Introduction
Ischemic heart disease (IHD) is a major cause of
death and disability worldwide (1,2).
Increasing evidence indicates that cardiomyocyte apoptosis is
closely associated with the pathogenesis of IHD (3). Although our understanding of the
molecular components involved in cardiomyocyte apoptosis has
rapidly improved, no treatment to inhibit this cellular process is
currently available (4).
Therefore, it is crucial to further elucidate the molecular
mechanisms underlying cardiomyocyte apoptosis and identify
effective therapeutic targets for the treatment of IHD.
Long non-coding RNAs (lncRNAs) have been reported to
have important roles in the development of cardiovascular diseases
(5). LncRNAs are defined as a
subgroup of RNAs lacking a protein-coding ability with a length of
>200 nucleotides (6). Through a
variety of mechanisms, lncRNAs are involved in numerous cellular
processes, including cell apoptosis (7,8).
Furthermore, the dysregulation of certain lncRNAs is associated
with the development of various diseases, including IHD (9). Mounting evidence has indicated that
lncRNAs may interact with microRNAs (miRNAs/miRs) and function as
competing endogenous RNAs (ceRNAs) to regulate gene expression
(10).
Nuclear paraspeckle assembly transcript 1 (NEAT1), a
structural component of paraspeckle, is a newly identified
nuclear-restricted lncRNA and acts as a transcriptional regulator
for numerous genes (11). Aberrant
expression of NEAT1 has been reported in multiple different types
of tumour, including lung cancer and acute promyelocytic leukaemia
(12,13). In addition, studies have indicated
that NEAT1 is involved in the pathogenesis of diverse diseases by
regulating the activity of miRNAs (14,15);
however, whether NEAT1 is involved in the regulation of
cardiomyocyte apoptosis has remained elusive.
The present study identified that NEAT1 is
downregulated in murine heart tissues subjected to
ischemia/reperfusion (I/R) injury and in
H2O2-induced cardiomyocytes. Further in
vitro study confirmed that ectopic overexpression of NEAT1
inhibits H2O2 treatment-induced cardiomyocyte
apoptosis. Furthermore, a search of the starBase database v2.0
(http://starbase.sysu.edu.cn) indicated
that miR-125a-5p binds to NEAT1. It was also revealed that NEAT1
serves as an miRNA sponge to sequester miR-125a-5p and increase the
expression of apoptosis repressor gene B-cell lymphoma-2-like 12
(BCL2L12) in cardiomyocytes. The present study provides novel
insight into the roles of NEAT1 in cardiomyocyte apoptosis, which
may be applied in the treatment of cardiomyocyte
apoptosis-associated heart diseases.
Materials and methods
Animal experiment
In total, 20 adult male C57BL/6 mice (age, 8 weeks;
weight, 18–20 g) were purchased from Shanghai Biomodel Organism
Science & Technology Development Co., Ltd. (Shanghai, China).
Mice had access to food and water, maintained under constant
temperature at 25±1°C, 55±5% humidity, under a 12-h light/dark
cycle. All animal experiments in this study were approved by the
Animal Ethics Committee of Guangdong Cardiovascular Research
Institute (Guangzhou, China). I/R surgery was performed as
previously described (16).
Cardiomyocyte culture and
treatment
Cardiomyocytes were isolated from the ventricular
myocardium of 2-day-old Sprague Dawley rats, as described
previously (17). In brief, hearts
were dissected and cells were subsequently dispersed through
enzymatic digestion. Cultured neonatal rat cardiomyocytes were
incubated in culture medium (control group) or treated with
H2O2 at 100 µM for 0, 6, 12 and 24 h.
(H2O2 group). Cell apoptosis was detected
when cardiomyocytes were treated with 100 µM for 24 h. Cells were
cultured in Dulbeccos Modified Eagles Medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and
antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin). Cells
were incubated at 37°C with 5% CO2.
