Breast cancer is one of the most common malignancies
in women worldwide and results in relatively high rates of
morbidity and mortality (1–4). In
China, the incidence rate of breast cancer has been increasing over
the past 20 years (5–7). Depending on the molecular
classification, therapies used to treat breast cancer, including
surgery, chemotherapy, radiation therapy, hormone (endocrine)
therapy and molecule-targeted therapy, vary in their survival rates
(8–13). As the prognosis for breast cancer
patients remains unsatisfying (14–18),
there is an urgent need to identify a more effective therapy.
Several chemical compounds used in traditional
Chinese medicine (TCM) have proven useful in some conventional
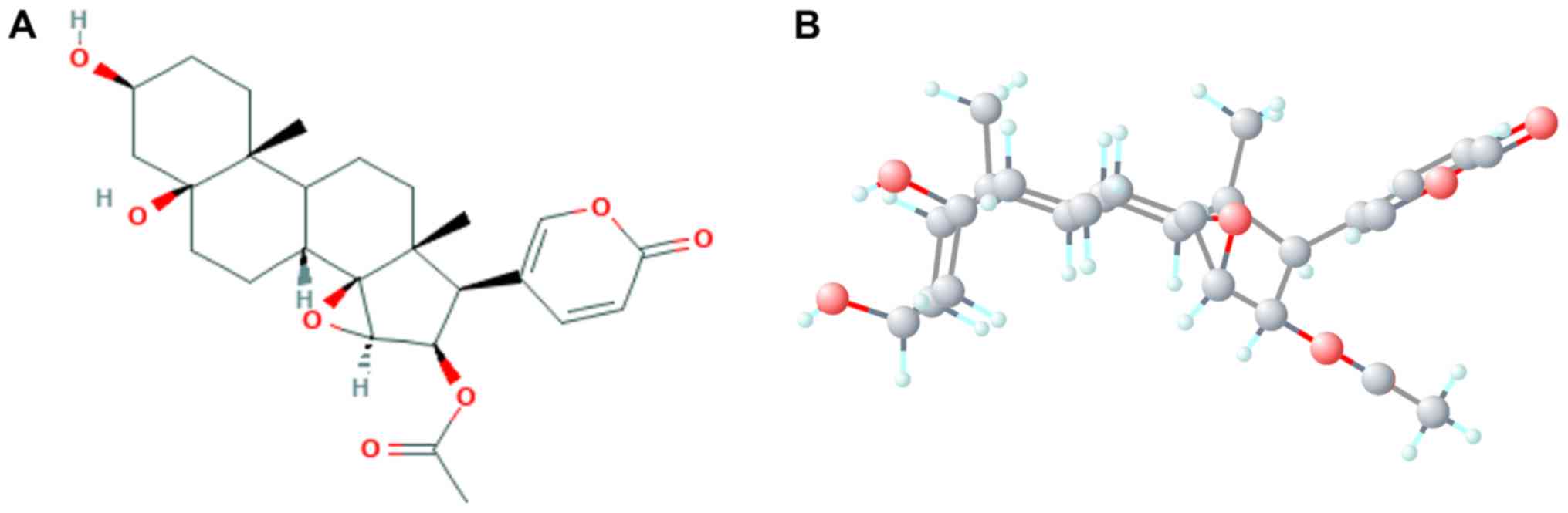
chemotherapies (19,20). Cinobufotalin, a member of the
bufadienolide family, is isolated from the skin parotoid glands of
toads, such as Bufo gargarizans and Duttaphrynus
melanostictus (21). The
broad-spectrum antineoplastic activity and chemosensitization of
bufadienolide has also been previously reported (22). Another study on cinobufotalin
revealed that it may serve as a cardiotonic, diuretic and
hemostatic agent (23). Previous
reports have also focused on the influences of cinobufotalin on
cancers such as hepatocellular carcinoma (HCC) and lung cancer
(24,25), but very few studies have examined
its mechanism in these malignancies and there are even fewer, if
any, reports on its functions in breast cancer. The mechanism of
cinobufotalin against breast cancer cells remain unknown.
In the present study, the GSE85871 microarray data
set from the Gene Expression Omnibus (GEO) database was used in an
optimized analysis to identify differentially expressed genes
(DEGs) in MCF-7 breast cancer treated with cinobufotalin.
Subsequently, the potential molecular mechanism of cinobufotalin in
breast cancer was explored through gene annotation, pathway
analysis and protein-protein interaction (PPI) analysis.
Connectivity Map (CMAP) analysis was used to identify drugs that
may exhibit similar curative properties as cinobufotalin. Based on
the mining of a large database, the present study comprehensively
revealed the roles of cinobufotalin and its potential molecular
