Introduction
Gastric cancers that develop from the lining of the
stomach are highly aggressive. Currently, gastric cancers are the
second leading cause of cancer-related deaths worldwide (1–3), and
development of novel therapeutic options is urgently required
(3).
Traditional herbal medicines have become
increasingly popular among cancer patients (4,5),
mainly because of minimal complications and side-effects from
treatment and improved quality of life (5–7).
Radix Sophorae Flavescentis (RSF) is an ancient Chinese herb
(8) that contains active
ingredients such as sophordine, matrine, and sophocarpine (8,9). RSF
has detoxification, diuretic, and insect repellent properties
(10). It is also one of the
important ingredients for the treatment of contact dermatitis and
local pruritus of the vagina (11,12).
It also has other pharmacological properties, such as
antibacterial, anti-tumour, and anti-viral (against hepatitis B
virus) effects (12–14). RSF induces apoptosis of laryngeal
neoplasm Hep2 cells (15),
esophageal carcinoma TE-8 cells (10), and PC-3 prostate cancer cells
(16). However, evidence is
minimal in order to recommend RSF for use in the treatment of
gastric cancers. Therefore, we investigated the mechanisms
underlying apoptosis induced by RSF in AGS cells to improve our
understanding and also expand the scope of its therapeutic
applications.
Materials and methods
Preparation of RSF
Radix Sophorae extract was purchased from the
Korea Plant Extract Bank (Ochang, Chungbuk, Korea). Following the
supplier instructions, Radix Sophorae was extracted in 95%
ethyl alcohol at 45°C for 3 days. The extracted solution was
filtered and evaporated at 45°C. The dried extract was dissolved in
methanol and diluted in water to obtain a final concentration 2
mg/ml. Matrine and oxymatrine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were dissolved in methanol and diluted in water
to obtain solutions with final concentrations of 100, 200, 300, 400
and 500 µg/ml. Solutions of Radix Sophorae, matrine, and
oxymatrine were filtered through a 0.45 µm regenerated cellulose
membrane filter (Sartorius AG, Goettingen, Germany). Analytical
high-performance liquid chromatography (HPLC) was performed in a
JASCO HPLC system (JASCO, Hachioji, Tokyo, Japan) comprising a
PU-980 pump, and an AS-950-10 autosampler equipped with MD-2010
Plus multi-wavelength detector. The chromatographic separation was
performed with a Waters Symmetry® C18 (4.6×250 mm,
particle size 5 µm) column. The ultraviolet (UV) detection was set
at 220 nm. A reverse-phase HPLC assay was performed using an
isocratic system with methanol:water containing 3% phosphoric acid
(4:96 v/v) as the mobile phase for a 30 min run. The flow rate was
set to 1 ml/min and the column was maintained at 30°C. Similar to
the previous HPLC run, the UV detection was set at 220 nm. The
injection volume was 10 µl. The standard calibration curve of
matrine and oxymatrine showed linearity (r2>0.999**)
in the range of 100–500 µg/ml. Quantitative analysis was repeated
three times, and all data are expressed as mean ± standard
deviation. The concentrations of matrine and oxymatrine were
determined to be 70.47±1.27 and 299.87±3.746 mg/g,
respectively.
Cell culture and reagents
The AGS human gastric adenocarcinoma cell line was
used for the experiments. These cells were propagated in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1% antibiotic mix
(penicillin and streptomycin) (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. SP600125 and PD98059 were purchased from
Tocris (Bristol, UK). All other reagents were obtained from
Sigma-Aldrich (Merck KGaA).
Cell viability assay
Cell viability was studied using the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. AGS cells were treated with MTT solution and incubated for 2
h at 37°C, following which, absorbance was measured at 570 nm.
Cell-cycle analysis
To AGS cells, ethyl alcohol was added and the cells
vortexed prior to overnight incubation at 4°C. Samples were
centrifuged for 5 min and the supernatant was discarded. Cell
pellets were resuspended in propidium iodine (PI) staining solution
(5 mg/ml; 2 µl) containing RNase (2 µl) (17,18)
and centrifuged at 20,000 × g for 10 sec. After incubation at room
temperature for 40 min in the dark, the samples were analysed using
a fluorescence-activated cell sorter (FACScan; Becton-Dickinson,
Mountain View, CA, USA) set at λ=488 nm and using the Cell-Quest
software (Becton-Dickinson, Franklin Lakes, NJ, USA).
Western blotting
Total cell extract was prepared using RIPA buffer
(Cell Signaling Technology Inc., Danvers, MA) containing 1 mM
phenylmethylsulfonyl fluoride (PMSF). Protein content was measured
using the Bradford method (Bio-Rad Laboratories, Hercules, CA).
Equal amounts of proteins were fractionated by SDS-PAGE and
transferred to a PVDF membrane (Bio-Rad Laboratories, Hercules,
CA). Membranes were blocked for at least 1 h with 5% non-fat dry
milk prior to incubating overnight with antibodies against Bcl-2
(cat. no. SC-783; Santa Cruz Biotechnology, Dallas, TX, USA), Bax
(cat. no. SC-493; Santa Cruz Biotechnology), JNK (cat. no. #9252;
Cell Signaling Technology, Beverly, MA, USA), p-JNK (cat. no.
#9255; Cell Signaling Technology), p38 MAPK (cat. no. #9228; Cell
Signaling Technology), p-p38 MAPK (cat. no. #9211; Cell Signaling
Technology, Beverly, MA, USA) and β-actin (cat. no. SC-47778; Santa
Cruz Biotechnology) at 4°C. The membrane was further incubated with
secondary antibodies conjugated to HRP at room temperature for 1 h.
Bands of interest were visualised by chemiluminescence (Super
Signal West Femto; Thermo Fisher Scientific, Inc.). Secondary
horseradish peroxidase-conjugated antibodies, including goat
anti-rabbit IgG (cat. no. sc-2004; Santa Cruz Biotechnology) and
goat anti-mouse IgG (cat. no. SC-2005; Santa Cruz Biotechnology),
were used. Relative intensities of protein (BCl-2 and Bax) bands
were analyzed with a GS-710 Image Densitometer (Bio-Rad
Laboratories, Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (PCR)
Total RNA was isolated using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and converted to cDNA
using AccuPower RT-PreMix (Bioneer Co., Daejeon, Korea). Specific
DNA sequences were amplified using AccuPower PCR-PreMix (Bioneer
Co.). PCR primers used in this study are: Fas
5′-ATGCTGGGCATCTGGACCCTCCTA-3′ forward and
5′-TCTGCACTTGGTATTCTGGGTCCG-3′ reverse; FasL
5′-ACTTCCGGGGTCAATCTTGC-3′ forward and 5′-TAGAACATCTCGGTGCCTGTA-3′
reverse; and β-actin 5′-CAAGAGATGGCCACGGCTGCT-3′ forward and
5′-TCCTTCTGCATCCTGTCGGCA-3′ reverse. Amplified products were
analysed in 1% agarose gel, and images were captured using the
GelDoc-It TS Imaging System (UVP, Upland, CA, USA).
