Introduction
The incidence of hepatocellular carcinoma (HCC),
which is the fifth most common cancer and the third most common
cause of cancer-associated mortality worldwide, has been observed
to have an increasing trend over the last decade. Although surgery
is the most efficient therapeutic method at present (1,2), the
5-year survival rate of HCC remains <5% due to the high rates of
recurrence and metastasis (3,4).
Therefore, determination of the risks and prognostic biomarkers is
required to tailor drug therapy and ultimately improve patient
prognosis following surgical therapies.
It has been indicated that the recurrence and
metastasis of tumors are associated with the tumor cells evading
immune surveillance by T cells in HCC (5). In particular, B7 family molecules, as
types of peripheral membrane proteins, can produce a co-stimulatory
or co-inhibitory signal to enhance or decrease the activity of
major histocompatibility complex-T cell receptor signaling
(6–10), which may subsequently induce T cell
dysfunction or apoptosis upon antigen recognition. Under
pathological conditions, B7 family molecules mainly interact with
the receptor on activating T cells, which provides subsequent
signals to weaken or even terminate the T-cell immune response
(11–14). Therefore, disorder in the
co-stimulatory or co-inhibitory signaling pathway serves an
important role in tumor immune evasion (5).
In previous decades, novel members of the B7 family,
including B7-1, B7-2, B7-H1, B7-H2, B7-H3, B7-H4 and B7-DC, have
been identified. Accumulating data have demonstrated that the
abnormal expression of B7 molecules is associated with the
development of HCC. In particular, Tu et al (15) determined that the upregulation of
B7-H2 can lead to an immunosuppressive HCC microenvironment and
result in unfavorable prognostic outcomes for patients with HCC.
B7-H3 is reported to promote the aggression and invasion of HCC via
the Janus kinase 2/signal transducer and activator of transcription
3/Slug signaling pathway (8).
Therefore, B7-H2 and B7-H3 molecules may useful as biological
markers for predicting the prognosis of patients with HCC in
clinical practice (3).
As B7-H2 and B7-H3 are likely to exert synergistic
effects on HCC, as they are from the same family of B7 molecules
and have similar immune regulatory functions in HCC, it is
considered necessary to evaluate the expression of B7-H2 and B7-H3
in the same HCC cohort to determine whether their combined
expression provides enhanced sensitivity and specificity of the
co-stimulatory molecules in predicting the prognosis of patients
with HCC. In the present study, investigations were performed on
the expression signatures of B7-H2 and B7-H3 in the same HCC tissue
set. It was revealed that the combination of B7-H2 with B7-H3 may
be a preferable prognostic biomarker for predicting recurrence and
overall survival rates in patients with HCC.
Materials and methods
Antibodies and reagents
Mouse anti-human ICOS (B7-H2) ligand monoclonal
antibody (cat. no. ab189052) and mouse anti-human CD276 (B7-H3)
monoclonal antibody (cat. no. ab105922) were obtained from Abcam
(Cambridge, UK). HRP-conjugated secondary antibodies (anti-mouse
IgG; cat. no. HS201-01) were purchased from Transgen Biotech Co.,
Ltd. (Beijing, China).
Patients and tissue samples
A total of 63 formalin-fixed and paraffin-embedded
HCC tissues from patients who underwent curative resection between
December 2002 and December 2009 at the First Affiliated Hospital of
Fujian Medical University (Fujian, China) were retrieved for
immunohistochemical staining. All patients were diagnosed as
negative for hepatitis C virus [HCV(−)].
The fresh HCC tissues and adjacent tissues were
collected at the time of surgery from patients, and were formalin
fixed and embedded for immunohistochemistry. Clinical information
was collected retrospectively from the electronic record of the
First Affiliated Hospital of Fujian Medical University. The
clinical information collected for each patient included
information on etiological factors, HCC recurrence and metastasis,
mortality (and the cause of mortality) and prognostic
clinicopathological characteristics, including tumor
differentiation stage, presence of vascular invasion, the number of
lesions, the largest tumor size and pre-surgical α-fetoprotein
(AFP) levels. Written consent was obtained from all participants
prior to surgery. The clinical and pathological diagnoses of
patients with HCC met the diagnostic criteria of the American
Association for the Study of Liver Diseases (16). The study was approved by the Ethics
Committee of the Mengchao Hepatobiliary Hospital of Fujian Medical
University (Fujian, China).
Construction of tissue microarrays
(TMAs)
The HCC samples and the adjacent tissues were
collected for the construction of TMAs. Two 1-mm cores were
obtained from the tumorous region and two 1-mm cores were obtained
from the corresponding adjacent tissue region via a manual tissue
arrayer method. The regions of vital tumor and adjacent tissues
were marked by experienced pathologists using a Hematoxylin and
Eosin staining kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) and the examination was performed using a routine
light microscope (Zeiss AG, Oberkochen, Germany). Subsequently, the
wax block (containing HCC tissues) was mixed together with a fresh
wax block at 52°C, cooled at room temperature for 30 min and
maintained at 4°C ready for further use. The cooled wax block was
fixed on an auto tissue slicer and sliced into 15 sections, each
section was 4-µm in thickness. The sections were then immersed in
distilled water for 2 min, and were finally adhered to slides.
Immunohistochemical staining
Immunohistochemical staining of B7-H2 and B7-H3 was
performed using a two-step Elivision plus staining technique.
Briefly, the 4-µm sections on the TMAs were dried at 60°C for 24 h,
de-paraffinized in xylene I for 10 min and de-paraffinized in
xylene II for another 10 min, and then rehydrated in graded ethanol
(100% ethanol for 5 min, 95% ethanol for 3 min, 80% ethanol for 3
min and 75% ethanol for 3 min). The slides were then incubated in 1
mol/l citric acid (OriGene Technologies, Inc., Beijing, China) for
antigen retrieval under 111.6°C and 150 KPa for 3 min, and cooled
to room temperature, followed by three washes with PBS (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA). Subsequently, the slides
were incubated in 3% hydrogen peroxide (OriGene Technologies, Inc.)
for 10 min to block endogenous peroxidase activity and then
rehydrated in PBS. The slides were incubated with primary
antibodies against B7-H2 (1:200 dilution) and B7-H3 (1:1,000
dilution) at 37°C for 1 h, rinsed several times with PBS, and
incubated with intensifier (OriGene Technologies, Inc.) at 37°C for
another 20 min. Following three rinses with PBS, the slides were
incubated with the HRP-conjugated secondary antibodies (goat
anti-mouse IgG, respectively) at 37°C for 30 min. Finally, the
slides were subjected to diaminobenzidine coloration (OriGene
Technologies, Inc.) for 5 min; following rinsing with distilled
water for 3 min, restaining with hematoxylin was performed.
The results were assessed by two double-blinded
pathologists independently. All tissues, at ×200 magnification,
were manually scored using the following criteria: i) According to
the proportion of positive cells (cytoplasm, cell membrane or
nucleus dyed yellow, brown or sepia), scored as 0 (≤5%), 1 (6–25%),
2 (26–50%), 3 (51–75%) and 4 (≥76%); and ii) according to the
intensity of staining, scored as 0 (blue), 1 (faint yellow), 2
(pale brown) and 3 (sepia). The final score was the score of the
first criterion multiplied by that of the second criterion. A final
score of 0–2 was considered as negative expression, whereas a score
of 3–12 was considered as positive expression. If the result of a
specimen contradicted between assessors, then a third pathologist
made the final decision. The histopathological examination was
performed using an orthotopic microscope (Zeiss AG, Oberkochen,
Germany).
