Introduction
Mastitis is the most common infectious disease in
dairy cows, and it remains a major challenge to the global dairy
industry (1). Mastitis is an
inflammation of the parenchyma of the mammary gland, and is caused
by different pathogens, including Staphylococcus aureus,
Escherichia coli and Streptococcus uberis (2,3).
Challenging or natural infection by different pathogens results in
an alteration in the mRNA expression of a subset of
immune-associated genes in cows (4–6).
Immune responses to infecting pathogens in the bovine mammary gland
are very complex. Therefore, a coordinated response exists between
the resident, recruited and inducible immune factors, including
chemokines (5). Upon bacterial
stimulation, bovine mammary epithelial cells are activated and are
able to release neutrophil-mobilizing chemokines, contributing to
different immune and inflammatory responses of the mammary gland
(7). C-C motif chemokine ligand 5
(CCL5), also termed RANTES, is upregulated in bovine mammary
epithelial cells following stimulation by E. coli and crude
lipopolysaccharide, but not by S. aureus culture supernatant
(8). By contrast, in a previous
study, it was identified that the CCL5 gene was downregulated in
S. aureus mastitis-infected mammary glands by 1.61-fold
compared with non-infected tissue in cows (6). CCL5 belongs to the C-C chemokine
family (9). Functional CCL5 serves
an active role in recruiting a variety of leukocytes, including
T-cells, macrophages, eosinophils and basophils to inflammatory
sites (10).
Alternative splicing (AS) of a gene may generate
numerous mature mRNA isoforms in higher eukaryotes (11). A limited number of genes are able
to generate vast numbers of proteins via AS of eukaryotic
transcripts in cells, which has notable physiological functions in
the different developmental processes in humans (12). According to the Alternative
Splicing Graph Server (ASGS), in a genome-wide analysis only 21%
(4,567 of 21,755) bovine genes were identified to be alternatively
spliced (13). Using Illumina
paired-end RNA sequencing and bioinformatics methods, a previous
study identified that 8,549 (52.62%) and 8,325 (51.24%) unigenes
were alternatively spliced, exhibiting 34,523 and 30,410 AS events
in terms of exon skipping, intron retention, alternative 5′
splicing and alternative 3′ splicing patterns in healthy and
mastitic tissues, respectively (6). Among them, CCL5 (ENSBTAG00000007191)
exhibited an exon-skipping splicing pattern, which was specific to
the mastitis-infected individual (6).
Single nucleotide polymorphisms (SNPs) are the most
frequent type of variation, and major contributors to natural
splice variants (14). Mutations
or variations in either cis-acting elements or trans-acting factors
result in aberrant splicing and abnormal protein production
(15), which are associated with
certain important diseases, including bovine mastitis (3,16,17).
However, alternative splicing and expression of the bovine CCL5
gene has not been elucidated, to the best of our knowledge. In the
present study, splice variants, transcript expression, and
splicing-associated SNPs of the CCL5 gene were investigated in
healthy and mastitic mammary glands in Holstein cows.
Materials and methods
Animals and experimental design
All experiments were conducted according to the
National Standards for Laboratory Animals Guideline for Ethical
Review of Animal Welfare of China (GB/T 35892-2018), and were
approved by the Animal Care and Use Committee of the Dairy Cattle
Research Center, Shandong Academy of Agricultural Sciences (Jinan,
China). Mammary tissue samples were collected from eight healthy
and eight mastitic Chinese Holstein cows (650–750 kg) at 2.8 to 3.0
years of age during their first lactation at a commercial slaughter
plant in Jinan, China. The duration of the experiment was 3 months,
and no animal succumbed during that time. Prior to the collection
of mammary tissues, the animal health and behavior were monitored
daily. The mastitic cows were selected according to their clinical
symptoms, and the mastitis infections were caused only by S.
aureus. The healthy cows were not affected by pathogen
infection and had no clinical symptoms (heat, pain, redness,
swelling of the udder and milk clotting). The mammary tissues were
sampled at the time of slaughter and frozen immediately in liquid
nitrogen.
Identification of the splice variants
of the bovine CCL5 gene
An E.Z.N.A® Mollusc RNA kit (Omega
Bio-Tek, Inc., Norcross, GA, USA) was used to extract the total RNA
from the mammary samples, and cDNA was synthesized using a
PrimeScript™ Reverse Transcription (RT) reagent kit with gDNA
Eraser (Perfect Real Time; Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's protocol. The primer
sequences of the CCL5 gene (GenBank accession no. NM_175827.2) and
KB-SNP1 were designed using Primer Premier 5.0 (Premier Biosoft
International, Palo Alto, CA, USA) and Primer-BLAST software
(www.ncbi.nlm.nih.gov/tools/primer-blast/; Table I), and synthesized by Beijing
TsingKe Biological Technology Co., Ltd. (Beijing, China). The
polymerase chain reaction (PCR) was performed as follows: 94°C for
4 min, 94°C for 30 sec, 68°C for 30 sec, 72°C for 35 sec (35
cycles), and 72°C for 10 min. DNA bands were separated by 1%
agarose gel electrophoresis and eluted using a Universal DNA
Purification kit (Tiangen Biotech Co., Ltd., Beijing, China). The
method of identifying the splice variants was performed as
described previously (18). The
PCR products were subcloned into the pEASY-T3 cloning vector
(TransGen Biotech Co., Ltd., Beijing, China) following
purification, and the mixture was transformed into E. coli
DH5α competent cells (Takara Biotechnology Co., Ltd.). The positive
clones were selected and sequenced by BGI LifeTech Co., Ltd.
