Introduction
Post-operative cognitive dysfunction (POCD)
generally refers to the decline in cognitive ability after
anesthesia and surgery when compared to preoperative cognitive
status (1). POCD can occur in
nearly 10% of all surgical patients and 40% of elderly patients at
the point of discharge, and the long-term impact of this is marked
by a significantly higher mortality rate than age and sex-matched
controls without POCD (1).
Although the causes of POCD are not fully understood, accumulating
evidence from experimental and clinical studies has shown that
general anesthesia alone is capable of causing cognitive
dysfunction (2). It is worth
noting that anesthesia-induced cognitive dysfunction may depend on
the types of anesthetic agents, drug doses, exposure duration and
patient age (3,4). In addition, the presence of a
pathological condition may increase the susceptibility to
developing POCD or exacerbate the pre-existing cognitive impairment
after anesthesia (5–7). Neuroinflammation in the brain,
particularly in the hippocampus, has been shown to play a
contributory role in the pathogenesis of cognitive dysfunction,
including POCD (8–10). Microglia, the resident innate
immune cells in the brain, are major sources of pro-inflammatory
cytokines (11). Activation of
microglia in the brain results in the release of pro-inflammatory
cytokines, which triggers the neuroinflammatory response and
subsequently leads to neuronal damage and losses (11–13).
Interventions that inhibit microglia activity and neuroinflammation
in the brain can significantly ameliorate cognitive dysfunction in
multiple neurodegenerative diseases (8,14).
Obstructive sleep apnea, characterized by repeated
occlusions of the pharyngeal airway during sleep, is a devastating
respiratory control disorder and is associated with serious and
adverse consequences including cognitive dysfunction and dementia
(15,16). It is widely accepted that chronic
intermittent hypoxia (CIH), a cardinal feature of obstructive sleep
apnea, plays an important role in cognitive dysfunction in
obstructive sleep apnea (17). CIH
can induce activation of microglia and subsequent neuroinflammation
in the hippocampus, a key region of the brain associated with
spatial learning and memory acquisition, contributing to cognitive
dysfunction (15,18).
Peroxisome proliferator-activated receptor-γ
(PPAR-γ) belongs to the PPAR family of ligand-activated
transcription factors that is well-known to regulate adipocyte
differentiation, fatty acid storage and glucose metabolism
(19). In addition, PPAR-γ plays
an important role in the immune response through its ability to
inhibit the expression of inflammatory cytokines and to direct the
differentiation of immune cells towards anti-inflammatory
phenotypes (20–22). In the brain, activation or
upregulation of PPAR-γ has been demonstrated to inhibit the
synthesis and release of pro-inflammatory cytokines in many central
nervous system diseases in which massive inflammation plays a
detrimental role (23–25). Notably, PPAR-γ is expressed in
multiple cell types in the brain including microglia, astrocytes
and neurons (26,27), and relatively high PPAR-γ
expression levels have been found in the brain areas associated
with learning and memory including the hippocampus (26,27).
Sevoflurane (SEV) is one of the most widely used anesthetic agents
for induction and maintenance of general anesthesia in surgical
patients, including patients with obstructive sleep apnea. Studies
from our laboratory and others have recently demonstrated that a
moderate duration of SEV (2–3% for 2 or 4 h) does not cause
neuroinflammation in the hippocampus and cognitive impairment in
either adult or aged animals (4,28–30).
However, SEV downregulates PPAR-γ expression and activity in the
hippocampus, which increase the sensitivity and susceptibility to
CIH insult, leading to aggravated microglia activation and
neuroinflammation as well as exaggeration of cognitive decline
under CIH conditions (28).
Pioglitazone (PIO) is a thiazolidinedione (TZD) class synthetic
PPAR-γ agonist and has been used for the treatment of patients with
type II diabetes mellitus. In addition, PIO exhibits
anti-inflammatory and neuroprotective effects in multiple
inflammatory central nervous system disorders (31,32).
PIO can cross the blood-brain barrier (33). The present study was designed to
examine whether treatment with PPAR-γ agonist PIO would upregulate
PPAR-γ expression and activity in the hippocampus, preventing
SEV-induced cognitive decline in a rat model of CIH.
Materials and methods
Animals
A total of 60 male Sprague-Dawley rats weighing
200–250 g (8–9 weeks of age) were purchased from Beijing Laboratory
Animal Research Center (Beijing, China) and maintained on a 12:12 h
light/dark cycle at room temperature (23±2°C) in 50–60% relative
humidity with ad libitum access to food and water. All
experimental protocols were approved by the Animal Care and Use
Committees of Shandong University and conducted in accordance with
the guidelines of the Animal Care and Use Committee at Shandong
University (Jinan, Shandong, China).
Protocol
Rats were placed into a plastic cage equipped with
the intermittent hypoxia apparatus and exposed to intermittent
hypoxia for 4 weeks, as previously described (28). The hypoxia/re-oxygenation profile
was run for eight consecutive hours during the 12 h of light cycle
each day to coincide with the animal sleep cycle. During each cycle
of intermittent hypoxia, the oxygen concentration in the cage
dropped from 21 to 5% over a 50-sec period and was then quickly
returned to 21% during the following 40 sec. Constant room air was
given to the cages during the 12 h of the dark cycle each day.
