Introduction
Circular RNAs (circRNAs) are an important type of
non-coding endogenous RNA molecules involved in regulating gene
expression (1). These molecules
were first identified in the 1970s in the RNA virus (2). In 1979, Hsu and Coca-Prados used
electron microscopy to observe, for the first time, that RNA could
be present in the cytoplasm of eukaryotic cells in a cyclic form
(3). Due to the restriction of the
technical capabilities at that time, circRNA was only considered to
be a type of low-abundance RNA molecule formed by the incorrect
splicing of exon transcripts. However, with the continuous
improvement of science and technology, especially widely used in
bioinformatics and RNA sequencing techniques, it hasbeen
established that transcripts of a number of exons can be used to
form circRNA through non-linear reverse splicing or gene
rearrangement (4).
In contrast to traditional linear RNA, circRNAs are
a type of non-coding RNA existing with 1–5 exons forming a closed
loop structure. With the rapid development of science and
technology circRNAs are now known to be extensively expressed in
mammals, have conserved sequences and exhibit structural stability
(5–8). More and more studies have
demonstrated that circRNAs have important roles in the expression
of various regulatory genes (9–11).
For example, circRNA-forkhead box protein O3 has been demonstrated
to form ternary complexes with P21 and cyclin dependent kinase 2 to
regulate cell cycle changes (12).
Additionally, hsa_circ-0067934 can promote the proliferation of
esophageal squamous cell carcinoma (13). However, the majority of previous
studies have indicated that the main function of circRNAs is to act
as an microRNA (miRNA/miR) sponge, achieving the effect of
controlling genes by inhibiting the activity of miRNAs (14,15).
Bone defects and bone shortening caused by trauma, inflammation and
surgical treatment of tumors remains a medical problem threatening
human health. Notably, in dental clinics, bone defects tend to
affect the efficiency of dental implant restoration, which can
reduce patients' quality of life; the quality and quantity of bone
is a key consideration following implant restoration (16). Gene expression and cellular
ultrastructures indicate that bone marrow mesenchymal stem cells
(BMSCs) have the potential for self-renewal and multi-directional
differentiation (17) into cells
of various tissues, including fat, bone and muscle, depending on
specific internal and external conditions. In addition, BMSCs are
crucial for normal bone development and maintenance of bone
metabolism (18). Therefore,
increasing the proliferation ability of BMSCs may be of great
importance for the repair of bone defects.
Calcitonin gene-related peptide (CGRP) is a
biologically active polypeptide consisting of 37 amino acids
expressed in the nervous system, respiratory system, digestive
system, cardiovascular system and skeleton (19). CGRP is currently the most potent
endogenous vasodilator peptide that has been identified (20). CGRP is also a type of neuropeptide,
with its highest expression level being in peripheral nerves.
Studies have revealed that the expression of CGRP in bone marrow
cells, osteoblasts and other cells in bone tissue has an important
role in bone repair and reconstruction. CGRP can promote the
differentiation and proliferation of osteoblasts, enhance the
proliferation of BMSCs, promote BMSC differentiation into
osteoblasts and inhibit the formation of osteoclasts (21–23).
Previous studies have reported that CGRP effectively promotes BMSC
proliferation and osteogenic differentiation (24,25).
However, whether CGRP regulates cell proliferation and
differentiation via circRNAs has not been previously investigated.
In the present study, the role of circRNAs in the CGRP-induced
proliferation of BMSCs was investigated to determine whether
circRNAs are part of the regulatory mechanism involved, aiming to
provide a basis for the further improvement of the osteogenic
ability of BMSCs.
Materials and methods
Cell culture and verification of
optimum concentration and time point for CGRP treatment of
BMSCs
BMSCs were purchased from Cyagen Biosciences Inc.,
Guangzhou, China (cat. no. MUBMX-01001) and cultured at 37°C with
5% CO2 in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.). BMSCs were seeded
on 6-well plates culture dishes at density of 1×105
cells per well. In the concentration experiments, BMSCs were
stimulated with different concentrations (10−7,
10−8, 10−9, 10−10,
10−11 or 0 M) of CGRP (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 1, 3 or 7 days. The cells were then treated
with Cell Counting Kit-8 (CCK8; Dojindo, Molecular Technologies,
Inc., Kumamoto, Japan) reagent for 2 h in the dark to assess the
BMSCs proliferation rate. The absorbance of the culture media was
measured with a microplate reader (Thermo Fisher Scientific, Inc.)
at 450 nm.
Microarray hybridization
The BMSCs were divided into 2 groups: The control
group and the subject group. In the subject group, the cells
(1×105) were treated with 10−9 M CGRP for 3
days. In the control, the cells were untreated. The total and
circular RNAs of the 2 groups was isolated using TRIpure (Aidalb
Biotechnologies Co., Ltd., Beijing, China). Total RNA and RNA in
each group were quantified using NanoDrop 2000 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). Sample labeling and array
hybridization were performed according to the protocol of the
manufacturer (Arraystar, Inc., Rockville, MD, USA). Briefly,
circRNA was treated with RNase R (Epicentre; Illumina, Inc., San
Diego, CA, USA) to remove linear RNA. Each sample was then
amplified and transcribed into fluorescent cRNA using the random
priming method (Arraystar Super RNA Labeling kit; Arraystar, lnc.).
The labeled cRNA was purified using an RNeasy Mini kit (Qiagen,
Inc., Valencia, CA, USA). The concentration and specific activity
of the labeled cRNA (pmol Cy3/µg cRNA) were measured using a
NanoDrop ND-1000. Each labeled cRNA (1 µg) was fragmented by the
addition of 5 µl 10X blocking agent and 1 µl 25X fragmented buffer
solution. Then, the mixture was heated at 60°C for 30 min. Finally,
25 µl 2X hybridization buffer was added to dilute the labeled cRNA.
Hybridization solution (50 µl) was dispensed into the gasket slides
and assembled onto the circRNA expression microarray slides which
were then incubated in an Agilent hybrid box at 65°C for 17 h, with
the hybrid array washed, scanned by the G2505C Agilent scanner
(version 11.0.1.1; Agilent Technologies, Inc., Santa Clara, CA,
USA).
Microarray data analysis
Quantile normalization of the original data and
subsequent data processing was performed using the R software
package (version 3.1.2) (25).
Following quantile normalization of the original data was
performed, low-intensity filtering was carried out and the circRNAs
with P or M (‘all target values’) tagged in at least one or two
samples were retained for further analysis. When comparing the two
sets of contours, the ‘fold-change’ (namely, the ratio of the group
mean) between groups of circRNAs was calculated. The statistical
significance of the differences between groups was determined using
a t-test. CircRNAs exhibiting a significant difference in
expression exhibited a fold-change >2 and a P-value <0.05.
The sorting and filtering function of Microsoft Excel was used to
filter data, analyze output and sort the circRNAs with differential
expression according to the fold-changes and P-values. In addition,
the interaction between circRNAs and miRNAs was predicted using The
relationship between circRNAs and miRNAs was predicted by miRNA
target prediction software based on TargetScan (http://www.targetscan.org/), miRanda (http://www.microrna.org/), miRDB (http://www.mirdb.org/). All differentially expressed
circRNAs were annotated in detail with information on the
interaction between the circRNAs and miRNAs. Cytoscape (version
3.6.0; http://cytoscape.org) software was used
to generate a network map, of upregulated circRNAs and their
corresponding miRNAs.
