Introduction
Breast cancer is the most common cancer in females
worldwide (1). Understanding of
the mechanism underlying tumour progression and the identification
of novel therapeutic methods are required to improve the prognosis
of patients with breast cancer. In previous decades, studies have
primarily focused on alterations in mRNA expression in cancer
cells; however, recent studies have suggested the important roles
for non-coding RNAs in the regulation of cell proliferation,
apoptosis, chemo-resistance and migration in cancer (2–5).
MicroRNAs (miRNAs/miRs) are non-coding endogenous
RNAs measuring ~22 nucleotides long. miRNAs bind to the partially
complementary sequences in the 3′-untranslated region (3′-UTR) of
the target mRNAs and stimulate mRNA degradation or translation
inhibition (6,7). Aberrant expression of multiple miRNAs
has been identified in breast cancer, and their expression levels
were associated with the extent of invasion or proliferation of
cancer cells (8–10). Subsequent exploration into the role
of the tumour-associated miRNAs in breast cancer may allow
identification of novel therapeutic targets to improve clinical
outcomes. miR-124 is significantly downregulated in various types
of cancer, including colorectal cancer (CRC) (11), non-small cell lung cancer (12), and nasopharyngeal carcinoma (NPC)
(13). It serves as a tumour
suppressor gene and affects apoptosis, proliferation and invasion
of cells. The expression of miR-124 was downregulated in CRC
tissues and its upregulated expression may suppress tumour
progression and decrease the drug sensitivity of CRC cells through
the negative regulation of the expression of DNA methyltransferase
(DNMT) 3 beta and DNMT1 (11).
Decreased expression of miR-124 was also observed in NPC tissues,
wherein it contributed to the initiation and development of NPC by
targeting forkhead box Q1, which is involved in tumour
proliferation and migration (13).
Downregulation of miR-124 expression has also been demonstrated in
human breast cancer tissues (14,15).
miR-124 serves a vital role in the modulation of proliferation and
invasion of breast cancer cells through the downregulation of the
oncogene Cbl proto-oncogene (16).
In addition, miR-124 mediated the oncogenic effects of
metastasis-associated lung adenocarcinoma transcript 1 in breast
cancer by directly interacting with the cyclin-dependent kinase
4/E2F transcription factor 1 signalling pathway (17). Therefore, miR-124 is an attractive
candidate as a therapeutic target in breast cancer. However, the
effects and underlying mechanisms of this miRNA in breast cancer
are incompletely characterized.
The present study aimed to clarify the functions of
miR-124 in breast cancer cells and to examine its effects on the
viability, proliferation and invasion of breast cancer cell lines.
Furthermore, these data were validated by performing in vivo
studies. Bioinformatics software was used to predict the potential
targets of miR-124 in breast cancer cells. According to the
results, signal transducer and activator of transcription 3 (STAT3)
was identified as the target. STAT3 is a key cytoplasmic
transcription factor involved in cell proliferation and invasion
and serves as an oncogenic gene in breast cancer (18–20).
STAT3 may bind to the promoter of the tumour protein p53 (p53) gene
and inhibit its expression, resulting in the inhibition of
p53-mediated apoptosis of cancer cells. In addition, miR-17-5p may
suppress the chemotherapy-induced apoptosis of breast cancer cells
through the deactivation of STAT3 (18). Liu et al (20) demonstrated that STAT3 may enhance
the transcription and expression of mitogen-activated protein
kinase kinase 5 to promote epithelial-mesenchymal transition (EMT)
in breast cancer cells. Previous data have suggested that STAT3 is
upregulated in breast cancer tissues, and the small-interfering RNA
(siRNA) and miRNA-mediated inhibition of STAT3 expression resulted
in suppression of the invasion of breast cancer cells (19,21,22).
The present study evaluated if miR-124 exerted its
anti-proliferation and anti-invasion effects by targeting STAT3.
The results indicated that miR-124 targeted STAT3 to decrease the
growth rate and invasiveness of breast cancer cells in vitro
and in vivo.
Patients and methods
Human tissues samples
Human breast tissue samples were obtained at the
Central Hospital of Wuhan (Wuhan, China) from September 2014 to
November 2015. A total of 10 patients (42–57 years old) with triple
negative breast cancer were included in the study. Cancer tissues
and matched normal breast tissues were collected. All patients from
whom samples were collected were treatment-naïve prior to surgical
resection. The study protocol followed the Declaration of Helsinki,
and all patients provided written informed consent. All protocols
and procedures were approved by the Ethics Committee of the Central
Hospital of Wuhan.
Cell culture
The human breast cancer cell lines MDA-MB-468 and
MDA-MB-231 were obtained from the American Type Culture Collection
(Manassas, VA, USA). MDA-MB-468 cells were routinely cultured at
37°C in a 5% CO2 environment with L15, 10% foetal bovine
serum (FBS), 100 U/ml penicillin and streptomycin (all Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). MDA-MB-231 cells
were cultured without CO2 in complete L15 medium (Gibco;
Thermo Fisher Scientific, Inc), according to the protocol of the
manufacturer.
Transfection
miRNA-124 mimics (miR-124), miRNA-124 inhibitor
(inh-124), STAT3 overexpression plasmid [pcDNA3.0-STAT3
(plasmid-STAT3)] and corresponding negative controls [miR-NC,
inh-NC and pcDNA3.0-empty vector (plasmid-NC)] were designed and
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
sequences of miR-124, miR-NC, inh-124 and inh-NC were
5′-CCGUAAGUGGCGCACGGAAU-3′, 5-UUCUCCGAACGUGUCACGUTT-3′,
5′-GGCAUUCACCGCGUGCCUUA-3′ and 5′-CAGUACUUUUGUGUAGUACAA-3′,
respectively. The sense and antisense of si-STAT3 were
5′-CAUCUGCCUAGAUCGGCUA-3′ and 5′-UAGCCGAUCUAGGCAGAUG-3′; the
sequences for negative control (si-NC) were
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′. Based
on these sequences, lentiviral vectors (pHelper 2.0) with siRNA
targeting human STAT3 (LV-si-STAT3), LV-miR-124, and corresponding
controls (LV-si-NC/LV-miR-NC) were purchased from Shanghai GeneChem
Co., Ltd. (Shanghai, China). A total of 5×104 cells were
seeded in 12-well plates and incubated overnight at 37°C in
Opti-MEM medium (Gibco; Thermo Fisher Scientific, Inc.), followed
by RNA/DNA transfection using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The final
concentrations of miR-124/miR-NC and inh-124/inh-NC were 50 and 100
nM, respectively. Cells were transfected with plasmid-STAT3 at a
concentration of 1.6 µg/well in a 12-well plate. For lentiviral
transfection, the concentration was set as 1×108
transducing units/ml in 6-well plates. Western blot analysis and
reverse transcription quantitative polymerase chain reaction
(RT-qPCR) assays were performed, as described subsequently, to
evaluate the transfection efficiency 48 h following transfection.