After 2 weeks of intermittent hypoxia, CIH rats underwent either
control (CON, room air) or a moderate duration of SEV (2.6%)
exposure for 4 h, and were treated with vehicle (VEH, distilled
water) or PPAR-γ agonist PIO for 2 weeks, starting one day prior to
CON or SEV exposure. The experimental groups were as follows (n=15
for each group): i) CIH-CON+VEH, ii) CIH-CON+PIO, iii) CIH-SEV+VEH,
and iv) CIH-SEV+PIO. SEV exposure was conducted as previously
described (28). Briefly, animals
assigned to SEV exposure were placed in a temperature-controlled
chamber that was equipped with an anesthesia device and a multi-gas
monitor. SEV (2.6%) was provided by a humidified 30% O2
carrier gas from a calibrated vaporizer for 4 h. Animals assigned
to CON exposure were also placed in the same chamber for 4 h but no
SEV was provided. After CON or SEV exposure, all animals were
returned to their original cages and underwent CIH for another 2
weeks. PIO (60 mg/kg) or VEH was administered twice daily by oral
gavage, as described previously (31). At the end of the study protocol,
spatial learning and memory in some animals (n=9 for each group)
were examined using the Morris Water Maze test. Immediately after
Morris Water Maze examination, some of the animals were sacrificed
by decapitation under deep pentobarbital anesthesia and brains were
quickly collected for molecular analyses. The rest of the animals
(n=6 for each group) were transcardially perfused with saline
containing heparin (1 U/ml) and then with 4% paraformaldehyde in
0.1 M phosphate buffer (PB) for immunofluorescent study. The
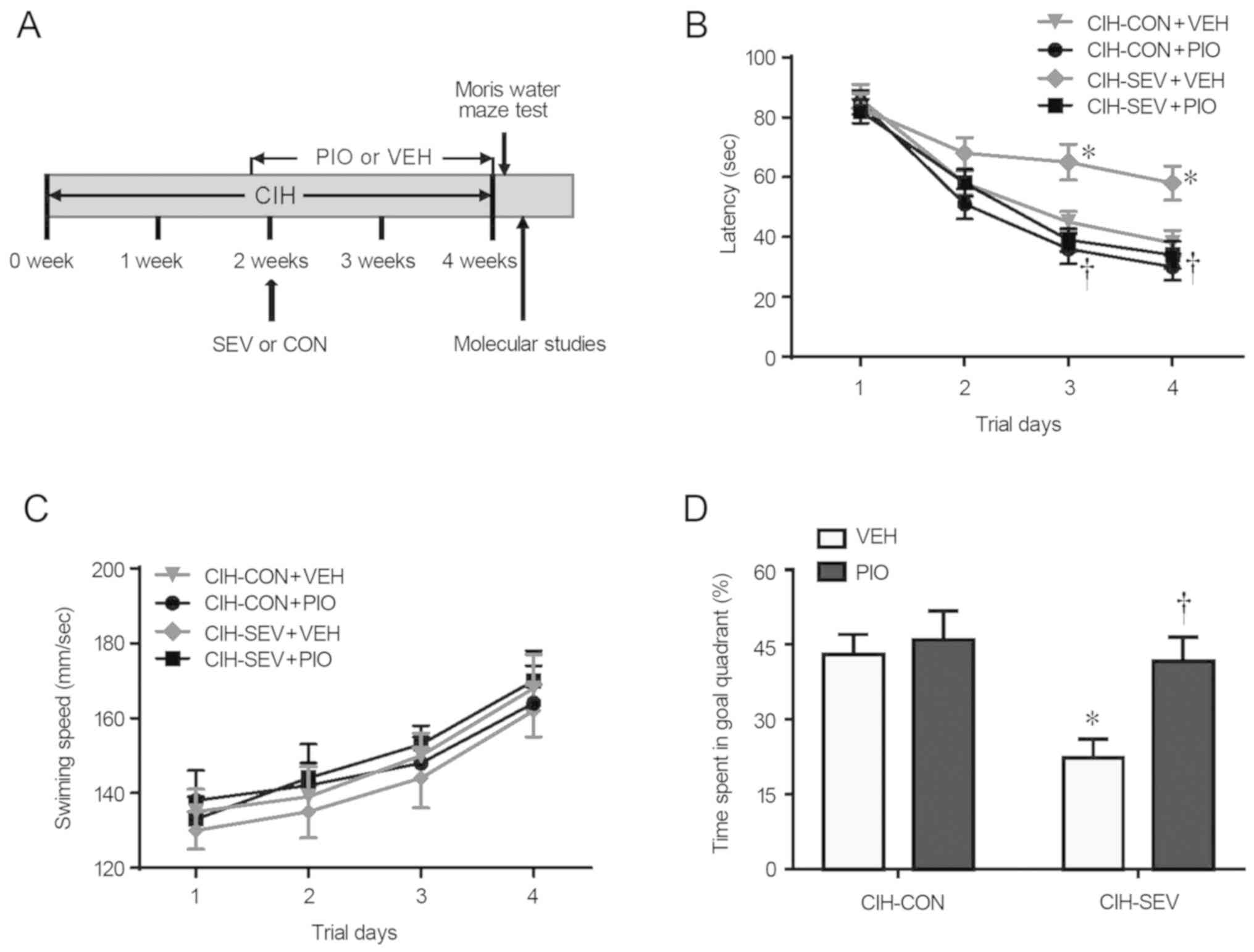
experimental procedures and the timeline are shown in Fig. 1A.
Morris water maze task
Spatial learning and memory were examined using the
Morris Water Maze after 4 weeks of intermittent hypoxia, as
previously described (28). The
spatial acquisition trial was performed over four consecutive days
and each acquisition trial section consisted of four trials with an
interval of 15 min. A rat was placed gently into the water facing
the wall of the pool and allowed 120 sec to find a hidden escape
platform submerged approximately 1 cm below the water surface. The
time for each rat spent to locate the submerged platform was
recorded as the escape latency. A probe trial was conducted 24 h
after completion of the spatial acquisition trial to assess the
spatial reference memory. During the probe test, the platform was
removed from the pool and rats were allowed to swim freely for 120
sec in any of the four quadrants of the swimming pool. The
percentage of time spent in the target quadrant where the platform
had been placed was calculated as a measure of memory for platform
position.
Western blot analysis
The rat brains were dissected and bilateral
hippocampal tissues were quickly removed. Hippocampal tissues were
homogenized in cold lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) containing protease inhibitors. The
proteins extracted from the hippocampal tissues were loaded onto
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel and then transferred to polyvinylidene fluoride
membranes. The membranes were immunoblotted with primary antibodies
to tumor necrosis factor (TNF)-α (1:200; sc-52746), interleukin
(IL)-1β (1:200; sc-52012), PPAR-γ (1:100; sc-7273), PPAR-α (1:100;
sc-398394), PPAR-β (1:200; sc-74517) and β-actin (1:1,000;
sc-47778) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
4°C overnight and then incubated with HRP-conjugated secondary
antibodies (1:5,000; sc-2031) (Santa Cruz Biotechnology, Inc.) for
1 h at room temperature. The immunoreactive bands were visualized
using enhanced chemiluminescence detection system (GE Healthcare,
Chicago, IL, USA) and analyzed with ImageJ software version 1.49v
(NIH; National Institutes of Health, Bethesda, MD, USA). All data
were normalized to β-actin.
