Introduction
Prostate cancer is the most common cancer to affect
men and is the second leading cause of cancer-associated mortality
in men (1). Present guidelines
suggest that treatment decisions should be made based on tumour
stage: Surgery and radiation therapy are common methods used to
treat early, localized cancer (2).
In addition, paclitaxel (a taxane antimitotic agent) is used as a
standard first-line chemotherapeutic agent (3,4).
Despite these combinatory approaches, a number of patients relapse
following primary treatment, and a substantial proportion of men
with metastatic, castration-resistant, prostate cancer develop
resistance towards paclitaxel and eventually succumb to the
disease. The mechanisms underlying acquired paclitaxel resistance
are hypothesised to involve different β-tubulin isoforms, mutations
of multi-drug resistance-associated genes and/or aberrant
activation of drug efflux pumps. Despite advances over previous
years in the understanding of the molecular basis of resistance,
treating patients with paclitaxel-resistant prostate cancer remains
a clinical challenge.
MicroRNAs (miRNAs) are short, highly conserved,
small non-coding RNA molecules that downregulate gene expression at
the post-transcriptional level by binding to the 3′-untranslated
regions (UTR) of mRNAs (5,6). miRNAs are involved in various
biological processes, including tumour proliferation, promotion,
invasion, angiogenesis and drug resistance (7,8).
Numerous miRNAs have been demonstrated to mediate chemo-resistance
in prostate cancer, including miR-148a, miR-200c, miR-205, miR-21,
miR-31, miR-34 and miR-375 (2,8–12).
The miR-302 miRNA family regulates cell proliferation and
differentiation, and the upregulation of miR-302 has been suggested
to lead to drug resistance (13–17);
however, the involvement of miR-302 in the chemotherapeutic
response in prostate cancer is unclear. A previous study of head
and neck cancer indicated that the miR-302-mediated regulation of
lysine demethylase 1B (AOF1), lysine demethylase 1A (AOF2) and DNA
methyltransferase 1 (DNMT1) is associated with chemo-resistance
(17). These data suggest that
miR-302 may be involved in chemo-resistance towards paclitaxel in
patients with prostate cancer.
The present study aimed to characterize in more
detail the molecular mechanisms that enhance chemo-resistance in
patients with prostate cancer. By performing a series of in
vitro assays, increased expression levels of miR-302a in the
human PC-3PR prostate cancer cell line were observed. Increased
miR-302a levels significantly increased the chemo-resistance of
prostate cancer cells exposed to paclitaxel. Additional analysis
demonstrated that miR-302a may confer chemo-resistance by
decreasing the expression levels of breast cancer resistance
protein (BCRP), permeability glycoprotein 1 (P-glycoprotein) and
AOF2.
Materials and methods
Cell culture and chemicals
The human prostate cancer PC-3 cell line [American
Type Culture Collection CRL1435TM; paclitaxel half maximal
inhibitory concentration (IC50)=2.30 nM] and the
paclitaxel-resistant PC-3 cells (PC-3PR; paclitaxel
IC50=97.87 nM) were purchased from the Guangxi Nanning
Longevity Biological Technology Co., Ltd. (Guangxi, China). The
cells were cultured in RPMI-1640 media (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% foetal
bovine serum and 10% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and maintained at 37°C in a humidified incubator
with a 5% CO2 atmosphere. PC-3 and PC-3PR cells were
seeded in 6-well plates (3×105 per well) and were incubated prior
to exposure to paclitaxel (PC-3: 2.3 nM and PC-3PR: 97.8 nM, Cayman
Chemical Company, Ann Arbor, MI, USA) for 48 h at 37°C in a
humidified incubator with a 5% CO2 atmosphere. The cells
were then harvested by 0.25% trypsinisation (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) for subsequent analyses.
Isolation of total RNA and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells harvested by
0.25% trypsinisation, according to the TRIzol (Gibco; Thermo Fisher
Scientific, Inc.) manufacturer's protocol. To detect miR-302a,
AOF2, BCRP and P-glycoprotein, 1 µg total RNA per sample was
converted to cDNA using a cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The cDNA was then amplified and detected
using a SYBR-Green PCR kit (Qiagen, Duesseldorf, Germany). β-actin
was used as an endogenous control. For detection of mRNAs, cDNA
products were synthesized using the miScript Reverse Transcription
kit (Qiagen GmbH, Hilden, Germany). Primers specific for miR-302a
and the endogenous control U6 were purchased from Qiagen (Table I). RT-qPCR was performed using the
miScript SYBR Green PCR kit (Qiagen GmbH). All reactions were
performed in triplicate on a Bio-Rad C1000 thermal cycler (CFX-96
real-time PCR detection systems; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The following thermocycling conditions were
used for the qPCR: 30 sec at 95°C; 40 cycles of 95°C for 5 sec and
60°C for 30 sec. Fold changes in miRNA or mRNA expression were
calculated using the 2−ΔΔCq method (18).
 | Table I.Primers used for reverse transcription
quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse transcription
quantitative polymerase chain reaction analysis.