For lentiviral infection, the cells were cultured at 37°C in
Enhanced Infection Solution and Polybrene (both Shanghai GeneChem
Co., Ltd.) for 5 h, and then this mixture was replaced with
complete medium.
Total RNA isolation and RT-qPCR
According to the manufacturer's protocol,
TRIzol® (Takara Bio, Inc., Otsu, Japan) was used to
extract total RNA from breast cancer cells 48 h after treatment.
mRNAs and miRNAs were reverse transcribed according to the
suppliers' protocols using PrimeScript® RT Master Mix
Perfect Real Time and One Step PrimeScript® microRNA
cDNA Synthesis kits (both Takara Bio, Inc.), respectively. SYBR
Premix Ex Taq II (Takara Bio, Inc.) was used for qPCR analysis. The
qPCR conditions were as follows: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. The 2−ΔΔCq
method was used to quantify expression (23). The expression levels of mRNAs and
miRNAs were normalized to β-actin and U6, respectively, and all
reactions were performed in triplicate. The primers for the RT-qPCR
were: miR-124 forward, 5′-TAAGGCACGCGGTGAATGCC-3′ and reverse,
5′-GATTGAATCGAGCACCAGTTA′-3; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; STAT3 forward,
5′-TGTGCGTATGGGAACACCTA-3′ and reverse, 5′-AGAAGGTCGTCTCCCCCTTA-3′;
β-actin forward, 5′-CTTTCTACAATGAGCTGCGTG-3′ and reverse,
5′-TCATGAGGTAGTCTGTCAGG-3′.
Western blot analysis
Transfected cells were washed with PBS three times
prior to protein collection. Protein lysis buffer was obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA), and a
bicinchoninic acid assay was performed to measure the concentration
of protein. Protein samples (30 µg/lane) were separated via 10%
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
Then, the membranes were blocked in 5% skimmed milk dissolved in
TBS-0.1% Tween-20 for 2 h at room temperature and further incubated
at 4°C overnight with the primary antibodies rabbit anti-STAT3
(1:1,000; cat. no. 12640) and mouse anti-β-actin (1:1,000; cat. no.
3700; both Cell Signaling Technology, Inc.). Following washing, the
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit and anti-mouse secondary antibodies (1:3,000; cat. nos.
7074 and 7076; Cell Signaling Technology, Inc.) at room temperature
for 2 h. Bands were visualized and quantified using enhanced
chemiluminescent substrate reagents (Thermo Fisher Scientific,
Inc.) and Image Lab version 4.1 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), respectively.
MTT assay
An MTT assay was conducted to measure the viability
of cancer cells. Cells were seeded at a density of 3×103
cells per well for a 12-well plate. Every group contained at least
6 wells. Cells were then cultured overnight and transfected as
aforementioned with mimics (50 nM)/inhibitor (100 nM) and
corresponding negative controls, respectively. Cells were cultured
for 2 days prior to the MTT assay to determine the effects of
treatment on cell viability. Cells were incubated with MTT (5
mg/ml) at 37°C for 4 h, and then 150 µl dimethyl sulfoxide was
added. The crystals were dissolved, and the absorbance was measured
using a spectrophotometer at 570 nm. For measuring cell viability
following transfection with lentiviruses, stably-transfected cells
were plated in 5 plates and MTT assays were conducted to detect the
relative absorbance every day; growth curves were calculated
following 5 days of observation. All procedures were repeated 3
times.
Cell invasion assay
Invasion assays in the present study were performed
in triplicate using Transwell chambers with 8 µm pore size (Costar;
Corning Incorporated, Corning, NY, USA), which were coated with
1:10 diluted Matrigel (BD Biosciences, San Jose, CA, USA) for 4 h
at 37°C. A total of ~5×104 cells were plated in 200 µl
of serum-free L15 medium in the upper chamber. Then, 700 µl L15
medium containing 30% FBS was placed in the lower chambers.
Following incubation for 48 h at 37°C, the cells on the top were
removed, and cells on the lower surface were stained at 37°C for 15
min with 0.1% crystal violet solution and counted in 10 microscopic
fields using a light microscope (magnification, ×400).
Dual-luciferase reporter assay
Putative binding sequences of miR-124 and STAT3 mRNA
were predicted using PITA version 6 bioinformatics (24). Wild type (WT) or mutant (MUT) dual
luciferase reporter plasmids (pmiR-RB-Report™) were designed to
contain the original binding sequence of STAT3 mRNA or a
non-functional MUT sequence (Guangzhou RiboBio Co., Ltd.). A total
of 5×103 cells/well were plated in a 96-well plate and
then co-transfected with luciferase reporter plasmids and miR-124
or miR-NC using Lipofectamine 2000. Then, 2 days post-transfection,
a Dual-Luciferase Reporter Assay System (Promega Corporation,
Madison, WI, USA) was applied to quantify the luciferase activity.
For data analysis, firefly luciferase activity was normalized to
the corresponding Renilla luciferase activity. All
experiments were performed 3 times with 5 duplicates.
Nude mouse models
A total of 20 male BALB/c nude mice (3 weeks old)
were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing,
China) and housed in a room with controlled temperature (26–28°C)
and humidity (40–60%), and access to food and water ad
libitum under a 10:14-h light:dark cycle. Animals were randomly
assigned into the following four groups (n=5/group): LV-miR-124;
LV-miR-NC; LV-si-STAT3; and LV-si-NC. A total of 5×106
stably transfected MDA-MB-468 cells were diluted in 200 µl PBS and
injected subcutaneously into the right back side of mice. Mice were
sacrificed after 3 weeks and tumour sizes were measured. All
protocols and procedures were approved by the Animal Care and Use
Committee of the Central Hospital of Wuhan.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism version 6.05 (GraphPad Software, Inc., La Jolla, CA, USA).