Determination of PPAR-γ activity
Nuclear extracts were prepared from the hippocampal
tissues with a Nuclear Extract Kit (Active Motif, Carlsbad, CA,
USA). PPAR-γ DNA binding activity was measured using Transcription
Factor Assay Kit (Active Motif) following the manufacturer's
instructions.
Immunofluorescence study
Immunofluorescence staining for microglia was
performed as previously described (28). Briefly, perfused brains were
removed and post-fixed in 4% paraformaldehyde at 4°C overnight
followed by incubation in 30% sucrose for 48 h at 4°C. After being
embedded in optimal cutting temperature (OCT), the brains were
serially sectioned at 20-µm intervals using a cryostat microtome.
The sections were immunostained at 4°C overnight with anti-CD11b
primary antibody (MCA275R; Chemicon, Temecula, CA, USA) that
recognizes both non-activated and activated microglia, and were
then incubated with a secondary antibody (Alex Fluor 488; A-11001,
Invitrogen Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2
h at room temperature. Fluorescence images were acquired using a
Zeiss LSM 510 confocal microscope at 40× magnification and the
number of total and activated microglia were counted in several
0.2×0.2 mm squares. Activated microglia were presented as a
percentage of the total number of microglia.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. A two-way analysis of variance followed by
Bonferroni's post hoc test was performed for statistical analysis
using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA).
Statistical significance was accepted at P<0.05.
Results
Effects of PIO treatment on
SEV-induced cognitive decline
Four weeks after intermittent hypoxia and 2 weeks
after CON or SEV exposure, the Morris Water Maze task was performed
to assess spatial learning and reference memory in each mouse
group. As shown in Fig. 1B,
CIH-SEV rats treated with VEH (CIH-SEV+VEH) exhibited significantly
longer latency to find the hidden platform on the last two trials
when compared with the CIH-CON rats treated with VEH (CIH-CON+VEH).
A 2-week PIO treatment, starting one day prior to CON or SEV
exposure, did not alter the escape latency in the CIH-CON rats, but
it significantly reduced the escape latency in the CIH-SEV rats to
an extent similar to that observed in the CIH-CON rats. Notably,
the swimming speed across four experimental groups was comparable
throughout 4 consecutive days (Fig.
1C). Probe trial showed that the percent time spent in the
target quadrant was markedly reduced in the CIH-SEV rats treated
with VEH (CIH-SEV+VEH) as compared to the CIH-CON rats treated with
VEH (CIH-CON+VEH) (Fig. 1D).
Compared with the respective VEH groups, PIO treatment
significantly increased the percent time spent in the target
quadrant in the CIH-SEV rats but not in the CIH-CON rats.
Effects of PIO treatment on
SEV-induced neuroinflammation in the hippocampus
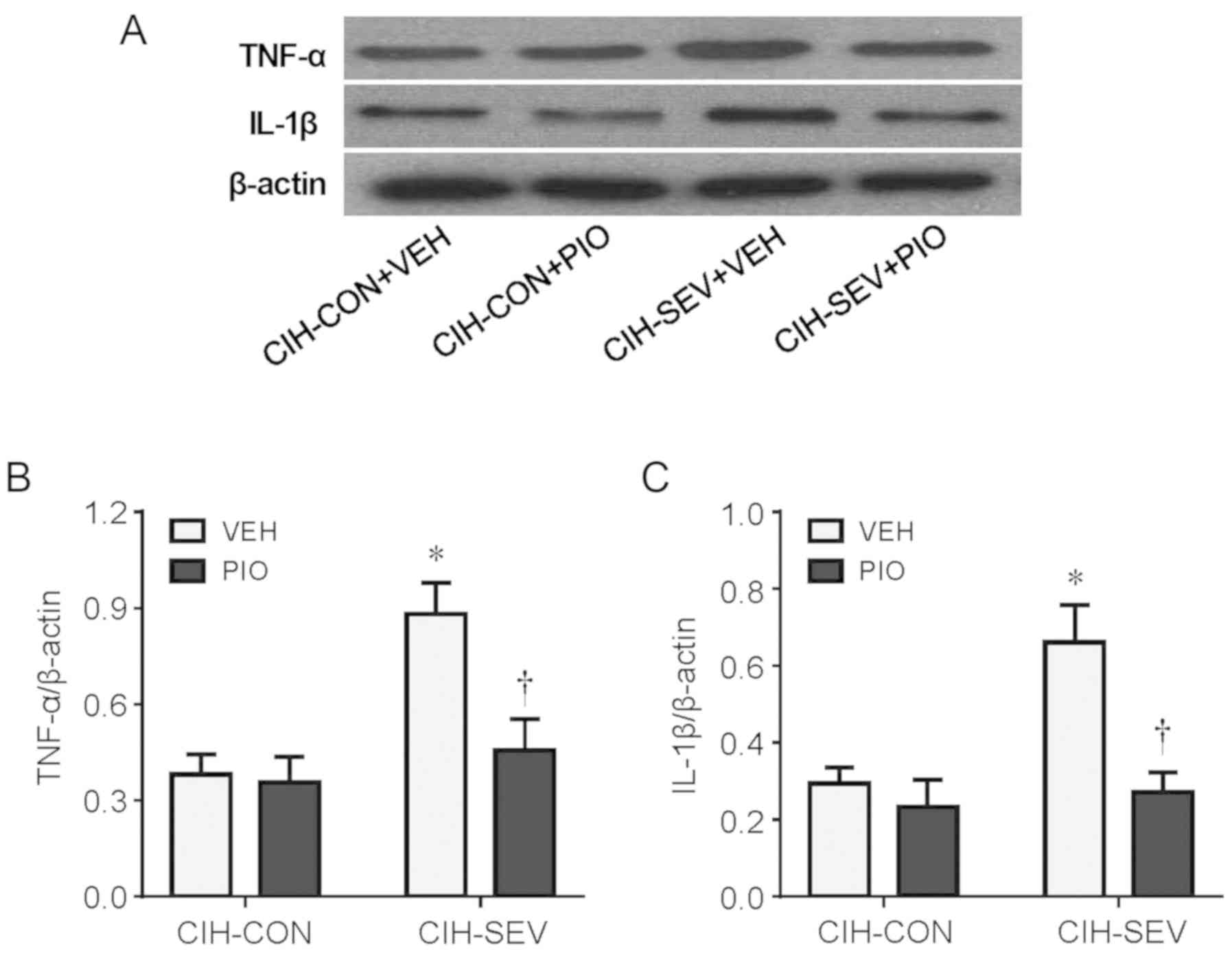
To examine the effect of PIO treatment on
neuroinflammation, the protein levels of TNF-α and IL-1β, two key
proinflammatory cytokines in the hippocampus, were assessed.