Cardiomyocyte apoptosis by terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) staining and flow cytometry
Cell apoptosis was determined by a TUNEL assay using
1×105 cells (DeadEnd™ Colorimetric TUNEL System; Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. In addition, 1×105 cardiomyocytes were stained with
Annexin V-fluorescein isothiocyanate and propidium iodide (BD
Pharmingen; Becton, Dickinson and Company, Franklin, Lakes, NJ,
USA), and then subjected to flow cytometry (CellQuest Pro software
(version 5.1; Becton, Dickinson and Company) to determine
apoptosis.
Dual-luciferase reporter assay
Luciferase reporter assay was performed according to
a published protocol (18). In
brief, the wild-type (wt) and mutant (mut) miR-125a-5p binding site
sequences in the NEAT1 3′-untranslated region were used to generate
Luc-NEAT1-wt vector and Luc-NEAT1-mut vector using pGL3 as backbone
(Promega Corporation). For reporter assays, 1×103 293 cells
(American Type Culture Collection, Manassas, VA, USA) were
transfected using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) with either NEAT1-wt or NEAT1-mut
construct (500 ng) with and without miR-125a-5p mimic. The
miR-125a-5p mimic and miR-negative control (NC) were transfected at
a concentration of 0.2 nmol per well and were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Luciferase activity
was measured 48 h after transfection using the Dual-Luciferase
Reporter Assay System (pRL vector; Promega Corporation). The
Renilla luciferase activity was normalized to the firefly
luciferase activity in the corresponding well.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RNA was reverse transcribed using the
PrimeScript RT Reagent Kit (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 15 min and at 85°C for 5 sec and qPCR was
performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.,
Dalian, China) with 35 cycles of 95°C for 1 min; 55°C for 1 min and
72°C for 1 min). The relative mRNA expression level was calculated
using the comparative cycle quantification (2−ΔΔCq)
method (19) with GAPDH as the
endogenous control to normalize the data. The primers used for
qRT-PCR were as follows: NEAT1 forward, 5′-TGGCTAGCTCAGGGCTTCAG-3′
and reverse, 5′-TCTCCTTGCCAAGCTTCCTT-3′; GAPDH forward,
5′-CGCTCTCTGCTCCTCCTGTTC-3′ and reverse,
5′-ATCCGTTGACTCCGACCTTCAC-3′.
RNA immunoprecipitation (RIP)
assays
RIP experiments were performed by using a Magna RIP
RNA-Binding Protein Immunoprecipitation Kit (EMD Millipore,
Billerica, MA, USA) according to the manufacturer's protocol. In
brief, cells were lysed in RIP lysis buffer, following incubation
with RIP buffer containing magnetic beads conjugated with
anti-argonaute 2, RISC catalytic component (AGO2) antibody.
Anti-small nuclear ribonucleoprotein U1 subunit 70 was used as a
positive control for the RIP procedure. The co-precipitated RNAs
were detected by RT-qPCR analysis performed as above-mentioned. The
primers used for miR-125a-5p was as follows: Forward:
5′-ACGGTGCTGGATGTGGCCTTT-3′, reverse:
5′-GGCCAACCGCGAGAAGATGTTTTTTTTT-3′.
Western blot analysis
Western blot analyses were performed using standard
methods (20). The primary
antibodies used were as follows: Anti-BCL2L12 (1:800 dilution;
Abcam, Cambridge, MA, USA) and anti-tubulin (1:1,000 dilution;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The signals were
detected by enhanced chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All data are expressed as the mean ± standard error.
