Introduction
Retinoblastoma (RB) is the most common intraocular
malignancy in infants and young children (1). RB accounts for 2–4% of all paediatric
cancer types, and the high-risk age of developing RB is 5 years old
(2,3). Defective RB tumour suppressor gene 1
(Rb1) is the main factor involved in the initiation and progression
of RB (4). However, the detailed
mechanisms underlying the pathogenesis and progression of RB remain
largely unknown. At present, the primary therapeutic techniques for
patients with RB include ophthalmectomy, laser photocoagulation,
cryotherapy, hyperthermia and chemoradiotherapy (5). The majority of patients with RB are
not diagnosed at an early stage, and their delayed diagnosis is
associated with the high incidence of mortality in this patient
group (6). The overall survival
rate of patients with RB is extremely poor, despite improvements in
the applied therapeutic methods (7). Therefore, the molecular mechanisms by
which RB is initiated and the factors that drive development must
be elucidated to develop effective diagnostic markers and
intervention approaches for managing patients with RB.
microRNAs (miRNAs) are a family of non-coding and
evolutionarily conserved RNAs. miRNAs contain 18–24 nucleotides,
and are associated with the regulation of genes expression
(8). miRNAs can inhibit gene
expression by binding miRNA ‘seed’ regions to complementary
sequences of 3′-untranslational regions (3′-UTRs) of their target
genes, thereby causing mRNA degradation and/or mRNA translation
suppression (9). Over the past
decade, studies have revealed that miRNAs are altered in nearly all
human cancer types, and that their alteration contributes toward
the initiation and progression of cancer (10–12).
An increasing volume of evidence has recently indicated that
numerous miRNAs, including miR-29a (13), miR-138 (14), miR-498 (15) and miR-655 (16), are aberrantly expressed in RB.
miRNA dysregulation participates in the regulation of various
biological behaviours, including cellular proliferation, cycle,
apoptosis and invasion, as well as metastasis and angiogenesis
(17–19). Therefore, miRNAs may serve as
potential diagnostic biomarkers and therapeutic targets for
patients with RB.
miR-492 is reportedly downregulated in cervical
cancer (20), osteosarcoma
(21) and clear cell renal cell
carcinoma (22). By contrast,
miR-492 is upregulated in hepatoblastoma, hepatic cancer (23) and breast cancer (24). However, the expression pattern and
detailed roles of miR-492 in RB and its regulation mechanisms
remain largely unclear. Therefore, the present study aimed to
detect miR-492 expression in RB, to investigate the detailed roles
of miR-492 in RB progression and to identify the molecular
mechanism underlying the action of miR-492 in RB.
Patients and methods
Patients and tissue collection
RB tissues were obtained from 27 patients with RB
(16 males and 11 females; age range, 13–46 years; mean age, 24
years) who had undergone surgery at Weifang People's Hospital
between February 2015 and March 2017. A total of 10 normal retinal
tissues were obtained from patients (6 males and 4 females; age
range, 24–58 years; mean age, 31 years) who had had a globe rupture
and had undergone an ophthalmectomy. All tissues were quickly
stored in liquid nitrogen until further use. None of the patients
had been treated with radiotherapy, chemotherapy or any other
treatments prior to surgical resection. The present study was
approved by the Ethics Committee of Weifang People's Hospital and
all subjects had provided written informed consent.
Cell lines
Three human RB cell lines (Weri-RB1, SO-RB50 and
Y79) and a normal retinal pigmented epithelial cell line (ARPE-19)
were acquired from American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and
100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and grown at 37°C under conditions of 100%
humidity, 95% air and 5% CO2.
miRNA inhibitor and short interfering
RNA (siRNA) transfection
miR-492 inhibitor and negative control miRNA
inhibitor (NC inhibitor) were designed and produced by GenePharma
Co., Ltd. (Shanghai, China). The sequences were as follows: miR-492
inhibitor, 5′AAGAAUCUUGUCCCGCAGGUCCU3′; NC inhibitor,
5′CAGUACUUUUGUGUAGUACAA3′; LATS2 siRNA targeting the expression of
LATS2 (LATS2-siRNA) and negative control siRNA (NC-siRNA) were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
LATS2-siRNA sequence was 5′GTTCGGACCTTATCAGAAA3′ and the NC-siRNA
sequence was 5′UUCUCCGAACGUGUCACGUTT3′. Cells were inoculated into
6-well culture plates one day prior to transfection. miRNA
inhibitor or siRNA was transfected into cells at a concentration of
100 nM using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis was performed at 48 and 72 h
post-transfection. Cell Counting kit-8 (CCK-8) and invasion assays
were carried out after 24 and 48 h culture, respectively.
RNA isolation and RT-qPCR
Total RNA was isolated from cells and tissue
specimens using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentration and purity of total RNA was
evaluated using a Nanodrop 2000 (Thermo Fisher Scientific, Inc.).
For quantification of miR-492 expression, complementary DNA (cDNA)
was synthesized by reverse transcription using the
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The temperature
protocol for reverse transcription was as follows: 16°C for 30 min,
42°C for 30 min and 85°C for 5 min. The TaqMan MicroRNA assay kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was utilized
to analyze miR-492 expression, using U6 snRNA as an internal
reference. The cycling conditions were as follows: 50°C for 2 min,
95°C for 10 min; 40 cycles of denaturation at 95°C for 15 sec; and
annealing/extension at 60°C for 60 sec.