Caspase assay
Caspase-3 assay kits (Cellular Activity Assay Kit
Plus; BioMol Plymouth, PA, USA) were used. After resuspending the
cells in ice-cold cell lysis buffer, the supernatant was removed.
Supernatant samples were incubated with caspase substrate (400-lM
Ac-DEVD-pNA; 50 µl) at 37°C. Each sample was read at 405 nm at
several time-points.
Measurement of ROS production
ROS generation in AGS cells was quantified using
DCF-DA (2′, 7′-dichlorodihydrofluorescein diacetate; Molecular
Probes, Eugene, OR, USA). The cells were treated with 20 µl DCF-DA
at 37°C for 30 min and washed with PBS. Fluorescence was measured
using an FACS system (Becton-Dickinson, Mountain View, CA, USA),
and at excitation/emission wavelength of 488/525 nm.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. N values refer to the number of cells used in the
experiments. One-way analysis of varaicne with Tukey's post hoc
comparison was used for multiple comparisons. Statistical analysis
was performed using Prism 6.0 (GraphPad Software, Inc., La Jolla,
CA, USA) and Origin 8.0 (OriginLab Corporation, Northampton, MA,
USA) software programs. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of standard components
of RSF
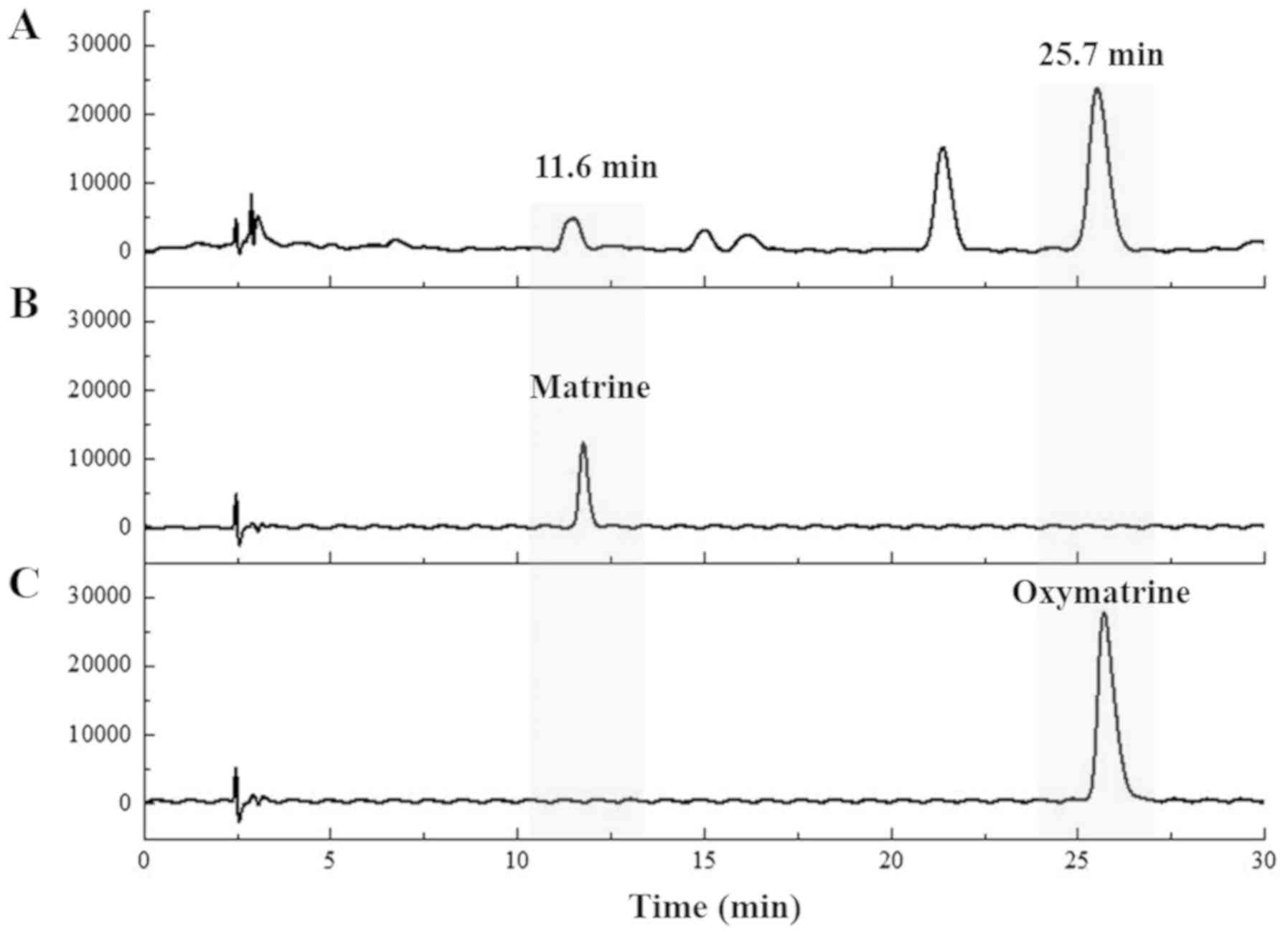
Matrine and oxymatrine were identified based on the
HPLC chromatogram of RSF with retention times of 11.6 and 25.7 min,
respectively (Fig. 1).