Differences between groups were analysed using a two-tailed
Student's t-test and One-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
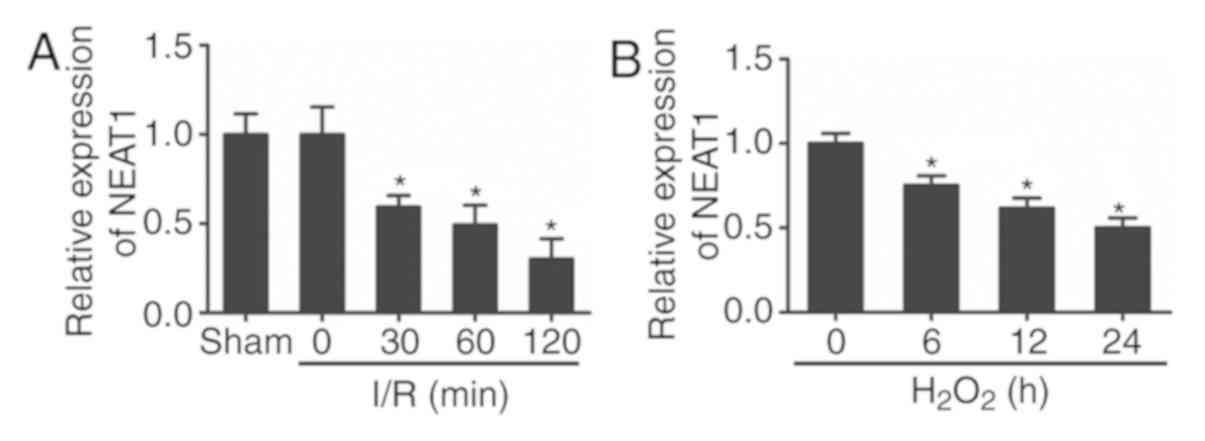
NEAT1 expression was downregulated in
heart tissues during I/R injury and
H2O2-induced cardiomyocytes
To investigate the expression and role of NEAT1 in
I/R-injured myocardium, a mouse model of I/R injury was
established. The expression of NEAT1 in the heart tissues of mice
with or without I/R injury was then assessed by RT-qPCR. As
presented in Fig. 1A, the RT-qPCR
analysis revealed that the expression of NEAT1 was significantly
downregulated in ischemic myocardium compared with sham-operated
controls.
Additionally, the primary cultured cardiomyocytes
were subjected to H2O2 treatment in
vitro. Consistent with the result obtained in the I/R-injured
hearts, the expression of NEAT1 was also downregulated in
cardiomyocytes following H2O2 treatment
(Fig. 1B). These results indicated
that the expression of NEAT1 was obviously decreased in I/R-injured
hearts and H2O2-treated cardiomyocytes.
Enhanced expression of NEAT1
attenuated H2O2-induced cardiomyocyte
apoptosis
To explore the potential biological function of
NEAT1 in cardiomyocyte apoptosis, the cultured cardiomyocytes were
transfected with a plasmid encoding NEAT1. As presented in Fig. 2A, cells transfected with NEAT1
expression plasmid exhibited obviously increased expression levels
of NEAT1 compared with those in the control and vector groups. The
TUNEL assay demonstrated that overexpression of NEAT1 inhibited
cardiomyocyte apoptosis in response to H2O2
treatment compared with that in the control and vector groups
(Fig. 2B and C). Consistent with
these results, the flow cytometry data also indicated that
overexpression of NEAT1 reduced H2O2-induced
cardiomyocyte apoptosis when compared with that in the control and
vector groups (Fig. 2D and E).
