Introduction
Alzheimer's disease (AD) is a common
neurodegenerative disorder in the elderly population worldwide
(1). Various biologically
plausible explanations for the pathogenesis of AD have been
proposed. These explanations include the endoplasmic reticulum
stress (ERS) hypothesis, the genetic factor hypothesis, the
cholinergic hypothesis and the lack of nerve growth factor
hypothesis (2,3) Among these hypotheses, the ERS
hypothesis appears to be the most probable as ERS is induced by
oxidative stress; ERS occurs in the earliest stages of AD
pathogenesis (4). Numerous studies
have attempted to ameliorate ERS as a therapy for AD (5–8);
however, the specific molecular mechanisms that lead to ERS during
the pathological progression of AD remain unknown. In the present
study, these mechanisms and potential targeting approaches were
investigated in an in vitro model of AD (6). The p38 mitogen-activated protein
kinase (p38 MAPK) signaling pathway is mainly involved in ERS
(9–11); however, the molecular mechanisms
underlying ERS, and the association between ERS and the p38 MAPK
signaling pathway in AD require further investigation. The present
study proposed that acetyl amyloid β (Aβ)25-35 may
induce oxidative stress via p38 MAPK, resulting in ERS-associated
apoptosis of PC12 cells.
Therapeutic agents for the treatment of AD have
numerous side effects, including increased likelihood of bone
fractures and heart failure (12).
Thus, it is important to find an alternative drug with minimal side
effects. Astragaloside IV (AST IV), a monomer extracted from
Astragalus, is a herbal remedy widely used for the treatment
of diabetes and diabetic nephropathy, and inhibits the effects of
oxidative stress (7–9). Li et al (10) reported that AST IV treatment
increased peroxisome proliferator-activated receptor γ and
β-secretase 1 expression, and reduced neuritic plaque formation and
Aβ levels in the brains of APP/PS1 mice. The present study
hypothesized that AST IV, as a p38 MAPK antagonist, may suppress
ERS by downregulating p38 MAPK expression and Aβ levels in patients
with AD. To evaluate this hypothesis, Aβ25-35 was
applied to PC12 cells to mimic an in vitro models of AD; AST
IV was applied to assess whether p38 MAPK expression is affected
and whether it may be a safe and effective drug for treating
patients with AD.
Materials and methods
Cell culture and administration
PC12 cells (Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences, Shanghai, China) were incubated in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences, Shanghai, China) supplemented with 10% fetal bovine
serum (FBS; HyClone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2. An in vitro model of AD was
established by incubating the cells with 20 mM Aβ25-35
at 37°C for 24 h to induce PC12 cell damage. The cells were treated
with AST IV (50, 100 and 200 µM; ShangHai YuanYe Biotechnology Co.,
Ltd., Shanghai, China) or SB203580 (20 µM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 24 h after the model was
established. Untreated cells served as the control.
Superoxide dismutase (SOD) and
malondialdehyde (MDA) assay
Following treatment with AST IV or SB203580, the
cells were centrifuged at 111 × g for 5 min at room temperature and
the supernatants from all groups were collected. SOD (cat. no.
A001-1) and MDA (cat. no. A003-1) (both from Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) assays were performed
according to the manufacturer's protocols. Optical densities were
measured at 570 nm using an Epoch microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Reactive oxygen species (ROS)
assay
Carboxy-2′, 7′-dichlorodihydrofluorescein diacetate
(H2DCFDA; Sigma-Aldrich; Merck KGaA) was used to detect
intracellular ROS production. Following the drug incubations, the
cells were incubated with 20 µM H2DCFDA at 37°C for 30
min in the dark. Then the cells were detached using 0.5 g/l trypsin
at 37°C for 2 min and washed with PBS three times. Trypsin was
deactivated with PBS supplemented with 3% FBS, and cells were
centrifuged (111 × g for 5 min at room temperature). The levels of
ROS in PC12 cells was measured by a FACScan flow cytometer and the
data were processed with FlowJo software 7.6 (FlowJo LLC, Ashland,
OR, USA).
RNA extraction and reverse
transcription semi-quantitative polymerase chain reaction
(RT-sqPCR)
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to extract total RNA from cells and a
First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.)
was utilized to reverse transcribe mRNA according to the
manufacturer's protocols. cDNA was used as the template for sqPCR
amplification using oligo primers; the internal control for the
analysis was GAPDH. The primer sequences of the binding
immunoglobulin protein (BIP)/glucose-regulated protein 78 (GRP78),
growth arrest- and DNA damage-inducible gene 153 (GADD153)/C/EBP
homologous protein (CHOP) and caspase-3 genes (Sangon Biotech Co.,
Ltd., Shanghai, China) are presented in Table I. PCR analysis was performed on the
BIO-RAD MyCycle thermocycler (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) with a total reaction volume of 12 µl in each well, which
contained 3 µl of PCR Master Mix (2X) (Thermo Fisher Scientific,
Inc.), 3 µl of cDNA, 1 µM forward primers, 1 µM reverse primers and
2 µl of DEPC ddH2O. Each group was assessed in
triplicate. The thermocycling conditions for PCR were as follows:
Initial heating at 95°C for 10 min, 35 cycles of denaturation at
94°C for 30 sec, annealing at 58°C for 1 min and extension at 72°C
for 1 min, and final extension at 72°C for 7 min. The PCR products
were separated on a 1.5% agarose gel and photographed using a gel
imaging system (SIM International Group Co., Ltd., Newark, DE,
USA). ImageJ Software 1.49 (National Institutes of Health,
Bethesda, MD, USA) was used to quantify the grey values of the DNA
bands.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Length (bp) |
|---|
| BIP |
CCTCAGAGTGGAGTTGAAAATGC |
CCCCAAGACACGTGAGCAA | 82 |
| CHOP |
GGAAGTGCATCTTCATACACCACC |
TGACTGGAATCTGGAGAGCGCGAGGG | 316 |
| Caspase-3 |
CTGGACTGCGGTATTGAG |
GGGTGCGGTAGAGTAAGC | 101 |
| β-actin |
AGGGAAATCGTGCGTGACAT |
AACCGCTCATTGCCGATAGT | 148 |
Western blot analysis
Following treatment, PC12 cells were lysed using
western lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) containing 1% PMSF on ice for 30 min for protein extraction.