Apoptosis by RSF in AGS cells
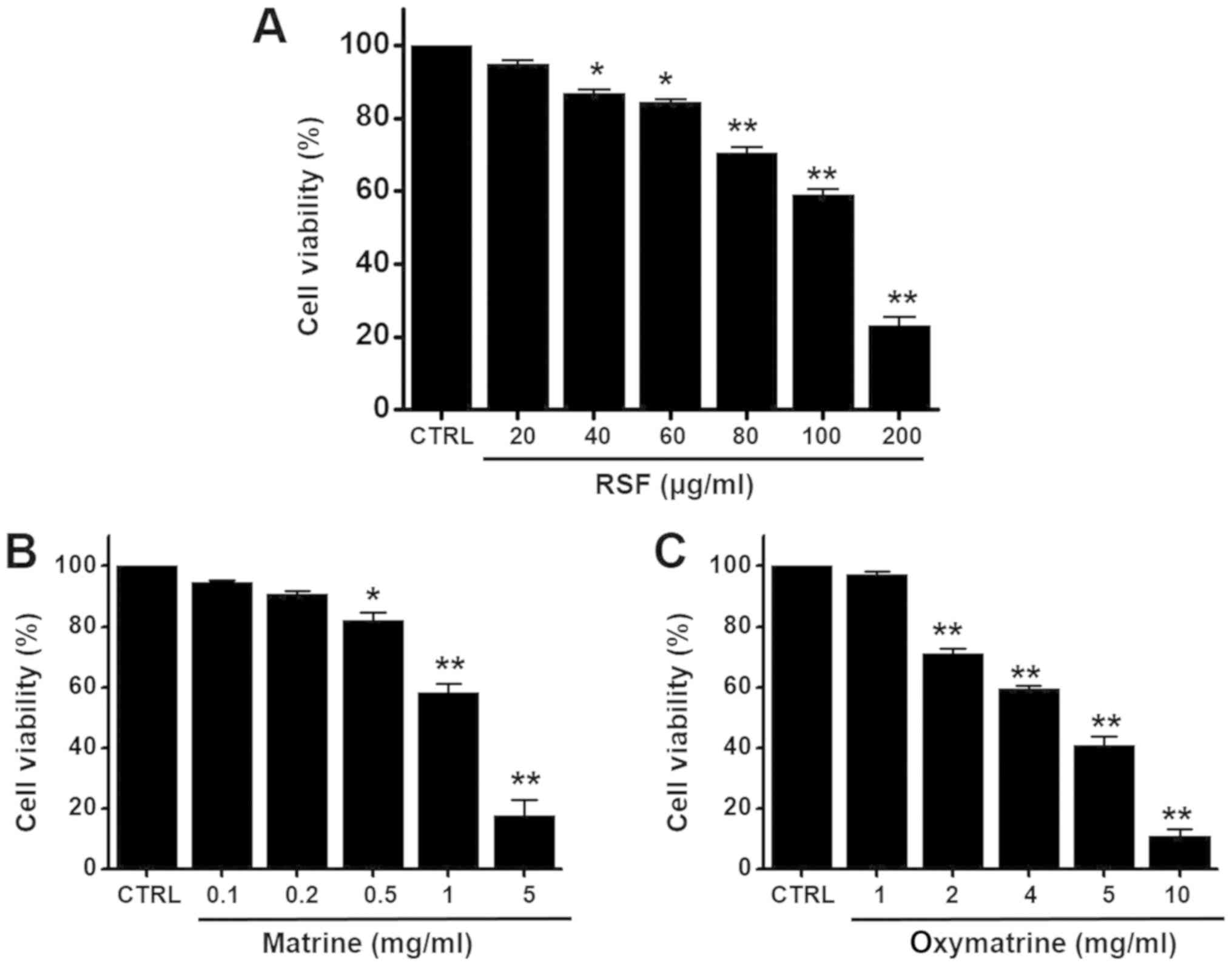
To determine whether RSF suppresses AGS cell growth,
MTT assays were performed after culturing cells with different
concentrations of RSF for 24 h. Cell viability decreased remarkably
following RSF treatment. Culturing in the presence of RSF
concentrations of 20, 40, 60, 80, 100, or 200 µg/ml inhibited AGS
survival by 94.9±1.1, 86.8±1.3, 84.4±0.9, 70.6±1.5, 59.0±1.6, or
23.2±2.5, respectively, as determined by MTT assay (n=6;
Fig. 2A). In addition, we
investigated the effects of matrine and oxymatrine, the major
ingredients in RSF, on cell viability by using the MTT assay. The
presence of matrine (0.1, 0.2, 0.5, 1, or 5 mg/ml) or oxymatrine
(1, 2, 4, 5, or 10 mg/ml) inhibited the survival of AGS cells
(n = 5; Fig. 2B and C). To
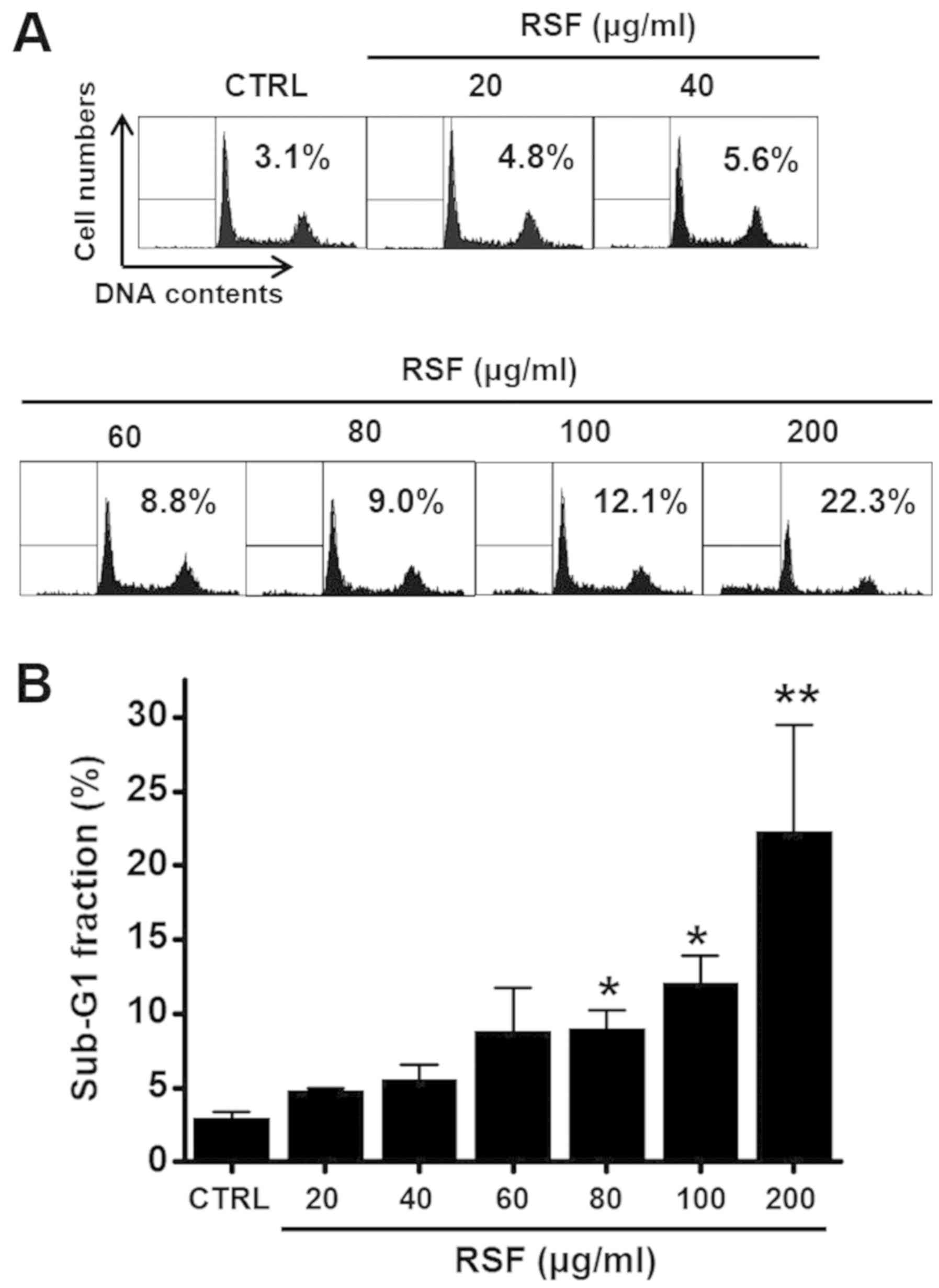
determine whether RSF induces apoptosis, cell cycle was studied by
using flow cytometry. Cells were treated with RSF for 24 h (with
concentrations ranging from 20 to 200 µg/ml; Fig. 3). The sub-G1 phase ratio was found
to be increased by RSF by 4.8±0.1 at 20 µg/ml, 5.6±1.0 at 40 µg/ml,
8.8±3.0 at 60 µg/ml, 9.0±1.3 at 80 µg/ml, 12.1±1.9 at 100 µg/ml,
and 22.3±7.2% at 200 µg/ml compared to that of the untreated cells
(n=6, respectively; Fig. 3A and
