Introduction
Atherosclerosis is a chronic inflammatory disorder
of the arterial vessel walls characterized by lipid deposition and
fibrous cap formation in the arterial intima. Atherosclerosis
contributes to cardiovascular disease (CVD), a major cause of
morbidity and mortality in Western countries. A total of ~80
million Americans have one or more forms of CVD (1). Growing evidence suggests that
dysfunction of endothelial cells (ECs) and vascular smooth muscle
cells (VSMC) is critical in the formation of atherosclerosis.
Abnormal proliferation and migration of these cells contribute to
the progression of atherosclerosis (2–4).
VSMCs migrate and proliferate to form a stabilizing fibrous cap
that encapsulates atherosclerotic plaques (5). During the initiation and development
of inflammation, ECs and VSMCs produce numerous types of cytokines,
including tumor necrosis factors (TNF), interleukin (IL), adhesion
molecules, interferon, and adventitium-derived relaxing factors
that are implicated in atherosclerosis (6–9).
Circular RNAs (circRNAs) are evolutionarily
conserved non-coding RNAs produced by the scrambling of exons at
the time of splicing. They are primarily produced in the brain and
are present inside the cell. Previous data have suggested that the
circRNA antisense non-coding RNA in the INK locus modulates
ribosomal RNA maturation and atherosclerosis in humans, indicating
that circularization of long non-coding RNAs may alter RNA function
and therefore protect against disease (10). The great potential of circRNAs as
biomarkers for the early detection of cardiovascular diseases has
been highlighted in several patents and previous studies (11). Certain circRNAs, including
circRNA-0003575 and circRNA-000595, are significantly associated
with atherosclerosis (12,13). Furthermore, the role of circRNAs in
atherosclerosis is associated with the regulation of the physiology
of ECs and smooth muscle cells (SMCs) (10). MicroRNAs (miRNAs) are a group of
highly conserved small non-coding RNA molecules measuring 18–22
nucleotides in length. By binding to the 3′-untranslated region of
their target mRNAs, miRNAs regulate expression levels of downstream
genes (14,15). In addition to participating in
regulating cell-cycle and cell proliferation (16,17),
miRNAs are associated with inflammatory processes involved in
atherosclerosis (18). miR-107 is
implicated in the pathogenesis of diseases, including renal
disease, Alzheimer's disease, gastric cancer, colon cancer and
hepatocellular carcinoma (19–22).
Previous studies suggested that miR-107 is a regulator of
atherosclerosis (23,24). In addition, binding of miR-107 to
the Clock circadian rhythm gene resulted in the deregulation of the
circadian rhythm of the cells (25,26).
However, the exact mechanism is largely unknown.
Certain circRNAs have been described as regulatory
transcripts as they behave like sponges to bind miRNAs. For
example, a previous study examining the role of circular RNA-7
demonstrated that the circRNA was an inhibitor of miR-7, an miRNA
known to regulate various diseases including, cancer,
neurodegenerative diseases, diabetes and atherosclerosis (27). In this context, the present study
aimed to investigate the role of circRNA in the regulation of
atherosclerosis and to understand the underlying mechanism
associated with miRNAs and signaling pathways.
Materials and methods
Patients and samples
Human blood cells were collected from 20 patients
with atherosclerosis and healthy controls at the Qi-Lu Hospital of
Shandong University (Jinan, China). In total, 13 males and seven
females (age range, 48–68 years) were enrolled in the study.
Following sonographic examination, the patients with a carotid
intima-media thickness ≥ 1.3 mm were considered to exhibit an
atherosclerotic plaque. Informed consent was obtained from all
patients and ethical approval was granted by The Ethics Committee
of the Qi-Lu Hospital of Shandong University.
Cell culture and treatment
All reagents for cell culture were obtained from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Human umbilical vein endothelial cells (HUVECs) were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA), and
human umbilical vein smooth muscle cells (HUVSMCs) were purchased
from iXCells Biotechnologies (San Diego, CA, USA). HUVECs and
HUVSMCs cells were maintained in minimum essential
medium-L-glutamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
smooth muscle cell growth medium and RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). The medium was supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin-amphotericin. The cells were
maintained in a humidified incubator with 5% CO2 at
37°C. The culture medium was changed twice weekly. HUVECs and
HUVSMCs were sub-cultured subsequent to reaching 80% confluency.
For the following assay, the cells initially received either 100
ng/ml lipopolysaccharide (LPS) or vehicle. The template DNA of
circRNA-0044073 was synthesized and then inserted into the multiple
cloning site in the pAd-Track-cmv vector (Addgene, Inc., Cambridge,
MA, USA). A total of 500 ng of plasmid DNA was subsequently
transfected into HUVECs or HUVSMCs in 24-well plates using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) to increase circRNA-0044073 expression, whereas,
the empty vector was transfected as the negative control (NC).
Following 48 h of incubation, the transfected cells were used in
the following experiments.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and was
reverse transcribed using the Bestar qPCR RT kit (DBI Bioscience,
Ludwigshafen, Germany) at 65°C for 5 min. qPCR amplification was
performed in triplicate using DBI Bestar® SybrGreen qPCR
MasterMix (DBI Bioscience, Ludwigshafen, Germany) in a total volume
of 20 µl containing 2 µl each cDNA. The amplification protocol
consisted of initial denaturation for 2 min at 94°C, followed by 40
cycles of denaturation (94°C for 20 sec), annealing for 20 sec at
58°C and elongation for 20 sec at 72°C. The primers for
circRNA-0044073, miR-107, U6, janus kinase 1 (JAK1), signal
transducer and activator of transcription 3 (STAT3), B-cell
lymphoma 2 (Bcl-2), v-myc avian myelocytomatosis viral oncogene
homolog (c-myc) and GAPDH are summarized in Table I. Using GAPDH or U6 as endogenous
control genes, the relative transcript levels were calculated using
the 2−ΔΔCq quantification method (28).
 | Table I.Primers used in the reverse
transcription quantitative polymerase chain reaction assay. |
Table I.
