Introduction
MicroRNAs (miRNAs/miRs) are highly conserved,
endogenous non-coding RNAs present in eukaryotes. miRNAs can
degrade target mRNA or suppress the translation of target mRNA via
binding to the 3′ untranslated region (UTR). Depending on their
targets, miRNAs can function as oncogenes or tumor suppressor genes
in different cancers (1).
Increasing research has suggested that aberrant
expression/functions of miRNAs are associated with the progression
of cancer and may serve as novel biomarkers for diagnosis and
prognosis in cancers, and may be potential novel therapeutic agents
for tumor suppression (2–5). miRNAs are involved in the regulation
of cellular functions, including cell proliferation and apoptosis
(6). For example, miRNA-101
inhibits cell proliferation and induces apoptosis, miRNA let-7c
suppresses cell proliferation and induces cell cycle arrest, and
miR-503 inhibits cell growth. These miRNAs all bind to the 3′UTR of
target genes and subsequently affect cellular functions (7–9).
miR-4530 is located on chromosome 19. In our
previous study (10), it was
revealed that miR-4530 is upregulated in the serum of patients with
diabetic retinopathy. Furthermore, it was demonstrated that
miR-4530 enhances angiogenesis in endothelial cells and breast
carcinoma cells. In addition, suppression of cell growth and
promotion of apoptosis, induced by overexpression of miR-4530, was
demonstrated in breast carcinoma cells (10). However, to the best of our
knowledge, the effects of miR-4530 in human umbilical vein
endothelial cells (HUVECs) has not yet been investigated.
Furthermore, the molecular mechanism of miR-4530 associated with
cell functions has not yet been determined. Therefore, the present
study aimed to investigate the role of miR-4530 in the cellular
function of HUVECs.
Analysis using TargetScan has suggested that Ras p21
protein activator 1 (RASA1) is a target gene of hsa-miR-4530 in
humans. The predominant function of RASA1 is to transform Ras from
its active guanosine-5′-triphospate (GTP)-bound form into its
inactive guanosine diphosphate-bound form by enhancement of the
endogenous GTPase activity of Ras via association with its
C-terminal GTPase activating protein (GAP) domain (11). In previous studies, miR-182, miR-31
and miR-223 were demonstrated to regulate cell proliferation,
apoptosis, migration and angiogenesis via association with RASA1
(12–14). Thus, as an important Ras GAP, a
mutation within the RASA1 gene may result in the progression of
numerous diseases. Furthermore, the enhancement of cell
proliferation and tube formation in endothelial cells resulting
from RASA1 depletion has been previously reported (15). The present study aimed to
investigate whether miR-4530 can also regulate cell growth and
apoptosis by targeting RASA1 in endothelial cells.
Materials and methods
Endothelial cell culture
The two cell lines used in the present study were
293T cells and HUVECs, purchased from the Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
Total 293T cells were cultured in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and 1% penicillin/streptomycin. RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc.), containing 10%
FBS and 1% penicillin/streptomycin, was used for culturing HUVECs.
All cells were placed in a humidified incubator at 37°C containing
5% CO2.
Construction of plasmids and
transfection into HUVEC cells
The pPG/miR/enhanced green fluorescent protein
(EGFP), pPG-miR4530-EGFP and pPG-miR4530sponge-EGFP plasmids were
purchased from Chang Jing Bio-Tech, Ltd. (Changsha, China). The
sequences inserted into the plasmids were as follows: miR-4530,
5′-AATTCGCCCAGCAGGACGGGAGCGGTTTTGGCCACTGACTGACCGCTCCCGCTGCTGGGCA-3′;
anti-miR-4530,
5′-AATTCCGCTCCCGTCCTGCTGGGCGATCGCTCCCGTCCTGCTGGGACCGGTCGCTCCCGTCCTGCTGGGTCACCGCTCCCGTCCTGCTGGGTTTTTTACGG-3′.
Furthermore, the vector upregulating RASA1 (GV230,
XhoI/KpnI) and the negative control (a random
sequence) were purchased from Shanghai GeneChem Co., Ltd.
(Shanghai, China). The full-length open reading frame of RASA1
(GeneBank accession no. NM_002890) was amplified using the
following primers: RASA1, forward,
5′-TACCGGACTCAGATCTCGAGCGCCACCATCGATGGCGGCCGAGGCCGGCAGTG-3′ and
reverse, 5′-GATCCCGGGCCCGCGGTACCGTCCTGACATCATTGGTTTTTGTATACTGG-3′.
The polymerase chain reaction (PCR) product was then inserted into
the expression vector GV230. In accordance with the manufacturer's
instructions, transfection was performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, cells were added to a 6-well plate,
cultured overnight until a ~70% convergence degree was reached, and
then resuspended in serum-free RPMI-1640 (Invitrogen; Thermo Fisher
Scientific, Inc.). Lipofectamine® 3000 was diluted in
serum-free RIPM-1640 and mixed with the diluted miRNA or plasmid
and P3000 (Invitrogen; Thermo Fisher Scientific, Inc.), incubated
at room temperature for 5 min, and then added to the cellular
suspension. Following 4–6 h of incubation, the medium was replaced
with fresh RPMI-1640 containing 10% FBS (Sigma-Aldrich; Merck
KGaA). According to the resistance of the different vectors,
blasticidin (for pPG-miR4530-EGFP and pPG-miR4530sponge-EGFP
plasmids; Sigma-Aldrich; Merck KGaA; 15 µg/ml) and G418 (for GV230;
Gibco; Thermo Fisher Scientific, Inc.; 20 µg/ml) were also used to
screen for stable cell lines.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from HUVECs
according to the manufacturer's instructions. Following this, 1,000
or 2,000 µg total RNA was reverse transcribed into miRNA-cDNA using
All-in-One miRNA First-Strand cDNA Synthesiskit (GeneCopoeia, Inc.,
Rockville, MD, USA) according to the manufacturer's protocol. A
total of 1,000 µg RNA was reverse-transcribed into mRNA-cDNA using
PrimeScriptRT Reagent kit with gDNA Eraser (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer's protocol.
In addition, qPCR was performed using the SYBR-Green PCR kit
(Takara Biotechnology Co., Ltd.) on the StepOne-Plus Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) to
detect miR-4530 and RASA1 mRNA expression. The following primers
were used: miR-4530 forward, 5′-TGCTGTCAACGATACGCTACG-3′ and
reverse, 5′-TGCTGTCAACGATACGCTACG-3′; RASA1 forward,
5′-ACTTGACAGAACGATAGCAGAAG-3′ and reverse,
5′-GCCTCCGATCACTCTCTCTTA-3′. Human U6 RNA was used as an internal
control for the normalization of miRNA expression. The internal
control used for the normalization of mRNA was GAPDH. The primers
used for GAPDH amplification were: GAPDH sense,
5′-GGAGTCCACTGGCGTCTT-3′ and antisense,
5′-ATCTTGAGGCTGTTGTCATAC-3′. The thermocycling conditions were:
95°C for 10 min, 95°C for 10 sec and 55°C for 10 sec, with a final
step of 72°C for 30 sec. Then steps 2–4 were repeated for 40 cycles
followed by a melt curve program for 60 min. The expression levels
were quantified using the 2−∆∆Cq method (16). Primers of U6 were purchased from
GeneCopoeia, Inc. (cat. no. HmiRQP9001; 2 µM).
Dual-luciferase reporter assay
TargetScan was used to predict the potential targets
of miR-4530 (9,17–19).
In TargetScanRelease 6.2 database, the human species was selected
and the miRNA name was entered as ‘has-miR-4530’. The potential
targets were selected. The analysis predicted that RASA1 is a
direct target gene of miR-4530. In order to verify the results of
this analysis, dual-luciferase reporter assays were performed.
