Introduction
The three essential elements of tissue engineering
are seed cells, scaffold materials and cytokines (1–3). The
selection of tissue engineering scaffold material is directly
associated with the effect of the material implanted in the animal
or human body. Most importantly, the scaffold materials should have
good biocompatibility (4–6). Scaffold materials can provide a good
adsorption interface for the adhesion of seed cells, in order to
facilitate cell proliferation. In addition, the scaffold material
must have a certain level of biomechanical strength (7,8) and
maintain a controlled degradation rate once implanted into the
animal or human body. The material should have no cytotoxic,
immunogenic, tumorigenic or teratogenic effects on animals or
humans. In addition, the scaffold materials are required to
integrate cytokines and to activate the expression of various
specific genes, Runt-related transcription factor 2, osteocalcin
(9), peroxisome
proliferator-activated receptor-γ (10) and α-smooth muscle actin (11) in order to maintain the normal
phenotype of the seed cells on the scaffold surface (12).
Classes of scaffold materials include natural
biological scaffold materials, synthetic biodegradable polymer
scaffolds materials (13–15), composite scaffold materials
(16–18) and nano-scaffold materials (16–18).
Natural biological scaffolds have good biocompatibility but poor
mechanical properties (19).
Synthetic biodegradable polymer scaffolds tend to have strong
mechanical properties (20), but
lack good cellular compatibility. Nano-scaffold materials have been
widely studied in recent years, but are expensive to produce
(21). Currently, the most widely
used scaffold materials are composite scaffold materials. These
benefit from the good cellular compatibility of natural biological
scaffolds and the strong mechanical properties of synthetic
biodegradable polymer scaffolds (22).
In our previous studies, human-like collagen I
(HLC-I) (23) was combined with
the acellular vascular matrix (ACVM) (23,24)
to construct the ACVM-0.25% HLC-I scaffold material (25). This process involved combining a
polymer material with natural degenerative biomaterials to
construct a composite tissue engineering scaffold material. This
material supports the growth of seed cells with a three-dimensional
ultrastructure of protein space to promote the formation of
functional tissue, but it also possesses compressive mechanical
properties required for vascular tissue engineering (25). A previous study demonstrated that
vascular endothelial cells and vascular smooth muscle cells are
induced and differentiated by human gingival fibroblasts (HGFs)
(25). Combining these cells with
ACVM-0.25% HLC-I scaffold materials supported successful tissue
engineering of vessels that possessed the morphological and immune
characteristics of vascular tissue. The structure of the
tissue-engineered vascular tissue was intact following 9 weeks in
nude mice (25). Thus, the
aforementioned results demonstrated the feasibility of ACVM-0.25%
HLC-I scaffold material for tissue engineering.
The present study compares the biological
characteristics of different ACVM-0.25% HLC-I scaffold materials
with Acellular Dermal Matrix (ADM) (26,27),
Small Intestinal Submucosa (SIS) (28,29)
and Bio-Gide scaffolds in tissue engineering. The aim of the
present study was to confirm the advantages of ACVM-0.25% HLC-I
composite scaffolds and lay a foundation for the research of tissue
engineering scaffold materials.
Materials and methods
Preparation of vascular scaffolds
Preparation of ACVM-HLC-I
scaffolds
Female 12-week-old New Zealand white rabbits,
weighing 1.8–2.0 kg, were provided by the Animal Center of Hebei
Medical University (Shijiazhuang, China; license no. SCXK
2013-1-003; certification no. 1505098). The rabbits were housed in
plastic cage (1 rabbit per cage) and were allowed free access to
food and water. The housing conditions were maintained at
temperature of 25°C, relative humidity of 40–70%, under a 12-h
light/dark cycle. Animal experiments were approved by the Ethics
Committee of Southwest Medical University (Sichuan, China).
ACVM-HLC-I scaffolds were prepared as described previously
(23). Briefly, the blood vessels
of New Zealand white rabbits were isolated and washed in saline
solution eight times. Sections of blood vessel 2 cm in length were
immersed in PBS containing 1% benzalkonium bromide (Lircon) for 1 h
at room temperature and then in PBS for 5 min. The vessels were
then placed in a liquid mixture with 1% trypsin and 0.01% EDTA for
24 h at 37°C and 5% CO2. Following washing with PBS for
5 min, samples were incubated in PBS containing 1% Triton-X-100 for
72 h at room temperature. Finally, blood vessels were immersed in
sterile PBS solution at 4°C for subsequent experiments.
Then, the ACVM scaffolds were prepared as follows:
The bio-activation of ACVM-HLC-I was performed by firstly using a
solution of acrylic acid to reduce the dissolved oxygen on the
surface of the ACVM scaffolds. The scaffolds were then exposed to
UV radiation for 30 min and placed in a Petri dish. The scaffolds
were washed with distilled water to remove the excess homo-polymer
and placed to dry in a vacuum desiccator. Immobilization of HLC-I
was performed once the surface of the scaffolds was modified with
acrylic acid. The ACVM-acrylic acid scaffolds were immersed at 4°C
for 1 h in PBS, which also contained 5 mg/ml water-soluble
carbodiimide in order to activate the carboxyl groups on the
surface of the ACVM scaffolds. Secondly, various concentrations of
HLC-I (0.10, 0.25, 0.50, 0.75 and 1.00 mg/ml) were added to combine
with the surface of the ACVM for 5 h at room temperature. Thirdly,
the scaffolds were washed with PBS at room temperature for 1 h to
remove the excess HLC-I that was physically adsorbed on the surface
of the scaffold. Finally, ACVM-HLC-I scaffolds were dried under
reduced pressure and stored at 4°C.
Preparation of SIS, ADM and Bio-Gide
scaffolds
Serosa and muscle of the jejunum were removed using
gauze. Then, the jejunum was immersed in PBS that contained 1%
penicillin and 1% streptomycin for 3 h at room temperature. Trypsin
(0.25%) was added to the jejunum for 24 h at room temperature and
then it was immersed in 0.5% SDS for 24 h. The jejunum was washed
with distilled water twice. Finally, the jejunum was sterilized in
sterile water containing 20% ethanol and 0.1% peracetic acid for 10
h at room temperature, then freeze-dried for use. The ADM scaffold
was purchased from Beijing Qingyuanweiye Bio-Tissue Engineering,
Co., Ltd., (Beijing, China), and the Bio-Gide scaffold was
purchased from Geistlich Pharma AG (Wolhusen, Switzerland).
Cell isolation and culture of primary
cells
HGFs were used for cell studies. HGFs were isolated
from healthy gingival tissue, which was harvested from clinical
donors undergoing mandibular third impact molar extraction as
described previously (30). The
acquisition of normal human gingival tissues from patients was
approved by the Hospital of Stomatology at Hebei Medical University
(Hebei, China), and written informed consent was obtained from all
patients. Following the removal of the epithelial layer, human
gingival tissues were cut into 1×1 mm2 pieces and placed
in low-glucose Dulbecco's modified Eagle medium (L-DMEM; Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin (Shenzhen Huayao Nanfang Pharmaceutical
Co., Ltd., Shenzhen, China), and 100 U/ml streptomycin (Shenzhen
Huayao Nanfang Pharmaceutical Co., Ltd.). Cells at the second or
the third passage were from 6 different donors in the present
study.
Immunofluorescence staining of
HGFs
HGFs were immersed in acetone and washed with PBS.
Then, HGFs were fixed in 4% paraformaldehyde at 4°C overnight and
washed with PBS three times. The following primary antibodies were
used for cell incubation at 37°C for 2 h: Vimentin (1:100; ZM-0260;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) and S100A4 (1:100; ZA-0257; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.). H2O2
(3%) was added to blocking protease for 20 min at room temperature
and then 30 µl sheep serum blocking buffer (ZC-02125; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) was added for 1 h
at room temperature. Following three washes with PBS, the cells
were incubated with the following secondary antibodies at 37°C for
1 h: Fluorescein isothiocyanate-conjugated goat anti-mouse
immunoglobulin G (IgG; 1:100, ZF-0312; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) and rhodamine-conjugated goat
anti-rabbit IgG (1:100; ZF-0316; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.). Cell nuclei were stained with
4′,6-diamidino-2-phenylindole for 10 min at room temperature.
Finally, the cells were photographed under a laser confocal
microscope (Olympus Corporation, Tokyo, Japan).
Optimal concentration of HLC-I
crosslinking with ACVM scaffolds measured by MTT
ACVM scaffolds with various concentrations of HLC-I
(ACVM-HLC-I scaffolds) were cut into smaller blocks of 10.0 mm
diameter. Scaffolds were placed in a 24-well culture plate. A
volume of 100 µl L-DMEM containing 1×105 HGFs was placed
on each scaffold. The seeded scaffolds were placed in a 37°C
incubator for 2 h and then transferred to another 24-well plate,
following which 2 ml culture medium was added to each well. The
culture medium was replaced every 2 days. The number of cells of
each scaffold were tested by MTT assay following culture for 1, 4
and 7 days.
For the MTT assay, HGFs on each scaffold were
incubated with L-DMEM for 12 h at 37°C. Then, 10 µl of 5 mg/ml MTT
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) was added
to each well. A well without cells was used as the blank control.
The plate was incubated at 37°C for 3.5 h. Then, 100 µl DMSO was
added to each well and incubated at room temperature for 10 min.
Absorbance at 490 nm was measured by microplate reader to calculate
the concentration of cells on ACVM-HLC-I scaffolds.
Observation analysis of the vascular
scaffolds
Histological analysis
Sections of scaffold (5 cm) were mounted on light
microscope slides and Masson Trichrome staining (HT15;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was performed. The
slides were stained with Masson complex staining for 5 min at room
temperature and washed with 0.2% acetic acid water. Then, the
slides were immersed in 5% phosphotunfstic acid for 5 min and
washed with 0.2% acetic acid water twice. Following
deparaffinization, the slides were stained with Bouin's solution at
56°C for 15 min. Hematoxylin and eosin (H&E) staining was used
to investigate the basic structure of ACVM-0.25% HLC-I scaffold
compared with SIS, ADM and Bio-Gide scaffolds, and to observe the
four different biodegradable scaffolds following seeding with HGFs.
After dewaxing, the slides were stained with hematoxylin for 5 min
at room temperature, following by washing with water for 2 min.
Then, the slides were stained with eosin for 30 sec, and next
washed with water for 2 min. Masson staining was performed to
evaluate the fiber composition of the ACVM-0.25% HLC-I, SIS, ADM
and Bio-Gide scaffolds.
Water absorption capacities
The water absorption capacities of the four
scaffolds were determined by the degree of swelling in PBS at room
temperature. A known weight of the scaffold material was placed in
PBS for 12 h. The wet weight of the scaffold was determined as the
weight of the scaffold after blotting the surface with filter paper
to remove the extra PBS. The percentage of water absorption of the
scaffolds was calculated using the following equation (23):
W1-W0/W0 ×100%, where
W1 represents the wet weight of the scaffolds after 24 h
and W0 is the initial weight of the scaffolds.