(Beijing, China) and DNAMAN v5.2.2 software (Lynnon LLC, San Ramon,
CA, USA) was used to identify the possible splice variants through
multiple sequence alignments with the CCL5-reference mRNA.
 | Table I.Primers used for screening SNPs. |
Table I.
Primers used for screening SNPs.
| Primers | Primer
sequences | Product size,
bp | Annealing
temperature, °C |
|---|
| CCL5 mRNA |
F:CTGCGCTCCTGCTTCTG | 566 | 58 |
|
|
R:CCAGTGAGGGACCGAGAT |
|
|
| KB-SNP1 |
F:GGCGGAGGAACTAAGACCAG | 598 | 58 |
|
|
R:CCTCCAGGAACACGTTGTCT |
|
|
Quantification of abundance of CCL5
transcript variants
An E.Z.N.A® Mollusc RNA kit was used to
extract the total RNA from the mammary samples, and cDNA was
synthesized as aforementioned. SYBR-Green PCR Master Mix (Tiangen
Biotech Co., Ltd.) was used for RT-quantitative (q)PCR in a BioRad
CFX96 Real-Time System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), according to the manufacturer's protocol. The primer
sequences for CCL5 and β-actin (GenBank accession no. NM_173979.3;
www.ncbi.nlm.nih.gov/genbank) were
designed using Primer Premier 5.0 and Primer-BLAST software, and
the primer sequences of CCL5-reference and CCL5-AS (Table II) were designed based on the
results for the splice variants of the CCL5 gene. The primer
sequences were synthesized by Beijing TsingKe Biological Technology
Co., Ltd. The relative expression values for the RT-qPCR assay were
calculated with β-actin as the endogenous control, and the template
that did not undergo the reverse transcription reaction served as
the negative control. qPCR was performed as follows: 95°C for 30
sec, 95°C for 15 sec, 60°C for 30 sec, 72°C for 35 sec (40
cycles).
 | Table II.Primers used in the reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers used in the reverse
transcription-quantitative polymerase chain reaction.
| Primers | Primer
sequences | Product size,
bp | Annealing
temperature, °C |
|---|
| β-actin |
F:CACAATGAAGATCAAGATCATC | 173 | 60 |
|
|
R:CTAACAGTCCGCCTAGAAGCA |
|
|
| CCL5-reference |
F:CACCCACGTCCAGGAGTATTT | 174 | 60 |
|
|
R:GCAAGTTCAGGTTCAAAACG |
|
|
| CCL5-AS |
F:GCTTCTGCCTCCCATCTTTAT | 142 | 60 |
|
|
R:AAAGTTGGCGCAAGTTCAGG |
|
|
Prediction of CCL5 splice variant
Exonic splice enhancer (ESE)finder 3.0 (http://rulai.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi)
was used to predict the alterations in the binding site of the
splicing factor, and the alternative splice site prediction was
achieved with ASSP (http://wangcomputing.com/assp/index.html).
CCL5 splicing mini-gene reporter
assay
The 598 bp genomic fragment of the CCL5 gene from
bovine genomic DNA, including intron 1, intron 2 and exon 2, was
amplified to evaluate the in vitro splicing with mini-genes,
and the primer sequences of KB-SNP1 are presented in Table I. The segment with the wild-type,
AA, or the mutant type, TA, of the CCL5 gene was cloned into the
pSPL3 vector (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) following digestion with EcoRI and XhoI. The
clones were transformed into Trans5a cells (TransGen Biotech Co.,
Ltd.) which were plated on agar containing 100 mg/ml ampicillin,
and incubated at 37°C overnight. The positive colonies were
cultured in lysogeny broth at 37°C overnight, and an Endo-free
Plasmid Mini kit II (Omega Bio-Tek, Inc.) was used to isolate the
plasmids according to the manufacturer's protocols. The presence of
the correct sequences was verified through sequencing constructs by
Beijing TsingKe Biological Technology Co., Ltd.
Bovine mammary epithelial cells (MAC-T) and 293T
cells were cultured in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) containing penicillin/streptomycin
(100 IU/ml; 0.1 mg/ml) with 10% fetal bovine serum in an atmosphere
of 5% CO2 in air at 37°C. The cells were transferred to
six-well culture plates, and transfected with 4 µg of wild-type or
mutant mini-gene constructs using Lipofectamine® 2000
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
for 5 h in Opti-MEM medium (Gibco; Thermo Fisher Scientific, Inc.),
according to manufacturer's protocol, when the cells had grown to
80–85% confluence. A subset of cells were transfected with pSPL3
lacking the CCL5 insert, and other cells were treated with only
transfection reagent as the control.
The cells were collected at 24 h post-transfection,
and the total RNA was extracted, reverse transcribed into cDNA, and
PCR was performed as described above. The pSPL3 vector specific
primers were SD6 (5′-TCTGAGTCACCTGGACAACC-3′) and SA2
(5′-ATCTCAGTGGTATTTGTGAGC-3′). The PCR products were separated on
2% agarose gels, and the DNA bands were extracted with a Gel
Extraction kit (Omega Bio-Tek, Inc.) and analyzed by direct
sequencing by Beijing TsingKe Biological Technology Co., Ltd.