| Name | Forward | Reverse |
|---|
| β-actin |
5′-AGCGAGCATCCCCCAAAGTT-3′ |
5′-GGGCACGAAGGCTCATCATT-3′ |
| BCRP |
5′-CAGGTGGAGGCAAATCTTCG-3′ |
5′-AGTTGTTGCAAGCCGAAGAG-3′ |
| AOF2 |
5′-TTTGATCGGGTGTTCTGGGA-3′ |
5′-ATCGGCCAACAATCACATCG-3′ |
| P-glycoprotein |
5′-GAGCCTACTTGGTGGCACAT-3′ |
5′-TCCTTCCAATGTGTTCGGCA-3′ |
| U6 |
5′-CGCTTCGGCAGCACATATAC-3′ |
5′-AAATATGGAACGCTTCACGA-3′ |
| miR-302 |
5′-TGCGCTAAGTGCTTCCATGTTTT-3′ |
|
| miR-302 loop
primer |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATT |
|
|
|
CGCACTGGATACGACTCACCAAA-3′ |
|
| miRNA universal
primer |
5′-CCAGTGCAGGGTCCGAGGTATT-3′ |
|
Transfection with miR-302a mimics,
inhibitors and negative controls
The miRNA mimics and inhibitors were transiently
transfected into PC-3 and PC-3PR cells using
Lipofectamine® RNAiMAX Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The miR-302a mimic (5′-ACUUAAACGUGGAUGUACUUGCU-3′), inhibitor
(5′-UCGUUCAUGUAGGUGCAAAUUCA-3′), and the respective negative
controls (NCs) were obtained from Ambion; Thermo Fisher Scientific,
Inc. The final concentration of miR-302a mimics, inhibitors and the
NC in the transfection system was 200 nM. Transfection efficiency
was assessed by fluorescence microscopy (magnification, ×40) and
RT-qPCR, as mentioned above after 48 h. The cells were collected 48
h following transfection for flow cytometry, western blot analysis
and RT-qPCR.
IC50 values assay
PC-3 (0, 1, 2, 4, 6 and 8 nM) and PC-3PR (0, 10, 20,
40, 80 and 120 nM) cells were treated with paclitaxel in various
concentrations. Following incubation of the cells (5×105
cells/well) in a 96-well plate at 37°C for 48 h with paclitaxel,
the cell culture medium was removed. A total of 10 µl Cell-Counting
Kit 8 (CCK-8) solution (Dojindo Molecular Technologies, Inc.)
dissolved in 100 µl medium (as described above), added to each well
and incubated at 37°C for 1 h in the dark. Then, the optical
density (OD) of each well at a wavelength of 450 nm was measured
using a microplate reader. The IC50 was calculated using
nonlinear regression modelling of the exponential data (SPSS
Software v22.0; IBM Corp., Armonk, NY, USA).
Cell proliferation assay
Cell proliferation was evaluated using a CCK-8
assay, according to the manufacturer's protocol (Dojindo Molecular
Technologies, Inc.). PC-3 and PC-3R cells were seeded in 96-well
plates (2,000 cells/well) and 10 µl CCK-8 solution was added to
each well at 48 h following transfection. The cells were then
incubated at 37°C for an additional 2 h. The absorbance values of
the cultures were then measured at 450 nm using a MultiskanTM FC
Microplate Photometer (Thermo Fisher Scientific, Inc.).
Apoptosis assay
Apoptosis was assessed using an Annexin V-FITC
Apoptosis Detection kit (556547; BD Pharmingen; BD Biosciences, San
Jose, CA, USA). Cells were treated with miR-302a mimics, inhibitors
or NCs, along with paclitaxel (PC-3=2.3 nM; PC-3PR=97.8 nM), then
harvested in the logarithmic growth phase and washed twice with
PBS. Following this, 1×106 cells, which had been washed twice with
PBS prior to resuspension in 1X Annexin V Binding Buffer (BD
Pharmingen; BD Biosciences), were incubated with Annexin V-PE (5
µl) and 7-aminoactinomycin (5 µl) on ice for 30 min to stain the
cells, followed by the addition of 400 µl 1X binding buffer to each
sample. Stained cells were measured by FACSCalibur flow cytometry
using Cell Quest Pro software (version 5.1) (both from BD
Biosciences). Data were then analysed using FlowJo v.10 software
(FlowJo LLC, Ashland, OR, USA).
Cell cycle assay
Cells were collected 48 h after transfection with
the miR-302a inhibitors, mimics or NCs and stained with propidium
iodide/RNase Staining Buffer (BD Pharmingen; BD Biosciences),
according to the manufacturer's protocol. Stained cells were
measured by flow cytometry on a FACSCalibur instrument using Cell
Quest Pro Software (version 5.1) (both from BD Biosciences). The
data ware analysed using FlowJo10 software (version 10.4.2; FlowJo
LLC).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS)
containing a Complete Protease Inhibitor Cocktail (Roche
Diagnostics, Indianapolis, IN, USA). Protein concentration was
determined using a Bio-Rad DC protein assay (Bio-Rad Laboratories,
Inc.). Total proteins (30 µg) from the cell lysate were separated
in 12% gel by SDS-PAGE and transferred to a nitrocellulose
membrane. The membrane was then blocked in 5% non-fat milk in PBS
overnight at 4°C and incubated with primary antibodies at 37°C for
2 h. Following washing three times with PBS + 0.05% Tween-20 for 30
min, the membrane was incubated with a 1:3,000 dilution of the
secondary goat anti-mouse antibody (Biotinylated; BA-9200-1.5;
Vector Laboratories, Inc., Burlingame, CA, USA) in TBS-0.05%
Tween-20 for 1 h at room temperature. Following additional washes
(three times), the proteins of interest were detected using a
Chemiluminescent Horseradish peroxidase Antibody Detection kit
(Denville Scientific, South Plainfield, NJ, USA) and the signals
were captured using an electrochemiluminescent system (PerkinElmer,
Inc., Waltham, MA, USA). The anti-cyclin-dependent kinase inhibitor
1 (p21) polyclonal antibody was used at a 1:1,000 dilution (cat.
no. SAB4500065; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), the
anti-β-actin antibody was used at a 1:5,000 dilution (cat. no.
3700; Cell Signaling Technology, Inc., Danvers, MA, USA), the
anti-B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax) protein
antibody was used at a 1:1,000 dilution (cat. no. 2772; Cell
Signaling Technology, Inc.), the anti-BCRP protein antibody was
used at a 1:1,000 dilution (cat. no. 4477; Cell Signaling
Technology, Inc.), the anti-Bcl-2 protein antibody was used at a
1:1,000 dilution (#15071, Cell Signaling Technology) and the
anti-mouse IgG (H+L) antibody was used at a 1:1,000 dilution (cat.
no. 14709; Cell Signaling Technology, Inc.).