Compared with the CIH-CON rats treated with VEH, CIH-SEV rats
treated with VEH had significantly increased protein levels of both
TNF-α (Fig. 2A and B) and IL-1β
(Fig. 2A and C) in the
hippocampus. Importantly, the increases in protein levels of TNF-α
and IL-1β observed in the CIH-SEV rats were inhibited by PIO
treatment, which did not alter these proinflammatory cytokines in
the CIH-CON rats.
Effects of PIO treatment on
SEV-induced microglial activity in the hippocampus
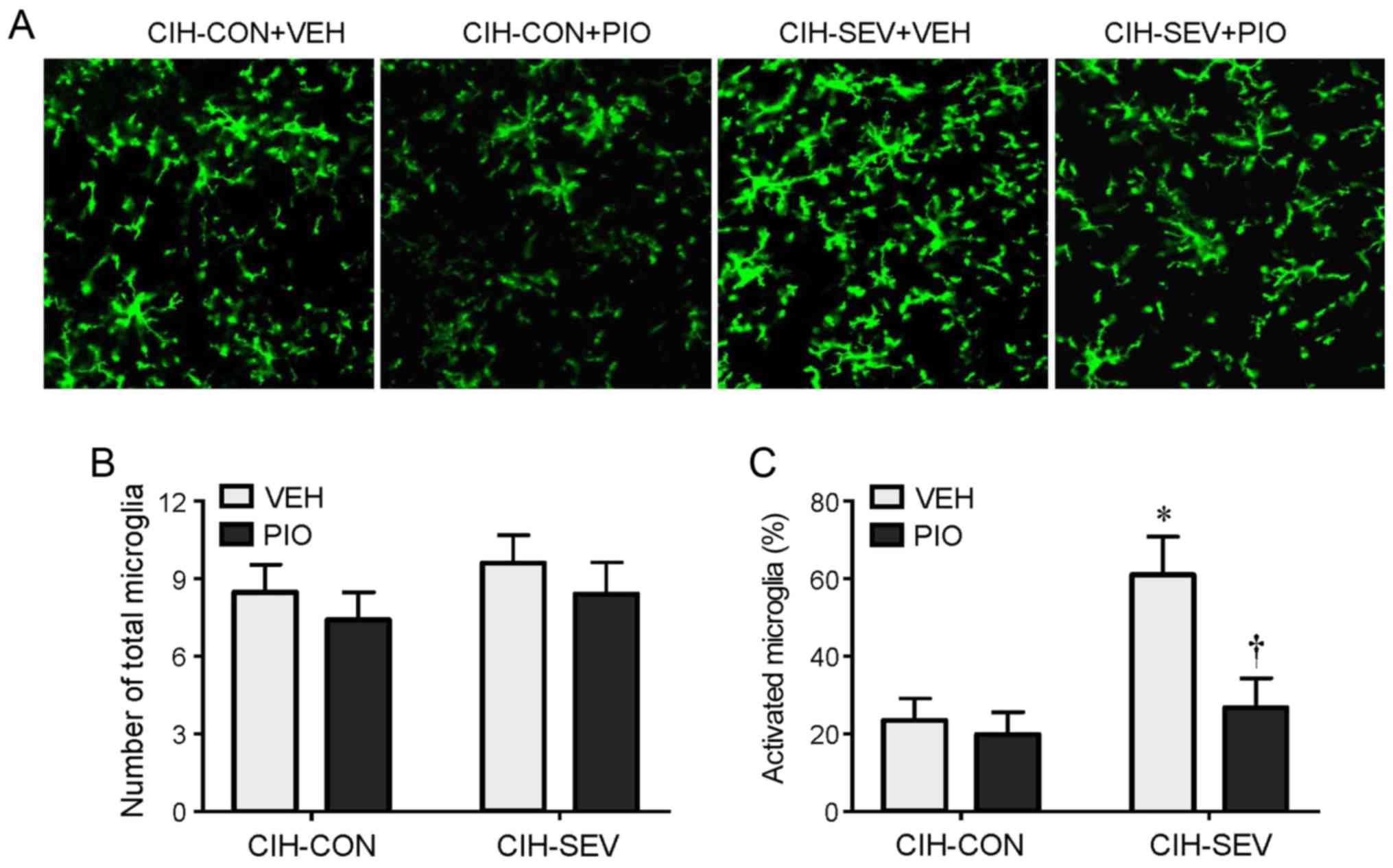
Immunofluorescence study revealed that the number of
total microglia in the hippocampus was comparable among the four
experimental groups (Fig. 3A and
B). However, the number of activated microglia that were
distinguished by strong CD11b immunoreactivity, an enlarged cell
body, and fewer and shorter hypertrophic processes extending from
the soma, was markedly higher in the hippocampus in the CIH-SEV
rats treated with VEH compared with CIH-CON rats treated with VEH
(Fig. 3A and C). Compared with the
respective VEH groups, PIO treatment did not change the number of
activated microglia in the CIH-CON rats, but it significantly
reduced the number of activated microglia in the CIH-SEV rats.
