Introduction
Lower back pain represents a significant
musculoskeletal disorder that is one of the primary causes of poor
quality of life and healthcare-associated expenditure worldwide
(1). The number of people
suffering from lower back pain has increased in previous years
(2). The cause of lower back pain
is complex and multifactorial. At present, the molecular mechanism
regulating lower back pain remains largely unknown. Emerging
evidence indicates that intervertebral disc degeneration (IDD) is a
major potential factor leading to lower back pain (3,4).
Nevertheless, no effective drug for IDD treatment has been
developed until recently.
Intervertebral discs are avascular and consist of a
gelatinous inner core, nucleus pulposus, the annulus fibrosus and
tough outer rings, which cooperatively endows specific mechanical
function of the disc and allows multi-axial flexibility of the
spine (5,6). It has been demonstrated that the
nucleus pulposus cells reside in the gelatinous nucleus pulposus,
are vital for the physiological functions of the disc and produce a
high level of extracellular matrix (ECM) proteins including
aggrecan, collagen II and other factors (7–9).
Adequate ECM is a prerequisite to ensure the internal pressure of
the intervertebral disc and the performance of normal physiological
disc functions (10,11). Abnormal apoptosis in nucleus
pulposus cells has been demonstrated to contribute to the process
of IDD (12–15).
Autophagy is a catabolic process by which
dysfunctional proteins or organelles are degraded to relieve the
cellular stresses (16). An
increasing number of studies have suggested that there is a close
association between autophagy and apoptosis in the pathological
processes of certain degenerative diseases, including IDD and
Alzheimer's disease (17,18). A previous study also revealed that
increasing autophagy levels in nucleus pulposus cells may decrease
apoptosis and alleviate IDD (19).
β-ecdysterone is a major component of Chinese herbal
medicines. β-ecdysterone has been demonstrated to exhibit a number
of functions, including anabolic and hepatoprotective effects
(20). Certain studies have
indicated that β-ecdysterone may increase the synthesis of collagen
protein and inhibit cell apoptosis by regulation of autophagy
(20–22). However, the roles of β-ecdysterone
on nucleus pulposus cells and IDD remain incompletely
characterized. We hypothesized that β-ecdysterone may have
protective functions on IDD via autophagy stimulation.
Increased oxidative stress is a pathological cause
of apoptosis in nucleus pulposus cells (23). Therefore, the present study used
tert-butyl hydroperoxide (TBHP) to induce oxidative stress and
explore the effects of β-ecdysterone on apoptosis of nucleus
pulposus cells under oxidative stress and IDD.
Patients and methods
Patient samples
All the subjects were patients (three males and two
females; Age, 59.3±7.2 years) undergoing lumbar spine surgery
admitted to Hubei Provincial Hospital of Traditional Chinese
Medicine (Wuhan, China) between May 2014 and December 2015. The IDD
tissues were harvested under sterile conditions and immediately
sent to the laboratory (within 30 min of harvesting). Written
informed consent was obtained from each patient. The study was
approved by the Ethics Committee of Hubei Provincial Hospital of
Traditional Chinese Medicine. Patients with ankylosing spondylitis
or diffuse idiopathic skeletal hyperostosis were excluded. Complete
Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) medium with serum at 4°C was
used as transport medium. The nucleus pulposus tissues were
carefully isolated from IDD tissues by a scalpel microscopically
under sterile conditions.
Reagents and antibodies
3-methyladenine (3-MA), TBHP and the type II
collagenases were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The primary antibodies against sequestosome-1
(p62; 1:2,000; cat. no. ab207305), horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. ab7090) and GAPDH (1:2,000; cat. no. ab9485; all Abcam,
Cambridge, UK). The microtubule-associated proteins 1A/1B light
chain 3A (LC-3; 1:2,000; cat. no. 12741), Beclin-1 (1:2,000; cat.
no. 3495), Bax (1:2,000; cat. no. 5023) and cleaved caspase 3
(1:2,000; cat. no. 9661) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). DAPI was obtained
from Beyotime Institute of Biotechnology (Haimen, China). The cell
culture reagents were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA).
Western blotting
Total protein was extracted from nucleus pulposus
cells by radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.). The protein lysates (40 µg per lane) were
separated using 10% SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane (Thermo Fisher Scientific, Inc.). The membrane
was blocked using 5% non-fat milk in PBS (Thermo Fisher Scientific,
Inc.) containing 0.1% Tween-20 (Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h. Subsequently, the membrane was incubated for 2
h at 25°C with specific primary anti-human antibodies, followed by
incubation for 1 h at 25°C with a goat horseradish
peroxidase-conjugated secondary antibody. Membranes were then
washed with PBS for 10 min at room temperature, and the protein
bands were visualized using an Enhanced Chemiluminescence Western
Blotting kit (Pierce; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's protocol. Protein densitometry
was performed using ImageJ software (version 1.41; National
Institutes of Health, Bethesda, MD, USA). GAPDH was used as a
control. The experiment was repeated 3 times.
Protein degradation detection
Chx (Cycloheximide, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany; 1 µg/ml) was added into cultured cell medium.
Nucleus pulposus cells were cultured for 0, 12, and 24 h. Then
cells were collected and Beclin-1 expression was measured using
western blotting.
Isolation and culture of human nucleus
pulposus cells
The human nucleus pulposus tissues were carefully
isolated from IDD tissues by a scalpel microscopically under
sterile condition as previously described (19). Then, they were washed with PBS
twice and cut into 1 mm3 fragments. The fragments of
nucleus pulposus tissues were digested in 0.25% trypsin solution
for 30 min at 37°C, following 3–4 h in 0.2% type II collagenase at
37°C. Tissue debris was removed by passing through a 200 µm filter
and then the nucleus pulposus cells were resuspended in Dulbecco's
modified Eagle's medium (DMEM)/F12 containing 15% fetal bovine
serum (FBS) and 1% penicillin-streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. When the cells grew to
80–90% confluence, they were digested by 0.25% trypsin solution and
sub-cultured in culture flasks. The third generation of nucleus
pulposus cells was used for all experiments.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the cells in a
6-well plate using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). The RNA quality and concentration was determined
using a Thermo Scientific NanoDrop ND-100 (Wilmington, DE, USA). A
total of 1 µg of total RNA was used to synthesize cDNA using a
Reverse Transcription System kit (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's protocol. The
thermocycling conditions were: 37°C for 25 min, incubated at 85°C
for 5 sec in 20 µl of reaction volume. For the PCR amplification, a
20 µl reaction volume was used, including 10 µl 2X SYBR Premix Ex
Taq mixture (Takara Bio, Inc., Otsu, Japan), 0.2 mmol/l primer, 2
µl 2-fold diluted cDNA and sterile distilled water. The reaction
and detection were conducted in a light cycler (Roche Diagnostics
GmbH, Mannheim, Germany). The thermocycling conditions were as
follows: Denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and elongation at 60°C for 1 min.