Human RASA1 mRNA 3′UTR WT and mutated miR-4530 human RASA1 mRNA
3′UTR sequences were amplified and then inserted into pmiR-reporter
plasmids. The following pmirGLO Dual-Luciferase miRNA target
expression vectors were purchased from Chang Jing Bio-Tech, Ltd.
(Changsha, China): PmirGLO-3′ UTR-WT,
5′-TGATGTGTGAGCTATGCAAACAAAATCCAAGATTCTGCTGGTGAATAACTATGC-3′ and
pmirGLO-3′ UTR-MUT,
5′-TGATGTGTGAGCTATGCAAACAAAATCCAAGATTCTTACGGTGAATAACTATGC-3′.
miRNA-4530 mimics (oligonucleotide sequence,
5′-CCCAGCAGGACGGGAGCG-3′) and NC mimics (random sequence,
5′-CAGUACUUUUGUGUAGUACAA-3′) were also purchased from Chang Jing
Bio-Tech, Ltd. (Changsha, China). miR-4530 mimics simulated the
endogenous and mature body of the miR-4530 sequence, and enhanced
its expression.
The following three vectors were co-transfected into
~1×105 293T cells in 24-well plates using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.): miR-4530 mimics + pmir-GLO-3′UTR wild-type
(Shanghai GenePharma Co., Ltd; 25 ng), miR-4530 mimics +
pmir-GLO-3′UTR-mutant (Shanghai GenePharma Co., Ltd; 25 ng) and
negative control (NC)-mimics + pmir-GLO-WT (25 ng). At 24 h
post-transfection, cells were lysed using a passive lysis buffer in
a dual-luciferase reporter assay kit (Promega Corporation, Madison,
WI, USA). Luciferase signals were subsequently determined using a
Tecan M1000 microplate reader (Thermo Fisher Scientific, Inc.). The
internal control used for normalization was comparison with
Renilla luciferase activity, and the strength of firefly
luciferase activity represented the expression of firefly
luciferase.
Colony formation assay
Then 3 groups [pPG/miR/enhanced green fluorescent
protein (EGFP), pPG-miR4530-EGFP and pPG-miR4530sponge-EGFP] of
cells were digested using pancreatin enzymes, and then 500 cells
were counted from each group and seeded into 6-well plates. Medium
was replaced with fresh RPMI-1640 containing 10% FBS every 2 days.
Following 14–16 days of incubation, cells were washed twice with
PBS and then fixed with 4% paraformaldehyde for 15 min at room
temperature. Following this, cells were stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) for 15
min and washed using high pressure water. The colony formation
assay was performed in triplicate and the results were imaged using
a digital camera.
Cell proliferation assay
Cell growth was determined using Cell Counting Kit-8
(CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
assays. Stable transfected cells were seeded into 96-well plates
(2,000 cells/well) and maintained at 37°C; the medium was replaced
with fresh RPMI-1640 every 2 days. Then, 3 wells were used for each
group and PBS was added to all other empty wells in order to
decrease error. At 24, 48, 72, 96 and 120 h time intervals, the
medium was replaced with 100 µl fresh serum-free RPMI-1640, 10 µl
CCK8 solution was added to each well, and plates were then
incubated at 37°C for 1 h. Following this, all plates were analyzed
at wavelength of 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.). To confirm that the miR-4530 promotes cell
apoptosis, PI3K/AKT inhibitor (LY294002; Cell Signaling Technology,
Danvers, MA, USA) was added to the stable cell lines and the cell
proliferation investigated by CCK8. First, stable transfected cells
were seeded into 96-well plates. After 24 h, the inhibitor was
diluted in concentrations of 5, 10, 20 and 40 µM using 1640 medium.
Then 100 µl was added to the cells. The specific steps of CCK8 are
the same as described above. To confirm that upregulation of
miR-4530 inhibited cell growth, a response experiment was required.
Stable transfected cells were seeded into 6-well plates and after
24 h, ERK/MAPK inhibitor (U126; Merck KGaA) was diluted to the
concentration of 5, 10, 20 or 40 µM using 1640 medium and 2 ml
added to the plates. Cell apoptosis was detected as below. Each
assay was performed in triplicate.
Cell cycle and cell apoptosis
analysis
Stably transfected cells were collected by
pancreatin enzymes and centrifuged at 1,200 × g for 5 min at room
temperature. Cells were washed twice in the process of cell
collection. Cells were fixed in 70% ethanol at 4°C overnight; that
cells did not cluster was crucial to the experiment. Cells were
washed twice using PBS, and the cells were then re-suspended in 160
µl 0.5 mg/ml RNase A (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) and incubated at 37°C for 30 min. Following this, cells were
stained using 50 µmol propidium iodide (Nanjing KeyGen Biotech Co.,
Ltd.) and then analyzed via flow cytometry using a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). The Annexin
V-allophycocyanin (APC)/Propidium Iodide kit (Nanjing KeyGen
Biotech Co., Ltd.) was used to analyze cell apoptosis. Cells were
collected using pancreatin enzymes, washed using PBS and stained
using the Annexin V-APC/Propidium Iodide kit (Nanjing KeyGen
Biotech Co., Ltd.) according to the manufacturer's protocols. The
cells were analyzed using flow cytometry (BD Biosciences). Data on
cell apoptosis were analyzed using FlowJo version 7.6 (FlowJo LLC,
Ashland, OR, USA) and the data of cell cycle was analyzed by ModFit
LT 3.1 (Verity Software House, Inc., Topsham, ME, USA).
Western blotting
Total protein was extracted using
radioimmunoprecipitation buffer (P0013D; Beyotime Institute of
Biotechnology, Haimen, China) containing 1% phenylmethane sulfonyl
fluoride. Lysates were transferred to clean Eppendorf tubes and
then centrifuged at 12,000 × g for 10 min at 4°C to remove cellular
debris. The total protein concentration was determined using the
Bradford assay (Beyotime Institute of Biotechnology) in accordance
with the manufacturer's instructions. Electrophoresis was used to
separate 30 µg protein samples using a 10% SDS-PAGE gel. Proteins
were then transferred to polyvinylidene fluoride membranes (Merck
KGaA). Proteins were then blocked with milk (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) for 2–3 h at room temperature to prevent
non-specific binding. Membranes were then incubated overnight at
4°C with primary antibodies against RASA1 (1:2,000; cat. no.
ab40677; Abcam, Cambridge, UK), phospho-Ser473 AKT serine threonine
kinase (p-AKT; 1:2,000; cat. no. 4060; Cell Signaling Technology,
Inc.), p-Ser308 AKT (1:1,000; cat. no. 2965; Cell Signaling
Technology, Inc.), B-cell lymphoma-2 (BCL-2; 1:2,000; cat. no.
ab182858; Abcam), BCL-2-like protein 4 (BAX; 1:1,000; cat. no.
ab32503; Abcam), AKT (1:1,000; cat. no. 9272; Cell Signaling
Technology, Inc.), GAPDH (1:6,000; cat. no. A0208; Beyotime
Institute of Biotechnology) and phosphorylated-extracellular
signal-regulated kinase (p-ERK; 1:2,000; cat. no. 9910; Cell
Signaling Technology, Inc.) Following this, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:10,000 in TBST; cat. no. A0208; Beyotime Institute of
Biotechnology) for 1 h at room temperature, and then washed with
Tris-buffered saline with 0.05% (v/v) Tween-20 for 1 h. Band
signals were visualized using an enhanced chemiluminescent kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. ImageJ version 1.44 software (National
Institutes of Health, Bethesda, MD, USA) was used to analyze the
gray level of every band.