TargetScan analysis
The 3′-UTR segments of AOF2, BCRP and P-glycoprotein
were predicted to interact with miR-302a using TargetScan software
(https://www.targetscan.org; release 7.2;
accessed March 2018).
Statistical analysis
Data are presented as the means ± standard
deviation. Student's t-tests were performed in Microsoft Excel
(version 15.00; Microsoft Corporation, Redmond, WA, USA) to perform
comparisons between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-302a is upregulated in prostate
cancer cells and underlies paclitaxel resistance
The expression of miR-302a was first analysed in
non-resistant prostate cancer PC-3 cells (Fig. 1A) and paclitaxel-resistant prostate
cancer PC3-PR cells (Fig. 1B), and
it was identified that miR-302a was significantly upregulated
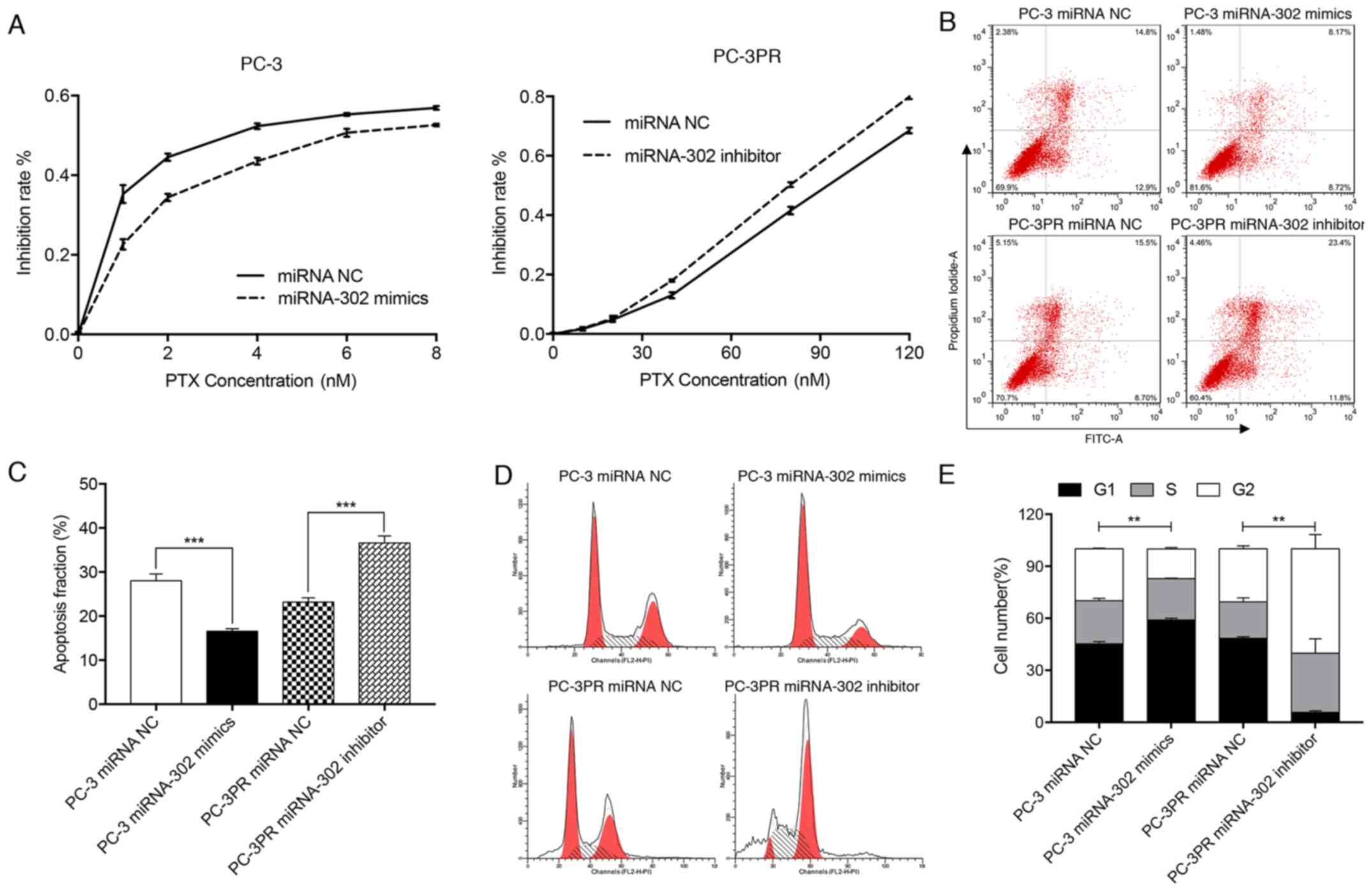
(114%) in the PC3-PR cells compared with the PC-3 cells (Fig. 1C). A CCK-8 assay of miR-302a mimic-
or miR-302a inhibitor-transfected PC-3 and PC3-PR cells was then
performed to determine whether miR-302a was associated with
proliferation in prostate cancer cells. As indicated in Fig. 2A, in PC-3 cells transfected with
miRNA NC, the IC30 and IC50 values for
paclitaxel (treated with 0, 1, 2, 4, 6 or 8 nM for 48 h at 37°C)
were 0.70 and 3.20 nM, respectively, while in PC-3 cells
transfected with the miR-302a mimic the IC30 and
IC50 values for paclitaxel (treated with 0, 1, 2, 4, 6
or 8 nM for 48 h at 37°C) were 1.58 and 6.12 nM, respectively.
