Introduction
Prostate cancer (PC) is among the top 10 cancer
types worldwide in terms of its high rates of prevalence and
mortality. Recent research has indicated that the estimated
mortalities associated with PC are higher in comparison with any
other cancer type, excluding lung carcinoma (1). PC is typically treated with a
combination of prostatectomy, radiation, chemotherapeutics and
androgen ablation (2). However,
the occurrence of distant invasion and migration of PC result in
poor prognosis (3). At present,
there is increasing research regarding PC invasion and migration,
which are the major malignant properties of PC and main causes of
mortality in patients (4–6).
MicroRNAs (miRs) are a class of small non-coding
RNAs (7). The update of the
miRBase in 2011, a biological database of miR sequences, currently
includes >30,000 known mature miR sequences described in 9
species (8). It is well
established that ectopic miR expression is strongly associated with
tumorigenesis. miRs may be oncogenic or tumor suppressors, and have
been widely reported as potential targets for cancer molecular
therapies (9). Furthermore, miRs
may be used as biomarkers or therapeutic targets for the diagnosis
and prognosis of multiple types of cancer. Studies on PC invasion
and metastasis have revealed a pivotal role of miRs during these
processes, as miRs post-transcriptionally regulate a variety of
invasion or metastasis-associated oncogenes (10,11).
The functional implication of miR-9 in evolution is
demonstrated by its conservation at the nucleotide level in several
different species, and it is known that the mature miR-9 sequence
is identical in insects and humans (12). miR-9 has been extensively
investigated in various biological processes, including neural
progenitor cell proliferation and migration (13), neuronal differentiation (14), apoptotic cell death (15), brain function, neurodegeneration
(16) and cancer (17). Aberrant miR-9 expression occurs in
several types of cancer, suggesting that miR-9 is involved in
tumorigenesis and cancer progression. However, through regulation
of various mRNA targets, miR-9 may have divergent effects on the
proliferation of various cancer cells (18–21).
Although numerous functions of miR-9 have been reported, the
mechanisms underlying its oncogenic or tumor suppressor functions
have yet to be fully revealed.
As a major signaling pathway participating in signal
transduction from the plasma to the nucleus, mitogen-activated
protein kinases (MAPKs) respond to cellular stressors and various
growth factors to mediate signaling (22). Mitogen-activated protein kinase
kinase kinase 3 (MEKK3), also known as MAP3K3, belongs to the MAPK
family. MEKK3 is a kinase that phosphorylates the serine or
threonine of c-Jun N-terminal kinase (JNK), p38 and extracellular
signal-regulated kinase (ERK). MEKK3 is an upstream mediator of
MAPK pathways regulating cell proliferation, tumorigenesis,
apoptosis and inflammatory responses (23). Recently, the ability of MEKK3 to
promote the transformation of breast epithelial cells has been
revealed, suggesting that it functions as an oncogene (24). However, while MEKK3 is essential
for endothelial function, it is not critical for tumorigenesis and
angiogenesis (25). Currently, the
role of MEKK3 in PC development and its underlying molecular
mechanism in tumorigenesis remain unclear.
The aim of the present study was to investigate the
effect of miR-9 on PC development. It was demonstrated that
alteration of miR-9 expression in different PC cells influenced
their proliferation and migration in vitro and in
vivo. In addition, alterations in E-cadherin, N-cadherin and
Vimentin expression levels were detected at the protein and mRNA
level, confirming the effect of miR-9 on the epithelial-mesenchymal
transition (EMT) of PC cells. It was also confirmed that miR-9
influenced PC cell behavior via targeting of the MEKK3
3′-untranslated region (3′-UTR). The present study expanded the
current understanding on the role of miR-9 as a PC suppressor, and
may facilitate the future development of miR-targeted cancer
diagnostics and therapeutics.
Materials and methods
Cell lines and cell culture
Several cell lines were purchased from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). These
included human normal prostate RWPE-1 cells, as well as DU145,
LNCaP and PC-3 cells, which are standard PC cell lines used in
therapeutic research (26). 22Rv1
was also obtained, which is a human prostate carcinoma epithelial
cell line derived from a xenograft that was serially propagated in
mice following castration-induced regression and relapse of the
parental, androgen-dependent CWR22 ×enograft (27). C4-2B cells, a subtype of the LNCaP
cell line, and VCaP cells, an adherent epithelial cell line with
high androgen receptor and prostate-specific antigen expression,
were also obtained (28,29). VCaP cell line is the only PC cell
model that expresses the androgen receptor splice variant, AR-V7,
with TMPRSS2-ERG gene fusion.
RWPE-1 cells were cultured in 1X defined
keratinocyte serum-free medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). LNCaP, 22Rv1, C4-2B and PC-3
cells were grown in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), while DU145 and VCaP
cells were grown in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. All
cells were incubated at 37°C in a humidified atmosphere with 5%
CO2 and routinely subcultured using 0.25% (w/v)
trypsin-ethylenediaminetetraacetic acid solution.
Patients
PC patients (diagnosed with National Institute for
Health and Clinical Excellence (NICE) criteria, n=31) (30) aged between 26 and 70 years, with a
mean age of 53 years, were recruited into the present study.
Clinicopathological information for these patients was obtained
from medical records. The corresponding PC paraneoplastic tissues
and their normal adjacent tissues were acquired at ≥1 cm from the
neoplastic tissue. All cases were initially treated by
prostatectomy and randomly selected at Qilu Hospital of Shandong
University (Jinan, China) between March 2011 and April 2016.
Specimens were obtained in agreement with the Declaration of
Helsinki and were preserved at Qilu Hospital. The experimental
protocol was approved by the Ethical Committee of Qilu Hospital of
Shandong University (Jinan, China). Written informed consent for
sample use in this research was obtained from both patients and
clinicians. All samples were reviewed and diagnosed by two
independent pathologists.
miR-9 expression vector
construction
A versatile expression vector of miR-9 was generated
by constructing the human miR-9 precursor region into minicircle
vectors. A transthyretin (TTR) gene promoter was inserted upstream
of the miR-9 precursor to guarantee liver-specific expression of
miR-9 (31). This construct was
referred to as TTR-miR-9. To exclude non-specific effects of the
plasmid, a miR-9 mismatched expression vector was generated by
mutating the seed region of miR-9, termed
transthyretin-miR-9-mut.
miR-9 mimic preparation and
transfection
miR-9 mimic and its scramble control [negative
control (NC) mimic] were purchased from GenScript (Piscataway, NJ,
USA). miR-9 mimic is an artificial chemically-modified
double-strand miRNA, which mimics mature endogenous miR-9
expression subsequent to transfection into cells. Transfection of
miRNA mimic was carried out by using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacture's protocol. All miRNA treatments proceeded for 48 h.
Both miR-9 mimic and miR-NC mimic were formulated in 0.9 % NaCl to
a final concentration of 10 mg/ml.
For enforced expression of MEKK3 in PC cells, open
reading frame region of MEKK3 gene was amplified and inserted into
the pcDNA3.1 eukaryotic expression vector (Invitrogen; Thermo
Fisher Scientific, Inc.). Vector empty pcDNA3.1 was served as
negative control (NC). Before transfection, cells were seeded into
6-well plates overnight. 3 µg/ml pcDNA3.1-MEKK3 or pcDNA3.1 plasmid
was then transfected into the PC cells by using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
In vivo tumorigenesis in a PC mouse
model
To detect whether upregulation of miR-9 reduced the
tumorigenic capacity of PC cells, an intratibial injection model
was established. A total of 6 male severe combined immunodeficiency
mice at 3–4 weeks old were purchased from Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). Mice were kept at
23°C in a humidified atmosphere (55–65% humidity) with food and
water ad libitum with a 12-h light/dark cycle. In order to
establish the PC animal model, each mouse was injected with
1×105 stably selected PC-3 cells into its right tibia,
while with PC-3 cells transfected with NC vector cells were
injected into the left tibia, serving as the NC group. Tumor length
and width were measured every 3 days after injection, and tumor
volume was calculated as follows: Length × (width2/2).
Mice were sacrificed at 33 days after the injection, and the tumors
were weighed. Ethical approval of the animal experiments was
provided by the Institutional Animal Care and Use Committee of
Shandong University Cancer Center (Jinan, China; approval no.
L102012016110D).