Statistical analysis
All statistical analyses were performed using SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Association
analyses of clinicopathological factors was performed with
Pearson's χ2 test. Survival curves were estimated using
the Kaplan-Meier method, and differences in survival rates were
detected using the log-rank test. Univariate and multivariate
analyses were used to identify prognostic factors in the patients
with HCC. Covariates with P<0.05 in univariate analysis were
further analyzed by multivariate analysis. In all tests, P<0.05
was considered to indicate a statistically significant difference.
Receiver operating characteristic (ROC) curves were used to compare
the diagnostic accuracy of the test molecules as prognostic factors
in patients with HCC.
Results
Expression of B7-H2 and B7-H3 in
patients with HCC
To determine the expression of B7-H2 and B7-H3 in
patients with HCC, TMAs were constructed of 63 HCC specimens and
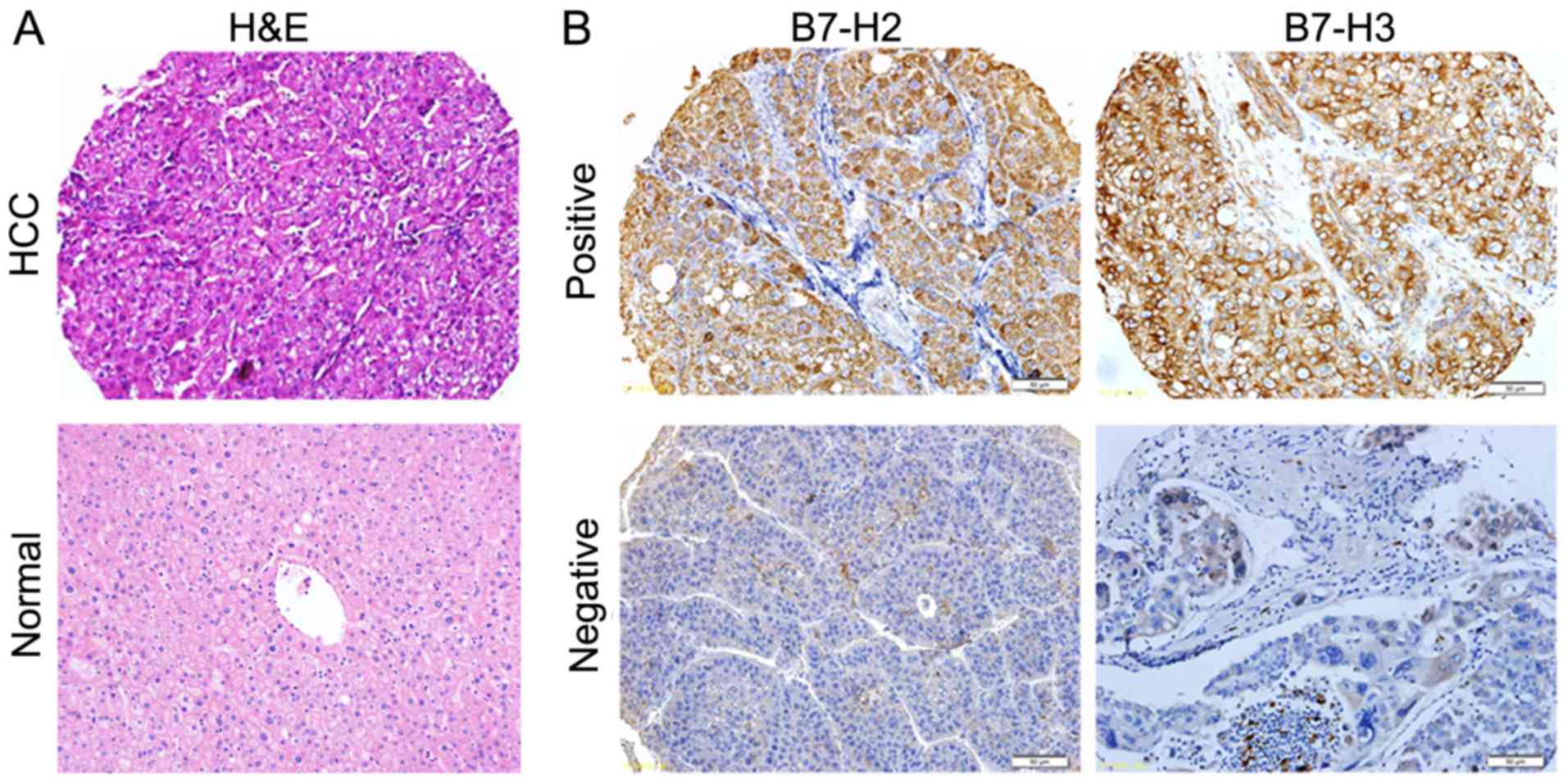
matched adjacent tissues. As presented in Fig. 1, B7-H2 and B7-H3 were positively
expressed in the HCC tissues. Considering the clinical history of
the patients, the association between the expression of B7-H2 or
B7-H3 and various clinical parameters was evaluated. As shown in
Table I, 29/40 cases that recurred
within 1 year were B7-H2+, whereas only 6/23 cases that
recurred after >1 year were B7-H2+ (P<0.01); 39/40
cases that recurred within 1 year were B7-H3+, whereas
only 14/23 cases that recurred after >1 year were
B7-H3+ (P<0.01); 96.0% (24/25) of the patients
presenting with metastasis were positive for B7-H3, whereas the
positive expression rate for non-metastatic cases was 76.3% (29/38;
P=0.036); and 94.3% (33/35) of the patients who had a survival time
of <2 years were positive for B7-H3, whereas the positive
expression rate in patients who had a survival time >2 years was
71.4% (20/28; P=0.016). These results suggested that the positive
expression of B7-H2 was associated with recurrence within 1 year in
patients with HCC, and that the positive expression of B7-H3 was
associated with recurrence within 1 year, metastasis and a survival
time of <2 years in patients with HCC.
 | Table I.Associations between clinical
parameters and the expression of B7-H2 and B7-H3 in patients with
hepatocellular carcinoma. |
Table I.
Associations between clinical
parameters and the expression of B7-H2 and B7-H3 in patients with
hepatocellular carcinoma.
