Introduction
Ovarian carcinoma (OC) is a type of malignancy that
originates in the ovaries, and annually affects >200,000
individuals and leads to >140,000 cases of OC-associated
mortality in females worldwide (1). Screening is widely used in the early
diagnosis of OC; however, at present, the majority of screening
methods have been demonstrated to be ineffective (2,3). The
survival of patients with OC markedly improved following treatment
with chemotherapy, olaparib maintenance therapy and radiation
therapy (4,5); however, no additional improvements in
patient survival were observed in subsequent decades (6). There is a lack of clear symptoms
during the early stages of OC; therefore, the majority of patients
with OC are diagnosed at advanced stages with metastasis, leading
to high mortality rates (7). At
present, early diagnosis and treatment remains critical for the
survival of patients with OC.
The Wnt/β-catenin pathway serves an important role
in the onset, development and progression of numerous types of
tumors, including OC (8).
Activation of Wnt/β-catenin signaling in epithelial ovarian cancer
regulates the expression of genes involved in cell apoptosis and
proliferation, thereby promoting the induction and progression of
cancer (8). Long noncoding RNAs
(lncRNAs) are a subgroup of noncoding RNAs comprising >200
nucleotides, a number of which are involved in the pathogenesis of
various types of malignancies via interactions with the
Wnt/β-catenin pathway (9,10). The lncRNA HOXA transcript at the
distal tip activates the Wnt/β-catenin pathway in osteosarcoma to
increase the chemoresistance of cancer cells (9). In non-small cell lung cancer, the
lncRNA small nucleolar RNA host gene (SNHG1) promotes the
progression of cancer via activation of the Wnt/β-catenin signaling
pathway (10). Associated with
poor prognosis of hepatocellular carcinoma (AWPPH) is a novel
lncRNA that serves an oncogenic role in hepatocellular carcinoma
(11) and bladder cancer (12). In the present study, the role of
AWPPH in OC was investigated and it was observed that the lncRNA
was upregulated in OC; AWPPH may serve to promote OC via activation
of the Wnt/β-catenin signaling pathway.
Materials and methods
Specimens
The present study was a retrospective analysis.
Tumor and adjacent healthy tissues within 2 cm of the tumor were
collected from 58 patients with OC. Blood was extracted from the 58
patients and stored at room temperature for 2 h, followed by
centrifugation at 1,000 × g for 20 min at 4°C to collect
supernatant for serum analysis. Patients were treated at Yantai
Yeda Hospital (Yantai, China) from June 2011 to June 2012. The age
of patients ranged from 30–69 years, with a mean age of 49.4±6.3
years. Inclusion criteria for the enrolment of patients were as
follows: i) Patients were pathologically diagnosed with OC; ii)
patients were initially diagnosed and treated at Yantai Yeda
Hospital; iii) clinical data was collected from patients; and iv)
patients completed follow-up care. Exclusion criteria were as
follows: i) Patients possessed a history of other malignancies; ii)
patients exhibited additional types of ovarian diseases; iii)
patients were treated prior to admission; and iv) patients
succumbed to mortality due to separate diseases during follow-up.
All patients possessed epithelial tumors. According to the American
Joint Committee on Cancer staging system (13), there were 6 cases in stage II, 8 in
stage III and 44 in stage IV.
Additionally, serum samples were obtained from 46
healthy individuals that received routine physiological
examinations at Yantai Yeda Hospital during the aforementioned time
period to serve as the control group. Controls were enrolled to
match the distributions of age and gender of cancer patients. The
age of healthy controls ranged from 33 to 69 years, with a mean age
of 49.9±6.1 years. No significant differences in age were
identified between the two groups. All patients and healthy
controls signed informed consent forms, and the study was approved
by the Ethics Committee of Yantai Yeda Hospital.
Cell culture and transfection
A total of two human OC cell lines, UWB1.289
(CRL-2945™) and UWB1.289 + BRCA1 (CRL-2946™), were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Cells were cultured with 50% ATCC-formulated RPMI-1640 medium and
50% Mammary Epithelial Cell Growth medium (ATCC) supplemented with
3% fetal bovine serum (ATCC) in an incubator (37°C, 5%
CO2).