For the detection of LATS2 mRNA expression, total
RNA was converted into cDNA using the PrimeScript® RT
reagent kit (Takara Bio, Inc., Otsu, Japan). The temperature
protocol for reverse transcription was as follows: 37°C for 15 min
and 85°C for 5 second. The expression of LATS2 mRNA was determined
by the SYBR Premix ExTaq kit (Takara Bio, Inc., Otsu, Japan), using
GAPDH as an internal control. The cycling conditions were as
follows: 5 min at 95°C, followed by 40 cycles of 95°C for 30 sec
and 65°C for 45 sec. Relative gene expression was calculated using
the 2−ΔΔCq method (25). The primers were designed as
follows: miR-492 forward,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAGAATCT-3′ and reverse,
5′-ACACTCCAGCTGGGAGGACCTGCGGACACA0-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; LATS2 forward,
5′-CCCGAGGAATGAGCAGATTG-3′ and reverse, 5′-GCTGGTGGTAGGACGCAAAC-3′;
and GAPDH forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′ and reverse,
5′-CCCCACTTGATTTTGGAGGGA-3′.
CCK-8 assay
The proliferation ability of RB cells was determined
using the CCK-8 assay. In brief, transfected cells were collected
at 24 h post-transfection, prepared into signal cell suspensions
and plated into 96-well culture plates at a density of 3,000
cells/well. Cells were then incubated at 37°C under conditions of
95% air and 5% CO2 for different time periods. At each
time point, 10 µl CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added into each well. After an additional 2 h
incubation, the optical density (OD) value of each well was
detected at a wavelength of 450 nm on an ELISA microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Invasion assay
Following 48 h incubation, transfected cells were
digested and then suspended into FBS-free DMEM. A total of
1×105 cells were added into the upper compartments of
Matrigel-coated Transwell chambers (both BD Biosciences, Franklin
Lakes, NJ, USA). The lower compartment of the Transwell chamber was
covered with 600 µl DMEM containing 20% FBS to serve as a
chemoattractant. After 24 h incubation at 37°C in a 5%
CO2 atmosphere, a cotton swab was utilized to wipe out
the non-invaded cells. The cells that had invaded through the
polycarbonate membrane, were fixed with 100% methanol at room