|
| B7-H2 |
|
| B7-H3 |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Clinical
parameter | − | + |
χ2-value | P-value | − | + |
χ2-value | P-value |
|---|
| Sex |
|
| 1.210 |
|
|
| 0.078 | 0.627 |
|
Male | 23 | 32 |
|
| 9 | 46 |
|
|
|
Female | 5 | 3 |
|
| 1 | 7 |
|
|
| Age (years) |
|
| 0.556 | 0.332 |
|
| 1.027 | 0.289 |
|
>60 | 5 | 9 |
|
| 1 | 13 |
|
|
|
≤60 | 23 | 26 |
|
| 9 | 40 |
|
|
| Size (cm) |
|
| 0.423 | 0.350 |
|
| 1.189 | 0.230 |
| ≤5 | 11 | 11 |
|
| 5 | 17 |
|
|
|
>5 | 17 | 24 |
|
| 5 | 36 |
|
|
|
Differentiation |
|
| 0.944 | 0.624 |
|
| 2.772 | 0.153 |
|
Poor | 10 | 9 |
|
| 2 | 17 |
|
|
|
Well | 12 | 19 |
|
| 4 | 27 |
|
|
|
Moderate | 6 | 7 |
|
| 4 | 9 |
|
|
| AFP (µg/l) |
|
| 0.213 | 0.437 |
|
| 1.310 | 0.238 |
|
<200 | 6 | 9 |
|
| 1 | 14 |
|
|
|
>200 | 22 | 25 |
|
| 9 | 38 |
|
|
| Liver
cirrhosis |
|
| 0.001 | 0.644 |
|
| 1.981 | 0.177 |
| − | 4 | 5 |
|
| 0 | 9 |
|
|
| + | 24 | 30 |
|
| 10 | 44 |
|
|
| HBV infection |
|
| 1.210 | 0.344 |
|
| 0.078 | 0.627 |
| − | 5 | 3 |
|
| 1 | 7 |
|
|
| + | 23 | 32 |
|
| 9 | 46 |
|
|
| TNM stage |
|
| 0.082 | 0.489 |
|
| 0.374 | 0.392 |
|
I–II | 17 | 20 |
|
| 5 | 32 |
|
|
|
III–IV | 11 | 15 |
|
| 5 | 21 |
|
|
| Recurrence |
|
| 12.740 |
0.007b |
|
| 14.674 |
<0.001b |
| <1
year | 11 | 29 |
|
| 1 | 39 |
|
|
| >1
year | 17 | 6 |
|
| 9 | 14 |
|
|
| Metastasis |
|
| 0.004 | 0.582 |
|
| 2.594 |
0.036a |
| − | 18 | 20 |
|
| 9 | 29 |
|
|
| + | 10 | 15 |
|
| 1 | 24 |
|
|
| Survival |
|
| 3.291 | 0.059 |
|
| 6.086 |
0.016a |
| <2
year | 12 | 23 |
|
| 2 | 33 |
|
|
| >2
year | 16 | 12 |
|
| 8 | 20 |
|
|
Positive expression of B7-H2 and B7-H3
is associated with poor prognosis in HCC
To evaluate the association between the expression
of B7-H2 or B7-H3 and the prognosis of HCC, the expression data on
B7-H2 and B7-H3 were analyzed further by considering
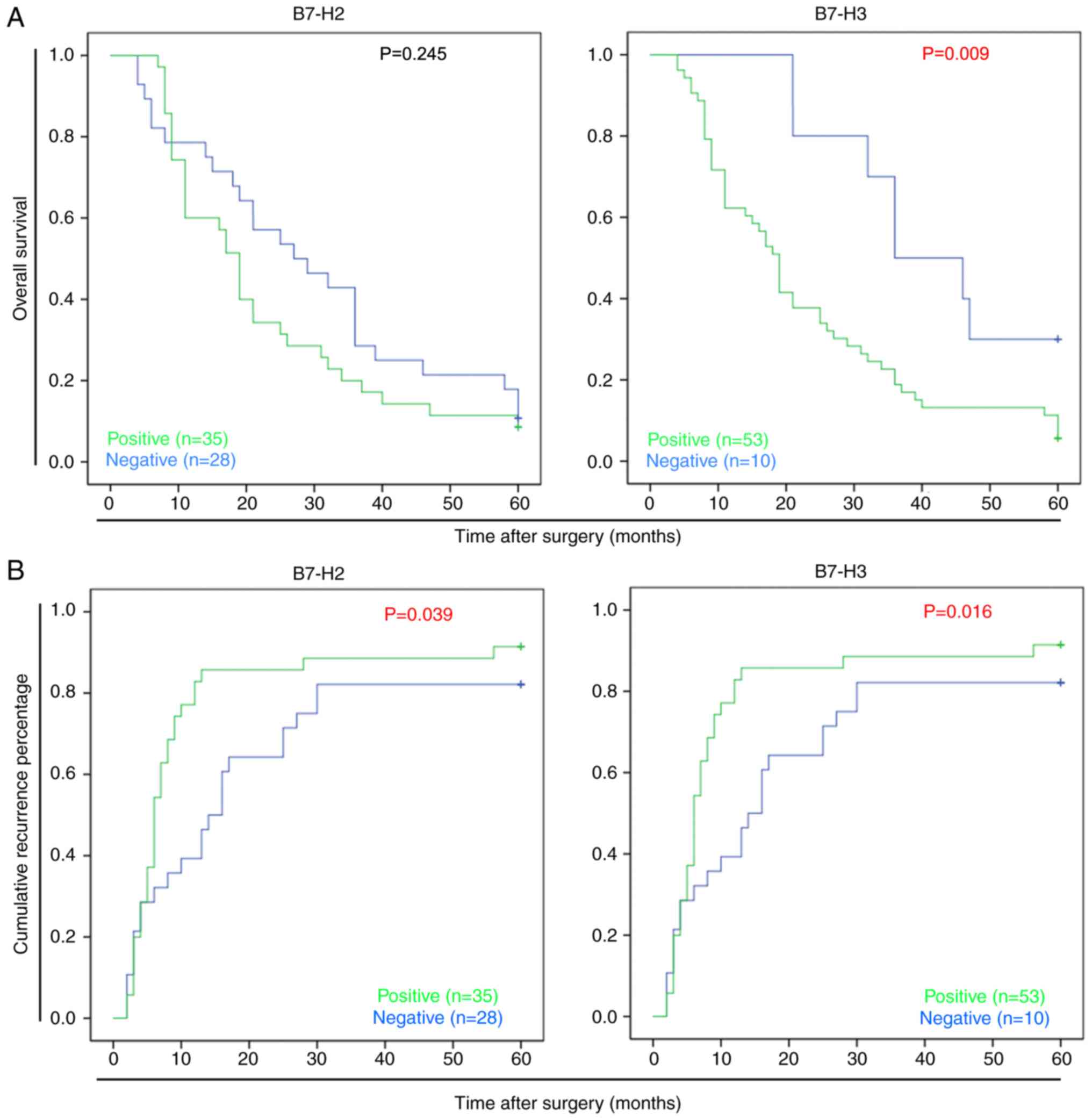
disease-related survival events. As shown in Fig. 2A and B, a Kaplan-Meier plot
indicated that the B7-H3− patients had significantly
longer survival rates following surgery than the B7-H3+
patients (P=0.009); the median survival time of the
B7-H3+ patients was 19.2 months compared with 37.3
months for the B7-H3− patients. In addition, it was
identified that B7-H3 may promote HCC recurrence; the median
disease-free survival time of the B7-H3+ patients was
5.9 months, compared with 18.1 months for the B7-H3−
patients (P=0.016). Similar results were observed in the
B7-H2+ patients, for whom the median disease-free
survival time was 6.8 months, compared with 12.6 months for the
B7-H2− patients (P=0.039). These data suggested that the
positive expression of B7-H2 or B7-H3 was indicative of poor
survival rates in patients with HCC.
Positive expression of B7-H2 and B7-H3
is associated with extrahepatic metastasis and early
recurrence/metastasis
To further determine the prognostic factors affected
by B7-H2 and B7-H3, the associations between the expression of
B7-H2 or B7-H3 and postoperative clinical features were assessed.