B). These results suggest that RSF has anti-cancer effects and
these effects are linked to apoptosis.
Mitochondria- and caspase-dependent
pathways in AGS cells
To determine whether RSF-induced apoptosis in AGS
cells is regulated by Bcl-2 (anti-apoptotic) and Bax
(pro-apoptotic), we performed western blotting after exposing the
cells to various concentrations of RSF (ranging from 20 to 200
µg/ml). While Bcl-2 expression was inhibited by RSF, Bax was
upregulated (Fig. 4A). Also, the
Fas/FasL system, a key player in the death receptor-mediated
apoptosis, was examined. Fas and FasL expression levels were both
up-regulated by RSF (Fig. 4B).
Caspase assays were performed to assess the activity of caspase-3
in the AGS cells. Caspase activity increased after treatment with
RSF (concentration ranging from 20 to 200 µg/ml), and that these
activities were suppressed by zVAD-fmk (Fig. 4C). These results suggest that
RSF-induced apoptosis is mediated by a mitochondrial- and
caspase-dependent pathway in AGS cells.
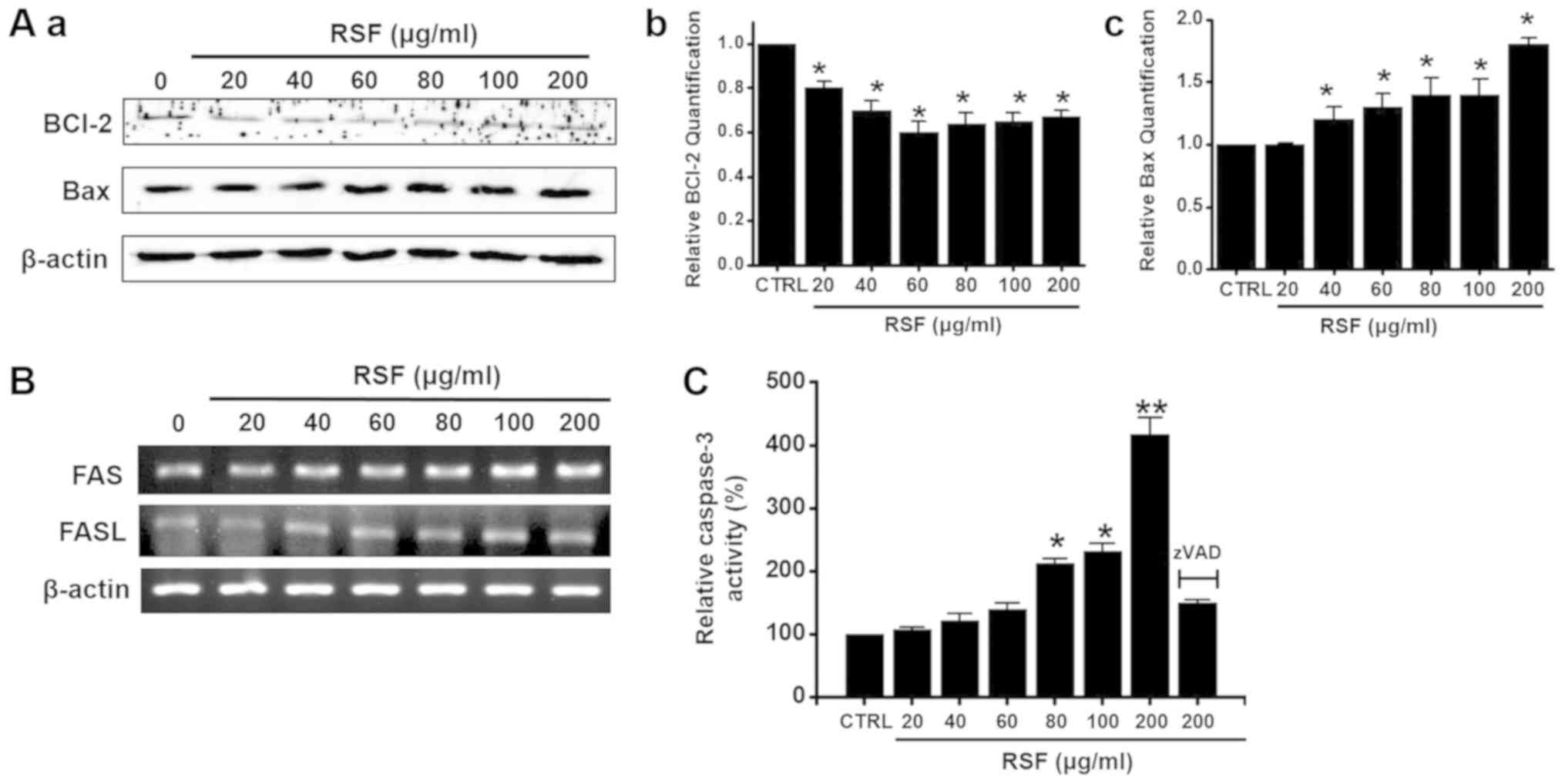 | Figure 4.Bcl-2, Bax protein regulation and
caspase-3 activities are shown in AGS cells after treatment with
RSF. (Aa) Western blotting was performed on AGS cells treated with
different RSF concentrations for 24 h. Bcl-2 expression was
downregulated by RSF, whereas, Bax expression was upregulated. Each