Measurement of scaffold mechanical
ability
The stress-time and stress-strain curves of the
scaffolds were tested using a universal material testing machine
(Zwick Roell Z020; Zwick Roell Group, Ulm, Germany). The scaffolds
were divided into four groups: ACVM-0.25% HLC-I scaffold, ADM
scaffold, Bio-Gide scaffold and SIS scaffold groups. During the
whole experiment, PBS was used to keep the samples moist. All
samples were pre-adjusted prior to testing. The length of the
specimen was 1 cm and it was stretched at a speed of 10 mm/min
until it snapped. The stress and strain were subsequently recorded.
Stress is the mechanical state describing every direction of every
point within an object. It is described as the force per unit area.
Strain is the amount of change in the shape of a material under
external force. The stress-strain curves were plotted on the basis
of stress as the ordinate and strain as the abscissa. The breaking
strength and elongation at break were also calculated. Breaking
strength = maximum stress when the material breaks /
cross-sectional area. Elongation at break (%) = displacement value
of material when it breaks/original length of material.
Scanning electron microscope (SEM)
imaging
The samples were fixed in a metal and coated with a
thin layer of gold, using an Edwards EXC 120 Turbo Pump Controller.
A SEM (JSM-5310; JEOL, Ltd., Tokyo, Japan) was used to investigate
the surface morphology of the four scaffolds.
Observation analysis of the vascular
scaffolds following seeding with HGFs
Histological analysis
Scaffold sections (5 cm) were mounted on light
microscope slides and Masson Trichrome staining was performed at
room temperature. The slides were stained with Masson complex
staining for 5 min and after washed with 0.2% acetic acid water.
Then, the slides were immersed in 5% phosphotunfstic acid for 5 min
and washed with 0.2% acetic acid water twice. Following dewaxing,
the slides were stained with Bouin's solution at 56°C for 15 min.
H&E staining was used to evaluate ACVM-0.25% HLC-I, SIS, ADM
and Bio-Gide scaffolds after seeding with HGFs. Tissue samples for
histology were fixed in 4% paraformaldehyde at 4°C for 24 h and
embedded in paraffin. After dewaxing, the slides were stained with
hematoxylin for 5 min at room temperature, following by washing
with water for 2 min. Then, the slides were stained with eosin for
30 sec, then with water for 2 min.
SEM imaging
The surface morphology of HGFs on the four different
biodegradable scaffolds was observed using an JSM-6320F SEM (JEOL,
Ltd.), as aforementioned.
Growth kinetics of HGFs on
scaffolds
Cell Counting Kit-8 (CCK-8) was used to evaluate the
growth kinetics of HGFs on the four scaffolds. A total of
2×103 HGFs/well were seeded on the four scaffolds
inoculated in 96-well microplates. CCK-8 solution (10 µl) was added
to each well and incubated at 37°C for 1 h. Subsequently, the
absorbance of each well was measured by a microplate reader at 450
nm on days 1, 4 and 7 after cell seeding. The absorbance values at
different time points were used to construct a growth curve to
indicate the growth kinetics of HGFs on the four scaffolds.
Changes in the diameter of the
scaffolds following HGF seeding
A vernier caliper was used to measure the diameter
of the ACVM-0.25% HLC-I, ADM, Bio-Gide and SIS scaffolds prior to
seeding with HGFs. The diameters of all scaffolds were measured to
be 10 mm. The density of 2×105 HGFs were placed on the
surface of each scaffold and cultured in a 37°C incubator for 48 h.
Then, the scaffolds were removed and the culture fluid was
aspirated with sterile filter paper. The diameter of each scaffold
was measured using a vernier caliper following compounding of
HGFs.
Statistical analysis
Statistical analysis was performed by using SPSS
software (v. 21.0; IBM Corp., Armonk, NY, USA). The measurement
data are presented as the mean ± standard deviation and were
analyzed by one-way analysis of variance, followed by a
Student–Newman–Keuls multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
General observations of the four
scaffold materials
The ACVM-HLC-I scaffold presented as an ivory-white,
translucent, non-elastic vascular wall with folded and damaged
lumen (Fig. 1A). The ADM scaffold
was ivory-white, translucent and exhibited a honeycomb structure
with a rough surface (Fig. 1B).
The thickness of ADM was 0.4–0.6 mm. Bio-Gide presented as pale
yellow-white, translucent, rectangular patches with a smooth
surface (Fig. 1C). SIS presented
as a pale ivory, translucent membrane (Fig. 1D).
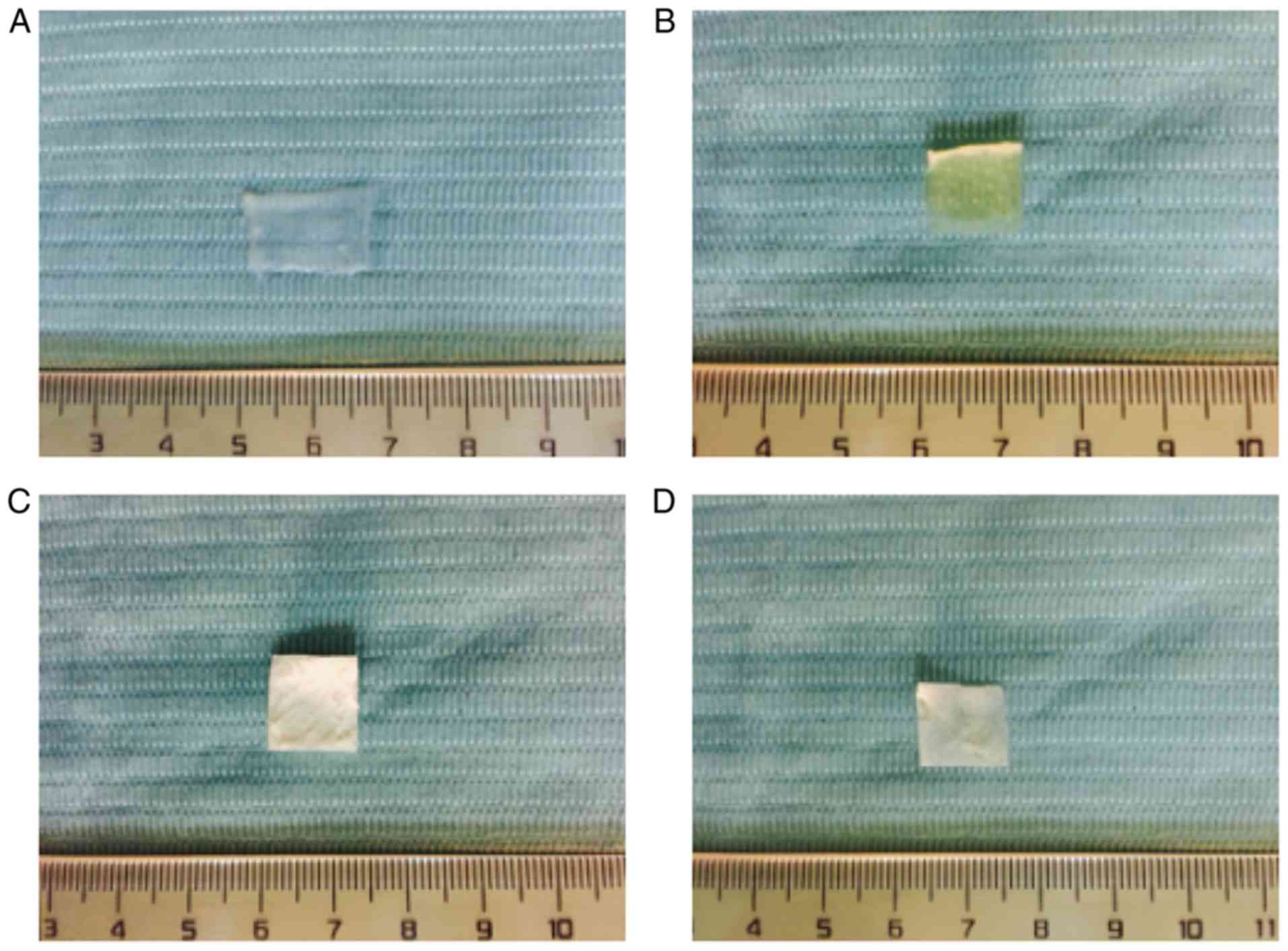 | Figure 1.Observation of scaffold materials.
(A) ACVM-HLC-I, (B) ADM, (C) Bio-Gide and (D) SIS. ACVM-HLC-I
scaffold presented as an ivory-white, translucent and non-elastic
vascular wall with a folded and damaged lumen. ADM presented with
an ivory-white, translucent, honeycomb structure with a rough
surface. Bio-Gide was pale yellow-white, with translucent
rectangular patches and a smooth surface. SIS presented as a pale
ivory, translucent membrane. ACVM-HLC-I, acellular vascular
matrix-human-like collagen I; ADM, acellular dermal matrix; SIS,
small intestinal submucosa. |
Observation of HGFs by inverted
microscope
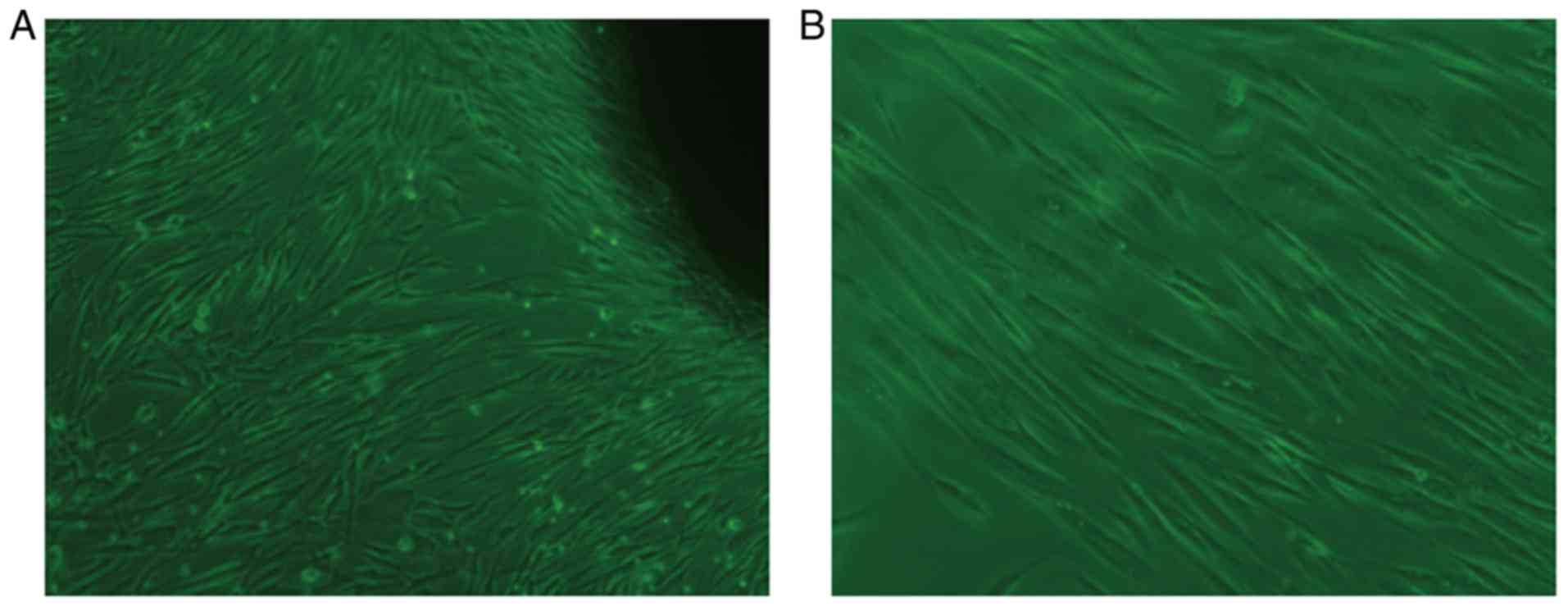
Following 7 days of primary culture of HGFs,
fibroblast-like cells with clear and large outlines, spherical or
elliptic nuclei were observed (Fig.