Results are expressed as the mean ± standard deviation of at least
three independent experiments. Comparisons were performed using
unpaired Student's t-tests, and one-way and two-way analyses of
variance followed by a Student-Newman-Keuls post hoc test. The
correlation between the expression of miR-124 and STAT3 was
analyzed by Spearman's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-124 inhibits the viability and
invasion of breast cancer cells in vitro
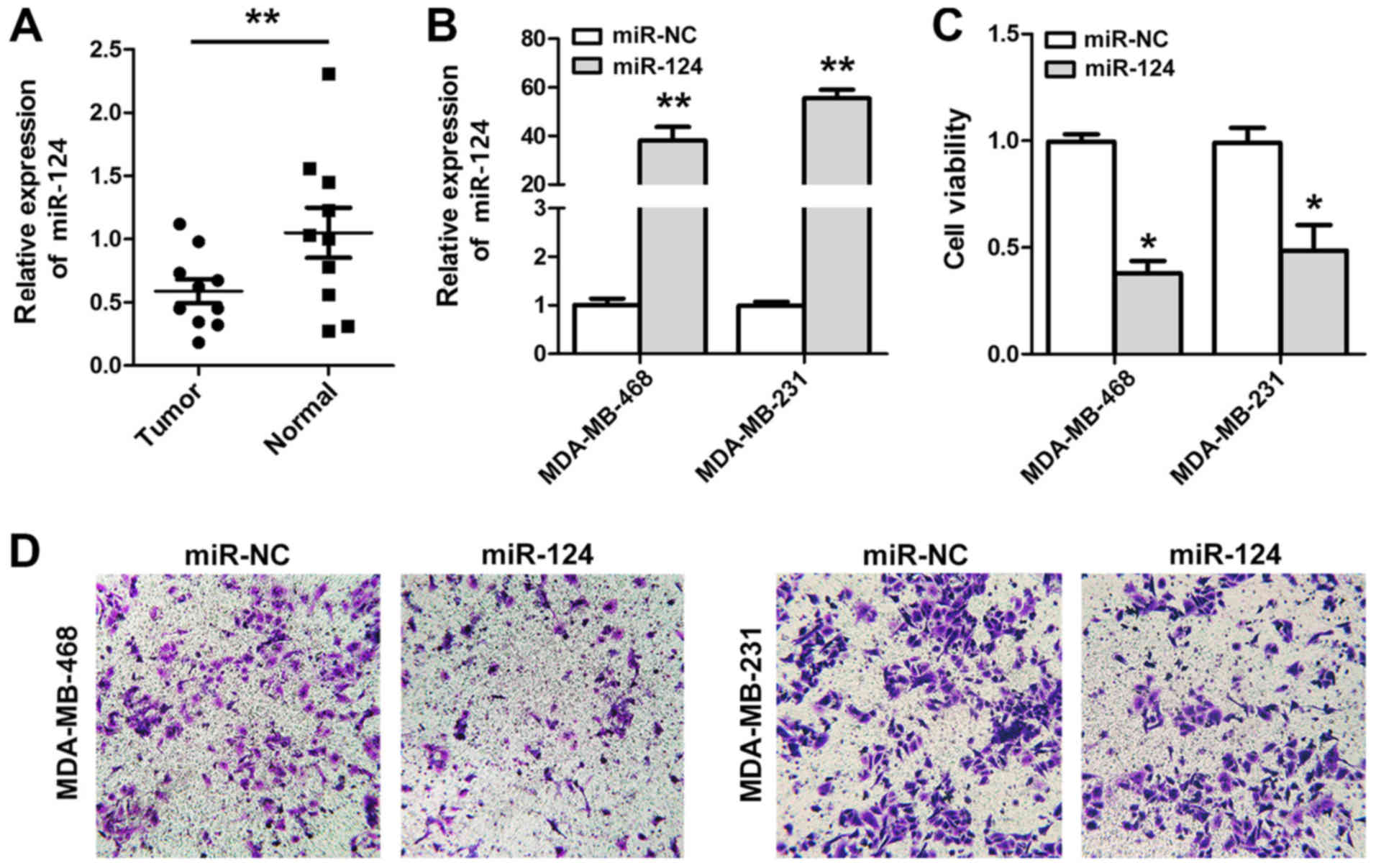
The downregulation of miR-124 expression in 10
breast cancer tissues and paired normal breast tissues was
confirmed by RT-qPCR (Fig. 1A). To
evaluate the effects of miR-124 expression on breast cancer cells,
MDA-MB-468 and MDA-MB-231 cell lines were transfected with miR-124
(Fig. 1B), and it was identified
that the restoration of miR-124 expression suppressed the viability
(Fig. 1C) and negatively affected
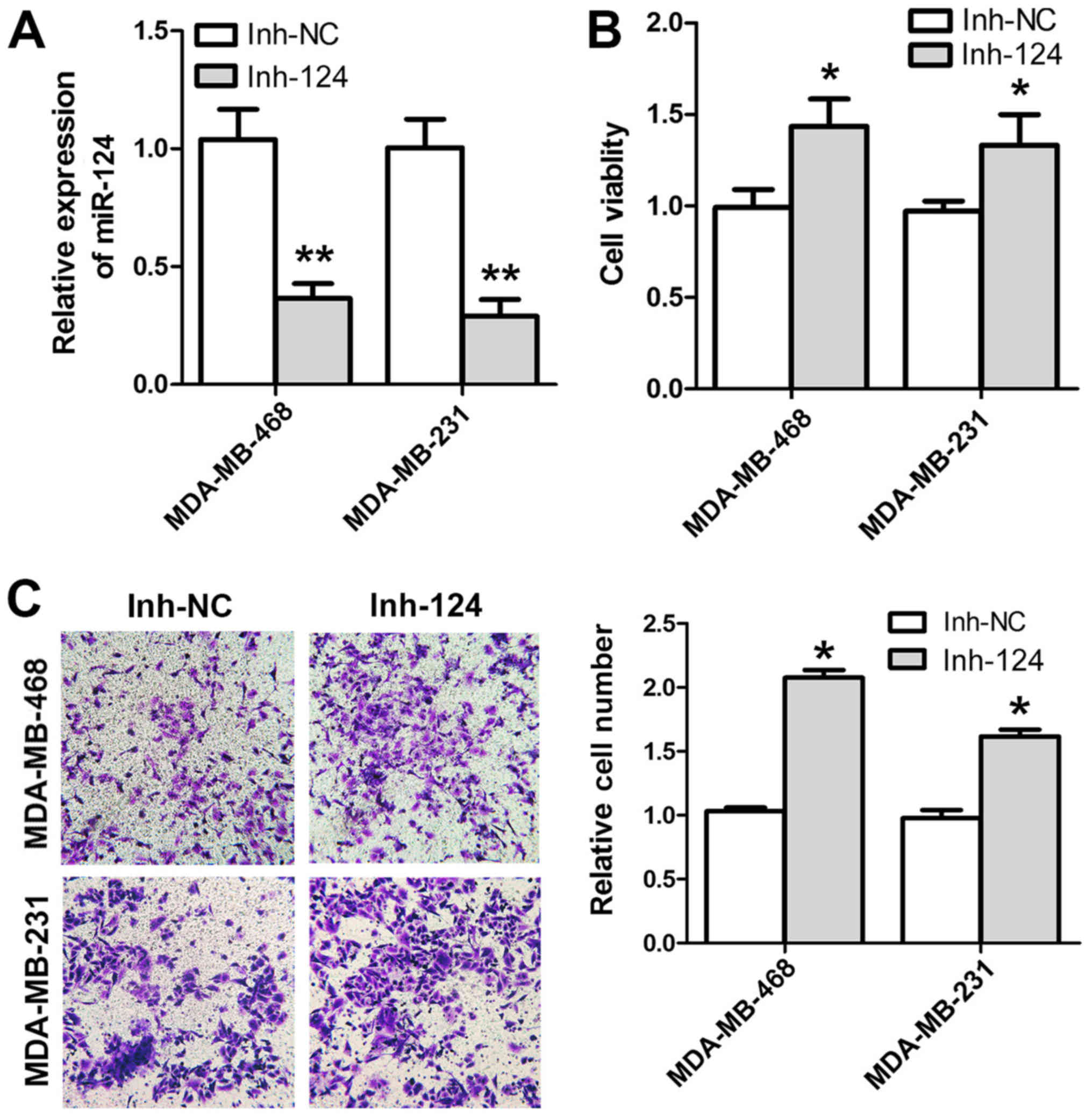
the invasive capacity of these breast cancer cell lines (Fig. 1D). Conversely, downregulation of
expression with inh-124 (Fig. 2A)
resulted in an increase in cell viability and invasion (Fig. 2B and C). Therefore, miR-124
expression was downregulated in human breast cancer cells and its
upregulation prohibited cancer cell invasion in MDA-MB-468 and
MDA-MB-231 cells.