level of (Ab) Bcl-2 or (Ac) Bax protein expression was normalized
to that of the corresponding β-actin, and the mean values are
presented as bar graphs. (B) Reverse transcription-polymerase chain
reaction was performed on AGS cells treated with different RSF
concentrations for 24 h. The Fas and FasL expression levels were
upregulated by RSF. (C) Caspase assays were performed following the
addition of the indicated RSF concentration for 24 h to the culture
cells. Cells were treated with zVAD-fm as a pan-caspase inhibitor.
β-actin was used as the loading control. Results are presented as
the mean ± standard error of the mean. *P<0.05, **P<0.01 vs.
untreated cells. RSF, Radix Sophorae Flavescentis; CTRL,
control; Bcl-2, B cell lymphoma 2; Bax, apoptosis regulator BAC;
FAS, apoptosis-mediating surface antigen FAS; FASL, Fas ligand. |
c-Jun N-terminal kinase (JNK) and
mitogen-activated protein kinase (MAPK) pathways in AGS cells
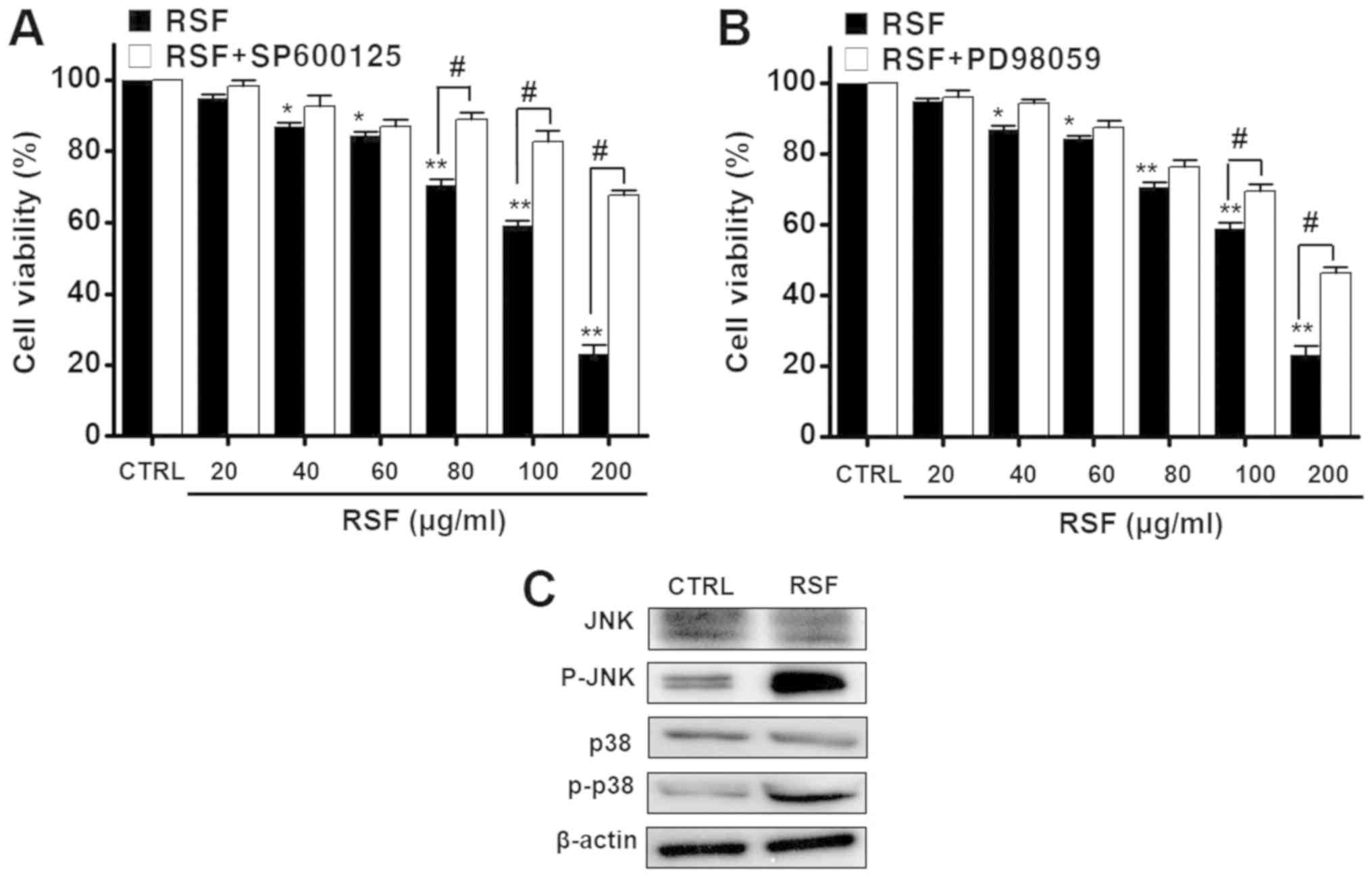
To investigate the involvement of MAPK pathways in
the inhibition of AGS cell proliferation by RSF, cell viability was
measured after treating the cells with different concentrations
(from 20 to 200 µg/ml) of RSF, with or without the JNK inhibitor or
MAPK inhibitor (SP600125 or PD98059) using the MTT assay.