As shown in Table II,
extrahepatic metastasis was associated with positive expression of
B7-H2 (P=0.041) and B7-H3 (P=0.036). Specifically, 25 patients
exhibited HCC metastasis, 17 of whom exhibited pulmonary metastasis
in B7-H3+ patients (68%). In addition, time of
recurrence in patients with HCC was analyzed, and the expression of
B7-H2 or B7-H3 was assessed in the 23 cases of later
recurrence/metastasis (>12 months) and the 40 cases of early
recurrence/metastasis (≤12 months). As shown in Table III, positive expression of B7-H2
was detected in 29 of the ≤12 months cases (29/40, 72.5%), and
positive expression of B7-H3 was detected in 39 of the ≤12 months
cases (39/40, 97.5%). Notably, lower expression rates of B7-H2
(26.09%, P=0.007) and B7-H3 (60.86%, P=0.001) were observed in the
>12 months cases than in the ≤12 months cases. These results
indicated that the positive expression of B7-H2 or B7-H3 was linked
with susceptibility to extrahepatic metastasis and early
recurrence/metastasis (within 1 year).
 | Table II.Associations between postoperative
clinical features and expression of B7-H2 and B7-H3 in patients
with hepatocellular carcinoma. |
Table II.
Associations between postoperative
clinical features and expression of B7-H2 and B7-H3 in patients
with hepatocellular carcinoma.
|
| B7-H2
expression |
| B7-H3
expression |
|
|---|
|
|
|
|
|
|
|---|
| Clinical
feature | − | + | P-value | − | + | P-value |
|---|
| Recurring type |
|
| NS |
|
| NS |
|
Regional recurring | 6 | 6 |
| 2 | 10 |
|
| Distant
recurring | 11 | 18 |
| 4 | 25 |
|
| Histological
grade |
|
| NS |
|
| NS |
|
Well-differentiated | 5 | 6 |
| 3 | 8 |
|
|
Moderately differentiated | 13 | 19 |
| 5 | 27 |
|
| Poorly
differentiated | 10 | 10 |
| 2 | 18 |
|
| Extrahepatic
metastasis |
|
| 0.041a |
|
| 0.036a |
|
Yes | 8 | 17 |
| 1 | 24 |
|
| No | 20 | 18 |
| 9 | 29 |
|
 | Table III.Associations between the time of
postoperative R/M and the expression of B7-H2 and B7-H3 in patients
with hepatocellular carcinoma. |
Table III.
Associations between the time of
postoperative R/M and the expression of B7-H2 and B7-H3 in patients
with hepatocellular carcinoma.
|
| B7-H2
expression |
| B7-H3
expression |
|
|---|
|
|
|
|
|
|
|---|
| R/M time | N | − | + | Positive expression
rate (%) | P-value | N | − | + | Positive expression
rate (%) | P-value |
|---|
| ≤12 months | 40 | 11 | 29 | 72.50 |
0.007a | 40 | 1 | 39 | 97.50 |
0.001a |
| >12 months | 23 | 17 | 6 | 26.09 |
| 23 | 9 | 14 | 60.87 |
|
Expression of B7-H2 is associated with
tumor capsule presence
To further determine the association of potential
tumor features with the expression of B7-H2 and B7-H3, associations
were analyzed using Pearson's χ2 test. As shown in
Table IV, the negative expression
of B7-H2 in HCC tissues was significantly associated with tumor
capsule presence (P=0.046). Additionally, larger tumor size and
faster progression of liver cirrhosis were more frequently observed
in the B7-H2- and B7-H3-positive expression groups, although not to
statistical significance. These data suggested that the positive
expression of B7-H2 was associated with the invasive features of
HCC, as the absence of tumor encapsulation is more likely to lead
to a further intrahepatic recurrence or metastasis.
 | Table IV.Associations between tumor features
and the expression of B7-H2 and B7-H3 in patients with
hepatocellular carcinoma. |
Table IV.
Associations between tumor features
and the expression of B7-H2 and B7-H3 in patients with
hepatocellular carcinoma.
|
| B7-H2
expression |
| B7-H3
expression |
|---|
|
|
|
|
|
|---|
| Tumor feature | − | + | P-value | − | + | P-value |
|---|
| Progression of
cirrhosis |