mechanism in breast cancer, and offered a possible avenue for
breast cancer treatment.
The expression data of the GSE85871 data set were
obtained from the National Center for Biotechnology Information GEO
database (26). The subject of
this microarray was Homo sapiens, and its research type was
expression profiling by array. The expression profile of this
microarray was provided by GPL571 (HG-U133A_2; Affymetrix Human
Genome U133A 2.0 Array). GSE85871 included the gene expression
profiles of 102 TCM compounds used to treat MCF-7 cells triple
positive for the presence of estrogen, progesterone and human
epidermal growth factor receptor-2 (HER-2) receptors. Additionally,
in this microarray, comparisons were made between the experimental
groups treated with each drug and their respective
dimethylsulphoxide (DMSO) controls; there were two expression
values for each drug. The expression profiles of genes in MCF-7
cells treated with cinobufotalin (GSM2286314 MCF-7_Cinobufotalin_1
µM_rep1 and GSM2286315 MCF-7_Cinobufotalin_1 µM_rep2) and the
profiles of respective controls [GSM2286316 MCF-7_vehicle
(DMSO)_rep1 and GSM2286317 MCF-7_vehicle (DMSO)_rep2] were
downloaded from the GSE85871 data set. Fold change (FC) was set as
the threshold for the mean value of gene expression in the
experimental groups and in the respective DMSO controls, as
previously described (26); DEGs
were identified as FC≥2 or FC≤0.5, and categorized as upregulated
or downregulated, respectively.
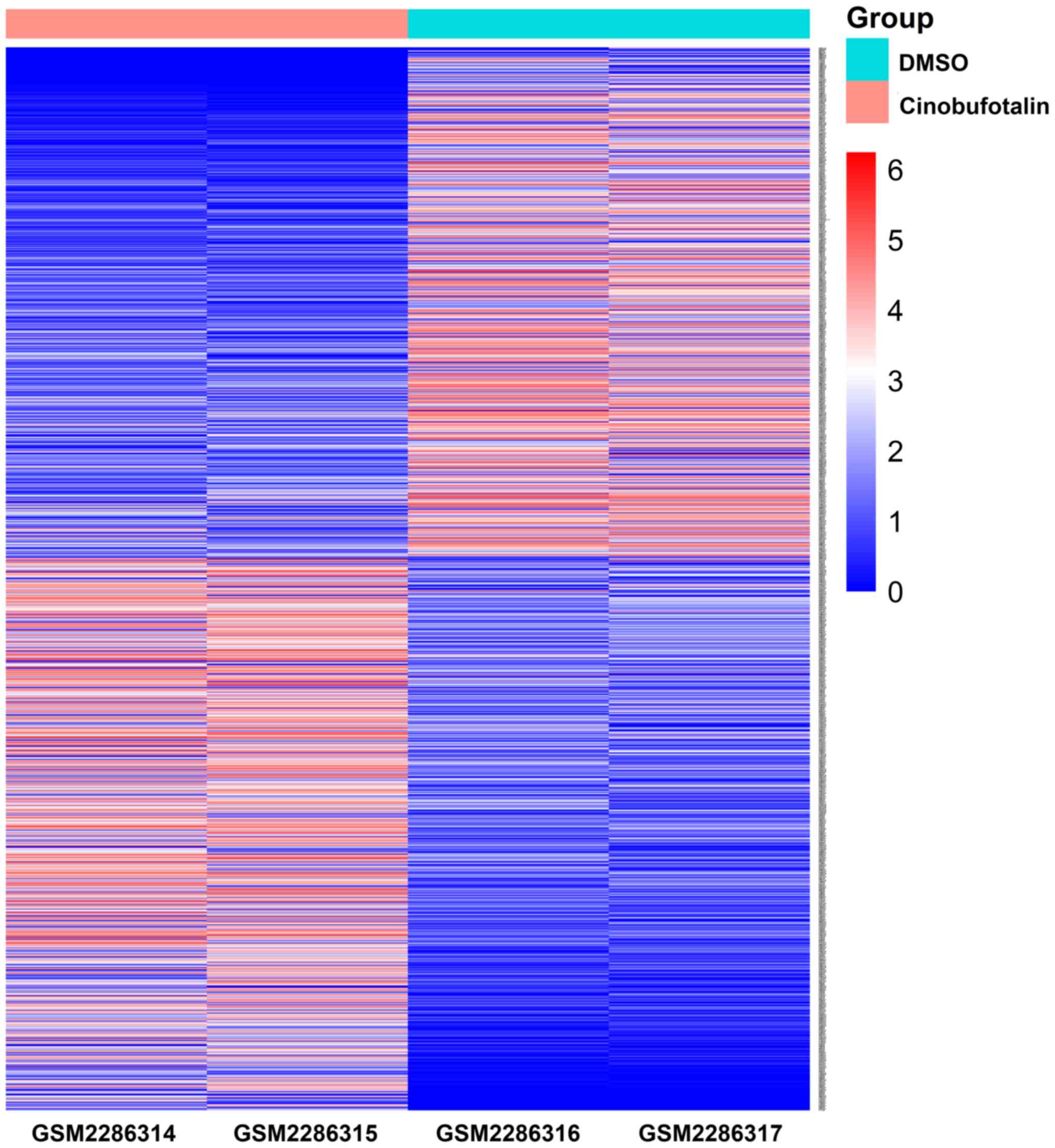
A total of 1,237 DEGs were identified in
cinobufotalin-treated MCF-7 cells compared with the DMSO-treated
controls. Of these, 641 genes were upregulated and 596 were
downregulated (Fig. 2). To
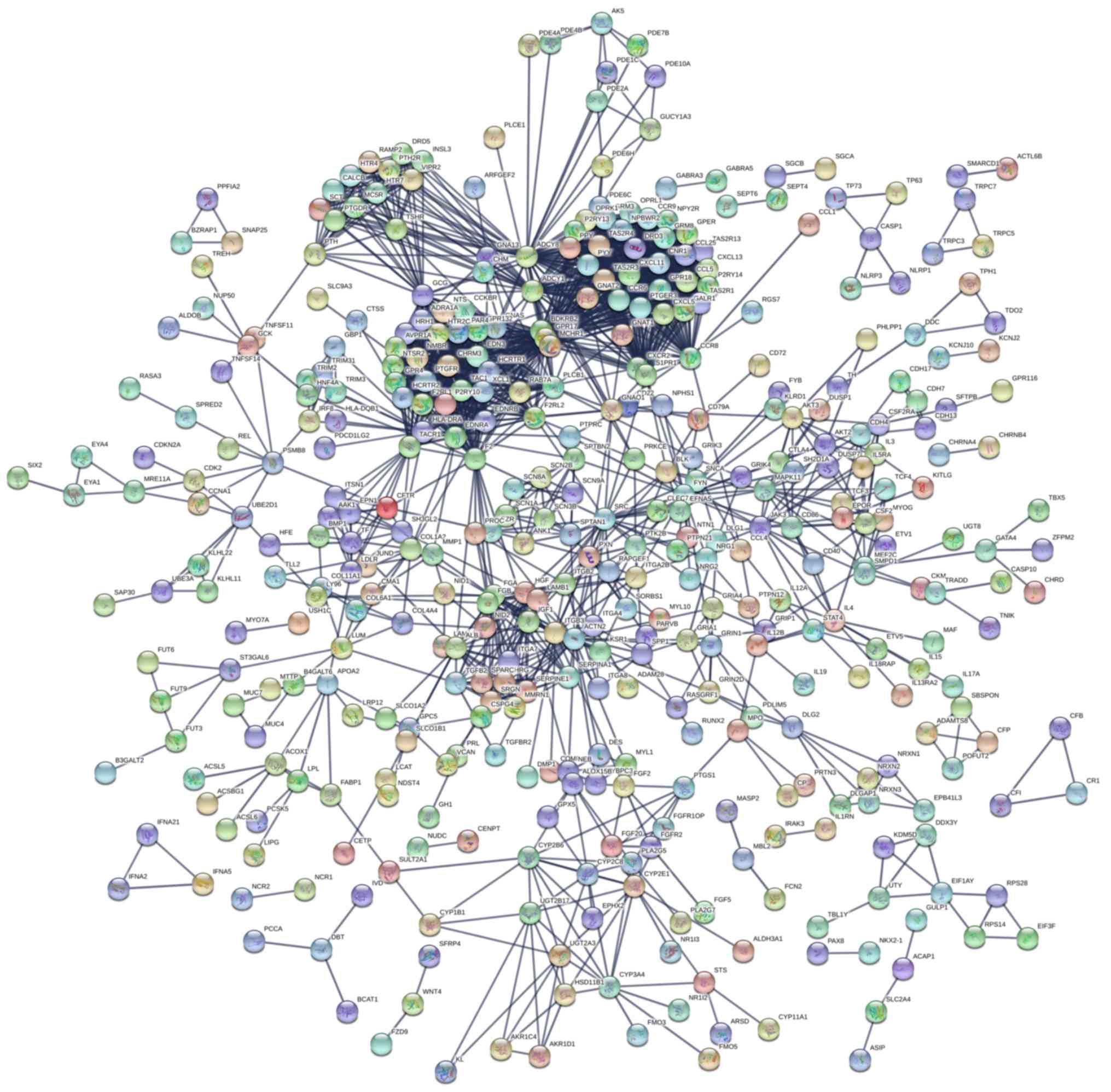
determine the protein interactions of these 1,237 DEGs, a PPI
network was constructed using STRING (Fig. 3). PPI network analysis revealed
several hub genes, including: Albumin (ALB); SRC proto-oncogene
non-receptor tyrosine kinase (SRC); glucagon; protein tyrosine
phosphatase receptor type C (PTPRC); spectrin α non-erythrocytic 1;
coagulation factor II, thrombin; FYN proto-oncogene, SRC family
tyrosine kinase (FYN); cyclin-dependent kinase inhibitor 2A