temperature for 20 min and stained with 0.1% crystal violet at room
temperature for 20 min. After being washed thrice, the number of
invaded cells was counted in five randomly selected visual fields
under an inverted microscope (magnification, ×200; Olympus
Corporation, Tokyo, Japan).
Bioinformatics prediction and
luciferase reporter assay
TargetScan (Release 7.2; http://www.targetscan.org/vert_72/), miRDB (www.mirdb.org) and microRNA.org
(www.microrna.org) were employed to screen the
putative target. LATS2 was identified as a major target of miR-492.
To experimentally verify this prediction, the 3′-UTR of LATS2
segments containing the wild-type (Wt) or mutant (Mut) miR-492
binding sequences were amplified by Shanghai GenePharma Co., Ltd.,
inserted into the pmirGLO luciferase reporter plasmid (Promega
Corporation, Madison, WI, USA), and named as
pmirGLO-LATS2-Wt-3′-UTR and pmirGLO-LATS2-Mut-3′-UTR, respectively.
Co-transfection with pmirGLO-LATS2-Wt-3′-UTR or
pmirGLO-LATS2-Mut-3′-UTR and miR-492 inhibitor or NC inhibitor into
cells was performed using Lipofectamine 2000, according to the
manufacturer's protocols. After 48 h of incubation, transfected
cells were collected and subjected to the detection of luciferase
activities using a Dual-Luciferase Reporter assay system (Promega
Corporation). Firefly luciferase activity was used for
normalization.
Western blot analysis
Cells and tissues were lysed using
radioimmunoprecipitation assay lysis buffer (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Total protein concentration was
detected using a BCA Protein assay kit (Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer's protocol. Equal amounts of
protein (30 µg) were loaded per lane, separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Subsequently, the membranes were blocked with
5% dried skimmed milk in TBS with 0.05% Tween-20 at room
temperature for 2 h, followed by incubation with primary antibodies
against LATS2 (cat. no. ab135794; dilution, 1:1,000 or GADPH (cat.
no. ab186930; dilution, 1:1,000; both Abcam, Cambridge, UK). The
membranes were washed three times with TBST and incubated with
horseradish peroxidase-conjugated secondary antibodies (cat. no.