The cycle threshold (Cq) values were collected and normalized to
the level of the housekeeping gene GAPDH according to the
2−ΔΔCq method (24).
The primer sequences were as follows: Collagen type II alpha 1
(Col2α1) forward (F), 5′-ACGCTCAAGTCGCTGAACAA-3′ and reverse (R),
5′-TCAATCCAGTAGTCTCCGCTCT-3′; Aggrecan F, 5′-TCCAAACCAACCCGACAAT-3′
and R, 5′-TCTCATAGCGATCTTTCTTCTGC-3′; a disintegrin and
metalloproteinase with thrombospondin motifs 5 (Adamts-5) F,
5′-CGACAAGAGTCTGGAGGTGAG-3′ and R, 5′-CGTGAGCCACAGTGAAAGC-3′;
matrix metalloproteinase-3 (MMP-3) F, 5′-ATGATGAACGATGGACAGATGA-3′
and R, 5′-CATTGGCTGAGTGAAAGAGACC-3′; Beclin-1 F,
5′-TCCGGGCTCCCGAGG-3′ and R, 5′-TTCCTCCTGGGTCTCTCCTG-3′; Bax F,
5′-TCATGGGCTGGACATTGGAC-3′ and R, 5′-GAGACAGGGACATCAGTCGC-3′; GAPDH
F, 5′-ATGTTGCAACCGGGAAGGAA-3′ and R,
5′-AGGAAAAGCATCACCCGGAG-3′.
Cell culture treatment protocols
To establish the apoptosis model of nucleus pulposus
cells, different concentrations of TBHP (50, 100, 200, 300 and 500
µM) were added into the culture medium of nucleus pulposus cells
for 24 h. Cells were pretreated with different concentrations of
β-ecdysterone (10, 50,100 and 200 µM) for 12 h prior to the
addition of TBHP (100 µM) to investigate its effect on cell
apoptosis. To examine the role of autophagy in
β-ecdysterone-mediated cell protection, nucleus pulposus cells were
pretreated with 10 µM 3-MA, an autophagy inhibitor, for 1 h prior
to β-ecdysterone administration. All experiments were performed in
triplicate. Small interfering (si)-Beclin-1 transfection
(5′-CGGAGAGGAGCCATTTATTGAA-3′; 50 nM) was performed using
Lipofectamine 2000 siRNA transfection reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
efficiency was confirmed 48 h after transfection, using
RT-qPCR.
Cell viability assay
Cell viability was assayed with the Cell Counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan)
according to the manufacturer's protocol. In brief, the nucleus
pulposus cells were plated in 96-well plates (5,000
cells/cm2) and incubated in DMEM with 10% FBS at 37°C
for 24 h. Then, the cells were treated with TBHP, β-ecdysterone and
3-MA, as aforementioned. Following treatment, the cells were washed
with PBS, and then 100 µl DMEM containing 10 µl CCK-8 solution was
added to each well, and the plate was incubated for an additional 1
h. The absorbance of the wells was then measured at 450 nm using a
microplate reader.
ELISA assay
Nucleus pulposus cells were pretreated with
β-ecdysterone for 12 h prior to TBHP stimulation for 24 h. Culture
supernatants were collected and stored at −20°C until analysis. The
concentration of Col2a1 (cat. no. DY7589-05), Aggrecan (cat. no.
DY1220), Adamts-5 (DY2198-05) and MMP-3 (cat. no. DMP300) in cell
culture supernatants was measured using ELISA kits (R&D Systems
Inc., Minneapolis, MN, USA) according to the manufacturer's