Primers used in the reverse
transcription quantitative polymerase chain reaction assay.
| Genes | Sequence
(5′-3′) |
|---|
|
hsa_circ_0044073F |
ACAGGGGTGTTTGTGTGTGT |
|
hsa_circ_0044073R |
CTTCACGTTGCAGGTGTAGC |
| miR-107 F |
ACACTCCAGCTGGGAGCAGCA UUGUACAGGG |
| miR-107 R |
CTCAACTGGTGTCGTGGA |
| U6 F |
CTCGCTTCGGCAGCACA |
| U6 R |
AACGCTTCACGAATTTGCGT |
| JAK1 F |
CACCAGGATGCGGATAAATAAT |
| JAK1 R |
CAAAGTTTCCAAGGTAGCCAAG |
| STAT3 F |
TGGTGTCTCCACTGGTCTATCTC |
| STAT3 R |
CATCAATGAATCTAAAGTGCGG |
| Bcl-2 F |
ATCGCCCTGTGGATGACTG |
| Bcl-2 R |
AGACAGCCAGGAGAAATCAAAC |
| c-myc F |
CATACATCCTGTCCGTCCA |
| c-myc R |
CGCACAAGAGTTCCGTAGC |
| GAPDH F |
TGTTCGTCATGGGTGTGAAC |
| GAPDH R |
ATGGCATGGACTGTGGTCAT |
Cell viability assay
Cell viabilities were assessed by the Cell Counting
Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). In
brief, cells were added into a 96-well plate at a density of 2×103
cells/well and incubated overnight at 37°C. Then, LPS was added to
each well and incubated at 37°C for 24, 48 and 72 h at the final
concentration of 100 ng/ml. Thereafter, 10 µl CCK-8 solution was
added into each well and incubated for 2 h at 37°C. The absorbance
was detected at 450 nm with the microplate reader. Optical density
was defined as the relative number of viable cells. The assay was
performed in triplicate.
Western blot analysis
Following treatments with LPS for 24 h, cells
were washed with PBS and subsequently harvested. Total cellular
proteins were extracted with radioimmunoprecipitation assay lysis
buffer containing 1% phenylmethanesulfonyl fluoride (Sigma-Aldrich;
Merck KGaA). Protein concentrations were determined with a BCA
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequent
to denaturation, a total of 20 µg protein per lane was separated by
electrophoresis by 10% SDS-PAGE and analyzed. Proteins were
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.) and then blocked with 5% dry milk in TBST (TBS
with 0.1% Tween 20) for 1 h at room temperature, washed, and
incubated overnight at 4°C with primary antibodies against JAK1
(1:1,000; cat. no. ab47435; Abcam, Cambridge, MA, USA), STAT3
(1:1,000; cat. no. ab68153; Abcam), phosphorylated (p)-STAT3
(1:800; cat. no. ab30647; Abcam), Bcl-2 (1:1,000; cat. no. 15071;
Cell Signaling Technology, Inc., Danvers, MA, USA), c-myc (1:1,000;
cat. no. 13987; Cell Signaling Technology, Inc.) and GAPDH
(1:1,000; cat. no. 2118; Cell Signaling Technology, Inc.). The
membranes were then washed with TBST and then incubated with the
secondary antibodies anti-rabbit and anti-mouse immunoglobulin G
conjugated with horseradish peroxidase (HRP; 1:10,000; cat. no.
7074 and 7076; Cell Signaling Technology, Inc.). Finally, the
immunoreactive bands were visualized by using an enhanced
chemiluminescent substrate (ECL-Plus; GE Healthcare, Chicago, IL
USA). Using ImageJ software (version 1.8.0; National Institutes of
Health, Bethesda, MD, USA; http://imagej.nih.gove/ij/), densitometric analysis of
bands was performed.
ELISA
The IL-1β (cat. no. CSB-E08053h), IL-6 (cat. no.
CSB-E04638h) and TNF-α (cat. no. CSB-E04740h) ELISA assay kits were
purchased from CUSABIO (Wuhan, China). After centrifugation at
1,000 × g for 15 min at 4°C, the concentrations in the supernatants
were assessed according to the manufacturer's protocols. In brief,
the samples were added to wells in duplicate and then incubated at
room temperature for 2 h. The liquid was removed, followed by the
addition of biotin-antibody and then the plates were incubated at
room temperature for 1 h. Following removal of the liquid and
washing, HRP-avidin was added and incubated at room temperature for
1 h. Following additional wash steps, visualization was achieved by
adding the TMB substrate. The absorbance was detected at 450 nm
with a microplate reader (Thermo Fisher Scientific, Inc.). The
experiments were performed in triplicate.
Cell cycle analysis by flow
cytometry
The cell cycle distribution was analyzed as
described previously (29).
Following treatment with LPS for 24 h, the cells were collected and
washed with PBS and then stained with 50 mg/ml propidium iodide
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 30 min at
4°C. The data were collected with a FACSCalibur flow cytometer
(Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for
cell-cycle distribution analysis using Cell Quest Software (version
5.1; Becton, Dickinson and Company).
Matrigel invasion assay
The cells were collected and suspended in medium
without serum. Transwell inserts (24-well insert; pore size, 8 mm;
Becton, Dickinson and Company) were inserted in the chamber and
incubated at 37°C overnight with serum-free RPMI-1640 with matrigel
(0.6 mg/ml; Becton, Dickinson and Company). Subsequently, 4×104
cells in 0.2 ml serum-free medium were seeded in the top chambers
of the transwell inserts, and 10% FBS was used as an attractant.
The cells were incubated with 1 mg/ml LPS or control treatment for
48 h at 37°C. The cells that did not invade through the pores were
removed, and the filters were stained at room temperature with
hematoxylin for 5 min, and with eosin for 1 min (cat. no. C0105;
Beyotime Institute of Biotechnology, Haimen, China). Samples were
visualized under a light microscope (magnification, ×200) and
counting.
RNA pull-down assay
The RNA pull-down assay was performed as previously
described (30). In brief, either
the pAd-Track-cmv-circRNA-0044073 or the control vectors were
transfected into 293 cells (ATCC). Subsequently, 100 µg total RNA
was extracted with TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) from the cells and incubated with 500 µg
streptavidin magnetic beads (cat. no. S1421S; New England BioLabs,
Inc., Ipswich, MA, USA), which were previously incubated for 2 h at
37°C with either 200 pmol biotin-miR-107 or 200 pmol
biotin-miR-107-mut (Shanghai GenePharma Co., Ltd., Shanghai,
China). Following RNA elution, RT-qPCR was performed to detect
circRNA-0044073 as aforementioned.
DNA construct and luciferase reporter
assay
As described previously (18), the psiCHECKTM-2 firefly luciferase
reporter plasmids (Promega Corporation, Madison, WI, USA) were
recombined with the potential miR-107 binding site sequences of
JAK1 or its mutated sequence. 293 cells were seeded into a 12-well
plate (3×105 cells/well) and transfected with 200 ng recombinant
luciferase reporter plasmid using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection with 10 nM miR-107 mimics
(5′-AGCAGCAUUGUACAGGGCUAUCA-3′) or NC miRNA
(5′UUCUCCGAACGUGUCACGUTT-3′) for 24 h, the cells were collected,
and luciferase activity was determined using the Dual-Luciferase
Reporter Gene Assay kit (Beyotime Institute of Biotechnology). The
relative luciferase activity was obtained by normalizing the
firefly luciferase activity to the internal control Renilla
luciferase activity.
Statistical analysis
Data in the present study are presented as the mean
± standard deviation of three independent tests. Using GraphPad
Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA),
two-tailed Student's t-tests were performed to compare data between
two groups, whereas, one-way analysis of variance followed by
Tukey's post hoc test was performed to compare data among three or
more groups.