Full-length AWPPH cDNA (Accession: NR_015395.2,
Sangon Biotech Co., Ltd., Shanghai, China) was obtained via
polymerase chain reaction (PCR) and inserted into a pIRSE2-EGFP
vector (Clontech Laboratories, Inc., Mountainview, CA, USA). The
restriction sites were BamH I and EcoR I. Empty vector was used as
the negative control (NC). AWPPH overexpression vectors were
transfected into 4×105 cells at a dose of 10 nmol using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cells were cultured in an
incubator (37°C, 5% CO2) following transfection, and
were collected at 24 h after transfection. The expression levels of
AWPPH were determined via reverse transcription-quantitative PCR
(RT-qPCR), and an overexpression rate of 150–200% was achieved
prior to subsequent experimentation. Control group was
non-transfected cells and cells transfected with the negative
control vector were negative control cells.
For Wnt Agonist treatment, 5×105 cells
were incubated with 10 ng/ml Wnt Agonist (CAS# 853220-52-7; Santa
Cruz Biotechnology Inc., Dallas, TX, USA) at 37°C (5%
CO2) for 12 h prior to experimentation. For Wnt
inhibitor treatment, 2.5 µmol inhibitor of Wnt production 2 (IWP-2;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into
culture medium containing 5×105 cells (37°C, 5%
CO2) and incubated for 12 h prior to experimentation;
1,000 X stocks of Wnt Agonist and IWP-2 were prepared in culture
medium.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was determined via a CCK-8 assay.
Briefly, a 96-well plate was seeded with 100 µl cells of UWB1.289
and UWB1.289 + BRCA1 cell lines suspended in a medium of 50%
ATCC-formulated RPMI-1640 medium and 50% Mammary Epithelial Cell
Growth medium supplemented with 3% fetal bovine serum at a density
of 4×103 cells/well. Cell culture was performed in an
incubator (37°C, 5% CO2), and 10 µl CCK-8 solution
(Sigma-Aldrich; Merck KGaA) was added to each well 24, 48, 72 and
96 h later. Cells were incubated for an additional 4 h at 37°C, and
the optical density (450 nm) was measured using a Fisherbrand™
accuSkan™ GO UV/Vis Microplate Spectrophotometer (Fisher
Scientific; Thermo Fisher Scientific, Inc.).
Transwell migration and invasion
assay
Transwell migration assays were performed by plating
4×103 cells in the upper chamber of Transwell plates in
0.1 ml serum-free culture medium, and RPMI-1640 medium (Thermo
Fisher Scientific, Inc.) containing 20% fetal calf serum
(Sigma-Aldrich; Merck KGaA) was added to the lower chamber. Cells
were incubated at 37°C for 24 h, and membranes were collected and
stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 15
min at room temperature. Cells were observed under an optical
microscope and 5 visual fields (magnification, ×40) were selected
from each membrane to count cell number. For invasion assays, the
upper chamber was coated with Matrigel® (Merck KGaA),
with all other steps performed as previously described.
RT-qPCR
Total RNA was extracted from tissues that were
ground in liquid nitrogen using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was also
extracted from OC cells. cDNA was synthesized via RT using
SuperScript III Reverse Transcriptase kit (Thermo Fisher
Scientific, Inc.) with the following conditions: 25°C for 5 min,
55°C for 10 min and 80°C for 5 min. qPCR was performed using
SYBR® Green Real-Time PCR Master Mixes (Thermo Fisher
Scientific, Inc.). The following primer pairs were used: AWPPH,
forward 5′-CTGGATGGTCGCTGCTTTTTA-3′, reverse,
5′-AGGGGGATGAGTCGTGATTT-3′; and β-actin, forward
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The following thermocycling conditions were used for PCR: 40 sec at
95°C, followed by 40 cycles at 95°C for 12 sec and 60°C for 40 sec.
The relative expression levels of AWPPH were normalized to β-actin
using the 2−ΔΔCq method (14).