Statistical analysis
Comparative analysis between groups was determined
by use of either the two-tailed Student's t-test or one way
analysis of variance followed by the Bonferroni test. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were conducted in triplicate, and data are expressed as
mean ± standard deviation.
Results
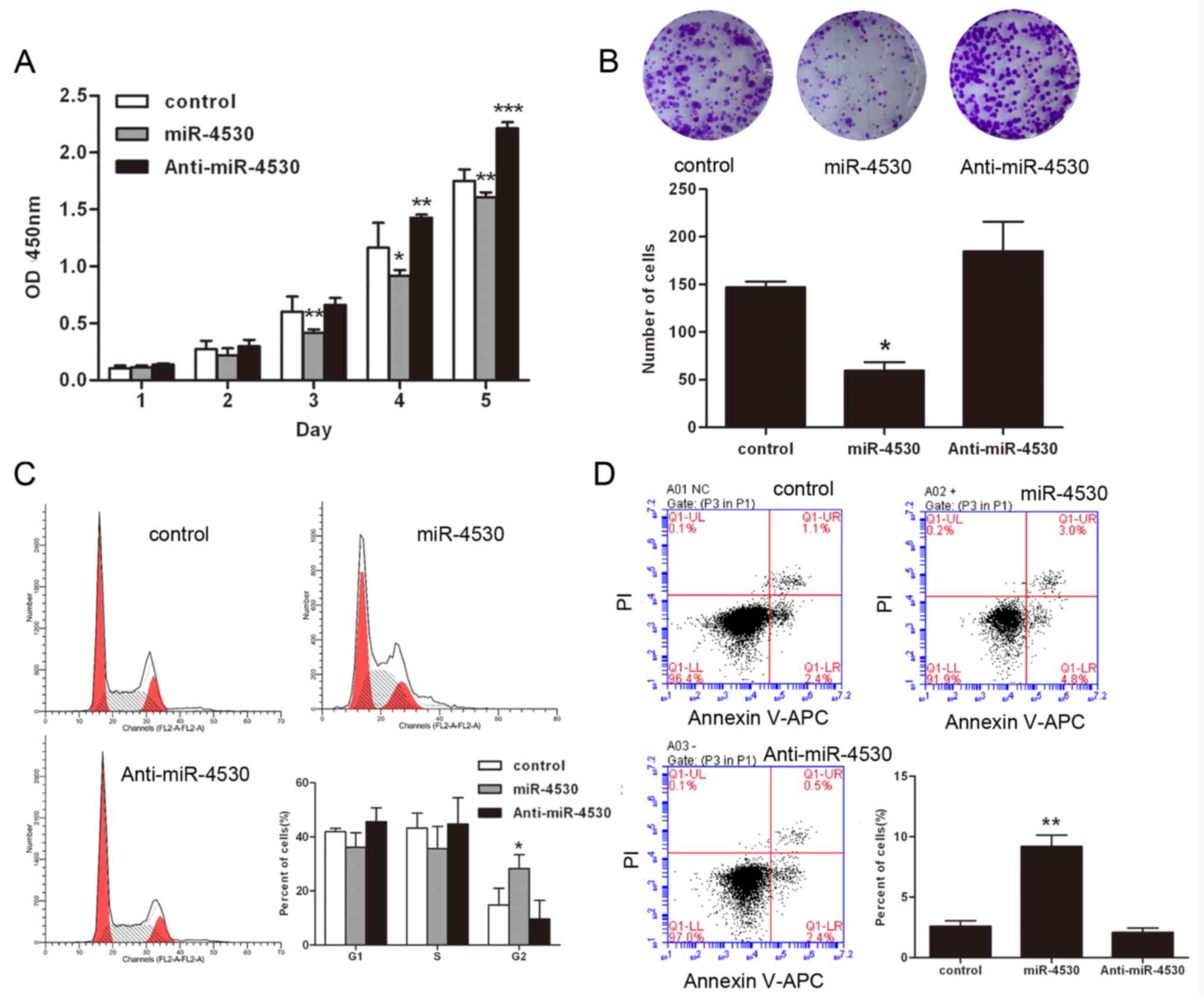
Overexpression of miR-4530 results in
suppression of cell proliferation and enhancement of apoptosis,
resulting in G2/M arrest in HUVECs
To investigate the role of miR-4530 in HUVECs, the
cell proliferation of stable transfected HUVECs was investigated
using CCK8 assays and colony formation assays. The results of the
CCK8 assays suggested that the proliferation rate of HUVECs was
significantly suppressed following overexpression of miR-4530
(Fig. 1A). Furthermore, colony
formation was significantly suppressed following overexpression of
miR-4530, and markedly enhanced following knockdown of miR-4530
(Fig. 1B). In order to investigate
the effects of miR-4530 on HUVECs, the cell cycle progression of
miR-4530 transfected cells was investigated. The results suggested
that overexpression of miR-4530 results in G2/M cell cycle arrest.
Furthermore, the results demonstrated that 28.26% of cells in the
overexpression miR-4530 group were in the G2/M phase, 14.80% cells
in the control group were in the G2/M phase and 9.64% cells of the
miR-4530 knockdown group were in the G2/M phase (Fig. 1C). Previous studies have suggested
that cells arrest in the G2/M phase and then undergo apoptosis
following treatment with miR-4530 (20). Thus, the results of the present
study suggest that overexpression of miR-4530 suppresses the
proliferation of HUVECs by blocking the G2/M stage transition.
Furthermore, cell apoptosis was also investigated using flow
cytometry. Stable transfected HUVECs were stained using the Annexin
V-APC/Propidium Iodide Kit. The results suggested that miR-4530
significantly enhances apoptosis in HUVECs cells compared with the
negative control (Fig. 1D).
However, anti-miR-4530 did not affect apoptosis in HUVEC and it was
hypothesized that the expression of miR-4530 was low in HUVEC. When
the expression of miR-4530 was decreased its effects on downstream
proteins were possibly less obvious and this is confirmed by the
results of subsequent experiments. In general, these results
suggest that miR-4530 is involved in apoptotic pathways.
RASA1 is a direct target gene of
miR-4530 in HUVECs
miRNA database (TargetScan) were analyzed to
identify potential targets and binding sites of miR-4530. Analysis
using TargetScan suggested that RASA1 is a target of miR-4530, with
a high degree of complementarity. The potential binding site is
located at bases 148–152 within the RASA1 3′UTR (Fig. 2A). A dual-luciferase reporter assay
was performed to further investigate the association between
miR-4530 and RASA1 in 293T cells. The sequences of Firefly
luciferase were followed by either the 3′UTR-WT or 3′UTR-MUT of
RASA1 in pmirGLO-3′UTR-WT or pmirGLO-3′UTR-MUT. Renilla
luciferase signal was used as an internal control, and the activity
of Firefly luciferase decreased following binding of miR-4530 to
the RASA1 3′UTR. The results of the dual-luciferase reporter assay
demonstrated that the relative luciferase activity of the WT group
was significantly enhanced compared with the other two groups, thus
suggesting that miR-4530 targets the 3′UTR of RASA1 and promotes
its expression. However, following mutation of the binding locus,
there was no significant change exhibited in the RASA1-MUT compared
with the control. The mutation of three bases within the RASA1
3′UTR was considered to sufficiently inhibit binding with miR-4530,
as there was no significant difference between the RASA1-MUT group
and the control group regarding relative luciferase activity, and
miRNAs regulate their target gene by binding to few genes of 3′UTR.
Therefore, the results suggested that residual binding does not
occur between miR-4530 and RASA1-MUT. In conclusion, the results
suggest that miR-4530 enhances the Firefly luciferase signal, and
this effect is reversible following mutation of the miR-4530
binding site in the RASA1 3′UTR (Fig.