Statistical analyses
Relative quantification of CCL5-reference and
CCL5-AS mRNA expression was performed using the 2−ΔΔCq
analysis method for relative real-time PCR (19). The data for the relative expression
levels of CCL5-reference and CCL5-AS mRNA were analyzed using a
completely randomized design with eight animals per group, via a
mixed-model analysis of variance using Proc Mixed in SAS (version
9.1; SAS Institute, Cary, NC, USA). The comparisons among the
relative expression levels of the different groups were performed
using the Duncan method, controlling the experiment wise type ±
error equal to 0.05. Data are presented as least squares means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Bovine CCL5 splice variants identified
in mammary glands
The cDNA from mammary tissues was used to amplify
the full-length CCL5 transcript with specific primers for CCL5 cDNA
(Table I). As a result, two PCR
amplification fragments were detected in the mastitis mammary gland
tissues, while only one fragment was detected in the healthy
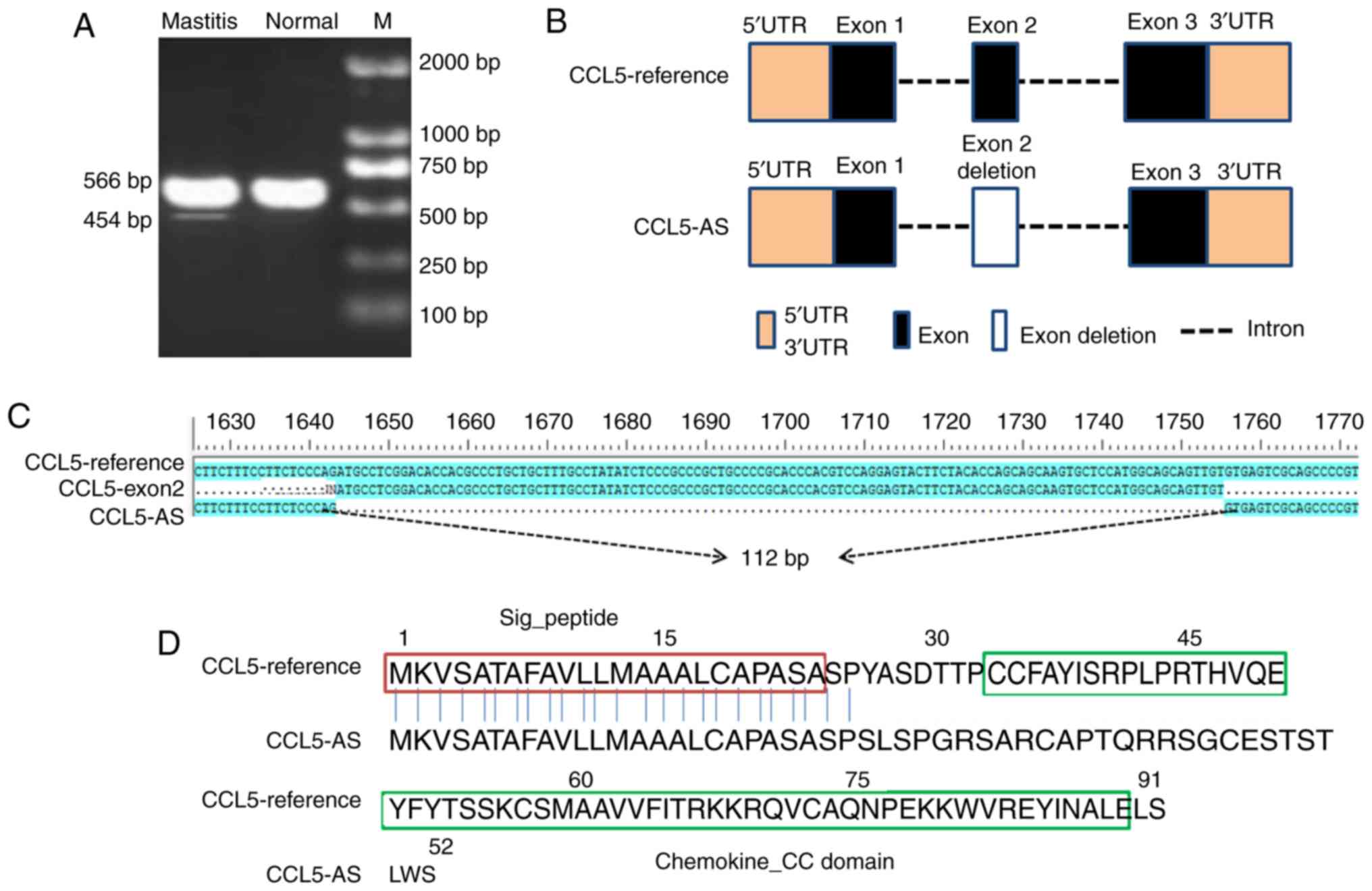
tissues (Fig. 1A). A total of two
PCR bands of different sizes were recovered and subsequently
sequenced. The common PCR fragment was the expected CCL5 product
(566 bp), and the shorter PCR fragment was a novel splice variant
with a size of 454 bp. By comparing with the reference genomic
sequence of the bovine CCL5 gene (GenBank accession no.
NC_007317.6) and the reference mRNA sequence (GenBank accession no.
NM_175827.2), it was observed that the shorter splice variant,
designated CCL5 transcript variant CCL5-AS, lacked the entirety of
exon 2 with a 112 bp deletion (Fig. 1B
and C). Furthermore, the putative protein of CCL5-AS was
missing the key Chemokine_CC domain when compared with the
CCL5-reference protein (Fig. 1D),
indicating the loss of the normal function of CCL5 in the immune
and inflammatory processes.