2A). At an average of 15 days, a large number of cells were
available to harvest the fibroblast-like cells. As shown in
(Fig. 2B), the second generation
of cells was tightly adhered, spindle-shaped and well-spread, and
fibroblast-like in appearance.
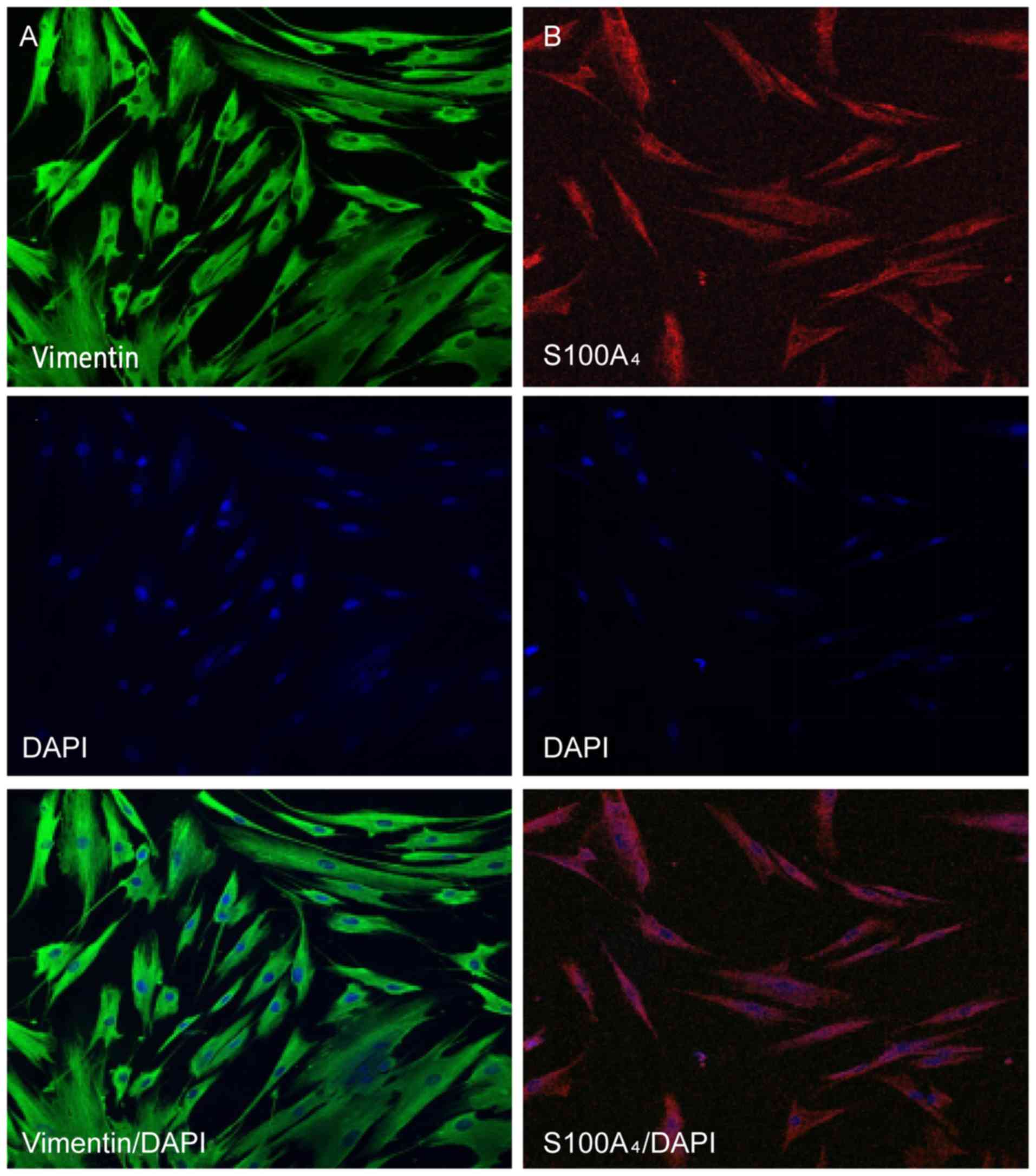
Identification of HGFs by
immunofluorescent staining
The characteristics of HGFs were identified by
immunofluorescent staining of vimentin and S100A4, markers for
mesenchymal cells. It was observed that the protein expression of
vimentin and S100A4 was positive in the cytoplasm of HGFs (Fig. 3).
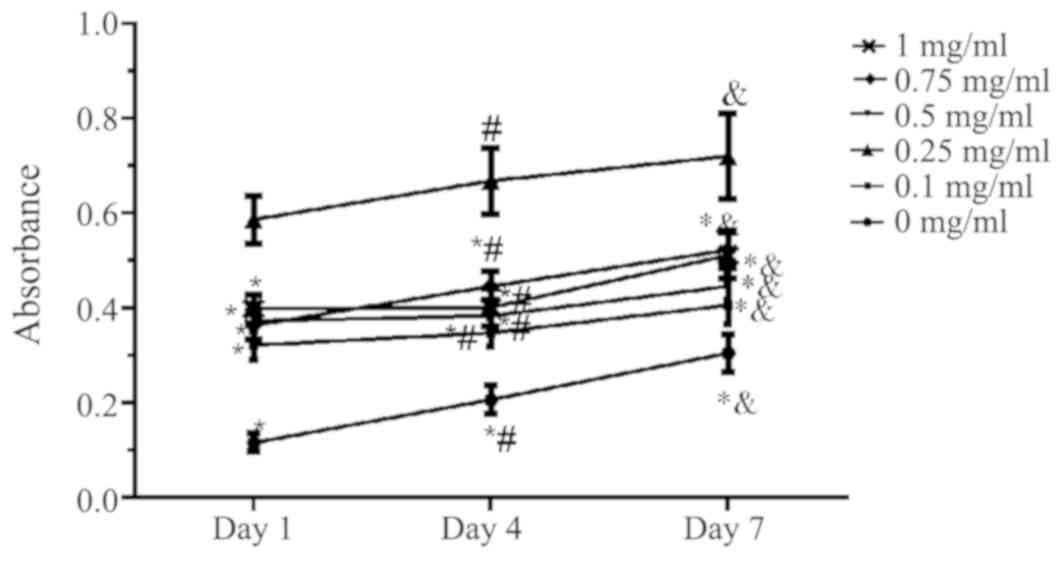
Cell adherence ability by MTT
assay
The results demonstrated that compared with the ACVM
scaffold without HLC-I, the ACVM-HLC-I scaffold had better cell
adherence. In addition, the ACVM scaffold coated with 0.25% HLC-I
presented the highest cell adherence at all time points (Fig. 4).