2B). To further investigate the increase of RASA1 expression in
HUVECs following treatment with miR-4530, stable transfected HUVEC
cell lines were constructed. Each cell line contained a stable
transfected miR-4530 precursor group, an anti-miR-4530 group and a
negative control group. Each group was confirmed by determining the
expression levels of miR-4530. It was demonstrated that the
expression level of miR-4530 was enhanced following transfection
with the miR-4530 precursor group compared with the negative
control group. In addition, the expression level of miR-4530 in the
anti-miR-4530 group was suppressed compared with the negative
control group (Fig. 2C).
Furthermore, RT-qPCR and western blot analysis suggested that RASA1
mRNA and protein expression were significantly increased following
miR-4530 overexpression in HUVECs (Fig. 2D and E). TargetScan analysis
suggested that miR-4530 has numerous target genes. Whether miR-4530
targets specific genes that negatively regulate RASA1, or whether
miR-4530 regulates the expression of RASA1 via alternative
mechanisms that promote the expression of RASA1, has not yet been
determined.
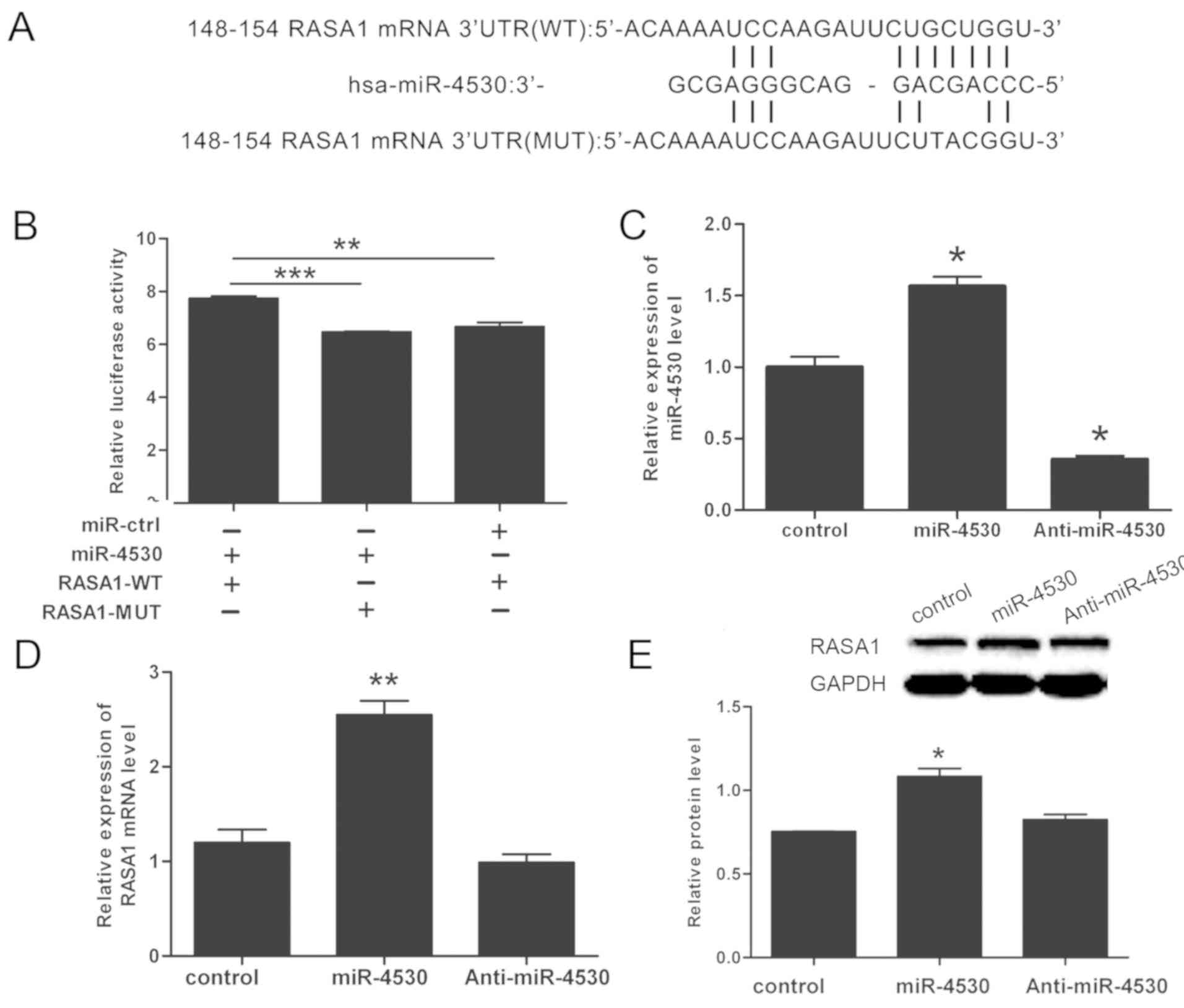 | Figure 2.miR-4530 upregulates RASA1 expression
in HUVECs. (A) Target sequence of miR-4530 in WT RASA1 3′UTR, and
the sequence of MUT 3′UTR of RASA1. (B) miR-4530 mimics enhanced
wild-type, but not mutant, RASA1 reporter activity in 293T cells.
(C) The expression of miR-4530 was increased by transfection with
miR-4530 precursor group compared with the negative control, and
transfection with anti-miR-4530 did not exhibit a significant
difference in the relative expression of miR-4530 compared with the
negative control. (D) Expression of RASA1 mRNA was increased
following overexpression of miR-4530. (E) miR-4530 upregulates
endogenous RASA1 protein expression HUVECs, and densitometric
analysis revealed that the difference in RASA1 expression between
the miR-4530 overexpression group and the negative control group
was statistically significant. *P<0.05 vs. control; **P<0.01
vs. control; ***P<0.001 vs. control. HUVEC, human umbilical vein
endothelial cell; RASA1, Ras p21 protein activator 1; 3′UTR, 3′
untranslated region; WT, wild-type; MUT, mutant; miR, microRNA. |
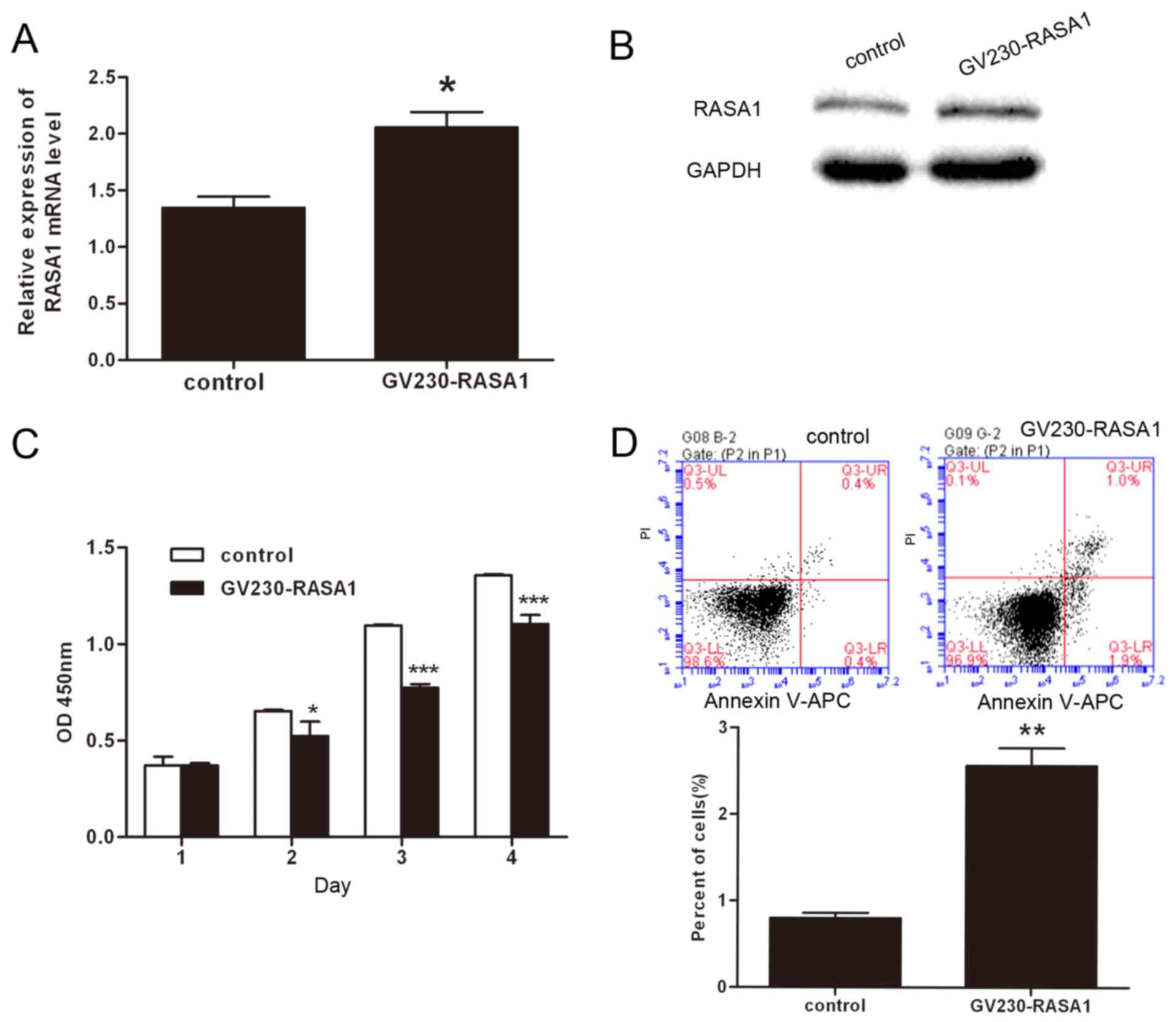
miR-4530-regulated RASA1 is involved
in the proliferation and apoptosis of HUVECs
In previous studies, numerous miRNAs have been
demonstrated to regulate cell proliferation, apoptosis, migration
and angiogenesis via association with RASA1 (12–14).
To investigate whether miR-4530-regulated RASA1 is involved in cell
proliferation and apoptosis, a RASA1 overexpression plasmid and a
negative control plasmid were transfected into HUVECs. The
expression of RASA1 mRNA and protein were significantly enhanced in
HUVECs transfected with the RASA1 overexpression plasmid (Fig. 3A and B). Furthermore, CCK8 assays
were performed to investigate cell growth. The results demonstrated