Western blotting
Radioimmunoprecipitation assay solution (Thermo
Fisher Scientific, Inc.) was used to extract total protein from
in vitro cultured OC cells. according to the manufacturer's
protocols. A bicinchoninic acid assay was performed to determine
protein concentration. Proteins (20 µg/lane) were separated via 10%
SDS-PAGE. Proteins were transferred to polyvinylidene difluoride
membranes, which were incubated with 5% skimmed milk at room
temperature for 1 h for blocking. Membranes were then incubated
with rabbit anti-β-catenin antibody (1:1,200; ab6302, Abcam,
Cambridge, UK) and anti-GAPDH primary antibody (1:1,400; ab8245,
Abcam) overnight at 4°C, followed by incubation with a horseradish
peroxidase-conjugated anti-rabbit IgG-HRP secondary antibody
(1:1,000; MBS435036, MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 4 h. An enhanced chemiluminescence kit
(Sigma-Aldrich; Merck KGaA) was then applied to visualize the
bands. Membranes were scanned using a MYECL™ Imager (Thermo Fisher
Scientific, Inc.), and β-catenin expression was normalized to GAPDH
expression using Image J V 1.6 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was performed on 3 biological
replicates. SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for all
statistical analyses. Data were presented as the mean ± standard
deviation. The diagnostic value of serum AWPPH for OC was
investigated by receiver operating characteristic (ROC) curve
analysis. Associations between the serum levels of AWPPH and the
clinicopathological data of patients with OC were analyzed using
χ2 tests. Comparisons between two groups and across
>2 groups were performed by unpaired t-tests and one-way
analyses of variance followed by post hoc least significant
difference tests, respectively. The 58 patients with OC were
divided into high- (n=27) and low- (n=31) AWPPH expression groups
according to Youden's index (13).
Kaplan-Meier analysis was performed to determine the survival of
patients in the two groups, and a log rank test was used to compare
survival curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of lncRNA AWPPH in tumor
and adjacent healthy tissues of patients with OC
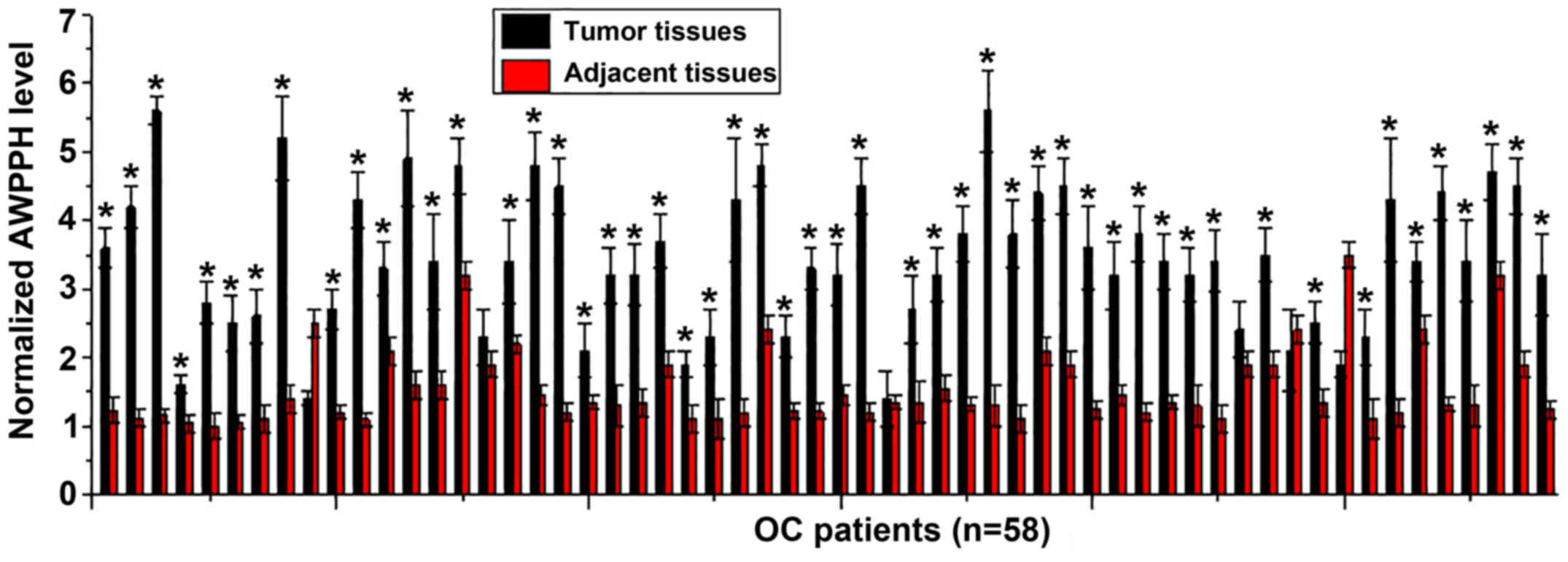
The expression levels of lncRNA AWPPH in tumor and
adjacent healthy tissues of 58 patients with OC were determined by
RT-qPCR. As presented in Fig. 1,
significantly increased expression of AWPPH in tumor tissues
compared with in adjacent healthy tissues was observed in 89.7%
(52/58) of patients with OC. The data suggested that upregulation
of AWPPH may be involved in the pathogenesis of OC.