ab6721; dilution, 1:5,000; Abcam). The protein signals were
visualized using an enhanced chemiluminescence-plus reagent (GE
Healthcare, Chicago, IL, USA) and quantified by densitometric
analysis of protein signals using ImageJ version 1.49 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
from at least three separate experiments. Differences between
groups were tested using Student's t-test or one-way analysis of
variance, followed by the Student-Newman-Keuls post hoc test. The
correlation between miR-492 and LATS2 mRNA expression levels was
assessed using Spearman's correlation analysis. All statistical
analyses were conducted using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
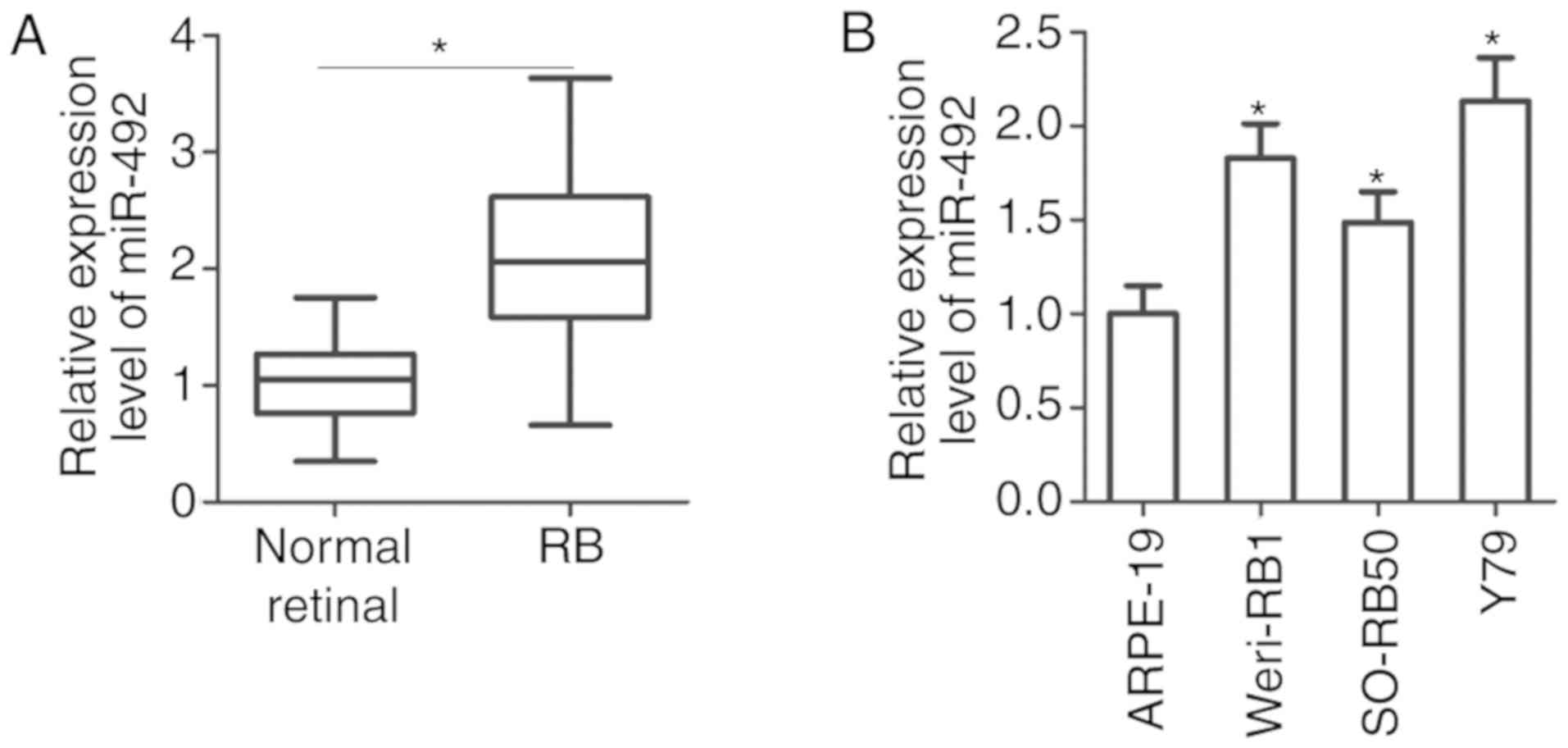
High expression of miR-492 in RB
tissues and cell lines
To understand the expression pattern of miR-492 in
RB, its expression was detected in 27 RB tissues and 10 normal
retinal tissues. RT-qPCR analysis revealed that miR-492 was
significantly upregulated in RB tissues compared with that in the
10 normal retinal tissues (Fig.
1A; P<0.05). In addition, miR-492 expression was detected in
three RB cell lines (Weri-RB1, SO-RB50 and Y79) and one normal
retinal pigmented epithelial cell line (ARPE-19). The results
indicated that the expression level of miR-492 was higher in the RB
cell lines than in ARPE-19 cell line (Fig. 1B; P<0.05). These results
suggested that the high miR-492 expression may be associated with
the malignant behaviour of RB.
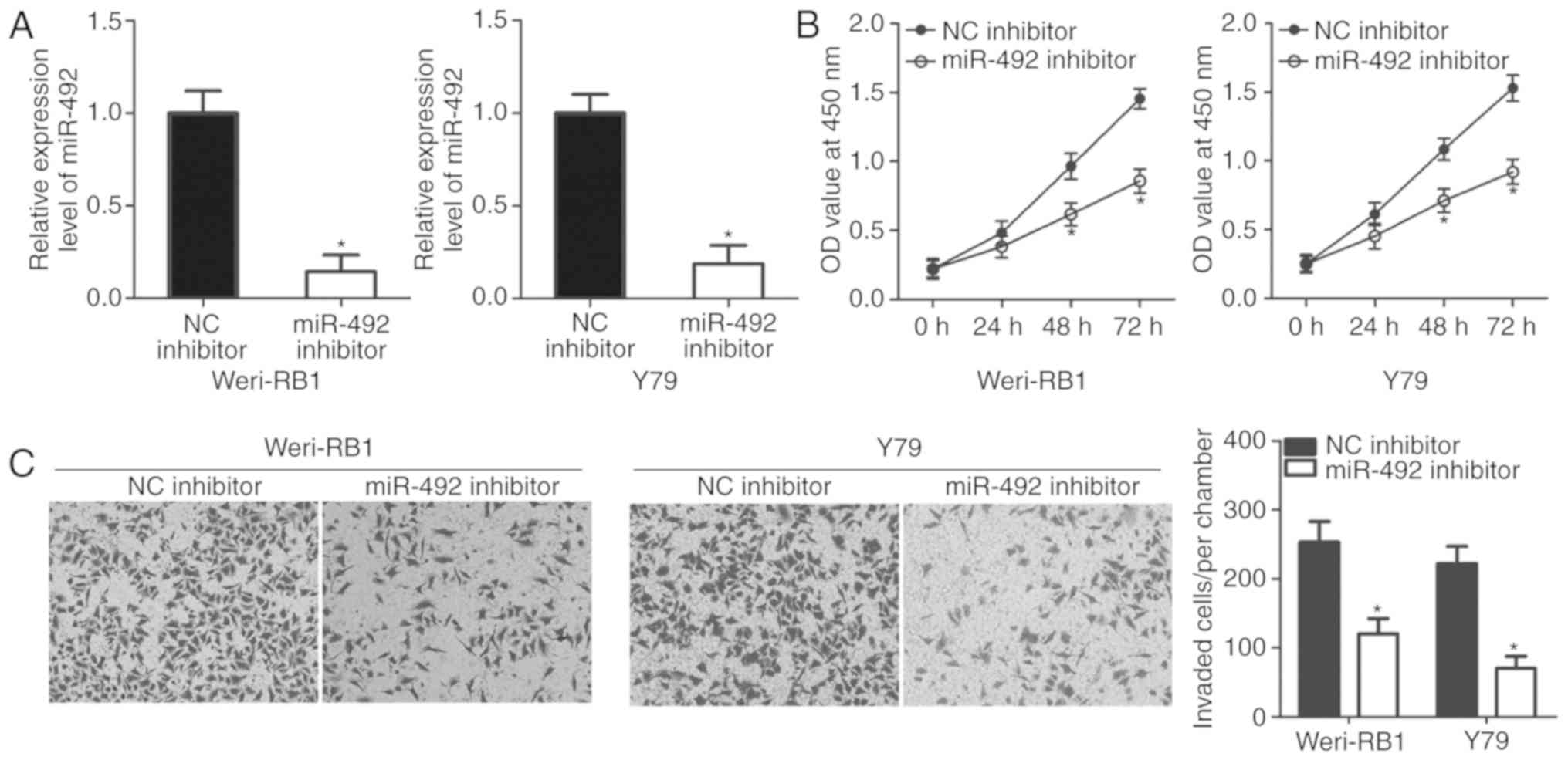
miR-492 downregulation inhibits cell
proliferation and invasion in RB
To elucidate the possible biological roles of
miR-492 in RB, miR-492 or NC inhibitor was transfected into
Weri-RB1 and Y79 cells, in which expression of miR-492 was
relatively high. The transfection efficiency was determined by
RT-qPCR. miR-492 was significantly downregulated in the Weri-RB1
and Y79 cells transfected with the miR-492 inhibitor (Fig. 2A; P<0.05). Next, the effect of
miR-492 inhibition on the proliferation of RB cells was detected
using a CCK-8 assay. As expected, miR-492 downregulation reduced
the proliferative ability of Weri-RB1 and Y79 cells, compared with
that in the cells transfected with NC inhibitor (Fig. 2B; P<0.05). Invasion assay