that cell proliferation was significantly suppressed following
overexpression of RASA1 compared with the negative control, which
is consistent with results of the miR-4530 overexpression analyses
(Fig. 3C). In addition, the
results of the Annexin V-FITC assay revealed that apoptosis was
significantly enhanced in HUVECs following upregulation of RASA1
(Fig. 3D). Therefore, the results
suggest that suppression of cell proliferation and enhancement of
apoptosis following miR-4530 overexpression are dependent upon the
association between miR-4530 and RASA1.
Phosphoinositide 3-kinase (PI3K)/AKT
signal transduction pathway is involved in cell apoptosis induced
by miR-4530 overexpression
To elucidate the signaling pathways involved in
miR-4530-associated regulation of cell growth in HUVECs, the
PI3K/AKT pathway, an anti-apoptosis signaling pathway, was
investigated. The level of p-Ser473 AKT and p-Thr308 AKT were
suppressed in the miR-4530 overexpression group compared with the
negative control. However, the levels of p-Ser473 AKT and p-Thr308
AKT in HUVECs did not exhibit a marked change following
transfection with the miR-4530 inhibitor compared with the negative
control (Fig. 4A). In addition,
the expression of BAX was significantly enhanced and the expression
of BCL-2 was significantly suppressed in HUVECs overexpressing
miR-4530 compared with the negative control (Fig. 4B). p-Ser473 AKT, p-Thr308 AKT, BAX
and BCL-2 are all downstream effectors of the PI3K/AKT signaling
pathway associated with cell apoptosis. These results suggest that
the PI3K/AKT signal transduction pathway is involved in cell
apoptosis resulting from miR-4530 overexpression; however, there
were no marked differences in the expression of proteins associated
with the PI3K/AKT pathway following the downregulation of miR-4530.
In order to further investigate the conclusion, stable transfected
cells were treated with a PI3K/AKT inhibitor at different
concentrations. Following treatment with LY294002, the percentage
of apoptotic cells increased in a dose-dependent manner, and the
difference between the miR-4530 overexpression group and control
group was decreased in a dose-dependent manner (Fig. 4C). In conclusion, the results
suggest that miR-4530 enhances cell apoptosis via the PI3K/AKT
pathway.
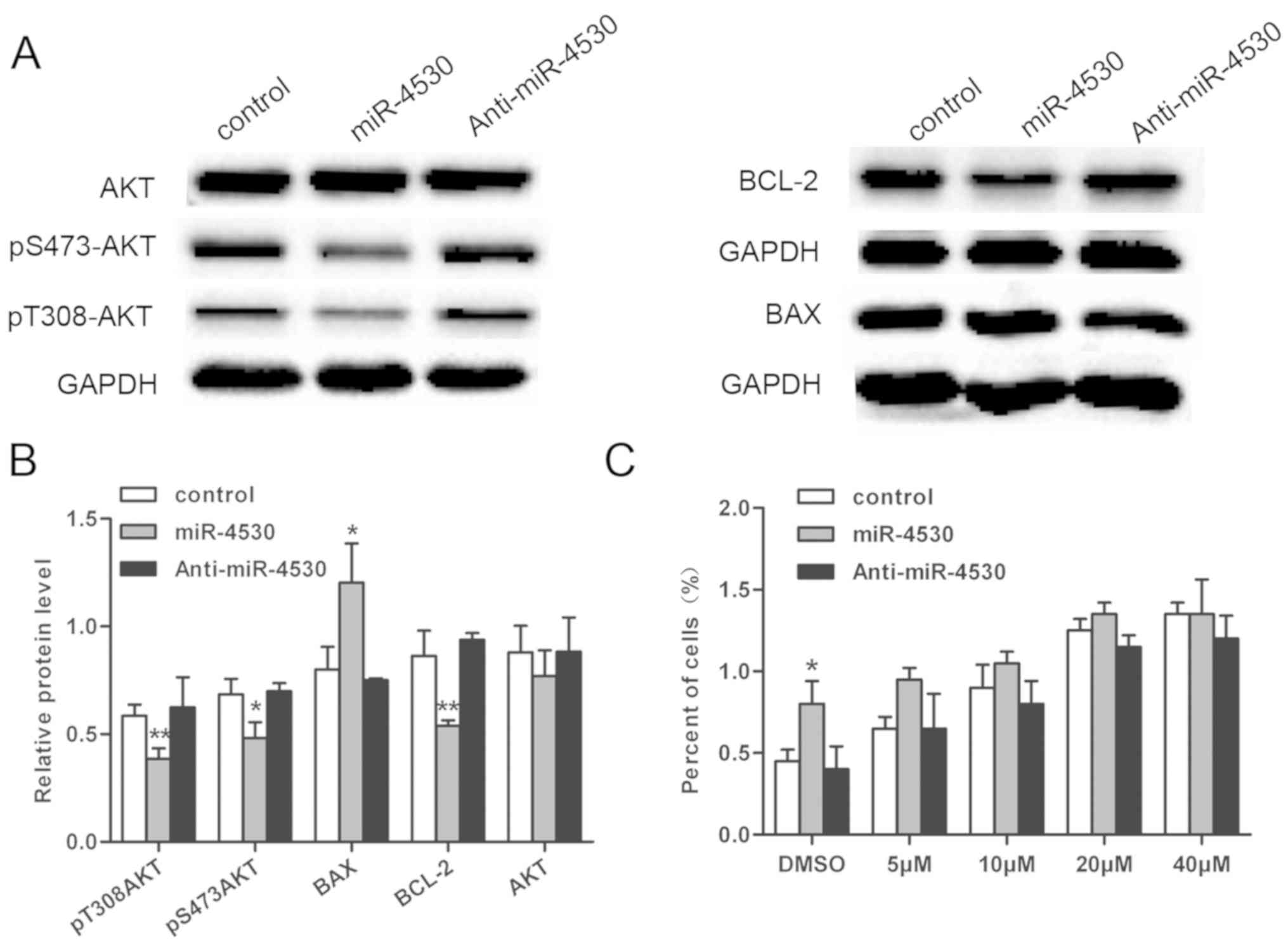 | Figure 4.Expression levels of AKT, p-Thr308
AKT, p-Ser473 AKT, BAX and BCL-2 were investigated. (A) The
expression levels of p-Thr308 AKT, p-Ser473 AKT, BCL-2 and AKT were
suppressed following miR-4530 overexpression compared with the
negative control; whereas expression of BAX was significantly
enhanced following miR-4530 overexpression compared with the
negative control. (B) Densitometric analysis of p-Thr308 AKT,
p-S473AKT, BAX and BCL-2. (C) Following addition of the PI3K/AKT
inhibitor, apoptosis was enhanced in a dose-dependent manner, and
the difference between the miR-4530 overexpression and control
groups was reduced in a dose-dependent manner. *P<0.05 vs.