Conversely, in PC3-PR cells treated with miRNA NC, the
IC30 and IC50 values for paclitaxel (treated
with 0, 10, 20, 40, 80 or 120 nM for 48 h at 37°C) were 64.80 and
91.44 nM, respectively, whereas in the PC3-PR cells treated with
the miR-302a inhibitor, the IC30 and IC50
values for paclitaxel (treated with 0, 10, 20, 40, 80 or 120 nM for
48 h at 37°C) were 55.50 and 79.64 nM, respectively. These data
indicate that high levels of expression of miR-302a may enhance
drug resistance in prostate cancer cells.
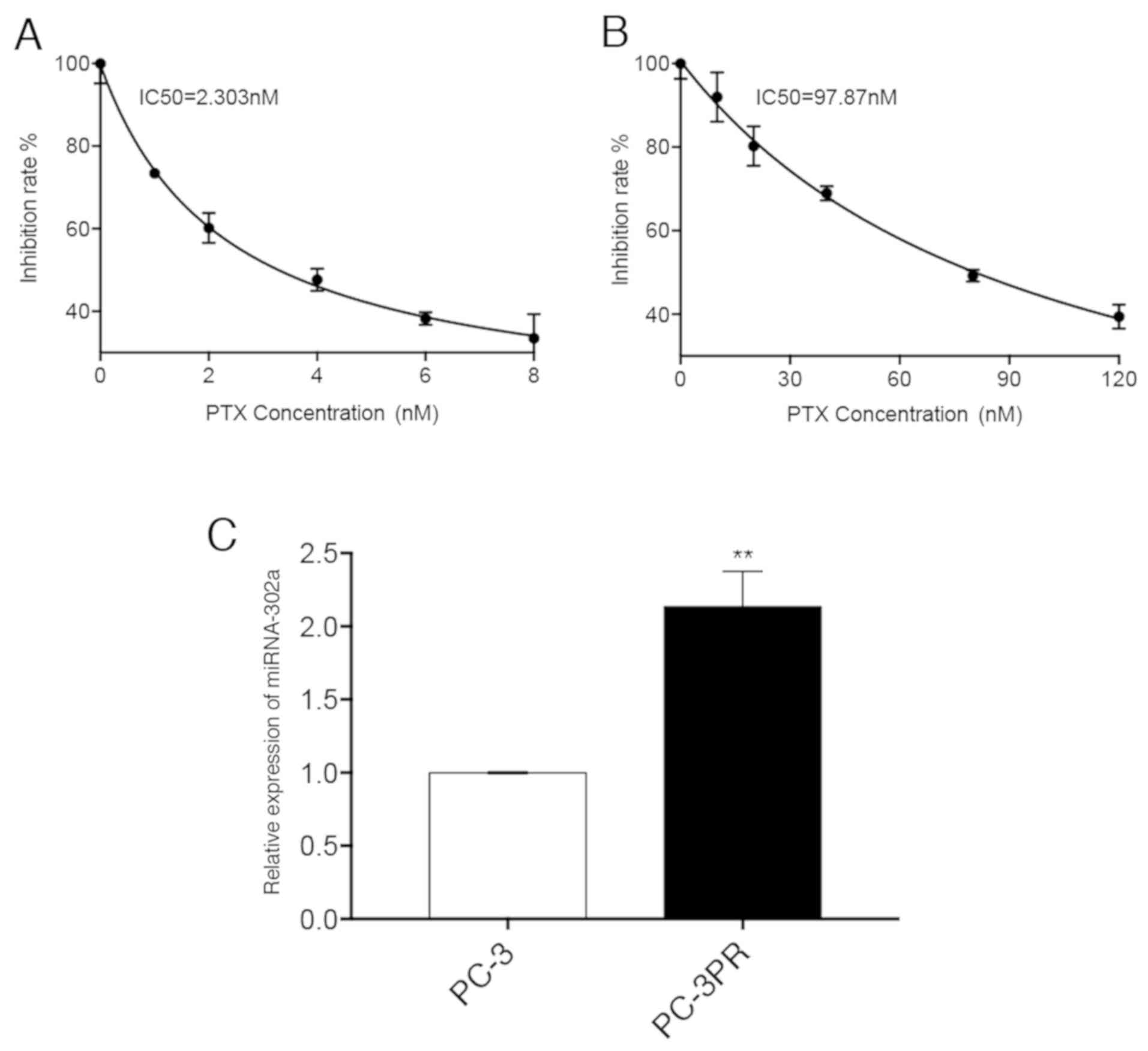 | Figure 1.Difference in miR-302a expression
levels between PC-3 and PC-3PR cells. (A) PC-3 cells were treated
with different concentrations (0, 1, 2, 4, 6 and 8 nM) of PTX for
48 h. (B) PC-3PR cells were treated with different concentrations
(0, 10, 20, 40, 80 and 120 nM) of PTX for 48 h. Inhibition of cell
proliferation was assessed using the CCK-8 assay. (C) The
expression levels of miR-302a were assessed by reverse
transcription quantitative polymerase chain reaction. **P<0.01
vs. PC-3. miR, microRNA; PTX, paclitaxel. |
High miR-302a levels inhibit apoptosis
in paclitaxel-resistant cells
Flow cytometry was used to quantify apoptosis in the
prostate cancer cell lines treated with paclitaxel. It was
identified that the apoptosis fraction in PC-3 cells transfected
with the miR-302a mimic was significantly decreased compared with
that of PC-3PR cells, whereas the apoptosis fraction in PC-3PR
cells transfected with the miR-302a inhibitor was significantly
increased compared with PC-3PR cells transfected with the NC
(P<0.001) (Fig. 2B and C).
These data indicate that high levels of miR-302a inhibit
apoptosis.