Co-treatment notably inhibited RSF-induced cell death when cells
were co-treated with 200 µg/ml of RSF. Co-treatment with RSF (20,
40, 60, 80, 100, or 200 µg/ml) and SP600125, inhibited cell
survival by 98.3±1.6, 92.6±3.1, 87.0±2.1, 88.9±2.1, 82.7±3.0, and
67.6±1.6%, respectively, also determined by the MTT assay
(n=6; Fig. 5A).
Co-treatment with RSF (20, 40, 60, 80, 100 or 200 µg/ml) and
PD98059 also inhibited cell survival, but by 96.2±2.1, 94.3±1.2,
87.6±2.0, 76.4±1.9, 69.5±2.1, and 46.3±1.7%, respectively
(n=6; Fig. 5B). The
expression of proteins corresponding to JNK, p-JNK, p38 MAPK and
p-p38 MAPK was observed using western blotting analysis. The
expression of p-JNK and p-p38 MAPK protein increased with RSF
addition (Fig. 5C). These results
suggest that both JNK and MAPK are involved in the RSF-induced
apoptosis of AGS cells.
Intracellular reactive oxygen species
(ROS) pathway in AGS cells
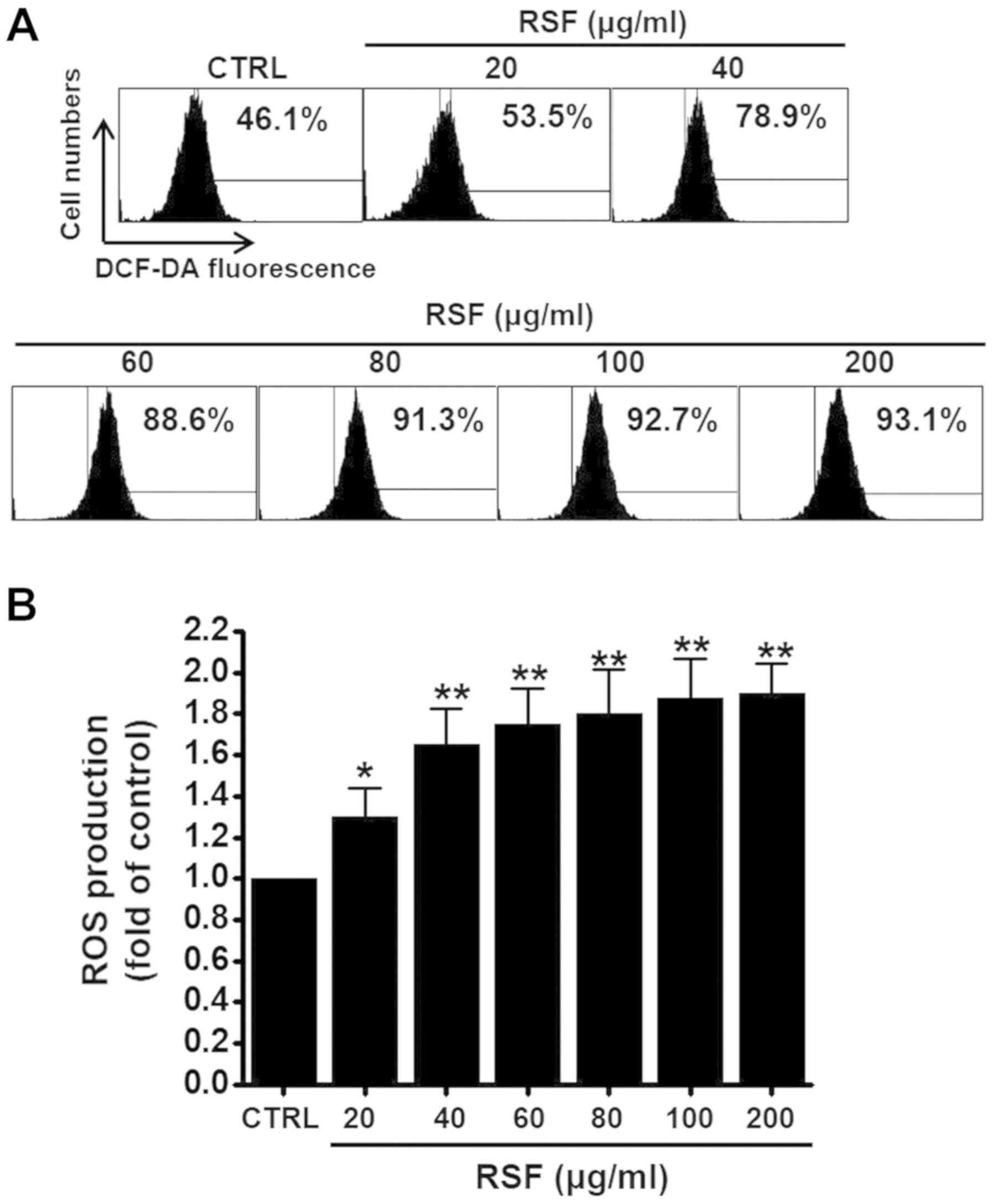
As ROS plays a key role in apoptosis, we studied
whether RSF can generate ROS in AGS cells. To check whether ROS
generation was associated with RSF-induced apoptosis, ROS was
investigated using a fluorescent dye, DCF-DA. As indicated in
Fig. 6A, when the cells were
exposed to RSF, ROS levels increased. Flow cytometry indicated that
ROS generation significantly increased in a dose-dependent manner
(RSF from 20 to 200 µg/ml) (Fig.
6B).
Discussion
RSF is extracted from the dried root of Sophora
Flavescens Ait, and it contains alkaloids including matrine and
oxymatrine that form its key constituents (15,19).