revealed that the invasion of miR-492 inhibitor-transfected
Weri-RB1 and Y79 cells was significantly lower than that in the NC
inhibitor group (Fig. 2C;
P<0.05). These results suggested that miR-492 serves oncogenic
roles in the development of RB.
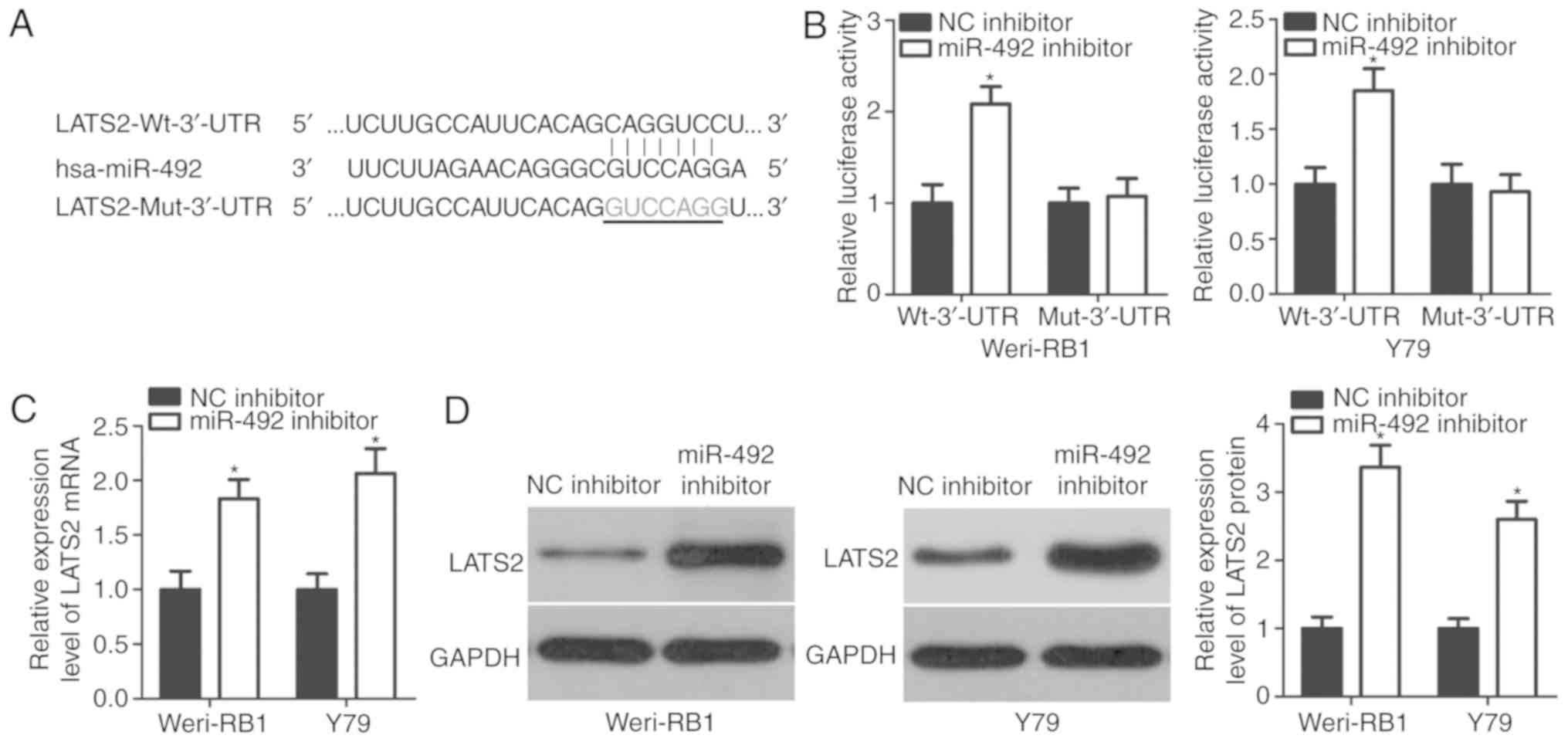
LATS2 is a direct target gene of
miR-492 in RB cells
To investigate the molecular mechanism of miR-492 in
the development of RB, putative targets of miR-492 were screened by
bioinformatics analysis. LATS2 was predicted to be a candidate of
miR-492 as the seed sequence of miR-492 was complementary to the
3′-UTR of LATS2 at positions 705–711 (Fig. 3A). To determine whether LATS2 is a
direct target of miR-492 in RB, luciferase reporter plasmids
containing either the wild-type or mutant 3′-UTR of LATS2, together
with miR-492 or NC inhibitor, were transfected into Weri-RB1 and
Y79 cells. Co-transfection of pmirGLO-LATS2-Wt-3′-UTR and miR-492
inhibitor into Weri-RB1 and Y79 cells resulted in significantly
increased luciferase activities (P<0.05). However, miR-492
inhibitor had none effect on the luciferase activities of the
plasmid carrying the mutant binding site in the 3′-UTR of LATS2
(Fig. 3B). In addition, the
present study investigated whether miR-492 can regulate endogenous
LATS2 expression in Weri-RB1 and Y79 cells. Compared with the NC
inhibitor group, miR-492-knockdown markedly increased the LATS2
mRNA (Fig. 3C; P<0.05) and
protein (Fig. 3D; P<0.05)
expression levels in Weri-RB1 and Y79 cells. These findings
confirmed that LATS2 is a direct target of miR-492 in RB cells.
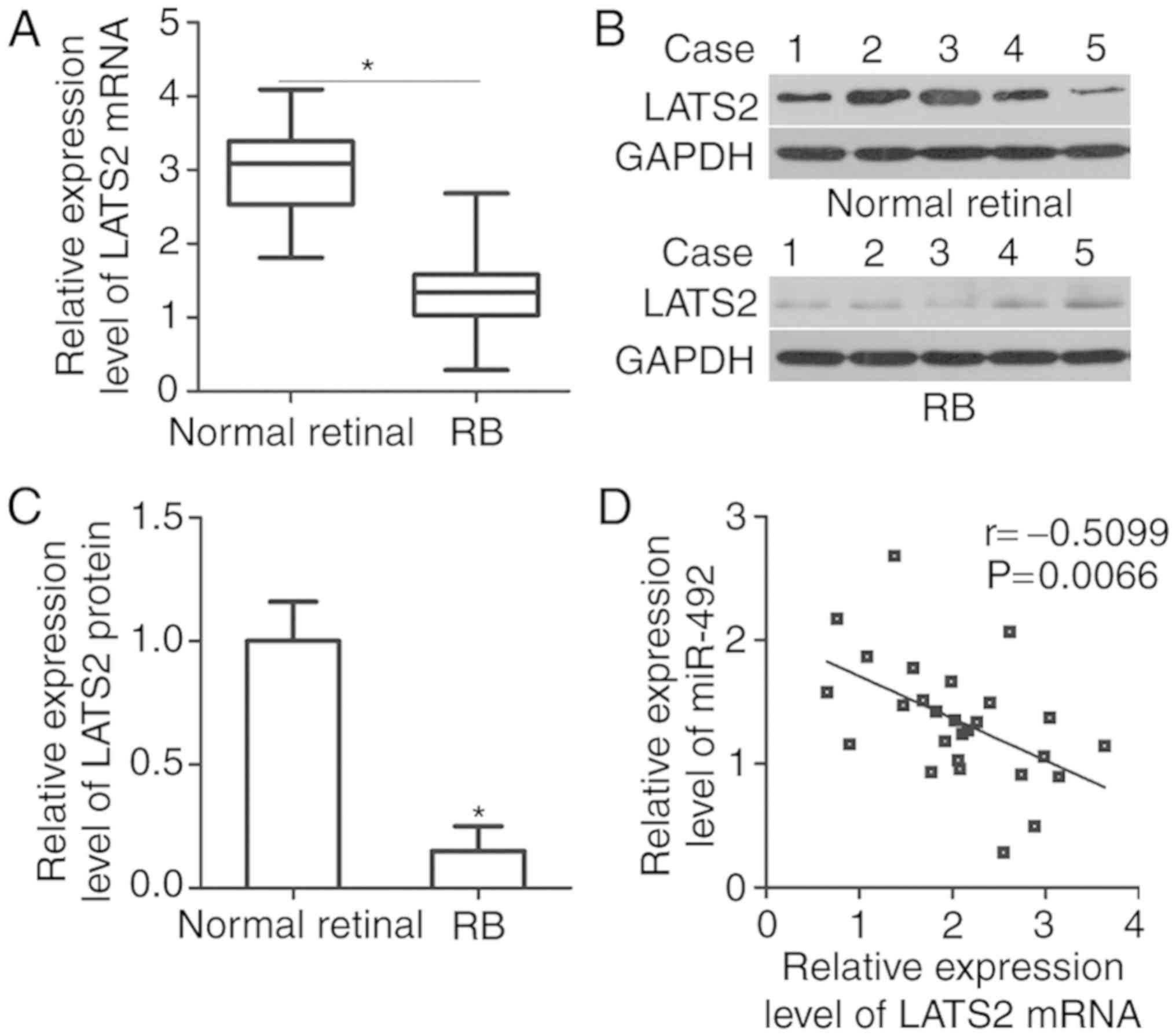
LATS2 underexpression in RB tissues is
negatively correlated with miR-492 expression
To elucidate the association between miR-492 and
LATS2 in RB, the expression of LATS2 in RB tissues and normal
retinal tissues was detected. The results of RT-qPCR and western
blot analysis revealed that LATS2 expression was downregulated in
RB tissues at mRNA (Fig. 4A;
P<0.05) and protein (Fig. 4B and
C; P<0.05) levels, compared with that in normal retinal
tissues. Spearman's correlation analysis was adopted to illustrate
the association between miR-492 and LATS2 mRNA levels in RB
tissues. As demonstrated in Fig.