|
| NS |
|
| NS |
|
Normal/early-stage | 26 | 33 |
| 9 | 50 |
|
|
Advanced-stage | 2 | 2 |
| 1 | 3 |
|
| Dissemination to
regional lymph nodes |
|
| NS |
|
| NS |
|
Yes | 3 | 1 |
| 1 | 3 |
|
| No | 25 | 34 |
| 3 | 50 |
|
| Tumor capsule |
|
|
0.046a |
|
| NS |
|
Absent | 23 | 34 |
| 9 | 48 |
|
|
Present | 5 | 1 |
| 1 | 5 |
|
| Tumor
boundaries |
|
| NS |
|
| NS |
|
Distinct | 24 | 32 |
| 10 | 46 |
|
|
Indistinct | 4 | 3 |
| 0 | 7 |
|
| PVTT |
|
| NS |
|
| NS |
|
Yes | 16 | 15 |
| 6 | 25 |
|
| No | 12 | 20 |
| 4 | 28 |
|
| Intraoperative
ascites |
|
| NS |
|
| NS |
|
Yes | 7 | 8 |
| 2 | 13 |
|
| No | 21 | 27 |
| 8 | 40 |
|
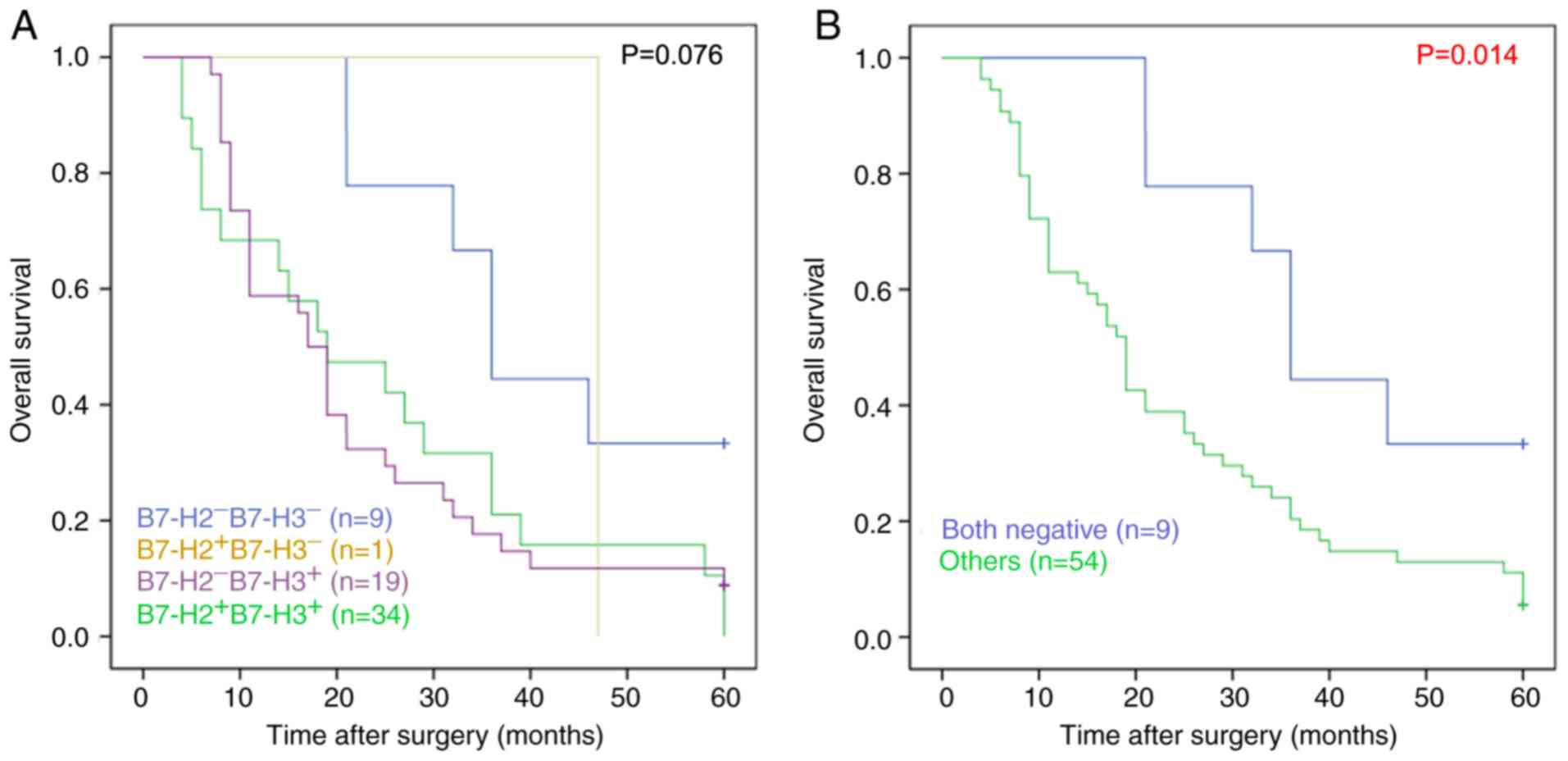
Combined expression of B7-H2 and B7-H3
is associated with recurrence and survival rates
Considering the similar expression characteristics
of B7-H2 and B7-H3 in patients with HCC, the associations between
clinical parameters and the combined expression of B7-H2 and B7-H3
were further investigated. As shown in Table V, the
B7-H2+/B7-H3+ patients were more likely to
exhibit recurrence within 1 year compared with the
B7-H2−/B7-H3− patients (P<0.001); and the
2-year survival rate of the B7-H2+/B7-H3+
patients was 38.9%, which was lower than that of the
B7-H2−/B7-H3− patients (77.8%; P=0.035).
Kaplan-Meier analysis further suggested that the overall survival
rate of the B7-H2−/B7-H3− patients was
significantly higher than that of the
B7-H2+/B7-H3−,
B7-H2−/B7-H3+ and
B7-H2+/B7-H3+ patients (P=0.014; Fig. 3A and B). Therefore, these data
suggested that the combined expression of B7-H2 and B7-H3 was
associated with recurrence and poor survival rate.
 | Table V.Associations between clinical
parameters and the combined expression of B7-H2 and B7-H3 in
patients with hepatocellular carcinoma. |
Table V.
Associations between clinical
parameters and the combined expression of B7-H2 and B7-H3 in
patients with hepatocellular carcinoma.
|
| Combination of
B7-H2 and B7-H3 |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
parameter | Both negative
(n) | Other (n) |
χ2-value | P-value |
|---|
| Sex |
|
| 0.064 | 0.680 |
|
Male | 8 | 47 |
|
|
|
Female | 1 | 7 |
|
|
| Age (years) |
|
| 0.075 | 0.354 |
|
>60 | 1 | 13 |
|
|
|
<60 | 8 | 41 |
|
|
| Size (cm) |
|
| 0.419 | 0.384 |
|
<5 | 4 | 18 |
|
|
|
>5 | 5 | 36 |
|
|
|
Differentiation |
|
| 1.088 | 0.580 |
|
Poor | 2 | 17 |
|
|
|
Well | 4 | 27 |
|
|
|
Moderate | 3 | 10 |
|
|
| AFP (µg/l) |
|
| 3.360 | 0.067 |
|
<200 | 0 | 15 |
|
|
|
>200 | 9 | 38 |
|
|
| Liver
cirrhosis |
|
| 1.750 | 0.225 |
| − | 0 | 9 |
|
|
| + | 9 | 45 |
|
|
| HBV infection |
|
| 0.024 | 0.680 |
| − | 1 | 7 |
|
|
| + | 8 | 47 |
|
|
| TNM stage |
|
| 0.884 | 0.280 |
|
I–II | 4 | 33 |
|
|
|
III–IV | 5 | 21 |
|
|
| Recurrence |
|
| 18.261 |
<0.001b |
| <1
year | 0 | 40 |
|
|
| >1
year | 9 | 14 |
|
|
| Metastasis |
|
| 2.063 | 0.146 |
| − | 8 | 35 |
|
|
| + | 1 | 19 |
|
|
| Survival |
|
| 4.725 |
0.035a |
| <2
years | 2 | 33 |
|
|
| >2
years | 7 | 21 |
|
|
Combined expression of B7-H2 and B7-H3
is associated with prognostic factors
In order to determine the association of combined
expression of B7-H2 and B7-H3 with prognosis, multivariate and
univariate analyses were performed. As shown in Table VI, tumor size (P<0.001) and
differentiation (P=0.003), tumor capsule presence (P=0.022) and,
most notably, the combined expression of B7-H2 and B7-H3 (P=0.022)
were significantly associated with overall survival rate, which
indicated that the combined expression of B7-H2 and B7-H3 was among
those factors associated with poor overall survival rate.
 | Table VI.Analysis of prognostic factors based
on Cox's proportional hazards model at 5 years of follow-up. |
Table VI.