Reverse transcription-quantitative
polymerase chain reaction (PCR) and nucleic acid
electrophoresis
Primer premier 5.0 software was used for
mmu_circRNA_003795 primer design. The sequence of the designed
primer and the sequence of mmu_circRNA_003795 were compared with
Blast database (https://blast.ncbi.nlm.nih.gov/) sequences, and the
forwards and reverse primer were matched with the sequence of
mmu_circRNA_003795. The total RNA of BMSCs was extracted using the
TRIzol method (Thermo Fisher Scientific, Inc.). The quantity and
quality of RNA were determined using a Nano Drop 2000 and by
electrophoresis on a 10 g/l agarose gel. Following RNA extraction,
SuperScript III reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to synthesize cDNA according to the
manufacturer's protocol. Subsequently, PCR was performed using 12.5
µl SYBR Premix EX Taq (Takara Bio, Inc., Otsu, Japan), 0.5 µl
forward primer (10 pmol/l), 0.5 µl reverse primer (10 pmol/l;
Table I), 1 µl cDNA and
ddH2O was added to a final volume of 25 µl. The reaction
conditions were as follows: 95°C for 30 sec; and 95°C for 5 sec,
55°C for 30 sec and 72°C for 30 sec for 40 cycles. The instrument
automatically generated the cycle quantification (Cq) value of mRNA
and the internal reference gene, GAPDH. Cq value difference (ΔCq)
indicated the relative expression of each mRNA investigate
(26). DNA samples were separated
by electrophoresis at 110 V for 30 min and observed using a gel
imager.
 | Table I.The primers of the detected
genes. |
Table I.
The primers of the detected
genes.
| Name | Primer |
|---|
| FOSL2 | F:
5′-AGCAGGGATGGACAAGAC-3′ |
|
| R:
5′-TGGGGTAGGTGAAGACAA-3′ |
| ALP | F:
5′-GAGGCATACGCCATCACATG-3′ |
|
| R:
5′-CCGATGGCACACCTGCTT-3′ |
| OCN | F:
5′-TCTGACAAAGCCTTCATGTCCA-3′ |
|
| R:
5′-AACGGTGGTGCCATAGAT-3′ |
| GAPDH | F:
5′-AAGAAGGTGGTGAAGCAGG-3′ |
|
| R:
5′-GAAGGTGGAAGAGTGGGAG-3′ |
| OSX | F:
5′-GCAAATGACTACCCACCCTT-3′ |
|
| R:
5′-ACGAGCCATAGGGATGAGTC-3′ |
| Runx2 | F:
5′-ACTTGTGGCTGTTGTGATG-3′ |
|
| R:
5′-TTGCTGTTGCTGTTGTTG-3′ |
| mmu_circRNA_ | F:
5′-CCTTAGCACCTGCCTTCTTG-3′ |
| 003795 |
|
| R:
5′-AGTTCACCCACTGTGCCTCC-3′ |
| miR-504-3p |
5′-AGCAGGGCAGGGTTTCAAA-3′ |
| U6 |
5′-GCGCGTCGTGAAGCGTTC-3′ |
| Inhibitor- |
5′-AGGGAGAGCAGGGCAGGGUUUC-3′ |
| miR-504-3p |
CircRNA and miRNA interference
The small interfering (si)RNAs targeting
mmu_circRNA_003795 were designed and synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). The sequences of siRNA were
as follows: (Sense 5′-GCUAGAACAGCAUGGUCCAdTdT-3′ and anti-sense
3′-dTdTCGAUCUUGUCGUACCAGGU-5′). The target sequence corresponding
to the sense and antisense siRNA oligonucleotides was as follows:
5′-GCTAGAACAGCATGGTCCA-3′. Negative control siRNA was designed and
synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China; the
sequence is confidential). The mmu-miR-504-3p inhibitor was
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The
sequence was as follows: 5′-AGGGAGAGCAGGGCAGGGUUUC-3′. BMSCs
(1×105) were transfected with 20 nM siRNA or inhibitor
using GenMute™ siRNA Transfection kit and Transfection Buffer
(SignaGen Laboratories, Rockville, MD, USA) according to the
manufacturer's protocol. Then the cells were incubated for 72 h at
37°C prior to the sequential tests.
Western blotting
Protein was extracted using radioimmunoprecipitation
assay buffer [50 mM Tris HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P40
and 0.1% sodium dodecyl sulfate] and phenylmethanesulfonyl fluoride
at 4°C. Bicinchoninic acid protein assay kit (BestBio, Shanghai,
China) was used to determine the concentration. Total protein (10
µg per well) was separated using 10% SDS PAGE and then transferred
to a polyvinylidene difluoride membrane. The membranes were blocked
using skimmed milk for 1 h at room temperature, then the blots were
incubated with primary antibody against FOS like 2 AP-1
transcription factor subunit (FOSL2; 1:1,000; ab124830; Abcam,
Cambridge, UK) overnight at 4°C. Following washing in 0.1% Tween
PBS-T, the blots were incubated in goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:5,000; ab6789; Abcam,
Cambridge, UK) for 1 h at room temperature. Proteins were detected
using electrochemiluminescence (ECL kit; Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). Image J (version k1.45;
National Institutes of Health, Bethesda, MD, USA) was used to
measure densitometry.
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK8; Dojindo, Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol.
Transfected cells were seeded in 96-well plates (3,000 cells per
well) with proliferation detected at 24 h intervals. Briefly, 10 µl
CCK-8 solution was added to each hole and incubated at 37°C for 2
h. Absorbance was then measured at 450 nm was measured using a
spectrophotometer.
Flow cytometry analysis
Cells were washed and resuspended in cold PBS and
incubated in ice-cold 70% ethanol for 3 h. The cells were then
centrifuged at 845 × g and 4°C for 10 min and resuspended in
PI/RNase Staining Buffer master mix (BD Pharmingen, BD Biosciences,
Franklin Lakes, NJ, USA; 40 mg/ml PI and 100 mg/ml RNase in PBS) at
a density of 5×105 cells/ml and incubated at 37°C for 30
min prior to flow cytometry analysis by flow cytometry (27). (BD FACSCanto II, BD Biosciences;
ModFit LT 3.2, Verity Software House, Inc., Topsham, ME, USA).
Statistical analysis
All data were analyzed using the SPSS 11.5 software
package (SPSS, Inc., Chicago, IL, USA). The nominal data, including
the genotype and allele frequency were calculated by a direct gene
counting method. Genotype and allele frequencies of each group were
analyzed using χ2. Additionally, the odds ratio and 95%
of confidence interval were used to indicate the relative risk.
Continuous data was expressed as the mean ± standard deviation and
the comparison of the data between groups was performed using
t-test or analysis of variance according to the nature of the data.