Serum levels of AWPPH in patients with
OC and healthy controls, and the diagnostic and prognostic
values
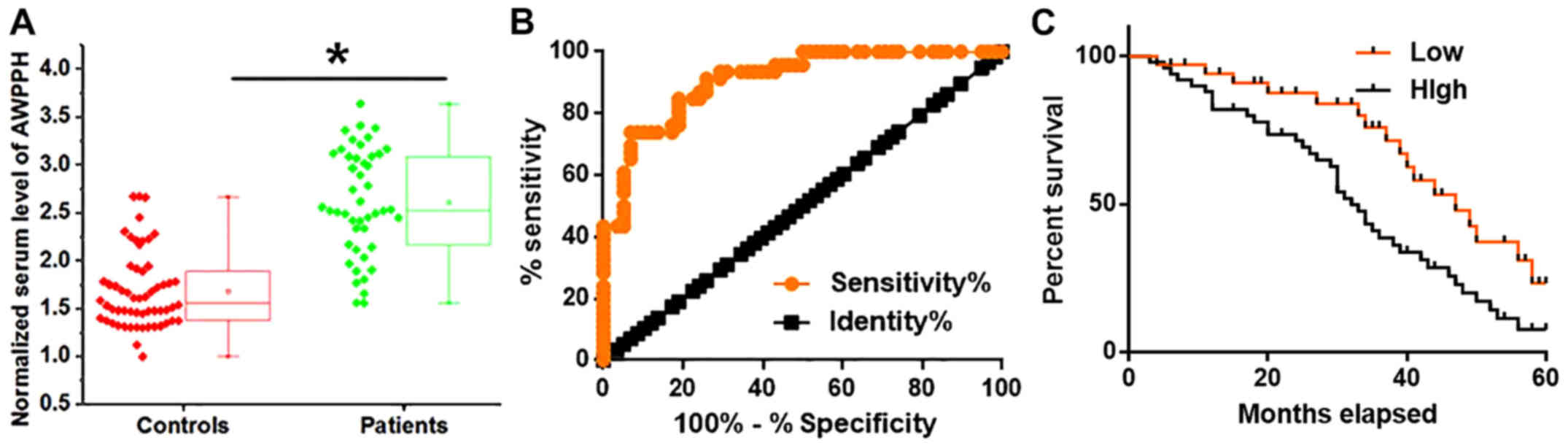
The serum expression levels of AWPPH in patients
with OC and healthy controls were determined by RT-qPCR. As
presented in Fig. 2A, the
expression levels of AWPPH were significantly increased in the
serum acquired from patients with OC compared with in healthy
controls (P<0.05). The diagnostic value of serum AWPPH for OC
was investigated by ROC curve analysis. As presented in Fig. 2B, the area under the curve was
0.9082, with a standard error value of 0.9082 and 95% confidence
interval from 0.8543 to 0.9620. The 58 patients with OC were
divided into high- (n=27) and low- (n=31) AWPPH expression groups
according to Youden's index (15).
Kaplan-Meier analysis was performed to determine the survival of
patients in the two groups, and a log rank test was used to compare
survival curves. As presented in Fig.
2C, the overall survival rate of patients with low serum levels
of AWPPH was significantly higher compared with patients with high
AWPPH serum levels (log rank test P=0.0322).
χ2 analysis of the
associations between the serum levels of AWPPH and the
clinicopathological data of patients
Patients were divided into high- (n=29) and
low-expression (n=29) groups according to the median value of
expression. A χ2 test was performed to analyze the
associations between the serum levels of AWPPH and the
clinicopathological data of patients with OC. As presented in
Table I, the serum levels of AWPPH
were not significantly associated with the age, or drinking and
smoking habits of patients; however, the serum levels of lncRNA
AWPPH exhibited a significant association with tumor size and tumor
distant metastasis.
 | Table I.χ2 analysis of the
association between the serum levels of long noncoding RNA
associated with poor prognosis of hepatocellular carcinoma and the
clinicopathological data of patients. |
Table I.