Protein concentration was determined using a BCA Protein
Quantification kit (cat. no. P0010; Beyotime Institute of
Biotechnology, Beijing, China). Equal amount of protein (10 µg) was
separated on an 8 or 10% SDS-PAGE gel and the proteins were
transferred to a polyvinylidene difluoride membrane (Merck KGaA).
Subsequently, the membrane was blocked with 5% non-fat milk for 1 h
at room temperature, and incubated with anti-p38 MAPK (1:500; cat.
no. ab32142; Abcam, Cambridge, UK), anti-phosphorylated (p)-p38
MAPK (1:500; cat. no. 4511P), anti-BIP/GRP78 (1:500; cat. no.
3183S) (both from Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-GADD153/CHOP (1:500; cat. no. sc-575; Santa Cruz
Biotechnology, Inc., USA), anti-caspase-3 (1:500; cat. no. 9665S;
Cell Signaling Technology, Inc.) and anti-β-actin (1:500; cat. no.
A1978; Sigma-Aldrich; Merck KGaA) primary antibodies overnight at
4°C. Then, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-mouse (1:2,000; cat. no. ZB2305) or
goat anti-rabbit secondary antibodies (1:2,000; cat. no. ZB2301)
(both from OriGene Technologies, Inc., Beijing, China) at room
temperature for 1 h. An enhanced chemiluminescence advanced western
blotting detection kit (Pierce; Thermo Fisher Scientific, Inc.) was
used to visualize the protein bands, which were then developed on
an X-ray film. Quantity One software 4.6.6 (Bio-Rad Laboratories,