Effect of miR-302a on
paclitaxel-induced G2/M arrest
Flow cytometry was used to assess the cell cycle
profile in the 2 prostate cancer cell lines. Compared with the PC-3
cells transfected with the miRNA NC, the proportion of cells in the
G1/G0 phase in PC-3 cells transfected with the miR-302a mimics was
significantly increased, the proportion of G2/M cells was
significantly decreased and the proportion of cells in S phase did
not change (Fig. 2D and E). In
PC-3PR cells transfected with the miR-302a inhibitor, the
proportion of cells in the G1/G0 phase was significantly decreased,
and the proportion of cells in the G2/M phase and S phase cells
were significantly increased compared with the PC-3PR cells
transfected with the miRNA NC. These data indicate that high levels
of miR-302a expression attenuate cell cycle arrest at G2/M
stage.
High levels of miR-302a promote AOF2,
BCRP and P-glycoprotein expression in paclitaxel-resistant
cells
To determine the effect of miR-302a expression
levels on paclitaxel-induced downstream gene expression, miRNA
target prediction algorithms (http://targetscan.org) were used to screen miR-302a
target genes, and AOF2, BCRP and P-glycoprotein were identified as
tentative targets of miR-302a (Fig.
3A-C). RT-qPCR was then used to quantify the relative
expression of miR-302a, AOF2, BCRP and P-glycoprotein. As indicated
in Fig. 3D-G, compared with
non-resistant prostate cancer cells (PC-3 cells transfected with
the miRNA NC), the levels of miR-302a, AOF2, BCRP and
P-glycoprotein were significantly upregulated in PC-3 cells
transfected with miR-302a, and in PC-3PR cells transfected with the
miRNA NC. The opposite effect was observed in PC-3PR cells
transfected with the miR-302a inhibitor. These results suggest that
miR-302a increases the expression of downstream targets AOF2, BCRP
and P-glycoprotein.
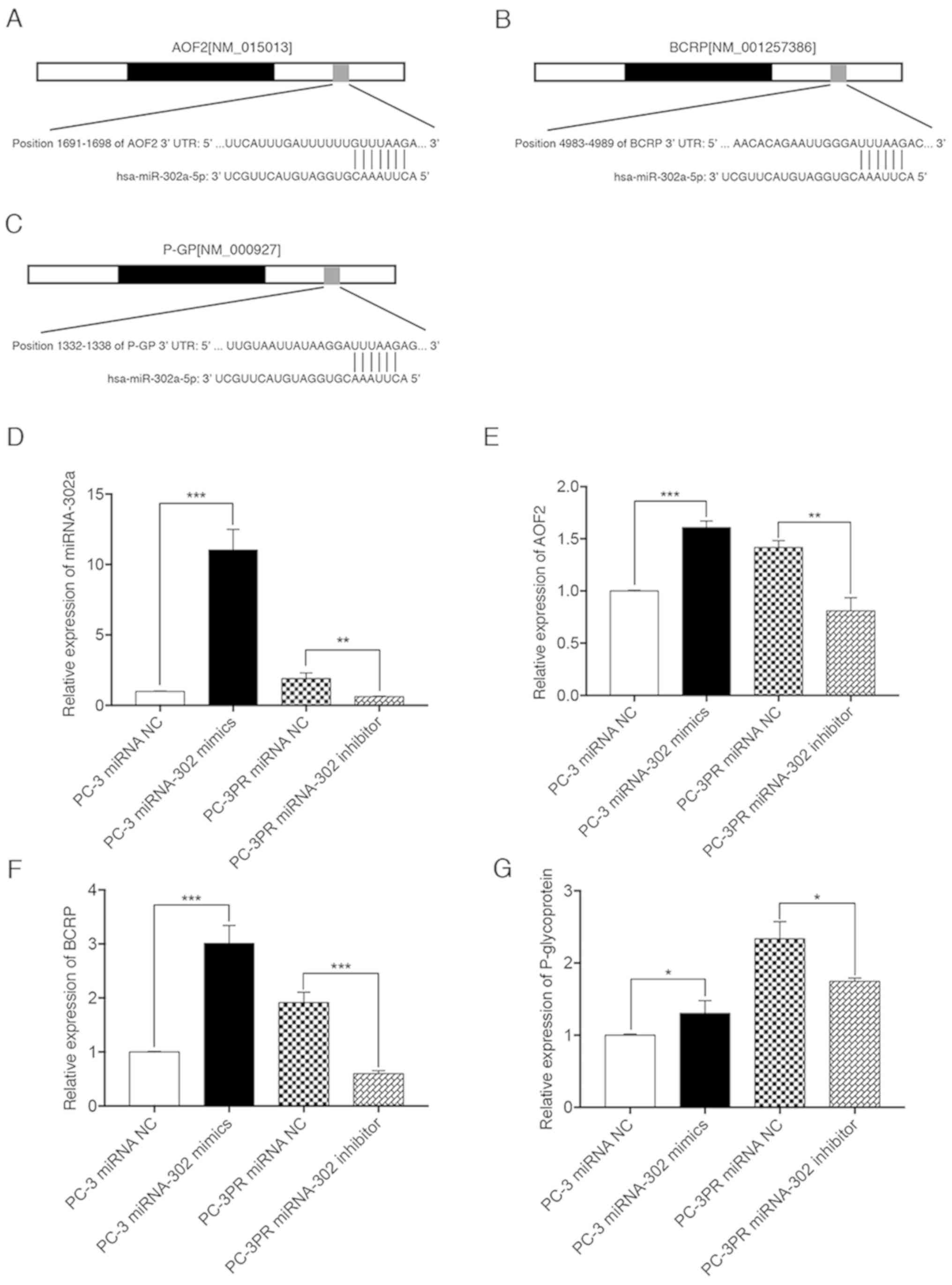 | Figure 3.miR-302a increases the expression of
downstream genes, AOF2, BCRP and P-glycoprotein. PC-3 cells were
transfected with the miR-302a or NC mimics in the presence of
paclitaxel (2.3 nM) for 48 h. PC-3PR cells were transfected with
the miR-302a inhibitor or NC inhibitors in the presence of
paclitaxel (97.8 nM) for 48 h. Binding sites of miR-302a in 3′-UTR
of human (A) AOF2, (B) BCRP and (C) P-glycoprotein mRNA were
predicted with miRNA target prediction algorithms. The expression
levels of (D) miR-302a, (E) AOF2, (F) BCRP and (G) P-glycoprotein
were assessed by qPCR. *P<0.05, **P<0.01 and ***P<0.01.