STAT3 may serve as the potential
target of miR-124 in breast cancer
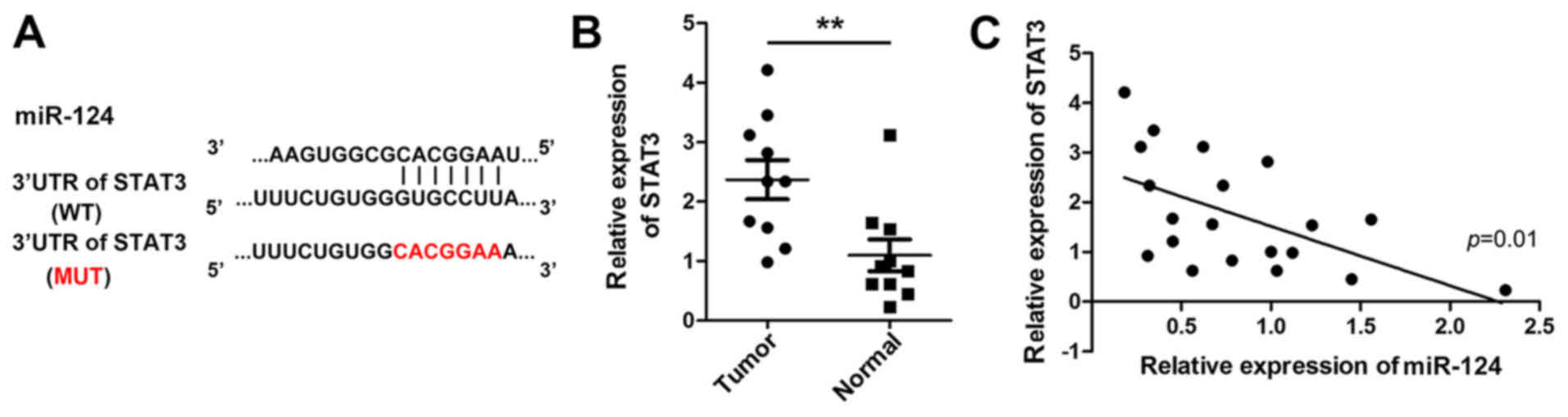
Bioinformatic studies suggested that STAT3 mRNA may
be a target of miR-124; the potential binding area is presented in
Fig. 3A. The software prediction
was validated by examining the association between miR-124 and
STAT3 in 10 pairs of human breast tissues. As demonstrated in
Fig. 3B and C, STAT3 was
overexpressed in breast cancer tissues, and its expression
exhibited a negative correlation with miR-124 expression
(r2=0.37; P=0.01). Therefore, STAT3 may serve as a
potential target of miR-124 in breast cancer cell lines; however,
additional in vitro studies are warranted to confirm this
hypothesis.
miR-124 negatively regulates the
expression of STAT3 via direct interaction in breast cancer
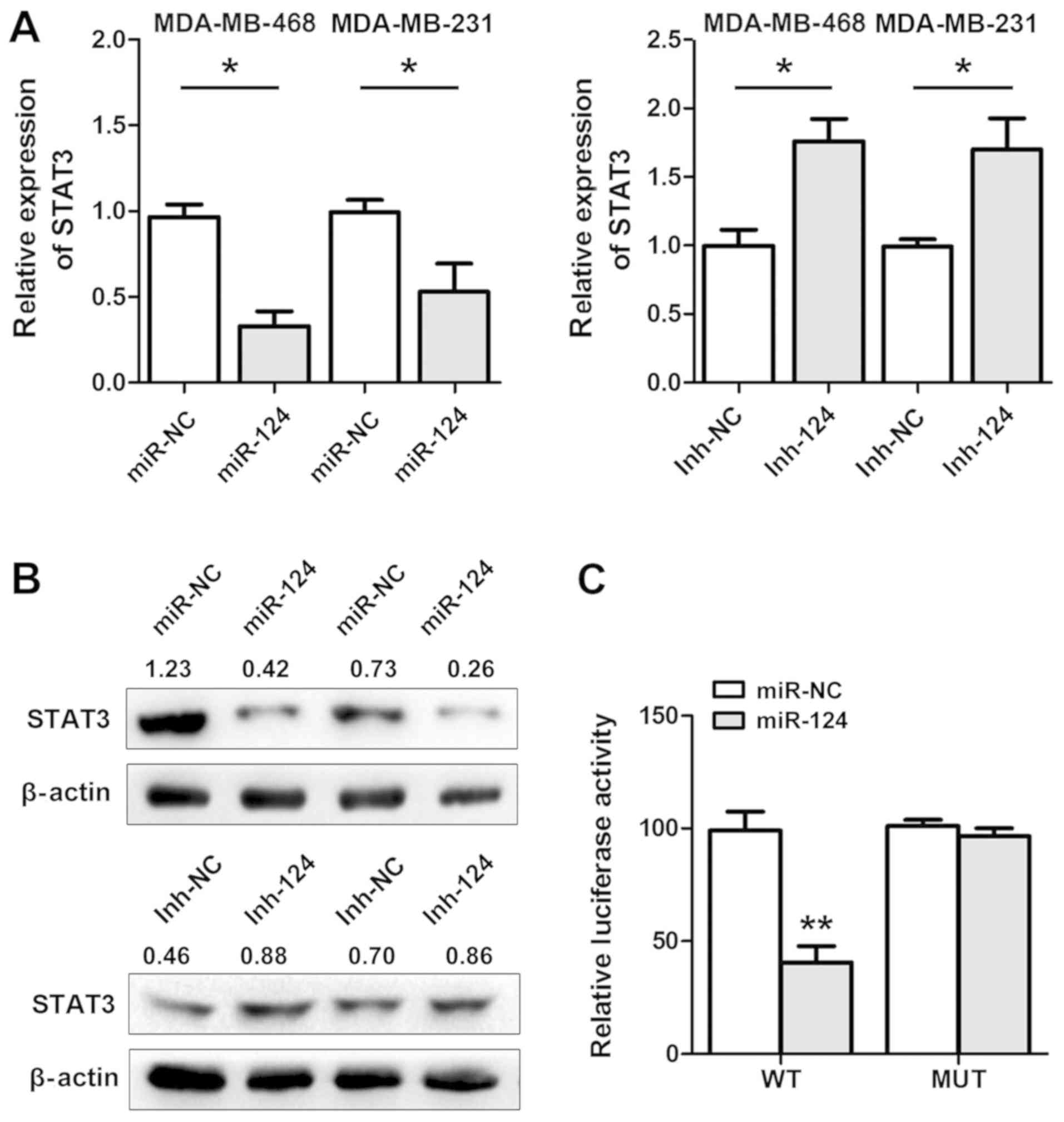
RT-qPCR and western blot analyses were performed to
demonstrate that STAT3 expression was highly downregulated in
miR-124-transfected cells. By contrast, inh-124 treatment
upregulated STAT3 expression (Fig. 4A
and B). Therefore, we hypothesised that miR-124 inhibited cell
invasion by regulating the expression of the STAT3 oncogene in