Each experiment was repeated three times. The data of three groups
were evaluated using analysis of variance and least significant
difference post hoc tests when the variance was normal, otherwise
Dunnett's T3 test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
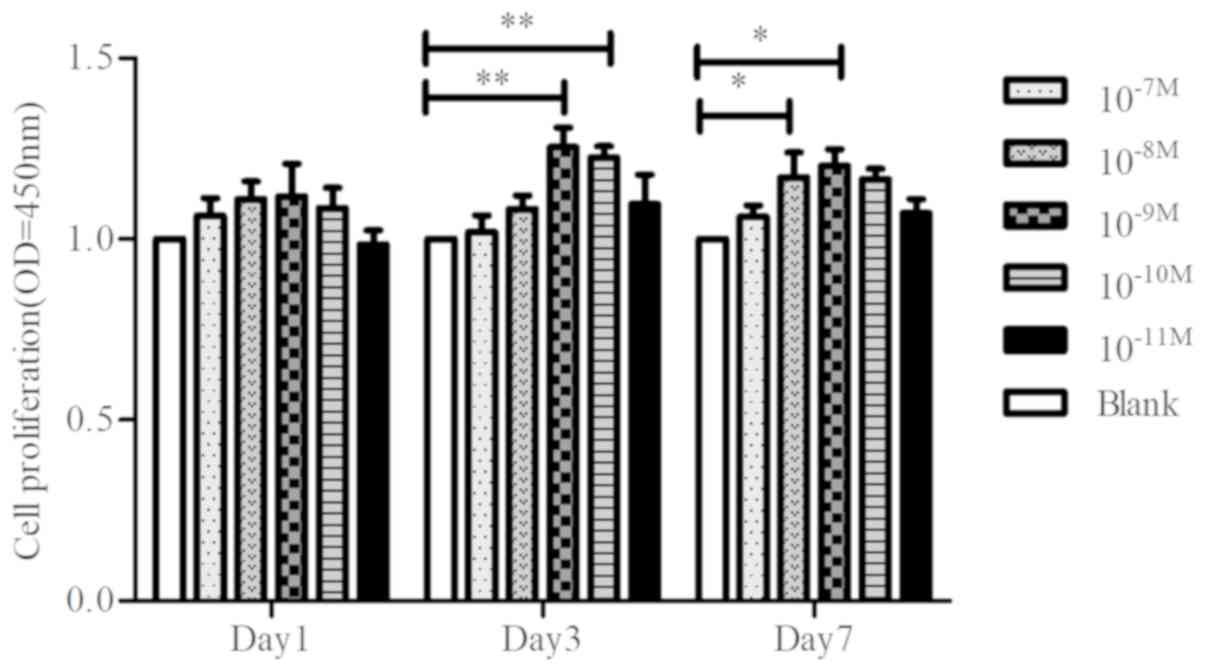
Verification of optimum concentration
of CGRP on cell proliferation of BMSCs
To determine the effect of CGRP on proliferation of
BMSCs, cells were stimulated with different concentrations
(10−7, 10−8, 10−9,
10−10, 10−11 or 0 M) of CGRP for 1, 3 or 7
days. The CCK-8 assay demonstrated that CGRP stimulated
proliferation at 10−9 M (Fig. 1).
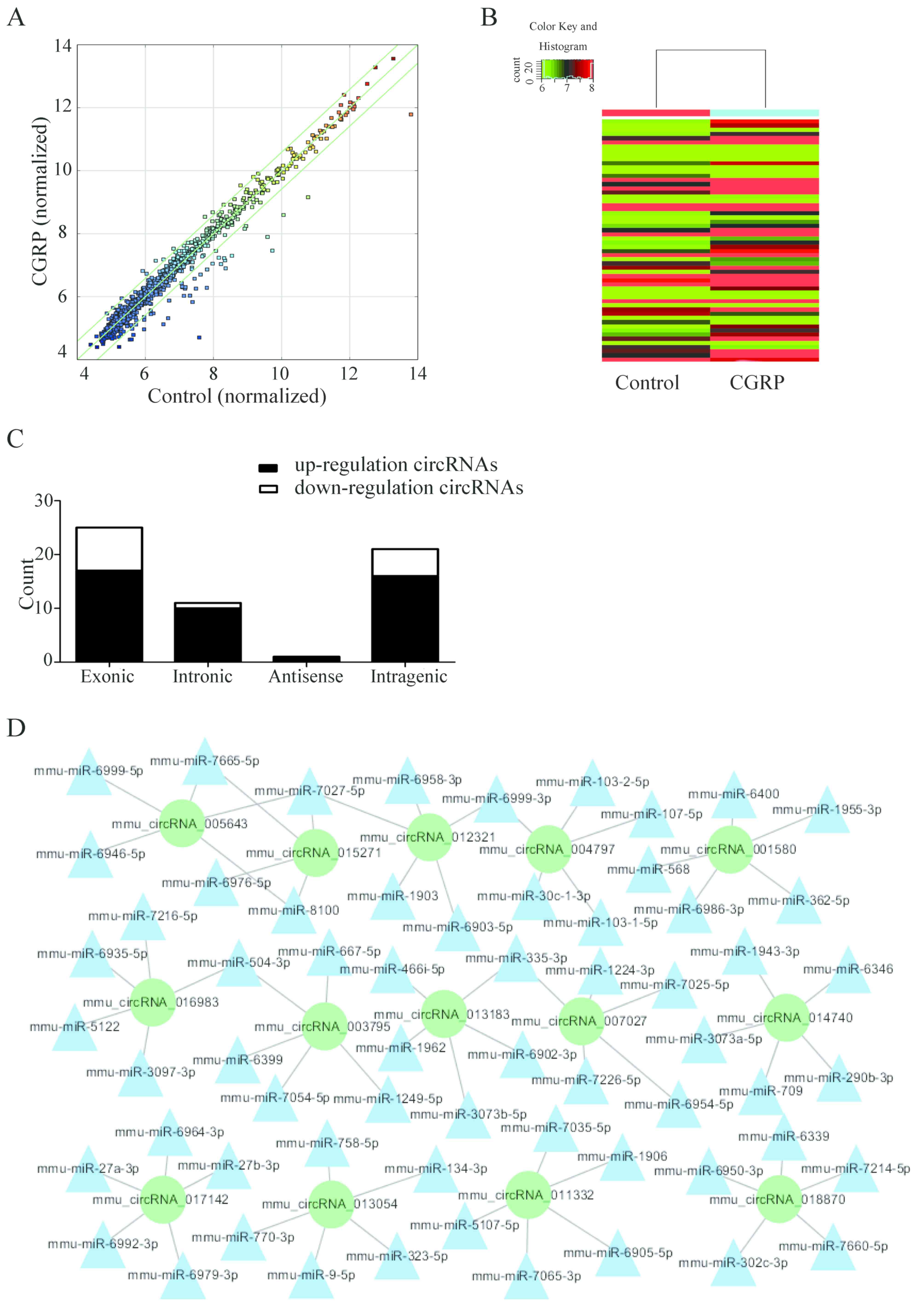
Microarray data analysis
High-throughput chip data analysis identified a
total of 58 differentially expressed circRNAs when comparing the
two treatment groups (the cells were treated with 10−9M
CGRP for 3 days and the control cells were untreated). The scatter
diagram and hierarchical clustering demonstrate that expression of
circRNAs of the CGRP-treated BMSCs was different compared with the
blank control group (Fig. 2A and
B). The differentially expressed circRNAs included 17 exonic,
16 intragenic and 10 intronic-derived RNAs (Fig. 2C). More circRNAs were downregulated
than upregulated (44 and 14, respectively). The seed sequences of
the circRNA were compared with miRNA sequences to identify the
miRNAs that potentially bind with circRNAs (Fig. 2D). Cytoscape software was used to
generate a network map, of upregulated circRNAs and their
corresponding miRNAs and target genes.
Microarray data analysis, identified the upregulated
and downregulated circRNAs, and those with more predicted target
area that could combine with miRNA were selected. Therefore, the
miRNAs that bind with the differentially expressed circRNA were
predicted. miRDB (http://www.mirdb.org/), TargetScan (http://www.targetscan.org/) and mirbases (http://www.mirbase.org/) were used to determine the
corresponding target genes of these miRNAs. Target genes with at
least two interrelated predictions in the above databases were
selected for further investigation. According to the analysis,
mmu_circRNA_003795, miR-504-3p and FOSL2 may have an influence on
the proliferation and differentiation of BMSCs.