Inc.) was used to quantify the grey values of the protein
bands.
Statistical analysis
All experiments were repeated three times. All
quantified results were expressed as the mean ± standard error of
the mean. Statistical analyses were performed with SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance followed by Student-Newman-Keuls post hoc test was used to
compare the mean values of the results from each group. P<0.05
was considered to indicate a statistically significant
difference.
Results
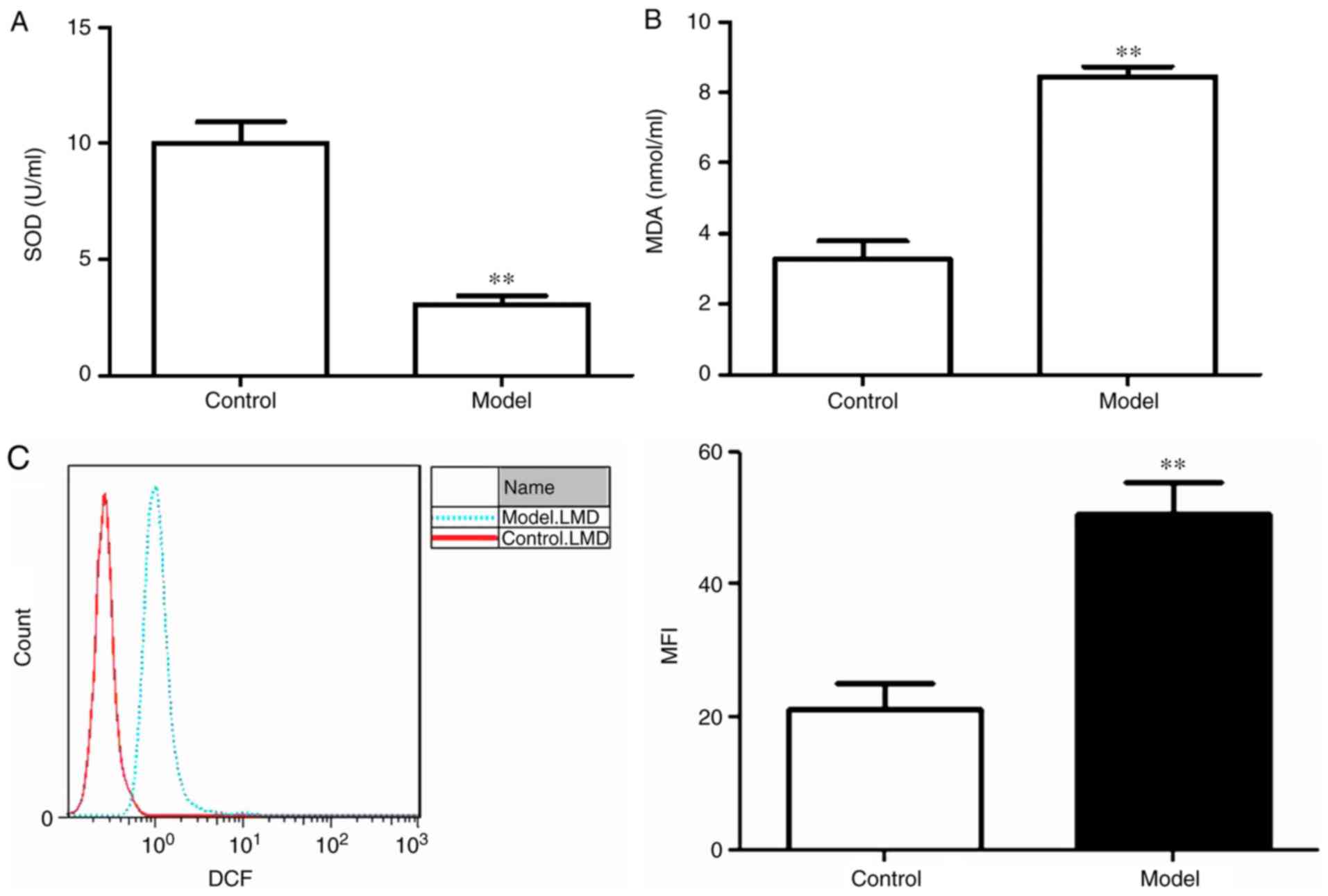
Oxidative stress is induced by
Aβ25-35 in PC12 cells
To assess whether treatment with 20 mM
Aβ25-35 for 24 h induced oxidative stress in PC12 cells,
SOD activity, MDA content and intercellular ROS levels were
assessed. SOD activity was significantly lower and MDA content was
significantly increased in the model group compared with the
control group (P<0.01; Fig. 1A and
B). Similarly, the mean fluorescence intensity of ROS in the
model group was significantly higher compared with the control
group (Fig. 1C).
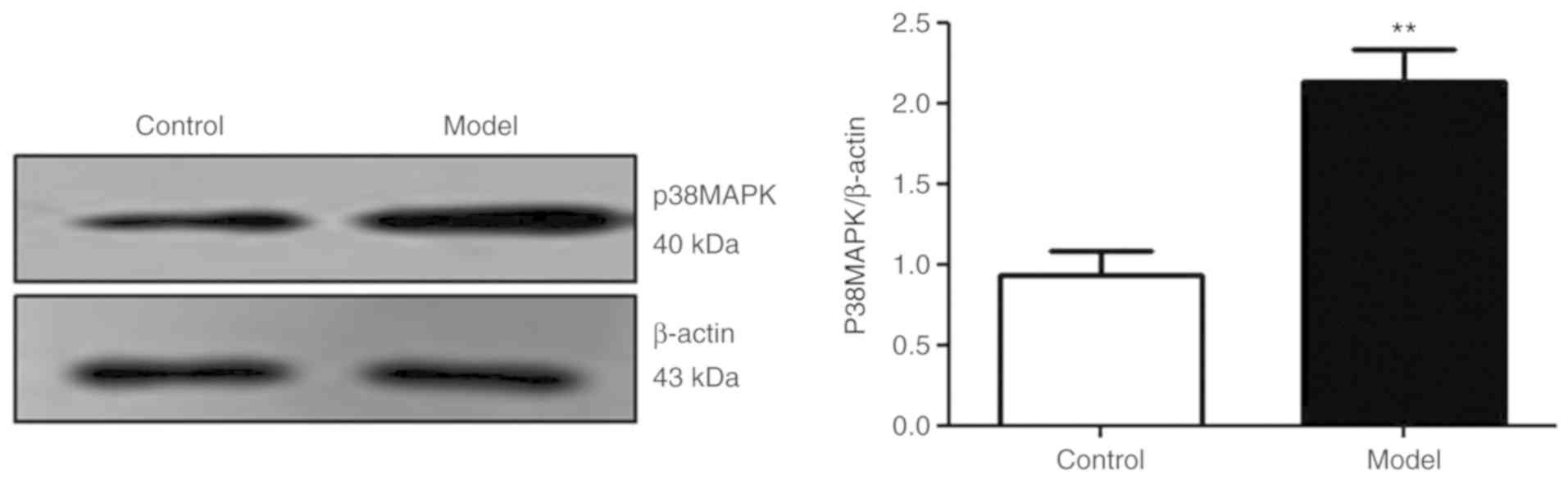
The p38 MAPK signaling pathway is
activated in Aβ25-35-treated PC12 cells
The results of the present study demonstrated that
the expression of p38 MAPK protein was significantly increased in
the model group compared with the control group (P<0.01;
Fig. 2). The results suggested
that Aβ25-35 activated the p38 signaling pathway.