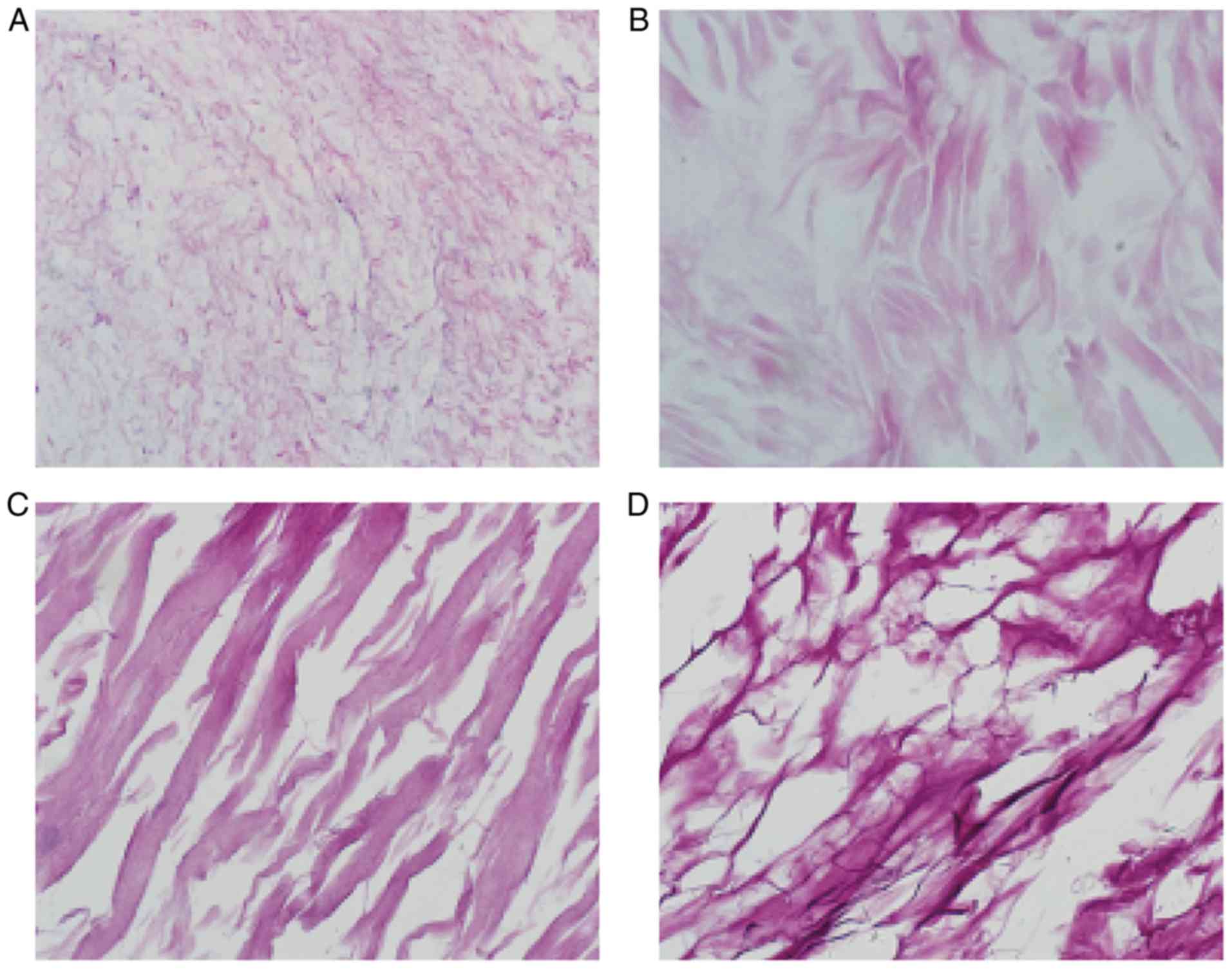
Observation of vascular scaffolds
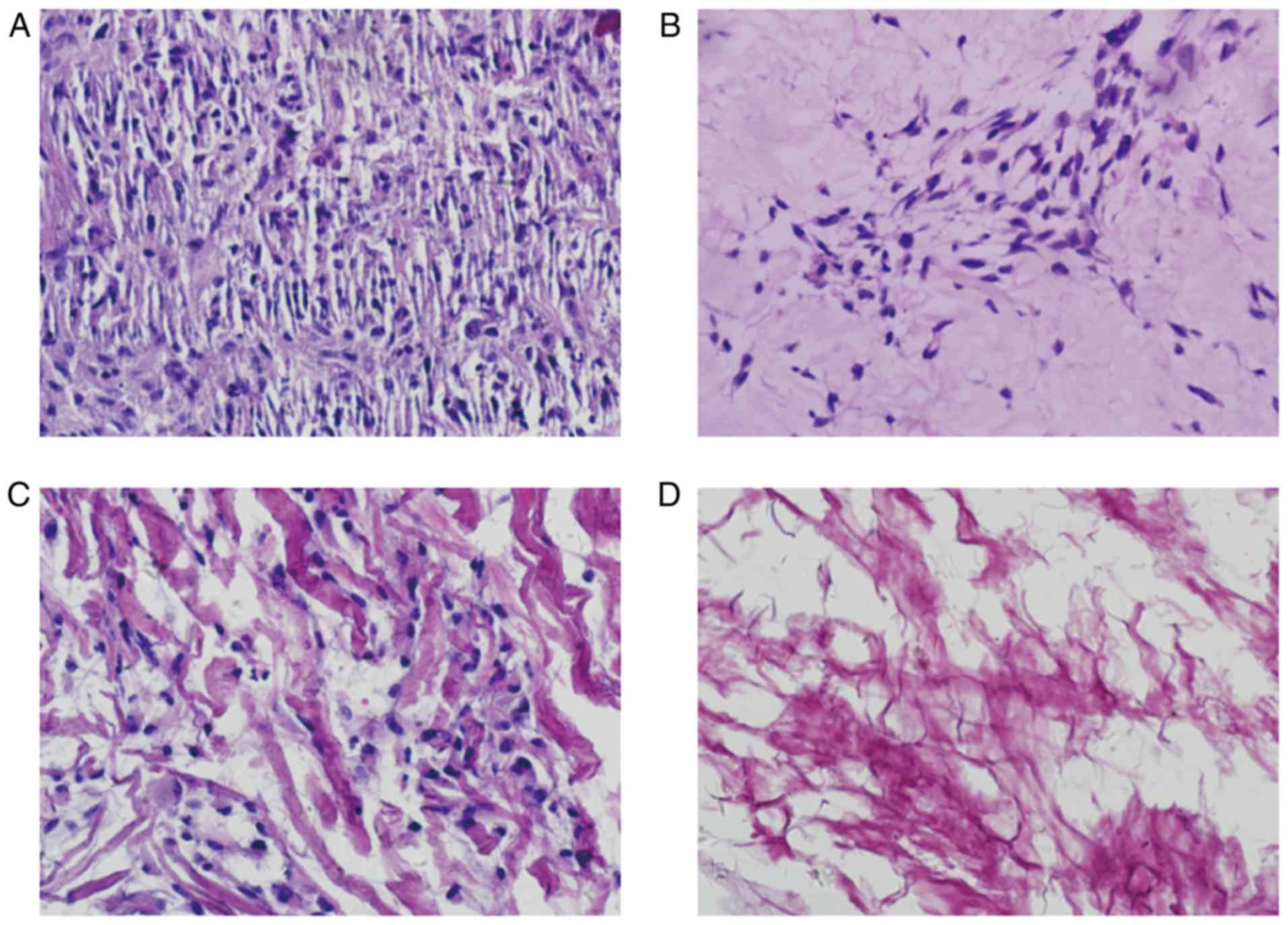
H&E staining
H&E staining of the ACVM-0.25% HLC-I scaffold
revealed the reticular and collagen fibers without cell nuclei,
cell debris and media layer smooth muscle cells. The inner membrane
was mainly distributed in the reticular fiber and media layer, the
outer membrane was mainly distributed in the collagen fibers. The
gap between collagen fibers was large, and collagen near the outer
membrane was relatively concentrated (Fig. 5A). H&E staining of the ADM
scaffolds revealed that the mesh structure of collagen fibers was
looser and fewer cells were present (Fig. 5B). H&E staining of the Bio-Gide
scaffolds exhibited a loose and thick mesh structure of collagen
fibers and complete absence of cells (Fig. 5C). In addition, it was observed
that in the SIS scaffold, interlaced irregular collagen fibers
produced a mesh with a large aperture and compact structure
(Fig. 5D).
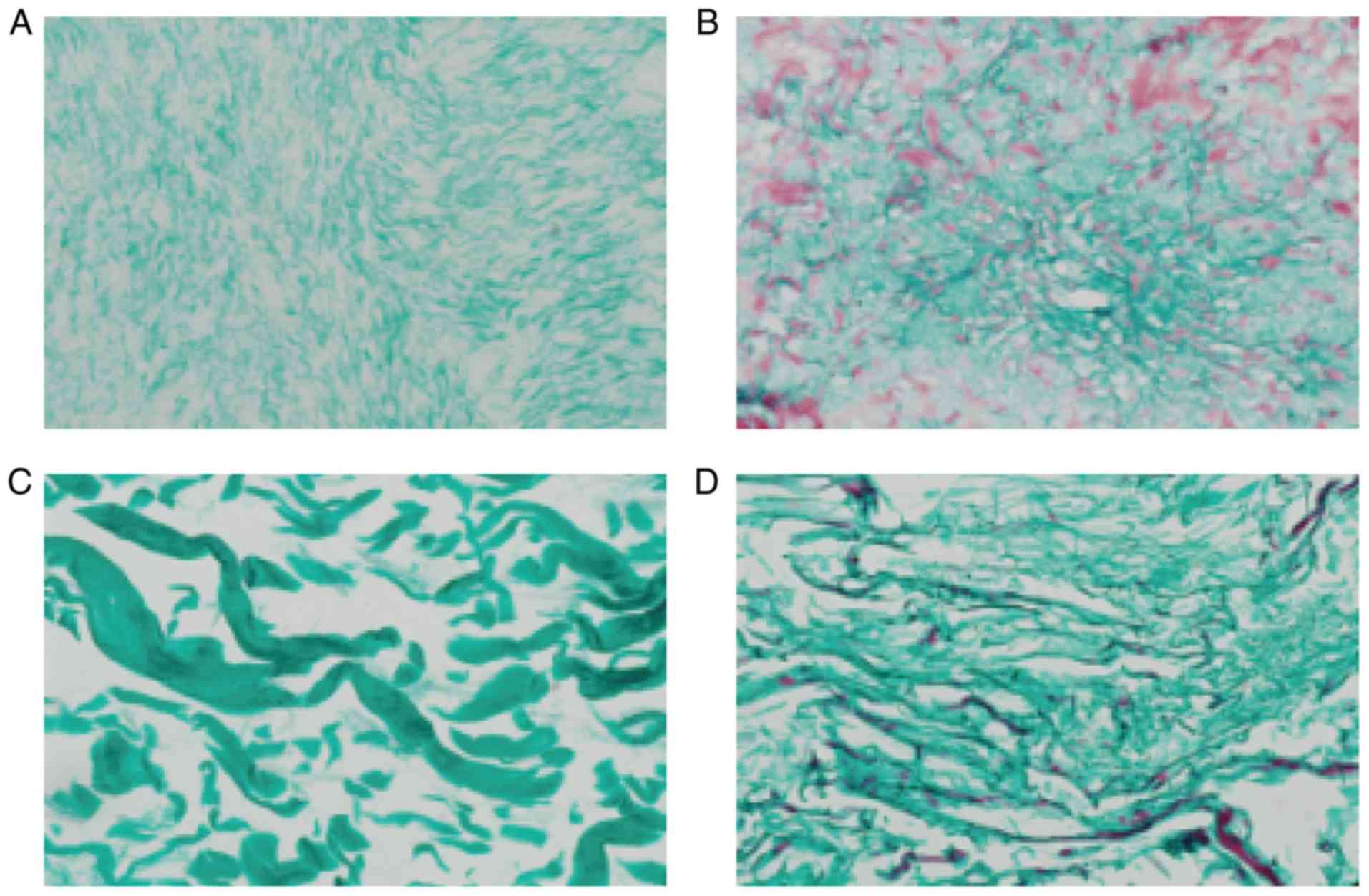
Masson staining
Masson staining of the four scaffolds suggested that
the majority of them were composed of collagen fibers and were dyed
green. In each layer of ADM, a large number of muscle fibers were
dyed red, and the structure of the collagen fiber was thin. In the
Bio-Gide group, the majority of the collagen fibers were dyed
green, and the collagen fibers were thick and sparse. In the SIS
scaffold only a small number of muscle fibers were dyed red
(Fig. 6).