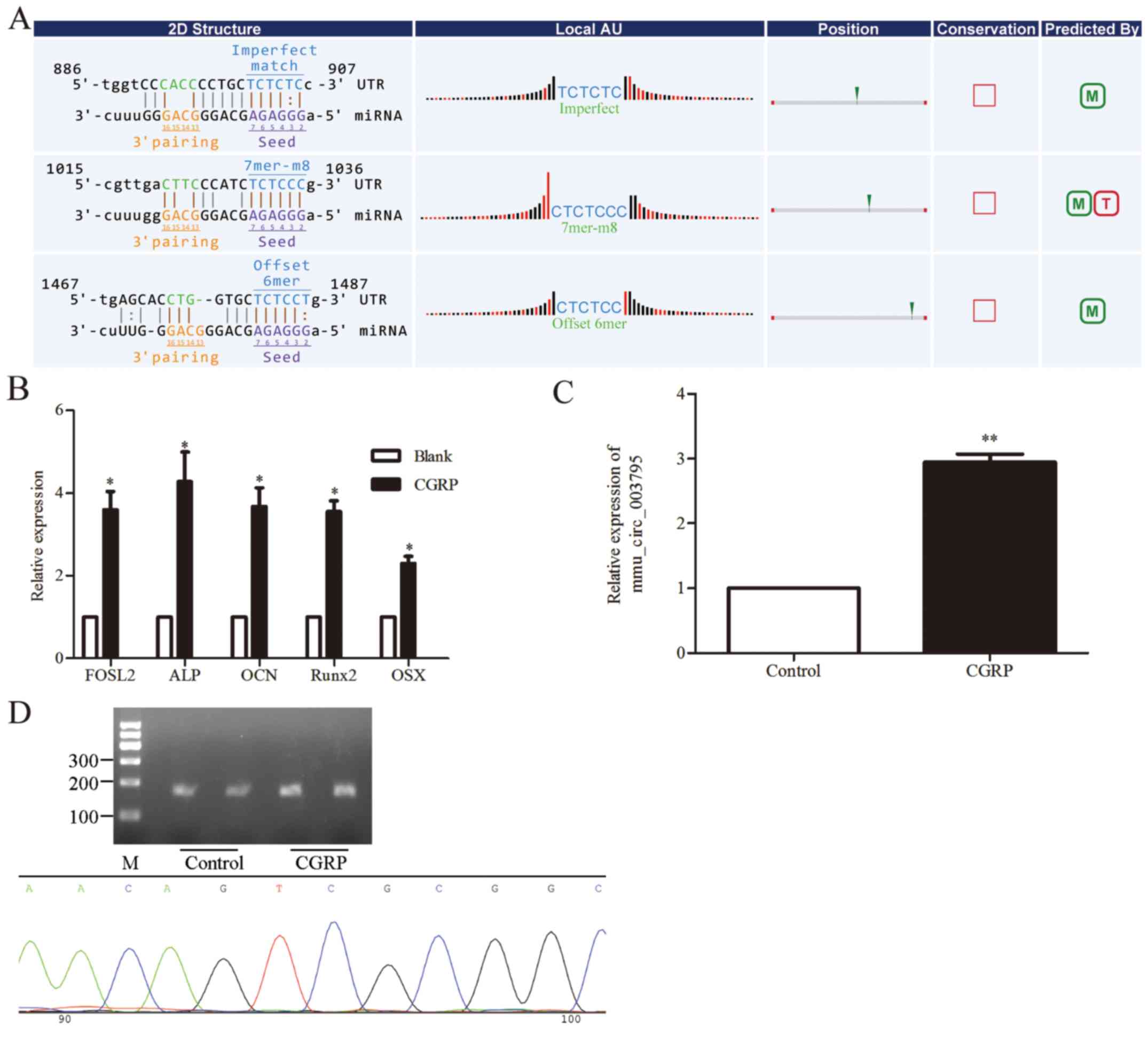
Verification of the expression of
mmu_circRNA_003795 in BMSCs
As demonstrated in Fig.
3A, the potential binding sites of mmu_circRNA_003795 and
miR-504-3p were predicted using bioinformatics tools. Furthermore,
RT-qPCR was used to analyze the expression of
osteogenesis-associated genes in cells treated with CGRP and the
blank control group. Osteogenic and proliferation associated genes
were significantly upregulated by CGRP, as demonstrated by the
following: FOSL2, 3.6-fold (P=0.027); alkaline phosphatase, tissue
nonspecific isozyme (ALP), 4.3-fold times (P=0.044); osteocalcin
(OCN), 3.7-fold (P=0.027); runt-related transcription factor 2
(Runx2), 3.6-fold (P=0.01) and Sp7 transcription factor (OSX),
2.3-fold (Fig. 3B; P=0.017).
Primer premier 5 software was used for mmu_circRNA_003795 primer
design. As part of the design, it was required that the product
should be able to cross the ring formation node. The sequence of
the designed primer and the sequence of mmu_circRNA_003795 were
compared with Blast database sequences, and the forwards and
reverse primer were perfectly matched with the sequence of
mmu_circRNA_003795. RT-qPCR was used for validation of the primers.
The expression of mmu_circRNA_003795 in the CGRP-stimulated cells
was significantly 2.9-fold higher compared with the control group;
the results were in accordance with the microarray data (P=0.004;
Fig. 3C). Additionally,
electrophoresis was used to test the PCR product, confirming the
upregulated expression of mmu_circRNA_003795 (Fig. 3D). Electrophoresis clearly
demonstrated that the PCR product was the expected size and a
single band. Furthermore, compared with the blank control group,
the mmu_circRNA_003795 expression in the CGRP-stimulated BMSC group
was upregulated. The PCR product was sequenced and matched the
mmu_circRNA_003795 sequence, including the ring formation node
(Fig. 3D). It was demonstrated
that the sequence of the product was almost an exact match with the
mmu_circRNA_003795 sequence (positive 97% and negative 100%) and
contained the ring formation node.