Modern pharmacological experiments show that RSF has a variety of
pharmacological properties. It is commonly used for the treatment
of viral hepatitis, cancer, viral myocarditis, and skin diseases
(20,21). The main components of RSF are
alkaloids, flavonoids, alkylxanthones, quinones, and triterpene
glycosides (20–22). Matrine and oxymatrine are the two
major alkaloids found in the roots of Sophora sp. (21). They inhibit the growth of various
tumour cell lines (21–24). Matrine inhibits the invasiveness
and metastasis of various tumour cells, such as A375, HeLa, and
K-562 (25,26). Oxymatrine has anti-cancer effects
against human gastric, breast, and pancreatic cancer cells
(27–29). Although, many reports suggest that
RSF inhibits the proliferation of several cancer cell lines
(23–29), its anti-cancer activity in human
gastric adenocarcinoma AGS cells remains unclear. Therefore, we
examined the mechanism behind this phenomenon, and found that RSF
induces apoptotic signalling via mitochondrial- and
caspase-dependent pathways mediated by ROS generation in the human
gastric cancer cells.
In the present study, AGS cell viability decreased
remarkably following RSF treatment (Fig. 2A). Also, matrine (Fig. 2B) or oxymatrine (Fig. 2C) inhibited the survival of AGS
cells. RSF is used in traditional Chinese medicine to treat various
diseases, and has the ability to clear heat and dampness from the
body (20,21). Matrine or oxymatrine is one of the
major bioactive compounds extracted from RSF (21). Therefore, matrine or oxymatrine may
be the major components of RSF induced AGS cell death. Matrine or
oxymatrine may have anti-cancer effects and in future, they will be
used as anti-cancer agents, especially in gastric cancer.
Apoptosis can be initiated via two signalling
pathways: The extrinsic and intrinsic pathway (30,31).
The extrinsic pathway is initiated by extracellular signals such
as, Fas ligand (FasL) (32). The
intrinsic pathway, on the other hand is regulated by Bcl-2 family
proteins (33). Caspases belong to
a group of enzymes called cysteine proteases that are known to
induce apoptotic processes (34).
In our study, Bcl-2 expression was inhibited by RSF, while Bax
expression was upregulated (Fig.
4A). Fas and FasL expression levels were up-regulated (Fig. 4B). Cell death is a complex
biological phenomenon regulated by multiple cellular processes,
including apoptosis and autophagy (35). Apoptosis and autophagy share common
molecular pathways and also exhibit synergistic or antagonistic
effects on each other during cell death (36–38).
The relationship between apoptosis and autophagy is largely unknown
in gastric cancer (39,40). Therefore, to develop anti-cancer
therapeutic agents, understanding the interactions between
apoptosis and autophagy is vital.
The induction of apoptosis leads to characteristic
cell changes and finally to death. These changes include blebbing,
cell shrinkage, nuclear fragmentation, chromatin condensation, and
chromosomal DNA fragmentation (30,31).
Apoptosis involves a complex cascade of reactions regulated by
specific proteases called caspases, and results in DNA degradation
(34). Autophagy describes the
fundamental catabolic mechanism during which cells degrade
dysfunctional and unnecessary cellular components (41,42).
This process is driven by the action of lysosomes and promotes
survival during starvation periods, as the cellular energy level
can thus be maintained (42). In
this study, RSF did not induce the autophagy (data not shown), but
the apoptosis. Therefore, these results suggest that RSF may induce
caspase-dependent AGS cell death, not lysosomal enzyme-dependent
cell death.
Medicinal herbs and their derivatives are
increasingly being used as a complementary treatment of cancer
(41). Many clinical studies have
highlighted the benefits of traditional herbal medicines on the
quality of life of cancer patients (43,44).
Therefore, we propose that when combined with chemotherapy, herbal
medicines could enhance the efficacy level and minimise toxic
reactions.
Activated transient receptor potential melastatin 7
(TRPM7) channels contribute to a number of physiological and
pathophysiological processes (45–47).
TRPM7 is a member of the large TRP channel superfamily expressed in
nearly every tissue and cell type (48). Human gastric adenocarcinoma cells
express the TRPM7 channel, which is essential for cell survival and
is a potential pharmacological target for the treatment of gastric
cancer (49). Therefore, we plan
to investigate the role of TRPM7 in RSF-treated gastric cancer
cells in the future, which we believe is a new research area.
The signalling pathway of RSF-induced apoptosis in
AGS cells can be summarised as follows. RSF promotes the expression
of the pro-apoptotic factor Bax, but decreases the expression of
the anti-apoptotic factor Bcl-2. These changes result in the
release of cytochrome c in the cytosol. Cytochrome c activates the
caspase-3 cascade, which leads to cell death via the intrinsic
apoptotic pathway. Another possible pathway is the extrinsic
apoptotic pathway, which acts through the ROS-mediated JNK/p38 MAPK
cascade. Therefore, we further propose that RSF-induced cell death
acts via a ROS-mediated JNK/p38 MAPK signalling pathway that
enhances the up-regulation of pro-apoptotic genes.
In conclusion, we have demonstrated that RSF
inhibits proliferation of AGS cells, and extends the sub-G1 phase
ratio. Additionally, RSF-induced apoptotic cell death is associated
with BCl-2 down-regulation and Bax up-regulation. RSF activates
caspase-3 and mitogen-activated protein kinase (MAPK) but the C-Jun
N-terminal kinase (JNK) inhibitors negate RSF-induced cell death.
RSF also increases the generation of reactive oxygen species (ROS).
Therefore, RSF may cause cell death via the intrinsic pathway in
AGS human gastric cancer cells, and these findings indicate that
RSF is a useful potential anticancer agent.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Korean National
Research Foundation Grant funded by the Korean Government (MSIP;
grant no. 2014R1A5A2009936).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSK and BJK designed the research. JSK, SJS, JNK,
MJK, EYL, YTK and HK performed the experiments. JSK, JNK and BJK
analyzed the data. JSK and BJK wrote the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boon HS, Olatunde F and Zick SM: Trends in
complementary/alternative medicine use by breast cancer survivors:
Comparing survey data from 1998 and 2005. BMC Womens Health.
7:42007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese medicine
in cancer care: A review of controlled clinical studies published
in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoder LH: Let's talk ‘cancer prevention’.
Medsurg Nurs. 14:195–198. 2005.PubMed/NCBI
|
|
7
|
Ernst E: Complementary and alternative
medicine (CAM) and cancer: The kind face of complementary medicine.