protocol.
Statistical analysis
Experiments were performed at least three times. The
results are presented as mean ± standard deviation. Statistical
analyses were performed using SPSS statistical software program
v.18.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed by
Student's t-test or one-way analysis of variance followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
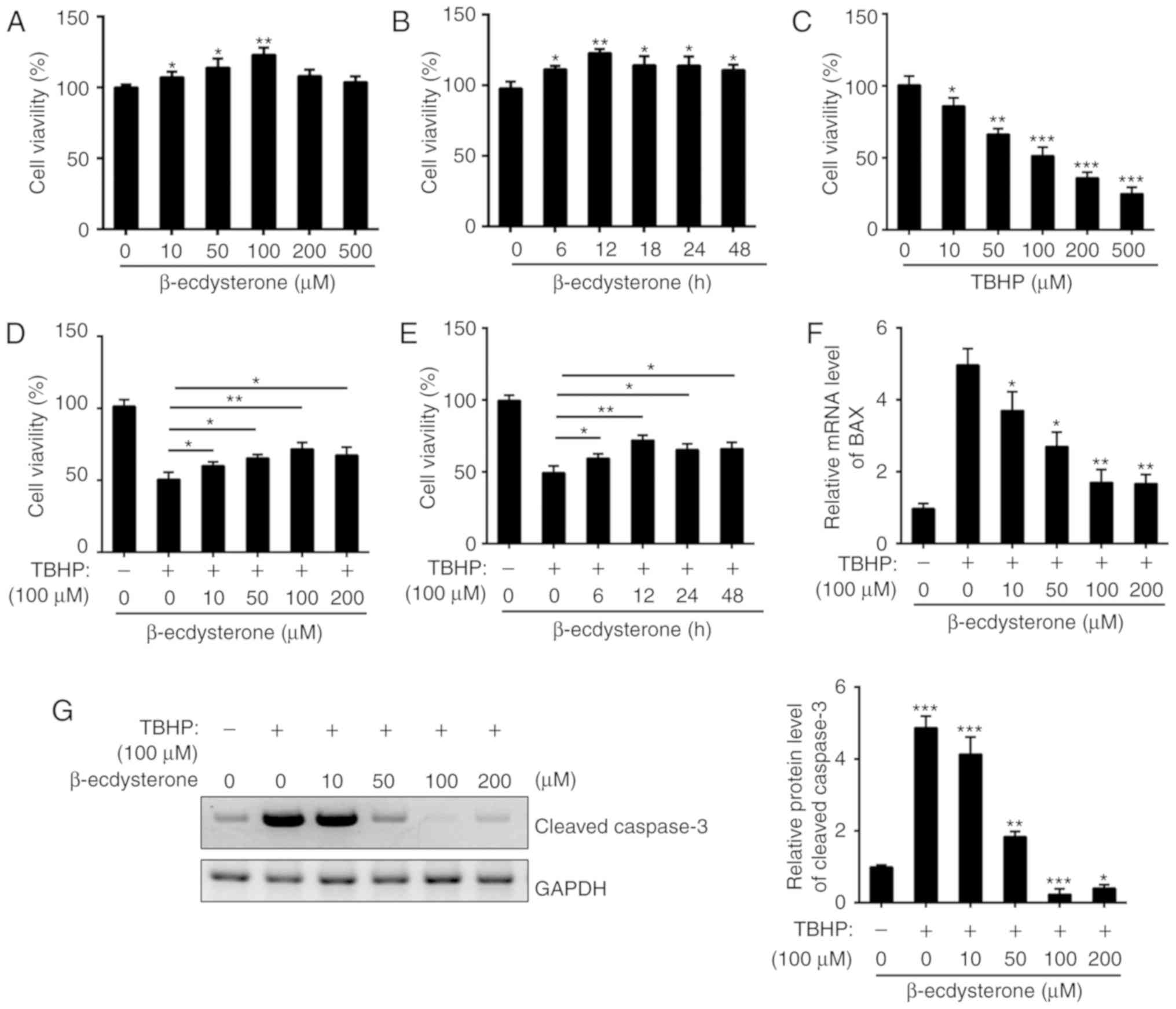
β-ecdysterone treatment decreases
apoptosis levels in nucleus pulposus cells
To investigate the effect of β-ecdysterone on
nucleus pulposus cells, the cytotoxic effect of β-ecdysterone was
first evaluated. Nucleus pulposus cells from patients with lumbar
vertebral fracture were treated with β-ecdysterone at different
concentrations (0, 10, 50, 100, 200 and 500 µM) or for different
times (0, 6, 12, 18, 24 and 48 h). As indicated in Fig. 1A and B, β-ecdysterone was not
cytotoxic to nucleus pulposus cells for 24 h or at concentrations
of ≤100 µM. However, the cell viability was significantly
downregulated following TBHP treatment in a dose-dependent manner
(Fig. 1C). Then, nucleus pulposus
cells were treated with β-ecdysterone and TBHP, and it was
identified that β-ecdysterone markedly protected nucleus pulposus
cells from TBHP-induced cell apoptosis (Fig. 1D and E). The expression of B cell
lymphoma 2-associated X protein (Bax) and cleaved caspase 3 were
also measured by RT-qPCR and western blot analysis. The results
indicated that pretreatment with β-ecdysterone decreased the mRNA
level of Bax and the protein level of cleaved caspase 3 induced by
TBHP in nucleus pulposus cells (Fig.
1F and G). Taken together, β-ecdysterone exhibited a marked
protective effect against TBHP-induced apoptosis in nucleus
pulposus cells.
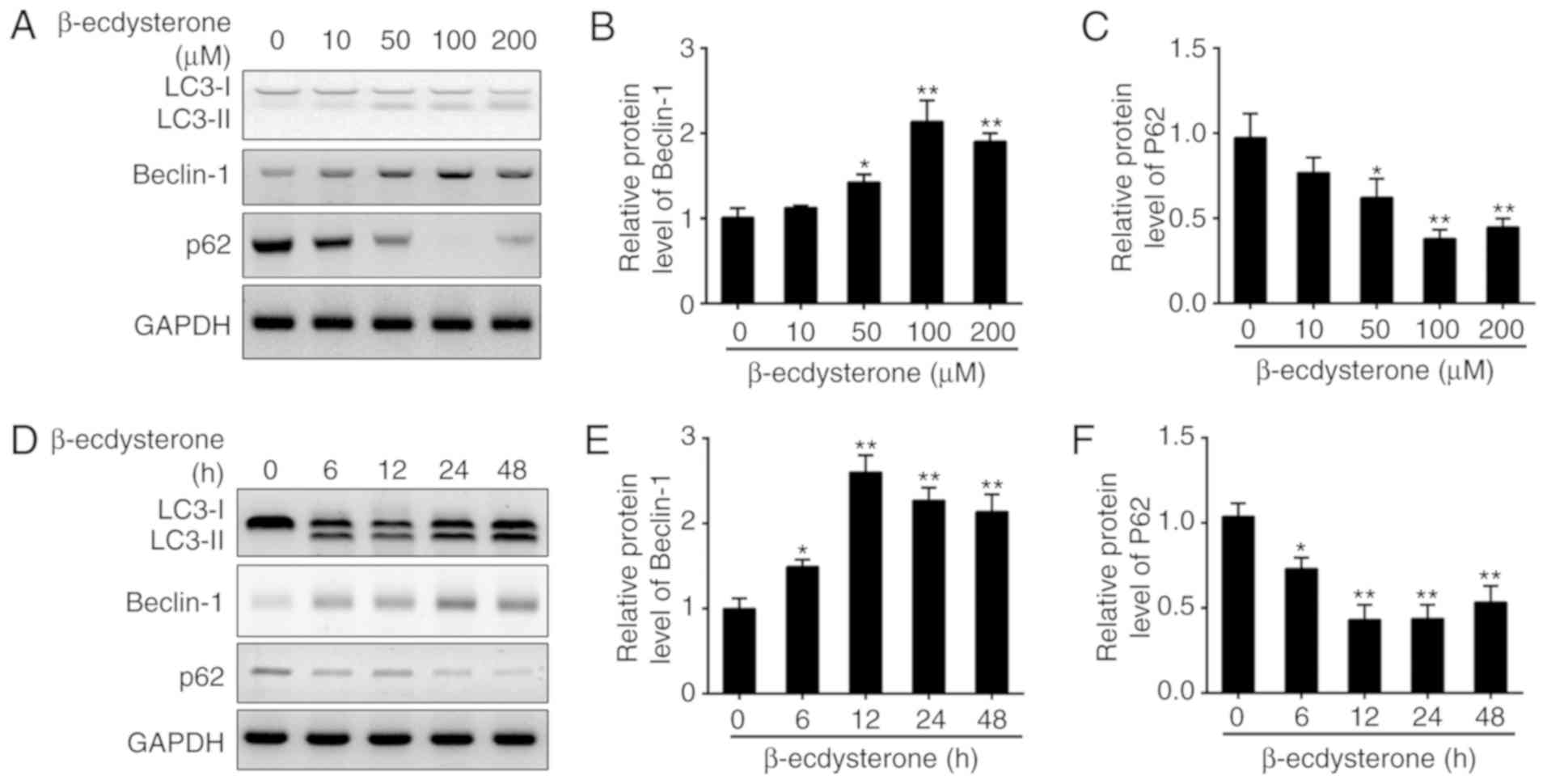
β-ecdysterone treatment induces
autophagy in nucleus pulposus cells
A previous study indicated that β-ecdysterone
inhibited cell apoptosis by induction of autophagy in osteoblasts
(20). To determine whether
β-ecdysterone has a role in autophagy in nucleus pulposus cells,
cells were treated with different concentrations of β-ecdysterone
or for different time intervals in the present study. LC3-II/LC3-I,
beclin-1 and p62 were recognized as indicators of autophagy
formation. Their levels in nucleus pulposus cells treated with
β-ecdysterone were assessed by western blot analysis, and it was
identified that the protein levels of LC3-II and beclin-1 were
significantly increased in a dose-dependent manner, while the
expression level of p62 was decreased following treatment (Fig. 2A-C). Their expression levels were
then determined at indicative time points following β-ecdysterone
treatment (100 µM). As demonstrated, the expression levels of
LC3-II and Beclin-1 were increased, while the expression of p62 was
significantly downregulated (Fig.