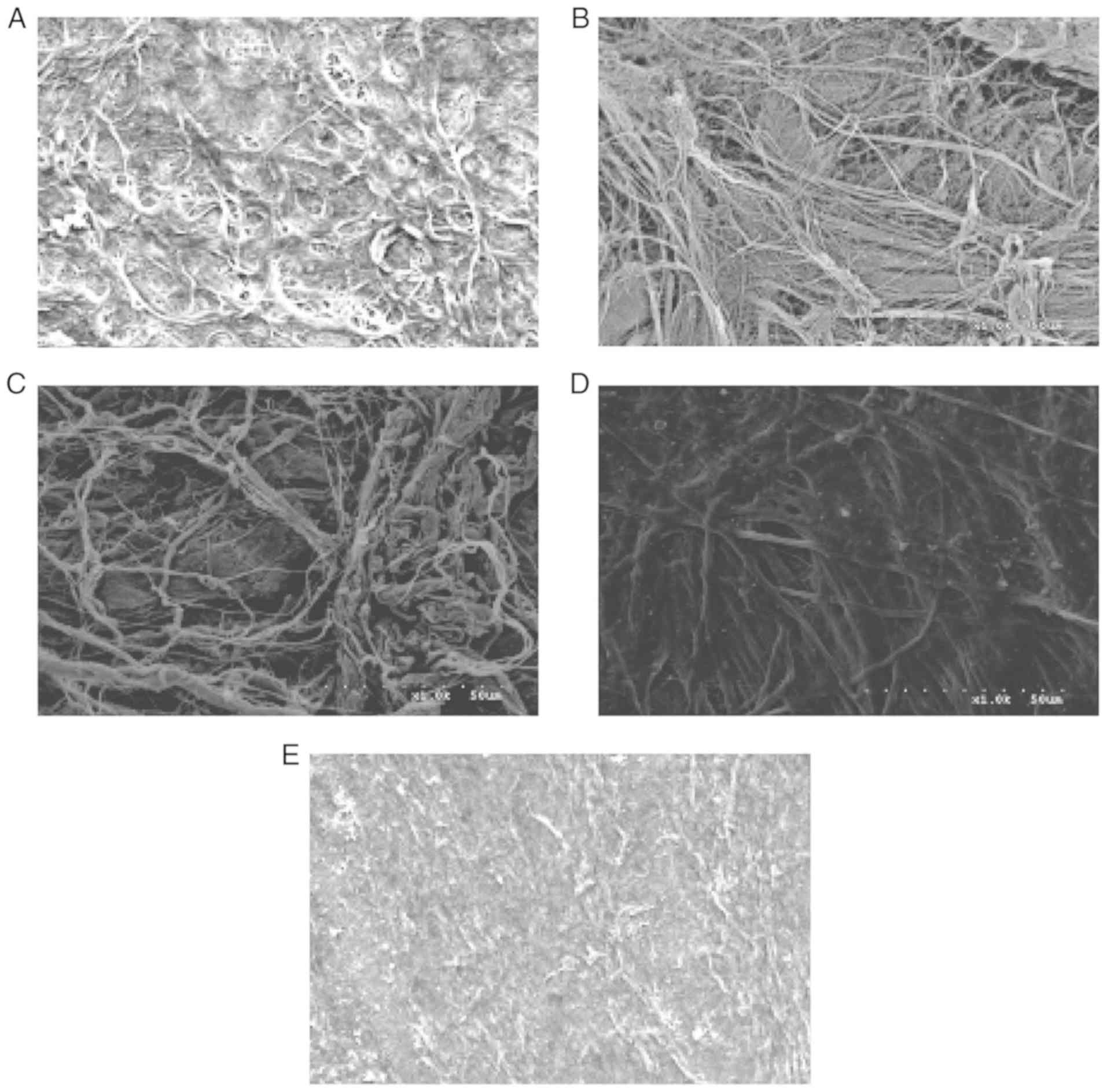
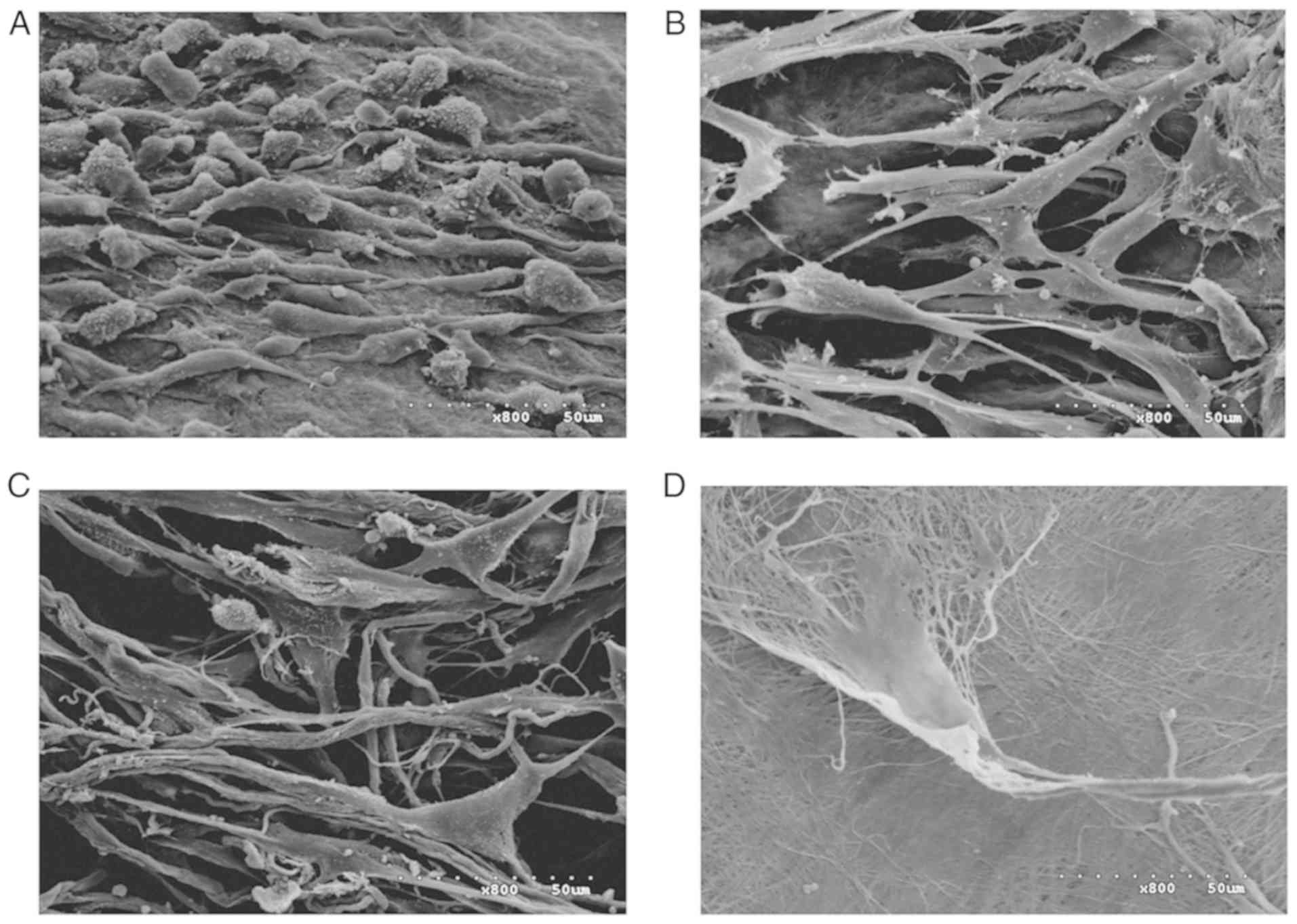
SEM
SEM images of the surfaces of the four scaffolds are
presented in Fig. 7. The surfaces
of the ADM, Bio-Gide, SIS and ACVM-0.25% HLC-I scaffolds exhibited
dense collagen fiber bundles. The material surface of the
ACVM-0.25% HLC-I scaffold was more slender and softer compared with
those of the SIS, ADM and Bio-Gide scaffolds. In addition, compared
with the Bio-Gide scaffold, the surface fiber structure of the ADM
scaffold was dense, while the surface fiber structure of the SIS
scaffold was relatively sparse and hard. The ACVM-0.25% HLC-I
scaffold surface appeared to have the smallest fibers, but no
statistically significant difference was observed between SIS and
ADM scaffolds.
Water absorption capacities of the
scaffold materials
Following 24 h, the ACVM-0.25% HLC-I scaffold
exhibited the best water absorption performance, followed by the
SIS and Bio-Gide scaffolds, and then the ADM scaffold. The water
absorption of the four scaffolds is presented in Table I. ACVM-0.25% HLC-I scaffolds had
significantly improved water absorption when compared with SIS,
Bio-Gide and ADM scaffolds (P<0.05).
 | Table I.Comparison of water absorption for
four different biodegradable scaffolds. |
Table I.
Comparison of water absorption for
four different biodegradable scaffolds.