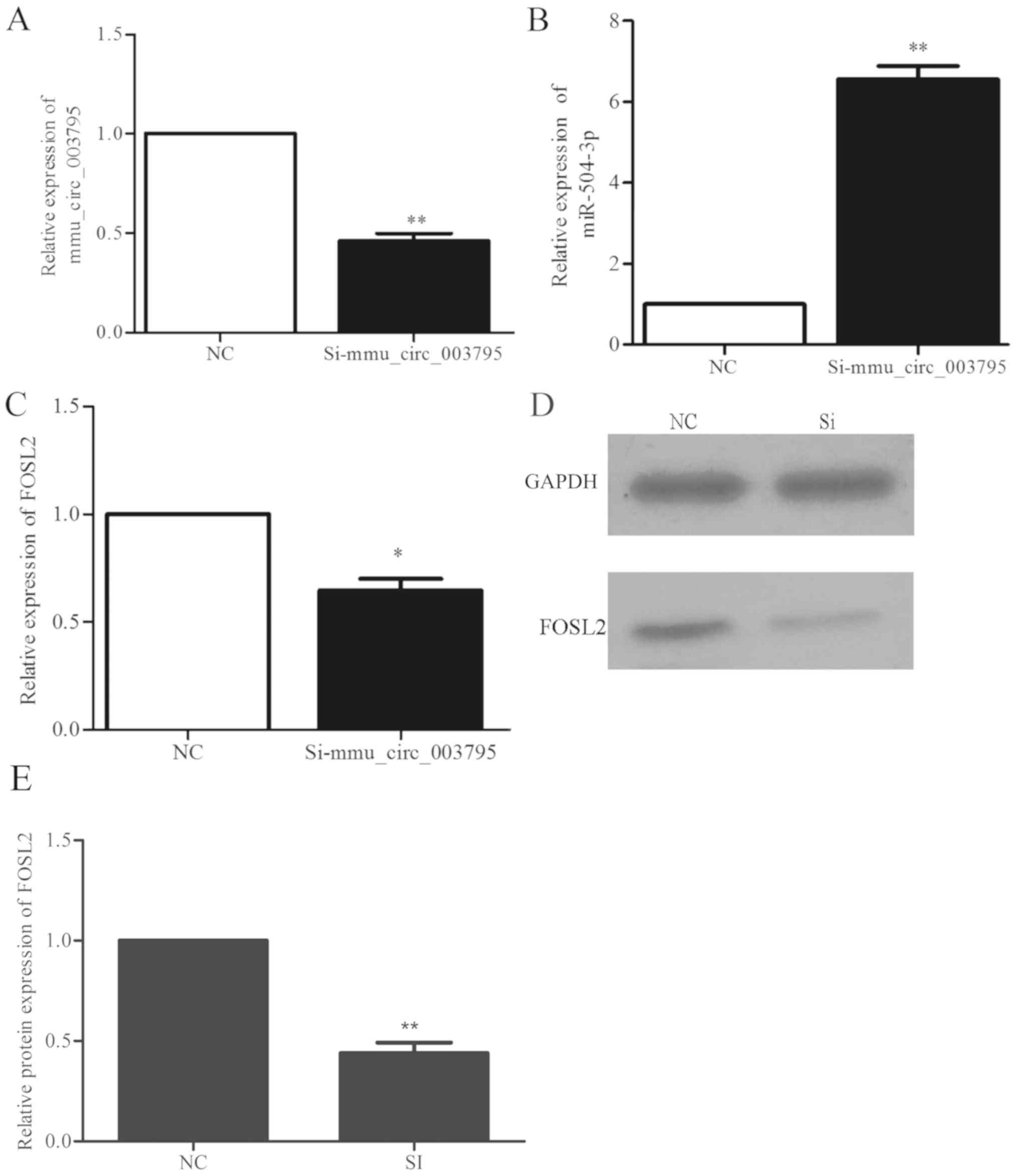
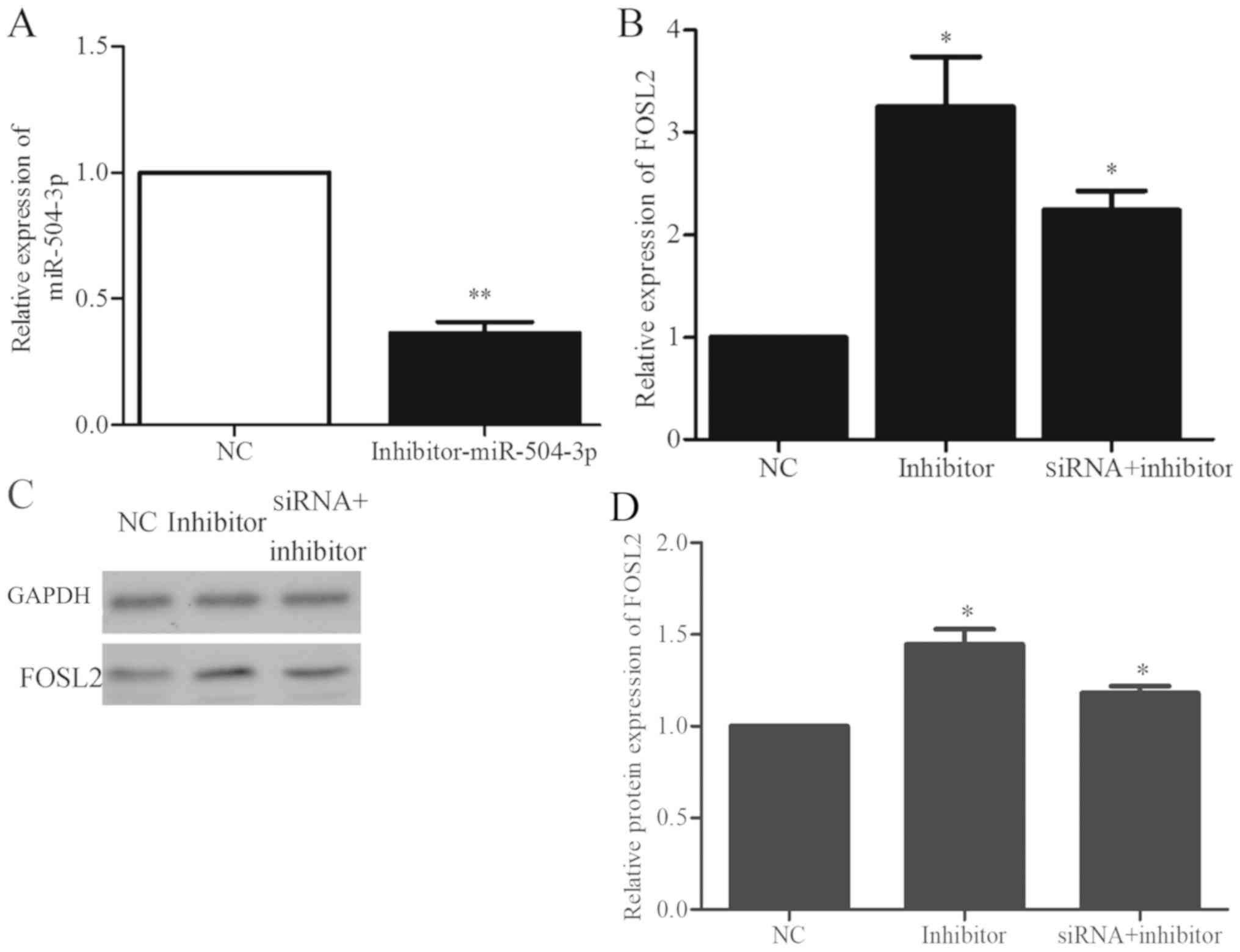
Alterations in the expression of
miR504-3p and FOSL2 following mmu_circRNA_003795 interference
The siRNA sequences were designed to silence
mmu_circRNA_003795. At 72 h following transfection, the
mmu_circRNA_003795 expression in the siRNA group and negative
control group were detected by RT-qPCR. The expression of
mmu_circRNA_003795 in the siRNA group was 0.46-fold decreased
compared with the negative control group (P=0.005), which indicated
that si-mmu_circRNA_003795 significantly inhibited the expression
of mmu_circRNA_003795 (Fig. 4A).
Following successful silencing of mmu_circRNA_003795, the
expression of miR-504-3P and FOSL2 was determined by RT-qPCR. The
results demonstrated that the expression of miR-504-3P was
significantly increased by 6.6-fold (P=0.004; Fig. 4B) and the expression of FOSL2 was
significantly decreased by 0.6-fold (P=0.024; Fig. 4C) following mmu_circRNA_003795
silencing. The western blot analysis demonstrated that silencing of
mmu_circRNA_003795 reduced the expression of FOSL2 protein
(Fig. 4D and E).
Alterations in the expression of FOSL2
following miR504-3p interference
An miRNA inhibitor sequence was used to block the
function of miR-504-3p. At 72 h following transfection, miR-504-3p
expression was detected by RT-qPCR. As demonstrated in Fig. 5A, the miRNA inhibitor significantly
decreased the expression of miR-504-3p by 0.363-fold (P=0.005).