4D, the miR-492 and LATS2 mRNA expression levels were
negatively correlated in RB tissues (Fig. 4D; r=−0.5099, P=0.0066). These
results suggested that the weak LATS2 expression in RB tissues is
partly due to miR-492 upregulation.
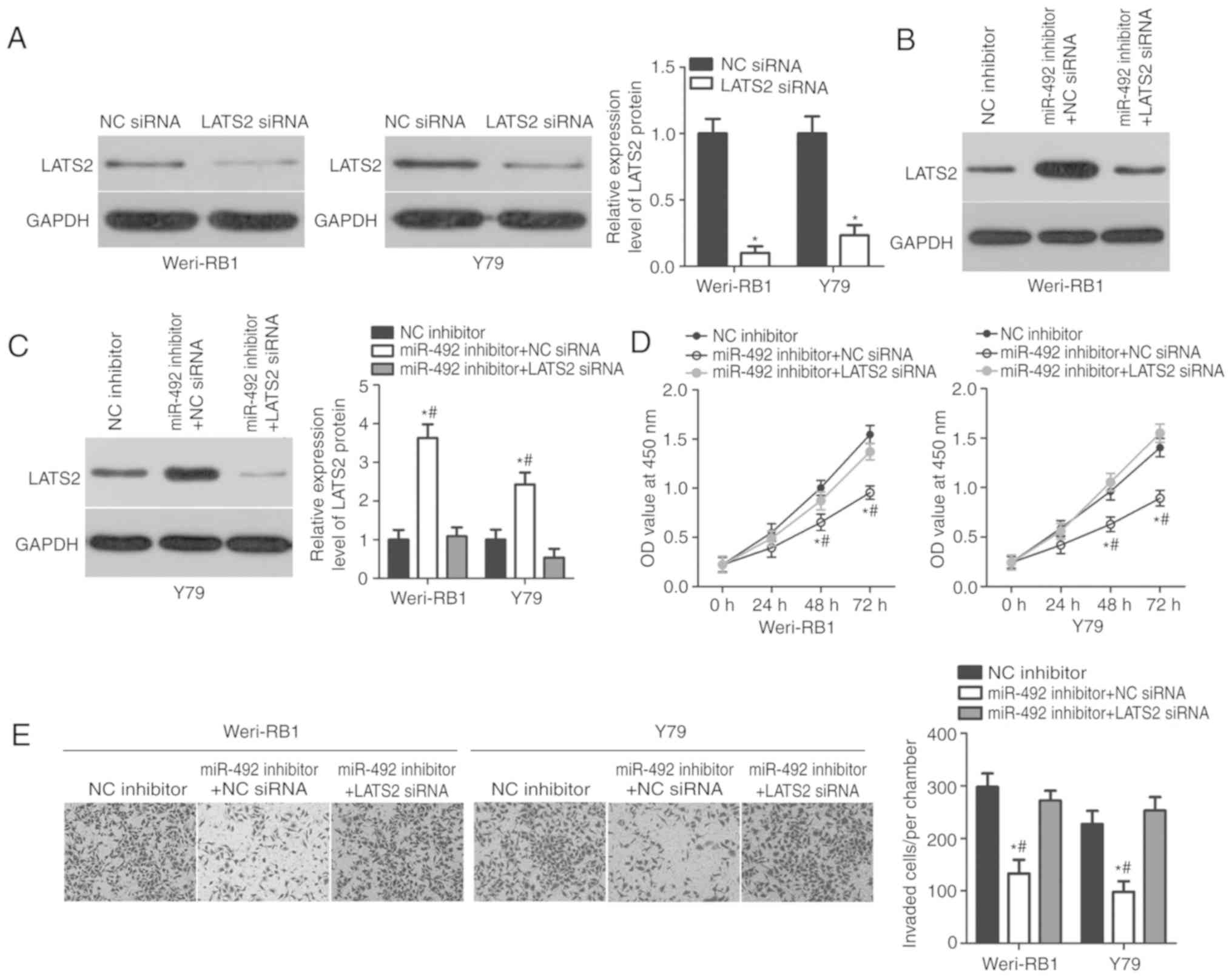
LATS2 downregulation abrogates the
effect of the miR-492 inhibitor in RB cells
Rescue experiments were performed to investigate
whether the roles of miR-492 inhibition in RB cells are mediated by
LATS2 upregulation. LATS2-siRNA or NC-siRNA was transfected into
Weri-RB1 and Y79 cells. Next, western blot analysis was used to
detect LATS2 protein expression. As demonstrated in Fig. 5A, LATS2 expression was efficiently
knocked down in LATS2-siRNA-transfected Weri-RB1 and Y79 cells,
compared with that in cells transfected with NC-siRNA (P<0.05).
Subsequently, LATS2-siRNA or NC-siRNA was co-transfected with
miR-492 inhibitor into Weri-RB1 and Y79 cells. The results
indicated that the co-transfection of LATS2-siRNA partially
abolished the miR-492 inhibitor-mediated upregulation of LATS2 in
Weri-RB1 and Y79 cells (Fig. 5B and
C; P<0.05). CCK-8 and invasion assays demonstrated that
LATS2 downregulation can reverse the suppression of proliferation
(Fig. 5D; P<0.05) and invasion
(Fig. 5E; P<0.05) induced by
the miR-492 inhibitor in Weri-RB1 and Y79 cells. Taken together,
these results indicated that the oncogenic roles of miR-492 in RB
cells are partly mediated by LATS2.