| Scaffold | n | Water absorption
(%) |
|---|
| ACVM-0.25%
HLC-I | 6 | 24.05±0.59 |
| SIS | 6 | 10.99±0.28 |
| Bio-Gide | 6 | 5.82±0.07 |
| ADM | 6 | 3.55±0.08 |
Mechanical test results of the
scaffold materials
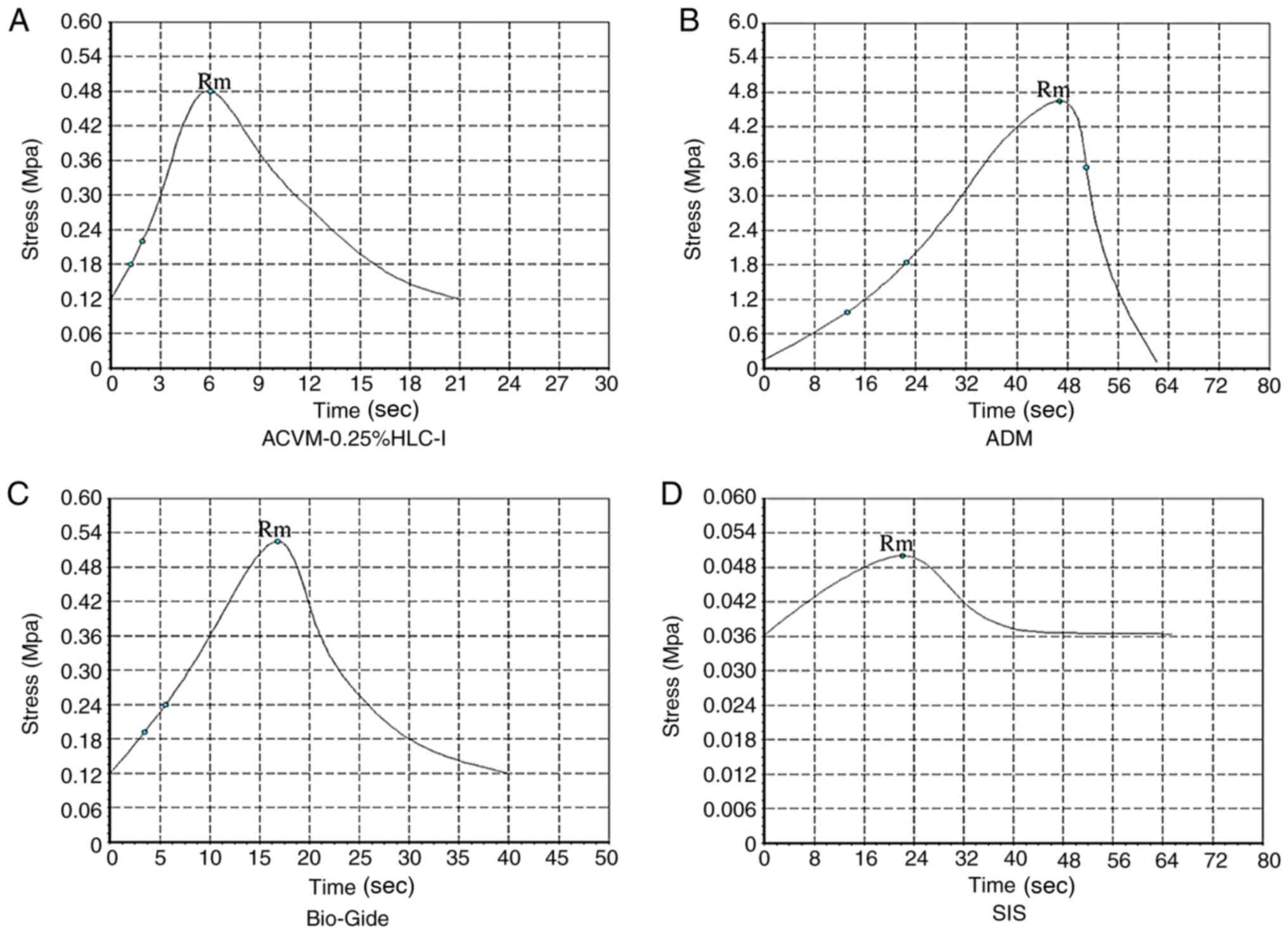
In terms of stress, the ADM scaffold material had
the largest tensile stress and reached a peak value of 4.65±0.78
Mpa at 46 sec, which was markedly higher when compared with the
tensile stress of the ACVM-0.25% HLC-I, Bio-Gide and SIS scaffold
materials (P<0.05; Fig. 8). The
tensile stress of ACVM-0.25% HLC-I, Bio-Gide and SIS reached their
peak values at 6, 16.8 and 22 sec (0.48±0.04, 0.52±0.09 and
0.05±0.0017 Mpa), respectively. There was no notable difference in
tensile stress between the ACVM-0.25% HLC-I and Bio-Gide scaffold
materials (P>0.05), but the tensile stress of the SIS scaffold
material was markedly lower when compared with the other three
types of scaffold (P<0.05). Therefore, the tensile stress of the
ACVM-0.25% HLC-I scaffold material was not as high as the ADM
scaffold material, but was similar to the Bio-Gide scaffold
material and was increased compared with the SIS scaffold material
(Fig. 8).
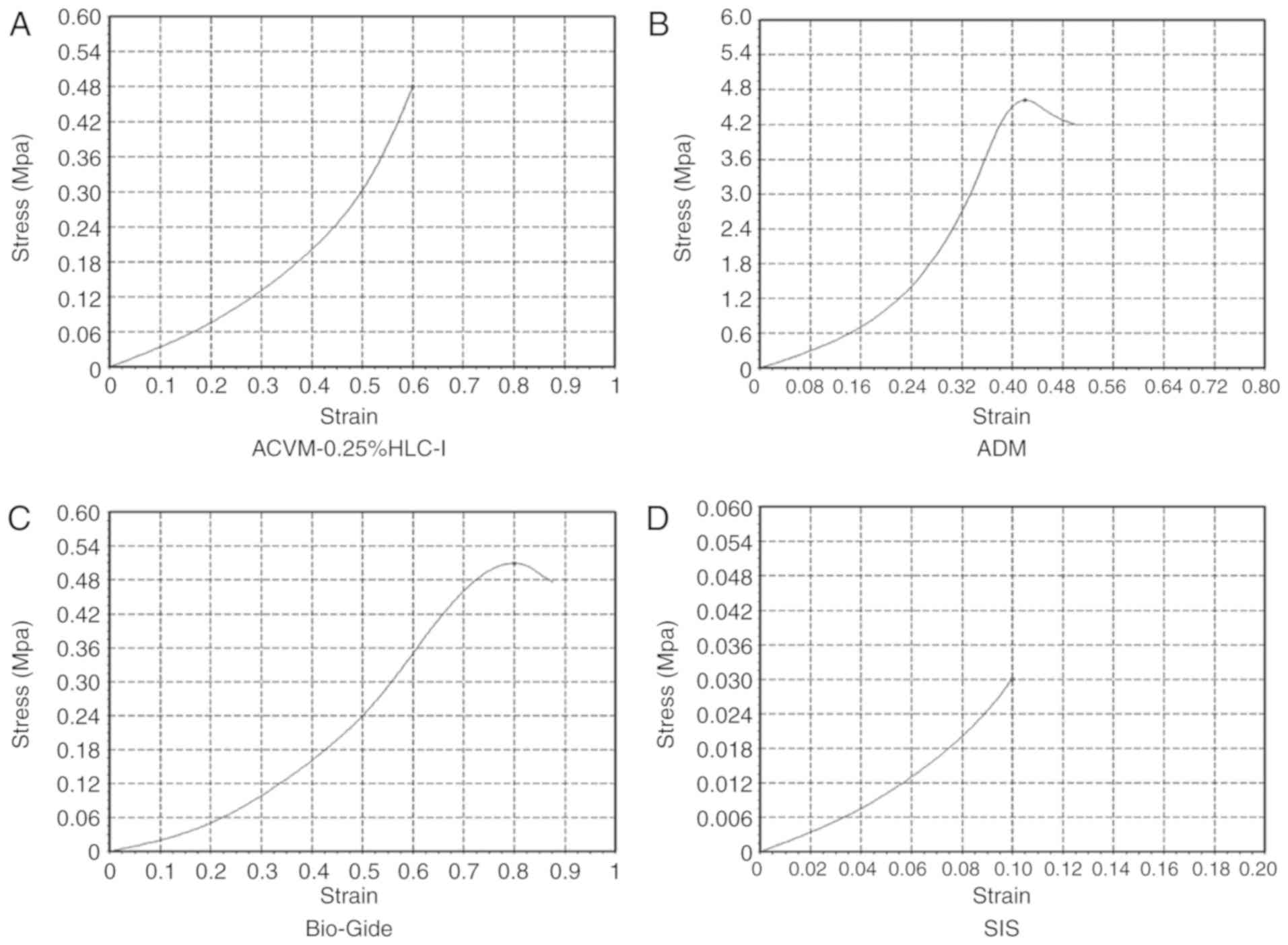
In terms of strain, ADM exhibited a notably higher
value compared with the ACVM-0.25% HLC-I, Bio-Gide and SIS scaffold
materials (P<0.05; Fig. 9).
There was no notable difference between ACVM-0.25% HLC-I and
Bio-Gide scaffold materials (P>0.05), but there was a notable
difference between ACVM-0.25% HLC-I and SIS scaffold materials
(P<0.05). The stress-strain curve indicated that the ADM
scaffold had the greatest tensile strength. The tensile strength of
the Bio-Gide scaffold was similar to that of the ACVM-0.25% HLC-I
scaffold, but the tensile strength of the SIS scaffold material was
relatively poor (Figs. 8 and
9). In terms of breaking strength,
the ADM scaffold material exhibited the highest strength, while the
SIS scaffold material exhibited the lowest. There was no marked
difference between the ACVM-0.25% HLC-I and Bio-Gide scaffold
materials (P>0.05). A negative association was observed between
the elongation at break and the breaking strength (Table II). The largest value of
elongation at break was observed in the SIS scaffold, while the
smallest value was observed in the ADM scaffold. There was no
notable difference in elongation at break between the Bio-Gide and
ACVM-0.25% HLC-I scaffolds (P>0.05; Table II).
 | Table II.Breaking strength and elongation at
break of the four scaffolds. |
Table II.
Breaking strength and elongation at
break of the four scaffolds.
| Group | Breaking strength
(MPa) | Elongation at break
(%) |
|---|
| ADM |
46.5±1.78a,b |
5.66±0.41a,b |
| ACVM-0.25%
HLC-I |
4.80±0.32a |
52.93±1.34a |
| Bio-Gide |
5.20±0.35a |
51.31±1.48a |
| SIS | 0.50±0.08 | 470.3±1.93 |
Observation of HGFs on vascular
scaffolds
HE staining
The histology of the ACVM-0.25% HLC-I scaffold
seeded with HGFs and cultured for 7 days is presented in Fig. 10. HGFs were abundant and
well-distributed in the central part of the ACVM-0.25% HLC-I
scaffold. The cells exhibited extending processes in various
directions, indicating that they had potential migration abilities
and cell activity. The histology of the HGFs seeded on the ADM and
Bio-Gide scaffolds are also presented in Fig. 10. Compared with the ACVM-0.25%
HLC-I scaffold, ADM and Bio-Gide scaffolds following 7 days
appeared to exhibit limited cell proliferation. The cells appeared
healthy with extended morphology. The HGFs seeded on the SIS
scaffold also presented and few cells grew on the surface of the
SIS scaffold following 7 days (Fig.
10).
SEM
SEM images of the four scaffolds seeded with HGFs
and cultured for 7 days are presented in Fig. 11. A large number of HGFs were
observed on the surface of the ACVM-0.25% HLC-I scaffold compared
with the ADM and Bio-Gide scaffolds. On the other hand, HGFs were
observed in limited numbers on the surface of the SIS scaffold.
This result was similar to the histology results of the four
different biodegradable scaffolds following seeding with HGFs.
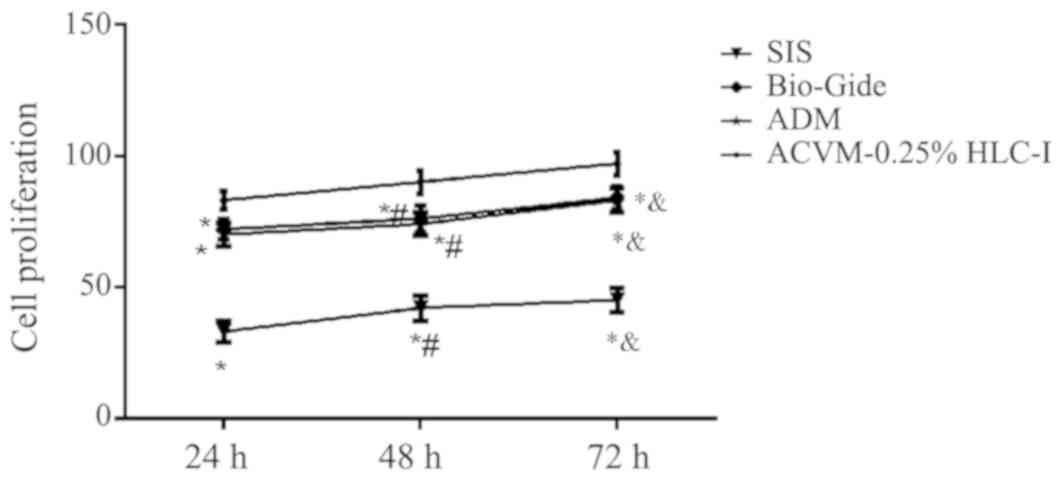
Growth kinetics of HGFs on
scaffolds
The number of HGFs in the ACVM-0.25% HLC-I, SIS, ADM
and Bio-Gide scaffolds were quantified by CCK-8 assay (Fig. 12). The ACVM-0.25% HLC-I scaffold
exhibited a larger number of cells when compared with the other
scaffolds. The ACVM-0.25% HLC-I scaffold had the fastest level of
proliferation compared with the ADM, Bio-Gide and SIS scaffolds,
with the majority of HGFs attaching following 1 h of incubation.