control; **P<0.01 vs. control. miR, microRNA; AKT, AKT
serine/threonine kinase; p, phospho; BCL-2, B-cell lymphoma-2; BAX,
BCL-2-like protein 4. |
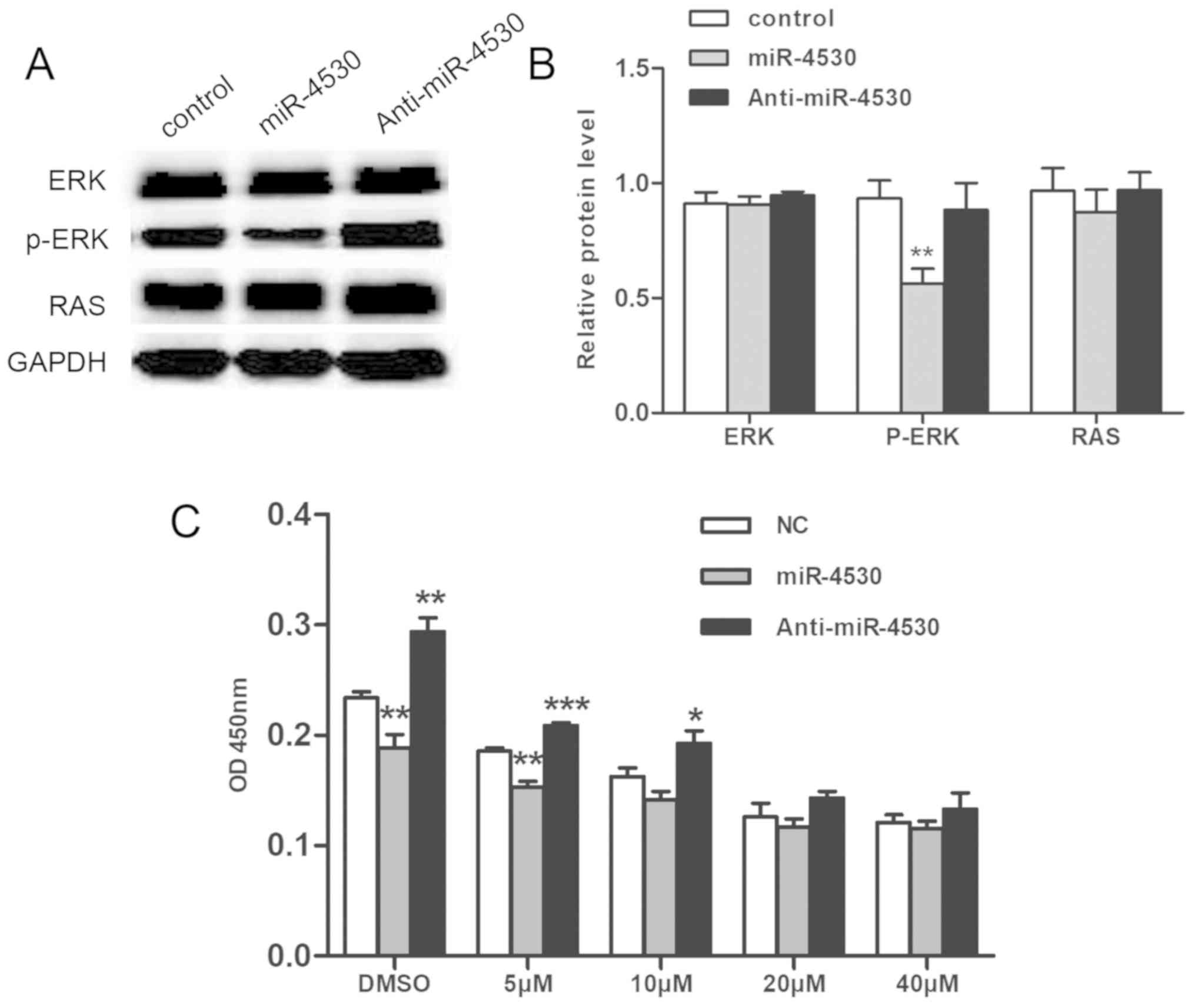
Suppression of cell proliferation via
miR-4530 overexpression is associated with the
ERK/mitogen-activated protein kinase (MAPK) pathway
To determine the pathway associated with the
suppression of cell proliferation by miR-4530 in HUVECs, the
ERK/MAPK pathway was investigated. The ERK/MAPK signaling pathway
regulates numerous important cellular progresses (21). Upregulation of the ERK/MAPK pathway
has previously been revealed to enhance cell proliferation
(22). In the present study, the
level of p-ERK was suppressed following overexpression of miR-4530
compared with the negative control, however, the expression of RAS
did not exhibit a marked difference following miR-4530
overexpression (Fig. 5A).
Densitometric analysis of western blot analyses demonstrated that
the difference in the level of p-ERK between the miR-4530
overexpression group compared with the negative control was
statistically significant (Fig.
5B). Furthermore, an ERK/MAPK inhibitor (U126) was added to
stable transfected cells. The growth of HUVECs was revealed to be
suppressed following treatment with U126 in a dose-dependent
manner, and the difference between the miR-4530 overexpression
group and the control group decreased in a dose-dependent manner
(Fig. 5C). Thus, the results
suggest that suppression of HUVEC cell proliferation following
miR-4530 overexpression is associated with the ERK pathway,
however, there were no marked differences associated with the
MAPK/ERK pathway following the downregulation of miR-4530.
Discussion
In our previous study, it was demonstrated that
miR-4530 is upregulated in the serum of patients with diabetic
retinopathy (10). Diabetic
retinopathy is a prevalent eye complication resulting from
diabetes. According to a number of previous studies, it has been
demonstrated that the occurrence and progression of diabetic
retinopathy is associated with angiogenesis and apoptosis in
numerous cell types, including capillary endothelial cells,
peripheral cells and nerve cells (23–26).