Discussion
Numerous studies have demonstrated that miRNAs are
upregulated or downregulated in RB, and this phenomenon is
associated with the modulation of various malignant behaviours
during RB occurrence and development (16–18).
Therefore, the mechanisms that associate deregulated miRNAs with RB
initiation and progression must be understood to identify effective
therapeutic techniques for patients with RB. The present study
detected miR-492 expression and investigated its biological roles
in RB development. It was revealed that miR-492 expression was
markedly overexpressed in RB tissues and cell lines, compared with
that in normal retinal tissues and the normal retinal pigmented
epithelial cell line ARPE-19, respectively. Functional experiments
revealed that miR-492 downregulation impeded the proliferation and
invasion of RB cells. In addition, LATS2 was identified as a direct
target gene of miR-492 in RB cells, and a negative association was
observed between the miR-492 and LATS2 mRNA levels in RB tissues.
LATS2 downregulation reversed the suppression of RB cell
proliferation and invasion caused by miR-492 inhibition. Therefore,
miR-492 inhibition may be a valuable treatment option for patients
with RB.
miR-492 is aberrantly expressed in several types of
human cancer. For example, miR-492 is downregulated in cervical
cancer tissue specimens and cell lines (20). miR-492 downregulation is
significantly correlated with the pelvic lymph node metastasis of
patients with cervical cancer (20). Weakly expressed miR-492 has also
been observed in osteosarcoma (21) and clear cell renal cell carcinoma
(22). By contrast, miR-492 is
highly expressed in hepatoblastoma tissues and cell lines. Retinal
cells in patients with hepatoblastoma who present with high miR-492
levels exhibit more high-risk or aggressive behaviours than those
with low miR-492 levels (26,27).
miR-492 is also upregulated in hepatic cancer, and this
upregulation is strongly associated with poor survival (23). The results of these conflicting
studies suggested that the expression pattern of miR-492 exhibits
tissue specificity, making it a promising biomarker for diagnosing
different types of human cancer.
miR-492 serves as a tumour suppressor in numerous
types of human cancer. miR-492 upregulation inhibits the
proliferation and invasion of cervical cancer cells, and increases
the sensitivity of cells to irradiation by inducing apoptosis
(20). In osteosarcoma, the
resumption of miR-492 expression suppresses cell growth and
metastasis in vitro, and decreases in vivo tumour
growth (21). In clear cell renal
cell carcinoma, miR-492 restoration restricts cell proliferation
and invasion, induces cell apoptosis and promotes cell adhesion
(22). Nonetheless, miR-492 serves
oncogenic roles in hepatoblastoma by promoting cell proliferation,
anchorage-independent growth and metastasis (26). In breast cancer, ectopic miR-492
expression significantly promotes cell proliferation and
anchorage-independent growth (24). In hepatic cancer, miR-492
downregulation attenuates cell proliferation in vitro and
restricts tumour growth in vivo (23). These conflicting findings indicated
that the biological roles of miR-492 exhibit tissue specificity.
Considering its crucial roles in tumorigenesis and tumor
development, a miR-492-based targeted therapy may be effective for
the treatment of patients with these caner types.
Researchers have identified numerous target genes of
miR-492, including TIMP2 (20) in
cervical cancer, PAK7 (21) in
osteosarcoma, CD44 (26) in
hepatoblastoma, SOX7 (24) in
breast cancer and PTEN (23) in
hepatic cancer. LATS2, which is a member of the serine/threonine
AGC kinase family, was validated as a direct target of miR-492 in
RB. It is located at chromosome 13q11-12 and is highly conserved
from flies to humans (28).
Decreased LATS2 expression has been reported in various cancer
types, including acute lymphoblastic leukaemia (29), breast cancer (30) and prostate cancer (31). LATS2 is downregulated in RB and is
associated with the formation and progression of RB (32). The present study demonstrated that
miR-492 inhibition directly targeted LATS2 to inhibit the
development of RB. Overall, these results confirmed that a
miR-492/LATS2-based targeted therapy may be a novel and efficient
therapeutic strategy for patients with RB.
In conclusion, the results of the present study
revealed that miR-492 was upregulated in RB tissues and cell lines,
and its downregulation significantly inhibited the proliferation
and invasion of RB cells. LATS2 was a direct target gene of miR-492
in RB. Considering these results, we hypothesized that
miR-492-knockdown or the restoration of LAST2 expression may be an
attractive therapeutic target for patients with RB. However, the
present study did not analyse the effects of miR-492
underexpression on RB cells in vivo. This is a limitation of
the present study and will be the focus of our future
experiments.
Acknowledgements
Not applicable.