Cell Counting Kit-8 (CCK-8) assay
PC cell viability was determined by a CCK-8/WST-8
assay (Dojindo Molecular Technologies, Inc., Shanghai, China),
which was performed following a standard procedure in a 96-well
plate, according to the manufacturer's protocol. Briefly, cells
(5×103 cells/well) were seeded in a 96-well plate and
grown to 80% confluence. miR-9 mimic or mimic NC was subsequently
transfected into PC cells. At 0, 24, 48 and 72 h post-transfection,
10 µl of CCK-8 reagent was added into each well. After 1 h of
incubation, cell viability was determined by measuring the
absorbance of the converted dye at 450 nm.
BrdU immunofluorescence assay
DU145, LNCaP, 22Rv1, PC-3, C4-2B and VCaP cells
(1~5×105) were seeded on glass coverslips placed in a
6-well plate. Following transfection with miR-9 mimic and miR-NC
for 48 h, 10 mg/ml BrdU stock solution in phosphate buffered saline
(PBS) was diluted to 1,000X in the culture medium and incubated for
60 min. Cells were washed with PBS, fixed for 20 min at 25°C in 4%
paraformaldehyde and permeabilized with 0.3% Triton X-100 for 10
min. Subsequent to blocking with 10% FBS for 1 h, cells were
incubated with a primary rabbit antibody against BrdU (1:200; cat.
no. ab6326, Abcam, Cambridge, UK) overnight at 4°C and then
incubated with the corresponding secondary antibody coupled to a
fluorescent marker, Cy3 (A10520, Thermo Fisher Scientific, Inc.),
at room temperature for 2 h. Following DAPI staining for 15 min at
37°C and a further wash with PBS, the cover slips were mounted onto
glass slides and visualized using a fluorescence microscope
(Olympus 600 auto-biochemical analyzer; Olympus Corporation, Tokyo,
Japan) with Image-Pro Plus software (Media Cybernetics, Inc.,
Rockville, MD, USA) for image analysis. A total of 10 microscopic
fields were analyzed to calculate the degree of BrdU staining.
Transwell assays
After 24 h of transfection, DU145, LNCaP, 22Rv1,
PC-3, C4-2B and VCaP cells were harvested by trypsinization and
washed once with D-Hank's solution. In order to measure cell
invasion, 8-µm pore size culture inserts or Matrigel-coated
inserts, respectively, were placed into the wells of 24-well
plates. In total, 1×105 cells were added to the upper
chamber, while 400 µl F12 medium containing 10% FBS and 20 ng/ml
Hepatocyte growth factor (HGF) was added to the lower chamber.
After 20 h of incubation, the cells that migrated through the pores
were stained with crystal violet and observed under a microscope
(Zeiss AG, Oberkochen, Germany).
Wound-healing assay
At 48 h post-transfection, 1.5 ml of DU145, LNCaP
and PC-3 cells (1×105 cells/ml) were grown to 100%
confluence in 12-wells plate. A scratch wound was then generated by
scraping the monolayer of the cells with a sterile pipette tip.
Next, samples were washed with PBS twice to remove the detached
cells, and subsequently incubated for 0 and 48 h prior to examining
and capturing images with an inverted microscope (Nikon
Corporation, Tokyo, Japan).
Bioinformatics analysis
The potential targets of miR-9 were predicted using
the miRDB database (http://www.mirdb.org). The putative genes that were
predicted by algorithms were accepted, and the candidates were
selected based on the gene function. MEKK3 was further chose as a
target is due to MEKK3 has been widely reported as an oncogene
(32,33).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40 and 0.1%
SDS, pH 8.0) supplemented with a protease inhibitor cocktail. The
protein concentration was measured using a BCA Protein Quantitation
kit. Protein samples were then separated by 10% SDS-PAGE and
electroblotted onto 0.45-µm Immobilon polyvinylidene difluoride
membranes. The membranes were then blocked with 5% bovine serum
albumin in PBS/Tween-20 for 1 h at room temperature and incubated
overnight at 4°C with the following antibodies: Rabbit anti-B-cell
lymphoma 2 (Bcl-2; 1:2,000, cat. no. ab59348, Abcam), rabbit
anti-Bcl-2-associated X protein (Bax; 1:1,000, cat. no. ab59348,
Abcam) and mouse anti-GAPDH (1:5,000, cat. no. ab8245, Abcam)
antibodies. Subsequently, membranes were incubated for 1 h at room
temperature with the following Amersham ECL horseradish
peroxidase-linked secondary antibodies: Goat anti-mouse IgG
(1:10,000) or goat anti-rabbit IgG (1:10,000; both from GE
Healthcare Life Sciences, Little Chalfont, UK). Western blot
immunoreactivity was detected using a SuperSignal West Femto
Maximum Sensitivity Substrate kit (Thermo Fisher Scientific, Inc.)
with a Chemiluminescent detection method of C-DiGit blot scanner
(LI-COR Biosciences, Lincoln, NE, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from PC cells following
different treatments or from homogenized PC tissues (stored at
−80°C) using TRIzol® reagent (Thermo Fisher Scientific,
Inc.), according to manufacturer's protocol. The RNA (10 mg) was
reverse transcribed into first-strand complementary DNA (cDNA)
using M-MLV reverse transcriptase (Promega Corporation, Madison,
WI, USA) for mRNA expression analysis. The levels of miR-9 were
determined by SYBR Green incorporation in a Roche Light-Cycler 480
Real-time PCR system (Roche Diagnostics GmbH, Mannheim, Germany).
GAPDH and U6 served as an internal control to determine the miR-9,
E-cadherin, N-cadherin, Vimentin and MEKK3 expression. The primer
sequences used in qPCR were as follows: miR-9,
5′-TGAGGTAGTAAGTTGTATTGTT-3′ (forward) and
5′-ACACACTTCCTTACATTCCATT-3′ (reverse); E-cadherin,
5′-GGGTTGTCTCAGCCAATGTT-3′ (forward) and 5′-CACCAACACACCCAGCATAG-3′
(reverse); N-cadherin, 5′-CGAGCCGCCTGCGCTGCCAC-3′ (forward) and
5′-CGCTGCTCTCCGCTCCCCGC-3′ (reverse); Vimentin,
5′-AGATCGATGTGGACGTTTCC-3′ (forward) and 5′-CACCTGTCTCCGGTATTCGT-3′
(reverse); MEKK3, 5′-AAGGGGTCAAAGGTGGAACC-3′ (forward) and
5′-TGCCTTGATGACGCCGTATT-3′ (reverse); GAPDH,
5′-GGAAAGCTGTGGCGTGAT-3′ (forward), 5′-AAGGTGGAAGAATGGGAGTT-3′
(reverse). U6, 5′-CTCGCTTCGGCAGCAC(forward)-3′ and
5′-ACGCTTCACGAATTTGC-3′ (reverse). qPCR was performed in a total
volume of 20 µl with SYBR Green PCR Master Mix (4309155, Thermo
Fisher Scientific, Inc.) at 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The target
expression, calculated using the 2−ΔΔCq method (34), was obtained by normalizing to the
endogenous reference and relative to a calibrator (Mean of the
control samples).
Dual-luciferase reporter assay
The MEKK3 gene was predicted to be a target of
miR-9, and their interaction was further examined by
dual-luciferase reporter assay. The entire MEKK3 gene 3′-UTR was
amplified, constructed and inserted downstream of the GV126
Luciferase gene. Based on the predicted target binding site of
miR-9 and the MEKK3 gene, site-directed mutagenesis was employed to
abolish the binding site, and the obtained mutant sequence was used
as a control. A Renilla luciferase-containing plasmid with a
thymidine kinase promoter (pRL-TK vector; Takara Bio, Inc., Otsu,
Japan) was used as a reporter control to adjust for transfection
efficiency. miR-9 mimic and miR-NC were respectively co-transfected
with the luciferase reporter vector into DU145, LNCaP, 22Rv1, PC-3,
C4-2B and VCaP cells. Luciferase assay was conducted according to
the Dual-Luciferase Assay kit protocol (Promega Corporation,
Madison, WI, USA). Briefly, 100 units of myllicin (T10326,
Transgenes, Beijing, China) was added to the cells, and then cells
were cultured in RPMI-1640/DMEM supplemented with 10% FBS in an
incubator with 5% CO2 at 37°C. The media were replaced
every 2 days, and cells were digested with 0.25% tryptase and
subcultured when 70–80% confluence had been reached in the culture.
After overnight culture, culture medium was removed and cells were
washed with PBS. Cells were then lysed, and cell lysates were
incubated with luciferin, followed by luminescence measurement.