Transcription pattern of the bovine
CCL5 splice variants in healthy and mastitic mammary gland
tissues
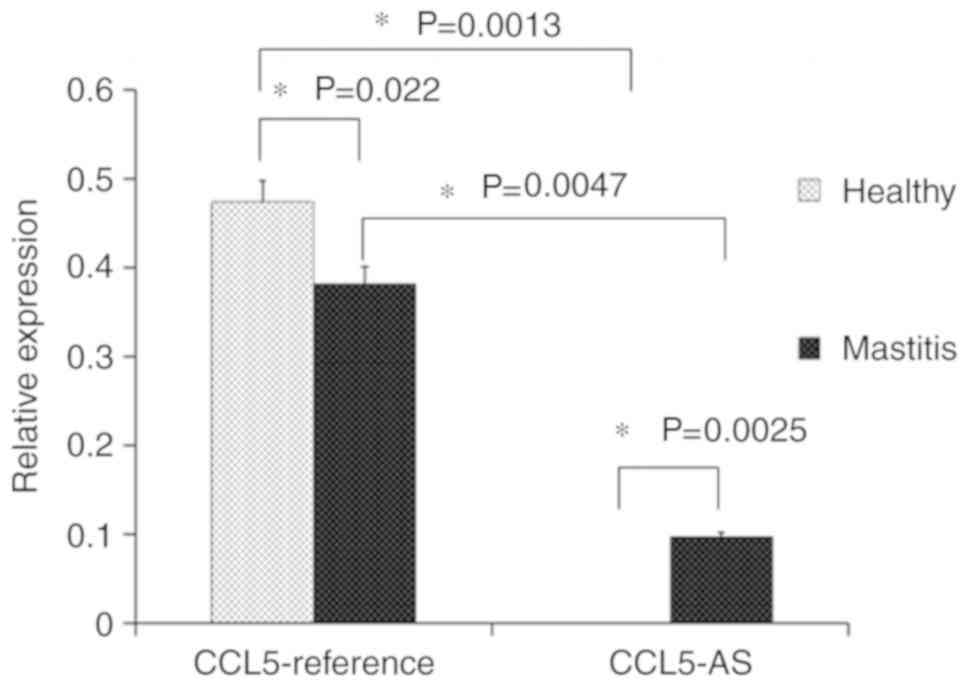
Through the RT-qPCR, the relative expression levels
of CCL5-reference and CCL5-AS were compared in the mammary gland
tissues of cows. The expression of CCL5-reference mRNA in the
healthy tissue was significantly higher compared with that in the
mastitic tissue. Moreover, it was demonstrated that the
CCL5-reference was the most abundant transcript in the healthy and
mastitic tissues, which is consistent with the RT-PCR results. The
novel transcript CCL5-AS was expressed in the mastitic mammary
tissues with a low abundance, whereas it was undetectable in the
normal tissues (Fig. 2). These
results supported the hypothesis that CCL5-AS was specific to
mastitic tissue.
Putative splicing-related SNPs
identified in exon 2 and intron 2 of CCL5
To analyze the molecular basis of the aberrant
CCL5-AS splice variant, a pair of primers (KB-SNP1; Table I) was designed to amplify a 598 bp
fragment harboring exon 2 of CCL5. A total of two novel SNPs, one
SNP (g.1647C>T) in exon 2 (a synonymous mutation) and the other
SNP (g.1804G>A) in the intron 2 region, were identified in the
Holstein cow population by sequencing the PCR amplification
products (Fig. 3A). To investigate
whether the SNPs affected the alternative splicing of exon 2,
ESEfinder 3.0 software was used to predict alterations in the
splice sites and factors in the ESE motif. The prediction
demonstrated that the introduction of allele T, relative to allele
C, in the locus g.1647C>T resulted in the deletion of two
binding sites of auxiliary splicing proteins, serine/arginine-rich
splicing factor 1 (SRSF1) and SRSF1 (IgM-BRCA1); however, the
introduction of allele A, relative to allele G, in the locus
g.1804G>A led to the deletion of two IgM-BRCA1 binding sites,
and increased the SRSF5 (SRp40) binding site (Fig. 3B). A total of two SNPs
(g.1647C>T and g.1804G>A) located in the ESE motif region
were in complete linkage disequilibrium, constituting two
haplotypes (C-G and T-A) of SNPs (g.1647C>T and g.1804G>A),
which may putatively induce the production of a novel splice
variant of the bovine CCL5 gene.