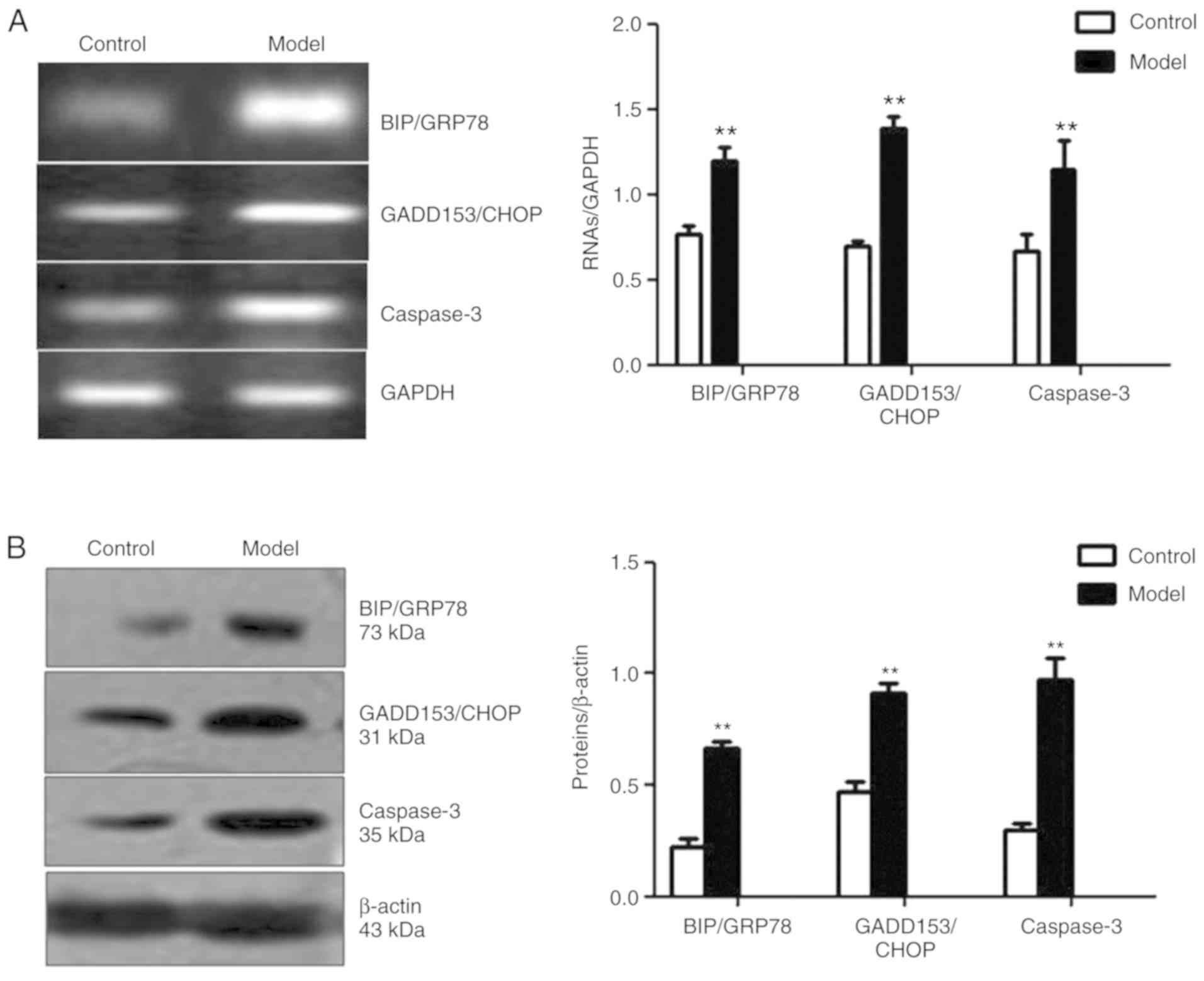
Aβ25-35 may contribute to
the ERS-induced apoptosis of PC12 cells
The expression levels of BIP/GRP78 and GADD153/CHOP,
two important ERS-associated proteins, and caspase-3, a terminal
apoptotic indicator (13), were
examined in Aβ25-35-treated PC12 cells. As presented in
Fig. 3A and B, the mRNA and
protein expression levels of BIP/GRP78, GADD153/CHOP and caspase-3
in the model group were significantly upregulated compared with the
control group (P<0.01).
AST IV inhibits oxidative stress in
Aβ25-35-treated PC12 cells in a concentration-dependent
manner
As presented in Fig.
4, Aβ25-35 significantly decreased the activity of
SOD, and increased the content of MDA and the level of ROS in PC12
cells compared with the control; however, treatment with 50, 100
and 200 µM AST IV significantly elevated SOD activity, and reduced
MDA content and ROS levels in a dose-dependent manner (P<0.01;
Fig. 4A-C). The middle dose of AST
IV (100 µM) was used for subsequent experiments.