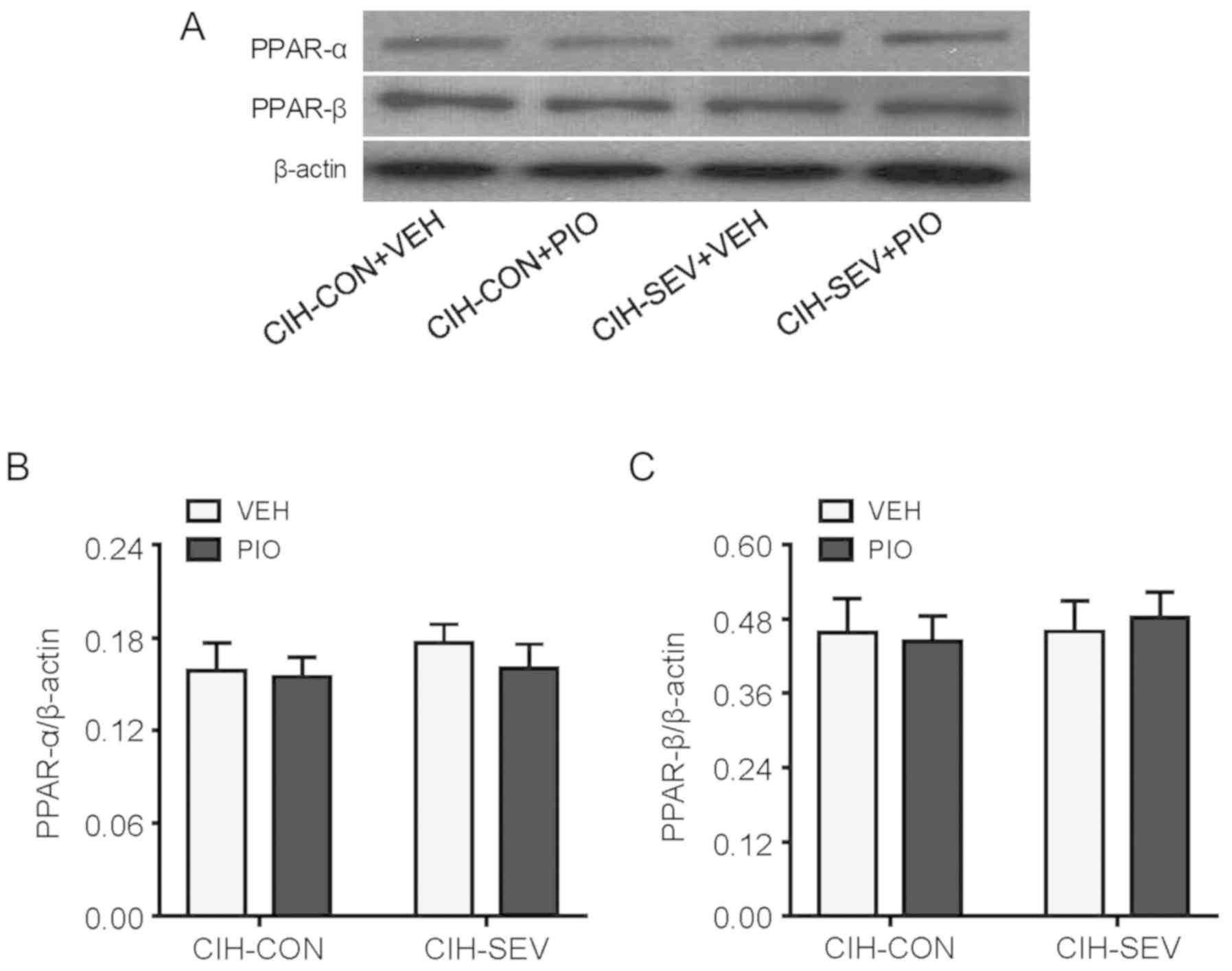
Effects of PIO treatment on PPAR
isoform expression and activity in the hippocampus
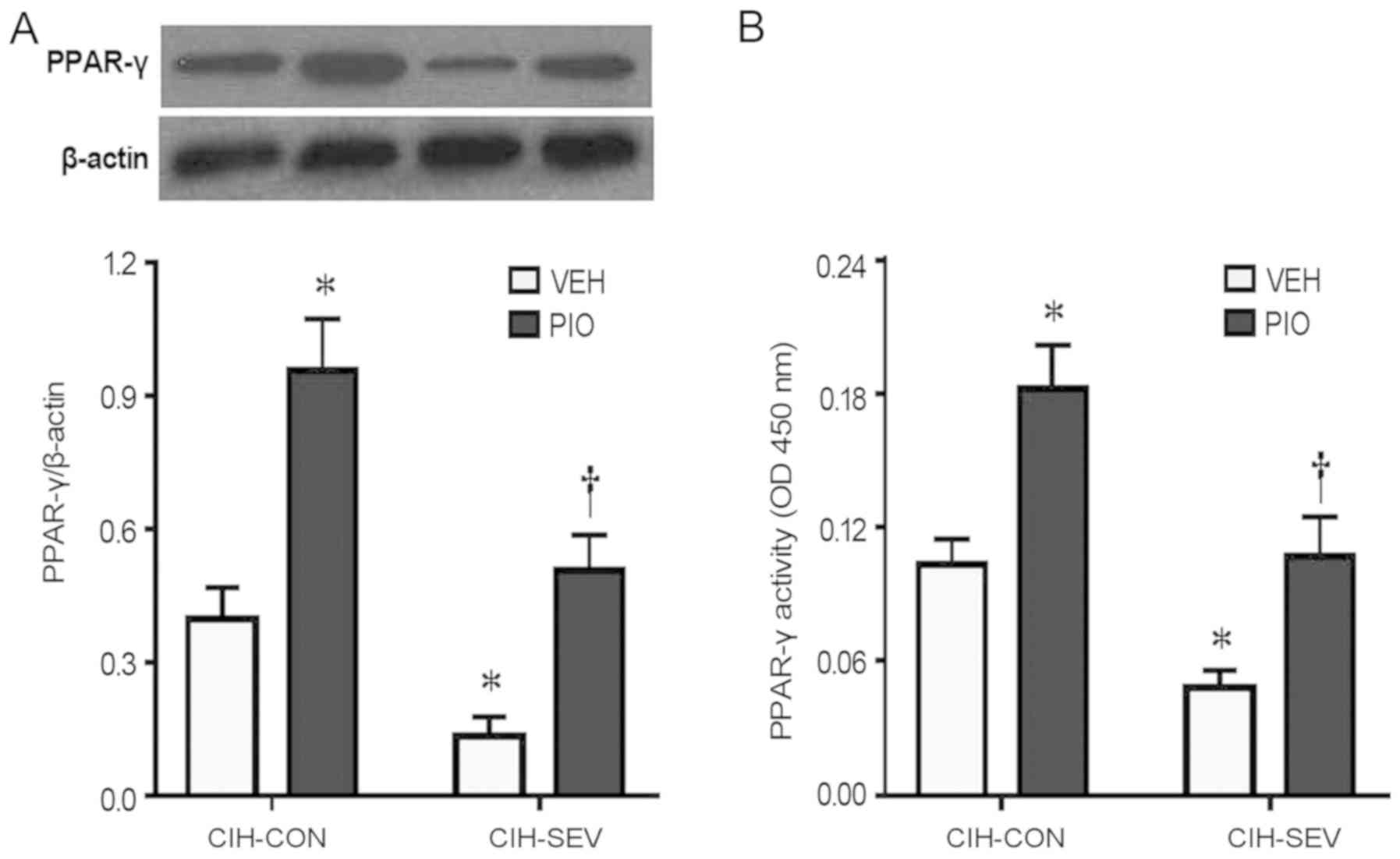
To determine whether PIO treatment prevented
exaggerated microglia activation and neuroinflammation by
upregulating hippocampal PPAR-γ, hippocampal PPAR-γ expression and
activity were further evaluated. PPAR-γ protein levels (Fig. 4A) and its DNA binding activity
(Fig. 4B) in the hippocampus were
significantly decreased in the CIH-SEV rats treated with VEH
compared with the CIH-CON rats treated with VEH. Interestingly, PIO
treatment markedly increased PPAR-γ expression and its DNA binding
activity in both the CIH-CON and CIH-SEV rats when compared with
their respective VEH groups. Of note, there were no significant
differences in expression of PPAR isoforms PPAR-α (Fig. 5A and B) or PPAR-β (Fig. 5A and C) among the four experimental
groups.
Discussion
The major finding of the present study is that
systemic administration of PPAR-γ agonist PIO upregulated
hippocampal PPAR-γ expression and activity, inhibited anesthetic
SEV-induced hippocampal microglia activation and neuroinflammation,
and prevented SEV-induced cognitive decline in a rat model of
CIH.
Obstructive sleep apnea is increasing in prevalence
although it still remains significantly underdiagnosed (34). Patients with obstructive sleep
apnea subjected to surgery are at increased risk for a wide variety
of postoperative complications including cognitive decline, which
should be recognized and managed in the perioperative period to
minimize postoperative complications. We and others have previously
reported that exposure to a moderate duration of SEV in animals did
not impair cognitive function under physiological conditions, but
it induced cognitive decline under CIH conditions (28–30).
In the present study, it was found that CIH-SEV rats treated with
VEH exhibited decline in both spatial learning and memory in the
Morris Water Maze task when compared with CIH-CON rats treated with
VEH. These observations are consistent with previous reports
(28), suggesting that a moderate
duration of SEV causes cognitive decline in animals under CIH
conditions.
Our previous study (28) demonstrated that SEV exaggerated
microglia activity and neuroinflammation in the hippocampus, which
were associated with reductions in hippocampal PPAR-γ expression
and activity. In the present study, the number of activated
microglia and proinflammatory cytokine expression were increased
but PPAR-γ expression and activity were decreased in the
hippocampus in CIH-SEV rats treated with VEH, compared with CIH-CON