There was no significant difference in the number of cells between
the ADM and Bio-Gide scaffolds (P>0.05). The number of cells in
the SIS scaffold was lower when compared with the other scaffolds
at all times. With the exception of the SIS scaffold, the number of
cells collected from the scaffolds at 72 h was significantly
increased from that at 24 h (P<0.05; Fig. 12).
Scaffold diameter prior to and
following seeding with HGFs
The diameter of all scaffolds was 10 mm prior to
seeding with HGFs. Following 7 days, the ACVM-0.25% HLC-I scaffold
had contracted significantly. The ADM and Bio-Gide scaffolds seeded
with HGFs were slightly larger when compared with prior to seeding.
There was no significant change in the SIS scaffold diameter
following seeding with HGFs. The diameters of the four scaffolds
prior to and following seeding with HGFs are presented in Table III.
 | Table III.Diameter of the four different
biodegradable scaffolds before and after seeding with HGFs. |
Table III.
Diameter of the four different
biodegradable scaffolds before and after seeding with HGFs.
|
| Diameter of
scaffold (mm) |
|---|
|
|
|
|---|
| Scaffold | Prior to
seeding | Following HGF
seeding for 7 days |
|---|
| ACVM-0.25%
HLC-I | 10.00 |
9.77a |
| SIS | 10.00 | 10.00 |
| Bio-Gide | 10.00 | 10.02 |
| ADM | 10.00 | 10.04 |
Discussion
The most widely used scaffold materials in tissue
engineering research are natural biological scaffold materials,
synthetic degradable polymer scaffolds and composite scaffold
materials. In the present study, the SIS (28,29)
and ADM scaffolds were used as natural biological scaffold
materials, and the Bio-Gide scaffold material was a synthetic
degradable polymer. The ACVM-HLC-I scaffold combined ACVM material
with HLC-I to form a composite scaffold material. Previous research
has indicated that composite scaffold materials possess the good
cellular compatibility of natural biological scaffolds and the
strong mechanical properties of synthetic degradable polymer
scaffolds (31). In the present
study, ACVM-HLC-I was compared with ADM, Bio-Gide and SIS, and the
aim of the study was to verify the feasibility of the composite
scaffold material ACVM-HLC-I for application as a tissue
engineering scaffold material. Preliminary findings had confirmed
that the ACVM-HLC-I composite scaffold material was feasible for
the construction of tissue-engineered blood vessels. ACVM-0.25%
HLC-I provides a three-dimensional ultrastructure space for the
growth of seeded cells and promotes the establishment of an
organized structure and function (32). It also has appropriate mechanical
properties for maintaining compressive resistance, which is
required for building blood vessel substitutes. A concentration of
0.25% HLC-I was demonstrated to be optimal (23). The preparation of ACVM-0.25% HLC-I
material supported the tissue engineering of blood vessels by
inducing vascular endothelial cells and smooth muscle cells
(33). In the present study, the
application feasibility of composite scaffold material ACVM-0.25%
HLC-I was explored further and compared with other tissue
engineering scaffold materials, ADM, Bio-Gide and SIS.
In the present study, the ADM material was a
transparent, milky-yellow film 0.4–0.6 mm thick, which was divided
into a coarse hairy surface and a translucent honeycomb smooth
surface with slightly elastic force. The Bio-Gide material was a
white membrane, which was slightly less thick than the ADM scaffold
material. Large collagen bundles could be seen protruding from the
surface of the Bio-Gide scaffold, and the elasticity was low. The
SIS scaffold material was a yellow and white translucent membrane
with low elasticity. The ACVM-0.25% HLC-I scaffold was a
homogeneous, translucent membrane with low thickness and high
elasticity. H&E staining, Masson staining and SEM indicated
that there were dense bundles of collagen fibers on the surface of
the ADM, Bio-Gide, SIS and ACVM-0.25% HLC-I scaffolds, but on the
ACVM-0.25% HLC-I scaffold these were more slender and soft compared
with those on the SIS, ADM and Bio-Gide scaffolds. In addition,
compared with the Bio-Gide scaffold, the surface fiber structure of
the ADM scaffold was dense, while the surface fiber structure of
the SIS scaffold was relatively sparse and hard.
Previous studies have indicated that scaffold
materials applied in tissue engineering should have a degree of
biomechanical strength (23,30),
also called mechanical strength, to ensure that they do not become
deformed or ruptured when implanted in the body of a human or
animal. For example, tissue-engineered blood vessels should have a
certain amount of compressive stress and shear stress to tolerate
blood flow impact without breaking. To compare the strength of ADM,
Bio-Gide, SIS and AVCM-0.25% HLC-I materials in the present study,
biomechanical test apparatus was used for various mechanical
experiments. In terms of stress, ADM scaffold materials reached a
maximum tensile stress of 4.65±0.78 Mpa at 46 sec, which was
significantly higher compared with the ACVM-0.25% HLC-I, Bio-Gide
and SIS scaffold materials. Among the groups, there was no
significant difference in tensile stress between the ACVM-0.25%
HLC-I and Bio-Gide scaffold materials, but the SIS scaffold
material had significantly lower tensile stress compared with other
three scaffold materials. There was a significant difference in
strain among the ADM, ACVM-0.25% HLC-I, Bio-Gide and SIS scaffold
materials. The ACVM-0.25% HLC-I was not significantly different
compared with the Bio-Gide scaffold, but was significantly
different compared with the SIS scaffold. A stress-strain curve was
constructed for the ADM, Bio-Gide, SIS and ACVM-0.25% HLC-I
scaffold materials. The ADM scaffold had the largest tensile
capacity. The resistance of the Bio-Gide scaffold was similar to
that of the ACVM-0.25% HLC-I, while the tensile strength of the SIS
scaffold material was relatively poor. According to the fracture
strength value, ADM scaffolds had the highest fracture strength.
The ACVM-0.25% HLC-I scaffold and Bio-Gide material had a similar
fracture strength and were weaker compared with the ADM scaffold.
The SIS scaffold had the lowest fracture strength. The elongation
at break value was highest for the SIS scaffold, followed by the
Bio-Gide and ACVM-0.25% HLC-I scaffolds. The ADM scaffold had the
lowest elongation at break value.
The aforementioned results indicated that the
breaking strength values of the ADM, Bio-Gide, ACVM-0.25% HLC-I and
SIS scaffolds were negatively associated with elongation at break.
Although ADM scaffolds had the highest breaking strength, their
elongation at break was the smallest. Although SIS scaffolds had
the smallest breaking strength, their elongation at break was the
largest. There was no significant difference between the Bio-Gide
and ACVM-0.25% HLC-I scaffolds in terms of breaking strength and
elongation at break. This suggested that although the tensile
stress, stress strain and breaking strength of the ACVM-0.25% HLC-I
scaffold were not as large as that of the ADM scaffold material, it
had similar properties to the Bio-Gide scaffold material. As
composite scaffolds, ACVM-0.25% HLC-I scaffolds have similar
mechanical properties and mechanical strength to synthetic
biodegradable polymer scaffolds; that is, ACVM-0.25% HLC-I
scaffolds have appropriate mechanical strength and other mechanical
properties to act as scaffolds for tissue engineering.
The results of the water absorption test indicated
that ACVM-0.25% HLC-I scaffolds had a significantly greater water
absorption capacity compared with the SIS, Bio-Gide and ADM
scaffolds. The SIS scaffold had the worst mechanical properties,
but its water absorption capacity was only inferior to that of the
ACVM-0.25% HLC-I scaffold. Although the mechanical capacity of
Bio-Gide scaffold was not significantly different to that of the
ACVM-0.25% HLC-I scaffold, its water absorption ability was
markedly lower compared with the ACVM-0.25% HLC-I scaffold. The
mechanical strength of the ADM scaffold was the largest, but its
water absorption capacity was the poorest. Based on the
aforementioned experimental results, it was concluded that the
ACVM-0.25% HLC-I scaffold possessed biomechanical properties and a
strong water absorption capability, further indicating its
feasibility as a tissue engineering scaffold material.
Furthermore, scaffold materials for tissue
engineering should have no cytotoxic effects in addition to
possessing biomechanical strength (34). The results indicated that the
cytotoxicity of ACVM-0.25% HLC-I scaffolds was significantly
different from that of the ADM, Bio-Gide and SIS scaffolds, while
there was no significant difference in cytotoxicity between ADM and
Bio-Gide scaffolds. The relative growth rate (RGR) of ACVM-0.25%
HLC-I, ADM and Bio-Gide scaffolds was >75% at 24, 48 and 72 h,
the cytotoxicity grade was 1, and the scaffolds were considered to
not be cytotoxic. The RGR of the SIS scaffolds was >40% at 24 h,
and the scaffolds were considered to be cytotoxic. The RGR was
>50% at 48 and 72 h, and the scaffolds were considered to not be
cytotoxic. This suggested that, as a scaffold for tissue
engineering, the ACVM-0.25% HLC-I scaffold had improved cellular
compatibility and lower cytotoxicity compared with the ADM,
Bio-Gide and SIS scaffolds, which provides a basis for screening of
the material in vivo implants. Subsequently, an experiment
was conducted to verify whether the diameter of the material
changed following seeding with HGFs. The results indicated that
following seeding with HGFs, the diameter of the ACVM-0.25% HLC-I
scaffold decreased and the diameter of the SIS scaffold had not
markedly changed. The diameters of the ADM and Bio-Gide scaffolds
increased slightly. This may be associated with good attachment and
growth of HGFs on the ACVM-0.25% HLC-I scaffold.
ACVM-0.25% HLC-I scaffold material, which combines
HLC-I (23) with ACVM (23,24),
is a composite tissue engineering scaffold formed by combining
natural acellular biomaterials with synthetic polymer materials.
Composite materials have the advantages of natural acellular
biomaterials (such as ADM scaffolds) and synthetic polymer
materials (such as Bio-Gide scaffolds). Therefore, they can provide
three-dimensional ultrastructural protein space for cell growth and
promote the formation of tissue function. They also possess
sufficient mechanical properties to maintain the compressive
ability of the tissue substitutes and the degradation rate of the
material, and can be designed and regulated (35,36).