Luciferase activity was calculated after normalization to the
Renilla luciferase activity.
Statistical analysis
Results are presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS 16.0
software (SPSS Inc., Chicago, IL, USA). Comparisons between groups
were investigated by two-tailed Student's t-test. Tukey's test was
used as a post hoc test following analysis of variance. Differences
were considered as statistically significant when the P-value was
<0.05.
Results
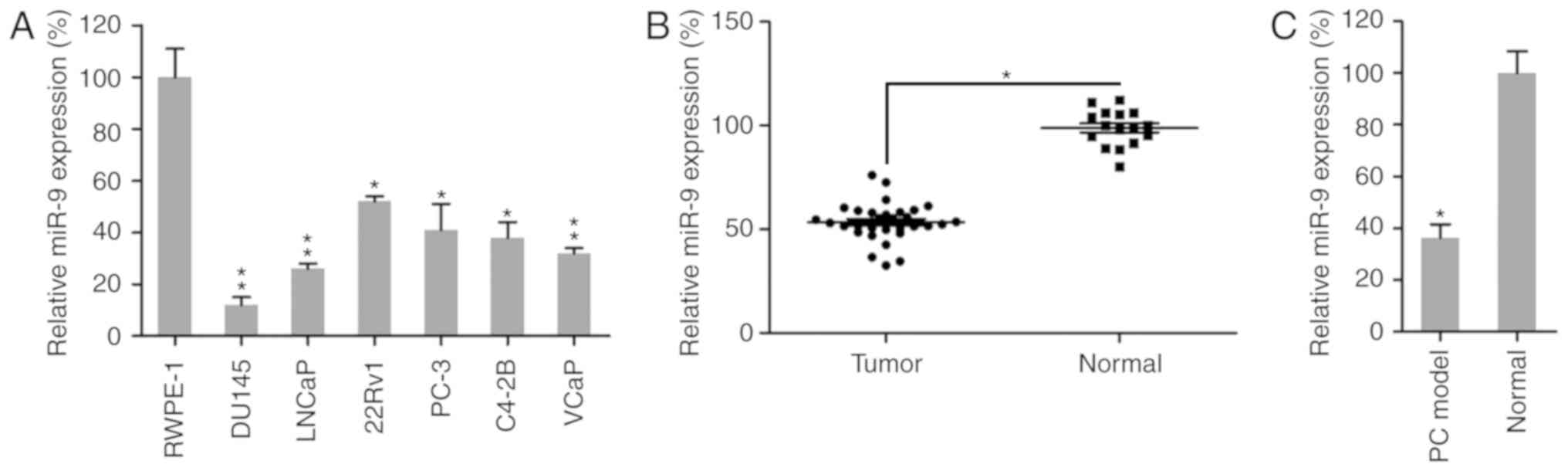
miR-9 expression is reduced in PC
cells and tissues
To identify whether miR-9 is an essential regulator
in PC progression and development, miR-9 expression was examined in
a PC mouse model, human PC tissues and six different PC cell lines,
including the brain metastatic PC cell line DU145, the lymph node
metastatic cell line LNCaP, the primary PC cell line 22Rv1, and the
bone metastatic PC cell lines PC-3, C4-2B and VCaP. Initially,
miR-9 expression was examined in normal prostate cells (RWPE-1)
(35,36) and the six PC cell lines. RT-qPCR
analysis revealed that miR-9 expression was significantly reduced
in the six PC cell lines compared with that in the normal cell line
(Fig. 1A). In addition, the
expression levels of miR-9 were evaluated in 31 human PC prostatic
tissues and 16 adjacent normal tissues. The results revealed that
miR-9 expression was significantly lower in human prostatic tissues
in comparison with that in normal tissues (Fig. 1B). Furthermore, the expression of
miR-9 was measured in normal mouse prostatic tissues and prostatic
tumor collected from a PC mouse model. It was observed that miR-9
expression was markedly reduced in prostate tumors of the mouse
model, as compared with that in the normal group (Fig. 1C). Taken together, these data
demonstrated that PC cells and tissues exhibited a marked decrease
in miR-9 expression, compared with that observed in normal cells or
tissues, respectively.
Effect of miR-9 on PC cell
viability
To assess the role of miR-9 on the growth and
viability of PC cells, miR-9-mimic or miR-NC were transfected into
DU145, LNCaP, 22Rv1, PC-3, C4-2B and VCaP cells. The expression
pattern of miR-9 was confirmed to be upregulated in these six PC
cell lines following transfection with miR-9-mimic, as compared
with cells transfected with miR-NC and untransfected cells, by
RT-qPCR assay (Fig. 2A). A CCK-8
assay was also performed to assess the proliferation of the six
cell lines. Compared with the miR-NC group, transfection with