breast cancer cells. A dual-luciferase assay was performed to
determine whether miR-124 directly interacted with STAT3 mRNA.
Dual-luciferase vectors with the STAT3 3′-UTR sequence containing
the potential miR-124 response element (WT) or a MUT sequence were
co-transfected with miR-124 into MDA-MB-468 cells. miR-124
significantly decreased the firefly luciferase activity of the WT
reporter but not the MUT reporter (Fig. 4C). These data indicated that
miR-124 may downregulate the expression of STAT3 by binding to its
3′-UTR sequence and modulating the malignant behaviour of breast
cancer cells.
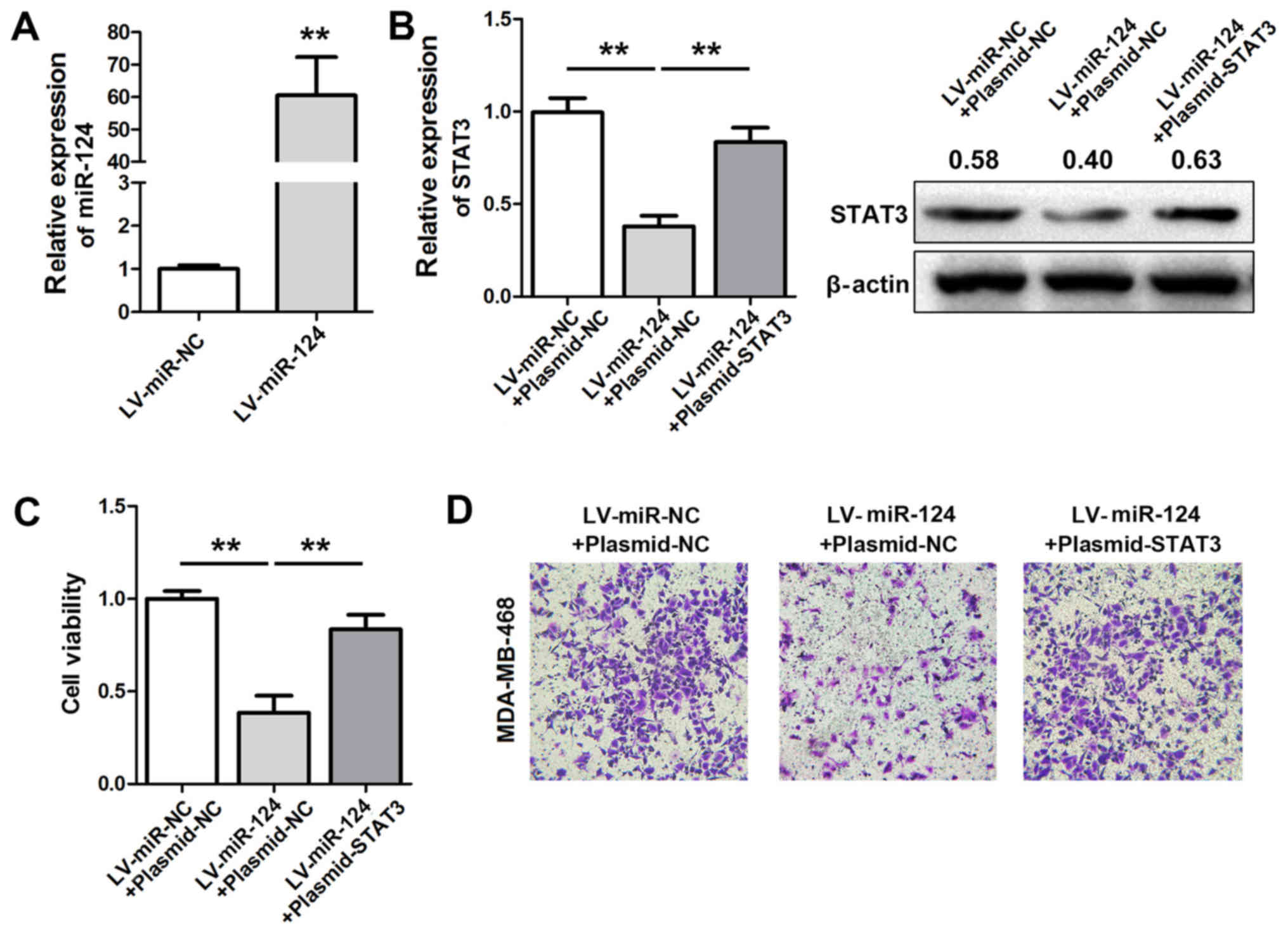
Restoration of STAT3 expression
rescues the miR-124-induced suppression of MDA-MB-468 cell
invasion
Stable expression of miR-124 via lentiviral
infection (LV-miR-124) in MDA-MB-468 cells (Fig. 5A) resulted in the downregulation of
STAT3 expression. STAT3 levels were restored and cell viability and
invasion activities were measured (Fig. 5B). The results indicated that the
suppressive effects of LV-miR-124 on cell viability and invasion
were partially abrogated following STAT3 overexpression (Fig. 5C and D).