χ2 analysis of the
association between the serum levels of long noncoding RNA
associated with poor prognosis of hepatocellular carcinoma and the
clinicopathological data of patients.
| Clinicopathological
factor | Groups | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Age (years) | >50 | 28 | 13 | 15 | 0.27 | 0.60 |
|
| <50 | 30 | 16 | 14 |
|
|
| Smoking | Yes | 16 | 7 | 9 | 0.35 | 0.56 |
|
| No | 42 | 22 | 20 |
|
|
| Drinking | Yes | 18 | 8 | 10 | 0.32 | 0.57 |
|
| No | 40 | 21 | 19 |
|
|
| Primary tumor
diameter | >2 cm | 27 | 19 | 8 | 8.35 | 0.01a |
|
| <2 cm | 31 | 10 | 21 |
|
|
| Distant tumor
metastasis | Yes | 34 | 22 | 12 | 7.11 | 0.01a |
|
| No | 24 | 7 | 17 |
|
|
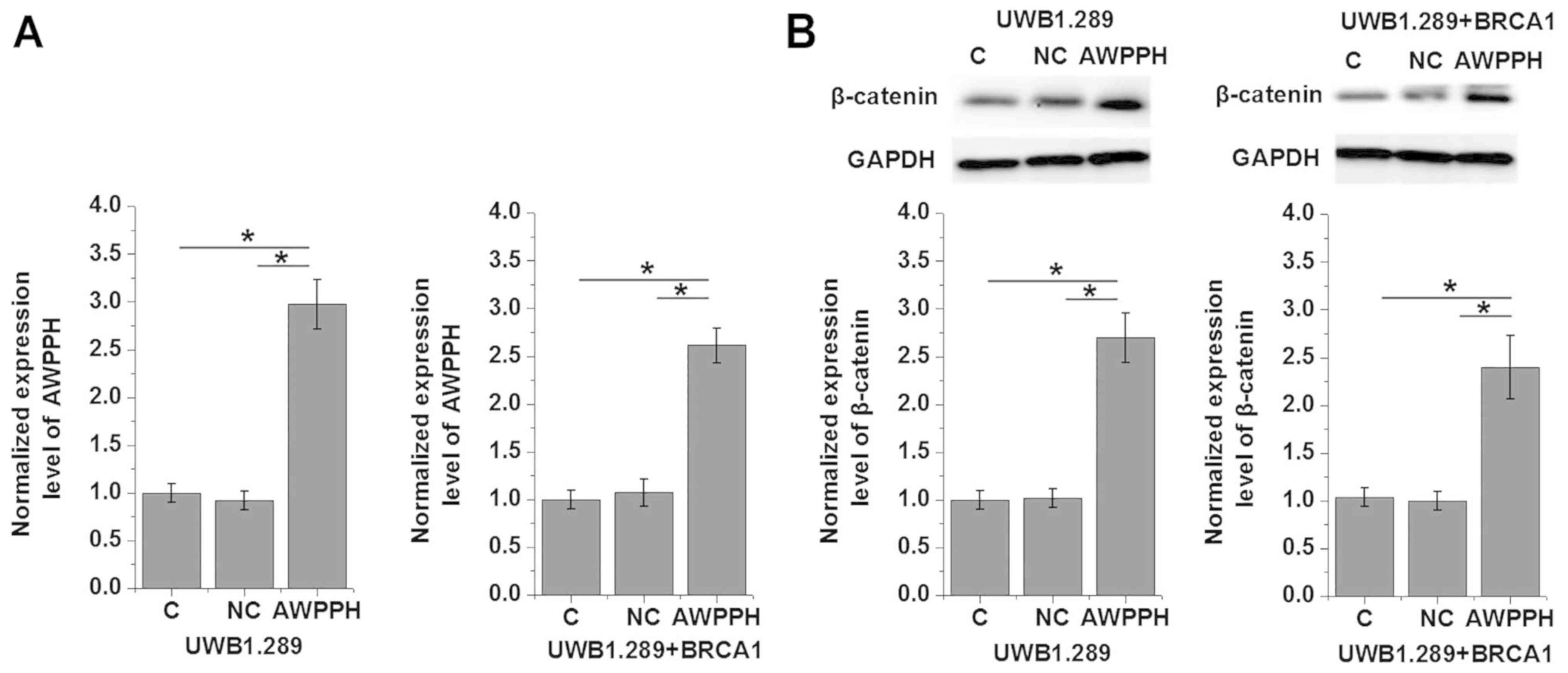
Effects of AWPPH overexpression on
β-catenin expression
The clinicopathological data presented in Table I indicated that AWPPH may be
involved in the regulation of tumor growth and metastasis of OC.