(CDKN2A); transferrin (TF) and insulin-like growth factor 1. The
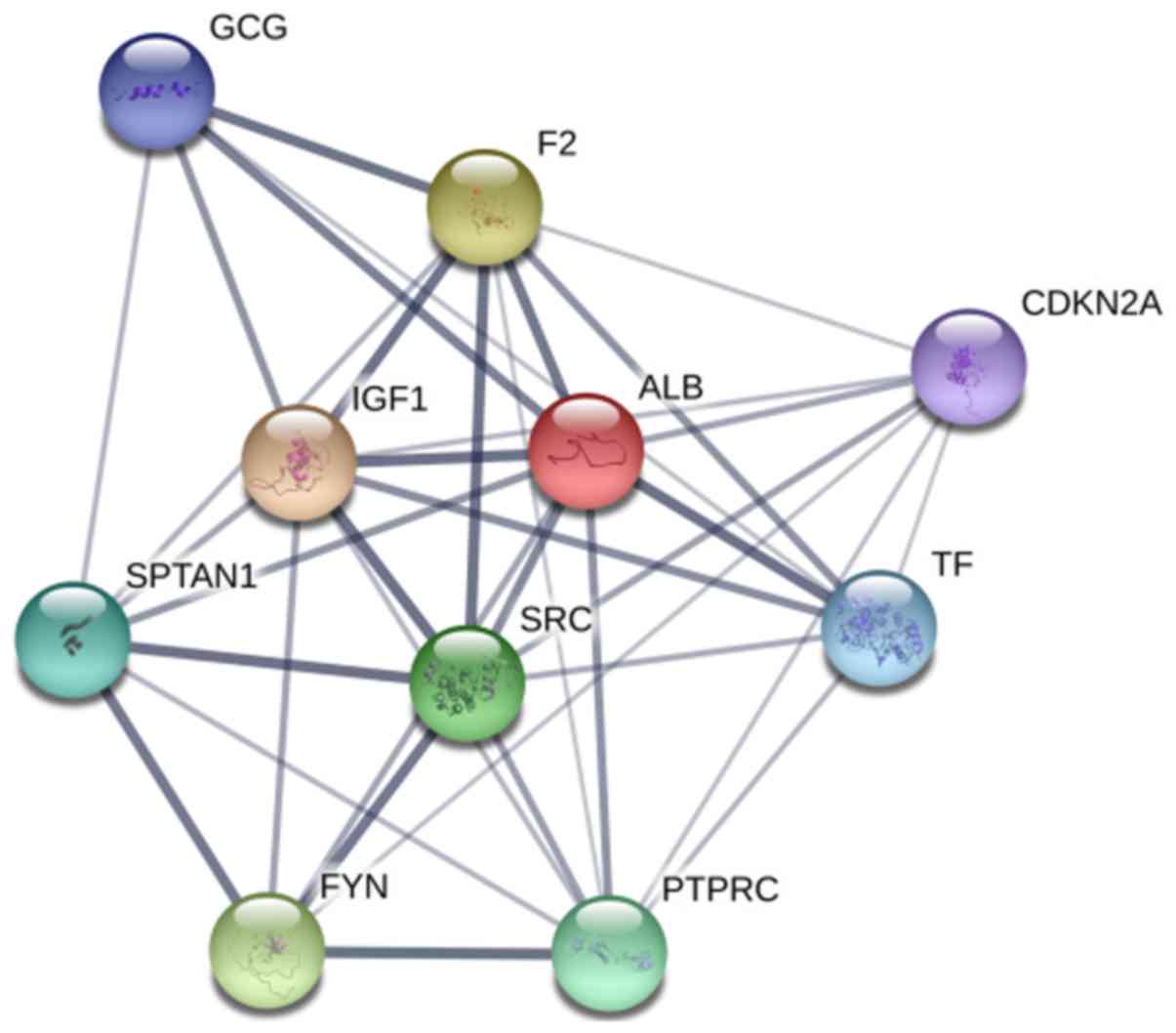
associations between these 10 genes are demonstrated in Fig. 4. As these genes could be the
targets of cinobufotalin treatment in breast cancer cells, the
clinical roles of these genes in breast cancer were next
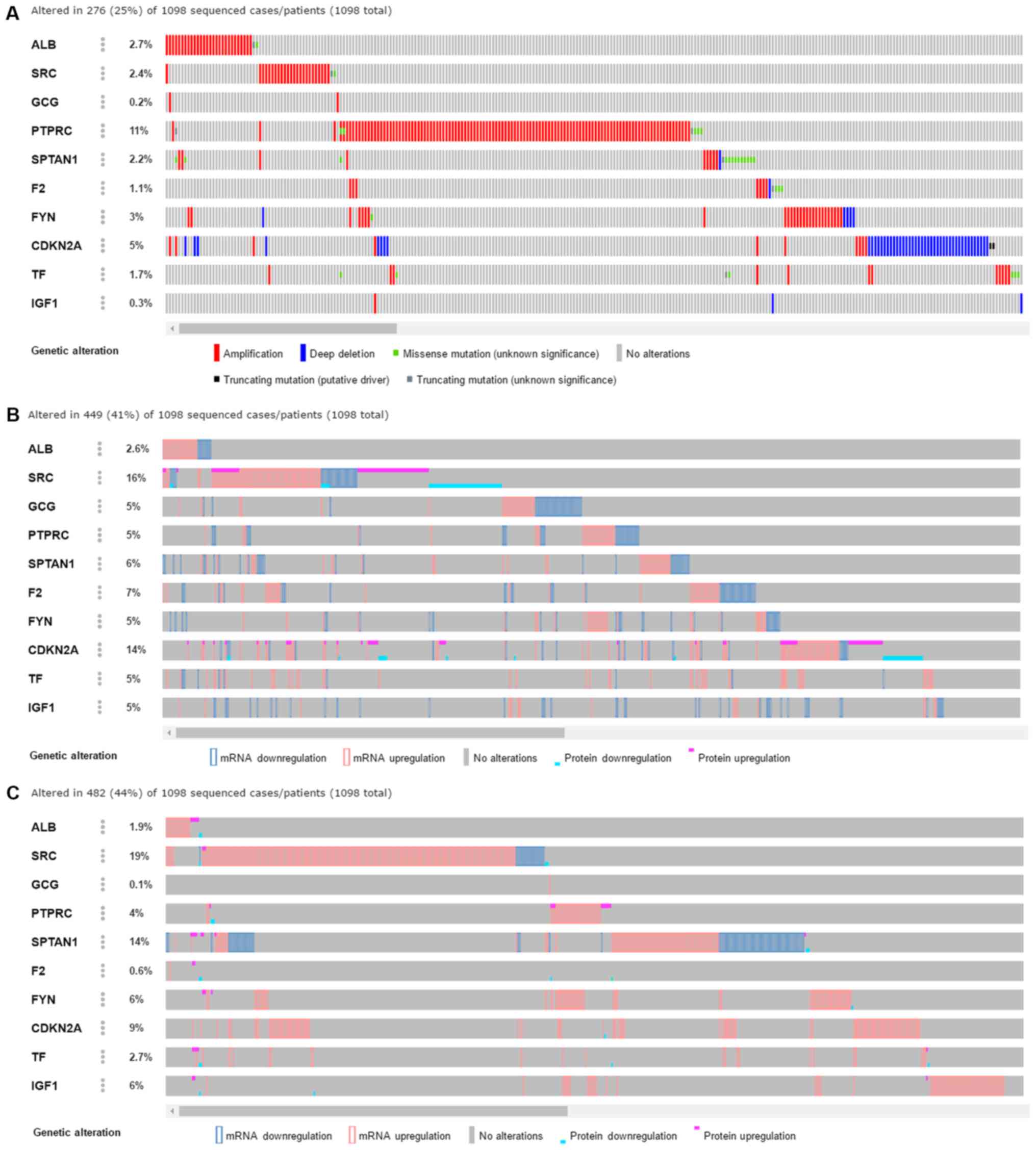
investigated using various approaches. Through cBioPortal data
mining (Fig. 5), varied genetic
alterations were observed in these genes in clinical breast cancer
tissue samples, including amplification, a number of different
mutations, and changes in mRNA and protein expression levels
detected by multiple approaches. These genetic alterations were
identified by three independent sources as provided by cBioPortal,
including TCGA RNA-sequencing, Provisional (Fig. 5A); GISTIC data set (Fig. 5B) and a microarray included in the
TCGA project (Fig. 5C). Based on
the TCGA data, the amplification of ALB, SRC and PTPRC were the
main genetic alteration events identified (Fig. 5A). In addition, the corresponding
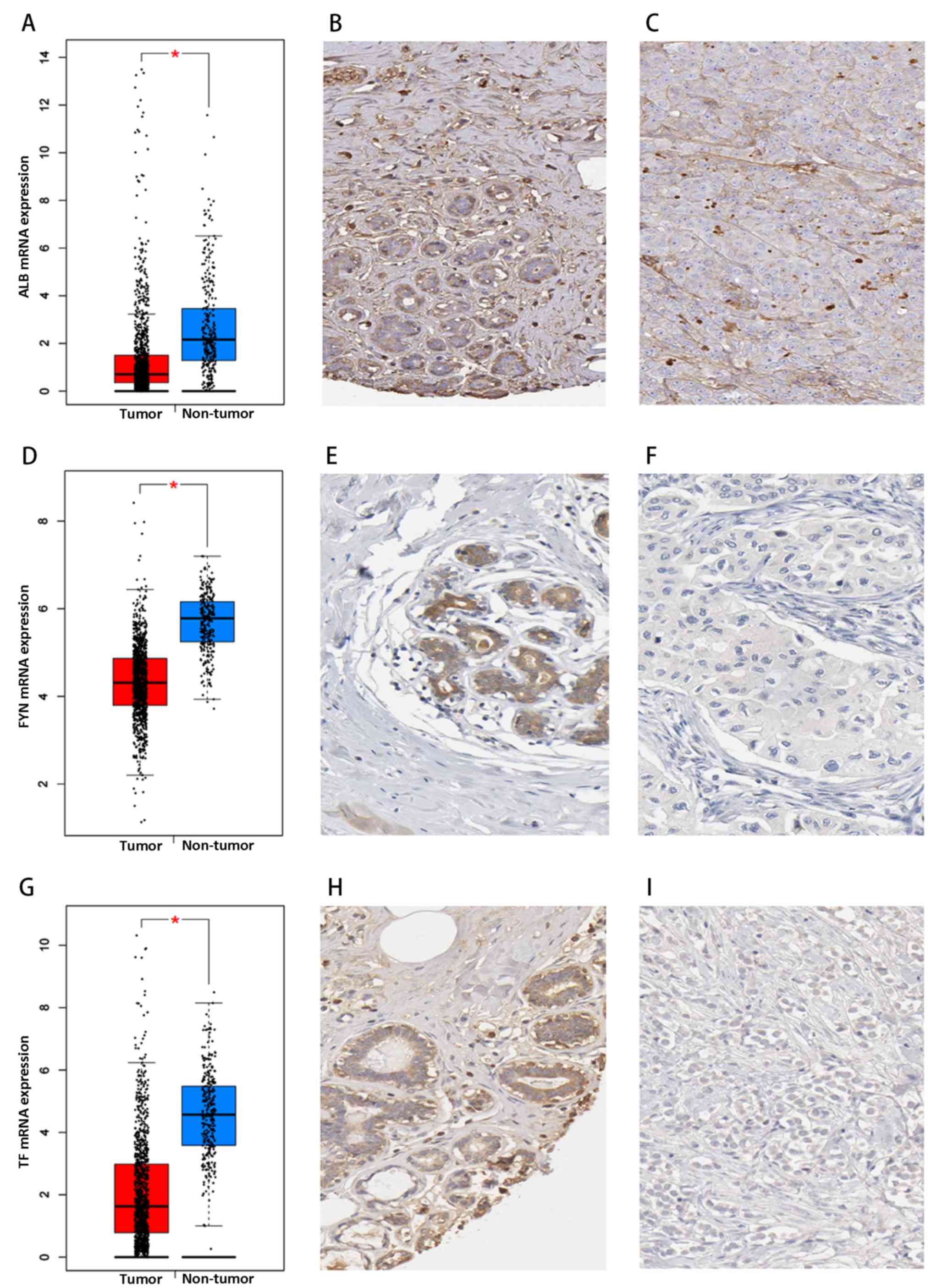
mRNA and protein expression levels of ALB, FYN and TF tended to
decrease in clinical breast cancer tissue samples (Fig. 6). The mRNA expression levels of
ALB, FYN and TF were predominantly downregulated based on
RNA-sequencing data with 1,085 cases of breast cancer and 291
non-cancerous breast tissues (all P<0.05), and the protein
expression levels of ALB, FYN and TF were also decreased in breast
cancer compared with the non-cancerous control tissues. The mRNA
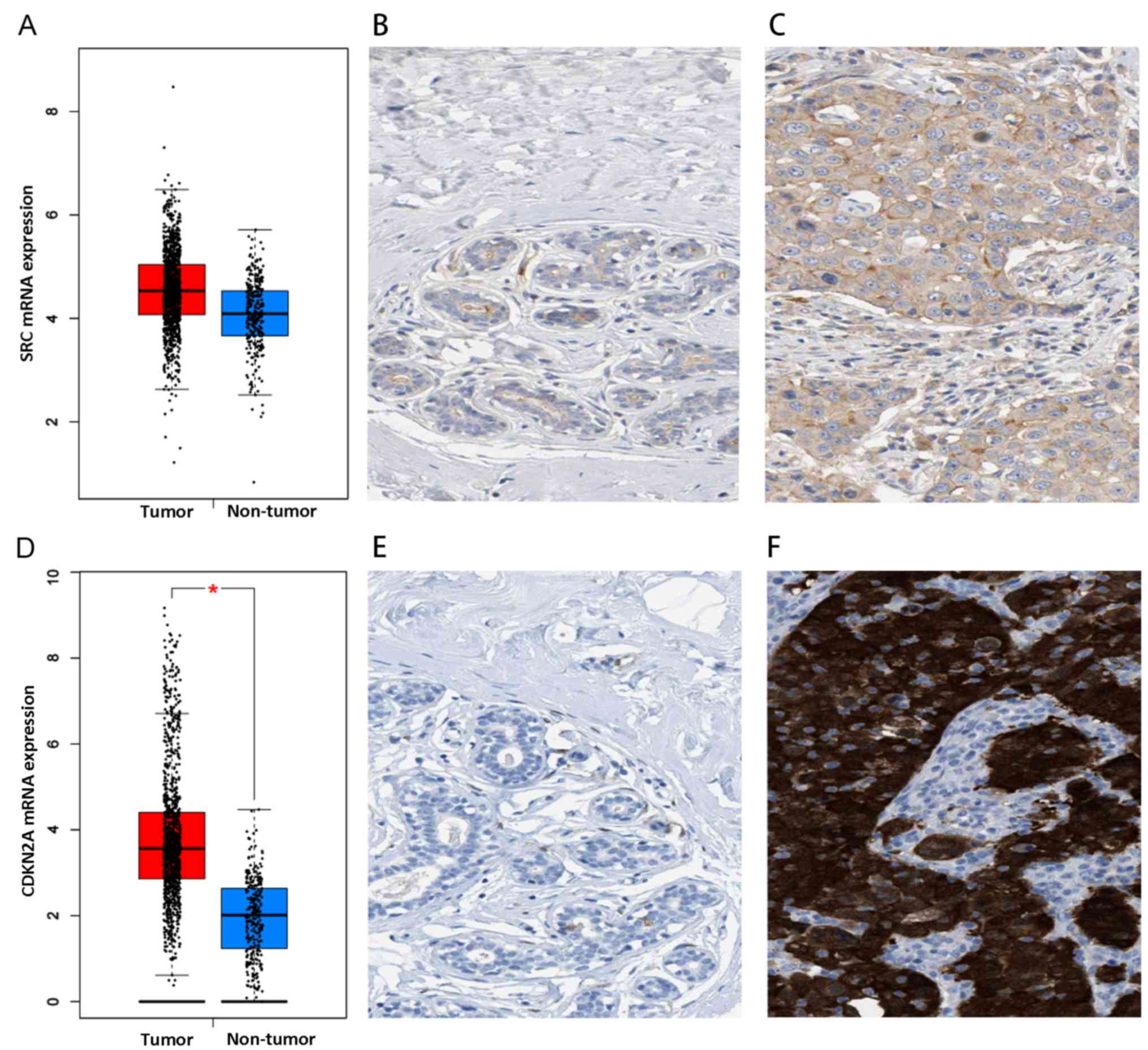
and protein expression levels of SRC and CDKN2A were increased in
breast cancer tissue samples, compared with the control tissue
(Fig. 7). However, owing to the
limited number of cases provided by The Human Protein Atlas,
statistical analysis was not possible. The protein expression
levels of the above genes need to be confirmed using a larger
cohort. Notably, following treatment with cinobufotalin, their
expressions were remarkably reduced, with CDKN2A expression
dropping to 42.2% and SRC plunging to 7.03% (data not shown). These
results indicated that cinobufotalin is more likely to target these
genes to exert its antitumor influences.