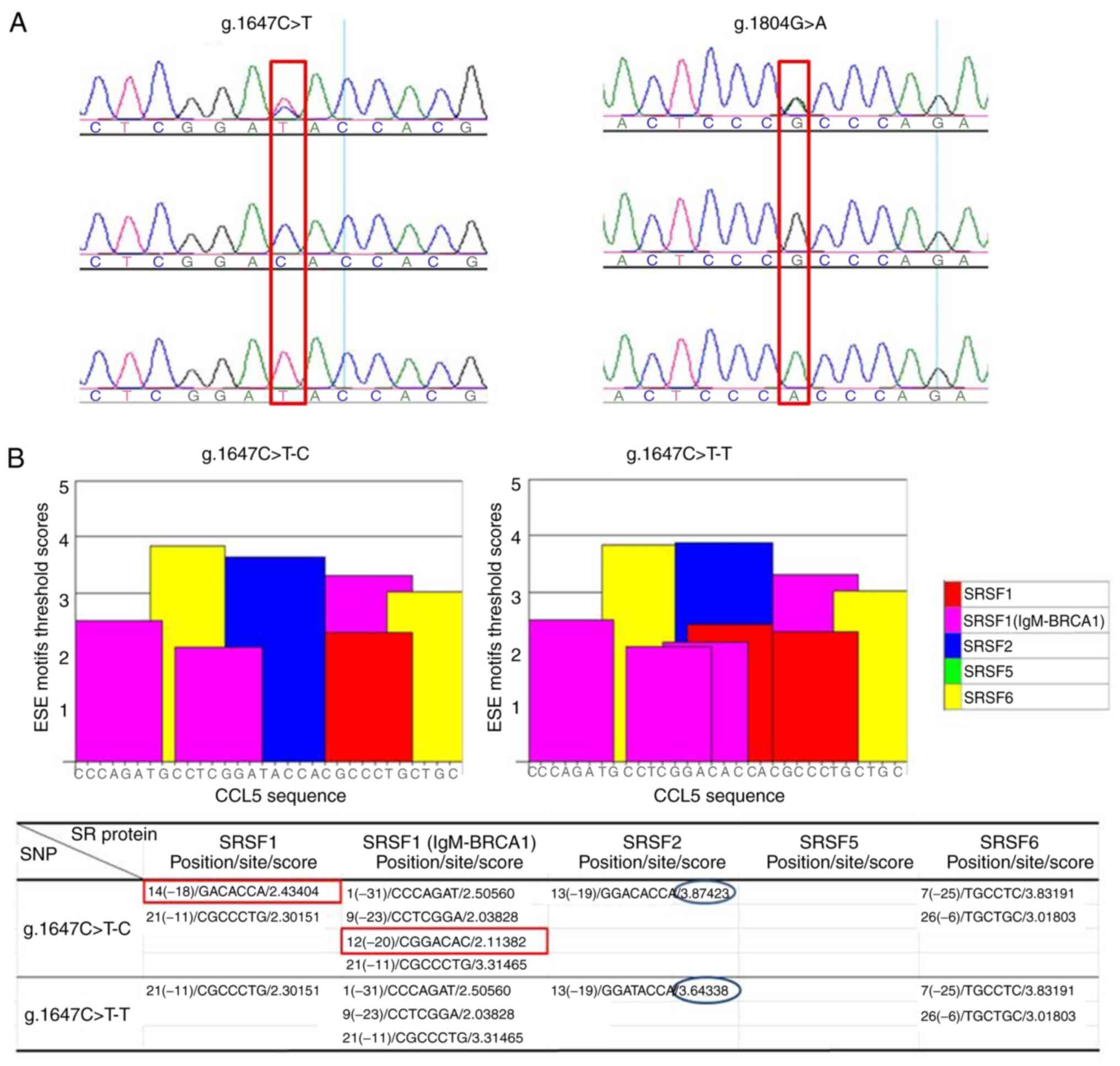 | Figure 3.ESE motif threshold scores associated
with CCL5 genotypes. (A) Sequencing identification of two SNPs
(g.1647 C>T and g.1804 G>A). (B) SNP (g.1647 C>T). The bar
graphs represent scores above the threshold for the ESE motifs in
the C or T allele of locus 1647. The red rectangles indicate the
introduction of allele T, relative to allele C, in the locus
g.1647C>T, and thus the deletion of binding sites to the
auxiliary splicing proteins SRSF1 and SRSF1 (gM-BRCA1). The blue
ellipses indicate the change in a score of SRSF2 binding site. (C)
SNP g.1804 G>A. The bar graphs represent scores above the
threshold for the ESE motifs in the G or A allele of locus 1804.
The red rectangles indicates the introduction of allele A, relative
to allele G, in the locus g.1804G>A, and thus the deletion of
two IgM-BRCA1 binding sites, and an increase in the SRSF5 (SRp40)
binding site. The blue ellipses indicate the changes in a
position/site/score of SRSF1 binding site, a score of IgM-BRCA1
binding site and a score of SRSF2 binding site. ESE, exonic splice
enhancer; CCL5, C-C motif chemokine ligand 5; SNP, single
nucleotide polymorphism; SRSF, serine/arginine-rich splicing
factor; BRCA1, BRCA1 DNA repair associated. |
SNPs in the putative ESEs do not
generate the novel splice transcript
To determine whether the two SNPs (g.1647C>T and
g.1804G>A) of CCL5 led to the abrogation of the predicted SR
protein motifs and caused AS, the 598 bp genomic fragment spanning
268 bp of intron 1, 112 bp of exon 2 and 218 bp of intron 2 was
amplified, which harbored the two haplotypes (C-G and T-A), and the
two fragments were cloned into the pSPL3 vector (Fig. 4A). The CCL5 splicing mini-gene
reporter assay demonstrated that transfecting the two plasmids,
with the wild haplotype (C-G) and mutant haplotype (TA),
respectively, into the 293T cells produced the same RT-PCR
amplification product with a size of 375 bp, while the negative
pSPL3-control generated a product of 263 bp (Fig. 4B). The results suggested that the
haplotypes of the two SNPs (g.1647C>T and g.1804G>A) were not
responsible for the production of CCL5-AS.