Results
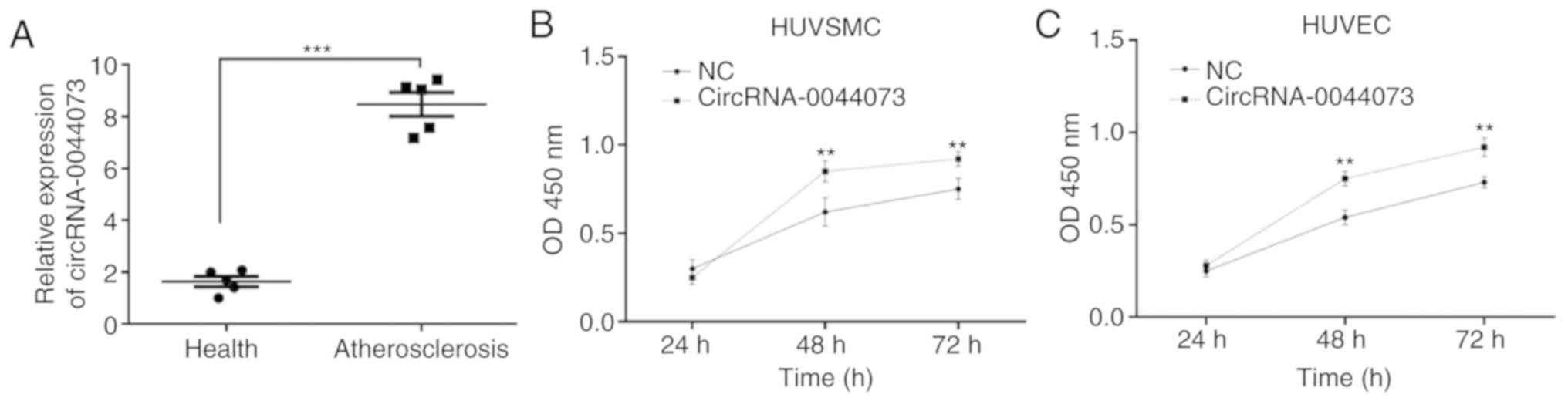
Levels of circRNA-0044073 are
upregulated in blood cells of patients with atherosclerosis and are
associated with the proliferation of HUVSMCs and HUVECs
The present study identified that the expression of
circRNA-0044073 in blood cells was significantly increased in
patients with atherosclerosis compared with that of healthy
controls (P<0.001; Fig. 1A).
Therefore, we hypothesized that circRNA-0044073 serves a key role
in atherosclerosis. As the abnormal proliferation of ECs, SMCs and
macrophages contribute to the progression of atherosclerosis, the
in vitro protocols of the present study included HUVECs and
HUVSMCs to determine the effect of circRNA-0044073 on cell growth.
It was observed that the overexpression of circRNA-0044073
significantly increased the proliferation of HUVECs and HUVSMCs,
compared with control groups, at 48 and 72 h (P<0.01; Fig. 1B and C). These results suggest a
potential association between the upregulation of circRNA-0044073
and proliferation of atherosclerosis-associated cells.
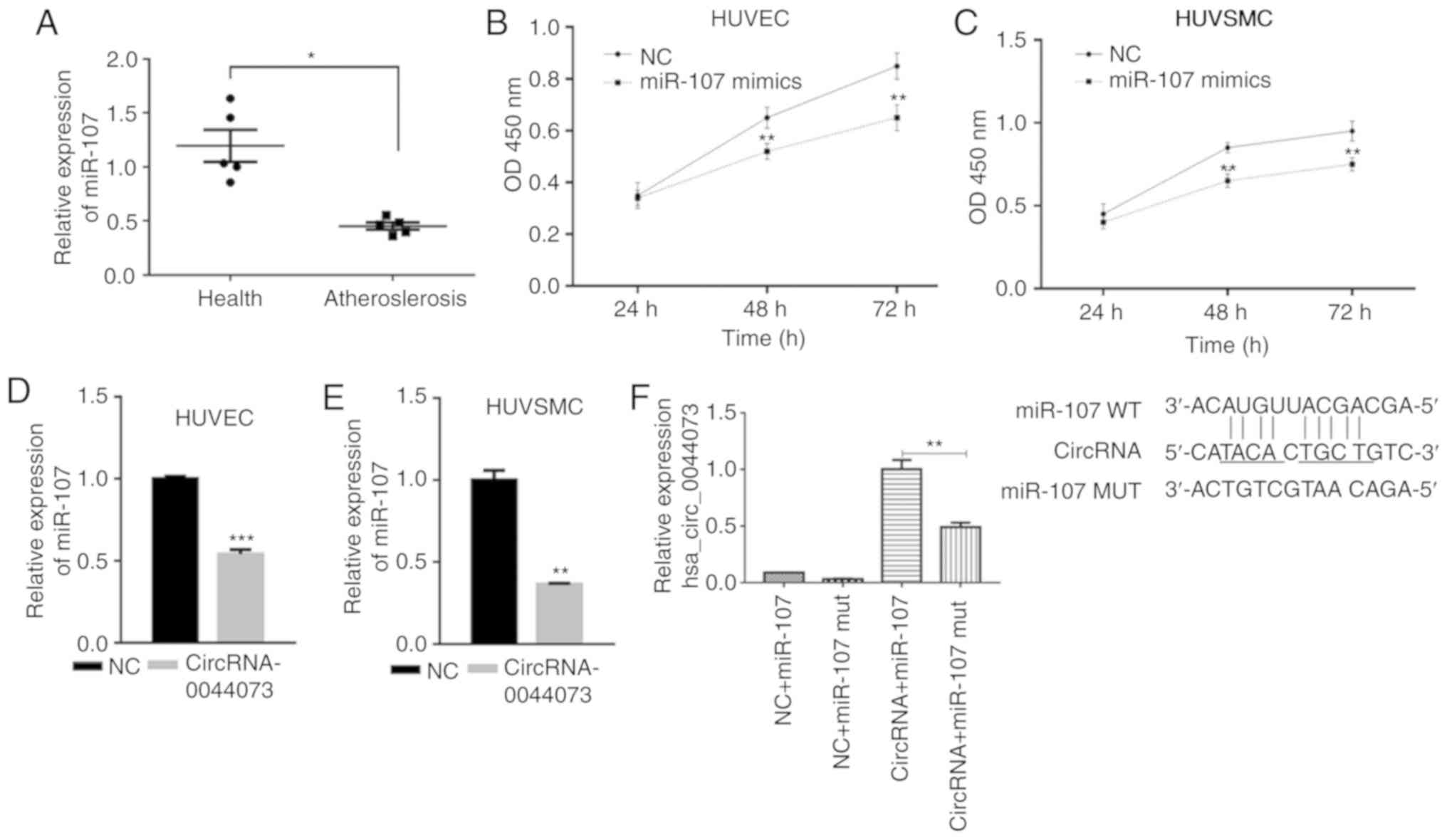
Levels of miR-107 are downregulated in
blood cells of patients with atherosclerosis and is targeted by
circRNA-0044073
A previous study has suggested the involvement of
miR-107 in the development of atherosclerosis (26). Therefore, the levels of miR-107
were detected and compared between patients with atherosclerosis
and healthy individuals. Notably, the expression of miR-107 was
significantly decreased in the blood cells of patients with
atherosclerosis compared with the controls (P<0.05; Fig. 2A). In addition, miR-107 mimics
significantly inhibited the proliferation of HUVECs and HUVSMCs,
compared with the control groups, at 48 and 72 h, which was in
contrast to the observed effects of circRNA-0044073 (P<0.01;
Fig. 2B and C). The association
between circRNA-0044073 and miR-107 was then investigated; the data
suggested that the overexpression of circRNA-0044073 significantly
downregulated the levels of miR-107 in HUVECs and HUVSMCs
(P<0.01; Fig. 2D and E).