miRNA, microRNA; NC, negative control; AOF2, lysine demethylase 1A;
BCRP, breast cancer resistance protein; P-glycoprotein,
permeability glycoprotein 1; UTR, untranslated region; hsa, Homo
sapiens. |
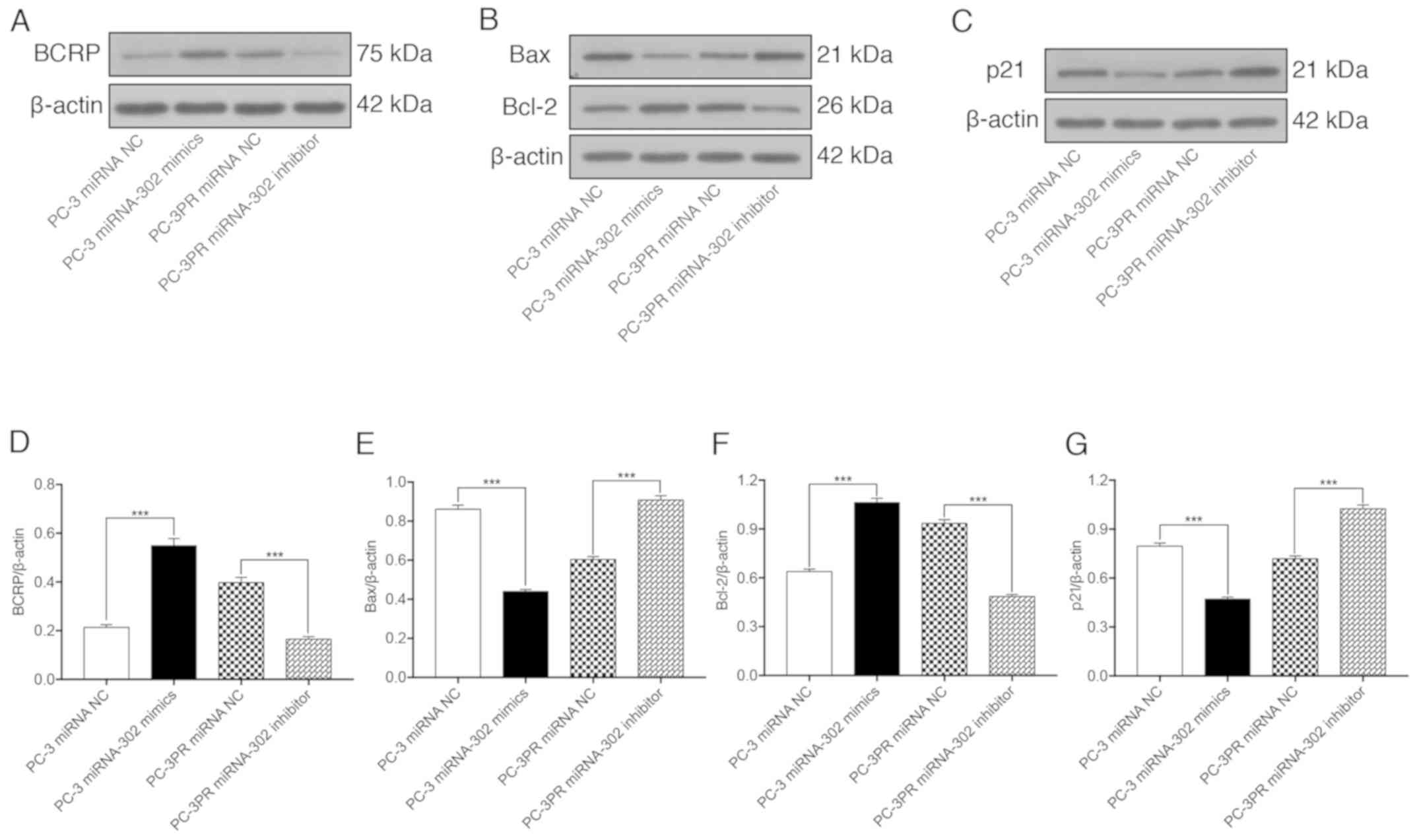
miR-302a regulates apoptosis and
cycle-associated proteins in paclitaxel-resistant cells
The effects of miR-302a on the paclitaxel-induced
expression of BCRP, the apoptosis-associated proteins Bax and Bcl-2
and the cell cycle regulator p21 were then assessed in prostate
cancer cells. As indicated in Fig.
4, compared with non-resistant prostate cancer cells (PC-3
cells transfected with the miRNA NC), the expression of BCRP and
Bcl-2 were significantly upregulated at the protein level in PC-3
cells transfected with miR-302a and in resistant PC-3PR cells
transfected with the miRNA NC, whereas the expression levels of Bax
and p21 were significantly downregulated. The opposite effect was
observed in the PC-3PR cells transfected with the miR-302a
inhibitor. These data support the hypothesis that high levels of
miR-302a increase the expression of BCRP and Bcl-2 and inhibit the
expression of the pro-apoptotic protein Bax and the cell cycle
regulator p21.