Forced expression of miR-124 and
downregulated STAT3 expression abrogates tumour formation in
vivo
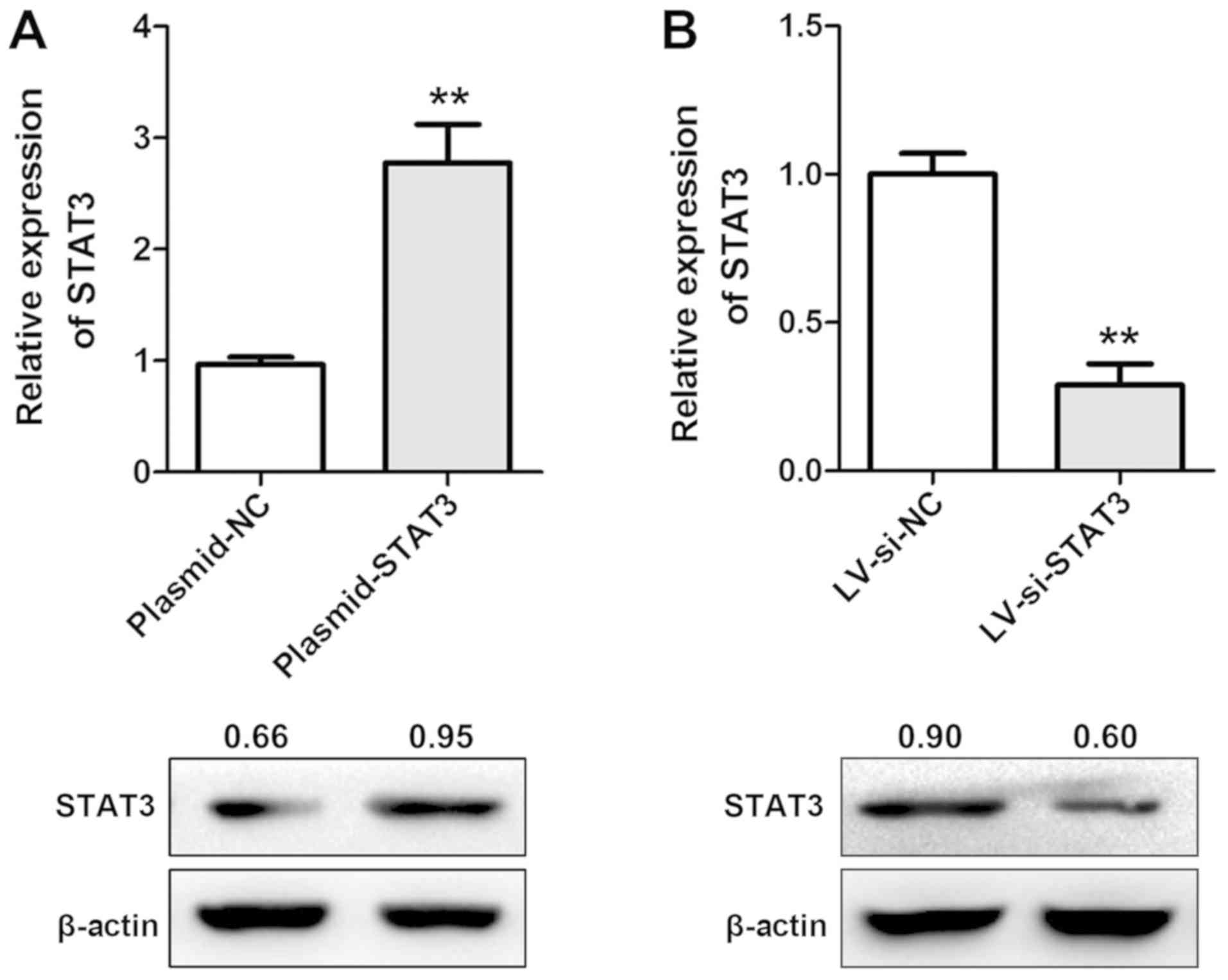
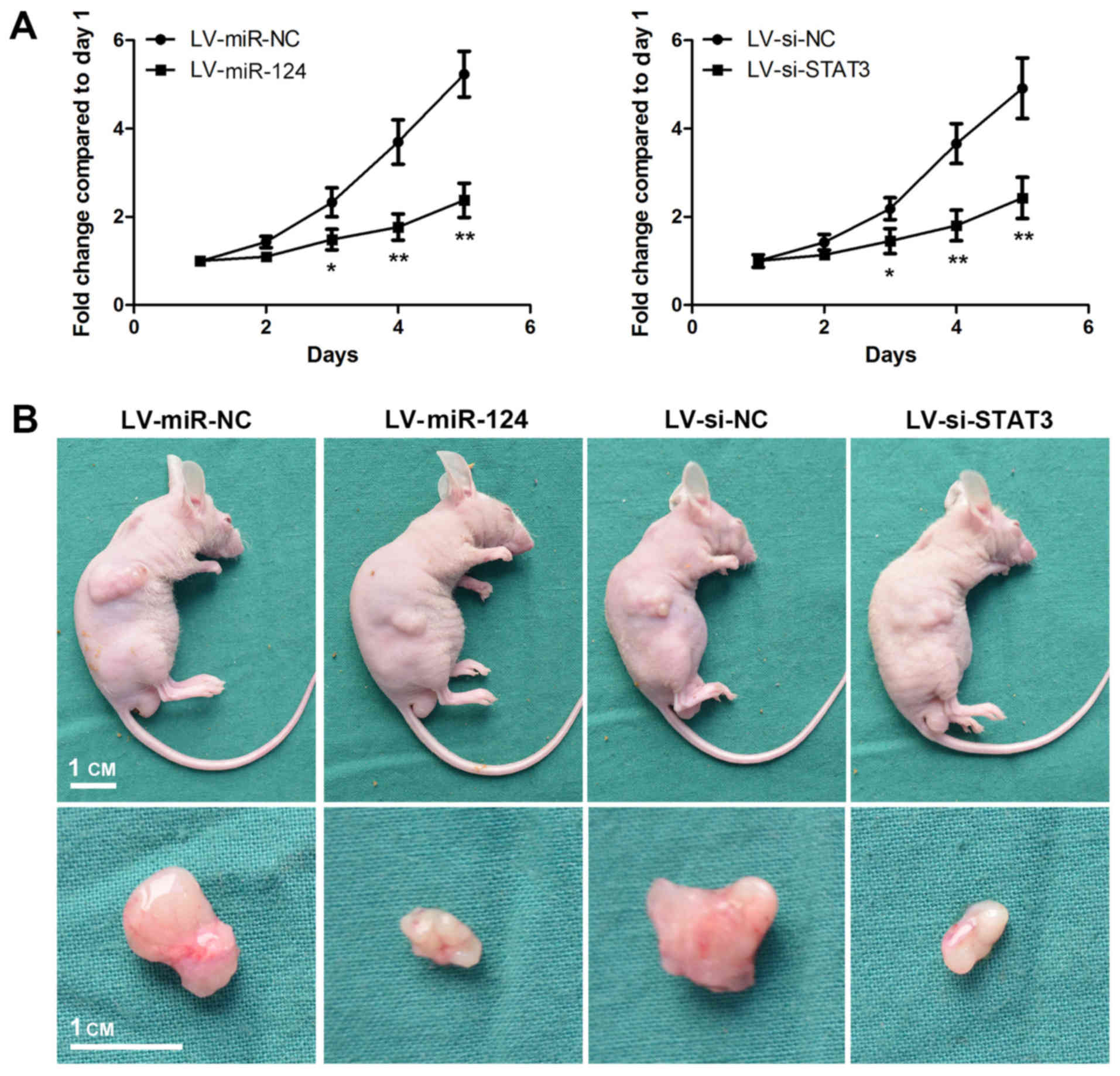
To determine the functional roles of miR-124 and
STAT3 on tumour proliferation, MDA-MB-468 cells were stably
transfected with LV-miR-124 and LV-si-STAT3 (Fig. 6). LV-si-STAT3 suppressed the
expression of STAT3 and decreased the growth rate of MDA-MB-468
cells (Fig. 7A). A similar result
was observed in LV-miR-124-transfected cells. MDA-MB-468 cells
stably transfected with LV-miR-124 or LV-si-STAT3 wee
subcutaneously injected into the right flanks of mice. Mice were
sacrificed 3 weeks later and images of representative tumours from
each group were captured (Fig.
7B). The sizes of tumours from mice in the LV-miR-124 and
LV-si-STAT3 groups were notably decreased compared with mice from
the control group. These results suggested that the overexpression
of miR-124 may suppress the proliferation of MDA-MB-468 cells in
vivo via the suppression of STAT3 expression.
Discussion
Cancer is a genetic disease that harbours various
gene alterations and mutations (25). Cancer cells exhibit replicative
immortality and activated invasion (26). STAT3, a member of the STAT protein
family, is activated by Janus kinases in response to cytokines and
growth factors. As a result, the activated STAT3 molecule
translocates to the cell nucleus and functions as a transcriptional
activator to modulate cell growth, apoptosis and invasion (27,28).
Constitutive activation of STAT3 has been suggested to contribute
to the carcinogenesis of human breast cancer and targeting STAT3
may serve as an effective strategy to suppress the growth and
invasion of breast cancer cells (29–31).
Liao et al (18) identified
that the downregulation of STAT3 expression mediated by miR-17-5p
resulted in an increase in chemotherapy-induced apoptosis of breast
cancer cells. Furthermore, miR520c-induced degradation of STAT3
resulted in the suppression of EMT progression in breast cancer
cells (19). For these reasons,
the STAT3 gene appears to be an attractive candidate for
mediating the effects of miR-124 in breast cancer.
Regulation of gene expression includes a wide range
of mechanisms, including DNA-RNA transcription to
post-translational modifications of the proteins (32–33).
Previous evidence has suggested that miRNAs bind to the
complementary sequences within the 3′-UTR of specific target mRNAs
to suppress the post-translational expression of genes either by
mRNA degradation or translational inhibition (6,7).
miRNAs have been associated with cancer through their regulatory
effects on tumour-relevant genes and effects on numerous
cancer-associated cellular behaviours, including cell invasion,
proliferation and chemoresistance (6,7).
Therefore, the evaluation of the regulatory effects of miRNAs is
valuable for the understanding of the underlying mechanism of STAT3
overexpression in breast cancer tissues.
Prediction of the association between novel miRNAs
and diseases is of clinical significance. Certain new valuable
computational tools for miRNA-disease association prediction are