Following the use of an inhibitor to silence miR-504-3p expression,
the expression of the FOSL2 mRNA was also detected. The expression
of FOSL2 was significantly increased by 3.3-fold (P=0.044; Fig. 5B) when the inhibitor of miR-504-3p
was used compared with the negative control. Additionally, BMSCs
were co-transfected with si-mmu_circRNA_003795 and miR-504-3p
inhibitor for 72 h. Compared with the control group, the mRNA
expression of FOSL2 was significantly increased by 2.24-fold
(P=0.022; Fig. 5B). Western blot
analysis demonstrated that inhibition of miR-504-3p increased the
expression of FOSL2 protein and expression was also increased in
the si-mmu_circRNA_003795 and miR-504-3p inhibitor-g groups
(Fig. 5C and D).
mmu_circRNA_003795 promotes cell
proliferation and cell cycle changes
The CCK-8 assay was used to detect cell
proliferation. The proliferation of BMSCs in the negative control
group was significantly increased compared with the
si-mmu_circRNA_003795 group (24 h, P=0.001; 48 h, P=0.014; 72 h,
P=0.042), which indicated that mmu_circRNA_003795 promotes the
proliferation of BMSCs (Fig. 6A).
Additionally, flow cytometry was used to analyze the cell cycle in
cells with silenced mmu_circRNA_003795. The si-mmu_circRNA_003795
group exhibited more cells in G0-G1 phase and
less cells in the G2-M and S phase compared with the
negative control group (G1, P=0.016; G2,
P=0.018; S, P=0.039; Fig. 6B),
indicating that mmu_circRNA_003795 may regulate the proliferation
of BMSCs cells by altering the cells cycles.
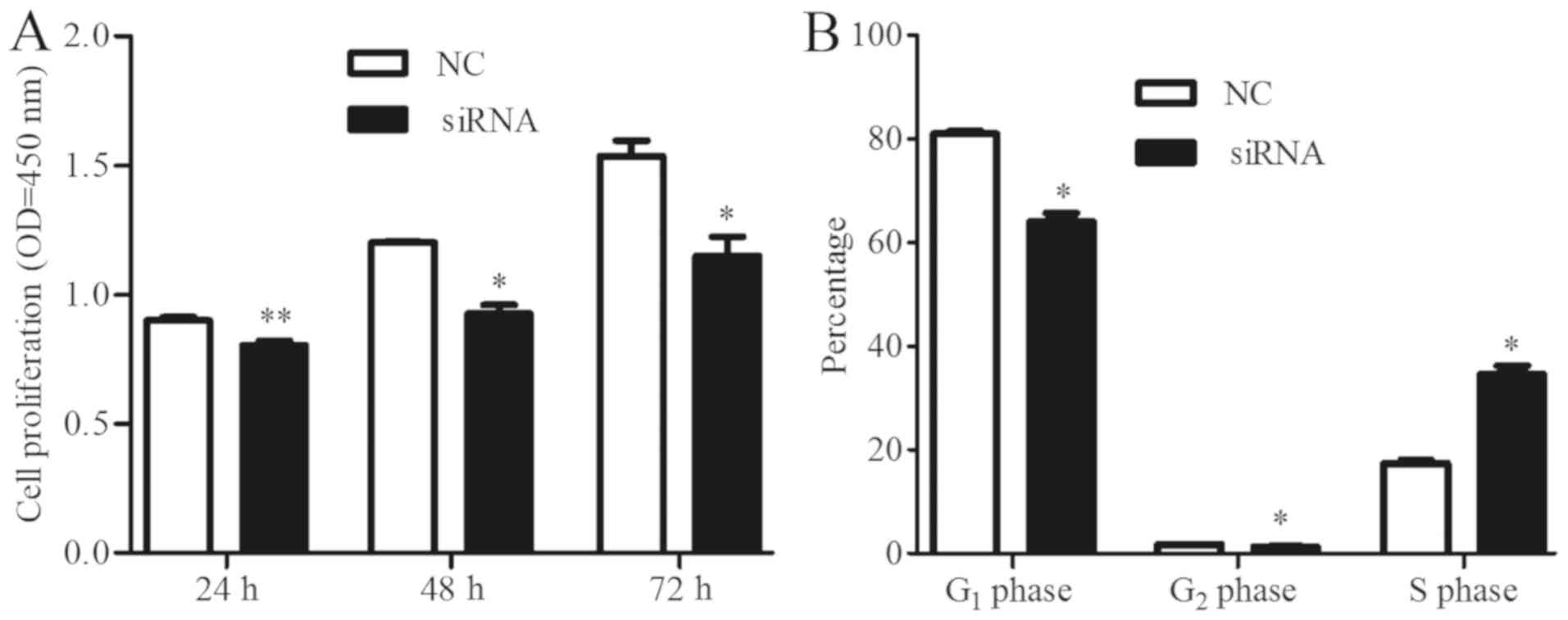 | Figure 6.mmu_circRNA_003795 promotes cells
proliferation and changes in the cell cycle. (A) Cell Counting
Kit-8 was used to detect cell proliferation of bone marrow stem
cells at 24, 48 and 72 h following transfection with
si-mmu_circRNA_003795 (24 h, P=0.001; 48 h, P=0.014; 72 h P=0.043).
(B) Flow cytometry analysis of the cell cycle in
si-mmu_circRNA_003795 cells and negative control cells (G1,
P=0.016; G2, P=0.018; S, P=0.039). *P<0.05 and **P<0.01 vs.
NC. circRNA, circular RNA; NC, negative control; si, small
interfering; OD, optical density. |
Discussion
circRNAs are circular RNA molecules usually composed
of more than one exon. These RNAs are present in eukaryotic cells,
with time and disease specificity (6). circRNAs are an increasingly important
research area with huge development potential, in addition to the
vast amount of studies that have investigated miRNA. Through
interaction with miRNAs associated with growth, development and
diseases, circRNAs can have important regulatory roles with the
potential to be used a novel type of clinical diagnostic marker and
as a tool to regulate cell growth and development (28,29).
CGRP was the first active polypeptide extracted using molecular
genetics and is widely distributed in bone tissue (30,31).
The effect of CGRP on the function of bone tissue and bone cells
has been widely investigated in previous years. According to the
studies of Wang et al (31)
CGRP promotes the expression of osteogenic genes and downregulates
tumor necrosis factor ligand superfamily member 11 to inhibit the
formation of osteoclasts, resulting in increased bone density. In
previous studies, 10−9 M CGRP was able to promote cell
proliferation (32,33). Wang et al (31) used different concentrations of CGRP
(10−8, 10−10 and 10−12 M) on BMSCs
and the proliferation activity was tested at day 4 post-seeding.
Their results indicated that 10−10 M was able to promote
cell proliferation. In the current study, stimulation of BMSCs with
10−9 M CGRP exerted the greatest effect on cell
proliferation.
The authors' previous study also demonstrated that
CGRP increases BMSC proliferation and upregulates the expression of
osteogenic genes. ALP, OCN, Runx2 and OSX are essential genes
required for osteogenic differentiation of BMSCs, and FOSL2 is
known to have significant influence on proliferation and osteogenic
differentiation of BMSCs (17).