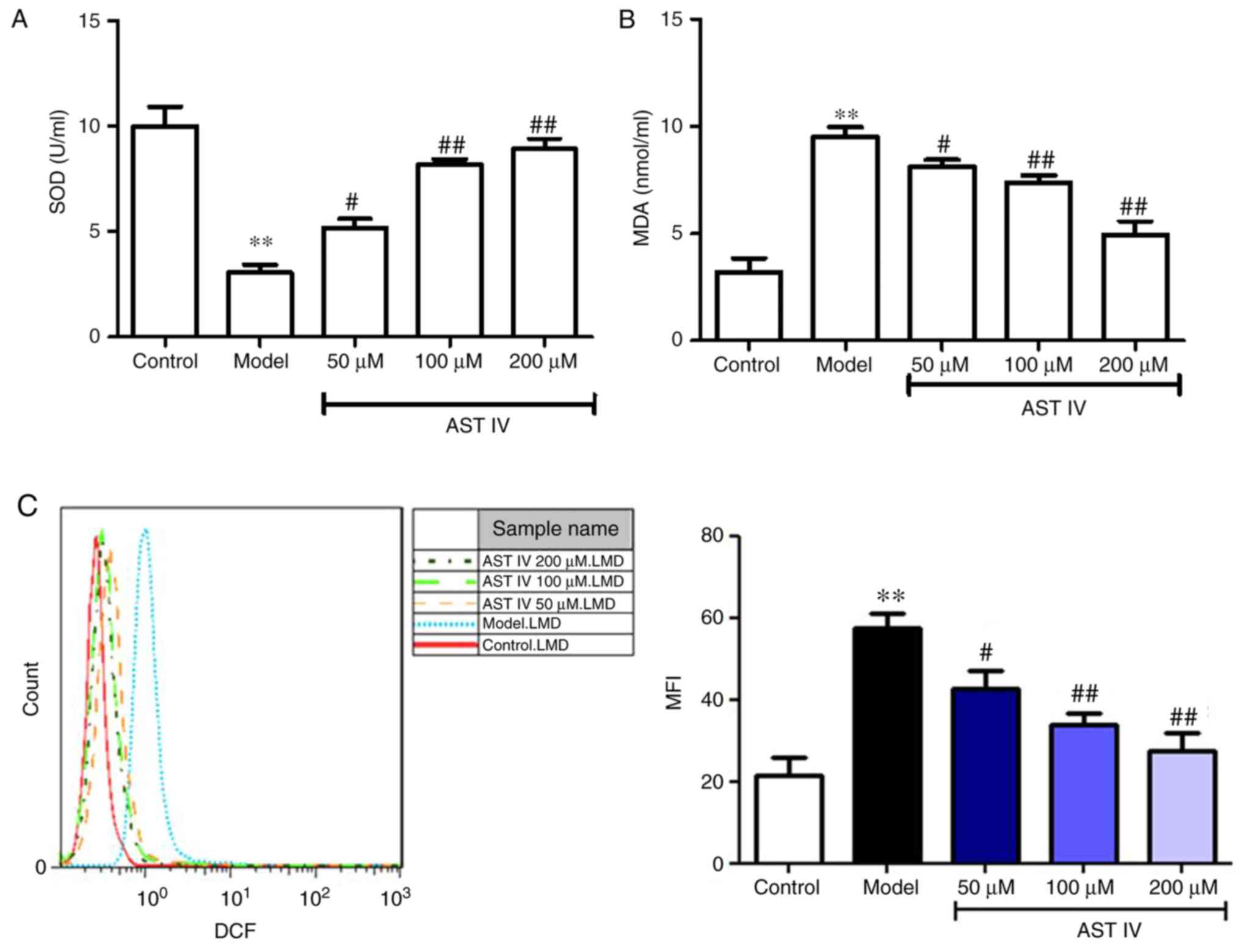 | Figure 4.AST IV alleviates oxidative stress in
amyloid β25-35-treated PC12 cells. (A) SOD activity and
(B) MDA content were measured from the supernatants of the control,
model, 50, 100 and 200 µM AST IV groups. (C) FACS was performed to
assess intercellular ROS levels in the control, model, 50, 100 and
200 µM AST IV groups. **P<0.01 vs. Control;
#P<0.05, ##P<0.01 vs. Model. AST IV,
astragaloside IV; MDA, malondialdehyde; SOD, superoxide
dismutase. |
AST IV ameliorates
Aβ25-35-induced ERS in PC12 cells via the p38 MAPK
signaling pathway
The present study reported that Aβ25-35
induced p38 MAPK expression in PC12 cells. SB203580, a specific
inhibitor of p38 MAPK, was incubated with PC12 cells to further
investigate whether oxidative stress induced ERS in PC12 cells via
the p38 MAPK signaling pathway. The results revealed that SB203580
treatment significantly increased SOD activity, and significantly
decreased MDA content and intercellular ROS levels compared with
the model group (P<0.01, Fig.
5A-C). In addition, it was observed that p-p38 MAPK protein
expression was significantly decreased in the SB203580 group
compared with in the model group (P<0.01; Fig. 5D). Furthermore, SB203580
significantly reduced the expression levels of BIP/GRP78,
GADD153/CHOP and caspase-3 compared with the model group
(P<0.01; Fig. 5E and F). AST IV
(100 µM) significantly ameliorated the effects induced by
Aβ25-35, potentially by alleviating oxidative stress
(P<0.01; Fig. 5A-C); a
significantly reduced p-p38 MAPK ratio and decreased caspase-3
expression in PC12 cells was also reported (P<0.05; Fig. 5D-F).