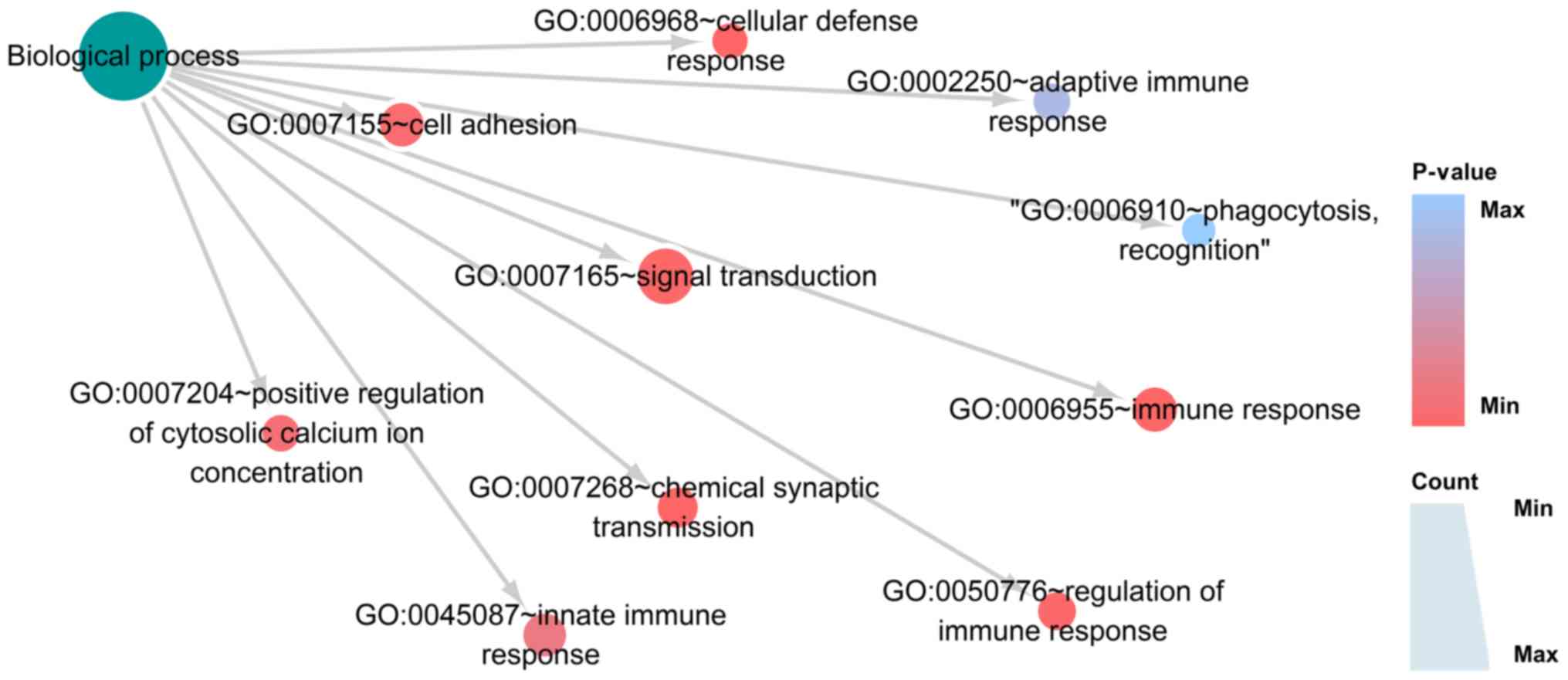
To explore the functions of these 1,237 DEGs, GO and
KEGG analyses were conducted using DAVID. In the GO analysis, the
genes were divided into three groups: i) Biological process (BP),
ii) cellular component (CC) and iii) molecular function (MF). In
BP, the three most significantly enriched processes were ‘immune
response’, ‘chemical synaptic transmission’ and ‘cellular defense
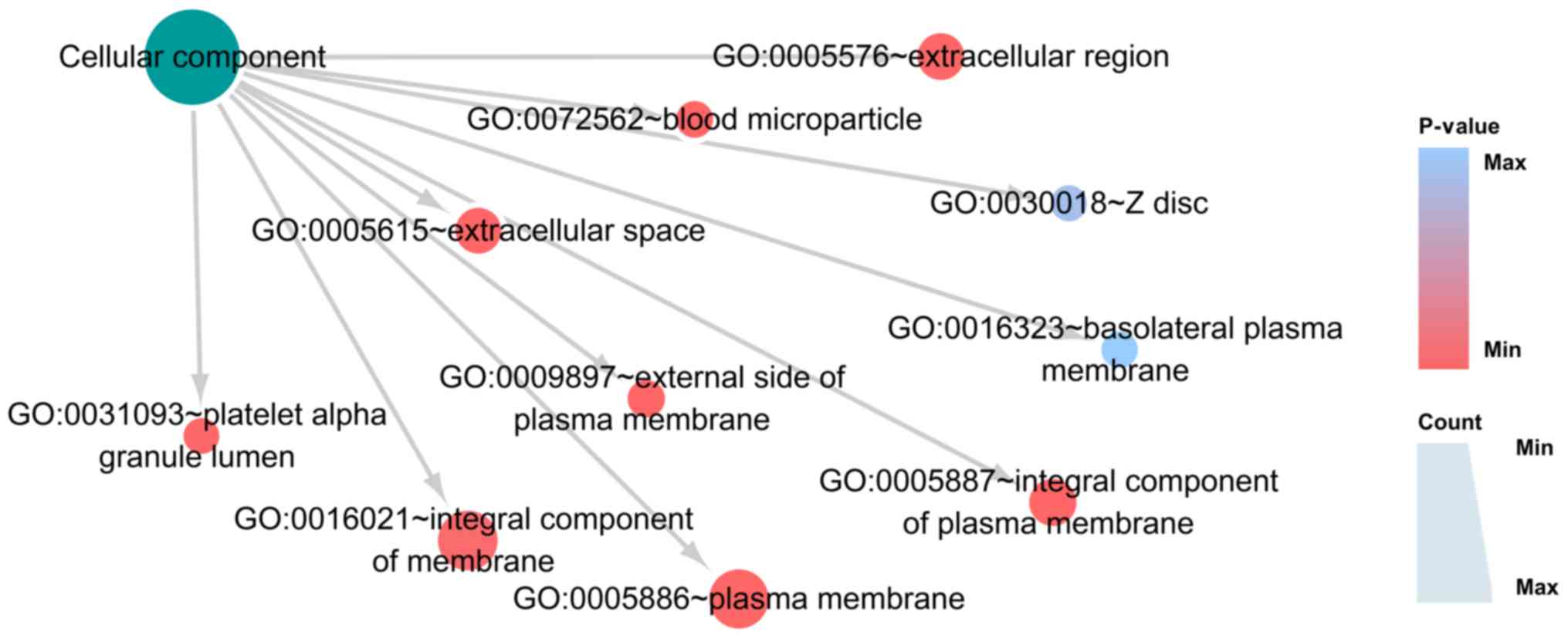
response’ (Fig. 8; Table IA). In CC, the three most
significant cellular components were ‘integral component of plasma
membrane’, ‘extracellular space’ and ‘plasma membrane’ (Fig. 9; Table