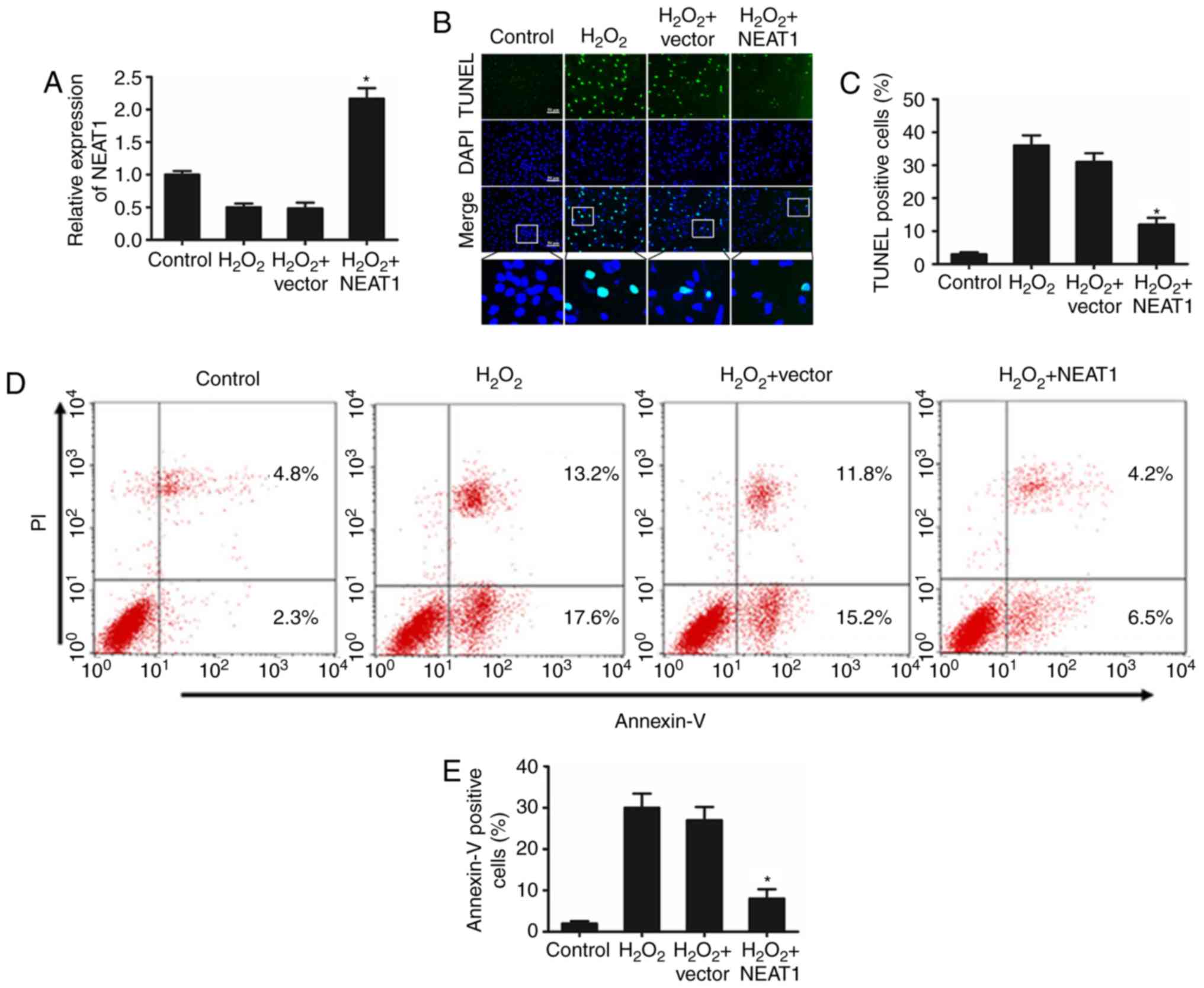 | Figure 2.Overexpression of NEAT1 inhibits
H2O2-induced cardiomyocyte apoptosis. (A)
Following transfection with a NEAT1 overexpression plasmid, the
cardiomyocytes were treated with 100 mM H2O2
for 24 h. Subsequently, the expression of NEAT1 was detected by
reverse transcription-quantitative polymerase chain reaction. Three
independent experiments were performed, and the data are presented
as the mean ± standard deviation. *P<0.05 vs. control group. A
TUNEL assay was used to (B) identify apoptotic cardiomyocytes, and
(C) the percentage of TUNEL-positive apoptotic cardiomyocytes was
quantitatively determined. Three independent experiments were
performed, and the data are presented as the mean ± standard
deviation. *P<0.05 vs. control group. Bar, 50 µm. (D) Apoptosis
analysis flow plots and (E) the percentage of the
Annexin-V-positive cell population was calculated. Three
independent experiments were performed, and the data are presented
as the mean ± standard deviation. *P<0.05 vs. control group.