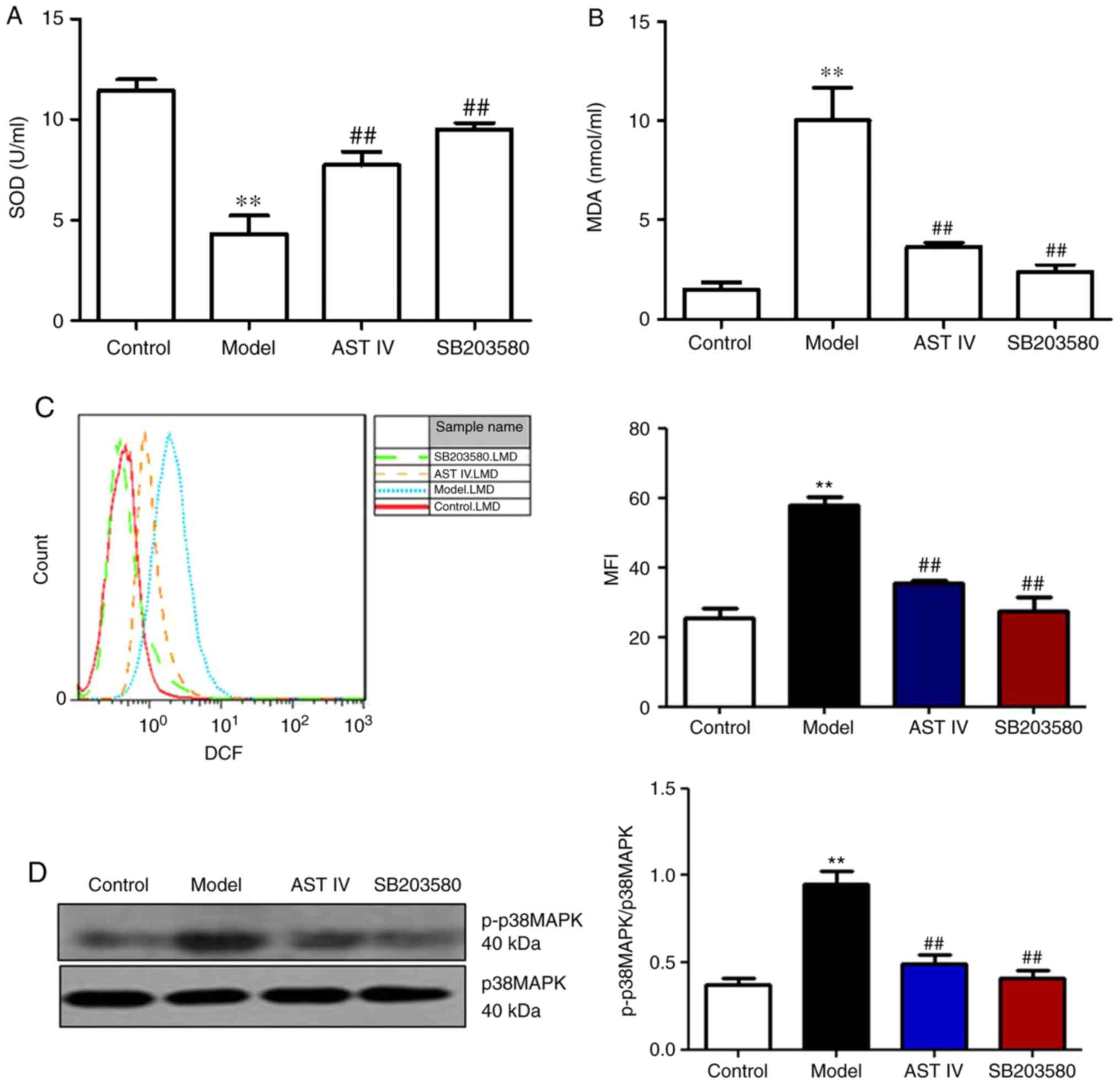 | Figure 5.AST IV (100 µM) and SB203580
ameliorate apoptosis in PC12 cells induced by amyloid
β25-35 via p38 MAPK. (A) SOD activity and (B) MDA
content were measured from the supernatants from the control,
model, AST IV and SB203580 groups. (C) FACS was performed to assess
intercellular ROS levels in the control, model, AST IV and SB203580
groups. (D) Western blot analyses of p38 MAPK and p-p38 MAPK. (E
and F) Reverse transcription semi-quantitative polymerase chain
reaction and western blot analyses of BIP/GRP78, GADD153/CHOP and
caspase-3 were performed of the control, model, AST IV and SB203580
groups. **P<0.01 vs. Control; #P<0.05,
##P<0.01 vs. Model. AST IV, astragaloside IV;
BIP/GRP78, binding immunoglobulin protein/glucose-regulated protein
78; GADD153/CHOP, growth arrest- and DNA damage-inducible gene
153/C/EBP homologous protein; MAPK, mitogen-activated protein
kinase; MDA, malondialdehyde; SOD, superoxide dismutase. |
Discussion
ERS serves a vital role in the earliest stages of AD
pathogenesis (6). Recent studies
have demonstrated that the toxic peptide, Aβ, induces ERS and then
activates the unfolded protein response (8,13).
It has been reported that ERS is involved in the cleavage of Aβ
protein precursor, thus promoting the production of Aβ and
facilitating the development of AD (14–16).
The results of the present study revealed that treatment with 20 mM
Aβ25-35 for 24 h contributed to the unbalancing of the
redox state by reducing SOD activity, and increasing MDA content
and ROS accumulation in PC12 cells, consistent with previous
studies (17,18). The results also indicated that the
expression levels of ERS-specific proteins, BIP/GRP78 and
GADD153/CHOP, were increased in PC12 cells in response to
Aβ25-35 treatment (19). Under particular conditions,
including lipid accumulation, glucose deprivation or oxidative
stress, ER homeostasis is dysregulated, which induces the
accumulation of misfolded proteins within the ER lumen (20,21).
Consequently, cells are unable to respond to unfolded proteins and
they succumb to apoptosis (15,22).
It was previously reported that oxidative stress activated
antiapoptotic ER chaperone proteins, including BIP/GRP78, but
severe ERS activated proapoptotic ER chaperone proteins, including
GADD153/CHOP (23). The
identification of these unfolded protein response markers indicated
an association between cell death and ERS. Apoptosis is known as a
possible cell death mechanism in age-associated diseases, and is
regulated by complex signaling pathways and enzymatic cascades.