It has previously been reported that overexpression of miR-4530
enhances angiogenesis in endothelial cells (27) and that the enhanced proliferation
and suppression of apoptosis exacerbates angiogenesis (28). However, the results of the present
study revealed that overexpression of miR-4530 enhances apoptosis
and suppresses cell growth in HUVECs. According to the results of
the present study, miRNA-4530 may have an important role in the
pathogenesis of diabetic retinopathy due to enhancement of
apoptosis. Further investigation into the mechanisms underlying the
association between miR-4530 and the suppression and enhancement of
cell proliferation and apoptosis, respectively, may identify novel
therapeutic approaches for the treatment of diabetic
retinopathy.
TargetScan analysis and the results of the
dual-luciferase reporter assay revealed that RASA1 is a direct
target gene of miR-4530 in HUVECs. RASA1 regulates numerous cell
functions, including migration, growth, apoptosis and angiogenesis,
via the Ras signaling pathway (29–31).
The results of the present study revealed that RASA1 has an
important role in the regulation of cell proliferation and
apoptosis. RT-qPCR was used to determine the expression level of
miR-4530 and RASA1 mRNA following transfection with pmiR-reporter
plasmids. It has previously been demonstrated that miRNAs can
suppress the expression of target genes by targeting associated
3′UTR sequences (32). However, in
the present study, the expression levels of RASA1 mRNA and protein
were enhanced following overexpression of miR-4530. An miRNA may
have numerous target genes, and a target gene can be regulated by
numerous miRNAs. miR-4530 regulates a number of proteins upstream
of RASA1, which subsequently negatively regulate the expression of
RASA1. Thus, miR-4530 enhances the expression of RASA1, however,
the exact molecular mechanism underlying this process remains
unclear. Previous studies have demonstrated that overexpression of
miRNA enhances the expression of target proteins in smooth muscle
cells (33). A further study
revealed that miRNAs can transform their inhibitory roles to
enhancer roles; for example, miR-369-3 enhances the translation of
its target gene. miRNAs that contain AU rich elements and conserved
sequence targets predominantly upregulate the translation of target
proteins, particularly in a period of cell cycle arrest following
cell processing (32). Following
overexpression of miR-4530, G2/M arrest was determined by flow
cytometry analysis. To determine the mechanism underlying the
regulatory functions of miR-4530, ERK/MAPK and PI3K/AKT signaling
pathways were investigated.
The activation of the PI3K/AKT pathway can suppress
cell apoptosis via the inactivation of BAX (34) and caspase-9 (35). In the present study, the levels of
p-S473 AKT and p-T308 AKT were suppressed following miR-4530
overexpression, and the expression of total AKT did not exhibit a
significant difference following miR-4530 overexpression.
Furthermore, it was revealed that the level of associated proteins
following downregulation of miR-4530 did not exhibit a significant
change compared with the negative control. The concentration of
exogenous miR-4530 has been demonstrated to be low, however, other
types of cells in vivo can also secrete miR-4530, which
subsequently acts on endothelial cells (10). Furthermore, it has previously been
demonstrated that cells can secrete miRNAs, which then target
recipient cells, and that exogenous miRNAs can subsequently
regulate target gene expression and recipient cell function
(36). BAX and BCL-2 are
downstream effectors of the PI3K/AKT pathway (34). In the present study, following the
upregulation of miR-4530 expression in HUVECs, the results
demonstrated that the expression of BAX was enhanced and the
expression of BCL-2 was suppressed. Therefore, it can be suggested
that the PI3K/AKT pathway is inactivated following upregulation of
miR-4530, thus resulting in an increase of apoptosis compared with
the negative control. Following the addition of a PI3K/AKT
inhibitor into stable transfected cells, flow cytometry analysis
revealed that the percentage of apoptotic cells increased in a
dose-dependent manner, and the difference between the percentage of
apoptotic cells of the miR-4530 overexpression and control groups
reduced in a dose-dependent manner. Thus, PI3K/AKT is an important
pathway associated with miR-4530 regulation of cell apoptosis.
Furthermore, it can be suggested that miR-4530 targets RASA1, RASA1
subsequently acts on the Ras pathway, and then the Ras pathway
targets the PI3K/AKT pathway in order to regulate cell apoptosis.
Considering this, our future studies will further investigate RASA1
and its association with miR-4530 and the PI3K/AKT pathway. Such
future studies will consist of a RASA1 knockdown assay, and an
investigation into the effects the downregulation of RASA1 on cell
proliferation and protein expression associated with the PI3K/AKT
pathway.
The ERK/MAPK pathway primarily functions as a linear
pathway responsible for the transmission of signals from cell
surface receptors to ERK/MAPK, which then transduce signals to
downstream effectors (37). The
ERK/MAPK pathway has been evolutionary conserved and regulates
numerous important cellular progresses, including cell growth,
survival, differentiation and motility (37). Furthermore, previous studies have
demonstrated that numerous proteins, including insulin-like growth
factor I and metastasis associated lung adenocarcinoma transcript
1, regulate cell proliferation via the ERK/MAPK signaling pathway
(38–40). Considering the association between
the ERK/MAPK pathway and cell proliferation, the key proteins of
the ERK/MAPK pathway were investigated in the present study. The
level of p-ERK1/2 was revealed to be suppressed following miR-4530
overexpression miR-4530 compared with the negative control, and
thus the results suggest that the activity of the ERK/MAPK pathway
is suppressed following miR-4530 upregulation. Furthermore, an ERK
inhibitor was added to the stable transfected cells. The results
suggested that the growth of HUVECs was suppressed in a
dose-dependent manner, and that the difference between the growth
of HUVECs in the miR-4530 overexpression and control groups
decreased in a dose-dependent manner following treatment with the
inhibitor. In conclusion, miR-4530 was revealed to regulate cell
growth via regulation of the ERK/MAPK pathway. The results
indicated that increased RASA1 inactivated Ras, which inhibited
ERK/MAPK signaling, resulting in reduced cell proliferation.
Studies have demonstrated that miR-4530 has an
important role as a molecular marker for the diagnosis of numerous
diseases. One study revealed that the expression of miR-4530 was
suppressed in patients with pancreatic and biliary-tract cancers
compared with healthy subjects, and that miR-4530 represents one of
eight significant diagnostic markers able to identify patients with
pancreatic and biliary-tract cancers (41). In addition to this, a further study
demonstrated that the expression of miR-4530 was upregulated in the
serum of patients with type 2 diabetes compared with healthy
subjects, and that the differential expression of miR-4530 may aid
future studies investigating type 2 diabetes (10). Furthermore, miRNA microarray
analysis has revealed that miR-4530 is also upregulated in
enterovirus 71-infected cells. Further investigation into the
prediction of miR-4530 target genes may aid future studies
investigating the regulatory mechanism of miRNAs underlying the
pathogenesis of enterovirus 71 infected cells (42). In conclusion, miR-4530 can be
considered to be an important marker for diagnosis and further
mechanistic studies should be conducted.
In conclusion, the function of miR-4530 in the
enhancement of apoptosis and the suppression of cell growth in
HUVECs suggests that miR-4530 possess value in the treatment of
cancer. Furthermore, the results of the present study provide a
foundation for future clinical drug research. In conclusion,
miR-4530 possesses a significant role in cellular functions and may
be applicable for use in targeted drug delivery for the treatment
of tumors.
Acknowledgements
Not applicable.
Funding
This project was supported by the Program for
Zhejiang Leading Team of S&T Innovation (grant no.