Wnt/β-catenin serves important roles in the progression of various
types of malignancies, such as ovarian cancer (8). In the present study, AWPPH
overexpression was induced via transfection with a pIRSE2-EGFP
plasmid containing AWPPH cDNA (Fig.
3A). Transfection significantly promoted the expression of
β-catenin in two human OC cell lines, UWB1.289 and UWB1.289 +
BRCA1, compared with the control groups of non-transfected cells
and cells transfected with the NC vector (P<0.05; Fig. 3B). Conversely, treatment with 10
ng/ml Wnt Agonist did not significantly affect AWPPH expression
(P>0.05; data not shown).
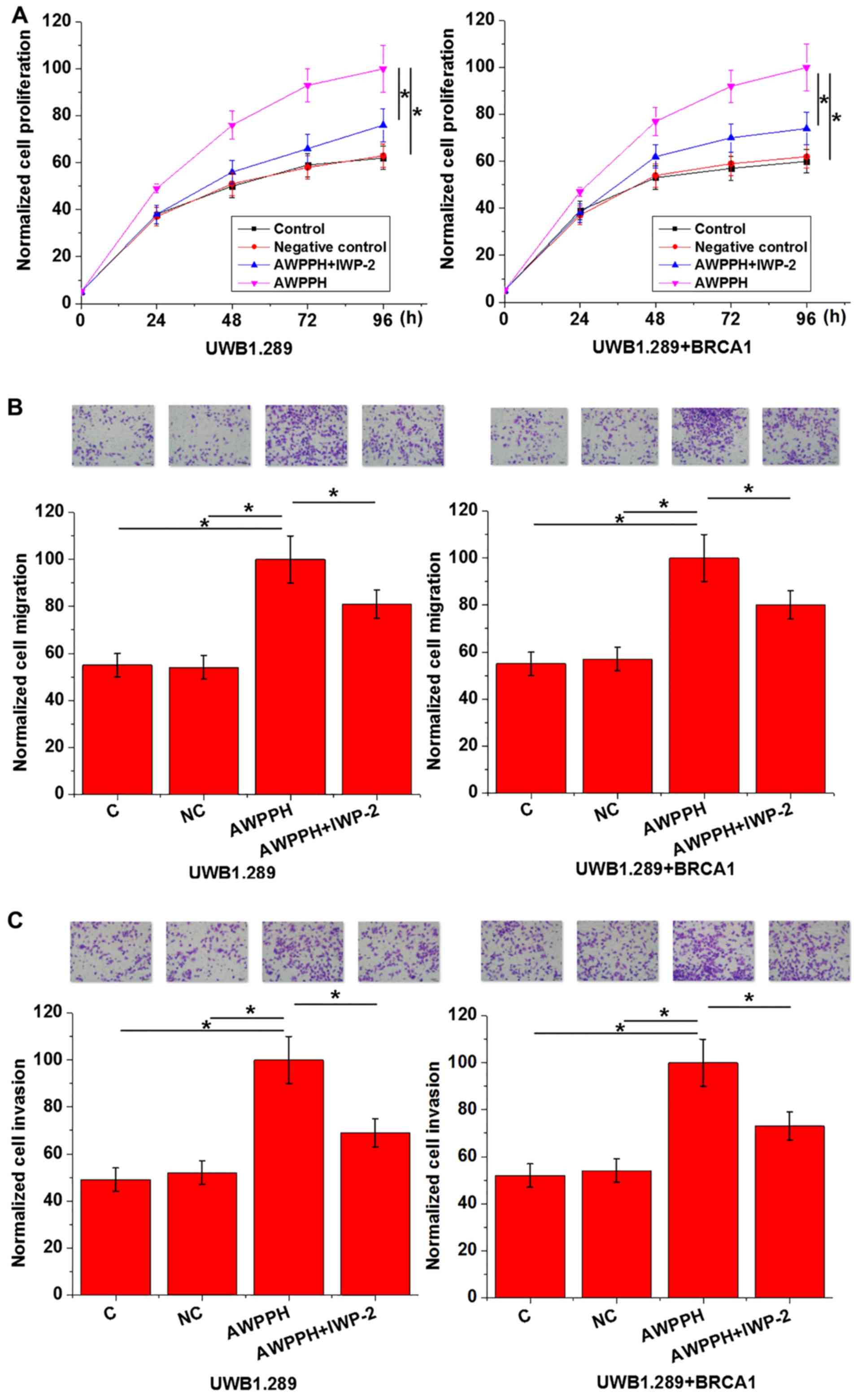
Effects of AWPPH overexpression and
Wnt inhibitor on cell proliferation, migration and invasion
As presented in Fig.