miR-9-mimic at a concentration of 100 nM resulted in a decreased
cell growth rate in the six PC cell lines at 0, 24, 48 and 72 h
after transfection (Fig. 2B).
Furthermore, BrdU immunofluorescence assay was performed to measure
the cell viability at 48 h after transfection with miR-9-mimic. The
BrdU staining results revealed that, compared with miR-NC and
untransfected groups, miR-9-mimic transfection inhibited viability
in DU145, LNCaP, 22Rv1, PC-3, C4-2B and VCaP cells by approximately
30, 50, 40, 50, 40 and 30%, respectively; however, the change in
DU145 cells was not statistically significant (Fig. 2C). Overall, these results revealed
that miR-9 overexpression displayed an inhibitory effect on cell
viability in a time-dependent manner in these six PC cell
lines.
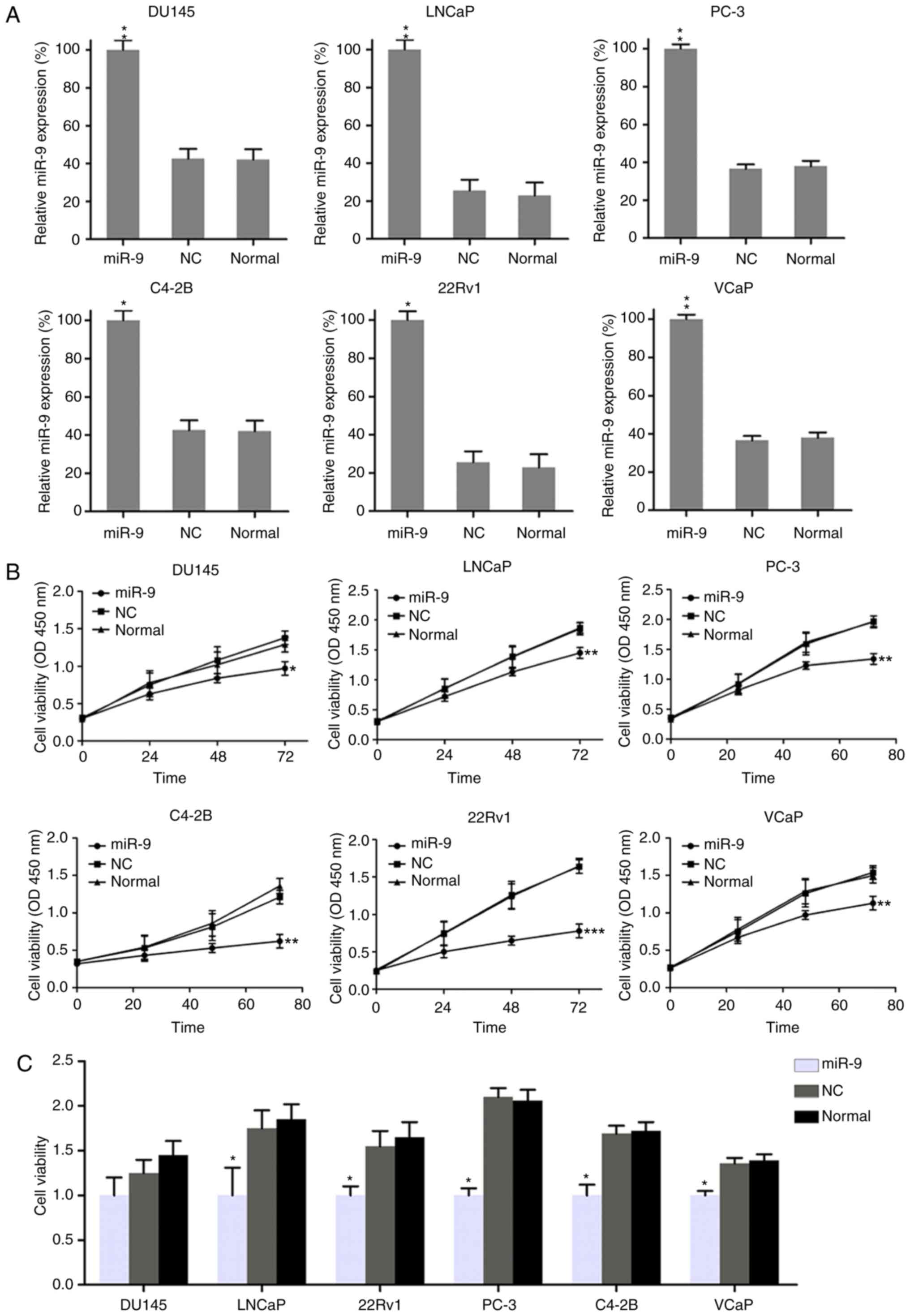 | Figure 2.Effect of miR-9 overexpression on PC
cell viability. (A) Quantitative polymerase chain reaction was used
to measure the expression of miR-9 in each PC cell line transfected
with miR-9 mimic and miR-NC mimic. (B) Viability of PC cell lines,
as measured by Cell Counting Kit-8 assay at 0, 24, 48 and 72 h post
transfection. (C) At 48 h post transfection, the viability of PC
cell lines was determined by a BrdU incorporation assay. Data
represent the mean ± standard deviation. *P<0.05, **P<0.01
and ***P<0.001, vs. normal group. miR, microRNA; PC, prostate
cancer; NC, negative control; OD, optical density. |
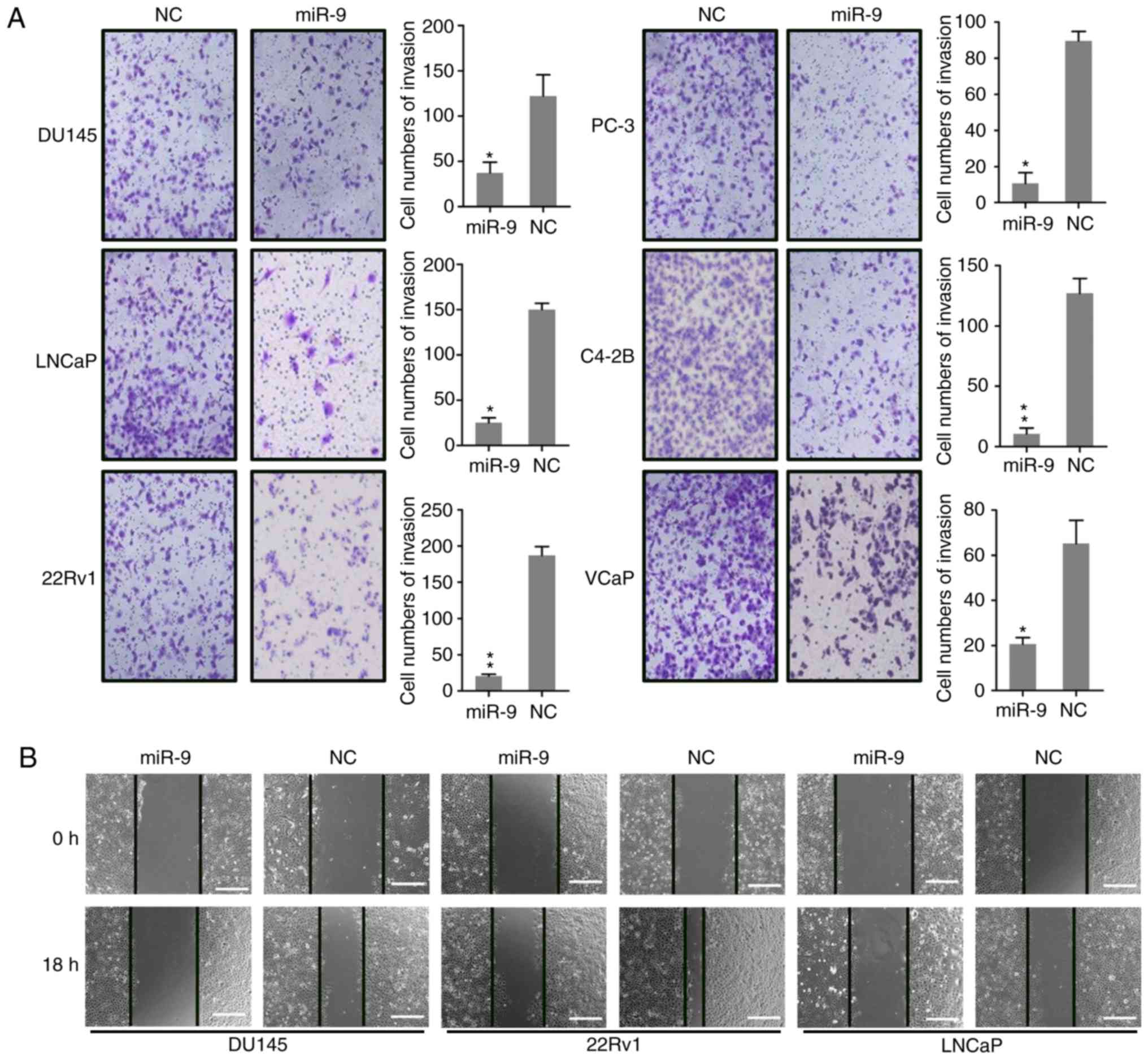
miR-9 inhibits PC cell migration and
invasion
Research has demonstrated that the invasion and
migration of PC cells is major cause for mortality during PC
development and metastasis into the bone. To determine whether
miR-9 expression impacted the invasion and migration of PC cells,
wound-healing and Transwell migration were conducted following the
transfection of DU145, LNCaP, 22Rv1, PC-3, C4-2B and VCaP cells
with miR-9 and miR-NC mimics. The Transwell assay demonstrated that
ectopic miR-9 expression had a significant inhibitory effect on
invasion of these cells (Fig. 3A),
which was consistent with the migration data obtained from the
wound-healing assay (Fig. 3B). The
wound-healing assay was also performed to detect the migratory
capacity of DU145, 22Rv1, and LNCaP cells, and the results revealed
that miR-9 overexpression caused marked suppression of these three
PC cells migration, particularly in 22Rv1 cells (Fig. 3B). The results suggested that miR-9
overexpression suppressed the migratory and invasive properties of
PC cells in vitro.