2D-F). Collectively, β-ecdysterone is an effective stimulus for
autophagy in nucleus pulposus cells.
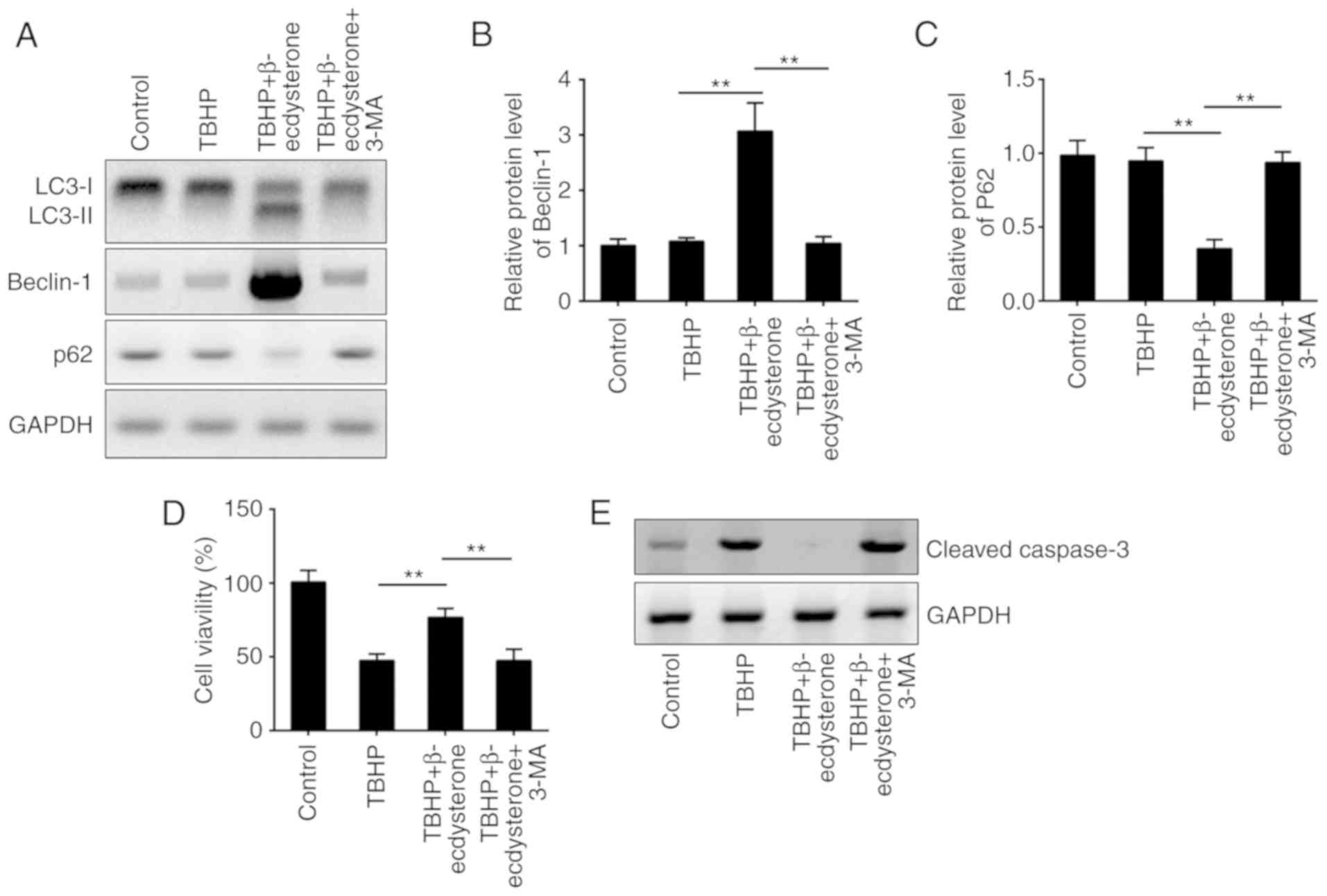
Autophagy induced by β-ecdysterone is
essential for protection against TBHP-induced apoptosis in nucleus
pulposus cells
Previous evidence indicates that autophagy protects
cells from apoptosis, including in nucleus pulposus cells (25). To determine whether
β-ecdysterone-induced autophagy is responsible for the protection
against TBHP-mediated apoptosis, autophagy was inhibited with 3-MA.
The administration of 3-MA abolished the autophagy induced by
β-ecdysterone in nucleus pulposus cells (Fig. 3A). As demonstrated, the protein
levels of LC3-II and beclin-1 were significantly decreased while
the expression of p62 was increased following 3-MA treatment
(Fig. 3A-C). These experiments
indicated that β-ecdysterone may effectively induce autophagy flux
in TBHP-treated nucleus pulposus cells, which may be completely
reversed by 3-MA treatment. To additionally determine whether
autophagy was involved in β-ecdysterone-mediated protection against
apoptosis in nucleus pulposus cells, the cell viability and the
expression level of cleaved caspase 3 were examined. As indicated,
β-ecdysterone increased the proportion of cell viability and
inhibited the protein level of cleaved caspase 3 in TBHP-treated
cells, while addition of 3-MA completely reversed this trend
(Fig. 3D and E).