Int J Surg. 7:499–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Zhu M, Shi R and Yang M: Radix
Sophorae flavescentis for chronic hepatitis B: A systematic
review of randomized trials. Am J Chin Med. 31:337–354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ
and Yu SQ: Attenuation of acute lung injury in mice by oxymatrine
is associated with inhibition of phosphorylated p38 mitogen
activated protein kinase. J Ethnopharmacol. 98:177–183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Cai W, Yang Q, Lu Z, Li J and Yu
J: Compound Radix Sophorae Flavescentis exerts antitumor
effects by inhibiting the proliferation and inducing the apoptosis
of esophageal carcinoma TE-8 cells. Oncol Lett. 10:2209–2213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drew AK, Bensoussan A, Whyte IM, Dawson
AH, Zhu X and Myers SP: Chinese herbal medicine toxicology database
monograph on Radix Sophorae Flavescentis, ‘ku shen’. J
Toxicol Clin Toxicol. 40:173–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong J, Liu Z, Zhou X and Xu J: Synergic
anti-pruritus mechanisms of action for the Radix Sophorae
Flavescentis and fructus cnidii herbal pair. Molecules.
22(pii): E14652017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang GB, Lee JE, Nho CW, Lee BU, Lee SJ,
Jung JH and Bae GN: Short-term effect of humid airflow on
antimicrobial air filters using Sophora flavescens nanoparticles.
Sci Total Environ. 421-422:273–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin JH, Kim JS, Kang SS, Son KH, Chang HW
and Kim HP: Anti-inflammatory and anti-arthritic activity of total
flavonoids of the roots of Sophora flavescens. J Ethnopharmacol.
127:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Han C, Fang X, Shi X, Feng A, He
K, Zhang S and Sun X: Effect of kushen (Radix Sophorae
Flavescentis) extract on laryngeal neoplasm Hep2 cells. J
Tradit Chin Med. 33:218–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Xu J, Li X, Zhang D, Han Y and
Zhang X: Comprehensive two-dimensional PC-3 prostate cancer cell
membrane chromatography for screening anti-tumor components from
Radix Sophorae Flavescentis. J Sep Sci. 40:2688–2693. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang BJ, Won SJ, Yu ZR and Su CL: Free
radical scavenging and apoptotic effects of Cordyceps sinensis
fractionated by supercritical carbon dioxide. Food Chem Toxicol.
43:543–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao BG: Study on alkaloids of Sophora
Alopecuroides. Yao Xue Xue Bao. 15:182–183. 1980.
|
|
20
|
Sun M, Han J, Duan J, Cui Y, Wang T, Zhang
W, Liu W, Hong J, Yao M, Xiong S and Yan X: Novel antitumor
activities of Kushen flavonoids in vitro and in vivo. Phytother
Res. 21:269–277. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun M, Cao H, Sun L, Dong S, Bian Y, Han
J, Zhang L, Ren S, Hu Y, Liu C, et al: Antitumor activities of
kushen: Literature review. Evid Based Complement Alternat Med 2012.
3732192012.
|
|
22
|
Cheng H, Xia B, Zhang L, Zhou F, Zhang YX,
Ye M, Hu ZG, Li J, Li J, Wang ZL, et al: Matrine improves
2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice.
Pharmacol Res. 53:202–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Wang B, Zhou C and Bi Y: Matrine
induces apoptosis in angiotensin II-stimulated hyperplasia of
cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3
activation. Basic Clin Pharmacol Toxicol. 101:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu XS, Jiang J, Jiao XY, Wu YE and Lin
JH: matrine induced apoptosis in leukemia U937 cells: Involvement
of caspases activation and MAPK-independent pathways. Planta Med.
72:501–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM,
Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL and Chen HC: Matrine
inhibits invasiveness and metastasis of human malignant melanoma
cell line A375 in vitro. Int J Dermatol. 47:448–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP families
and releasing of cytochrome c. J Exp Clin Cancer Res. 30:662011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28 Suppl 1:S99–S107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wajant H: The Fas signaling pathway: More
than a paradigm. Science. 296:1635–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thorburn A: Apoptosis and autophagy:
Regulatory connections between two supposedly different processes.
Apoptosis. 13:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SH, Park EJ, Lee CR, Chun JN, Cho NH,
Kim IG, Lee S, Kim TW, Park HH, So I and Jeon JH: Geraniol induces
cooperative interaction of apoptosis and autophagy to elicit cell
death in PC-3 prostate cancer cells. Int J Oncol. 40:1683–1690.
2012.PubMed/NCBI
|
|
39
|
Bento CF, Renna M, Ghislat G, Puri C,
Ashkenazi A, Vicinanza M, Menzies FM and Rubinsztein DC: Mammalian
autophagy: How does it work? Annu Rev Biochem. 85:685–713. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun T, Liu H and Ming L: Multiple roles of
autophagy in the sorafenib resistance of hepatocellular carcinoma.
Cell Physiol Biochem. 44:716–727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang F, Wang BR and Wang YG: Role of
autophagy in tumorigenesis, metastasis, targeted therapy and drug
resistance of hepatocellular carcinoma. World J Gastroenterol.
24:4643–4651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wilde L, Tanson K, Curry J and
Martinez-Outschoorn U: Autophagy in cancer: A complex relationship.
Biochem J. 475:1939–1954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin SY, Wei WC, Jian FY and Yang NS:
Therapeutic applications of herbal medicines for cancer patients.
Evid Based Complement Alternat Med 2013. 3024262013.
|
|
44
|
Li HC, Xia ZH, Chen YF, Yang F, Feng W,
Cai H, Mei Y, Jiang YM, Xu K and Feng DX: Cantharidin inhibits the
growth of triple-negative breast cancer cells by suppressing
autophagy and inducing apoptosis in vitro and in vivo. Cell Physiol
Biochem. 43:1829–1840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin J, Desai BN, Navarro B, Donovan A,
Andrews NC and Clapham DE: Deletion of Trpm7 disrupts embryonic
development and thymopoiesis without altering Mg2+ homeostasis.
Science. 322:756–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang J, Li MH, Inoue K, Chu XP, Seeds J
and Xiong ZG: Transient receptor potential melastatin 7-like
current in human head and neck carcinoma cells: Role in cell
proliferation. Cancer Res. 67:10929–10938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schmitz C, Perraud AL, Johnson CO, Inabe
K, Smith MK, Penner R, Kurosaki T, Fleig A and Scharenberg AM:
Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell.
114:191–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ
and So I: Suppression of transient receptor potential melastatin 7
channel induces cell death in gastric cancer. Cancer Sci.
99:2502–2509. 2008. View Article : Google Scholar : PubMed/NCBI
|