Med Pharmacol Sci. 22:486–491.
2018.PubMed/NCBI
|
|
5
|
Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D,
Liu F and Guo Z: miR-34b-5p inhibition attenuates lung inflammation
and apoptosis in an LPS-induced acute lung injury mouse model by
targeting progranulin. J Cell Physiol. 233:6615–6631. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao D, Xie J, Zhang J, Feng C, Yao B, Ma
K, Li J, Wu X, Huang S and Fu X: MSC attenuate diabetes-induced
functional impairment in adipocytes via secretion of insulin-like
growth factor-1. Biochem Biophys Res Commun. 452:99–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matthay MA: Therapeutic potential of
mesenchymal stromal cells for acute respiratory distress syndrome.
Ann Am Thorac Soc. 12 Suppl:S54–S57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Huang S, Wu Y, Gu C, Gao D, Feng C,
Wu X and Fu X: Paracrine factors from mesenchymal stem cells: A
proposed therapeutic tool for acute lung injury and acute
respiratory distress syndrome. Int Wound J. 11:114–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie J, Liu B, Chen J, Xu Y, Zhan H, Yang
F, Li W and Zhou X: Umbilical cord-derived mesenchymal stem cells
alleviated inflammation and inhibited apoptosis in interstitial
cystitis via AKT/mTOR signaling pathway. Biochem Biophys Res
Commun. 495:546–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XX, Tang L, Fu YM, Wang Y, Han ZH and
Meng JG: Paralemmin-3 contributes to lipopolysaccharide-induced
inflammatory response and is involved in
lipopolysaccharide-toll-like receptor-4 signaling in alveolar
macrophages. Int J Mol Med. 40:1921–1931. 2017.PubMed/NCBI
|
|
11
|
Molteni M, Gemma S and Rossetti C: The
role of toll-like receptor 4 in infectious and noninfectious
inflammation. Mediators Inflamm 2016. 69789362016.
|
|
12
|
Wang X, Sun Y, Yang H, Lu Y and Li L:
Oxidized low-density lipoprotein induces apoptosis in cultured
neonatal rat cardiomyocytes by modulating the TLR4/NF-κB pathway.
Sci Rep. 6:278662016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scotti L, Abramovich D, Pascuali N, de
Zúñiga I, Oubiña A, Kopcow L, Lange S, Owen G, Tesone M and
Parborell F: Involvement of the ANGPTs/Tie-2 system in ovarian
hyperstimulation syndrome (OHSS). Mol Cell Endocrinol. 365:223–230.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hille A, Grüger S, Christiansen H, Wolff
HA, Volkmer B, Lehmann J, Dörr W and Rave-Fränk M: Effect of
tumour-cell-derived or recombinant keratinocyte growth factor (KGF)
on proliferation and radioresponse of human epithelial tumour cells
(HNSCC) and normal keratinocytes in vitro. Radiat Environ Biophys.
49:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YL, Hui YN, Guo B and Ma JX:
Strengthening tight junctions of retinal microvascular endothelial
cells by pericytes under normoxia and hypoxia involving
angiopoietin-1 signal way. Eye (Lond). 21:1501–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hostanska K, Melzer J, Amon A and Saller
R: Suppression of interleukin (IL)-8 and human beta defensin-2
secretion in LPS-and/or IL-1β-stimulated airway epithelial A549
cells by a herbal formulation against respiratory infections (BNO
1030). J Ethnopharmacol. 134:228–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kucukgul A and Erdogan S: Low
concentration of oleic acid exacerbates LPS-induced cell death and
inflammation in human alveolar epithelial cells. Exp Lung Res.
43:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao G, Ling L, Luan J, Ye D and Zhu P:
Nonylphenol induces apoptosis of Jurkat cells by a caspase-8
dependent mechanism. Int Immunopharmacol. 7:444–453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi L, Wang JS, Liu XM, Hu XY and Fang Q:
Upregulated functional expression of toll like receptor 4 in
mesenchymal stem cells induced by lipopolysaccharide. Chin Med J
(Engl). 120:1685–1688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shim DW, Han JW, Sun X, Jang CH, Koppula
S, Kim TJ, Kang TB and Lee KH: Lysimachia clethroides duby extract
attenuates inflammatory response in raw 264.7 macrophages
stimulated with lipopolysaccharide and in acute lung injury mouse
model. J Ethnopharmacol. 150:1007–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu
H, Zhang X and Jiang H: Complement C5a exacerbates acute lung
injury induced through autophagy-mediated alveolar macrophage
apoptosis. Cell Death Dis. 5:e13302014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ming J, Liu XS, Liu L, Xu H, Ran XZ and
Cheng TM: Effect of lipopolysacharide on the biological features
and growth factor secretion power of U937 cell line. Zhonghua Shao
Shang Za Zhi (In Chinese). 20:92–94. 2004.
|
|
24
|
Yin Q, Jiang D, Li L, Yang Y, Wu P, Luo Y,
Yang R and Li D: LPS promotes vascular smooth muscle cells
proliferation through the TLR4/Rac1/Akt signalling pathway. Cell
Physiol Biochem. 44:2189–2200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Liew LN, Kuo IC, Huang CH, Goh DL
and Chua KY: The modulatory effects of
lipopolysaccharide-stimulated B cells on differential T-cell
polarization. Immunology. 125:218–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Pan X, Zhao J, Chi C, Wu G, Wang Y,
Liao S, Wang C, Ma J and Pan J: Bone marrow mesenchymal stem cells
suppress acute lung injury induced by lipopolysaccharide through
inhibiting the TLR2, 4/NF-κB pathway in rats with multiple trauma.
Shock. 45:641–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu D, Liang M, Dang H, Fang F, Xu F and
Liu C: Hydrogen protects against hyperoxia-induced apoptosis in
type II alveolar epithelial cells via activation of PI3K/Akt/Foxo3a
signaling pathway. Biochem Biophys Res Commun. 495:1620–1627. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirzaei H, Sahebkar A, Sichani LS,
Moridikia A, Nazari S, Sadri Nahand J, Salehi H, Stenvang J,
Masoudifar A, Mirzaei HR and Jaafari MR: Therapeutic application of
multipotent stem cells. J Cell Physiol. 233:2815–2823. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Qin Y and Mi X: The protective
effects of bone marrow-derived mesenchymal stem cell (BMSC) on
LPS-induced acute lung injury via TLR3-mediated IFNs, MAPK and
NF-κB signaling pathways. Biomed Pharmacother. 79:176–187. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan H, Wu M, Yuan Y, Wang ZZ, Jiang H and
Chen T: Priming of toll-like receptor 4 pathway in mesenchymal stem
cells increases expression of B cell activating factor. Biochem
Biophys Res Commun. 448:212–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horie S, Masterson C, Devaney J and Laffey
JG: Stem cell therapy for acute respiratory distress syndrome: A
promising future. Curr Opin Crit Care. 22:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang X, Neyrinck AP, Matthay MA and Lee
JW: Allogeneic human mesenchymal stem cells restore epithelial
protein permeability in cultured human alveolar type II cells by
secretion of angiopoietin-1. J Biol Chem. 285:26211–26222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ware LB and Matthay MA: Keratinocyte and
hepatocyte growth factors in the lung: Roles in lung development,
inflammation, and repair. Am J Physiol Lung Cell Mol Physiol.
282:L924–L940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernard O, Jeny F, Uzunhan Y, Dondi E,
Terfous R, Label R, Sutton A, Larghero J, Vanneaux V, Nunes H, et
al: Mesenchymal stem cells reduce hypoxia-induced apoptosis in
alveolar epithelial cells by modulating HIF and ROS hypoxic
signaling. Am J Physiol Lung Cell Mol Physiol. 314:L360–L371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thorley AJ, Ford PA, Giembycz MA,
Goldstraw P, Young A and Tetley TD: Differential regulation of
cytokine release and leukocyte migration by
lipopolysaccharide-stimulated primary human lung alveolar type II
epithelial cells and macrophages. J Immunol. 178:463–473. 2007.
View Article : Google Scholar : PubMed/NCBI
|