4, AWPPH overexpression significantly promoted cell
proliferation, migration and invasion of the two human OC cell
lines compared with the controls. In addition, treatment with 2.5
µmol IWP-2 significantly reduced the enhancing effects of AWPPH
overexpression on each cellular property. The data suggested that
AWPPH may promote cell proliferation, migration and invasion in OC
via activation of the Wnt/β-catenin pathway.
Discussion
The key finding of the present study is that lncRNA
AWPPH, previously identified as an oncogene in hepatocellular
carcinoma (11) and bladder cancer
(12), may serve a similar role in
OC. The oncogenic effects of AWPPH in OC may be achieved via
activation of the Wnt/β-catenin signaling pathway. Furthermore, the
results revealed that AWPPH may be involved in regulating the
growth and metastasis of OC.
The development of OC is accompanied with
alterations in the expression profiles of numerous lncRNAs
(16). Various lncRNAs exhibit
altered expression profiles and serve separate roles in OC to
inhibit or promote tumor progression. Decreased expression levels
of lncRNA maternally expressed 3 were reported in OC tissues
compared with adjacent healthy tissues, and upregulation of this
lncRNA suppressed tumor progression (17). Conversely, SNHG1 expression is
significantly upregulated in OC tissues, indicating an oncogenic
role in OC (18). Upregulation of
AWPPH was observed in hepatocellular carcinoma (11) and bladder cancer (12). In the present study, significantly
increased levels of AWPPH expression were reported in tumor tissues
compared with adjacent healthy tissues in the majority of patients
with OC, indicating a potentially oncogenic role for the lncRNA in
the pathogenesis of OC.
Tumor metastasis is the main obstacle in the
treatment of OC, and early diagnosis and treatment is important for
the survival of patients with OC. The onset of disease in humans is
usually associated with alterations in the levels of certain
substances in the blood, the detection of which may aid diagnosis
and improve prognosis of human diseases (19). In the present study, ROC curve
analysis revealed that serum AWPPH was able to effectively separate
patients with OC from healthy controls. Additionally, increased
serum levels of AWPPH were associated with shorter survival time.
The serum levels of AWPPH did not correlate with the age, or
smoking and drinking habits of patients, which have been
demonstrated to affect the expression of certain lncRNAs (19–22).
Therefore, AWPPH may serve as a potential diagnostic and prognostic
biomarker for OC; however, as a novel lncRNA, the expression
profile of AWPPH in other human diseases have not yet been
reported. Therefore, the inclusion of additional biomarkers may
improve the accuracy of diagnosis and prognosis of patients.
The present study also revealed that the serum
levels of AWPPH were associated with distant tumor metastasis and
tumor size. The Wnt/β-catenin pathway serves important roles in
tumor progression in various types of malignancies, including
ovarian cancer (8). The results
demonstrated that AWPPH overexpression significantly promoted the
expression of β-catenin in two human OC cell lines. Conversely,
Wnt/β-catenin activation exhibited no significant effects on the
expression of AWPPH in the cell lines, indicating that AWPPH may be
an upstream activator of the Wnt/β-catenin pathway. In vitro
cell proliferation, migration and invasion assays demonstrated the
potential involvement of AWPPH in the regulation of growth and
metastasis in OC. Additionally, treatment with the Wnt/β-catenin
inhibitor IWP-2 eliminated the effects of AWPPH overexpression in
these assays, indicating that the roles of AWPPH in OC may involve
the Wnt/β-catenin pathway.