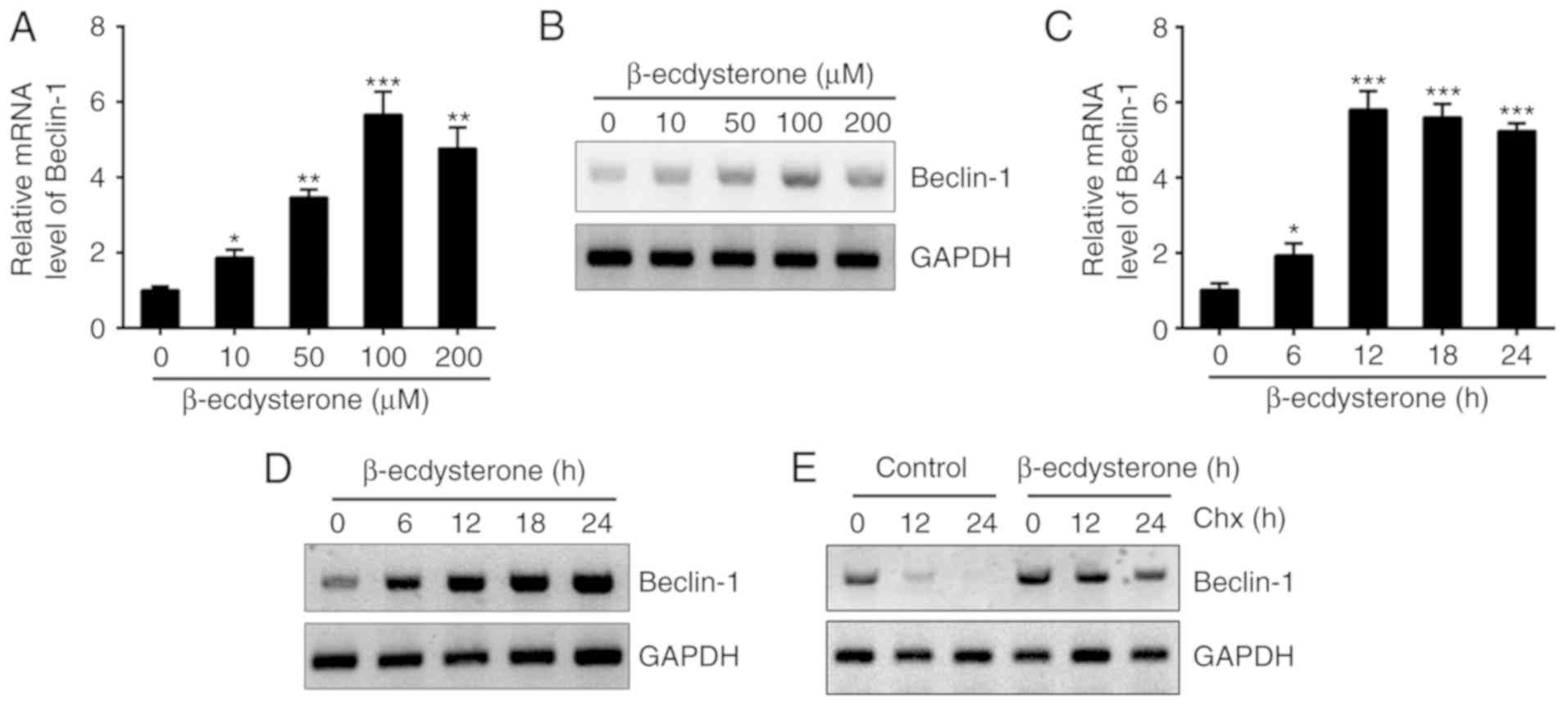
β-ecdysterone promotes the mRNA and
protein levels of Beclin-1 in nucleus pulposus cells
Subsequently, the present study aimed to determine
the molecular mechanism by which β-ecdysterone regulated autophagy
in nucleus pulposus cells. To examine whether β-ecdysterone may
promote the expression of Beclin-1; cells were treated with
β-ecdysterone at different concentrations and it was identified
that the Beclin-1 mRNA protein levels were markedly upregulated
(Fig. 4A and B). Similarly,
administration with β-ecdysterone for different time intervals also
promoted the mRNA and protein levels of Beclin-1 (Fig. 4C and D). In addition, it was
identified that β-ecdysterone significantly improved the stability
of Beclin-1 in nucleus pulposus cells. As demonstrated in Fig. 4E, following the addition of Chx,
the degradation of Beclin-1 in β-ecdysterone-treated cells was
slower.
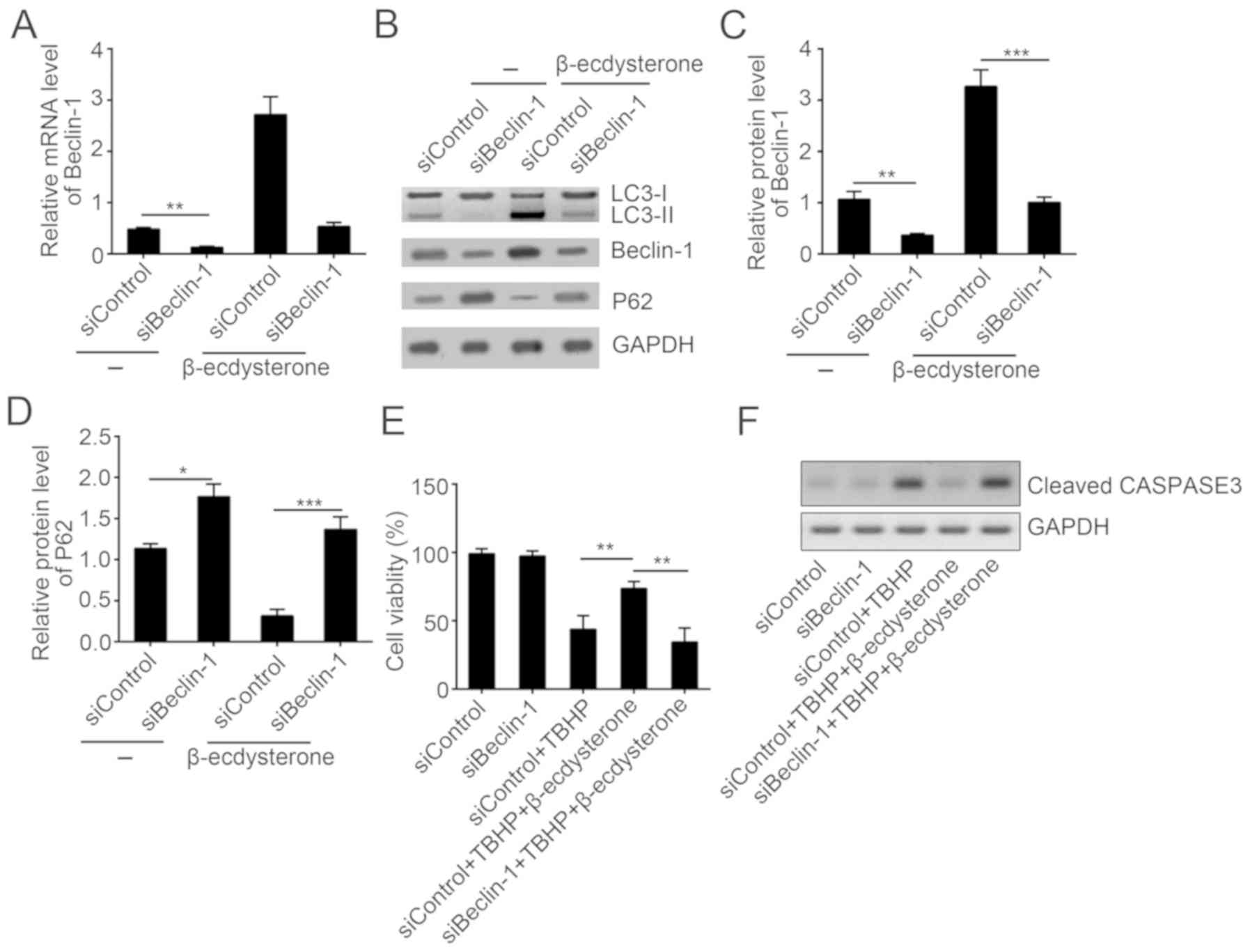
β-ecdysterone induces autophagy in a
Beclin-1-dependent manner
To additionally investigate the role of Beclin-1 in
the process of β-ecdysterone-induced autophagy, nucleus pulposus
cells were transfected with Beclin-1-siRNA (Fig. 5A). By western blot analysis, it was
demonstrated that depletion of Beclin-1 protein levels by siRNA
abolished the increased autophagy in nucleus pulposus cells
(Fig. 5B-D). Then, the effect of
Beclin-1 knockdown on the protective effect of β-ecdysterone was
also evaluated; it was identified that β-ecdysterone improved the
cell activity and inhibited the expression of cleaved caspase 3 in
nucleus pulposus cells while Beclin-1 knockdown abolished this
effects (Fig. 5E and F), which
indicated that β-ecdysterone-mediated autophagy and protective
function against apoptosis relied on upregulation of Beclin-1.