rats treated with VEH. These results confirm previous findings,
suggesting that downregulation of PPAR-γ in the hippocampus by SEV
might contribute to exaggerated neuroinflammation and consequently
cognitive decline by increasing the sensitivity and susceptibility
to CIH insult.
Experimental studies in animals have reported that
systemic treatment with PIO attenuates brain microglial activation
and neuroinflammation, improves neuronal survival, and prevents
cognitive impairment in many inflammatory central nervous system
disorders, such as chronic stress (32), alcohol-induced neuronal damage
(31), traumatic brain injury
(35) and forebrain
ischemia/reperfusion injury (36).
In the present study, systemic treatment with PIO, starting one day
prior to SEV exposure, markedly increased PPAR-γ expression and
activity in the hippocampus of CIH-SEV rats, which were associated
with significant reductions in microglia activity and
proinflammatory cytokine expression. Moreover, systemic treatment
with PIO also improved cognitive decline in CIH-SEV rats as
indicated by decreased latency to find the hidden platform and
increased the percent time spent in the target quadrant, suggesting
that SEV exposure causes a decline in spatial learning and memory
abilities under CIH conditions, which can be prevented by treatment
with PPAR-γ agonist PIO. Of note, swimming speed was similar among
the 4 experimental groups, indicating that no alteration in
sensory-motor activity that would influence the Morris Water Maze
performance was produced by either SEV exposure or PIO treatment.
Importantly, systemic treatment with PIO also markedly increased
PPAR-γ expression and activity in the hippocampus of CIH-CON rats
but did not alter microglia activity and proinflammatory cytokine
expression as well as cognitive function in these animals,
suggesting that microglia activity and proinflammatory cytokine
expression in the hippocampus of CIH-CON rats without SEV exposure
are mediated by other mechanisms rather than PPAR-γ. However, SEV
exposure reduced PPAR-γ expression and activity, which increased
sensitivity to inflammatory stimuli, resulting in exaggerated
inflammatory responses. Collectively, these results indicate that
systemic treatment with PIO inhibits SEV exposure-induced
exaggeration of microglia-mediated neuroinflammation and cognitive
decline in CIH rats via upregulation hippocampal PPAR-γ. PPAR-γ can
physically bind to NF-κB p65 to inhibit NF-κB activity (37,38),
and SEV exposure has been reported to induce microglia activation
and neuroinflammation through the NF-κB pathway (39,40).