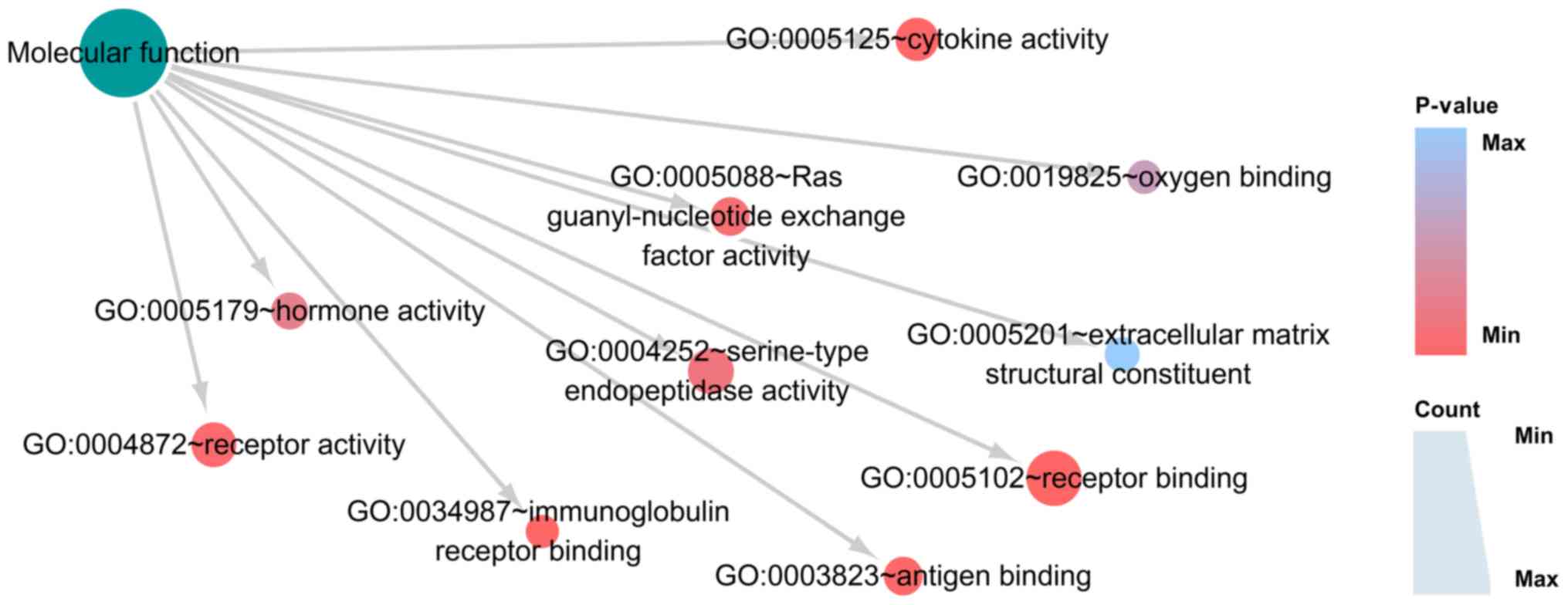
IB). In MF, the top three functions were ‘receptor binding’,
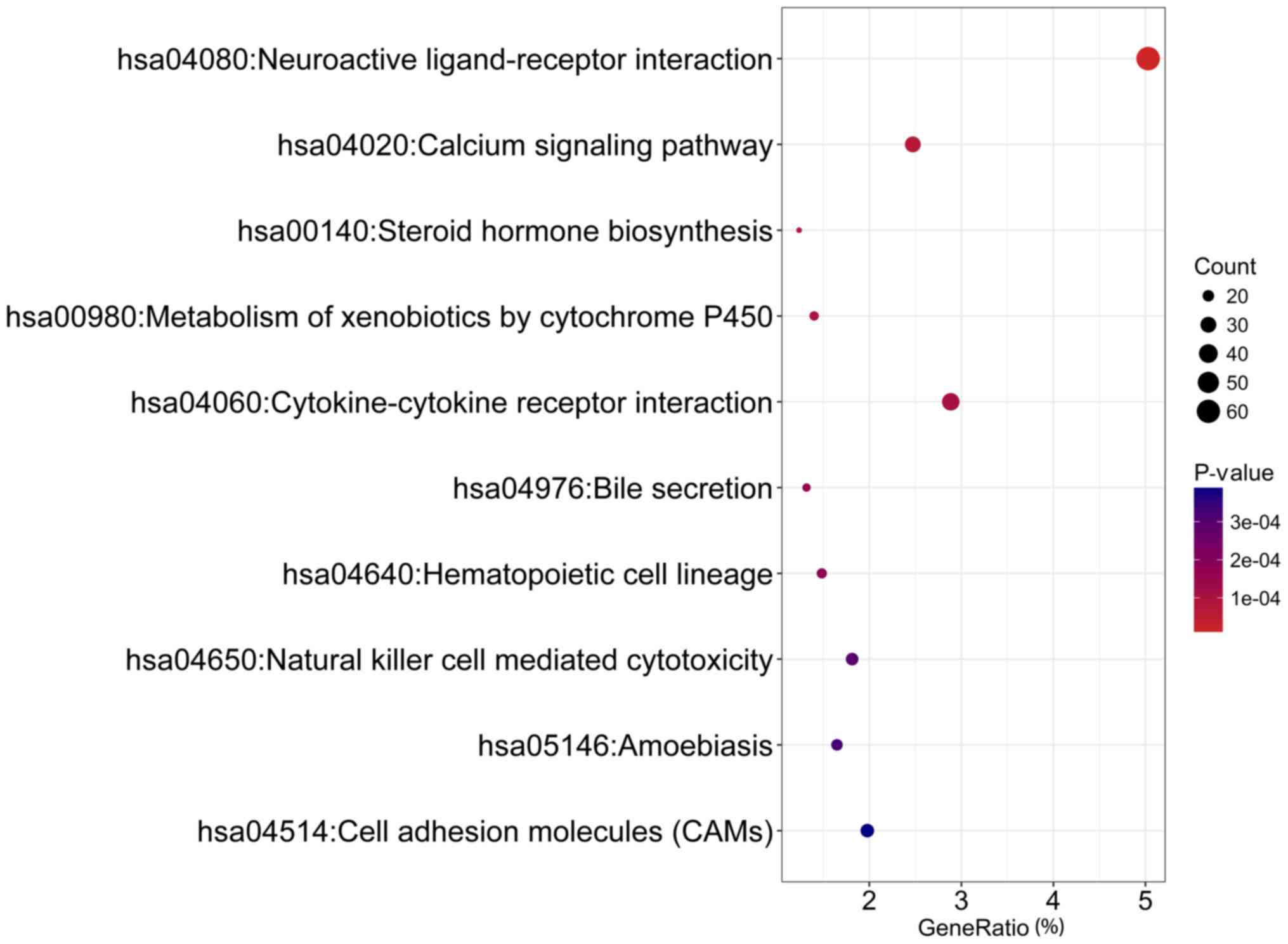
‘immunoglobulin receptor binding’ and ‘cytokine activity’ (Fig. 10; Table IC). KEGG pathway analysis confirmed
that DEGs were remarkably enriched in pathways of ‘neuroactive
ligand-receptor interaction’, ‘calcium signaling pathway’ and
‘steroid hormone biosynthesis’ (Fig.
11; Table ID). The top five GO
terms for each category and KEGG pathways concerning cinobufotalin
treatment on breast cancer cells in GSE85871 are displayed in
Table I.