β-ecdysterone regulates the expression
of degeneration-associated genes via autophagy
Decrease in ECM proteins including aggrecan,
collagen II and other factors often results in IDD (26,27).
To additionally explore the degeneration of nucleus pulposus cells,
the expression levels of major ECM synthesis genes (Col2a1 and
aggrecan) and ECM degrading genes (MMP-3 and Adamts5) in nucleus
pulposus cells were examined. As demonstrated, the mRNA levels of
Col2a1 and aggrecan were downregulated following TBHP treatment,
while TBHP upregulated the mRNA levels of MMP-3 and Adamts5 in
nucleus pulposus cells (Fig. 6A).
Notably, β-ecdysterone reversed the effects of TBHP on the mRNA
levels of Col2a1, aggrecan, MMP-3 and Adamts5 via activation of
autophagy (Fig. 6A), which was
validated by the addition of 3-MA. Furthermore, the ELISA results
also exhibited a similar trend. β-ecdysterone promoted the protein
levels of collagen-II and aggrecan but inhibited that of MMP-3 and
Adamts-5 (Fig. 6B). Finally,
nucleus pulposus cells were isolated from patients with IDD, and it
was identified that β-ecdysterone upregulated the protein levels of
collagen-II and aggrecan but inhibited that of MMP-3 and adamts-5
by ELISA (Fig. 6C). In addition,
β-ecdysterone also increased the autophagy of nucleus pulposus
cells from patients with IDD and promoted their survival by
inhibiting apoptosis (Fig.
6D-F).
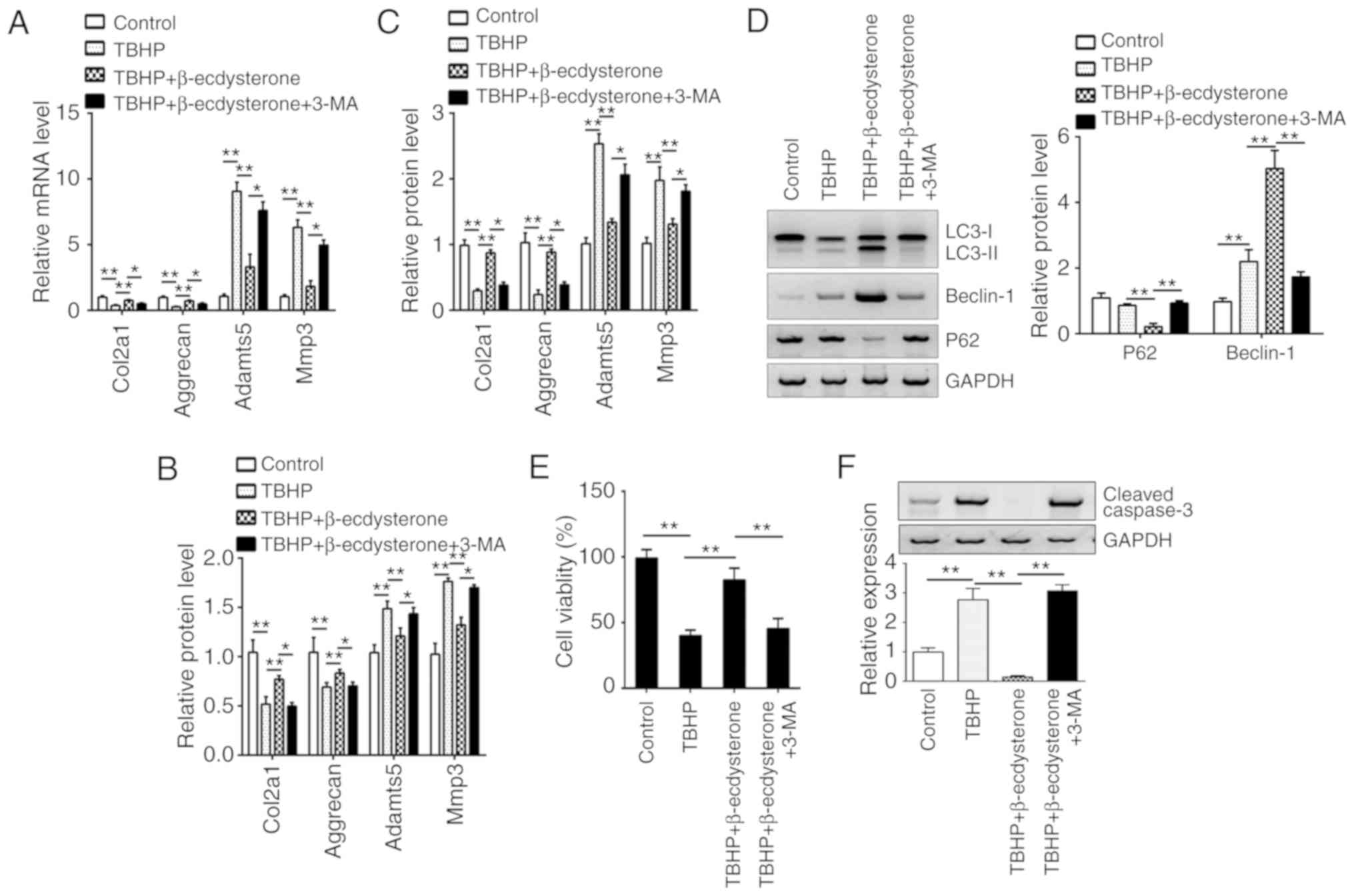 | Figure 6.β-ecdysterone regulates the
expression of degeneration-associated genes via autophagy. (A) The
mRNA expression of Col2a1, Aggrecan, Adamts-5 and MMP-3 were
measured by reverse transcription quantitative polymerase chain
reaction in the nucleus pulposus cells from normal human
intervertebral discs. (B) The protein expression levels of Col2a1,
Aggrecan, Adamts-5 and MMP-3 were measured by ELISA in the nucleus
pulposus cells from normal human intervertebral discs. (C) The
protein expression levels of Col2a1, Aggrecan, Adamts-5 and MMP-3
were measured by ELISA in the nucleus pulposus cells from patients
with IDD. (D) The protein levels of LC3, beclin-1 and p62 were
determined by western blot analysis in nucleus pulposus cells from
patients with IDD. Cell viability and apoptosis were determined by
(E) CCK8 and (F) western blot analysis assays in nucleus pulposus
cells from patients with IDD. *P<0.05 and **P<0.01 vs.