Caspase-3 is a terminal indicator of apoptosis and cytokine
processing (24); the present
study indicated that the expression levels of Caspase3 mRNA and
proteins were upregulated as previously reported.
Jian et al reported that treatment with
SB203580, a p38 MAPK inhibitor, inhibited ROS generation in
myocardial cells, thus protecting the cells from
doxorubicin-induced apoptosis (25). The results of the present study
also demonstrated that treatment with 20 mM Aβ25-35
induced p38 MAPK activation, consistent with recent studies
(26,27). To investigate the role of p38 MAPK
in an in vitro model of AD, SB203580 was administered. The
results revealed that SB203580 administration could decrease ROS
levels, ERS and caspase-3 expression in Aβ25-35-treated
PC12 cells, which were consistent with previous studies (28–30).
The present study indicated that AST IV exhibited similar effects
to SB203580; it is possible that the mechanism of AST IV is
associated with the enhancement of SOD activity, which is an
important enzyme in cellular oxidative injury (31), decreased MDA content and inhibition
of ROS aggregation. It was suggested that the p38 MAPK signaling
pathway serves a role in the pathogenesis of AD in
Aβ25-35-treated PC12 cells. By suppressing the p38 MAPK
signaling pathway and treating cells with AST IV, the protein
expression of BIP/GRP7, GADD153/CHOP and caspase-3 may be
downregulated.
It remains unknown whether other oxidative
stress-associated signaling pathways are involved in ERS, which is
a limitation of our study. Further investigation is required to
determine the molecular mechanism underlying the pharmacological
effects of AST IV in patients with AD and to understand the role of
other signaling pathways. In conclusion, these data suggested that
PC12 cells treated with 20 mM Aβ25-35 for 24 h induced
ERS-associated apoptosis, which may be partly due to oxidative
stress, as ERS is induced by activating the p38 MAPK signaling
pathway (32). The results of the
present study suggest that AST IV may possess therapeutic potential
in the treatment of AD.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Anhui (grant no. 1508085SMH236) and the Introduction
and Cultivation Project of Leading Talents in Universities and
Colleges of Anhui Province-Visiting Scholar (grant no.
gxfxZD2016162).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX performed the experiments, including cell
culture, western blot analysis and polymerase chain reaction. YM
made substantial contributions to the conception and design of the
study, and was involved in drafting the manuscript and revising it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ide K, Matsuoka N and Kawakami K: Is the
use of proton-pump inhibitors a risk factor for Alzheimer's
disease? Molecular mechanisms and clinical implications. Curr Med
Chem. 25:2166–2174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lista S, Khachaturian ZS, Rujescu D,
Garaci F, Dubois B and Hampel H: Application of systems theory in
longitudinal studies on the origin and progression of Alzheimer's
disease. Methods Mol Biol 1303. 49–67. 2016. View Article : Google Scholar
|
|
3
|
Ellis B, Hye A and Snowden SG: Metabolic
modifications in human biofluids suggest the involvement of
sphingolipid, antioxidant, and glutamate metabolism in Alzheimer's
disease pathogenesis. J Alzheimers Dis. 46:313–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Zhang X, Zhang S, Huang H, Wu J,
Wang Y, Yuan L, Liu C, Zeng X, Cheng X, et al: Oxidative stress
mediates microcystin-LR-induced endoplasmic reticulum stress and
autophagy in KK-1 cells and C57BL/6 mice ovaries. Front Physiol.
9:10582018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adzic M, Mitic M and Radojcic M:
Mitochondrial estrogen receptors as a vulnerability factor of
chronic stress and mediator of fluoxetine treatment in female and
male rat hippocampus. Brain Res 1671. 77–84. 2017. View Article : Google Scholar
|
|
6
|
Chen W, Chan Y, Wan W, Li Y and Zhang C:
Aβ1-42 induces cell damage via RAGE-dependent
endoplasmic reticulum stress in bEnd.3 cells. Exp Cell Res.
362:83–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraga FJ, Mamani GQ, Johns E, Tavares G,
Falk TH and Phillips NA: Early diagnosis of mild cognitive
impairment and Alzheimer's with event-related potentials and
event-related desynchronization in N-back working memory tasks.
Comput Methods Programs Biomed. 164:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song X, Liu B, Cui L, Zhou B, Liu L, Liu
W, Yao G, Xia M, Hayashi T, Hattori S, et al: Estrogen receptors
are involved in the neuroprotective effect of silibinin in
Aβ1-42-treated rats. Neurochem Res. 43:796–805. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kheiri G, Dolatshahi M, Rahmani F and
Rezaei N: Role of p38/MAPKs in Alzheimer's disease: Implications
for amyloid beta toxicity targeted therapy. Rev Neurosci. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Ma X, Wang Y, Chen C, Hu M, Wang L,
Fu J, Shi G, Zhang D and Zhang T: Methyl salicylate lactoside
protects neurons ameliorating cognitive disorder through inhibiting
amyloid beta-induced neuroinflammatory response in Alzheimer's
disease. Front Aging Neurosci. 10:852018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melone MAB, Dato C, Paladino S, Coppola C,
Trebini C, Giordana MT and Perrone L: Verapamil inhibits
Ser202/Thr205 phosphorylation of Tau by blocking TXNIP/ROS/p38 MAPK
pathway. Pharm Res. 35:442018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong BL, Rybalsky I, Shellenbarger KC,
Tian C, McMahon MA, Rutter MM, Sawnani H and Jefferies JL:
Long-term outcome of interdisciplinary management of patients with
duchenne muscular dystrophy receiving daily glucocorticoid
treatment. J Pediatr. 182:296–303.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen H, Pan XD, Zhang J, Zeng YQ, Zhou M,
Yang LM, Ye B, Dai XM, Zhu YG and Chen XC: Endoplasmic reticulum
stress induces the early appearance of pro-apoptotic and
anti-apoptotic proteins in neurons of five familial Alzheimer's
disease mice. Chin Med J (Engl). 129:2845–2852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobylewski SE, Henderson KA, Yamada KE and
Eckhert CD: Activation of the EIF2α/ATF4 and ATF6 pathways in
DU-145 cells by boric acid at the concentration reported in men at
the US mean boron intake. Biol Trace Elem Res. 176:278–293. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu XJ, Wei J, Shang YH, Huang HC and Lao
FX: Modulation of AβPP and GSK3β by endoplasmic reticulum stress
and involvement in Alzheimer's disease. J Alzheimers Dis.