Discussion
Paclitaxel is a common chemotherapeutic agent that
is efficacious in a number of types of cancer, but in certain
patients, its efficacy is limited due to the development of drug
resistance. Therefore, it is critical to determine optimal
chemotherapeutic protocols and identify predictive markers of
resistance to assist in stratifying patients at risk. Increasing
evidence suggests that various miRNAs are involved in the
development of chemo-resistance. miRNA screening may be useful to
identify the subgroups of patients who are paclitaxel-resistant and
determine the molecular mechanisms of chemo-resistance. By directly
targeting protein-coding genes, miRNAs may inhibit genes that are
required for paclitaxel-induced apoptosis, cell cycle arrest or the
signalling pathways that render cells resistant to therapy.
Numerous miRNAs have been implicated in controlling
chemo-resistance in prostate cancer. The present study addressed
the effects of miR-302a on paclitaxel resistance and its downstream
targets using in vitro assays.
miRNAs are the most commonly studied class of
non-coding RNAs (~22 nucleotides); these RNAs cause
post-transcriptional gene silencing by regulating the translation
of mRNAs into proteins. The miR-302/367 cluster is formed from 4
highly homologous miRNAs, miR-302b, miR-302c, miR-302a, miR-302d,
and miR-367, in a 5′-to-3′ direction (15). The present study demonstrated that
the upregulation of miR-302a leads to chemo-resistance and tumour
progression; however, this result contradicts other studies
indicating that in other tumour types it targets key oncogenes
mediating chemo-sensitivity (19,20).
Previous studies have revealed that miR-302/367 is involved in
maintaining stemness and reprogramming somatic cells into induced
pluripotent stem cells (13,14,21,22).
Other studies have suggested that miR-302 maintains the
tumorigenesis of human pluripotent stem cells by inhibiting cyclin
dependent kinase 4/6 (CDK4/6) and CDK2 (15,23).
miR-302b mediates cell proliferation by inhibiting
the epidermal growth factor receptor/RAC-beta serine/threonine
protein kinase/G1/S-specific cyclin-D1 signalling pathway in
hepatocellular carcinoma cells (24,25).
A previous study in head and neck cancer demonstrated that miR-302
was associated with chemo-resistance by regulating AOF1/AOF2/DNMT1
(17). These data suggest that
chemo-resistance in prostate cancer may also be associated with
changes in the expression of these candidate genes. Therefore,
RT-qPCR and western blot analysis of a sub-set of these genes in
paclitaxel resistant and non-resistant prostate cancer cell lines
was performed in the present study, and it was confirmed that there
was an association between elevated miR-302a expression levels and
increased expression of AOF2, BCRP, P-glycoprotein and Bcl-2, along
with decreased levels of Bax and p21 expression.
Overexpression of AOF2, also known as
lysine-specific histone demethylase 1A, correlates with poor
survival and castration-resistant prostate cancer (26). Conversely, AOF2 inhibition
decreases v-myc avian myelocytomatosis viral oncogene homolog
expression in poorly differentiated prostate cancer cell lines and
exhibits a therapeutic benefit in paclitaxel-resistant prostate
cancer (27). BCRP and
P-glycoprotein are drug efflux transporters that belong to the
adenosine 5′-triphosphate binding cassette family of proteins. BCRP
and P-glycoprotein are located in the apical membrane of epithelial
cells, where they transport drug substrates including paclitaxel
out of cancer cells (28–30). Proteins of the Bcl-2 family,
particularly Bcl-2 and Bax, are also involved in
mitochondria-mediated apoptotic pathways (31). In terms of cell-cycle kinetics, the
kinase inhibitor protein p21 binds to the CDK4 or 6 complex to
inhibit progression through the G1 phase of the cell cycle
(32).
In summary, the data from the present study
suggested that increased miR-302a expression serves an important
role in mediating resistance to paclitaxel in prostate cancer
cells. AOF2, BCRP and P-glycoprotein are all miR-302a targets and
may mediate miR-302a-induced chemo-resistance to paclitaxel in
PC-3PR cells. In addition, miR-302a may also participate in drug
resistance by regulating the expression of the apoptosis-associated
proteins Bcl-2 and Bax and the cell cycle-associated protein p21.
This novel avenue of study, based on miR-302a targeting AOF2, BCRP
and P-glycoprotein, will assist in improving the understanding of
the molecular basis of chemo-resistance, and will guide the
development of novel therapeutics for prostate cancer in the
future.
A limitation of the present study is that although
TargetScan analysis was performed, a luciferase assay was not
conducted due to funding limitations, which would have determined
whether the regulation of AOF2, BCRP and P-glycoprotein luciferase
expression was dependent on the binding of complementary 3′UTR
sequences to the miR-302a seed sequence. The other limitation is
that only one prostate cancer cell line was analysed in the present
study, as cancer resistant cells are difficult to obtain. In future
studies, other prostate cancer paclitaxel-resistant cell lines will
be included, to explore the role of miR-302a in paclitaxel
resistance in prostate cancer in more detail.
Acknowledgements
The authors would like to thank Professor Jessica
Tamanini (Department of Basic Medicine, Shenzhen University) for
editing the manuscript prior to submission.