control. Col2a1, collagen type II alpha 1; Adamts-5, a disintegrin
and metalloproteinase with thrombospondin motifs 5; MMP-3, matrix
metalloproteinase 3; IDD, intervertebral disc degeneration; LC3,
Microtubule-associated proteins 1A/1B light chain 3A; p62,
sequestosome-1. |
Discussion
Autophagy is a catabolic process by which
dysfunctional proteins or organelles are degraded to relieve the
cellular stresses (28). Emerging
evidence has indicated that there is a closely association between
autophagy and various diseases including neurodegeneration,
infection and aging (29–31). An increasing number of studies have
demonstrated that autophagy serves an important role in the
pathology of disc degeneration (17,32,33).
For example, NAD-dependent protein deacetylase sirtuin-1 protects
against apoptosis in degenerative human disc nucleus pulposus cells
via promoting autophagy (28).
Zhao et al (32) revealed
that microRNA (miRNA)-129-5P modulates nucleus pulposus cell
autophagy by targeting Beclin-1 in IDD. In addition, glucosamine
exhibited a protective effect on nucleus pulposus cells via
activation of autophagy in an mechanistic target of
rapamycin-dependent manner (34).
Diverse factors have been demonstrated to regulate autophagy,
including miRNAs (35). A previous
study also revealed the association between autophagy and oxidative
stress-induced apoptosis in nucleus pulposus cells (36). Diverse factors may contribute to
IDD, including genetic predisposition, infection, excessive
biomechanical loading and aging (37,38).
At present, no effective drug for IDD treatment has been developed.
The present study identified that β-ecdysterone may regulate
autophagy in nucleus pulposus cells and inhibit cell apoptosis. In
addition, it was demonstrated that β-ecdysterone may be a potential
therapy drug for IDD treatment.
A previous study has demonstrated that oxidative
stress may lead to degenerated intervertebral discs (38). Emerging evidence has suggested that
increased concentration of oxidation products may induce cell
apoptosis by the mitochondrial pathway (36). Other studies have also revealed
that various pathogenic factors may induce cell apoptosis via
reactive oxygen, which leads to dysfunction of the mitochondria
(39,40). A previous study demonstrated that
TBHP induces the production of reactive oxygen species and then
leads to apoptosis in nucleus pulposus cells (19). In the present study, a TBHP-induced
apoptosis model was used to investigate the role of β-ecdysterone
in the process of IDD. Autophagy is a process used to degrade
useless proteins or organelles to maintain cellular functions
(41). Increasing evidence has
indicated that moderate autophagy exhibits a protective function
against cellular pathologies, including IDD (42). In addition, a specific study
revealed that autophagy-associated genes were downregulated in IDD
tissues compared with healthy tissues, including Beclin-1 (28). Therefore, modulating autophagy in
disc cells may be an effective therapeutic method for the treatment
of patients with IDD. In addition, there is great clinical
importance and an urgent requirement to develop novel drugs
targeting autophagy for the therapy of IDD. To the best of our
knowledge, the present study demonstrated for the first time that
β-ecdysterone regulated autophagy in a Beclin-1 dependent manner in
nucleus pulposus cells.
A large number of studies have demonstrated that
apoptosis promotes the development of IDD (12). In the present study, it was
identified that pretreatment with β-ecdysterone markedly inhibited
the expression of Bax and the activation of caspase 3 in nucleus
pulposus cells under oxidative stress, which indicated that
β-ecdysterone protects nucleus pulposus cells by inhibiting
apoptosis, at least in part. In addition, it was demonstrated that
β-ecdysterone administration promoted the protein expression of ECM
components including Col2A and aggrecan, whilst inhibiting the
expression of catabolism of ECM components including MMP-3 and
Adamts-5, which implied that β-ecdysterone may be of use in
preventing IDD. Finally, it was also identified that β-ecdysterone
may inhibit apoptosis by activating autophagy in nucleus pulposus
cells isolated from patients with IDD, which suggests that
β-ecdysterone may be a potential drug to ameliorate disc
degeneration.
The present study demonstrated that β-ecdysterone
may upregulate the mRNA and protein levels of Beclin-1, and
stabilize Beclin-1. However, how β-ecdysterone regulates beclin-1
expression remains unknown. In addition, the molecular mechanism by
which β-ecdysterone stabilizes beclin-1 remains to be
investigated.
In conclusion, the present study provides evidence
that treatment with β-ecdysterone induces autophagy in a
Beclin-1-dependent manner in the nucleus pulposus cells, which
confers an anti-apoptosis role against oxidative stress. These data
revealed the therapeutic potential of β-ecdysterone in the
prevention of the disc degeneration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW and A-FY initiated and designed the present
study, performed the experiments and wrote the manuscript. JY, C-JH
and Z-WZ performed the western blot analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol for the present study was approved by
the Institutional Ethics Committee of Hubei Provincial Hospital of
Traditional Chinese Medicine and all enrolled patients signed a
written informed consent document approving the use of their
samples.
Patient consent for publication
Patients have provided written informed consent for
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X,
Zhang X, Shen J, Fang J and Jiang W: SIRT1 inhibits apoptosis of
degenerative human disc nucleus pulposus cells through activation
of Akt pathway. Age (Dordr). 35:1741–1753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frymoyer JW and Cats-Baril WL: An overview
of the incidences and costs of low back pain. Orthop Clin North Am.
22:263–271. 1991.PubMed/NCBI
|
|
5
|
Pattappa G, Li Z, Peroglio M, Wismer N,
Alini M and Grad S: Diversity of intervertebral disc cells:
Phenotype and function. J Anat. 221:480–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayes AJ, Benjamin M and Ralphs JR:
Extracellular matrix in development of the intervertebral disc.