Furthermore, RNA pull down assay demonstrated that miR-107 was a
target of circRNA-0044073 (P<0.01; Fig. 2D-F). These results indicate that
circRNA-0044073 counteracts the effect of miR-107 via a sponge
mechanism.
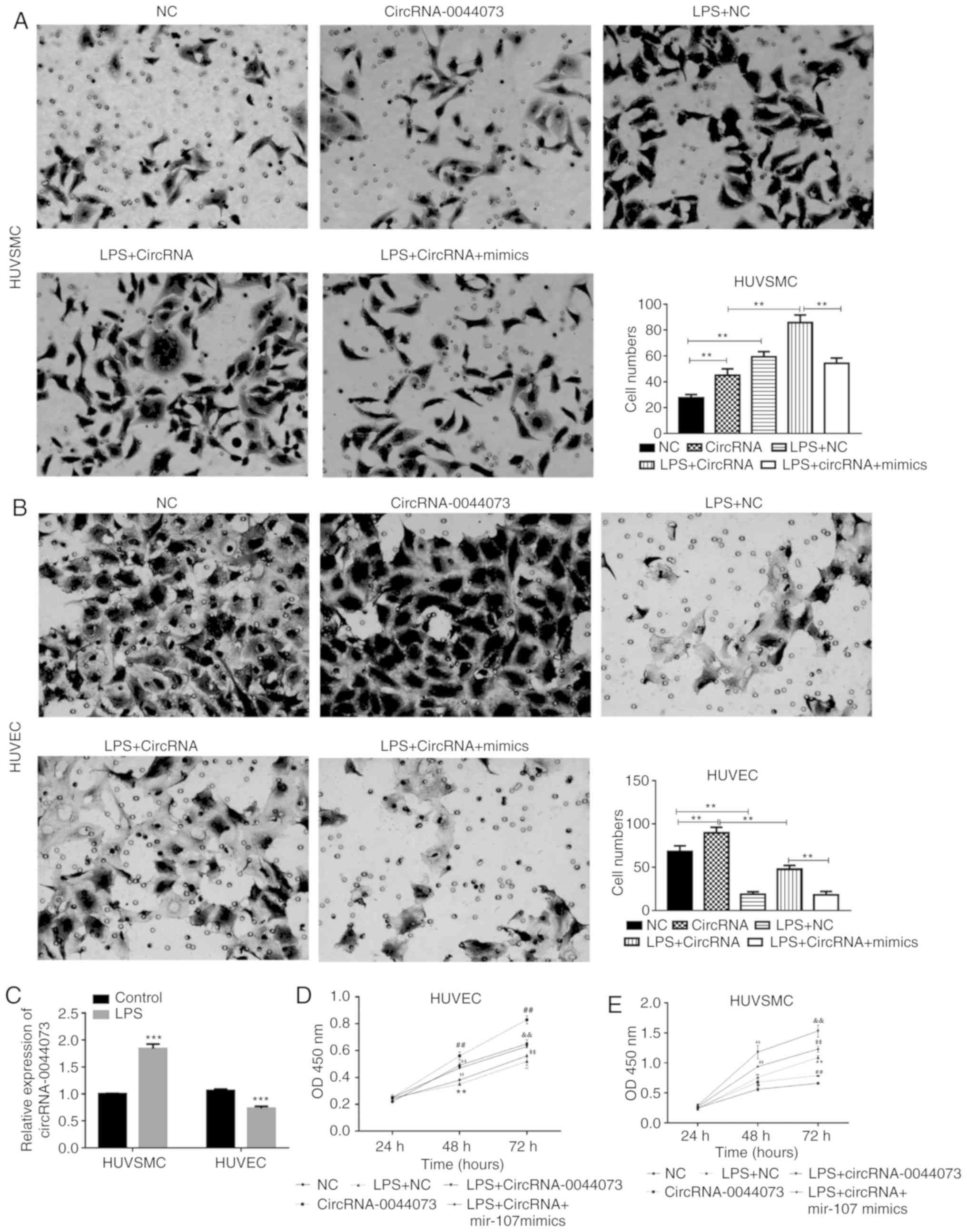
CircRNA-0044073 increases the
proliferation and invasion of cells by regulating miR-107
The regulatory mechanism of circRNA-0044073 on the
invasive activity of HUVSMCs and HUVECs was then explored. As an
endotoxin, LPS is a potentially important stimulator and
atherosclerosis risk factor. Therefore, an in vitro
atherosclerosis model was established by treating cells with LPS.
Compared with the control groups, treatment with LPS significantly
increased the number of invasive HUVSMCs and inhibited the
proliferation of HUVECs, respectively (P<0.01; Fig. 3A and B). Additionally, LPS
treatment significantly increased the levels of circRNA-0044073 in
HUVSMCs but downregulated the levels of circRNA-0044073 in HUVECs
(P<0.001; Fig. 3C). It was
additionally identified that in HUVSMCs, the combined use of LPS
and circRNA-0044073 significantly increased the proliferation of
cells, compared with a single treatment of LPS or circRNA-0044073
overexpression vector (P<0.01; Fig.