2012R10048-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and JL conceived and designed the experiments,
which were performed by LJ and HL. LJ and TZ analyzed the data and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lynam-Lennon N, Maher SG and Reynolds JV:
The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos
Soc. 84:55–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vandenboom Ii TG, Li Y, Philip PA and
Sarkar FH: MicroRNA and cancer: Tiny molecules with major
implications. Curr Genomics. 9:97–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rachagani S, Kumar S and Batra SK:
MicroRNA in pancreatic cancer: Pathological, diagnostic and
therapeutic implications. Cancer Lett. 292:8–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Findlay VJ: MicroRNAs and breast cancer.
Open Cancer J. 3:55–61. 2010.
|
|
6
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan H, Dai Z, Ma Y, Wang Z, Liu X and
Wang X: MicroRNA-101 inhibits cell proliferation and induces
apoptosis by targeting EYA1 in breast cancer. Int J Mol Med.
37:1643–1651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao Y, Tian Q, He J, Huang M, Yang C and
Gong L: MiR-503 inhibits hepatocellular carcinoma cell growth via
inhibition of insulin-like growth factor 1 receptor. Onco Targets
Ther. 9:3535–3544. 2016.PubMed/NCBI
|
|
9
|
Zhu X, Wu L, Yao J, Jiang H, Wang Q, Yang
Z and Wu F: MicroRNA let-7c inhibits cell proliferation and induces
cell cycle arrest by targeting CDC25A in human hepatocellular
carcinoma. PLoS One. 10:e01242662015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding L, Ai D, Wu R, Zhang T, Jing L, Lu J
and Zhong L: Identification of the differential expression of serum
microRNA in type 2 diabetes. Biosci Biotechnol Biochem. 80:461–465.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lapinski PE, Doosti A, Salato V, North P,
Burrows PE and King PD: Somatic second hit mutation of, RASA1, in
vascular endothelial cells in capillary malformation-arteriovenous
malformation. Euro J Med Genetics. 61:11–16. 2018. View Article : Google Scholar
|
|
12
|
Du C, Weng X, Hu W, Lv Z, Xiao H, Ding C,
Gyabaah OA, Xie H, Zhou L, Wu J and Zheng S: Hypoxia-inducible
MiR-182 promotes angiogenesis by targeting RASA1 in hepatocellular
carcinoma. J Exp Clin Cancer Res. 34:672015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J,
Zhang CY, Chen J and Zhang J: MicroRNA-31 activates the RAS pathway
and functions as an oncogenic MicroRNA in human colorectal cancer
by repressing RAS p21 GTPase activating protein 1 (RASA1). J Biol
Chem. 288:9508–9518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Wang C, Long S, Ma Y, Guo Y, Huang
Z, Chen X, Zhang C, Chen J and Zhang J: C/EBP-β-activated
microRNA-223 promotes tumour growth through targeting RASA1 in
human colorectal cancer. Br J Cancer. 112:1491–1500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung H, Kanchi KL, Wang X, Hill KS,
Messina JL, Lee JH, Kim Y, Dees ND, Ding L, Teer JK, et al:
Inactivation of RASA1 promotes melanoma tumorigenesis via R-Ras
activation. Oncotarget. 7:23885–23896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sui Y, Zheng X and Zhao D: Rab31 promoted
hepatocellular carcinoma (HCC) progression via inhibition of cell
apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol.
36:8661–8670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teodoro JG, Heilman DW, Parker AE and
Green MR: The viral protein Apoptin associates with the
anaphase-promoting complex to induce G2/M arrest and apoptosis in
the absence of p53. Genes Dev. 18:1952–1957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mochizuki N, Ohba Y, Kiyokawa E, Kurata T,
Murakami T, Ozaki T, Kitabatake A, Nagashima K and Matsuda M:
Activation of the ERK/MAPK pathway by an isoform of rap1GAP
associated with G alpha(i). Nature. 400:891–894. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guangda L, Jianbo W and Yingjun N:
Research on the relationship between apoptosis and diabetic
retinopathy. J Clin Ophthalmol. 13:381–383. 2005.
|
|
24
|
Funatsu H, Hori S, Ohashi Y and Ishigaki
T: Risk factors for occurrence and progression of diabetic
retinopathy. Nippon Ganka Gakkai Zasshi. 97:939–946. 1993.(In
Japanese). PubMed/NCBI
|
|
25
|
Hauser D, Katz H, Pokroy R, Bukelman A,
Shechtman E and Pollack A: Occurrence and progression of diabetic
retinopathy after phacoemulsification cataract surgery. J Cataract
Refract Surg. 30:428–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raman R, Ganesan S, Pal SS, Gella L,
Kulothungan V and Sharma T: Incidence and progression of diabetic
retinopathy in urban India: Sankara Nethralaya-Diabetic Retinopathy
Epidemiology and Molecular Genetics Study (SN-DREAMS II), Report 1.
Ophthal Epidemiol. 24:294–302. 2017. View Article : Google Scholar
|
|
27
|
Zhang T, Jing L, Li H, Ding L, Ai D, Lyu J
and Zhong L: MicroRNA-4530 promotes angiogenesis by targeting VASH1
in breast carcinoma cells. Oncol Lett. 14:111–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2015. View Article : Google Scholar
|
|
29
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: MiR-21/RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Li D, Wikstrom JD, Pivarcsi A,
Sonkoly E, Ståhle M and Landén NX: MicroRNA-132 promotes fibroblast
migration via regulating RAS p21 protein activator 1 in skin wound
healing. Sci Rep. 7:77972017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma SB, Lin CC, Farrugia MK, McLaughlin
SL, Ellis EJ, Brundage KM, Salkeni MA and Ruppert JM: MicroRNAs 206
and 21 cooperate to promote RAS-extracellular signal-regulated
kinase signaling by suppressing the translation of RASA1 and
SPRED1. Mol Cell Biol. 34:4143–4164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: MicroRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
34
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shultz JC, Goehe RW, Wijesinghe DS,
Murudkar C, Hawkins AJ, Shay JW, Minna JD and Chalfant CE:
Alternative splicing of caspase 9 is modulated by the
phosphoinositide 3-kinase/Akt pathway via phosphorylation of
SRp30a. Cancer Res. 70:9185–9196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Liu D, Chen X, Li J, Li L, Bian
Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150
enhances targeted endothelial cell migration. Mol Cell. 39:133–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolch W: Coordinating ERK/MAPK signalling
through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 6:827–837.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi YS, Cho HY, Hoyt KR, Naegele JR and
Obrietan K: IGF-1 receptor-mediated ERK/MAPK signaling couples
status epilepticus to progenitor cell proliferation in the
subgranular layer of the dentate gyrus. Glia. 56:791–800. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jaiswal K, Lopez-Guzman C, Souza RF,
Spechler SJ and Sarosi GA Jr: Bile salt exposure increases
proliferation through p38 and ERK MAPK pathways in a non-neoplastic
Barrett's cell line. Am J Physiol Gastrointest Liver Physiol.
290:G335–G342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu XS, Walbladng XA, Wu WG, Hu YP, Li ML,
Ding Q, Weng H, Shu YJ, Liu TY, Jiang L, et al: MALAT1 promotes the
proliferation and metastasis of gallbladder cancer cells by
activating the ERK/MAPK pathway. Cancer Biol Ther. 15:806–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kojima M, Sudo H, Kawauchi J, Takizawa S,
Kondou S, Nobumasa H and Ochiai A: MicroRNA markers for the
diagnosis of pancreatic and biliary-tract cancers. PLoS One.
10:e01182202015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xun M, Ma CF, Du QL, Ji YH and Xu JR:
Differential expression of miRNAs in enterovirus 71-infected cells.
Virol J. 12:562015. View Article : Google Scholar : PubMed/NCBI
|