NEAT1, nuclear paraspeckle assembly transcript 1; TUNEL, terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling; PI,
propidium iodide. |
NEAT1 is targeted by miR-125a-5p in an
AGO2-dependent manner
To investigate the molecular mechanisms of NEAT1 in
regulating cardiomyocyte apoptosis, the present study explored
which miRNAs are able to bind to NEAT1. A bioinformatics prediction
with Starbase v2.0 software revealed that miR-125a-5p has
complementary base pairing with NEAT1. Subsequently, a luciferase
vector containing the binding sequence of NEAT1, Luc-NEAT1-wt, and
its mutated form, Luc-NEAT1-mut, were constructed (Fig. 3A). It was confirmed that the
expression of miR-125a-5p was increased in 293 cells transfected
with miR-125a-5p mimics (Fig. 3B).
As presented in Fig. 3C, the
luciferase activity was obviously inhibited in the presence of
miR-125a-5p and was recovered when the binding site for miR-125a-5p
was mutated. These results confirmed that NEAT1 directly interacts
with miR-125a-5p.
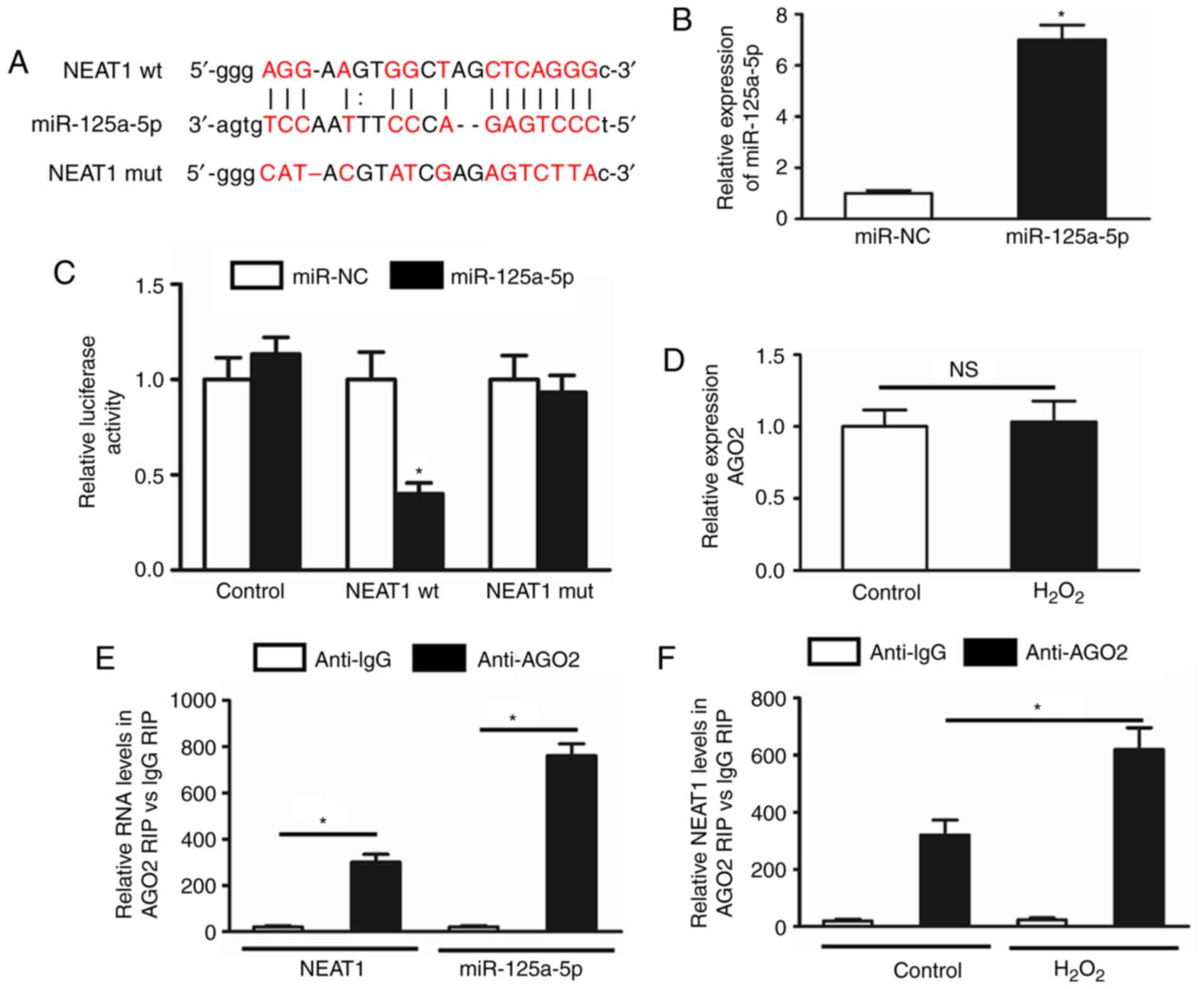 | Figure 3.NEAT1 is targeted by miR-125a-5p in
an AGO2-dependent manner. (A) Putative complementary sites between
NEAT1 and miR-125a-5p. Mutation was generated in the NEAT1 sequence
at the complementary sites of miR-125a-5p, as indicated. (B) The
miR-125a-5p expression was increased in 293 cells transfected with
miR-125a-5p mimics. (C) Luciferase activity in 293 cells
co-transfected with miR-125a-5p mimics or NC, and luciferase
reporters containing no insert (control), NEAT1 wt or NEAT1 mut.
Three independent experiments were performed, and the data are
presented as the mean ± standard deviation. *P<0.05 vs. miR-NC
group. (D) The expression of AGO2 was detected in cardiomyocytes
subjected to H2O2 treatment. (E) A RIP assay
was performed to confirm whether NEAT1 and miR-125a-5p directly
bind to AGO2 in cardiomyocytes. (F) The RIP experiments were