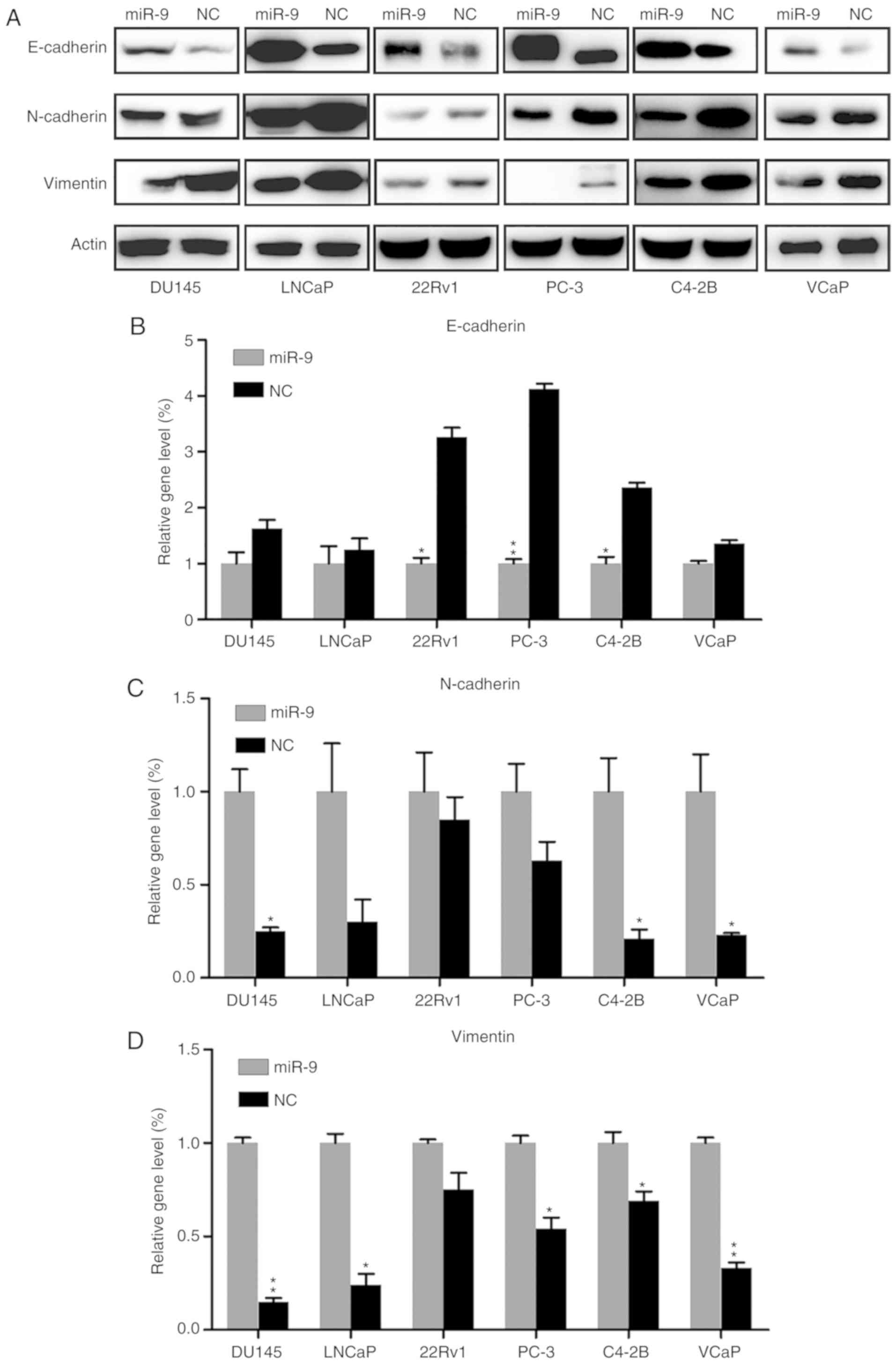
Effect of miR-9 on EMT
Epithelial-mesenchymal transition (EMT) serves an
essential role in tumor invasion and migration. Western blotting
and RT-qPCR were performed to reveal the effect of miR-9 on EMT,
through examining the protein and gene expression levels of
epithelial marker E-cadherin, mesenchymal marker N-cadherin and
Vimentin. E-cadherin protein expression in each PC cell line of the
miR-9 mimic-transfected group was markedly upregulated, compared
with that in the NC group. By contrast, N-cadherin and Vimentin
expression levels were notably decreased by miR-9 overexpression
when compared with those in the NC group (Fig. 4A). In addition, E-cadherin,
N-cadherin and Vimentin mRNA expression levels in the miR-9
mimic-transfected groups were significantly changed, compared with
those in the NC group, in the majority of cell lines (P<0.05).
More specifically, E-cadherin mRNA was significantly increased,
whereas N-cadherin and Vimentin mRNA levels were markedly reduced
in the miR-9 groups (Fig.
4B-D).
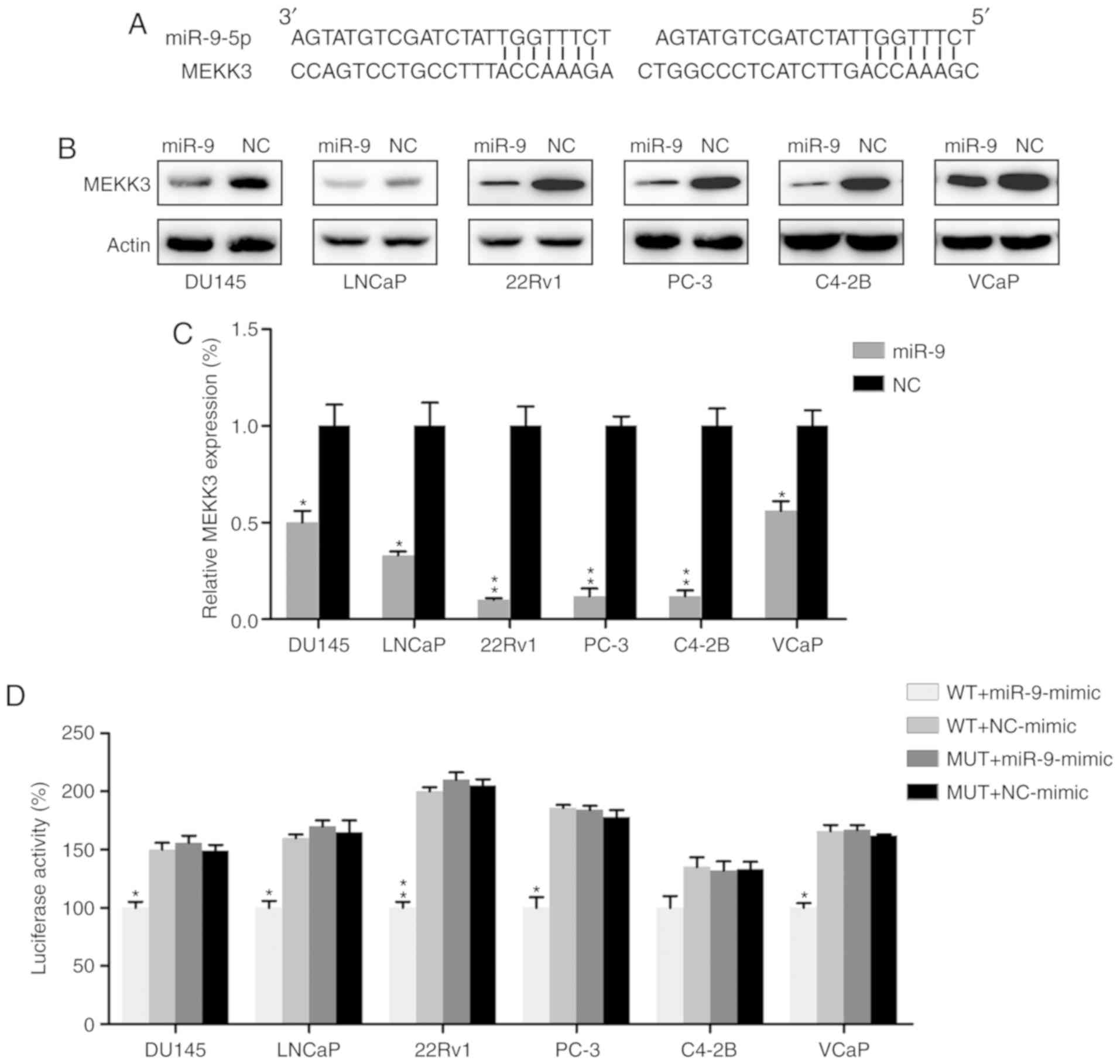
MEKK3 is a target of miR-9
MEKK3, which has been well-documented as a mediator
of development and migration of other cancers (37–39),
was identified as a functional candidate target for miR-9 by
bioinformatics analysis (Fig. 5A).
MEKK3 expression was thus examined in PC cells transfected with
miR-9 mimic or miR-NC mimic by western blot analysis and RT-qPCR.
MEKK3 expression was markedly suppressed in the miR-9
mimic-transfected group at the protein and mRNA levels (Fig. 5B and C). A dual-luciferase reporter
assay, which is widely applied to confirm the binding of miRs to
the promoter region of genes (40–42),
was also performed in PC cell lines to determine if there was a
direct interaction between MEKK3 and miR-9. The data demonstrated
that miR-9 mimic transfection decreased luciferase activity, which
was fused with the MEKK3 3′-UTR, as compared with in the control
group (Fig. 5D).
Overexpression of MEKK3 protein
suppresses the inhibitory effect of miR-9 on PC cell viability and
migration
To further determine whether the inhibitory effect
of miR-9 on PC development was exerted through targeting MEKK3, the
expression of MEKK3 was we restored in six PC cell lines (DU145,
LNCaP, 22Rv1, PC-3, C4-2B and VCaP cells) expressing miR-9. By
performing a BrdU immunofluorescence assay, it was identified that
increased expression of MEKK3 inhibited the miR-9-mediated
viability decrease of DU145, LNCaP, 22Rv1, PC-3, C4-2B and VCaP
cells (Fig. 6A). Transwell
migration assays for DU145 and LNCaP cells, which have showed best
invasive capacity in above mentioned experiments, were performed to
further assess the effect of MEKK3 overexpression on these PC cells
that were overexpressing miR-9. As expected, the MEKK3 +
miR-9-overexpressing cells displayed enhanced invasive capacity,
compared with in the group transfected with miR-9 mimic alone
(Fig. 6B). MEKK3 overexpression
also inhibited the miR-9-induced E-cadherin upregulation and
N-cadherin downregulation (Fig.