A gene expression profile comparison was conducted
using the aforementioned 1,237 DEGs post-cinobufotalin treatment
and those genes related to the known drugs from the whole CMAP
database and 9 compounds yielded scores >0.962 (Table II), including trichostatin A
(appearing twice), esculetin, metixene, niclosamide, 15-delta
prostaglandin J2, pimethixene, acetohexamide, allantoin and
pregnenolone. The 1,237 DEGs generated after cinobufotalin
treatment in MCF-7 cells were also compared with the genes
following treatment with other drugs from the CMAP project in MCF-7
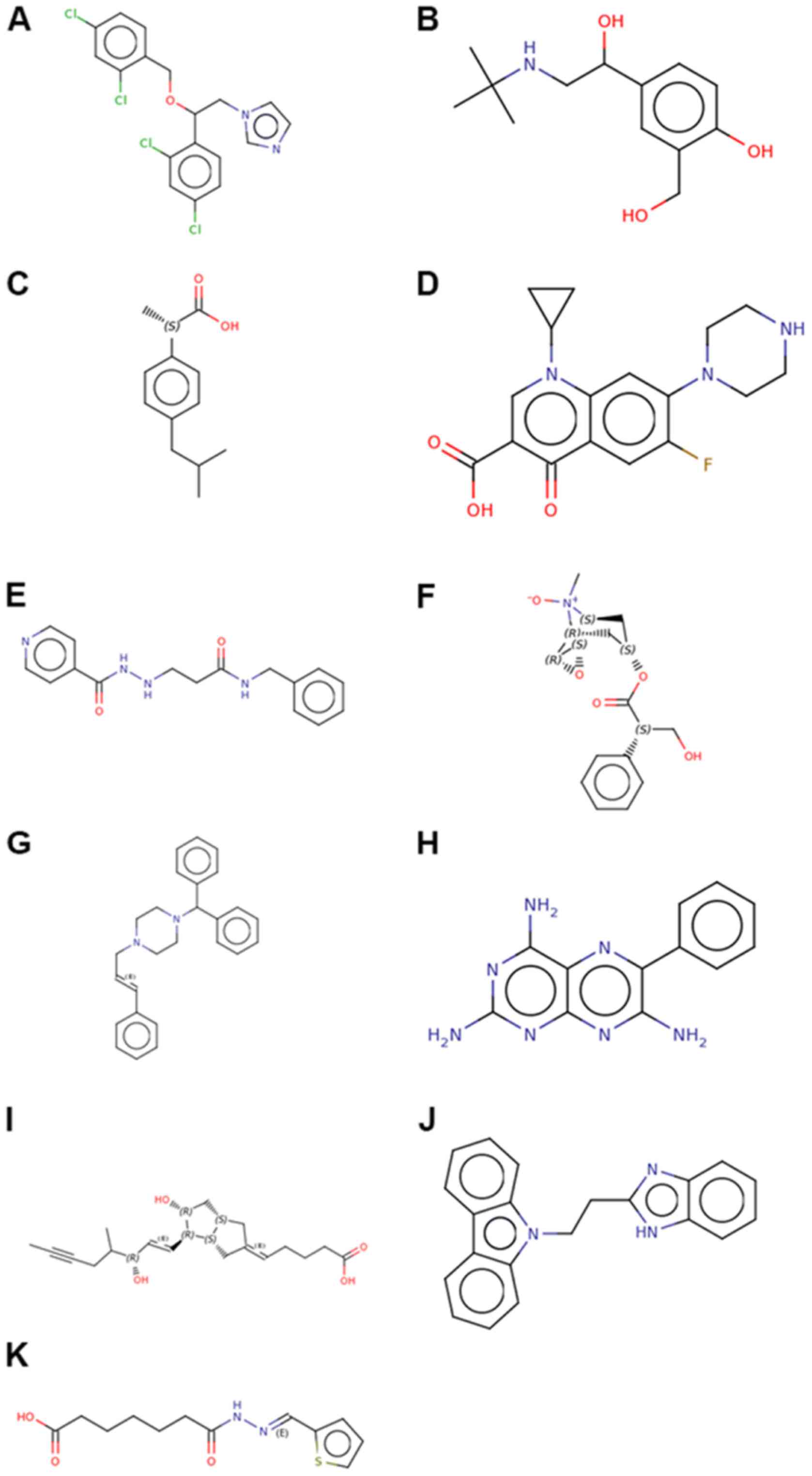
and PC3 cell lines. A total of 11 compounds were chosen with
P<0.05. The results revealed that the compounds exhibiting
similar roles to cinobufotalin included miconazole, salbutamol,
dexibuprofen, ciprofloxacin, nialamide, scopolamine N-oxide and
cinnarizine, whereas those exhibiting an opposite effect included
triamterene, iloprost, BCB000040 and BCB000038 (Table III; Fig. 12). Among these drugs, most of them
were generated from the same cell line, MCF-7, which enhanced the
power of drug prediction, e.g. miconazole, salbutamol, iloprost,
dexibuprofen, ciprofloxacin, nialamide, scopolamine N-oxide and
cinnarizine may have comparable target genes with cinobufotalin in
breast cancer cells.
Currently, to the best of the authors' knowledge,
there have been no reports on the antitumor mechanisms of
cinobufotalin in breast cancer cells through large data mining
analyses. Microarrays and RNA-sequencing have facilitated research
on functions and mechanisms of TCM (49–52).
The present study was conducted by combining microarray analysis
and RNA-sequencing data in breast cancer tissues. For the potential
target gene of cinobufotalin, several genes were selected for
confirmation and demonstrated that ALB, FYN, TF, SRC and CDKN2A may
serve pivotal roles in the onset and development of breast cancer.
These genes also were affected by cinobufotalin in treated MCF-7
cells, which may shed light on the potential mechanism of
cinobufotalin on breast cancer cells. In the present study, the
pathways of ‘neuroactive ligand-receptor interaction’ and ‘calcium
signaling’ appeared to be significant pathways for cinobufotalin in
MCF-7 breast cancer cells. In addition, connectivity mapping
demonstrated that cinobufotalin had similar molecular mechanisms to
drugs such as miconazole as they target consistent genes in breast
cancer cells, which may provide a theoretical foundation for
research on the anticancer mechanism of cinobufotalin in breast
cancer cells.
The anticancer ability of cinobufotalin has been
previously documented in hepatoblastoma (24) and lung cancer cells (25), based on in vitro models. In
HepG2 hepatoblastoma cells, cinobufotalin was reported to
inactivate Akt-S6K1 signaling, and in A549, H460 and HTB-58 lung
cancer cells, cinobufotalin mainly induced Cyclophilin D-dependent
non-apoptotic death. Data from PubChem also revealed that
cinobufotalin exhibited effects on other cancer cells. For
instance, cinobufotalin cytotoxicity against human Bel7402 cells,
which have been identified as being derived from Hela cells, was
detected by MTT assay (BioAssay AID: 343717) and the activity value
(IC50) was 1.21 mM. Another BioAssay (AID: 1221865)
indicated an activity value (IC 50) of 8.62 mM when cytotoxicity
against human Bel7402 cells was assessed after treatment of 72 h.
Interestingly, a phase I clinical trial sponsored by Shanghai
University of TCM is now at recruitment stage and will use
cinobufotalin injection as intervention to treat malignant tumor of
small intestine metastatic to liver (https://clinicaltrials.gov; ClinicalTrials.gov Identifier: NCT03189992). However,
no previous study has examined the effects and mechanism of
cinobufotalin on breast cancer cells. From the expression data
provided by the GSE85871 data set in MCF-7 cells following
cinobufotalin treatment (26), the
present study identified 1,237 DEGs, and subsequently conducted
further analysis of the core genes disclosed by PPI. Additional
analysis demonstrated that some of these core genes, to some
extent, may influence the onset and development of breast cancer
through their abnormal expression and genetic alteration. According
to the data in TCGA and The Human Protein Atlas, the mRNA and
protein expression levels of SRC and CDKN2A were increased in
breast cancer tissues compared with non-cancerous tissues. Previous
studies have also reported that increased SRC and CDKN2 expression
levels correlated with the onset, metastasis and prognosis of
breast cancer (53–59). Therefore, SRC and CDKN2 may be the
most important hub genes in the biological function of
cinobufotalin on breast cancer MCF-7 cells, as in this study,
cinobufotalin was observed to inhibit the overexpression of SRC and
CDKN2A (data not shown). The potential targeting of these genes
suggested that cinobufotalin may have anticancer potential.
In addition, potential mechanisms of cinobufotalin
in MCF-7 breast cancer cells were elucidated from the prospective
signaling pathways. The 1,237 DEGs were annotated to examine how
cinobufotalin functioned on breast cancer cells. Notably, the GO
term results of the 1,237 DEGs were mainly linked to immunity,
including ‘immune response’, ‘regulation of immune response’,
‘innate immune response’ and ‘adaptive immune response’. It was
reported previously that cinobufotalin activated the nuclear
factor-κB pathway and decreased expression levels of brain-derived
neurotrophic factor to induce neuroinflammation in rats (60). Nonetheless, no direct evidence has
revealed that cinobufotalin was associated with number of immune
cells, organism immunity or tumor immunity. On a molecular level,
the present study hypothesized that in treating breast cancer,
cinobufotalin may exert its antitumor influences by activating the
immune response; however, additional studies are required for
verification.
To further interpret the potential mechanism of
cinobufotalin in MCF-7 breast cancer cells, KEGG pathway enrichment
was performed on the identified DEGs, which revealed that several
pathways were connected, not only to immunity after cinobufotalin
was applied to treat breast cancer samples but also to other
pathways. ‘Neuroactive ligand-receptor interaction’, which contains
numerous G protein-coupled receptors, continued to be the most
significantly enriched pathway. As one of the most common pathways
of malignancies, the pathway of ‘Neuroactive ligand-receptor
interaction’ ranks fifth in genes with mutation in the central
nervous system (61). A previous
study reported that low expression of cannabinoid receptor-1
(CNR1), part of the neuroactive ligand-receptor interaction
pathway, indicated that breast cancer patients may benefit from
chemotherapy (62). Notably, this
CNR1 was among the 1,237 DEGs following cinobufotalin treatment in
breast cancer cells. In addition, this pathway was reported to
serve a vital role in tumorigenesis and chemotherapy for breast
cancer (62,63), which suggested that cinobufotalin
may exhibit its curative potential for breast cancer by influencing
the neuroactive ligand-receptor interaction pathway. The
co-treatment of cinobufotalin to chemotherapeutics may induce a
synergistic effect by suppressing the neuroactive ligand-receptor
interaction pathway.
The second most enriched KEGG pathway was the
‘calcium signaling pathway’, the mechanism of which is rather
complicated in breast cancer. Previous studies have reported that
the calcium signaling pathway interacts with other pathways to
contribute to the onset and progression of breast cancer (64–68).
In invasive ductal carcinoma the calcium signaling pathway was
reported to interact with pathways in cancer, including the
pathways of glyoxylate and dicarboxylate metabolism, basal
transcription factors, tyrosine metabolism, FcγR-mediated
phagocytosis, metabolism of xenobiotics by cytochrome P450 and
phagosome (69). Wnt5a in the
noncanonical Wnt pathway was considered a possible anti-oncogene in
breast cancer, as it was demonstrated to serve an essential role
via the calcium signaling pathway (70). In addition, the calcium signaling
pathway was strongly linked with the proliferation, migration and
invasion of breast cancer cells (71). Based on these results,
cinobufotalin may affect breast cancer cells by inhibiting the
calcium signaling pathway.
TCMs differ from other chemical compounds owing to
their natural ingredients and low toxicity (72–75).
CMAP analysis revealed that cinobufotalin and miconazole shared
similar mechanisms, as the varied genes post-miconazole treatment
were comparable to those following cinobufotalin treatment in the
same breast cancer cell line MCF-7. It was previously demonstrated
that miconazole was able to induce apoptosis in bladder cancer
cells through the death receptor 5-dependent and
mitochondrial-mediated pathways (76); thus, cinobufotalin may also cause
the death of cancer cells (24,25).