Analysis of prognostic factors based
on Cox's proportional hazards model at 5 years of follow-up.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Clinical
parameter | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male/female | 0.611 | 0.274–1.360 | 0.227 |
|
|
|
| Tumor size |
|
|
|
|
|
|
| <5
cm/>5 cm | 2.982 |
1.662–5.349 |
<0.001b | 4.343 |
2.153–8.763 |
<0.001b |
|
Differentiation |
|
|
|
|
|
|
|
Poor/moderate/well | 0.578 |
0.403–0.829 |
0.003b | 0.576 |
0.385–0.863 |
0.007b |
| TNM stage |
|
|
|
|
|
|
|
I–II/III–IV | 1.661 | 0.972–2.836 | 0.063 |
|
|
|
| Tumor capsule |
|
|
|
|
|
|
|
Present/absent | 0.367 |
0.156–0.864 |
0.022a | 0.376 |
0.146–0.970 |
0.043a |
| B7-H2 and B7-H3
combination |
|
|
|
|
|
|
| Both
low/other | 2.703 |
1.155–6.328 |
0.022a | 6.784 |
2.522–18.245 |
<0.001b |
Combined expression of B7-H2 and B7-H3
increases sensitivity and specificity in predicting HCC
prognosis
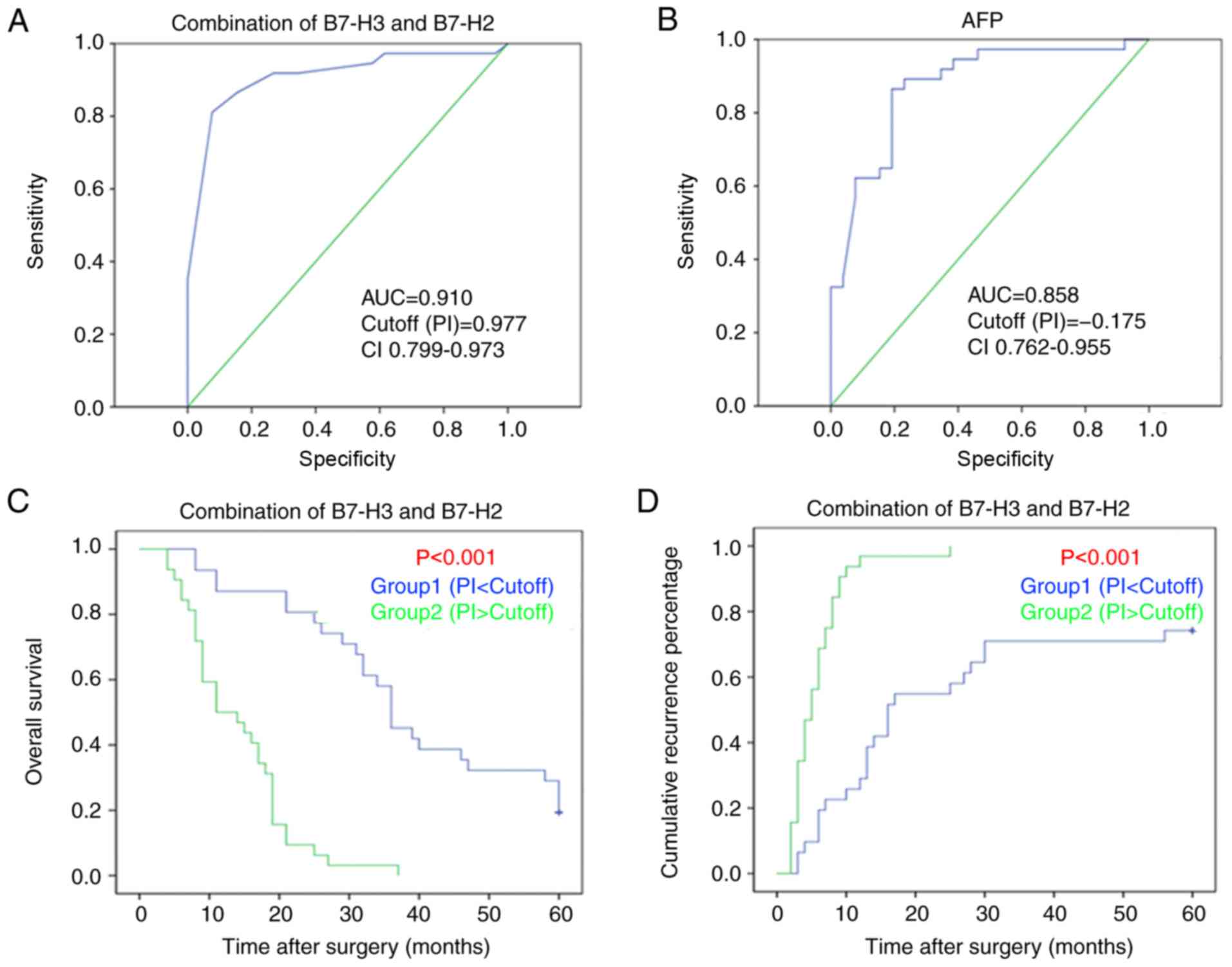
On the basis of the results on associations with
prognostic factors, ROC curves were evaluated for prognostic
indicator (PI). PI was calculated as follows: PI = (β1 × score of
combined B7-H2 and B7-H3) + (β2 × score of tumor size) + (β3 ×
score of tumor differentiation) + (β4 × score of tumor capsule).
The values of β1–4 represented the regression coefficients of the
these variables from the COX regression model. To further determine
the sensitivity and specificity of combined expression in
predicting 2-year survival rate, the recognized indicator of HCC,
AFP, was used as the control. As shown in Fig. 4A and B, the area under the curve
(AUC) for the combined expression of B7-H2 and B7-H3 was 0.910 [95%
confidence interval (CI), 0.833–0.986, P<0.001], whereas the AUC
for the AFP model was 0.858 (95% CI, 0.762–0.955, P<0.001),
indicating greater sensitivity and specify for predicting
HCC-related survival with use of the combined expression of B7-H2
and B7-H3. In addition, the samples were re-divided into two groups
according to the value of PI: Group 1 [PI<Cutoff (0.977)] and
Group 2 [PI>Cutoff (0.977)] to further confirm the prediction of
HCC-related survival and recurrence rates following the use of the
combined expression of B7-H2 and B7-H3. As shown in Fig. 4C and D, the combination of B7-H2
and B7-H3 predicted poor overall survival and recurrence rates of
HCC. Therefore, these data demonstrated that the combined
expression of B7-H2 and B7-H3 may be used to enhance the
sensitivity and specificity of predicting prognosis in HCC.
Discussion
A large proportion of patients with HCC present with
intrahepatic recurrence or extra-hepatic metastasis within 5 years
following curative liver resection performed according to the Milan
criteria (17), and 50–70% of the
recurrence/metastasis occurs within 1 year following the surgical
procedure (18,19); the median survival time following
presentation of recurrence/metastasis is only 13 months (20). Therefore, the determination of
novel prognostic biomarkers is urgently required in order to
enhance the prediction of the disease course of HCC at diagnosis.
Previous findings have suggested that disorder in co-stimulatory or
co-inhibitory signaling pathways serves a critical role in tumor
cell evasion of adaptive immune surveillance (21–24).
In particular, the dysregulation of B7-H2 and/or B7-H3 may
deregulate T lymphocyte function in the HCC microenvironment, and
even promote recurrence following surgery in patients with HCC
(8,15). Considering the apparently similar
pathological expression profiles of B7-H2 and B7-H3 in HCC, it was
logical to investigate the combined expression of B7-H2 and B7-H3
in the same set of patients with HCC to assess whether this
provided enhanced prediction of HCC prognosis. On evaluating the
expression of B7-H2 and B7-H3 in the same group of patients with
HCC, it was determined that the combination of B7-H2 and B7-H3 may
serve as a potential prognostic indicator for the prediction of
disease-associated survival events post-liver resection in HCC.
Although B7-H3 and B7-H2 are abundantly expressed in
patients with HCC and have been indicated to possess similar immune
regulation effects in the progression of HCC, there are several
differences in their prognostic value in HCC. In the present study,
the B7-H3+ patients were more likely to develop
pulmonary metastasis (17/25, 68%), whereas the B7-H2+
patients were associated with absence of a tumor capsule,
indicating an application in predicting intrahepatic recurrence or
metastasis. However, it was determined that both B7-H3 and B7-H2
were associated with prognostic factors, including recurrence
(within 1 year), metastasis and survival (within 2 years),
indicating the accordance of B7-H3 and B7-H2 for predicting
prognosis in HCC. On the basis of these results, the subsequent aim
was to evaluate the associations between the combined expression of
B7-H3 and B7-H2 and prognostic factors. As expected, it was
identified that the combined expression of B7-H3 and B7-H2 was also
associated with prognostic factors, including recurrence (within 1
year) and survival (within 2 years). Therefore, the combination of
B7-H3 and B7-H2 may provide a novel prognostic biomarker in
HCC.