There are certain limitations of the present study.
A small sample size was employed. Additionally, the expression of
β-catenin was only investigated at the protein level; thus, its
expression at the mRNA level in OC cells remains unknown.
Furthermore, the expression of other genes involved in the
Wnt/β-catenin signaling pathway was not investigated. Therefore,
the molecular mechanisms underlying the regulatory effects of AWPPH
on Wnt/β-catenin signaling remain unknown. Further investigation of
the components of the Wnt/β-catenin signaling pathway is required
to provide greater insight into the oncogenic properties of
AWPPH.
In conclusion, AWPPH expression was upregulated in
OC in the present study. The serum expression levels of AWPPH may
serve as a potential diagnostic and prognostic biomarker for OC.
AWPPH overexpression promoted the proliferation, migration and
invasion of OC cells and upregulated β-catenin expression.
Treatment with Wnt Agonist markedly affected AWPPH expression;
however, IWP-2 reduced the effects of AWPPH overexpression on
proliferation, migration and invasion of OC cells. Therefore, the
results suggested that lncRNA AWPPH may be involved in the
pathogenesis of OC, possibly via activation of the Wnt/β-catenin
signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GY, WW, JD and SD made substantial contributions to
the conception and design of the present study. GY and WW performed
the experiments. GY, WW and JD analyzed and interpreted the data.
GY and WW drafted the article. GY, WW and SD were responsible for
the revision of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of Yantai Yeda Hospital (Yantai, China). All patients
provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi H, Yamada Y, Sado T, Sakata M,
Yoshida S, Kawaguchi R, Kanayama S, Shigetomi H, Haruta S, Tsuji Y,
et al: A randomized study of screening for ovarian cancer: A
multicenter study in Japan. Int J Gynecol Cancer. 18:414–420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buys SS, Partridge E, Black A, Johnson CC,
Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B,
et al: Effect of screening on ovarian cancer mortality: The
Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening
randomized controlled trial. JAMA. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote IB, Witteveen P,
Bamias A, et al: AURELIA: A randomized phase III trial evaluating
bevacizumab combined with chemotherapy for platinum-resistant
recurrent ovarian cancer. J Clin Oncol. 30:327s2012. View Article : Google Scholar
|
|
5
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinum-sensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ovarian Cancer Research Program of BC and
Cheryl Brown: Ovarian Cancer Outcomes Unit. OVCARE. Research
platforms, 2013. http://www.ovcare.caFeb 6–2014
|
|
7
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
10
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017.PubMed/NCBI
|
|
11
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
American Joint Committee on Cancer, .
Ovary[M]//AJCC cancer staging manual. Springer; New York, NY: pp.
pp.275–283. 2002
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fluss R, Faraggi D and Reiser B:
Estimation of the Youden Index and its associated cutoff point.
Biom J. 47:458–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou M, Sun Y, Sun Y, Xu W, Zhang Z, Zhao
H, Zhong Z and Sun J: Comprehensive analysis of lncRNA expression
profiles reveals a novel lncRNA signature to discriminate
nonequivalent outcomes in patients with ovarian cancer. Oncotarget.
7:32433–32448. 2016.PubMed/NCBI
|
|
17
|
Xiu YL, Sun KX, Chen X, Chen S, Zhao Y,
Guo QG and Zong ZH: Upregulation of the lncRNA Meg3 induces
autophagy to inhibit tumorigenesis and progression of epithelial
ovarian carcinoma by regulating activity of ATG3. Oncotarget.
8:31714–31725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding
F, Li D and Yang T: Long noncoding RNA SNHG1 predicts a poor
prognosis and promotes hepatocellular carcinoma tumorigenesis.
Biomed Pharmacother. 80:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moore RG, Brown AK, Miller MC, Skates S,
Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P,
Granai CO and Bast RC Jr: The use of multiple novel tumor
biomarkers for the detection of ovarian carcinoma in patients with
a pelvic mass. Gynecol Oncol. 108:402–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grammatikakis I, Panda AC, Abdelmohsen K
and Gorospe M: Long noncoding RNAs(lncRNAs) and the molecular
hallmarks of aging. Aging (Albany NY). 6:992–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|