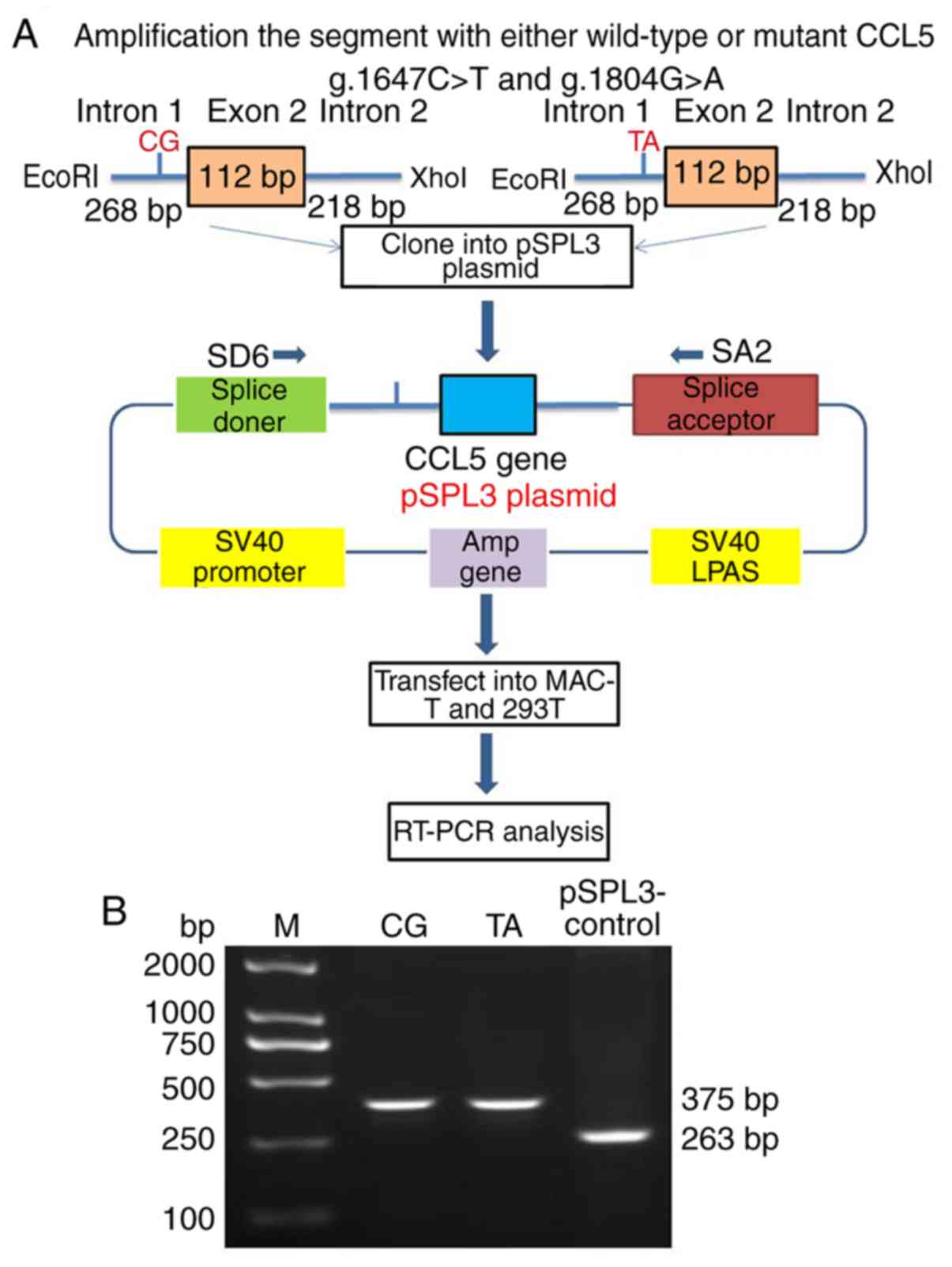 | Figure 4.Protocol of splicing mini-gene
reporter assay. (A) A total of two haplotypes (the wild haplotype
C-G, g.1647 C>T-C and g.1804 G>A-G; and the mutant haplotype
TA with two SNPs, g.1647 C>T-T and g.1804 G>A-A) generated
the same splicing pattern of CCL5 in MAC-T and 293T cells. (B)
RT-PCR analysis of the CCL5 spliced transcripts on a 2% agarose
gel. The wild haplotype, CG, and the mutant type, TA, mini-gene
reporters gave rise to the same 375 bp fragment, while there was a
263 bp fragment of pSPL3 vector in the MAC-T and 293T cells.
Nonspecific amplification products were visible and sequenced.
CCL5, C-C motif chemokine ligand 5; RT-PCR, reverse
transcription-polymerase chain reaction; M, marker. |
Discussion
The present study identified two splice variants and
SNPs of CCL5, and confirmed its downregulation in the mammary
glands from cows with clinical mastitis caused by natural infection
with S. aureus. Furthermore, using the exon splicing
mini-gene reporter assay, it was demonstrated that the candidate
SNPs were not the cause of the generation of the aberrant
transcript, CCL5-AS, in the mammary glands.
Mammary inflammation is a normal defensive response
to bacterial invasion, and part of the subsequent direct immune
response. When inflammation cannot be controlled in the mammary
glands, mastitis occurs and leads to tissue damage. During the
inflammatory response, processes including pathogen recognition,
the release of pro-inflammatory cytokines and chemokines, rapid
recruitment of immune cells from the vasculature to the infection
site, and the subsequent removal of pathogens from the mammary
glands, are of importance (4,20).