available, including Laplacian Regularized Sparse Subspace Learning
for miRNA-disease association prediction (34), Matrix decomposition and
Heterogeneous Graph Interference for miRNA-disease association
prediction (35) and Bipartite
Network Projection for miRNA-disease association prediction
(36). These software programmes
offer the advantages of high levels of efficiency and accuracy. A
total of >95% of the top 50 predictions associated with various
tumors are validated by conventional open databases (35). In the present study, the
bioinformatic analysis predicted a physical interaction between
miR-124 and the 3′-UTR of STAT3 mRNA, suggesting that STAT3 may be
a target candidate of miR-124. miR-124 expression is downregulated
in numerous cancer tissues, including breast cancer (14,15),
and ectopic expression of miR-124 resulted in the attenuation of
cell growth and invasion in the present study. Together these data
support the tumour-suppressive role of miR-124 in human breast
cancer.
In the present study, miR-124 was transfected into
the breast cancer MDA-MB-468 and MDA-MB-231 cell lines, and its
effects on cancer phenotypes were evaluated. Expression of miR-124
significantly suppressed the viability, proliferation and invasion
of breast cancer cells through the downregulation of STAT3
expression. Similar results were also observed in the in
vivo studies, wherein the overexpression of miR-124 resulted in
a decrease in tumour sizes. These data suggest that the loss of
miR-124 expression results in an increase in STAT3 expression,
leading to promotion of cell viability, growth rate and enhanced
invasive capacity. Therefore, we hypothesise that the manual
restoration of miR-124 expression may be an effective therapeutic
strategy for the treatment of patients with breast cancer. In
comparison with normal breast tissues, breast cancer tissues
exhibited a significantly decreased expression of miR-124. In
addition, the data obtained from the in vitro and in
vivo studies demonstrated the antitumour function of miR-124
was mediated through the downregulation of the expression of the
oncogene STAT3 in breast cancer cell lines, consistent with the
prediction of the bioinformatics analysis. The direct interaction
between miR-124 and the 3′-UTR of STAT3 in MDA-MB-468 cells was
verified using a dual-luciferase assay. The restoration of STAT3
expression upon transfection with the plasmid-STAT3 into the cells
stably overexpressing miR-124 abolished the suppressive effects of
miR-124 on cell growth and invasion. Taken together, these data
indicate that miR-124 suppresses the growth and invasion of breast
cancer cells via downregulation of STAT3 expression.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science
Foundation Committee of China (grant nos. 81600366 and
81702332).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL made substantial contributions towards the design
of the study and analyzed the data. PS, CC and ZW performed the
in vitro and in vivo experiments. XL and ZL collected
the clinical and histologic data. PS drafted the manuscript.