Therefore, the expression of the five genes, namely ALP, OCN,
Runx2, OSX and FOSL2, in BMSCs were detected with CGRP stimulation
and a blank control group. In the CGRP-treated group, FOSL2 was
upregulated by 3.6-fold; ALP was upregulated by 4.3-fold, Runx2 was
upregulated by 3.6-fold, OSX was upregulated by 2.3-fold and OCN
was upregulated by 3.7-fold. The results clearly demonstrated that
CGRP has an important role in BMSC proliferation and
differentiation. The results are similar to the previous findings
(31). According to the study of
Qu et al (34), circRNA
microarrays are a reliable and convenient method to research
circRNAs and their target miRNAs and genes. Subsequently,
high-throughput microarray detection of circRNAs was performed in
BMSCs stimulated with CGRP. There was a total of 58 circRNAs with
differential expression, of which 14 were upregulated and 44 were
downregulated. Furthermore, several bioinformatics tools were used
to identify miRNAs that potentially bind to the conserved seed
sequence in the circRNAs and analyzed the possible target genes of
these miRNAs. Subsequently, Cytoscape software was used to create a
network map of the interactions between circRNAs and miRNAs. The
results of the microarray analysis and the corresponding
alterations in gene expression and proliferation and
differentiation indicated that mmu_circRNA_003795 may have a role
as an miR504-3p absorber, which results in upregulation of FOSL2
expression to ultimately promote the proliferation of BMSCs.
mmu_circRNA_003795 was differentially expressed in
CGRP-treated BMSCs and the control group. The expression of
mmu_circRNA_003795 in the CGRP-treated group was 2.9-fold compared
with the blank control group, which indicated that
mmu_circRNA_003795 may be associated with the proliferation of
BMSCs. Electrophoresis was then used to examine the PCR product and
verify the upregulation of mmu_circRNA_003795. Visualization of the
DNA on the agarose gel clearly demonstrated that the size of the
PCR product was the same as the expected product size and was a
single band. The PCR product was sequenced and a Blast search was
performed to compare the sequencing data and to establish the
sequence of mmu_circRNA_003795. This also demonstrated that a
circular RNA was detected, rather than a linear RNA molecule.
Notably, although the expression of mmu_circRNA_003795, which was
validated by RT-qPCR, exhibited the same trend in the microarray
and RT-qPCR assays, the alteration in expression detected by PCR
was much higher compared with the microarray. This may be due to
the small sample number used for the microarray. According to the
studies of Deng et al (35), the results obtained by the circRNA
microarray can only be a reference method and should be confirmed
by further experiments. Subsequently, an si-RNA targeting
mmu_circRNA_003795 was designed to further verify its role in the
proliferation of BMSCs. According to the RT-qPCR results, the
expression of mmu_circRNA_003795 was inhibited by
si-mmu_circRNA_003795, reduced to 0.5-fold of the level of the
negative control. This indicated that si-mmu_circRNA_003795
effectively inhibited the expression of mmu_circRNA_003795. In
cells transfected with si-mmu_circRNA_003795, the expression of
miR-504-3p was increased by 6.6-fold compared with the control
group, which indicated that the downregulation of
mmu_circRNA_003795 upregulated miR504-3p levels.
As a member of the activator factor protein 1
transcription factor family, FOSL2 is expressed in a variety of
tissues. These transcription factors are associated with regulation
of cell proliferation, differentiation, transformation and cell
apoptosis, and with the regulation of the immune system in
vivo (36,37). Further study has demonstrated that
FOS proteins have important roles in cell proliferation,
differentiation and metabolism, growth traits, immune system
responses and other physiological activities (38). FOSL2 is involved in fat metabolism,
bone development and the occurrence and development of cancer, and
is considered to be very important in the transcription of genes
involved in osteogenic differentiation (39–42).
Certain research indicates that FOSL2 exhibited an important effect
on the treatment of bone loss (42,43).
FOSL2 is a target gene of miR-504-3p. Therefore, the expression
quantity of FOSL2 in the cells transfected with
si-mmu_circRNA_003795 and a negative control was detected. The
results demonstrated that the expression of FOSL2 in
si-mmu_circRNA_003795 group was 0.6-fold of that of the control
group. A similar result was produced by western blot analysis,
indicating that mmu_circRNA_003795 indirectly regulates the
expression of FOSL2.
To further verify whether miR504-3p is capable of
acting on FOSL2 mRNA, an miR504-3p inhibitor was designed. The
experimental results demonstrated that the expression of miR504-3p
was 0.4-fold of the negative control group. Subsequently, the
expression quantity of FOSL2 in the BMSCs transfected with the
miR-504-3p inhibitor and the control groups were detected. The
expression of FOSL2 in the experimental group was 3.3-fold of that
of the control group suggesting that miR-504-3p affects the
expression of FOSL2. In conjunction with the previous conclusions,
it was hypothesized that mmu_circRNA_003795 may indirectly regulate
the expression of FOSL2 by acting as a sponge for miR-504-3p. In
order to validate this, BMSCs were simultaneously transfected with
the si-mmu_circRNA_003795 and miR-504-3p inhibitor for 72 h, and
the results demonstrated that the expression of FOSL2 was increased
by 2.2-fold. In addition, the western blot analysis demonstrated
that compared with the control group, the expression of FOSL2 was
increased slightly by co-transfection with si-mmu_circRNA_003795
and miR-504-3p; this was because the miR-504-3p inhibitor is more
effective at inhibiting its target than si-mmu_circRNA_003795.
The effect of mmu_circRNA_003795 on the
proliferation of BMSCs was also analyzed. The results demonstrated
that the cell proliferation rate of BMSCs was significantly reduced
by si-mmu_circRNA_003795 compared with the negative control group;
the change was most significant at 72 h following transfection.
However, the rate of change reached its peak at 48 h, which may
indicate the interference effect of the siRNA gradually weakened
following 2 days in culture. Additionally, flow cytometry analysis
of the cell cycle demonstrated that si-mmu_circRNA_003795 increased
the proportion of cells in the G0-G1 phase and reduced the
proportion of cells in the G2-M and S phase compared with the
negative control group. This indicated that mmu_circRNA_003795 may
affect cell proliferation by influencing the cell cycle. Notably,
previous studies on FOSL2 have reported that FOSL2 mainly affects
osteogenic differentiation of cells and there are few reports
arguing that FOSL2 is involved in cell proliferation (43,44).
Therefore, it was hypothesized that FOSL2 may promote cell
osteogenesis by influencing cell proliferation; this will be
investigated further in additional experiments. Furthermore, CGRP
function on bone generation in vivo also should be detected
and the tomography of bone following the CGRP treating will be
conducted in vivo in future studies.
In conclusion, the preliminary observations of the
study demonstrated that CGRP regulates miR504-3p via
mmu_circRNA_003795, which results in increased expression of FOSL2
and enhanced proliferation of BMSCs. However, the role of
proliferation promotion during differentiation still requires
further investigation.
Acknowledgements
The authors thank Dr Zhichao Zheng (Key Laboratory
of Oral Medicine, Guangzhou Institute of Oral Disease, Stomatology
Hospital of Guangzhou Medical University, Guangzhou, Guangdong,
China) for his great help in writing this paper.