3D and E). By contrast, miR-107 mimics significantly alleviated
the effect of LPS + circRNA-0044073 treatment on the promotion the
proliferation of cells (P<0.01; Fig. 3D-E). Although the invasive activity
of HUVECs was suppressed by LPS, circRNA-0044073 transfection
partially reversed the effect of LPS and induced the invasion of
HUVECs. Similar to the effect in HUVSMCs, miR-107 impeded the role
of circRNA-0044073 in promoting cell proliferation. The effect of
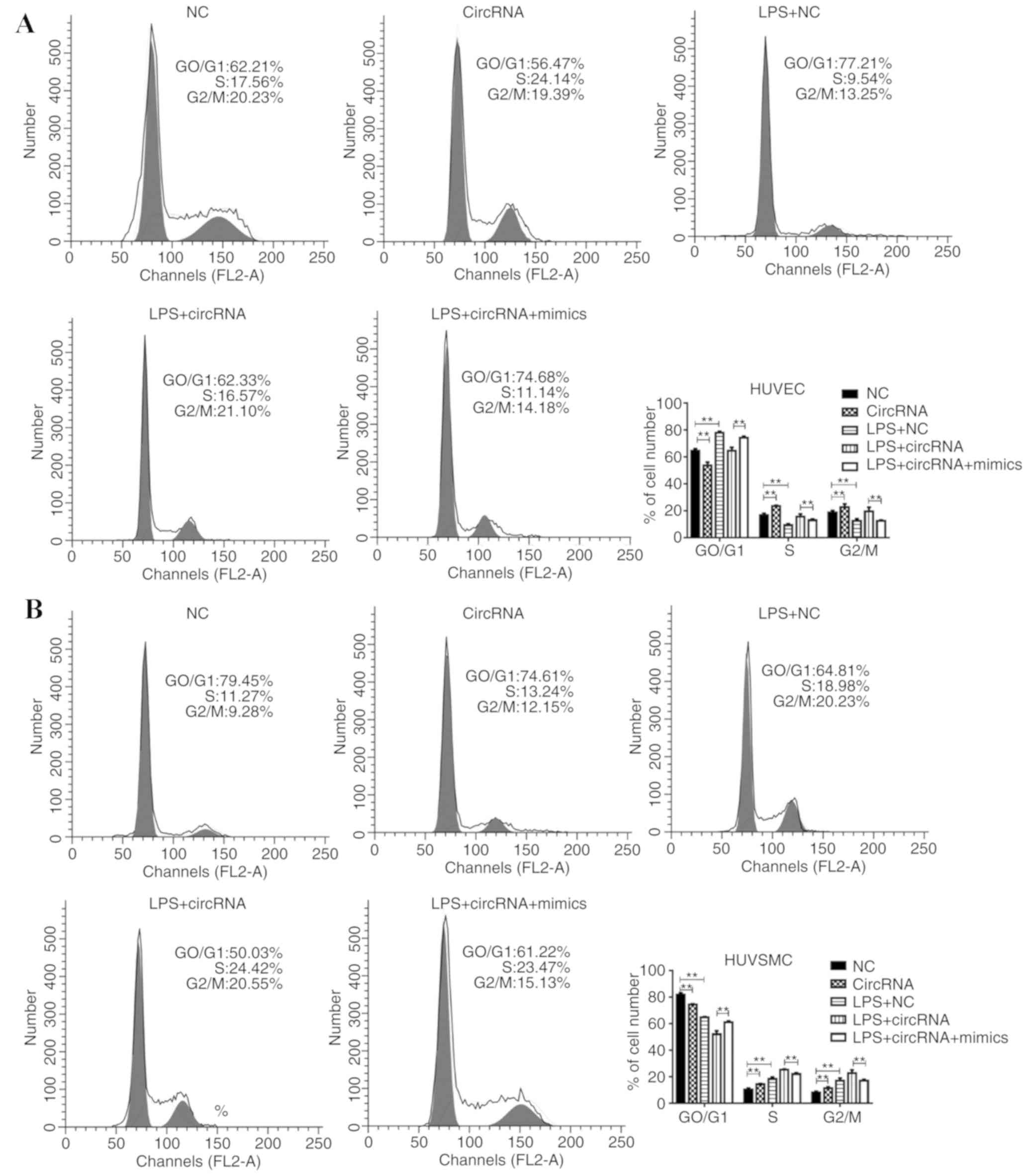
circRNA-0044073 on the cell cycle distribution was also examined.
Despite the observation that LPS treatment affected the percentages
of G2/M and S phase cells, with the addition of circRNA-0044073
transfection, the percentage of cells in G2/M phase was
additionally increased, and was partially impaired by using miR-107
mimics (Fig. 4). These results
support the hypothesis that circRNA-0044073 facilitates the
proliferation and invasion of cells by inhibiting miR-107 in the
development of atherosclerosis.
CircRNA-0044073 activates the JAK/STAT
signaling pathway of HUVSMCs and HUVECs
JAK1 is a critical contributor to the pathogenesis
of a number of inflammatory diseases and has been identified as a
potential therapeutic target (31,32).
The expression levels of JAK1 and STAT3 were significantly
increased in blood cells in patients with atherosclerosis, in
addition to an increase in Bcl-2 and c-myc levels, compared with
those in the healthy controls (P<0.001; Fig. 5A-D). Notably, in the LPS treatment
group, the Bcl-2 and c-myc levels were markedly increased in
HUVSMCs and decreased in HUVECs, which is consistent with the
distinct effect of LPS on the proliferation of these two types of
cells (33,34). However, the overexpression of
miR-107 significantly inhibited the levels of JAK1 in HUVSMCs and
HUVECs (Fig. 5E-F). The luciferase
reporter assay additionally confirmed that JAK1 was a target of
miR-107 (Fig. 5G). In contrast to
the inhibitory role of miR-107 on JAK1 expression, overexpression
of circRNA-0044073 activated the JAK/STAT signaling pathway, along
with upregulation of Bcl-2, c-myc, IL-1β, IL-6 and TNF-α levels,
compared with those in control group, which is in accordance with
the in vivo data concerning the changes in expression of
JAK1, p-STAT3, Bcl-2 and c-myc (Fig.
5H-N).