Several important risk factors of HCC have been
identified, including chronic hepatitis B virus (HBV) infection,
chronic HCV, nonalcoholic steatohepatitis and excessive consumption
of alcohol; however, the heterogeneity and the geographic
variability of the incidence of HCC have been widely associated to
the different distribution of HCV and HBV infections worldwide
(25). In particular, it has been
suggested that HBV accounts for ~80% of virus-infective HCC cases
globally and is the main causal factor in regions with a high
incidence of HCC, particularly in Africa and East Asia; whereas HCV
is a major etiological factor of HCC in regions with a low
incidence, including America and Western Europe (25–28).
Considering chronic infections with HBV and HCV are key risk
factors for patients with HCC, the association of the expression of
B7-H3/B7-H2 with HBV and HCV was examined. In the present study,
although there was no significant association between the
expression of B7-H2/B7-H3 and HBV infection, it remained unclear
whether the expression of B7-H3/B7-H2 was associated with the
prognosis of HCV(+) patients with HCC, as all cases in the present
study were HCV(−). Further investigations are necessary to
ascertain this information.
In view of the fact that diabetes mellitus is
another risk factor for patients with HCC, the association between
the expression of B7-H3/B7-H2 and body mass index (BMI) was also
investigated in the present study. Although there was no
significant association between the expression of B7-H2/B7-H3 and
BMI, whether the expression of B7-H3/B7-H2 was associated with
other diabetes-related factors, including insulin resistance and
expression of SH2 domain-containing inositol phosphatase 2, in
patients with HCC remains unclear. Previously, it has been
suggested that BMI and insulin resistance are risk factors for
patients with HCC with HCV infection (27). In particular, an antidiabetic agent
exhibited efficacy in reducing the spontaneous regression of HCC
(28). Further investigations are
necessary to determine the link between the expression of
B7-H2/B7-H3 and diabetes mellitus in HCC, particularly in those
patients with HCV infection.
At present, AFP, having been established as an
indicator for HCC, is widely used in the diagnosis of HCC. However,
its specificity and sensitivity are below what is usually required
in clinical practice, with the result being that it is responsible
for a high rate of misdiagnosis in HCC. More recently, combination
with B7 family molecules has been used to enhance the sensitivity
and specificity of prediction. Previous studies have determined
that the combination of B7-H3 and B7-H4 may be used as a novel
prognostic marker for esophageal cancer (29), and that T lymphocytes plus
enoblituzumab (B7-H3 antibody) can inhibit tumor growth in renal
and bladder carcinoma xenografts (30). Furthermore, the B7-H2 antibody
JTX-2011 has shown success in the phase 1 ICONIC clinical trial
(NCT02904226) (31). Therefore, in
the present study, ROC analysis was used to evaluate the prediction
value of the combination of B7-H3 and B7-H2. Notably, an increased
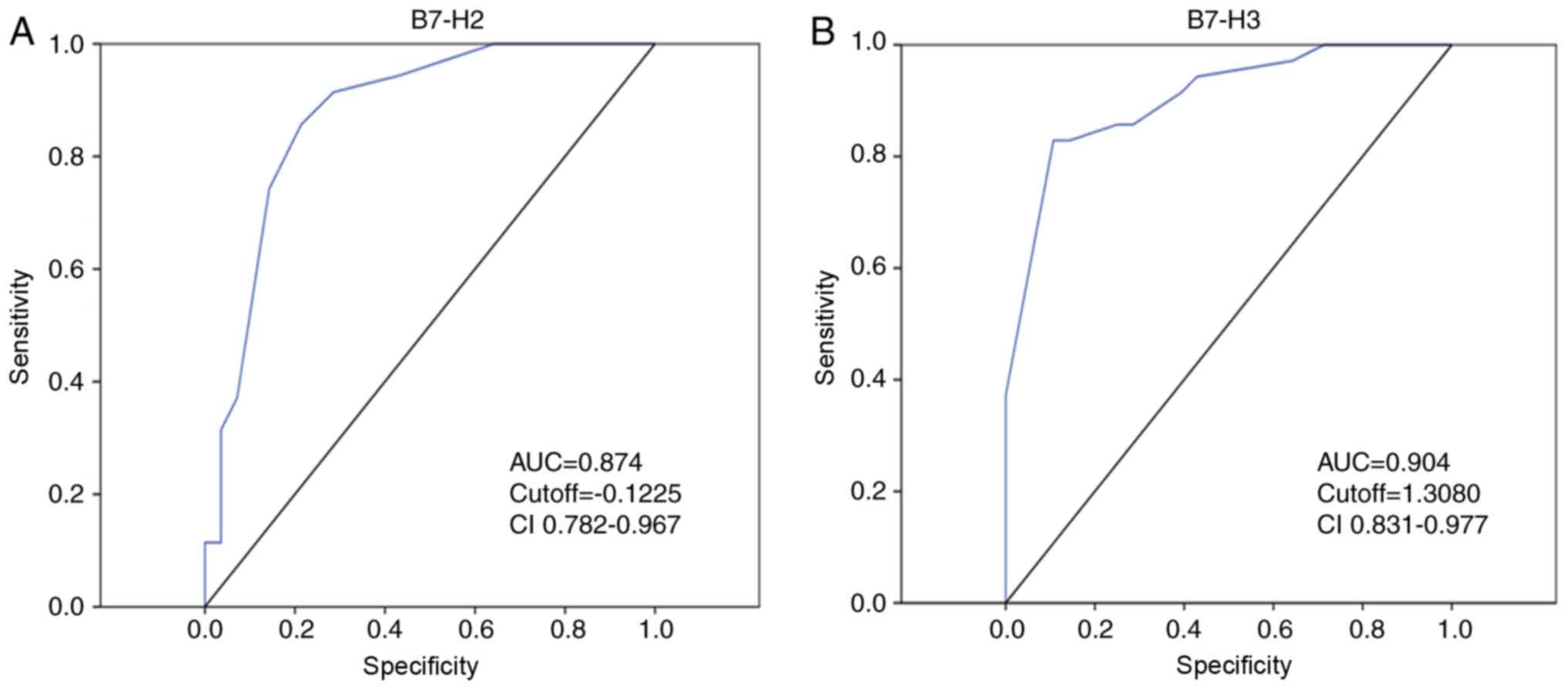
AUC was observed for B7-H3 or B7-H2 alone compared with that for
AFP alone (Fig. 5A and B),
suggesting greater specificity and sensitivity for predicting
prognosis in HCC. More notably, the higher specificity and
sensitivity was further improved using the combination of B7-H3 and
B7-H2.
To conclude, TMA-based immunohistochemistry staining
was applied in the present large-scale cohort study, and it was
observed that combined expression of B7-H2 and B7-H3 was
significantly associated with the prognosis of HCC. Based on these
findings, the combination of B7-H2 and B7-H3 may be a potential
prognostic biomarker for predicting recurrence and overall survival
rates in HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81472830, 31600616 and
81672376), the Natural Science Foundation of Fujian Province (grant
nos. 2017J 05142 and 2016J01417), the Youth Research Project of
Fujian Provincial Health and Family Planning Commission (grant no.
2018-1-93), the Scientific Foundation of Fuzhou Health Department
(grant no. 2015-s-143-19), the Medical Innovation Project of Fujian
Province (grant no.2016-CX-48) and the Fuzhou Health and Family
Planning Science and Technology Project (grant no.2017-S-wt2).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
YZ and NL were involved in study design and drafted
the manuscript. YWu and JG were involved in TMA. ZL, WL and YWa
were involved in the statistical analysis. ML, XL and LC were
involved in clinical data collection. WZ and BZ were involved in
the study design, financial support and proof-reading of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of the Mengchao Hepatobiliary Hospital of Fujian Medical
University. Patients provided informed consent and agreed to the
use of their tissues for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z,
Yang L, Chen Y, Hu Z, Chen Z, et al: Cancer incidence and
mortality: A cohort study in China, 2008–2013. Int J Cancer.