57:1157–1170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan YL, Zhao J and Li S: Update on the
neuroprotective effect of estrogen receptor alpha against
Alzheimer's disease. J Alzheimers Dis. 43:1137–1148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei H, Gao Z, Zheng L, Zhang C, Liu Z,
Yang Y, Teng H, Hou L, Yin Y and Zou X: Protective effects of
fucoidan on Aβ25-35 and d-Gal-induced neurotoxicity in PC12 cells
and d-Gal-induced cognitive dysfunction in mice. Mar Drugs.
15:E772017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu P, Wang H, Li Z and Yang Z: Triptolide
attenuated injury via inhibiting oxidative stress in
Amyloid-Beta25-35-treated differentiated PC12 cells. Life Sci.
145:19–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Wu J, Zeng W, Zhao Y and Zu H:
Exendin-4, a glucagon-like peptide-1 receptor agonist, inhibits
Aβ25-35-induced apoptosis in PC12 cells by suppressing the
expression of endoplasmic reticulum stress-related proteins. Int J
Clin Exp Pathol. 8:12784–12792. 2015.PubMed/NCBI
|
|
20
|
Basseri S and Austin RC: Endoplasmic
reticulum stress and lipid metabolism: Mechanisms and therapeutic
potential. Biochem Res Int 2012. 8413622012.
|
|
21
|
E L, Cheng Y and Zhao X: Protective
effects of high-density lipoprotein on mice cardiomyocytes induced
by oxygen and glucose deprivation through Akt signaling pathway.
Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 30:795–799. 2018.(In
Chinese). PubMed/NCBI
|
|
22
|
Chen M, Liu Q, Chen L, Zhang L and Gu E:
Remifentanil postconditioning ameliorates histone H3 acetylation
modification in H9c2 cardiomyoblasts after hypoxia/reoxygenation
via attenuating endoplasmic reticulum stress. Apoptosis.
22:662–671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Q, Gao H, Dong R and Wu YQ:
Sequential changes of endoplasmic reticulum stress and apoptosis in
myocardial fibrosis of diabetes mellitus-induced rats. Mol Med Rep.
13:5037–5044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sánchez-Rodríguez C, Cuadrado E,
Riestra-Ayora J and Sanz-Fernández R: Polyphenols protect against
age-associated apoptosis in female rat cochleae. Biogerontology.
19:159–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jian CY, Ouyang HB, Xiang XH, Chen JL, Li
YX, Zhou X, Wang JY, Yang Y, Zhong EY, Huang WH and Zhang HW:
Naringin protects myocardial cells from doxorubicininduced
apoptosis partially by inhibiting the p38MAPK pathway. Mol Med Rep.
16:9457–9463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Wang ZF, Li W, Hong H, Chen J,
Tian Y and Liu ZY: Protective effects of microRNA-330 on amyloid
β-protein production, oxidative stress, and mitochondrial
dysfunction in Alzheimer's disease by targeting VAV1 via the MAPK
signaling pathway. J Cell Biochem. 119:5437–5448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo J, Chang L, Li C, Li M, Yan P, Guo Z,
Wang C, Zha Q and Wang Q: SB203580 reverses memory deficits and
depression-like behavior induced by microinjection of
Aβ1-42 into hippocampus of mice. Metab Brain Dis.
32:57–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Werner E, Wang H and Doetsch PW: Opposite
roles for p38MAPK-driven responses and reactive oxygen species in
the persistence and resolution of radiation-induced genomic
instability. PLoS One. 9:e1082342014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang G, Yang W, Wu L and Wang R: H2S,
endoplasmic reticulum stress, and apoptosis of insulin-secreting
beta cells. J Biol Chem. 282:16567–16576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JY, Kim EJ, Kwon KJ, Jung YS, Moon
CH, Lee SH and Baik EJ: Neuroprotection by
fructose-1,6-bisphosphate involves ROS alterations via p38
MAPK/ERK. Brain Res 1026. 295–301. 2004. View Article : Google Scholar
|
|
31
|
Sun Y, Xun L, Jin G and Shi L: Salidroside
protects renal tubular epithelial cells from hypoxia/reoxygenation
injury in vitro. J Pharmacol Sci. 137:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinceti E, Shults CL, Rao YS and Pak TR:
Differential effects of E2 on MAPK activity in the brain and heart
of aged female rats. PLoS One. 11:e01602762016. View Article : Google Scholar : PubMed/NCBI
|