Thus, the salutary effects of PIO treatment in the brain of CIH-SEV
rats may be explained by the ability of PPAR-γ to inhibit
SEV-induced NF-κB activity.
It is worth noting that PPAR-α and PPAR-β, two other
PPAR isotypes, are also expressed in the brain and have been shown
to exert anti-inflammatory and neuroprotective actions (41). However, expression of PPAR-α and
PPAR-β in the hippocampus was similar among the 4 groups, excluding
the possibility that PIO treatment inhibited microglia-mediated
neuroinflammation in CIH-SEV rats by modulation of PPAR-α and
PPAR-β.
One limitation of the study should be acknowledged.
In the present study, we measured PPAR-γ expression and activity in
the hippocampus but did not examine any PPAR-γ downstream targets.
Further studies are necessary to determine whether PPAR-γ
downstream targets are involved in the beneficial effects of PIO
treatment on SEV-induced cognitive dysfunction under CIH conditions
and provide detailed mechanisms.
In summary, the present study demonstrated that
treatment with PPAR-γ agonist PIO prevents anesthetic SEV-induced
cognitive decline in CIH rats and that these beneficial effects are
mediated by upregulation of PPAR-γ, which inhibits SEV-induced
neuroinflammation in the hippocampus. These findings may provide
new insight into the potential use of PIO for preventing anesthetic
SEV-induced cognitive decline in surgical patients with obstructive
sleep apnea.
Acknowledgements
Not applicable.
Funding
This study was supported by the Major Project of
Science and Technology of Shandong Province (no. 2016GSF201070 to
DL).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contribution
XZ and JF designed the experiments and wrote the
manuscript. XZ, NL, LLu, QL, LLi, PD, BY and DL performed the
experiments, collected and analyzed the data. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Care and Use Committees of Shandong University and conducted
in accordance with the guidelines of the Animal Care and Use
Committee at Shandong University (Jinan, Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd
S, Lewis M, Marriott A, Moore EM, Morris G, Page RS and Gray L:
Post-operative cognitive dysfunction: An exploration of the
inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev.
84:116–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jevtovic-Todorovic V, Absalom AR, Blomgren
K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold
KF, Loepke AW, et al: Anaesthetic neurotoxicity and
neuroplasticity: An expert group report and statement based on the
BJA Salzburg Seminar. Br J Anaesth. 111:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Callaway JK, Jones NC, Royse AG and Royse
CF: Memory impairment in rats after desflurane anesthesia is age
and dose dependent. J Alzheimers Dis. 44:995–1005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng X, Degos V, Koch LG, Britton SL, Zhu
Y, Vacas S, Terrando N, Nelson J, Su X and Maze M: Surgery results
in exaggerated and persistent cognitive decline in a rat model of
the metabolic syndrome. Anesthesiology. 118:1098–1105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang C, Zhu B, Ding J and Wang ZG:
Isoflurane anesthesia aggravates cognitive impairment in
streptozotocin-induced diabetic rats. Int J Clin Exp Med.
7:903–910. 2014.PubMed/NCBI
|
|
7
|
Yue T, Shanbin G, Ling M, Yuan W, Ying X
and Ping Z: Sevoflurane aggregates cognitive dysfunction and
hippocampal oxidative stress induced by β-amyloid in rats. Life
Sci. 143:194–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wadhwa M, Prabhakar A, Ray K, Roy K,
Kumari P, Jha PK, Kishore K, Kumar S and Panjwani U: Inhibiting the
microglia activation improves the spatial memory and adult
neurogenesis in rat hippocampus during 48 h of sleep deprivation. J
Neuroinflammation. 14:2222017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Ni C, Xia C, Jaw J, Wang Y, Cao Y,
Xu M and Guo X: Calcineurin/nuclear factor-κB signaling mediates
isoflurane-induced hippocampal neuroinflammation and subsequent
cognitive impairment in aged rats. Mol Med Rep. 15:201–209. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai JC, Liu W, Lu F, Kong WB, Zhou XX,
Miao P, Lei CX and Wang Y: Resveratrol attenuates neurological
deficit and neuroinflammation following intracerebral hemorrhage.
Exp Ther Med. 15:4131–4138. 2018.PubMed/NCBI
|
|
11
|
Block ML: Neuroinflammation: Modulating
mighty microglia. Nat Chem Biol. 10:988–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Huang F, Wu H, Zhang B, Shi H, Wu X
and Hu Z: Isoastragaloside I inhibits NF-κB activation and
inflammatory responses in BV-2 microglial cells stimulated with
lipopolysaccharide. Int J Mol Med. 40:1270–1276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin M, Park SY, Shen Q, Lai Y, Ou X, Mao
Z, Lin D, Yu Y and Zhang W: Anti-neuroinflammatory effect of
curcumin on Pam3CSK4-stimulated microglial cells. Int J Mol Med.
41:521–530. 2018.PubMed/NCBI
|
|
14
|
Zhang Z, Yuan H, Zhao H, Qi B, Li F and An
L: PPARγ activation ameliorates postoperative cognitive decline
probably through suppressing hippocampal neuroinflammation in aged
mice. Int Immunopharmacol. 43:53–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gagnon K, Baril AA, Gagnon JF, Fortin M,
Décary A, Lafond C, Desautels A, Montplaisir J and Gosselin N:
Cognitive impairment in obstructive sleep apnea. Pathol Biol
(Paris). 62:233–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin F, Liu J and Zhang X, Cai W, Zhang Y,
Zhang W, Yang J, Lu G and Zhang X: Effect of continuous positive
airway pressure therapy on inflammatory cytokines and
atherosclerosis in patients with obstructive sleep apnea syndrome.