Notably, miconazole activates the release of phospholipase
C-dependent Ca2+ from the endoplasmic reticulum by
influencing the elevation of calcium ions, thus inducing ZR-75-1
breast cancer cell apoptosis (77). Based on these previous studies and
the present CMAP analysis, it was hypothesized that cinobufotalin
may induce the apoptosis of cancer cells in breast cancer MCF-7
cells via the calcium signaling pathway, thus resembling the
curative properties of miconazole. However, this hypothesis
requires additional experiments for confirmation.
Several limitations exist in the present study.
First, the cellular model MCF-7 only represents a specific subtype
of breast cancer. It is probable that cinobufotalin may serve
different functions through various genes and pathways in distinct
subtypes of breast cancer. Therefore, additional cell lines should
be examined in future studies. Second, the current findings are
based on in silico analyses and verification with in
vitro and in vivo experiments are needed, including the
biological effect and potential molecular mechanism.
In conclusion, cinobufotalin is likely to act as an
effective compound to treat this subtype of breast cancer, triple
positive for estrogen, progesterone and HER-2 receptors, and its
mechanism may correlate to various pathways. In addition,
cinobufotalin may have anticancer functions in MCF-7 cells similar
to those of miconazole.
The authors are grateful for the public microarray
and RNA-sequencing data.
The present study was supported by The Guangxi
Provincial Health Bureau Scientific Research Project (grant no.
Z2013424).
All data generated or analyzed during this study are
included in this published article.
JL, MHR, YWD, RQH, JCZ, JM and GC designed the
study. JL, MHR, XJL, DDX, LJZ, HQ, CXF and GC conceived and
performed the experiments. JL, MHR, XJL, DDX, LJZ, HQ, CXF, QL and
HY performed the statistical analysis and data interpretation. JL,
MHR and RQH wrote the manuscript, HY, JCZ and GC corrected the
paper. All authors approved the final version of the manuscript for
publication.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kolak A, Kamińska M, Sygit K, Budny A,
Surdyka D, Kukielka-Budny B and Burdan F: Primary and secondary
prevention of breast cancer. Ann Agric Environ Med. 24:549–553.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yedjou CG, Tchounwou PB, Payton M, Miele
L, Fonseca DD, Lowe L and Alo RA: Assessing the racial and ethnic
disparities in breast cancer mortality in the United States. Int J
Environ Res Public Health. 14:E4862017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Song XW and Zhao CH: Roles of
programmed cell death protein 5 in inflammation and cancer
(Review). Int J Oncol. 49:1801–1806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan Z, Jing W, He K, Zhang L and Long X:
SATB1 is correlated with progression and metastasis of breast
cancers: A meta-analysis. Cell Physiol Biochem. 38:1975–1983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Zhang C, Song Y, Wang Z, Wang Y,
Luo F and Xu Y, Zhao Y, Wu Z and Xu Y: Mechanism of immune evasion
in breast cancer. Onco Targets Ther. 10:1561–1573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Chen Y, Wu S, Song F, Zhang H and
Tian M: Molecular imaging using PET and SPECT for identification of
breast cancer subtypes. Nucl Med Commun. 37:1116–1124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing L, He Q, Wang YY, Li HY and Ren GS:
Advances in the surgical treatment of breast cancer. Chin Clin
Oncol. 5:342016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De A, Kuppusamy G and Karri VVSR: Affibody
molecules for molecular imaging and targeted drug delivery in the
management of breast cancer. Int J Biol Macromol. 107:906–919.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matos Do Canto L, Marian C, Varghese RS,
Ahn J, Da Cunha PA, Willey S, Sidawy M, Rone JD, Cheema AK, Luta G,
et al: Metabolomic profiling of breast tumors using ductal fluid.
Int J Oncol. 49:2245–2254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quispe-Soto ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang N, Zhou TC, Lei XX, Wang C, Yan M,
Wang ZF, Liu W, Wang J, Ming KH, Wang BC, et al: Inhibition of
Sonic Hedgehog signaling pathway by thiazole antibiotic
thiostrepton attenuates the CD44+/CD24-stem-like population and
sphere-forming capacity in triple-negative breast cancer. Cell
Physiol Biochem. 38:1157–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canfeld K, Wells W, Geradts J, Kinlaw WB,
Cheng C and Kurokawa M: Inverse association between MDM2 and HUWE1
protein expression levels in human breast cancer and liposarcoma.
Int J Clin Exp Pathol. 9:6342–6349. 2016.PubMed/NCBI
|
|
13
|
Santa Mina D, Brahmbhatt P, Lopez C, Baima
J, Gillis C, Trachtenberg L and Silver JK: The case for
prehabilitation prior to breast cancer treatment. PM R. 9
(Suppl):S305–S316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mo CH, Gao L, Zhu XF, Wei KL, Zeng JJ,
Chen G and Feng ZB: The clinicopathological significance of UBE2C
in breast cancer: A study based on immunohistochemistry, microarray
and RNA-sequencing data. Cancer Cell Int. 17:832017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomulkiewicz A, Jablonska K, Pula B,
Grzegrzolka J, Borska S, Podhorska-Okolow M, Wojnar A, Rys J,
Ambicka A, Ugorski M, et al: Expression of metallothionein 3 in
ductal breast cancer. Int J Oncol. 49:2487–2497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong DD, Lv J, Wei KL, Feng ZB, Chen JT,
Liu KC, Chen G and Luo DZ: A nine-miRNA signature as a potential
diagnostic marker for breast carcinoma: An integrated study of
1,110 cases. Oncol Rep. 37:3297–3304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen SQ, Huang LS, Xiao XL, Zhu XF, Xiong
DD, Cao XM, Wei KL, Chen G and Feng ZB: miR-204 regulates the
biological behavior of breast cancer MCF-7 cells by directly
targeting FOXA1. Oncol Rep. 38:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li CY, Xiong DD, Huang CQ, He RQ, Liang
HW, Pan DH, Wang HL, Wang YW, Zhu HW and Chen G: Clinical value of
miR-101-3p and biological analysis of its prospective targets in
breast cancer: A study based on The Cancer Genome Atlas (TCGA) and
bioinformatics. Med Sci Monit. 23:1857–1871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Penchala S, Prabhu S, Wang J and
Huang Y: Molecular basis of traditional Chinese medicine in cancer
chemoprevention. Curr Drug Discov Technol. 7:67–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M and Tang W: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen KK, Anderson RC and Henderson FG:
Comparison of cardiac action of bufalin, cinobufotalin, and
telocinobufagin with cinobufagin. Proc Soc Exp Biol Med.
76:372–374. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng L, Chen YZ, Peng Y, Yi N, Gu XS, Jin
Y and Bai XM: Ceramide production mediates cinobufotalin-induced
growth inhibition and apoptosis in cultured hepatocellular
carcinoma cells. Tumour Biol. 36:5763–5771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kai S, Lu JH, Hui PP and Zhao H:
Pre-clinical evaluation of cinobufotalin as a potential anti-lung
cancer agent. Biochem Biophys Res Commun. 452:768–774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv C, Wu X, Wang X, Su J, Zeng H, Zhao J,
Lin S, Liu R, Li H, Li X and Zhang W: The gene expression profiles
in response to 102 traditional Chinese medicine (TCM) components: A
general template for research on TCMs. Sci Rep. 7:3522017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harryman WL, Pond E, Singh P, Little AS,
Eschbacher JM, Nagle RB and Cress AE: Laminin-binding integrin gene
copy number alterations in distinct epithelial-type cancers. Am J
Transl Res. 8:940–954. 2016.PubMed/NCBI
|
|
29
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thul PJ and Lindskog C: The human protein
atlas: A spatial map of the human proteome. Protein Sci.
27:233–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He RQ, Li XJ, Liang L, Xie Y, Luo DZ, Ma
J, Peng ZG, Hu XH and Chen G: The suppressive role of miR-542-5p in
NSCLC: The evidence from clinical data and in vivo validation using
a chick chorioallantoic membrane model. BMC Cancer. 17:6552017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357(pii): eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Panosyan EH, Lin HJ, Koster J and Lasky JL
III: In search of druggable targets for GBM amino acid metabolism.
BMC Cancer. 17:1622017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petrizzo A, Caruso FP, Tagliamonte M,
Tornesello ML, Ceccarelli M, Costa V, Aprile M, Esposito R,
Ciliberto G, Buonaguro FM and Buonaguro L: Identification and
validation of HCC-specific gene transcriptional signature for tumor
antigen discovery. Sci Rep. 6:292582016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu X, Wang X, Fu B, Meng L and Lang B:
Differentially expressed genes and microRNAs in bladder carcinoma
cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp
Pathol. 8:12678–12687. 2015.PubMed/NCBI
|
|
36
|
Cao L, Zhang Q, Cheng S, Chen Z, Hua Z,
Yang J, Liu D and Cui N: Long non-coding RNAs and genes
contributing to the generation of cancer stem cells in
hepatocellular carcinoma identified by RNA sequencing analysis.
Oncol Rep. 36:2619–2624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang DW, Yu SY, Cao Y, Yang L, Liu W, Er
XQ, Yao GJ and Bi ZG: Identification of CD20, ECM, and ITGA as
biomarkers for osteosarcoma by integrating transcriptome analysis.