141:1315–1323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zheng RS and Zhang SW: Liver
cancer incidence and mortality in China, 2009. Chin J Cancer.
32:162–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A and Bruix J: Biomarkers for early
diagnosis of hepatocellular carcinoma. Lancet Oncol. 13:750–751.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braillon A: Hepatocellular carcinoma.
Lancet. 380:469author reply 470, 471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Worns MA and Galle PR: Immune oncology in
hepatocellular carcinoma-hype and hope. Lancet. 389:2448–2449.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Z, Shen J, Wang MH, Yi T, Yu Y, Zhu Y,
Chen B, Chen J, Li L, Li M, et al: Comprehensive molecular
profiling of the B7 family of immune-regulatory ligands in breast
cancer. Oncoimmunology. 5:e12078412016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong B, Qian Y, Zhang H, Sang YW, Cheng
LF, Wang Q, Gao S, Zheng M and Yao HP: Expression of B7-H4 and
hepatitis B virus X in hepatitis B virus-related hepatocellular
carcinoma. World J Gastroenterol. 22:4538–4546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang FB, Wang L, Jia HC, Li D, Li HJ,
Zhang YG and Sun DX: B7-H3 promotes aggression and invasion of
hepatocellular carcinoma by targeting epithelial-to-mesenchymal
transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int.
15:452015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung J and Suh WK: The CD28-B7 Family in
anti-tumor immunity: Emerging concepts in cancer immunotherapy.
Immune Netw. 14:265–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao Y, Chen L, Wang F, Zhu D, Ge X, Hua D
and Sun J: Cancer cell-expressed B7-H3 regulates the
differentiation of tumor-associated macrophages in human colorectal
carcinoma. Oncol Lett. 6177–6183. 2017.PubMed/NCBI
|
|
12
|
Jung HI, Jeong D, Ji S, Ahn TS, Bae SH,
Chin S, Chung JC, Kim HC, Lee MS and Baek MJ: Overexpression of
PD-L1 and PD-L2 is associated with poor prognosis in patients with
hepatocellular carcinoma. Cancer Res Treat. 49:246–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C1, Costentin C, Luciani A, Zafrani ES, Laurent A,
Azoulay D, et al: Programmed death ligand 1 expression in
hepatocellular carcinoma: Relationship with clinical and
pathological features. Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vigdorovich V, Ramagopal UA, Lazar-Molnar
E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG and Almo
SC: Structure and T cell inhibition properties of B7 family member,
B7-H3. Structure. 21:707–717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu JF, Ding YH, Ying XH, Wu FZ, Zhou XM,
Zhang DK, Zou H and Ji JS: Regulatory T cells, especially ICOS+
FOXP3+ regulatory T cells, are increased in the hepatocellular
carcinoma microenvironment and predict reduced survival. Sci Rep.
6:350562016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pahwa A, Beckett K, Channual S, Tan N, Lu
DS and Raman SS: Efficacy of the American association for the study
of liver disease and Barcelona criteria for the diagnosis of
hepatocellular carcinoma. Abdom Imaging. 39:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang M, Jayakrishnan TT, Green DE, George
B, Thomas JP, Groeschl RT, Erickson B, Pappas SG, Gamblin TC and
Turaga KK: Systematic review of outcomes of patients undergoing
resection for colorectal liver metastases in the setting of extra
hepatic disease. Eur J Cancer. 50:1747–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng KM, Yan TD, Black D, Chu FC and Morris
DL: Prognostic determinants for survival after resection/ablation
of a large hepatocellular carcinoma. HPB (Oxford). 11:311–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi GH, Han DH, Kim DH, Choi SB, Kang CM,
Kim KS, Choi JS, Park YN, Park JY, Kim DY, et al: Outcome after
curative resection for a huge (>or=10 cm) hepatocellular
carcinoma and prognostic significance of gross tumor
classification. Am J Surg. 198:693–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH
and Ahn CS: Outcome of patients with huge hepatocellular carcinoma
after primary resection and treatment of recurrent lesions. Br J
Surg. 94:320–326. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wang YR, Dingi GH, Yang TS, Jiang
SL, Wang L, Xun LJ, Song RM, Song ZS and Zhou B: Influence of
surgical resection of hepatocellular carcinoma(HCC) for
hematogenous dissemination of HCC cells and its effect on
recurrence and metastasis: 3 years prospective study. Neoplasma.
62:635–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu B, Cai Z, Zeng Y, Chen L, Du X, Huang
A, Liu X and Liu J: Alpha-Methylacyl-CoA racemase (AMACR) serves as
a prognostic biomarker for the early recurrence/metastasis of HCC.
J Clin Pathol. 67:974–979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang YF, Yang ZH and Hu JQ: Recurrence or
metastasis of HCC: Predictors, early detection and experimental
antiangiogenic therapy. World J Gastroenterol. 6:61–65. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang TW, Yevsa T, Woller N, Hoenicke L,
Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova
A, et al: Senescence surveillance of pre-malignant hepatocytes
limits liver cancer development. Nature. 479:547–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petruzziello A: Epidemiology of hepatitis
B virus (HBV) and hepatitis C virus (HCV) related hepatocellular
carcinoma. Open Virol J. 12:26–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu CJ and Kao JH: Hepatitis B
virus-related hepatocellular carcinoma: Epidemiology and pathogenic
role of viral factors. J Chin Med Assoc. 70:141–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blachier M, Leleu H, Peck-Radosavljevic M,
Valla DC and Roudot-Thorav F: The burden of liver disease in
Europe: A review of available epidemiological data. J Hepatol.
58:593–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sumie S, Kawaguchi T, Komuta M, Kuromatsu
R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N, et
al: Significance of glucose intolerance and SHIP2 expression in
hepatocellular carcinoma patients with HCV infection. Oncol Rep.
18:545–552. 2007.PubMed/NCBI
|
|
29
|
Kawaguchi T, Nakano D, Okamura S, Shimose
S, Hayakawa M, Niizeki T, Koga H and Torimura T: Spontaneous
regression of hepatocellular carcinoma with reduction in
angiogenesis-related cytokines after treatment with sodium-glucose
cotransporter 2 inhibitor in a cirrhotic patient with diabetes
mellitus. Hepatol Res. 2018 Sep;4.Doi: 10.1111/hepr.13247.
|
|
30
|
Chen L, Xie Q, Wang Z, Shi L, Wu C and
Jiang J: Assessment of combined expression of B7-H3 and B7-H4 as
prognostic marker in esophageal cancer patients. Oncotarget.
7:77237–77243. 2016.PubMed/NCBI
|
|
31
|
Burris HA, Callahan MK, Tolcher AW, Kummar
S, Falchook GS, Pachynski RK, Tykodi SS, Gibney GT, Seiwert TY,
Gainor JF, et al: Phase 1 safety of ICOS agonist antibody JTX-2011
alone and with nivolumab (nivo) in advanced solid tumors; predicted
vs. observed pharmacokinetics (PK) in ICONIC. J Clin Oncol.
15:3033. 2017. View Article : Google Scholar
|