6C). Taken together, the data indicated that increased MEKK3
expression reduced the miR-induced inhibition of PC cell growth and
invasion. The data also strongly suggested that the inhibitory
effect observed in PC cells by miR-9 overexpression was exerted
through cross-talk between miR-9 and MEKK3.
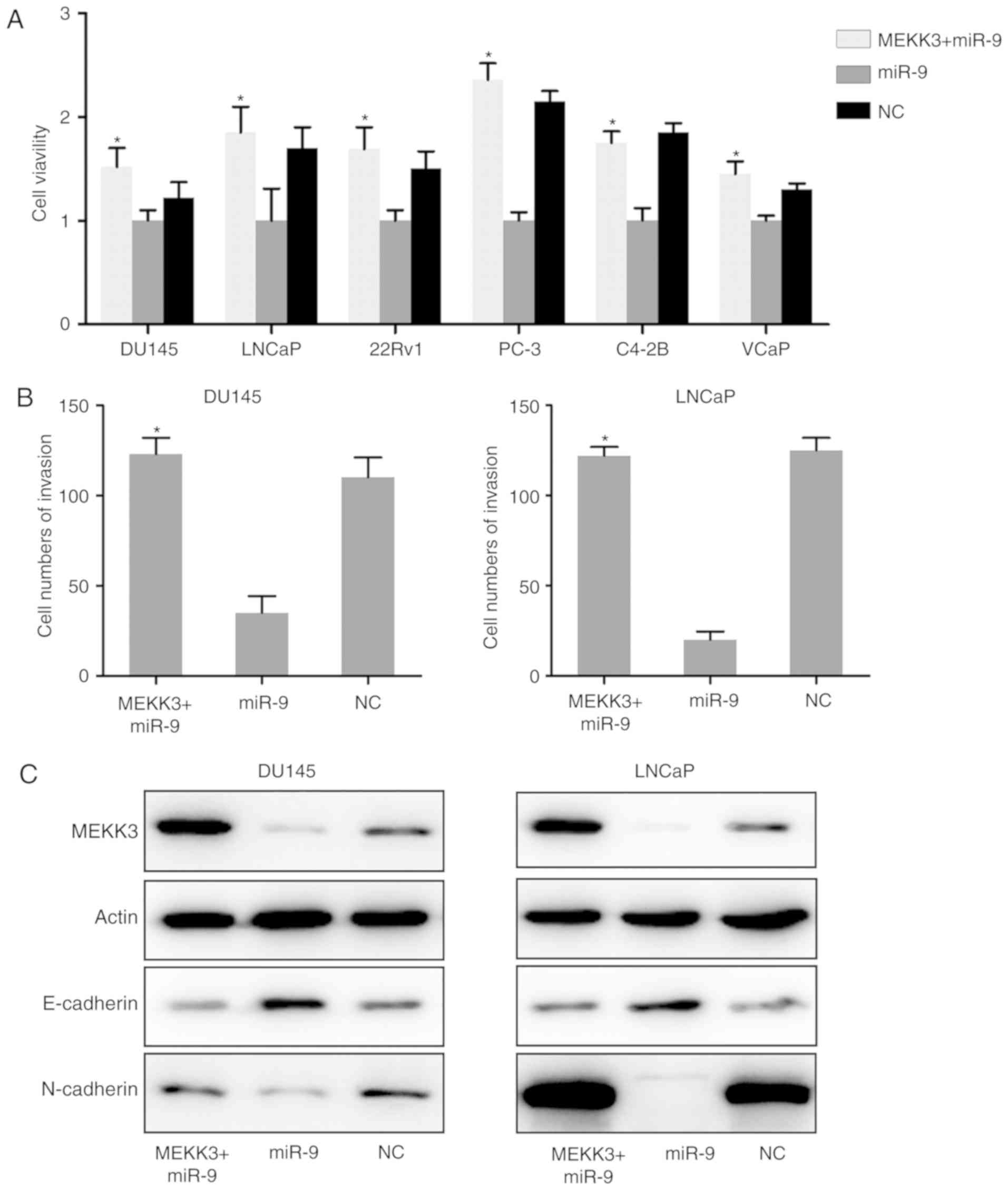 | Figure 6.MEKK3 overexpression restored the
inhibitory effect of miR-9 on the PC cell properties. (A) Viability
of PC cells overexpressing MEKK3 + miR-9 or miR-9 alone was
measured by Cell Counting Kit-8 assay at 0, 24, 48, and 72 h post
transfection. (B) After miR-9 mimic and MEKK3-expressing plasmid
transfection, the invasion ability of DU145 and LNCaP cells was
measured by Transwell assay. (C) Western blot analysis was
performed to assess the MEKK3, E-cadherin and N-cadherin expression
levels, which were regulated by miR-9 mimic and MEKK3 plasmid
co-transfection in DU145 and LNCaP cells. Data represent the mean ±
standard deviation. *P<0.05 vs. miR-9 group. miR, microRNA; PC,
prostate cancer; NC, negative control; MEKK3, mitogen-activated
protein kinase kinase kinase 3. |
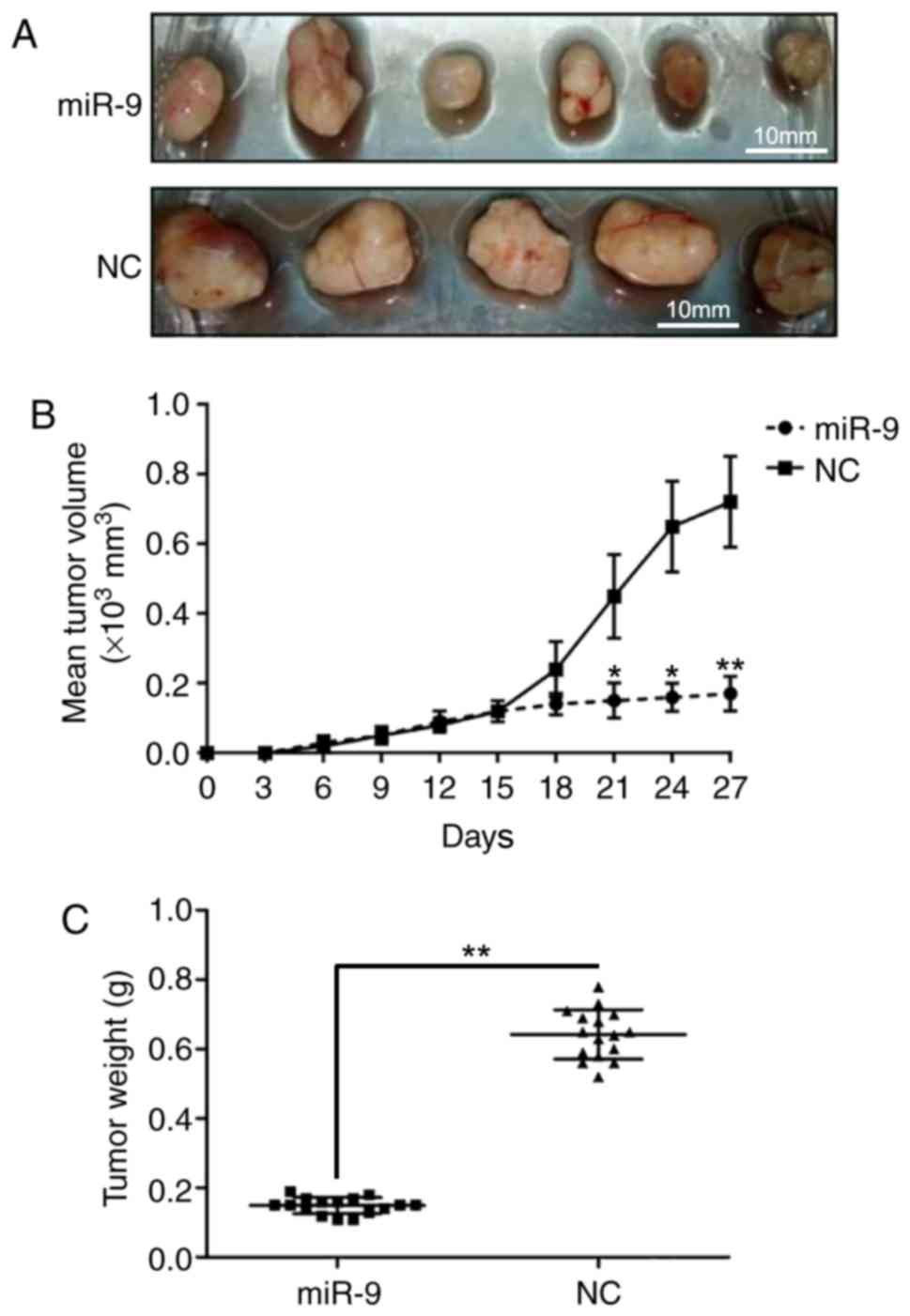
miR-9 inhibits prostate tumor growth
in vivo
To further demonstrate the effect of miR-9 on PC
development in vivo, a mouse model was established through
intratibial injection. The mice were sacrificed, and tumors were
excised and weighed at 30 days after injection. As presented in
Fig. 7A, upregulation of miR-9
inhibited the xenograft prostate tumor development ability in
vivo. In addition, mice injected with PC cells that were
transfected with miR-9 mimics exhibited a marked reduction in tumor
volumes compared with those inoculated with control PC cells
(Fig. 7B). Furthermore,
overexpression of miR-9 greatly reduced the weight of tumors
(Fig. 7C). These observations
indicated that the upregulation of miR-9 repressed prostate tumor
formation.