Matrix Biol. 20:107–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rutges JP, Nikkels PG, Oner FC, Ottink KD,
Verbout AJ, Castelein RJ, Creemers LB and Dhert WJ: The presence of
extracellular matrix degrading metalloproteinases during fetal
development of the intervertebral disc. Eur Spine J. 19:1340–1346.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayes AJ, Benjamin M and Ralphs JR: Role
of actin stress fibres in the development of the intervertebral
disc: Cytoskeletal control of extracellular matrix assembly. Dev
Dyn. 215:179–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Setton LA and Chen J: Mechanobiology of
the intervertebral disc and relevance to disc degeneration. J Bone
Joint Surg Am. 88 Suppl 2:S52–S57. 2006. View Article : Google Scholar
|
|
11
|
Wu B, Meng C, Wang H, Jia C and Zhao Y:
Changes of proteoglycan and collagen II of the adjacent
intervertebral disc in the cervical instability models. Biomed
Pharmacother. 84:754–758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones P, Gardner L, Menage J, Williams GT
and Roberts S: Intervertebral disc cells as competent phagocytes in
vitro: Implications for cell death in disc degeneration. Arthritis
Res Ther. 10:R862008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agrawal L, Sahu S, Ghosh S, Shiga T,
Fujita D and Bandyopadhyay A: Inventing atomic resolution scanning
dielectric microscopy to see a single protein complex operation
live at resonance in a neuron without touching or adulterating the
cell. J Integra Neurosci. 15:435–462. 2016. View Article : Google Scholar
|
|
16
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu
Y, Tian N, Huang Y, Xue E, Wang X and Xu H: Apoptosis, senescence,
and autophagy in rat nucleus pulposus cells: Implications for
diabetic intervertebral disc degeneration. J Orthop Res.
31:692–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salminen A, Kaarniranta K, Kauppinen A,
Ojala J, Haapasalo A, Soininen H and Hiltunen M: Impaired autophagy
and APP processing in Alzheimer's disease: The potential role of
Beclin 1 interactome. Prog Neurobiol. 106-107:33–54. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y,
Cai N, Tang Q, Wang C, Yan M, et al: Metformin protects against
apoptosis and senescence in nucleus pulposus cells and ameliorates
disc degeneration in vivo. Cell Death Dis. 7:e24412016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang YH, Yue ZS, Li GS, Zeng LR, Xin DW,
Hu ZQ and Xu CD: Effect of β-ecdysterone on glucocorticoidinduced
apoptosis and autophagy in osteoblasts. Mol Med Rep. 17:158–164.
2018.PubMed/NCBI
|
|
21
|
Syrov VN, Khushbaktova ZA and Nabiev AN:
An experimental study of the hepatoprotective properties of
phytoecdysteroids and nerobol in carbon tetrachloride-induced liver
lesion. Eksp Klin Farmakol. 55:61–65. 1992.(In Russian). PubMed/NCBI
|
|
22
|
Sass M and Kovács J: The effect of
ecdysone on the fat body cells of the penultimate larvae of
Mamestra brassicae. Cell Tissue Res. 180:403–409. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Zhang ZZ, Yang W, Ouyang ZH, Xue
JB, Li XL, Zhang J, Chen WK, Yan YG and Wang WJ: MiR-210
facilitates ECM degradation by suppressing autophagy via silencing
of ATG7 in human degenerated NP cells. Biomed Pharmacother.
93:470–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo W, Zhang B, Li Y, Duan HQ, Sun C, Xu
YQ and Feng SQ: Gene expression profile identifies potential
biomarkers for human intervertebral disc degeneration. Mol Med Rep.
16:8665–8672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang S, Liu C, Sun Z, Yan P, Liang H,
Huang K, Li C and Tian J: IL-1β increases asporin expression via
the NF-kB p65 pathway in nucleus pulposus cells during
intervertebral disc degeneration. Sci Rep. 7:41122017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang W, Zhang X, Hao J, Shen J, Fang J,
Dong W, Wang D, Zhang X, Shui W, Luo Y, et al: SIRT1 protects
against apoptosis by promoting autophagy in degenerative human disc
nucleus pulposus cells. Sci Rep. 4:74562014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kizilarslanoğlu MC and Ülger Z: Role of
autophagy in the pathogenesis of Alzheimer disease. Turk J Med Sci.
45:998–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cybulsky AV: The intersecting roles of
endoplasmic reticulum stress, ubiquitin-proteasome system, and
autophagy in the pathogenesis of proteinuric kidney disease. Kidney
Int. 84:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryter SW and Choi AM: Autophagy in lung
disease pathogenesis and therapeutics. Redox Biol. 4:215–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao K, Zhang Y, Kang L, Song Y, Wang K,
Li S, Wu X, Hua W, Shao Z, Yang S and Yang C: Methylation of
microRNA-129-5P modulates nucleus pulposus cell autophagy by
targeting Beclin-1 in intervertebral disc degeneration. Oncotarget.
8:86264–86276. 2017.PubMed/NCBI
|
|
33
|
Ye W, Xu K, Huang D, Liang A, Peng Y, Zhu
W and Li C: Age-related increases of macroautophagy and
chaperone-mediated autophagy in rat nucleus pulposus. Connect
Tissue Res. 52:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang L, Jin Y, Wang H, Jiang Y and Dong
J: Glucosamine protects nucleus pulposus cells and induces
autophagy via the mTOR-dependent pathway. J Orthop Res.
32:1532–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Geng Z, Xu F and Zhang Y:
MiR-129-5p-mediated Beclin-1 suppression inhibits endothelial cell
autophagy in atherosclerosis. Am J Transl Res. 8:1886–1894.
2016.PubMed/NCBI
|
|
36
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: Implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roberts S, Evans H, Trivedi J and Menage
J: Histology and pathology of the human intervertebral disc. J Bone
Joint Surg Am. 88 Suppl 2:S10–S14. 2006. View Article : Google Scholar
|
|
38
|
Battié MC, Videman T, Kaprio J, Gibbons
LE, Gill K, Manninen H, Saarela J and Peltonen L: The Twin Spine
Study: Contributions to a changing view of disc degeneration. Spine
J. 9:47–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong JG, Park JB, Lee D and Park EY:
Effect of high glucose on stress-induced senescence of nucleus
pulposus cells of adult rats. Asian Spine J. 9:155–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang L, Zhu L, Dong W, Cao Y, Lin L, Rong
Z, Zhang Z and Wu G: Reactive oxygen species-mediated mitochondrial
dysfunction plays a critical role in high glucose-induced nucleus
pulposus cell injury. Int Orthop. 2013. View Article : Google Scholar
|
|
41
|
Dutta D, Xu J, Kim JS, Dunn WA Jr and
Leeuwenburgh C: Upregulated autophagy protects cardiomyocytes from
oxidative stress-induced toxicity. Autophagy. 9:328–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen K, Lv X, Li W, Yu F, Lin J, Ma J and
Xiao D: Autophagy is a protective response to the oxidative damage
to endplate chondrocytes in intervertebral disc: Implications for
the treatment of degenerative lumbar disc. Oxid Med Cell Longev
2017. 40417682017.
|