Mol Med Rep. 16:6334–6339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sforza E and Roche F: Chronic intermittent
hypoxia and obstructive sleep apnea: An experimental and clinical
approach. Hypoxia (Auckl). 4:99–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yaffe K, Laffan AM, Harrison SL, Redline
S, Spira AP, Ensrud KE, Ancoli-Israel S and Stone KL:
Sleep-disordered breathing, hypoxia, and risk of mild cognitive
impairment and dementia in older women. JAMA. 306:613–619. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moore-Carrasco R, Poblete Bustamante M,
González Guerra O, Leiva Madariaga E, Mujica Escudero V, Aranguez
Arellano C and Palomo I: Peroxisome proliferator-activated
receptors: Targets for the treatment of metabolic illnesses
(Review). Mol Med Rep. 1:317–324. 2008.PubMed/NCBI
|
|
20
|
Martin H: Role of PPAR-gamma in
inflammation. Prospects for therapeutic intervention by food
components. Mutat Res. 690:57–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang T, Shao B and Liu GA: Rosuvastatin
promotes the differentiation of peripheral blood monocytes into M2
macrophages in patients with atherosclerosis by activating PPAR-γ.
Eur Rev Med Pharmacol Sci. 21:4464–4471. 2017.PubMed/NCBI
|
|
22
|
Wang Q, Su YY, Li YQ, Zhang YF, Yang S,
Wang JL and Li HY: Atorvastatin alleviates renal
ischemia-reperfusion injury in rats by promoting M1-M2 transition.
Mol Med Rep. 15:798–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Culman J, Zhao Y, Gohlke P and Herdegen T:
PPAR-gamma: Therapeutic target for ischemic stroke. Trends
Pharmacol Sci. 28:244–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicolakakis N and Hamel E: The nuclear
receptor PPARgamma as a therapeutic target for cerebrovascular and
brain dysfunction in Alzheimer's disease. Front Aging Neurosci.
2:212010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Domi E, Uhrig S, Soverchia L, Spanagel R,
Hansson AC, Barbier E, Heilig M, Ciccocioppo R and Ubaldi M:
Genetic deletion of neuronal PPARγ enhances the emotional response
to acute stress and exacerbates anxiety: An effect reversed by
rescue of amygdala PPARγ Function. J Neurosci. 36:12611–12623.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bernardo A and Minghetti L: Regulation of
glial cell functions by PPAR-gamma natural and synthetic agonists.
PPAR Res. 2008:8641402008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong P, Zhao J, Li N, Lu L, Li L, Zhang X,
Yang B, Zhang L and Li D: Sevoflurane exaggerates cognitive decline
in a rat model of chronic intermittent hypoxia by aggravating
microglia-mediated neuroinflammation via downregulation of PPAR-γ
in the hippocampus. Behav Brain Res. 347:325–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Callaway JK, Jones NC, Royse AG and Royse
CF: Sevoflurane anesthesia does not impair acquisition learning or
memory in the Morris water maze in young adult and aged rats.
Anesthesiology. 117:1091–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Liu L, Li L, Li X, Huang B, Zhou C,
Zhang Z, Wang C, Dong P, Zhang X, et al: Sevoflurane induces
exaggerated and persistent cognitive decline in a type II diabetic
rat model by aggregating hippocampal inflammation. Front Pharmacol.
8:8862017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cippitelli A, Domi E, Ubaldi M, Douglas
JC, Li HW, Demopulos G, Gaitanaris G, Roberto M, Drew PD, Kane CJ
and Ciccocioppo R: Protection against alcohol-induced neuronal and
cognitive damage by the PPARγ receptor agonist pioglitazone. Brain
Behav Immun. 64:320–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang
J, Peng C and You Z: The antidepressant-like effects of
pioglitazone in a chronic mild stress mouse model are associated
with PPARγ-mediated alteration of microglial activation phenotypes.
J Neuroinflammation. 13:2592016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grommes C, Karlo JC, Caprariello A,
Blankenship D, Dechant A and Landreth GE: The PPARγ agonist
pioglitazone crosses the blood-brain barrier and reduces tumor
growth in a human xenograft model. Cancer Chemother Pharmacol.
71:929–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garvey JF, Pengo MF, Drakatos P and Kent
BD: Epidemiological aspects of obstructive sleep apnea. J Thorac
Dis. 7:920–929. 2015.PubMed/NCBI
|
|
35
|
Liu M, Bachstetter AD, Cass WA, Lifshitz J
and Bing G: Pioglitazone attenuates neuroinflammation and promotes
dopaminergic neuronal survival in the nigrostriatal system of rats
after diffuse brain injury. J Neurotrauma. 34:414–422. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Collino M, Aragno M, Mastrocola R,
Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C and
Fantozzi R: Modulation of the oxidative stress and inflammatory
response by PPAR-gamma agonists in the hippocampus of rats exposed
to cerebral ischemia/reperfusion. Eur J Pharmacol. 530:70–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen F, Wang M, O'Connor JP, He M,
Tripathi T and Harrison LE: Phosphorylation of PPARgamma via active
ERK1/2 leads to its physical association with p65 and inhibition of
NF-kappabeta. J Cell Biochem. 90:732–744. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruan H, Pownall HJ and Lodish HF:
Troglitazone antagonizes tumor necrosis factor-alpha-induced
reprogramming of adipocyte gene expression by inhibiting the
transcriptional regulatory functions of NF-kappaB. J Biol Chem.
278:28181–28192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Chen X, Zhang J, Zhao Y, Li S, Tan
L, Gao J, Fang X and Luo A: Glycyrrhizin attenuates
isoflurane-induced cognitive deficits in neonatal rats via its
anti-inflammatory activity. Neuroscience. 316:328–336. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Zhang J, Yang L, Dong Y, Zhang Y
and Xie Z: Isoflurane and sevoflurane increase interleukin-6 levels
through the nuclear factor-kappa B pathway in neuroglioma cells. Br
J Anaesth. 110 (Suppl 1):i82–i91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Warden A, Truitt J, Merriman M, Ponomareva
O, Jameson K, Ferguson LB, Mayfield RD and Harris RA: Localization
of PPAR isotypes in the adult mouse and human brain. Sci Rep.
6:276182016. View Article : Google Scholar : PubMed/NCBI
|