Discussion
Tumor development is a complex synergistic process,
involving the activation of oncogenic and suppression of tumor
suppressor signaling networks, which may be associated with
epigenetic alterations. Although PC has been extensively
investigated in previous studies, the underlying mechanisms of PC
remain poorly understood due to its complexity. In the present
study, a specific miRNA was identified, namely miR-9, which was
downregulated in six different PC cell lines (DU145, LNCaP, 22Rv1,
PC-3, C4-2B, and VCaP). In addition, it was observed that
overexpression of miR-9 in these cell lines inhibited cell
viability and migration. In vivo experiments demonstrated
that the inoculation of PC-3 cells expressing miR-9 into an
immune-deficient mouse model resulted in a robust inhibitory effect
on prostate tumor growth. The ability of miR-9 to inhibit the
expression of MEKK3, a key diagnostic and therapeutic target of PC,
was also identified. Further investigation demonstrated that miR-9
directly bound to the 3′-UTR of MEKK3 and abrogated its expression.
Restoration of MEKK3 expression prevented the inhibitory effects of
miR-9 in PC cells. The findings of the present study strongly
suggested that miR-9 suppressed PC development via the regulation
of cell viability through MEKK3 regulation, and provided evidence
for the use of this miRNA as a potential target in PC therapy.
Accumulating studies have focused on the effects of
miRNAs in PC therapy, due to their stability and relatively high
uptake by the prostate (43). The
identified inhibitory effect of miR-9 on PC was not unpredictable,
since its role as an oncogene or tumor suppressor, depending on the
cancer type, has been previously established. Several studies have
demonstrated that miR-9 is upregulated in breast cancer, brain
cancer, and melanoma (17,44,45).
By contrast, a number of other studies have indicated that miR-9 is
downregulated in certain types of cancer, including cervical
adenocarcinoma, and hepatocellular, ovarian and gastric carcinoma
(20,46–49),
suggesting a bidirectional role of miR-9 in cancer. Accordingly,
miR-9 may have different gene targets depending on the tumor type.
In the current study, miR-9 expression in PC cell lines and tumor
tissues obtained from 31 PC patients was significantly
downregulated compared with that in normal cells and tissues.
However, restoration of miR-9 expression in PC cells inhibited the
cell viability in vitro and tumor development in
vitro. Furthermore, the data demonstrated that miR-9 expression
was inversely associated with MEKK3 expression in PC cell
lines.
MEKK3, a member of the MEKK family, is ubiquitously
expressed in various human tissues; however, increased MEKK3
expression in cancer indicates a high risk of tumor progression and
is correlated with poor prognosis. MEKK is an upstream regulator of
the MAPK cascade and activates multiple MAPKs, including ERK1/2,
p38, JNK, ERK5 and nuclear factor (NF)-κB (50). The role of MAPKs in tumorigenesis
has been extensively investigated over the last decade,
particularly in the cases of ERK1/2 and NF-κB (51). Recently, the focus of research has
shifted to the potential role of MEKKs in tumor development and
progression. For instance, MEKK3 has been reported to contribute to
the development of cervical (37),
breast (24) and esophageal cancer
(32). It also has the ability to
induce tumor cell migration and invasion via MAPK pathway
activation. In the present study, MEKK3 was identified to be a
direct target of miR-9. Previously, it has been proven that
positive results in dual-luciferase reporter assay, combined with
the identification of the miR-downregulated target protein, are
sufficient to verify the interaction between miR and the gene of
the target protein (40–42). This provides evidence for the
function of miR-9 in the regulation of PC cell proliferation,
migration, invasion and EMT, at least partially through targeting
the 3′-UTR of MEKK3.
EMT is the transformation process of epithelial
cells into mesenchymal cells. It has also been demonstrated to be
involved in tumor development and progression, particularly in
tumor metastasis (52). In the
present study, MEKK3 overexpression markedly reduced the inhibitory
effect of miR-9 transfection on PC viability, invasion and EMT
marker expression. The present data demonstrated that miR-9 may
suppress the neoplastic capacity of PC cells.
In conclusion, the data presented in the current
study suggested that miR-9 exerted a multifunctional anti-tumor
effect against the major malignant properties of PC cell lines. In
addition, it was revealed that miR-9 reduced tumor development in a
PC mouse model. These effects were demonstrated to be, at least
partially, mediated by MEKK3 regulation. Nevertheless, it cannot be
excluded that miR-9 may target other factors and thus contribute to
PC pathogenesis. Therefore, a modified HITS-CLIP method combined
with the miR-TRAP technique, which was introduced by Baigude et
al (53), will be applied in
our future investigations to screen and identify the targets of
miR-9 in PC cell lines and the prostate tissue of a PC mouse model.
This method may provide a more precise and comprehensive
interpretation for the role of miR-9 in PC development.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
Shandong Science and Technology Research Program (no.
2014GSF121026).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XJ performed the CCK-8 assay. ZS performed all other
assays. LG analyzed the data and wrote the manuscript. GY conceived
the study and designed the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The human specimen experiments were approved by the
Ethical Committees of Qilu Hospital of Shandong University (Jinan,
China), and written informed consent for sample use was obtained
from patients and clinicians. Ethical approval for the animal
experiments was provided by the Institutional Animal Care and Use
Committee of Shandong University Cancer Center (approval no.
L102012016110D).
Patient consent for publication
Written informed consent for sample use and
publication in this research was obtained from both patients and
clinicians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomella LG, Singh J, Lallas C and Trabulsi
EJ: Hormone therapy in the management of prostate cancer:
Evidence-based approaches. Ther Adv Urol. 2:171–181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39:D152–D157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim S, Lee UJ, Kim MN, Lee EJ, Kim JY, Lee
MY, Choung S, Kim YJ and Choi YC: MicroRNA miR-199a* regulates the
MET proto-oncogene and the downstream extracellular
signal-regulated kinase 2 (ERK2). J Biol Chem. 283:18158–18166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuva-Aydemir Y, Simkin A, Gascon E and Gao
FB: MicroRNA-9: functional evolution of a conserved small
regulatory RNA. RNA Biol. 8:557–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata M, Kurokawa D, Nakao H, Ohmura T
and Aizawa S: MicroRNA-9 modulates cajal-retzius cell
differentiation by suppressing foxg1 expression in mouse medial
pallium. J Neurosci. 28:10415–10421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leucht C, Stigloher C, Wizenmann A, Klafke
R, Folchert A and Bally-Cuif L: MicroRNA-9 directs late organizer
activity of the midbrain-hindbrain boundary. Nat Neurosci.
11:641–648. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delaloy C, Liu L, Lee JA, Su H, Shen F,
Yang GY, Young WL, Ivey KN and Gao FB: MicroRNA-9 coordinates
proliferation and migration of human embryonic stem cell-derived
neural progenitors. Cell Stem Cell. 6:323–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pietrzykowski AZ, Friesen RM, Martin GE,
Puig SI, Nowak CL, Wynne PM, Siegelmann HT and Treistman SN:
Posttranscriptional regulation of BK channel splice variant
stability by miR-9 underlies neuroadaptation to alcohol. Neuron.