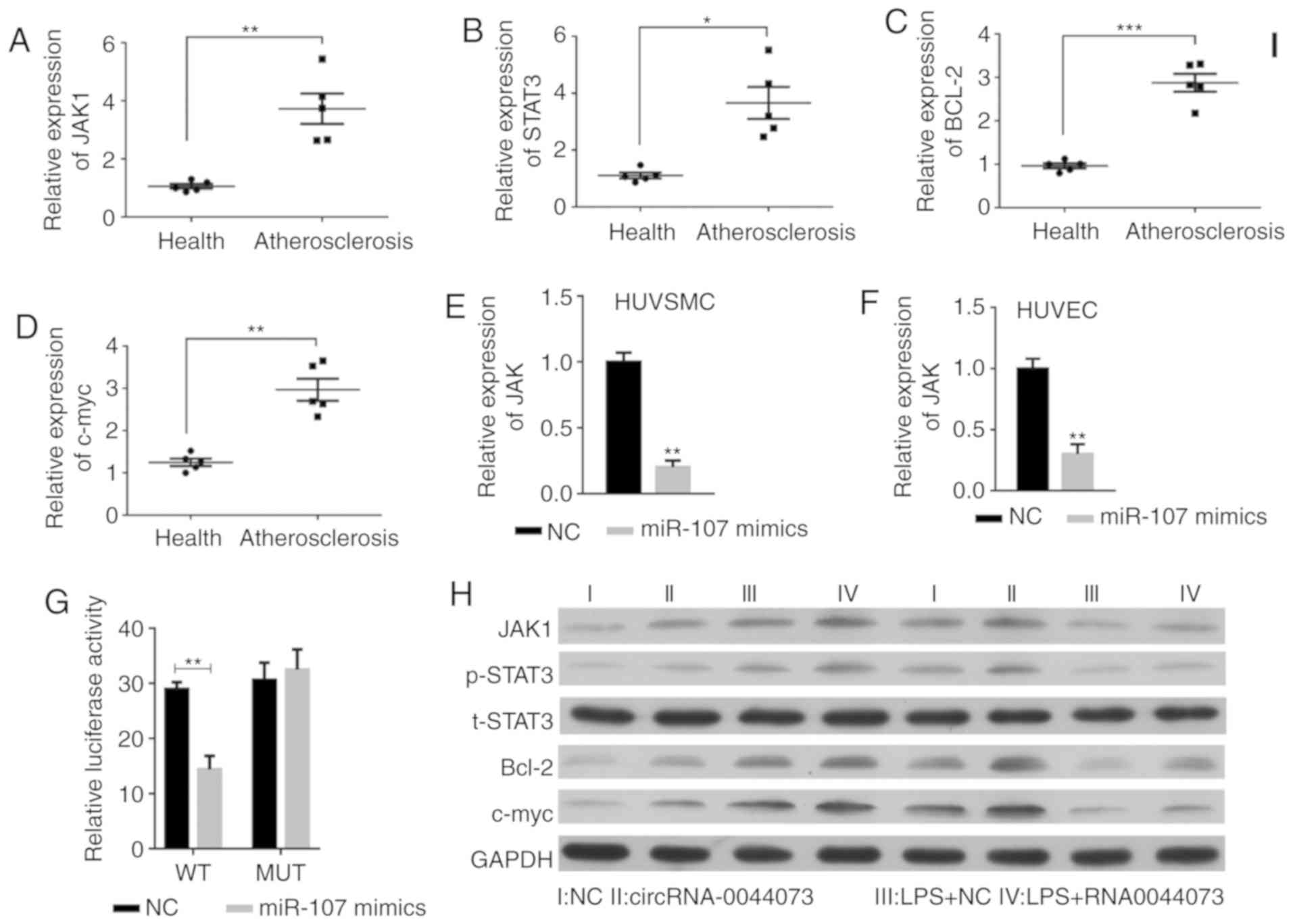 | Figure 5.Effects of circRNA-0044073 on the
JAK/STAT pathway and inflammation in atherosclerosis. Expression
levels of (A) JAK1, (B) STAT3, (C) Bcl-2 and (D) c-myc from blood
cells from 5 randomly selected patients with atherosclerosis and 5
healthy donor livers were detected. miR-107 significantly
downregulated the expression levels of JAK1 in (E) HUVSMCs and (F)
HUVECs. (G) The role of JAK1 as a target of miR-107 was identified
by using a luciferase reporter assay. (H) JAK1, t-STAT3, p-STAT3,
Bcl-2, c-myc levels were analyzed by western blot analysis.
Expression levels of (I) IL-1β in HUVSMCs, (J) IL-1β in HUVECs, (K)
IL-6 in HUVSMCs, (L) IL-6 in HUVECs, (M) TNF-α in HUVSMCs and (N)
TNF-α in HUVECs were analyzed by ELISA. *P<0.05, **P<0.01 and
***P<0.001 vs. NC. circRNA, circular RNA; HUVSMCs, human
vascular smooth muscle cells; HUVECs, human vascular endothelial
cells; NC, negative control; Il, interleukin; TNF-α, tumor necrosis
factor α; JAK1, janus kinase 1; STAT3, signal transducer and
activator of transcription 3; Bcl-2, B-cell lymphoma 2; c-myc,
v-myc avian myelocytomatosis viral oncogene homolog; t-STAT3, total
STAT3; p, phosphorylated; Health, health donor samples. |
Discussion
As the primary cause of myocardial infarction, heart
failure, myocardial ischemia and stroke, atherosclerosis accounts
for high mortality and morbidity rates worldwide (35). Although a number of treatments have
been developed and widely applied for atherosclerosis, certain
subgroups of patients remain at high risk for the development and
progression of atherosclerosis, creating an urgent requirement to
explore novel therapeutic targets or/and more effective treatments
for atherosclerosis. A previous study demonstrated that circRNAs
are crucial contributors to the pathogenesis of atherosclerosis
(36). Therefore, increasing
interest in the treatment of atherosclerosis is being focused on
circRNAs at present. In the present study, to the best of our
knowledge, it was observed for the first time that circRNA-0044073
levels were negatively associated with atherosclerosis and that
cirRNA-0044073 may directly target miR-107 and thereby lead to the
upregulation of JAK1/STAT3 signaling.
As a chronic inflammatory disease, atherosclerosis
constitutes a dysregulation of a variety of cytokines. A previous
study demonstrated that the JAK/STAT signaling pathway promotes
vascular cell inflammation, proliferation, migration and adhesion
(37). JAK1 is expressed in all
cell types, including ECs and SMCs (38,39).
Among the STAT proteins, STAT3 is an activator of systemic
inflammatory genes and is present in the inflammatory regions of
human atherosclerotic lesions in an activated form. Previous data
demonstrated an important role for the JAK/STAT pathway in oxidized
1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine-induced IL-8
transcription in vitro and in atherosclerosis in vivo
(1). It has also been indicated
that suppressors of cytokine signaling modulate the
JAK/STAT-dependent responses in vascular cells, which are
associated with atherosclerotic plaque development (40). In the present study, it was
observed that the expression levels of JAK1 and STAT3 were
significantly increased in the blood cells from patients with
atherosclerosis, which was consistent with previous studies
(18,41). Bcl-2 proteins represent the major
regulators of extrinsic and intrinsic apoptosis signaling pathways
and target apoptosis of vascular cells in atherosclerotic lesions.
The decrease in c-myc oncogene levels alleviates the proliferation
in rat VSMCs and induces apoptosis (42). Therefore, Bcl-2 and c-myc serve
important roles in the deregulation of cell apoptosis and
proliferation, contributing to the pathogenesis of atherosclerosis
(43). The data presented in the
present study indicated that the expression levels of Bcl-2 and
c-myc were significantly increased in the blood cells from patients
with atherosclerosis compared with the healthy controls. Consistent
with these results, a previous study revealed that c-myc was
overexpressed in plaque SMCs (44). However, a small number of studies
contradict the Bcl-2 data from the present study (45,46).
This may be due to differences in the test samples and the stage of
atherosclerosis investigated.