Funding
The study was sponsored by grants from the Shenzhen
Science and Technology Innovation Committee (grant no. 20180077)
and from Shenzhen University General Hospital Science and
Technology Talent Promotion Program (grant no. SUGH-2018-001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and XW conceived and designed the study. YW, LH
and ZQ performed the experiments. YW wrote the paper. YW, LH, ZQ
and XW reviewed and edited the manuscript. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhatnagar N, Li X, Padi SK, Zhang Q, Tang
MS and Guo B: Downregulation of miR-205 and miR-31 confers
resistance to chemotherapy-induced apoptosis in prostate cancer
cells. Cell Death Dis. 1:e1052010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17-5p promotes chemotherapeutic drug
resistance and tumour metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 5:2974–2987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi N, Park J, Lee JS, Yoe J, Park GY,
Kim E, Jeon H, Cho YM, Roh TY and Lee Y:
miR-93/miR-106b/miR-375-CIC-CRABP1: A novel regulatory axis in
prostate cancer progression. Oncotarget. 6:23533–23547. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujita Y, Kojima K, Ohhashi R, Hamada N,
Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T and Ito
M: MiR-148a attenuates paclitaxel resistance of hormone-refractory,
drug-resistant prostate cancer PC3 cells by regulating MSK1
expression. J Biol Chem. 285:19076–19084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puhr M, Hoefer J, Schäfer G, Erb HH, Oh
SJ, Klocker H, Heidegger I, Neuwirt H and Culig Z:
Epithelial-to-mesenchymal transition leads to docetaxel resistance
in prostate cancer and is mediated by reduced expression of
miR-200c and miR-205. Am J Pathol. 181:2188–2201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi GH, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ and Ma CG: Involvement of
microRNA-21 in mediating chemo-resistance to docetaxel in
androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin.
31:867–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kojima K, Fujita Y, Nozawa Y, Deguchi T
and Ito M: MiR-34a attenuates paclitaxel-resistance of
hormone-refractory prostate cancer PC3 cells through direct and
indirect mechanisms. Prostate. 70:1501–1512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Lieberman R, Pan J, Zhang Q, Du M,
Zhang P, Nevalainen M, Kohli M, Shenoy NK, Meng H, et al: miR-375
induces docetaxel resistance in prostate cancer by targeting SEC23A
and YAP1. Mol Cancer. 15:702016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Card DA, Hebbar PB, Li L, Trotter KW,
Komatsu Y, Mishina Y and Archer TK: Oct4/Sox2-regulated miR-302
targets cyclin D1 in human embryonic stem cells. Mol Cell Biol.
28:6426–6438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Deng S, Zhao Z, Zhang H, Xiao J,
Song W, Gao F and Guan Y: Oct4 regulates the miR-302 cluster in P19
mouse embryonic carcinoma cells. Mol Biol Rep. 38:2155–2160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu
DT, Chen DT and Ying SY: Mir-302 reprograms human skin cancer cells
into a pluripotent ES-cell-like state. RNA. 14:2115–2124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bourguignon LY: Matrix hyaluronan promotes
specific MicroRNA upregulation leading to drug resistance and tumor
progression. Int J Mol Sci. 17:5172016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Zhao L, Xiao Q, Jiang L, He M, Bai
X, Ma M, Jiao X and Wei M: miR-302a/b/c/d cooperatively inhibit
BCRP expression to increase drug sensitivity in breast cancer
cells. Gynecol Oncol. 141:592–601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Wang Y, Jiang L, He M, Bai X, Yu L
and Wei M: MiR-302a/b/c/d cooperatively sensitizes breast cancer
cells to adriamycin via suppressing P-glycoprotein(P-gp) by
targeting MAP/ERK kinase kinase 1 (MEKK1). J Exp Clin Cancer Res.
35:252016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanyam D, Lamouille S, Judson RL, Liu
JY, Bucay N, Derynck R and Blelloch R: Multiple targets of miR-302
and miR-372 promote reprogramming of human fibroblasts to induced
pluripotent stem cells. Nat Biotechnol. 29:443–448. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lipchina I, Studer L and Betel D: The
expanding role of miR-302-367 in pluripotency and reprogramming.
Cell Cycle. 11:1517–1523. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin SL and Ying SY: Mechanism and method
for generating tumor-free iPS cells using intronic microRNA miR-302
induction. Methods Mol Biol. 936:295–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Yao J, Shi X, Hu L, Li Z, Song T
and Huang C: MicroRNA-302b suppresses cell proliferation by
targeting EGFR in human hepatocellular carcinoma SMMC-7721 cells.
BMC Cancer. 13:4482013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Yao J, Zhang X, Guo B, Le X,
Cubberly M, Li Z, Nan K, Song T and Huang C: miRNA-302b suppresses
human hepatocellular carcinoma by targeting AKT2. Mol Cancer Res.
12:190–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Y, Ahmed M, Guo H, Soares F, Hua JT,
Gao S, Lu C, Poon C, Han W, Langstein J, et al: LSD1-mediated
epigenetic reprogramming drives CENPE expression and prostate
cancer progression. Cancer Res. 77:5479–5490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta S, Weston A, Bearrs J, Thode T,
Neiss A, Soldi R and Sharma S: Reversible lysine-specific
demethylase 1 antagonist HCI-2509 inhibits growth and decreases
c-MYC in castration- and docetaxel-resistant prostate cancer cells.
Prostate Cancer Prostatic Dis. 19:349–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noguchi K, Katayama K, Mitsuhashi J and
Sugimoto Y: Functions of the breast cancer resistance protein
(BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 61:26–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Kolk DM, Vellenga E, Scheffer GL,
Müller M, Bates SE, Scheper RJ and de Vries EG: Expression and
activity of breast cancer resistance protein (BCRP) in de novo and
relapsed acute myeloid leukemia. Blood. 99:3763–3770. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Breedveld P, Beijnen JH and Schellens JH:
Use of P-glycoprotein and BCRP inhibitors to improve oral
bioavailability and CNS penetration of anticancer drugs. Trends
Pharmacol Sci. 27:17–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benadiba M, Dos Santos RR, Silva Dde O and
Colquhoun A: Inhibition of C6 rat glioma proliferation by
[Ru2Cl(Ibp)4] depends on changes in p21, p27, Bax/Bcl2 ratio and
mitochondrial membrane potential. J Inorg Biochem. 104:928–935.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sa G and Das T: Anti cancer effects of
curcumin: Cycle of life and death. Cell Div. 3:142008. View Article : Google Scholar : PubMed/NCBI
|