During the early stages of E. coli mastitis, the chemokine
response is considered to be a decisive mechanism and serves a
critical role in the inflammatory process (4). Following the challenge with E.
coli, chemokine levels in milk whey and the density of
polymorphonuclear neutrophils are markedly elevated in udder tissue
(21). However, expression changes
of the chemokine CCL5 gene in bovine mammary gland explants in
response to peptidoglycan combined with lipotechoic acid,
representing gram-positive bacteria (for example, S.
aureus), have not been observed (20). In this study, the chemokine CCL5
was downregulated in the mammary glands in the late stages of
natural mastitis infection. Differences in the CCL5 expression
pattern may be due to different infection stages and model systems
of cells, contributing to different immune responses to pathogens.
CCL5 interacts with the G-protein-coupled receptors C-C motif
chemokine receptor (CCR)1, CCR3 and CCR5 in various types of cells
(22). Novel therapies for chronic
inflammatory diseases target CCL5, which may decrease inflammatory
responses and fibrosis (23). CCL5
is an immune-associated gene, which is expressed by T-lymphocytes,
macrophages, platelets, synovial fibroblasts and the tubular
epithelium (10). Therefore, CCL5
expression and its regulation in the mammary gland may be
implicated in bovine mastitis infection and prevention.
AS may enhance transcriptomic and proteomic
diversity in species ranging from bacteria to humans (24), and the regulation of AS is
important for the determination of complex traits (25). In total, 92–94% of human genes
undergo AS, which leads to a single gene producing multiple mRNA
and protein isoforms that have similar, distinct or even opposing
functions (26). In the present
study, a novel CCL5 transcript lacking exon 2 was expressed in the
mammary gland tissue and was mastitis-specific, confirming a recent
study and hypothesis (6). The
results suggested that AS is one of the regulatory mechanisms
underlying the repression of mRNA transcription and the loss of
function of CCL5 at the late stage of mastitis infection,
contributing to the severity of inflammation and tissue damage.
Different SNPs result in a variety of SRSF1 binding
sites (27), and variations in the
intronic SRSF1-binding site influences binding affinity and leads
to the alteration of splicing, according to global cross-linking
immunoprecipitation anlaysis (28). A number of studies have reported
that SNPs in the exons or introns of immune-associated genes result
in aberrant splicing and are associated with susceptibility to
bovine mastitis (3,16,17).
In the present study, the in silico predicted results
indicated that two SNPs may result in the deletion of two binding
sites for SRSF1 and SRSF1 (IgM-BRCA1), and increase the SRSF5
(SRp40) binding site. SRSF proteins are a battery of splicing
regulatory proteins, which are implicated in numerous cellular
functions under pathological and physiological conditions (29). Copy number variations (CNVs) may
result in sporadic chromosomal microdeletion syndromes and
Mendelian diseases (30).
Insertions/deletions (indels) exert a strong influence on gene
function, thus indels may be the ‘driver mutations’ in oncogenesis
(31). CNVs and microRNA (miRNA)
variants are associated with inherited retinal diseases in humans
(32,33). In the present study, a pSPL3 exon
trapping system was used to verify the two SNPs, but the SNPs were
not able to induce the production of CCL5-AS, which may be caused
by indels, miRNA variants or CNVs. The molecular basis of the
production of CCL5-AS remains to be elucidated in a future
study.
In conclusion, the expression levels of CCL5-AS were
lower than that of CCL5-reference in normal and mastitis mammary
tissues, and a novel transcript of CCL5-AS was detected with a
deletion of 112 bp compared with CCL5-reference. Two novel SNPs,
SNP g.1647C>T and SNP g.1804G>A were identified, but the CCL5
alternative splicing was not associated with SNP g.1647C>T and
SNP g.1804G>A.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 31672397), Shandong
Provincial Natural Science Foundation for Distinguished Young
Scholars of China (grant no. JQ201709), Major Project of National
Transgene in China (grant no. 2018ZX08007001-002), Shandong
Provincial Natural Science Foundation (grant no. ZR2016CM37),
Shandong Science and Technological Development Plan (grant no.
2013GNC11023), and China Agriculture Research System (grant no.
CARS-36).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH, LY and RG conceptualized the study design. RG,
ZJ, XW and QJ performed the experiments. YL, HZ, KH and JL analyzed
the data. LY and JH wrote the paper.
Ethics approval and consent to
participate
This study was approved by the Animal Care and Use
Committee from the Dairy Cattle Research Center, Shandong Academy
of Agricultural Sciences (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eshetu E: An overview on the epidemiology
and diagnosis of bovine mastitis. Adv Life Sci Technol. 35:23–27.
2015.
|
|
2
|
Olde Riekerink RG, Barkema HW, Kelton DF
and Scholl DT: Incidence rate of clinical mastitis on Canadian
dairy farms. J Dairy Sci. 91:1366–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Zhong J, Gao Y, Ju Z and Huang J:
A SNP in intron 8 of CD46 causes a novel transcript associated with
mastitis in Holsteins. BMC Genomics. 15:6302014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bannerman DD, Paape MJ, Lee JW, Zhao X,
Hope JC and Rainard P: Escherichia coli and Staphylococcus aureus
elicit differential innate immune responses following intramammary
infection. Clin Diagn Lab Immunol. 11:463–472. 2004.PubMed/NCBI
|
|
5
|
Swanson KM, Stelwagen K, Dobson J,
Henderson HV, Davis SR, Farr VC and Singh K: Transcriptome
profiling of Streptococcus uberis-induced mastitis reveals
fundamental differences between immune gene expression in the
mammary gland and in a primary cell culture model. J Dairy Sci.