HUVECs have been used as in vitro models for
the study of atherosclerosis (47). As an endotoxin, LPS is a
potentially important stimulator and risk factor for
atherosclerosis (48). LPS has
been implicated in endothelial injury and has induced apoptosis and
proliferation in endothelial and VSMCs, respectively (34,35,49).
Consistently, the present study observed that LPS exposure resulted
in the apoptosis of HUVECs and the proliferation of HUVSMCs, and
the increased expression of circRNA-0044073 in the cells. With the
proliferative effect of circRNA-0044073, the apoptosis of HUVECs by
LPS was significantly attenuated, and the proliferation of HUVSMCs
was significantly improved. Furthermore, it was also observed that
the invasion levels of HUVECs and HUVSMCs were promoted on
treatment with LPS. Therefore, we hypothesized that circRNA-0044073
is associated with the initiation and development of the disease.
However, additional studies are required to validate this.
miR-107 is associated with inflammation (23). Previously, it was demonstrated that
the expression of miR-107 is decreased in macrophages following LPS
stimulation (23,50). A consistent change in miR-107
expression in the blood cells from patients with atherosclerosis
was observed in the present study. However, it was also
demonstrated that the miR-107 expression was upregulated in HUVECs
and HUVSMCs following LPS treatment. This variation may be due to
the different cells examined. Based on the actual pro-inflammation
function of miR-107, the decrease in miR-107 expression may be a
result of the regulative feedback (23). Consistent with a previous study,
the present study confirmed that miR-107 directly targeted JAK1
(51). Additionally, it was
demonstrated that miR-107 is also a direct target of
circRNA-0044073. Furthermore, it was observed that in HUVSMCs and
HUVECs, circRNA-0044073 overexpression decreased miR-107 levels and
resultantly increased the levels of JAK1 and p-STAT3, and the
levels of downstream proteins including Bcl-2 and c-myc. As
JAK1/STAT3 serve critical roles in inflammation, we hypothesized
that cirRNA-0044073 may be associated with inflammation, which is a
key contributor to atherosclerosis. In the present study, it was
observed that circRNA-0044073 significantly induced the levels of
IL-1β, IL-6 and TNF-α. These pro-inflammatory cytokines are
pro-atherogenic. However, the underlying mechanisms by which
circRNA-0044073 regulates these inflammatory cytokines requires
additional investigation.
Taken together, the data from the present study
suggests that circRNA-0044073 is a potential therapeutic target for
the treatment of atherosclerosis, as it directly targets miR-107
and resultantly increases STAT3 activation, and upregulates the
expression of the downstream proteins that contribute to
atherosclerosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Key Research and
Develop-ment Plan of Shandong Province (grant no. 2017GSF218012)
and Shandong Provincial Natural Science Foundation (grant no.
ZR2015HM052).
Authors' contributions
LS, YH and WWu designed the study. LS, YH, JL, SY,
WWa and YW performed the experiments. LS, YX and WWa performed the
data analysis and drafted the manuscript. All authors read and
approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
Informed consent was obtained from all patients and
ethical approval was granted by The Ethics Committee of the Qi-Lu
Hospital of Shandong University.
Patient consent for publication
Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gharavi NM, Alva JA, Mouillesseaux KP, Lai
C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, et al:
Role of the Jak/STAT pathway in the regulation of interleukin-8
transcription by oxidized phospholipids in vitro and in
atherosclerosis in vivo. J Biol Chem. 282:31460–31468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gorenne I, Kumar S, Gray K, Figg N, Yu H,
Mercer J and Bennett M: Vascular smooth muscle cell sirtuin 1
protects against DNA damage and inhibits atherosclerosis.
Circulation. 127:386–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng Y, Li Y, Liu G, Qi X and Cao X:
MicroRNA-24 inhibits the proliferation and migration of endothelial
cells in patients with atherosclerosis by targeting importin-α3 and
regulating inflammatory responses. Exp Ther Med. 15:338–344.
2018.PubMed/NCBI
|
|
5
|
Baumer Y, McCurdy S, Alcala M, Mehta N,
Lee BH, Ginsberg MH and Boisvert WA: CD98 regulates vascular smooth
muscle cell proliferation in atherosclerosis. Atherosclerosis.
256:105–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luc G, Bard JM, Juhan-Vague I, Ferrieres
J, Evans A, Amouyel P, Arveiler D, Fruchart JC and Ducimetiere P;
PRIME Study Group, : C-reactive protein, interleukin-6, and
fibrinogen as predictors of coronary heart disease: The PRIME
study. Arterioscler Thromb Vasc Biol. 23:1255–1261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunetti ND, Salvemini G, Cuculo A,
Ruggiero A, De Gennaro L, Gaglione A and Di Biase M: Coronary
artery ectasia is related to coronary slow flow and inflammatory
activation. Atherosclerosis. 233:636–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zernecke A and Weber C: Chemokines in the
vascular inflammatory response of atherosclerosis. Cardiovasc Res.
86:192–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barton M: Prevention and endothelial
therapy of coronary artery disease. Curr Opin Pharmacol.
13:226–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holdt LM, Stahringer A, Sass K, Pichler G,
Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou
A, et al: Circular non-coding RNA ANRIL modulates ribosomal RNA
maturation and atherosclerosis in humans. Nat Commun. 7:124292016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayoumi AS, Aonuma T, Teoh JP, Tang YL and
Kim IM: Circular noncoding RNAs as potential therapies and
circulating biomarkers for cardiovascular diseases. Acta Pharmacol
Sin. 39:1100–1109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CY, Ma L and Yu B: Circular RNA
hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells
proliferation and angiogenesis. Biomed Pharmacother. 95:1514–1519.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng C, Niu H, Li M, Zhang H, Yang Z,
Tian L, Wu Z, Li D and Chen X: Cyclic RNA has-circ-000595 regulates
apoptosis of aortic smooth muscle cells. Mol Med Rep. 12:6656–6662.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diniz GP and Wang DZ: Regulation of
skeletal muscle by microRNAs. Compr Physiol. 6:1279–1294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu C, Xie Z and Peng Q: MiRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
16
|
Chen Z, Zhao L, Zhao F, Yang G and Wang J:
MicroRNA-26b regulates cancer proliferation migration and cell
cycle transition by suppressing TRAF5 in esophageal squamous cell
carcinoma. Am J Transl Res. 8:1957–1970. 2016.PubMed/NCBI
|
|
17
|
Undi RB, Kandi R and Gutti RK: MicroRNAs
as haematopoiesis regulators. Adv Hematol. 2013:6957542013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Han Z, Fan Y, Zhang J, Chen K, Gao
L, Zeng H, Cao J and Wang C: MicroRNA-9 Inhibits NLRP3 inflammasome
activation in human atherosclerosis inflammation cell models
through the JAK1/STAT signaling pathway. Cell Physiol Biochem.