Ethics approval and consent to
participate
The study protocol followed the Declaration of
Helsinki, and all patients provided written informed consent. All
protocols and procedures were approved by the Ethics Committee of
the Central Hospital of Wuhan and the Animal Care and Use Committee
of the Central Hospital of Wuhan.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S,
Liu S, Ma S, Ma PX and Chen J: MiRNA-29b suppresses tumor growth
through simultaneously inhibiting angiogenesis and tumorigenesis by
targeting Akt3. Cancer Lett. 397:111–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun G, Sun L, Liu Y, Xing H and Wang K:
Her-2 expression regulated by downregulation of miR-9 and which
affects chemotherapeutic effect in breast cancer. Cancer Gene Ther.
24:194–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Li X, Zhu S, Zhang JG, Yang M, Qin
Q, Deng SC, Wang B, Tian K, Liu L, et al: Ectopic expression of
miR-494 inhibited the proliferation, invasion and chemoresistance
of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther.
22:729–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Deng SJ, Zhu S, Jin Y, Cui SP, Chen
JY, Xiang C, Li QY, He C, Zhao SF, et al: Hypoxia-induced
lncRNA-NUTF2P3-001 contributes to tumorigenesis of pancreatic
cancer by derepressing the miR-3923/KRAS pathway. Oncotarget.
7:6000–6014. 2016.PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Zou A, Ma L, Chen X, Wang L, Zeng
X and Tan T: miR-455 inhibits breast cancer cell proliferation
through targeting CDK14. Eur J Pharmacol. 807:138–143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Xu T, Zhou X, Liao L, Pang G, Luo W,
Han L, Zhang J, Luo X, Xie X and Zhu K: Downregulation of miRNA-141
in breast cancer cells is associated with cell migration and
invasion: Involvement of ANP32E targeting. Cancer Med. 6:662–672.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao M, Ang L, Huang J and Wang J:
MicroRNAs regulate the epithelial-mesenchymal transition and
influence breast cancer invasion and metastasis. Tumour Biol.
39:10104283176916822017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Z, Liu S, Tian L, Wu M, Ai F, Tang W,
Zhao L, Ding J, Zhang L and Tang A: miR-124 and miR-506 inhibit
colorectal cancer progression by targeting DNMT3B and DNMT1.
Oncotarget. 6:38139–38150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Ai X, Shen S and Lu S:
NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung
cancer by targeting MYO10. Oncotarget. 6:8244–8254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng XH, Huang HR, Lu J, Liu X, Zhao FP,
Zhang B, Lin SX, Wang L, Chen HH, Xu X, et al: MiR-124 suppresses
tumor growth and metastasis by targeting Foxq1 in nasopharyngeal
carcinoma. Mol Cancer. 13:1862014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai WL, Huang WD, Li B, Chen TR, Li ZX,
Zhao CL, Li HY, Wu YM, Yan WJ and Xiao JR: microRNA-124 inhibits
bone metastasis of breast cancer by repressing Interleukin-11. Mol
Cancer. 17:92018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du S, Li H, Sun X, Li D, Yang Y, Tao Z, Li
Q and Liu K: MicroRNA-124 inhibits cell proliferation and migration
by regulating SNAI2 in breast cancer. Oncol Rep. 36:3259–3266.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang
N, Hu X, Liu Z, Zhang CY, Zen K, et al: miR-124-3p functions as a
tumor suppressor in breast cancer by targeting CBL. BMC Cancer.
16:8262016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian
K, Xie Y, Wang S, Xu N, Wang Y and Qi C: miR-124 downregulation
leads to breast cancer progression via LncRNA-MALAT1 regulation and
CDK4/E2F1 signal activation. Oncotarget. 7:16205–16216.
2016.PubMed/NCBI
|
|
18
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. 8:15763–15774. 2016.
|
|
19
|
Wang N, Wei L, Huang Y, Wu Y, Su M, Pang
X, Wang N, Ji F, Zhong C, Chen T and Li B: miR520c blocks EMT
progression of human breast cancer cells by repressing STAT3. Oncol
Rep. 37:1537–1544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu F, Zhang H and Song H: Upregulation of
MEK5 by Stat3 promotes breast cancer cell invasion and metastasis.
Oncol Rep. 37:83–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McDaniel JM, Varley KE, Gertz J, Savic DS,
Roberts BS, Bailey SK, Shevde LA, Ramaker RC, Lasseigne BN, Kirby
MK, et al: Genomic regulation of invasion by STAT3 in triple
negative breast cancer. Oncotarget. 8:8226–8238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang Y, Liao XH, Yu CX, Yao A, Qin H, Li
JP, Hu P, Li H, Guo W, Gu CJ and Zhang TC: MiR-93-5p inhibits the
EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp Cell
Res. 357:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watson IR, Takahashi K, Futreal PA and
Chin L: Emerging patterns of somatic mutations in cancer. Nat Rev
Genet. 14:703–718. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ilamathi M, Prabu PC, Ayyappa KA and
Sivaramakrishnan V: Artesunate obliterates experimental
hepatocellular carcinoma in rats through suppression of
IL-6-JAK-STAT signalling. Biomed Pharmacother. 82:72–79. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
29
|
Tkach M, Rosemblit C, Rivas MA, Proietti
CJ, Díaz Flaqué MC, Mercogliano MF, Beguelin W, Maronna E, Guzmán
P, Gercovich FG, et al: p42/p44 MAPK-mediated Stat3Ser727
phosphorylation is required for progestin-induced full activation
of Stat3 and breast cancer growth. Endocr Relat Cancer. 20:197–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X and Huang L: LRSSLMDA: Laplacian
regularized sparse subspace learning for MiRNA-disease association
prediction. PLoS Comput Biol. 13:e10059122017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Yin J, Qu J and Huang L: MDHGI:
Matrix decomposition and heterogeneous graph inference for
miRNA-disease association prediction. PLoS Comput Biol.
14:e10064182018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Xie D, Wang L, Zhao Q, You ZH and
Liu H: BNPMDA: Bipartite network projection for MiRNA-disease
association prediction. Bioinformatics. 34:3178–3186. 2018.
View Article : Google Scholar : PubMed/NCBI
|