In conclusion, the results of the present study indicate that
ACVM-0.25% HLC-I promotes cell adhesion, cell proliferation and
cell migration, and possesses good mechanical and biomechanical
properties. This suggests that the composite tissue engineering
scaffold material could be successfully applied in tissue
regeneration.
The limitations in the present study must also be
mentioned. Firstly, during the experiment, the authors did not
consider to evaluate the potential change of the scaffold material
following 7 days in mechanical properties, only testing the
mechanical properties of the scaffold material prior to planting
the cells. Secondly, no in vivo tests were performed on
animals, which was a flaw in our experimental design, thus this
will be addressed in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by Applied basic
research found of Luzhou Science and Technology Bureau of Sichuan
province (grant no. 2018-JYJ-36); City-School-Stomatological
Hospital Introduces Doctoral Research Fund (grant no. 20186-2);
Scientific Research Fund of Sichuan Medical Association (grant no.
S17073)
Availability of data and materials
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
All authors conceived and designed the experiments.
YLQ, XC and YLH performed the experiments and analyzed the data.
YJH, SBT and YHC wrote the manuscript. LY, MHN and XQL modified the
manuscript and designed the experiments.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethics
Committee of Southwest Medical University (Sichuan, China). The
acquisition of normal human gingival tissues from patients was
approved by the Hospital of Stomatology at Hebei Medical University
(Hebei, China), and written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao P, Jiang D and Li Z: The application
progress of human urine derived stem cells in bone tissue
engineering. Zhonghua Wai Ke Za Zhi. 54:317–320. 2016.(In Chinese).
PubMed/NCBI
|
|
2
|
Sun Z and Li J: Research progress of
tissue engineered ligament. Zhongguo Xiu Fu Chong Jian Wai Ke Za
Zhi. 29:1160–1166. 2015.(In Chinese). PubMed/NCBI
|
|
3
|
Yang Q, Xu HW, Hurday S and Xu BS:
Construction strategy and progress of whole intervertebral disc
tissue engineering. Orthop Surg. 8:11–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ge L, Cao C, Chen S, Shao B, Li Q, Li Q,
Liu L, Liang Z and Huang Y: Preparation of laminin/nidogen adsorbed
urinary bladder decellularized materials via a mussel-inspired
polydopamine coating for pelvic reconstruction. Am J Transl Res.
9:5289–5298. 2017.PubMed/NCBI
|
|
5
|
Mi HY, Jing X, Napiwocki BN, Hagerty BS,
Chen G and Turng LS: Biocompatible, degradable thermoplastic
polyurethane based on
polycaprolactone-block-polytetrahydrofuran-block-polycaprolactone
copolymers for soft tissue engineering. J Mater Chem B.
5:4137–4151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Yang J, Ran B, Yang X, Zheng W,
Long Y and Jiang X: Small molecular TGF-β1-inhibitor-loaded
electrospun fibrous scaffolds for preventing hypertrophic scars.
ACS Appl Mater Interfaces. 9:32545–32553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faulk DM, Londono R, Wolf MT, Ranallo CA,
Carruthers CA, Wildemann JD, Dearth CL and Badylak SF: ECM hydrogel
coating mitigates the chronic inflammatory response to
polypropylene mesh. Biomaterials. 35:8585–8595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Deng D, Wang B, Zhou G, Zhang W,
Cao Y, Zhang P and Liu W: * Comparison of autologous, allogeneic,
and cell-free scaffold approaches for engineered tendon repair in a
rabbit model-a pilot study. Tissue Eng Part A. 23:750–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie J, Peng C, Zhao Q, Wang X, Yuan H,
Yang L, Li K, Lou X and Zhang Y: Osteogenic differentiation and
bone regeneration of iPSC-MSCs supported by a biomimetic
nanofibrous scaffold. Acta Biomater. 29:365–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baumgartner W, Schneider I, Hess SC, Stark
WJ, Märsmann S, Brunelli M, Calcagni M, Cinelli P and Buschmann J:
Cyclic uniaxial compression of human stem cells seeded on a bone
biomimetic nanocomposite decreases anti-osteogenic commitment
evoked by shear stress. J Mech Behav Biomed Mater. 83:84–93. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu XW and Wang JH: Efficacy of dermal
scaffold for promoting repair of acute full-thickness skin defects
in pigs. Nan Fang Yi Ke Da Xue Xue Bao. 38:363–368. 2018.(In
Chinese). PubMed/NCBI
|
|
12
|
Ramanathan G, Muthukumar T and
Tirichurapalli Sivagnanam U: In vivo efficiency of the collagen
coated nanofibrous scaffold and their effect on growth factors and
pro-inflammatory cytokines in wound healing. Eur J Pharmacol.
814:45–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farkas B, Rodio M, Romano I, Diaspro A,
Intartaglia R and Beke S: Fabrication of hybrid nanocomposite
scaffolds by incorporating ligand-free hydroxyapatite nanoparticles
into biodegradable polymer scaffolds and release studies. Beilstein
J Nanotechnol. 6:2217–2223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nasonova MV, Glushkova TV, Borisov VV,
Velikanova EA, Burago AY and Kudryavtseva YA: Biocompatibility and
structural features of biodegradable polymer scaffolds. Bull Exp
Biol Med. 160:134–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perry L, Flugelman MY and Levenberg S:
Elderly patient-derived endothelial cells for vascularization of
engineered muscle. Mol Ther. 25:935–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu WW, Wu YC and Hu ZC: The development of
an alginate/polycaprolactone composite scaffold for in situ
transfection application. Carbohydr Polym. 183:29–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun T, Liu M, Yao S, Ji Y, Xiong Z, Tang
K, Chen K, Yang H and Guo XD: Biomimetic composite scaffold
containing small intestinal submucosa and mesoporous bioactive
glass exhibits high osteogenic and angiogenic capacity. Tissue Eng
Part A. 24:1044–1056. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tohamy KM, Mabrouk M, Soliman IE, Beherei
HH and Aboelnasr MA: Novel alginate/hydroxyethyl
cellulose/hydroxyapatite composite scaffold for bone regeneration:
In vitro cell viability and proliferation of human mesenchymal stem
cells. Int J Biol Macromol. 112:448–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Awad NK, Niu H, Ali U, Morsi YS, Lin T, et
al: Electrospun fibrous scaffolds for small-diameter blood vessels:
A review. Membranes (Basel). 8:E152018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Wu J, Wang H, Li H, Di N, Song L, Li
S, Li D, Xiang Y, Liu W, et al: Fabrication of electrospun
poly(L-lactide-co-ε-caprolactone)/collagen nanoyarn network as a
novel, three-dimensional, macroporous, aligned scaffold for tendon
tissue engineering. Tissue Eng Part C Methods. 19:925–936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng C, Hu J, Liu C, Liu S, Liao G, Song L
and Zeng X: Association of 17-β estradiol with adipose-derived stem
cells: New strategy to produce functional myogenic differentiated
cells with a nano-scaffold for tissue engineering. PLoS One.
11:e01649182016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lech CJ and Phan AT: Ball with hair:
Modular functionalization of highly stable G-quadruplex DNA
nano-scaffolds through N2-guanine modification. Nucleic Acids Res.
45:6265–6274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Wang J, Dong F, Song P, Tian S, Li
H and Hou Y: Cytocompatibility and biologic characteristics of
synthetic scaffold materials of rabbit acellular vascular matrix
combining with human-like collagen I. J Biomater Appl. 32:463–471.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lalka SG, Oelker LM, Malone JM, Duhamel
RC, Kevorkian MA, Raper BA, Nixon JC, Etchberger KJ, Dalsing MC,
Cikrit DF, et al: Acellular vascular matrix: A natural endothelial
cell substrate. Ann Vasc Surg. 3:108–117. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Wang J, Dong F, Song P, Li H and
Hou Y: Study of composite vascular scaffold combining with
differentiated VSMC- and VEC-like cells in vitro and in vivo. J
Biomater Appl. 32:219–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heidemann LN, Gunnarsson GL, Salzberg CA,
Sørensen JA and Thomsen JB: Complications following nipple-sparing
mastectomy and immediate acellular dermal matrix implant-based
breast reconstruction-a systematic review and meta-analysis. Plast
Reconstr Surg Glob Open. 6:e16252018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sobti N, Ji E, Brown RL, Cetrulo CL Jr,
Colwell AS, Winograd JM, Austen WG Jr and Liao EC: Evaluation of
acellular dermal matrix efficacy in prosthesis-based breast
reconstruction. Plast Reconstr Sur. 141:541–549. 2018. View Article : Google Scholar
|
|
28
|
Pitman MJ, Kurita T, Powell ME, Kimball
EE, Mizuta M, Chang S, Garrett CG and Rousseau B: Vibratory
function and healing outcomes after small intestinal submucosa
biomaterial implantation for chronic vocal fold scar. Laryngoscope.
128:901–908. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q, Teng F, Zhang Y, Sun Q and Meng G:
Evaluation of transventricular placement of porcine small
intestinal submucosa stent valves in the pulmonary position in
juvenile sheep model. Interact Cardiovasc Thorac Surg. 27:295–300.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Wang J, Dong F, Li H and Hou Y:
Induced differentiation of human gingival fibroblasts into
VSMC-like cells. Differentiation. 95:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi HJ, Lee JJ, Lee JB, Sung HJ, Shin JW,
Shin JW, Wu Y and Kim JK: MG-63 cells proliferation following
various types of mechanical stimulation on cells by auxetic hybrid
scaffolds. Biomater Res. 20:322016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Choi JK and He X: Engineering
microvascularized 3D tissue using alginate-chitosan microcapsules.
J Biomater Tissue Eng. 7:170–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Yin P, Bian GL, Huang HY, Shen H,
Yang JJ, Yang ZY and Shen ZY: The combination of stem cells and
tissue engineering: An advanced strategy for blood vessels
regeneration and vascular disease treatment. Stem Cell Res Ther.
8:1942017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saberi EA, Karkehabadi H and Mollashahi
NF: Cytotoxicity of various endodontic materials on stem cells of
human apical papilla. Iran Endod J. 11:17–22. 2016.PubMed/NCBI
|
|
35
|
Wu T, Huang C, Li D, Yin A, Liu W, Wang J,
Chen J, Ei-Hamshary H, Al-Deyab SS and Mo X: A multi-layered
vascular scaffold with symmetrical structure by bi-directional
gradient electrospinning. Colloids Surf B Biointerfaces.
133:179–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang B, Akar B, Waller TM, Larson JC,
Appel AA and Brey EM: Design of a composite biomaterial system for
tissue engineering applications. Acta Biomater. 10:1177–1186. 2014.
View Article : Google Scholar : PubMed/NCBI
|