92:117–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XG, Ju ZH, Hou MH, Jiang Q, Yang CH,
Zhang Y, Sun Y, Li RL, Wang CF, Zhong JF and Huang JM: Deciphering
transcriptome and complex alternative splicing transcripts in
mammary gland tissues from cows naturally infected with
Staphylococcus aureus mastitis. PLoS One. 11:e01597192016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lahouassa H, Moussay E, Rainard P and
Riollet C: Differential cytokine and chemokine responses of bovine
mammary epithelial cells to Staphylococcus aureus and Escherichia
coli. Cytokine. 38:12–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilbert FB, Cunha P, Jensen K, Glass EJ,
Foucras G, Robert-Granié C, Rupp R and Rainard P: Differential
response of bovine mammary epithelial cells to Staphylococcus
aureus or Escherichia coli agonists of the innate immune system.
Vet Res. 44:402013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aldinucci D and Colombatti A: The
inflammatory chemokine CCL5 and cancer progression. Mediators
Inflamm. 2014:2923762014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, Akerman M, Sun S, McCombie WR,
Krainer AR and Zhang MQ: SpliceTrap: A method to quantify
alternative splicing under single cellular conditions.
Bioinformatics. 27:3010–3016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baralle FE and Giudice J: Alternative
splicing as a regulator of development and tissue identity. Nat Rev
Mol Cell Biol. 18:437–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chacko E and Ranganathan S: Genome-wide
analysis of alternative splicing in cow: Implications in bovine as
a model for human diseases. BMC Genomics. 10 (Suppl 3):S112009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montgomery SB, Sammeth M,
Gutierrez-Arcelus M, Lach RP, Ingle C, Nisbett J, Guigo R and
Dermitzakis ET: Transcriptome genetics using second generation
sequencing in a Caucasian population. Nature. 464:773–777. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia-Blanco MA, Baraniak AP and Lasda
EL: Alternative splicing in disease and therapy. Nat Biotechnol.
22:535–546. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ju Z, Wang C, Wang X, Yang C, Sun Y, Jiang
Q, Wang F, Li M, Zhong J and Huang J: Role of an SNP in alternative
splicing of bovine NCF4 and mastitis susceptibility. PLoS One.
10:e01437052015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Wang X, Li R, Ju Z, Qi C, Zhang
Y, Guo F, Luo G, Li Q, Wang C, et al: Genetic mutations potentially
cause two novel NCF1 splice variants up-regulated in the mammary
gland, blood and neutrophil of cows infected by Escherichia coli.
Microbiol Res. 174:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju Z, Wang C, Li Q, Hou M, Gao S, Hou Q,
Li J, Huang J and Zhong J: Alternative splicing and mRNA expression
analysis of bovine SLAMF7 gene in healthy and mastitis mammary
tissues. Mol Biol Rep. 39:4155–4161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mount JA, Karrow NA, Caswell JL, Boermans
HJ and Leslie KE: Assessment of bovine mammary chemokine gene
expression in response to lipopolysaccharide, lipotechoic acid +
peptidoglycan, and CpG oligodeoxynucleotide 2135. Can J Vet Res.
73:49–57. 2009.PubMed/NCBI
|
|
21
|
Sipka A, Klaessig S, Duhamel GE, Swinkels
J, Rainard P and Schukken Y: Impact of intramammary treatment on
gene expression profiles in bovine Escherichia coli Mastitis. PLoS
One. 9:e855792014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pakianathan DR, Kuta EG, Artis DR, Skelton
NJ and Hébert CA: Distinct but overlapping epitopes for the
interaction of a CC-chemokine with CCR1, CCR3 and CCR5.
Biochemistry. 36:9642–9648. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marques RE, Guabiraba R, Russo RC and
Teixeira MM: Targeting CCL5 in inflammation. Expert Opin Ther
Targets. 17:1439–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keren H, Lev-Maor G and Ast G: Alternative
splicing and evolution: Diversification, exon definition and
function. Nat Rev Genet. 11:345–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Swami M: Alternative splicing: Deciding
between the alternatives. Nat Rev Genet. 10:712009. View Article : Google Scholar
|
|
26
|
Wang ET, Sandberg R, Luo S, Khrebtukova I,
Zhang L, Mayr C, Kingsmore SF, Schroth GP and Burge CB: Alternative
isoform regulation in human tissue transcriptomes. Nature.
456:470–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manning KS and Cooper TA: The roles of RNA
processing in translating genotype to phenotype. Nat Rev Mol Cell
Biol. 18:102–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsiao YH, Bahn JH, Lin X, Chan TM, Wang R
and Xiao X: Alternative splicing modulated by genetic variants
demonstrates accelerated evolution regulated by highly conserved
proteins. Genome Res. 26:440–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Latorre E and Harries LW: Splicing
regulatory factors, ageing and age-related disease. Ageing Res Rev.
36:165–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stankiewicz P and Lupski JR: Structural
variation in the human genome and its role in disease. Annu Rev
Med. 61:437–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H, Zhong Y, Peng C, Chen JQ and Tian
D: Important role of indels in somatic mutations of human cancer
genes. BMC Med Genet. 11:1282010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang XF, Huang ZQ, Fang XL, Chen ZJ,
Cheng W and Jin ZB: Retinal miRNAs variations in a large cohort of
inherited retinal disease. Ophthalmic Genet. 39:175–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang XF, Mao JY, Huang ZQ, Rao FQ, Cheng
FF, Li FF, Wang QF and Jin ZB: Genome-wide detection of copy number
variations in unsolved inherited retinal disease. Invest Ophthalmol
Vis Sci. 58:424–429. 2017. View Article : Google Scholar : PubMed/NCBI
|