Funding
This study was supported by Science & Technology
Bureau of Guangdong Province (grant no. 2017A050501041) and Health
Department of Guangdong Province (grant no. B2015089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WR, LY, TD, CW, YL, JW, ZH and FD performed the
experiments and analyzed the data. LG designed the study and wrote
the article. All authors approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelly S, Greenman C, Cook PR and
Papantonis A: Exon skipping is correlated with exon
circularization. J Mol Biol. 427:2414–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Li T, Zhao Q, Xiao B and Guo J:
Using circular RNA hsa_circ_0000190 as a new biomarker in the
diagnosis of gastric cancer. Clin Chim Acta. 466:167–171. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang ZG, Zhang JA, Luo HL, Liu GB, Lu
YB, Ge NH, Zheng BY, Li RX, Chen C, Wang X, et al: The circular RNA
of peripheral blood mononuclear cells: Hsa_circ_0005836 as a new
diagnostic biomarker and therapeutic target of active pulmonary
tuberculosis. Mol Immunol. 90:264–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia W, Qiu M, Chen R, Wang S, Leng X, Wang
J, Xu Y, Hu J, Dong G, Xu PL and Yin R: Circular RNA
has_circ_0067934 is upregulated in esophageal squamous cell
carcinoma and promoted proliferation. Sci Rep. 6:355762016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J
and Ao Y: Circular RNA related to the chondrocyte ECM regulates
MMP13 expression by functioning as a MiR-136 ‘Sponge’ in human
cartilage degradation. Sci Rep. 6:225722016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: circRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G,
Li Q, Chen X, Ji J, Zhang Y and OuYang HW: The promotion of bone
regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by
effects on integrin-BMP/Smad signaling pathway in BMSCs.
Biomaterials. 34:4404–4417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative effect of Mir-205 on osteogenic differentiation of bone
mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and
ERK/MAPK pathway. Int J Mol Sci. 16:10491–10506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Özdal-Kurt F, Tuğlu I, Vatansever HS, Tong
S and Deliloğlu-Gürhan SI: The effect of autologous bone marrow
stromal cells differentiated on scaffolds for canine tibial bone
reconstruction. Biotech Histochem. 90:516–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Novikova LN, Brohlin M, Kingham PJ,
Novikov LN and Wiberg M: Neuroprotective and growth-promoting
effects of bone marrow stromal cells after cervical spinal cord
injury in adult rats. Cytotherapy. 13:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russell FA, King R, Smillie SJ, Kodji X
and Brain SD: Calcitonin gene-related peptide: Physiology and
pathophysiology. Physiol Rev. 94:1099–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang W, Zhuo X, Tang Z, Wei X and Li B:
Calcitonin gene-related peptide stimulates proliferation and
osteogenic differentiation of osteoporotic rat-derived bone
mesenchymal stem cells. Mol Cell Biochem. 402:101–110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi N, Matsuda Y, Sato K, de Jong
PR, Bertin S, Tabeta K and Yamazaki K: Neuronal TRPV1 activation
regulates alveolar bone resorption by suppressing
osteoclastogenesis via CGRP. Sci Rep. 6:292942016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He H, Chai J, Zhang S, Ding L, Yan P, Du W
and Yang Z: CGRP may regulate bone metabolism through stimulating
osteoblast differentiation and inhibiting osteoclast formation. Mol
Med Rep. 13:3977–3984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang W, Li L, Cui X, Tang Z, Wei X, Pan H
and Li B: Enhanced proliferation and differentiation effects of a
CGRP- and Sr-enriched calcium phosphate cement on bone mesenchymal
stem cells. J Appl Biomater Funct Mater. 14:e431–e440.
2016.PubMed/NCBI
|
|
25
|
R Core Team: R: A language and environment
for statistical computing. version 3.1.2. R foundation for
statistical computing, Vienna, 2014. http://www.R-project.org/
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu C, Zheng Z, Ren W, Deng T, Li Y, Yang
L, Wu J, Huang Z, Li Z and Guo L: Mm9_circ_009056 enhances
osteogenesis by targeting BMP7 via CGRP-mediated miR-22-3p. Biochem
Biophys Res Commun. 501:199–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2014. View Article : Google Scholar
|
|
29
|
Peng Y, Song X, Zheng Y, Wang X and Lai W:
Circular RNA profiling reveals that circCOL3A1-859267 regulate type
I collagen expression in photoaged human dermal fibroblasts.
Biochem Biophys Res Commun. 486:277–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sample SJ, Hao Z, Wilson AP and Muir P:
Role of calcitonin gene-related peptide in bone repair after cyclic
fatigue loading. PLoS One. 6:e203862011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin-gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cornish J, Callon KE, Bava U, Kamona SA,
Cooper GJ and Reid IR: Effects of calcitonin, amylin, and
calcitonin gene-related peptide on osteoclast development. Bone.
29:162–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Yang L, Zheng Z, Li Z, Deng T, Ren
W, Wu C and Guo L: Bio-Oss® modified by calcitonin
gene-related peptide promotes osteogenesis in vitro. Exp Ther Med.
14:4001–4008. 2017.PubMed/NCBI
|
|
34
|
Qu S, Song W, Yang X, Wang J, Zhang R,
Zhang Z, Zhang H and Li H: Microarray expression profile of
circular RNAs in human pancreatic ductal adenocarcinoma. Genom
Data. 5:385–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng T, Yang L, Zheng Z, Li Y, Ren W, Wu C
and Guo L: Calcitonin gene-related peptide induces IL-6 expression
in RAW264.7 macrophages mediated by mmu_circRNA_007893. Mol Med
Rep. 16:9367–9374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weekes D, Kashima TG, Zandueta C, Perurena
N, Thomas DP, Sunters A, Vuillier C, Bozec A, El-Emir E, Miletich
I, et al: Regulation of osteosarcoma cell lung metastasis by the
c-Fos/AP-1 target FGFR1. Oncogene. 35:2852–2861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kushibiki T, Tu Y, Abu-Yousif AO and Hasan
T: Photodynamic activation as a molecular switch to promote
osteoblast cell differentiation via AP-1 activation. Sci Rep.
5:131142015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jahangiri L, Sharpe M, Novikov N,
González-Rosa JM, Borikova A, Nevis K, Paffett-Lugassy N, Zhao L,
Adams M, Guner-Ataman B, et al: The AP-1 transcription factor
component Fosl2 potentiates the rate of myocardial differentiation
from the zebrafish second heart field. Development. 143:113–122.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Sun D, Wang Y, Ren F, Pang S, Wang
D and Xu S: FOSL2 positively regulates TGF-β1 signalling in
non-small cell lung cancer. PLoS One. 9:e1121502014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luther J, Ubieta K, Hannemann N, Jimenez
M, Garcia M, Zech C, Schett G, Wagner EF and Bozec A: Fra-2/AP-1
controls adipocyte differentiation and survival by regulating PPARγ
and hypoxia. Cell Death Differ. 21:655–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hasenfuss SC, Bakiri L, Thomsen MK,
Hamacher R and Wagner EF: Activator protein 1 transcription factor
Fos-related antigen 1 (Fra-1) is dispensable for murine liver
fibrosis, but modulates xenobiotic metabolism. Hepatology.
59:261–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Li S, Hu Y, Cao G, Wang S, Rai P,
Wang X and Sun K: The expression level of mRNA, protein, and DNA
methylation status of FOSL2 of Uyghur in XinJiang in type 2
diabetes. J Diabetes Res. 2016:1–7. 2016. View Article : Google Scholar
|
|
43
|
Bozec A, Bakiri L, Jimenez M, Schinke T,
Amling M and Wagner EF: Fra-2/AP-1 controls bone formation by
regulating osteoblast differentiation and collagen production. J
Cell Biol. 190:1093–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bozec A, Bakiri L, Jimenez M, Rosen ED,
Catalá-Lehnen P, Schinke T, Schett G, Amling M and Wagner EF:
Osteoblast-specific expression of Fra-2/AP-1 controls adiponectin
and osteocalcin expression and affects metabolism. J Cell Sci.
126:5432–5440. 2013. View Article : Google Scholar : PubMed/NCBI
|