59:274–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehmann U, Hasemeier B, Christgen M,
Müller M, Römermann D, Länger F and Kreipe H: Epigenetic
inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J
Pathol. 214:17–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laios A, O'Toole S, Flavin R, Martin C,
Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, et al:
Potential role of miR-9 and miR-223 in recurrent ovarian cancer.
Mol Cancer. 7:352008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nie K, Gomez M, Landgraf P, Garcia JF, Liu
Y, Tan LH, Chadburn A, Tuschl T, Knowles DM and Tam W:
MicroRNA-mediated down-regulation of PRDM1/blimp-1 in
hodgkin/reed-sternberg cells: A potential pathogenetic lesion in
hodgkin lymphomas. Am J Pathol. 173:242–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Q, Shen W, Huang H, Liu J, Zhang J,
Huang X, Wu J and Shi Y: Insight into the binding properties of
MEKK3 PB1 to MEK5 PB1 from its solution structure. Biochemistry.
46:13478–13489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura K, Kimple AJ, Siderovski DP and
Johnson GL: PB1 domain interaction of p62/sequestosome 1 and MEKK3
regulates NF-kappaB activation. J Biol Chem. 285:2077–2089. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan Y, Ge N, Wang X, Sun W, Mao R, Bu W,
Creighton CJ, Zheng P, Vasudevan S, An L, et al: Amplification and
over-expression of MAP3K3 gene in human breast cancer promotes
formation and survival of breast cancer cells. J Pathol. 232:75–86.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng Y, Yang J, McCarty M and Su B: MEKK3
is required for endothelium function but is not essential for tumor
growth and angiogenesis. Am J Physiol Cell Physiol.
293:C1404–C1411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alimirah F, Chen J, Basrawala Z, Xin H and
Choubey D: DU-145 and PC-3 human prostate cancer cell lines express
androgen receptor: Implications for the androgen receptor functions
and regulation. FEBS Lett. 580:2294–2300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sramkoski RM, Pretlow TG II, Giaconia JM,
Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D and
Jacobberger JW: A new human prostate carcinoma cell line, 22Rv1. In
Vitro Cell Dev Biol Anim. 35:403–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korenchuk S, Lehr JE, Mclean L, Lee YG,
Whitney S, Vessella R, Lin DL and Pienta KJ: VCaP, a cell-based
model system of human prostate cancer. In Vivo. 15:163–168.
2001.PubMed/NCBI
|
|
29
|
Thalmann GN, Anezinis PE, Chang SM, Zhau
HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC and Chung LW:
Androgen-independent cancer progression and bone metastasis in the
LNCaP model of human prostate cancer. Cancer Res. 54:2577–2581.
1994.PubMed/NCBI
|
|
30
|
Claxton K, Martin S, Soares M, Rice N,
Spackman E, Hinde S, Devlin N, Smith PC and Sculpher M: Methods for
the estimation of the National Institute for Health and Care
Excellence cost-effectiveness threshold. Health Technol Assess.
19:1–503. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayandharan GR, Zhong L, Sack BK, Rivers
AE, Li M, Li B, Herzog RW and Srivastava A: Optimized
adeno-associated virus (AAV)-protein phosphatase-5 helper viruses
for efficient liver transduction by single-stranded AAV vectors:
Therapeutic expression of factor IX at reduced vector doses. Hum
Gene Ther. 21:271–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hasan R, Sharma R, Saraya A, Chattopadhyay
TK, DattaGupta S, Walfish PG, Chauhan SS and Ralhan R: Mitogen
activated protein kinase kinase kinase 3 (MAP3K3/MEKK3)
overexpression is an early event in esophageal tumorigenesis and is
a predictor of poor disease prognosis. BMC Cancer. 14:22014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samanta AK, Huang HJ, Bast RC Jr and Liao
WS: Overexpression of MEKK3 confers resistance to apoptosis through
activation of NFkappaB. J Biol Chem. 279:7576–7583. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Xu X, Xu X, Li S, Liang Z, Hu Z, Wu
J, Zhu Y, Jin X, Wang X, et al: MicroRNA-193a-3p inhibits cell
proliferation in prostate cancer by targeting cyclin D1. Oncol
Lett. 14:5121–5128. 2017.PubMed/NCBI
|
|
36
|
Huang S, Wa Q, Pan J, Peng X, Ren D, Huang
Y, Chen X and Tang Y: Downregulation of miR-141-3p promotes bone
metastasis via activating NF-κB signaling in prostate cancer. J Exp
Clin Cancer Res. 36:1732017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao XQ, Lu HS, Zhang L, Chen LL and Gan
MF: MEKK3 and survivin expression in cervical cancer: Association
with clinicopathological factors and prognosis. Asian Pac J Cancer
Prev. 15:5271–5276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Q, Lu HS, Gan MF, Chen LX, He K, Fan
GM and Cao XQ: Expression and prognostic role of MEKK3 and pERK in
patients with renal clear cell carcinoma. Asian Pac J Cancer Prev.
16:2495–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santoro R, Zanotto M, Carbone C, Piro G,
Tortora G and Melisi D: MEKK3 sustains EMT and stemness in
pancreatic cancer by regulating YAP and TAZ transcriptional
activity. Anticancer Res. 38:1937–1946. 2018.PubMed/NCBI
|
|
40
|
Wa Q, Li L, Lin H, Peng X, Ren D, Huang Y,
He P and Huang S: Downregulation of miR19a3p promotes invasion,
migration and bone metastasis via activating TGFβ signaling in
prostate cancer. Oncol Rep. 39:81–90. 2018.PubMed/NCBI
|
|
41
|
Yang Y, Ji C, Guo S, Su X, Zhao X, Zhang
S, Liu G, Qiu X, Zhang Q, Guo H and Chen H: The miR-486-5p plays a
causative role in prostate cancer through negative regulation of
multiple tumor suppressor pathways. Oncotarget. 8:72835–72846.
2017.PubMed/NCBI
|
|
42
|
Yamada Y, Nishikawa R, Kato M, Okato A,
Arai T, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N:
Regulation of HMGB3 by antitumor miR-205-5p inhibits cancer cell
aggressiveness and is involved in prostate cancer pathogenesis. J
Hum Genet. 63:195–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanwal R, Plaga AR, Liu X, Shukla GC and
Gupta S: MicroRNAs in prostate cancer: Functional role as
biomarkers. Cancer Lett. 407:9–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nass D, Rosenwald S, Meiri E, Gilad S,
Tabibian-Keissar H, Schlosberg A, Kuker H, Sion-Vardy N, Tobar A,
Kharenko O, et al: MiR-92b and miR-9/9* are specifically expressed
in brain primary tumors and can be used to differentiate primary
from metastatic brain tumors. Brain Pathol. 19:375–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shiiyama R, Fukushima S, Jinnin M,
Yamashita J, Miyashita A, Nakahara S, Kogi A, Aoi J, Masuguchi S,
Inoue Y and Ihn H: Sensitive detection of melanoma metastasis using
circulating microRNA expression profiles. Melanoma Res. 23:366–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J, Jia J, Zhao L, Li X, Xie Q, Chen
X, Wang J and Lu F: Down-regulation of microRNA-9 leads to
activation of IL-6/Jak/STAT3 pathway through directly targeting
IL-6 in HeLa cell. Mol Carcinog. 55:732–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohammadi-Yeganeh S, Mansouri A and Paryan
M: Targeting of miR9/NOTCH1 interaction reduces metastatic behavior
in triple-negative breast cancer. Chem Biol Drug Des. 86:1185–1191.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Higashi T, Hayashi H, Ishimoto T, Takeyama
H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, et al:
miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in
hepatocellular carcinoma cells. Br J Cancer. 113:252–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo H, Zhang H, Zhang Z, Zhang X, Ning B,
Guo J, Nie N, Liu B and Wu X: Down-regulated miR-9 and miR-433 in
human gastric carcinoma. J Exp Clin Cancer Res. 28:822009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Di Y, Li S, Wang L, Zhang Y and Dorf ME:
Homeostatic interactions between MEKK3 and TAK1 involved in
NF-kappaB signaling. Cell Signal. 20:705–713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baigude H, Ahsanullah, Li Z, Zhou Y and
Rana TM: miR-TRAP: A benchtop chemical biology strategy to identify
microRNA targets. Angew Chem Int Ed Engl. 51:5880–5883. 2012.
View Article : Google Scholar : PubMed/NCBI
|