41:1555–1571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang ZP and Zhou TB: Role of miR-107 and
its signaling pathways in diseases. J Recept Signal Transduct Res.
34:338–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu F, Liu S, Ai F, Zhang D, Xiao Z, Nie X
and Fu Y: miR-107 promotes proliferation and inhibits apoptosis of
colon cancer cells by targeting prostate apoptosis response-4
(Par4). Oncol Res. 25:967–974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nelson PT and Wang WX: MiR-107 is reduced
in Alzheimer's disease brain neocortex: Validation study. J
Alzheimers Dis. 21:75–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su SG, Yang M, Zhang MF, Peng QZ, Li MY,
Liu LP and Bao SY: miR-107-mediated decrease of HMGCS2 indicates
poor outcomes and promotes cell migration in hepatocellular
carcinoma. Int J Biochem Cell Biol. 91:53–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Foley NH and O'Neill LA: miR-107: A
Toll-like receptor-regulated miRNA dysregulated in obesity and type
II diabetes. J Leukoc Biol. 92:521–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W
and Zhang A: Upregulation of MicroRNA-107 induces proliferation in
human gastric cancer cells by targeting the transcription factor
FOXO1. FEBS Lett. 588:538–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norata GD, Sala F, Catapano AL and
Fernandez-Hernando C: MicroRNAs and lipoproteins: A connection
beyond atherosclerosis? Atherosclerosis. 227:209–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daimiel-Ruiz L, Klett-Mingo M,
Konstantinidou V, Micó V, Aranda JF, García B, Martínez-Botas J,
Dávalos A, Fernández-Hernando C and Ordovás JM: Dietary lipids
modulate the expression of miR-107, an miRNA that regulates the
circadian system. Mol Nutr Food Res. 59:552–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong X, Moran MS, Zhao Y and Yang Q:
Inhibition of metadherin sensitizes breast cancer cells to AZD6244.
Cancer Biol Ther. 13:43–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang CM, Zhang M, Huang L, Hu ZQ, Zhu JN,
Xiao Z, Zhang Z, Lin QX, Zheng XL, Yang M, et al: CircRNA_000203
enhances the expression of fibrosis-associated genes by
derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac
fibroblasts. Sci Rep. 7:403422017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Shea JJ and Plenge R: JAK and STAT
signaling molecules in immunoregulation and immune-mediated
disease. Immunity. 36:542–550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maschalidi S, Sepulveda FE, Garrigue A,
Fischer A and de Saint Basile G: Therapeutic effect of JAK1/2
blockade on the manifestations of hemophagocytic
lymphohistiocytosis in mice. Blood. 128:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi KB, Wong F, Harlan JM, Chaudhary PM,
Hood L and Karsan A: Lipopolysaccharide mediates endothelial
apoptosis by a FADD-dependent pathway. J Biol Chem.
273:20185–20188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang D, Yang Y and Li D:
Lipopolysaccharide induced vascular smooth muscle cells
proliferation: A new potential therapeutic target for proliferative
vascular diseases. Cell Prolif. 50:e123322017. View Article : Google Scholar
|
|
35
|
Liu M, Tao G, Liu Q, Liu K and Yang X:
MicroRNA let-7g alleviates atherosclerosis via the targeting of
LOX-1 in vitro and in vivo. Int J Mol Med. 40:57–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z
and Sharpless NE: Expression of linear and novel circular forms of
an INK4/ARF-associated non-coding RNA correlates with
atherosclerosis risk. PLoS Genet. 6:e10012332010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai
L, Chen Y, Zhao S, Fan J, Cheng X and Liu E: Protein inhibitor of
activated STAT3 suppresses oxidized LDL-induced cell responses
during atherosclerosis in apolipoprotein E-deficient mice. Sci Rep.
6:367902016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rane SG and Reddy EP: Janus kinases:
Components of multiple signaling pathways. Oncogene. 19:5662–5679.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Verma A, Kambhampati S, Parmar S and
Platanias LC: Jak family of kinases in cancer. Cancer Metastasis
Rev. 22:423–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ortiz-Muñoz G, Martin-Ventura JL,
Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O,
Muñoz-Garcia B, Fernandez-Vizarra P, Ortega L, Egido J and
Gomez-Guerrero C: Suppressors of cytokine signaling modulate
JAK/STAT-mediated cell responses during atherosclerosis.
Arterioscler Thromb Vasc Biol. 29:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hiltunen MO, Tuomisto TT, Niemi M, Bräsen
JH, Rissanen TT, Törönen P, Vajanto I and Ylä-Herttuala S: Changes
in gene expression in atherosclerotic plaques analyzed using DNA
array. Atherosclerosis. 165:23–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bennett MR, Evan GI and Newby AC:
Deregulated expression of the c-myc oncogene abolishes inhibition
of proliferation of rat vascular smooth muscle cells by serum
reduction, interferon-gamma, heparin, and cyclic nucleotide
analogues and induces apoptosis. Circ Res. 74:525–536. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kutuk O and Basaga H: Bcl-2 protein
family: Implications in vascular apoptosis and atherosclerosis.
Apoptosis. 11:1661–1675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marin ML, Gordon RE, Veith FJ, Tulchin N
and Panetta TF: Distribution of c-myc oncoprotein in healthy and
atherosclerotic human carotid arteries. J Vasc Surg. 18:170–177.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kockx MM, De Meyer GR, Muhring J, Jacob W,
Bult H and Herman AG: Apoptosis and related proteins in different
stages of human atherosclerotic plaques. Circulation. 97:2307–2315.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saxena A, McMeekin JD and Thomson DJ:
Expression of Bcl-x, Bcl-2, Bax, and Bak in endarterectomy and
atherectomy specimens. J Pathol. 196:335–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi Y and Tokunaga O: Chlamydia pneumoniae
(C. pneumoniae) infection upregulates atherosclerosis-related gene
expression in human umbilical vein endothelial cells (HUVECs).
Atherosclerosis. 177:245–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Anikhovskaia IA, Kubatiev AA and Iakovlev
Mlu: Endotoxin theory of atherosclerosis. Fiziol Cheloveka.
41:106–116. 2015.(In Russian). PubMed/NCBI
|
|
49
|
Wort SJ and Evans TW: The role of the
endothelium in modulating vascular control in sepsis and related
conditions. Br Med Bull. 55:30–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hennessy EJ, Sheedy FJ, Santamaria D,
Barbacid M and O'Neill LA: Toll-like receptor-4 (TLR4)
down-regulates microRNA-107, increasing macrophage adhesion via
cyclin-dependent kinase 6. J Biol Chem. 286:25531–25539. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kumar V and Mahato RI: Delivery and
targeting of miRNAs for treating liver fibrosis. Pharm Res.
32:341–361. 2015. View Article : Google Scholar : PubMed/NCBI
|