Med Sci Monit. 22:2075–2085. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu X and Li H: Integrated microRNA-gene
analysis of coronary artery disease based on miRNA and gene
expression profiles. Mol Med Rep. 13:3063–3073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Zhang J, Li L, Xu X, Zhang Y, Teng
Z and Wu F: Identification of molecular targets for predicting
colon adenocarcinoma. Med Sci Monit. 22:460–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen W, Liu Q, Lv Y, Xu D, Chen W and Yu
J: Special role of JUN in papillary thyroid carcinoma based on
bioinformatics analysis. World J Surg Oncol. 15:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian W, Liu J, Pei B, Wang X, Guo Y and
Yuan L: Identification of miRNAs and differentially expressed genes
in early phase non-small cell lung cancer. Oncol Rep. 35:2171–2176.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Yang X, Guan H, Mizokami A, Keller
ET, Xu X, Liu X, Tan J, Hu L, Lu Y and Zhang J: Exosome-derived
microRNAs contribute to prostate cancer chemoresistance. Int J
Oncol. 49:838–846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song B, Du J, Feng Y, Gao YJ and Zhao JS:
Co-expressed differentially expressed genes and long non-coding
RNAs involved in the celecoxib treatment of gastric cancer: An RNA
sequencing analysis. Exp Ther Med. 12:2455–2468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang H, Zhang X, Huang J and Fan X:
Identification of key genes and pathways for peri-implantitis
through the analysis of gene expression data. Exp Ther Med.
13:1832–1840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu H, Pei D, Chen L, Zhou X and Zhu H:
Identification of key genes and molecular mechanisms associated
with dedifferentiated liposarcoma based on bioinformatic methods.
Onco Targets Ther. 10:3017–3027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nicolau CA, Prorock A, Bao Y,
Neves-Ferreira AGDC, Valente RH and Fox JW: Revisiting the
therapeutic potential of Bothrops jararaca venom: Screening for
novel activities using connectivity mapping. Toxins (Basel).
10:E692018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thillaiyampalam G, Liberante F, Murray L,
Cardwell C, Mills K and Zhang SD: An integrated meta-analysis
approach to identifying medications with potential to alter breast
cancer risk through connectivity mapping. BMC Bioinformatics.
18:5812017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Busby J, Murray L, Mills K, Zhang SD,
Liberante F and Cardwell CR: A combined connectivity mapping and
pharmacoepidemiology approach to identify existing medications with
breast cancer causing or preventing properties. Pharmacoepidemiol
Drug Saf. 27:78–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu Y, Zhao Y, Wei Z, Tao L, Sheng X, Wang
S, Chen J, Ruan J, Liu Z, Cao Y, et al: Targeting thioredoxin
system with an organosulfur compound, diallyl trisulfide (DATS),
attenuates progression and metastasis of triple-negative breast
cancer (TNBC). Cell Physiol Biochem. 50:1945–1963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liao KF, Chiu TL, Huang SY, Hsieh TF,
Chang SF, Ruan JW, Chen SP, Pang CY and Chiu SC: Anti-cancer
effects of radix angelica sinensis (Danggui) and
N-butylidenephthalide on gastric cancer: Implications for REDD1
activation and mTOR inhibition. Cell Physiol Biochem. 48:2231–2246.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hao J, Jin Z, Zhu H, Liu X, Mao Y, Yang X,
Gao L, Liu D, Chen D and Wu X: Antiestrogenic activity of the
Xi-huang formula for breast cancer by targeting the estrogen
receptor α. Cell Physiol Biochem. 47:2199–2215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ding X, Yang Q, Kong X, Haffty BG, Gao S
and Moran MS: Radiosensitization effect of Huaier on breast cancer
cells. Oncol Rep. 35:2843–2850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Browne AL, Charmsaz S, Vareslija D, Fagan
A, Cosgrove N, Cocchiglia S, Purcell S, Ward E, Bane F, Hudson L,
et al: Network analysis of SRC-1 reveals a novel transcription
factor hub which regulates endocrine resistant breast cancer.
Oncogene. 37:2008–2021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hamurcu Z, Delibasi N, Gecene S, Sener EF,
Dönmez-Altuntas H, Ozkul Y, Canatan H and Ozpolat B: Targeting LC3
and Beclin-1 autophagy genes suppresses proliferation, survival,
migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin
β1/Src signaling in triple negative breast cancer cells. J Cancer
Res Clin Oncol. 144:415–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abdullah C, Korkaya H, Iizuka S and
Courtneidge SA: SRC increases MYC mRNA expression in ER+
breast cancer via mRNA stabilization and inhibition of p53
function. Mol Cell Biol. (pii): MCB.00463-172017.(Epub ahead of
print). View Article : Google Scholar
|
|
56
|
Ocana A, Gil-Martin M, Martin M, Rojo F,
Antolin S, Guerrero A, Trigo JM, Munoz M, Pandiella A, et al: A
phase I study of the SRC kinase inhibitor dasatinib with
trastuzumab and paclitaxel as first line therapy for patients with
HER2-overexpressing advanced breast cancer. GEICAM/2010-04 study.
Oncotarget. 8:73144–73153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y, Zhou W, Tang K, Chen X, Feng Z and
Chen J: Silencing Aurora A leads to re-sensitization of breast
cancer cells to Taxol through downregulation of SRC-mediated ERK
and mTOR pathways. Oncol Rep. 38:2011–2022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Subash-Babu P, Li DK and Alshatwi AA: In
vitro cytotoxic potential of friedelin in human MCF-7 breast cancer
cell: Regulate early expression of Cdkn2a and pRb1, neutralize
mdm2-p53 amalgamation and functional stabilization of p53. Exp
Toxicol Pathol. 69:630–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Spitzwieser M, Entfellner E, Werner B,
Pulverer W, Pfeiler G, Hacker S and Cichna-Markl M:
Hypermethylation of CDKN2A exon 2 in tumor, tumor-adjacent and
tumor-distant tissues from breast cancer patients. BMC Cancer.
17:2602017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bi QR, Hou JJ, Qi P, Ma CH, Shen Y, Feng
RH, Yan BP, Wang JW, Shi XJ, Zheng YY, et al: Venenum Bufonis
induces rat neuroinflammation by activiating NF-κB pathway and
attenuation of BDNF. J Ethnopharmacol. 186:103–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Engel SR and Grant KA: Neurosteroids and
behavior. Int Rev Neurobiol. 46:321–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Y, Liu X, Tang H, Yang H and Meng X:
RNA sequencing uncovers molecular mechanisms underlying
pathological complete response to chemotherapy in patients with
operable breast cancer. Med Sci Monit. 23:4321–4327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou L, Gao HF, Liu DS, Feng JY, Gao DD
and Xia W: Gene expression profiling of brain metastatic cell from
triple negative breast cancer: Understanding the molecular events.
Gene. 640:21–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi HY, Park N, Na JB, Ko ES, Park JY and
Yoo JC: Direct binding of Copine3 with Jab1 activates downstream
ErbB2 signaling and motility in SKBr3 breast cancer cells. Oncol
Rep. 35:1147–1152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Liu X, Tang H, Yang H and Meng X:
RNA sequencing uncovers molecular mechanisms underlying
pathological complete response to chemotherapy in patients with
operable breast cancer. Med Sci Monit. 23:4321–4327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang M, Li H, Li Y, Ruan Y and Quan C:
Identification of genes and pathways associated with MDR in
MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol Med Rep.
17:6211–6226. 2018.PubMed/NCBI
|
|
67
|
Dang YW, Lin P, Liu LM, He RQ, Zhang LJ,
Peng ZG, Li XJ and Chen G: In silico analysis of the potential
mechanism of telocinobufagin on breast cancer MCF-7 cells. Pathol
Res Pract. 214:631–643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Varga K, Hollósi A, Pászty K, Hegedűs L,
Szakács G, Tímár J, Papp B, Enyedi Á and Padányi R: Expression of
calcium pumps is differentially regulated by histone deacetylase
inhibitors and estrogen receptor alpha in breast cancer cells. BMC
Cancer. 18:10292018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen WY, Wu F, You ZY, Zhang ZM, Guo YL
and Zhong LX: Analyzing the differentially expressed genes and
pathway cross-talk in aggressive breast cancer. J Obstet Gynaecol
Res. 41:132–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Trifa F, Karray-Chouayekh S, Jmal E, Jmaa
ZB, Khabir A, Sellami-Boudawara T, Frikha M, Daoud J and
Mokdad-Gargouri R: Loss of WIF-1 and Wnt5a expression is related to
aggressiveness of sporadic breast cancer in Tunisian patients.
Tumour Biol. 34:1625–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Di J, Huang H, Qu D, Tang J, Cao W, Lu Z,
Cheng Q, Yang J, Bai J, Zhang Y and Zheng J: Rap2B promotes
proliferation, migration, and invasion of human breast cancer
through calcium-related ERK1/2 signaling pathway. Sci Rep.
5:123632015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lou JS, Yao P and Tsim KWK: Cancer
treatment by using traditional Chinese medicine: Probing active
compounds in anti-multidrug resistance during drug therapy. Curr
Med Chem. 25:5128–5141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jiang Y and Gao H: Pharmacophore-based
drug design for potential AChE inhibitors from traditional chinese
medicine database. Bioorg Chem. 76:400–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng X, Wu F, Lin X, Shen L and Feng Y:
Developments in drug delivery of bioactive alkaloids derived from
traditional Chinese medicine. Drug Deliv. 25:398–416. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xia X, Cole SPC, Cai T and Cai Y: Effect
of traditional Chinese medicine components on multidrug resistance
in tumors mediated by P-glycoprotein. Oncol Lett. 13:3989–3996.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yuan SY, Shiau MY, Ou YC, Huang YC, Chen
CC, Cheng CL, Chiu KY, Wang SS and Tsai KJ: Miconazole induces
apoptosis via the death receptor 5-dependent and
mitochondrial-mediated pathways in human bladder cancer cells.
Oncol Rep. 37:3606–3616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Roan CJ, Chou CT, Liang WZ, Chang HT, Kuo
DH, Kuo CC, Chen FA, Shieh P and Jan CR: Effect of miconazole on
[Ca2+]i and cytotoxicity in ZR-75-1 human
breast cancer cells. Chin J Physiol. 58:377–384. 2015. View Article : Google Scholar : PubMed/NCBI
|