Funding
No funding was recieved.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AZ designed the present study. ZS, AZ and LZ
performed the functional experiments. All authors have read and
approved the final draft.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital and was performed in
accordance with the Declaration of Helsinki and the guidelines of
the Ethics Committee of Weifang People's Hospital. Written informed
consent was obtained from all patients for the use of their
clinical tissues.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H, Corson TW, Cobrinik D, White A,
Zhao J, Munier FL, Abramson DH, Shields CL, Chantada GL, Njuguna F
and Gallie BL: Retinoblastoma. Nat Rev Dis Primers. 1:150212015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacCarthy A, Draper GJ, Steliarova-Foucher
E and Kingston JE: Retinoblastoma incidence and survival in
European children (1978–1997). Report from the Automated Childhood
Cancer Information System project. Eur J Cancer. 42:2092–2102.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benavente CA and Dyer MA: Genetics and
epigenetics of human retinoblastoma. Annu Rev Pathol. 10:547–562.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shields CL and Shields JA: Retinoblastoma
management: Advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimaras H, Dimba EA and Gallie BL:
Challenging the global retinoblastoma survival disparity through a
collaborative research effort. Br J Ophthalmol. 94:1415–1416. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting CyclinD2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pratap P, Raza ST, Abbas S and Mahdi F:
MicroRNA-associated carcinogenesis in lung carcinoma. J Cancer Res
Ther. 14:249–254. 2018.PubMed/NCBI
|
|
11
|
Vannini I, Fanini F and Fabbri M: Emerging
roles of microRNAs in cancer. Curr Opin Genet Dev. 48:128–133.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou W, Liu J, Gao Y, Zhong G, Chen D, Shen
J, Bao C, Xu L, Pan J, Cheng J, et al: MicroRNAs in cancer
metastasis and angiogenesis. Oncotarget. 8:115787–115802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Zhang X, Hu C, Wang Y and Xu C:
miR-29a inhibits human retinoblastoma progression by targeting
STAT3. Oncol Rep. 39:739–746. 2018.PubMed/NCBI
|
|
14
|
Wang Z, Yao YJ, Zheng F, Guan Z, Zhang L,
Dong N and Qin WJ: Mir-138-5p acts as a tumor suppressor by
targeting pyruvate dehydrogenase kinase 1 in human retinoblastoma.
Eur Rev Med Pharmacol Sci. 21:5624–5629. 2017.PubMed/NCBI
|
|
15
|
Yang L, Wei N, Wang L, Wang X and Liu QH:
miR-498 promotes cell proliferation and inhibits cell apoptosis in
retinoblastoma by directly targeting CCPG1. Childs Nerv Syst.
34:417–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang M, Li Q, Pan Y, Wang H, Liu G and
Yin H: MicroRNA-655 attenuates the malignant biological behaviours
of retinoblastoma cells by directly targeting PAX6 and suppressing
the ERK and p38 MAPK signalling pathways. Oncol Rep. 39:2040–2050.
2018.PubMed/NCBI
|
|
17
|
Singh U, Malik MA, Goswami S, Shukla S and
Kaur J: Epigenetic regulation of human retinoblastoma. Tumour Biol.
37:14427–14441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golabchi K, Soleimani-Jelodar R, Aghadoost
N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H and
Mirzaei H: MicroRNAs in retinoblastoma: Potential diagnostic and
therapeutic biomarkers. J Cell Physiol. 233:3016–3023. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Ge S, Jia R, Zhou Y, Song X, Zhang H
and Fan X: Hypoxia-induced miR-181b enhances angiogenesis of
retinoblastoma cells by targeting PDCD10 and GATA6. Oncol Rep.
33:2789–2796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, An J, Huang M, Wang L, Tu B, Song
Y, Ma K, Wang Y, Wang S, Zhu H, et al: MicroRNA-492 overexpression
involves in cell proliferation, migration, and radiotherapy
response of cervical squamous cell carcinomas. Mol Carcinog.
57:32–43. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song X, Xie Y, Liu Y, Shao M and Yang W:
MicroRNA-492 overexpression exerts suppressive effects on the
progression of osteosarcoma by targeting PAK7. Int J Mol Med.
40:891–897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu A, Wu K, Li M, Bao L, Shen X, Li S, Li
J and Yang Z: Upregulation of microRNA-492 induced by epigenetic
drug treatment inhibits the malignant phenotype of clear cell renal
cell carcinoma in vitro. Mol Med Rep. 12:1413–1420. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J, Zhang Y, Yu C, Li Z, Pan Y and
Sun C: MicroRNA-492 expression promotes the progression of hepatic
cancer by targeting PTEN. Cancer Cell Int. 14:952014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen F, Cai WS, Feng Z, Li JL, Chen JW,
Cao J and Xu B: MiR-492 contributes to cell proliferation and cell
cycle of human breast cancer cells by suppressing SOX7 expression.
Tumour Biol. 36:1913–1921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Frowein J, Hauck SM, Kappler R, Pagel
P, Fleischmann KK, Magg T, Cairo S, Roscher A, von Schweinitz D and
Schmid I: MiR-492 regulates metastatic properties of hepatoblastoma
via CD44. Liver Int. 38:1280–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Frowein J, Pagel P, Kappler R, von
Schweinitz D, Roscher A and Schmid I: MicroRNA-492 is processed
from the keratin 19 gene and up-regulated in metastatic
hepatoblastoma. Hepatology. 53:833–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pearce LR, Komander D and Alessi DR: The
nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol.
11:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jimenez-Velasco A, Roman-Gomez J, Agirre
X, Barrios M, Navarro G, Vazquez I, Prosper F, Torres A and
Heiniger A: Downregulation of the large tumor suppressor 2
(LATS2/KPM) gene is associated with poor prognosis in acute
lymphoblastic leukemia. Leukemia. 19:2347–2350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi Y, Miyoshi Y, Morimoto K,
Taguchi T, Tamaki Y and Noguchi S: Low LATS2 mRNA level can predict
favorable response to epirubicin plus cyclophosphamide, but not to
docetaxel, in breast cancers. J Cancer Res Clin Oncol. 133:501–509.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Powzaniuk M, McElwee-Witmer S, Vogel RL,
Hayami T, Rutledge SJ, Chen F, Harada S, Schmidt A, Rodan GA,
Freedman LP and Bai C: The LATS2/KPM tumor suppressor is a negative
regulator of the androgen receptor. Mol Endocrinol. 18:2011–2023.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chakraborty S, Khare S, Dorairaj SK,
Prabhakaran VC, Prakash DR and Kumar A: Identification of genes
associated with tumorigenesis of retinoblastoma by microarray
analysis. Genomics. 90:344–353. 2007. View Article : Google Scholar : PubMed/NCBI
|