performed under H2O2 treatment, and the
expression of NEAT1 was then assessed by reverse
transcription-quantitative polymerase chain reaction analysis.
Three independent experiments were performed, and the data are
shown as the mean ± standard deviation. *P<0.05 vs IgG. NEAT1,
nuclear paraspeckle assembly transcript 1; wt, wild-type; miR,
microRNA; mut, mutant; NC, negative control; AGO2, argonaute 2,
RISC catalytic component; RIP, RNA immunoprecipitation. |
Subsequently, to confirm the pivotal roles of
miR-125a-5p in this effect, an RIP assay was performed on AGO2,
which is the core component of the RNA-induced silencing complex.
First, it was indicated that H2O2 treatment
had no effect on the expression of AGO2 in cardiomyocytes (Fig. 3D). Furthermore, the RIP assay
indicated that NEAT1 and miR-125a-5p were preferentially enriched
in the AGO2-containing miRNA ribonucleoprotein complex when
compared with the immunoglobulin G immunoprecipitates (Fig. 3E). In addition, the binding of
NEAT1 to AGO2 in cardiomyocytes was enhanced under
H2O2 treatment (Fig. 3F).
NEAT1 regulates cardiomyocyte
apoptosis through targeting miR-125a-5p and BCL2L12
To investigate the role of miR-125a-5p in
cardiomyocyte apoptosis, the expression of miR-125a-5p was examined
in I/R-injured mouse hearts and in H2O2
treated cardiomyocytes. The results indicated that the expression
levels of miR-125a-5p were significantly upregulated in I/R-injured
myocardium and in H2O2-induced cardiomyocytes
(Fig. 4A and B). The effects of
miR-125a-5p on cardiomyocyte apoptosis following
H2O2 treatment were then investigated. The
expression level of miR-125a-5p was examined following transfection
with miR-125a-5p inhibitor and mimic (Fig. 4C). As expected, the results
demonstrated that knockdown of miR-125a-5p significantly suppressed
cardiomyocyte apoptosis induced by H2O2
(Fig. 4D and E).
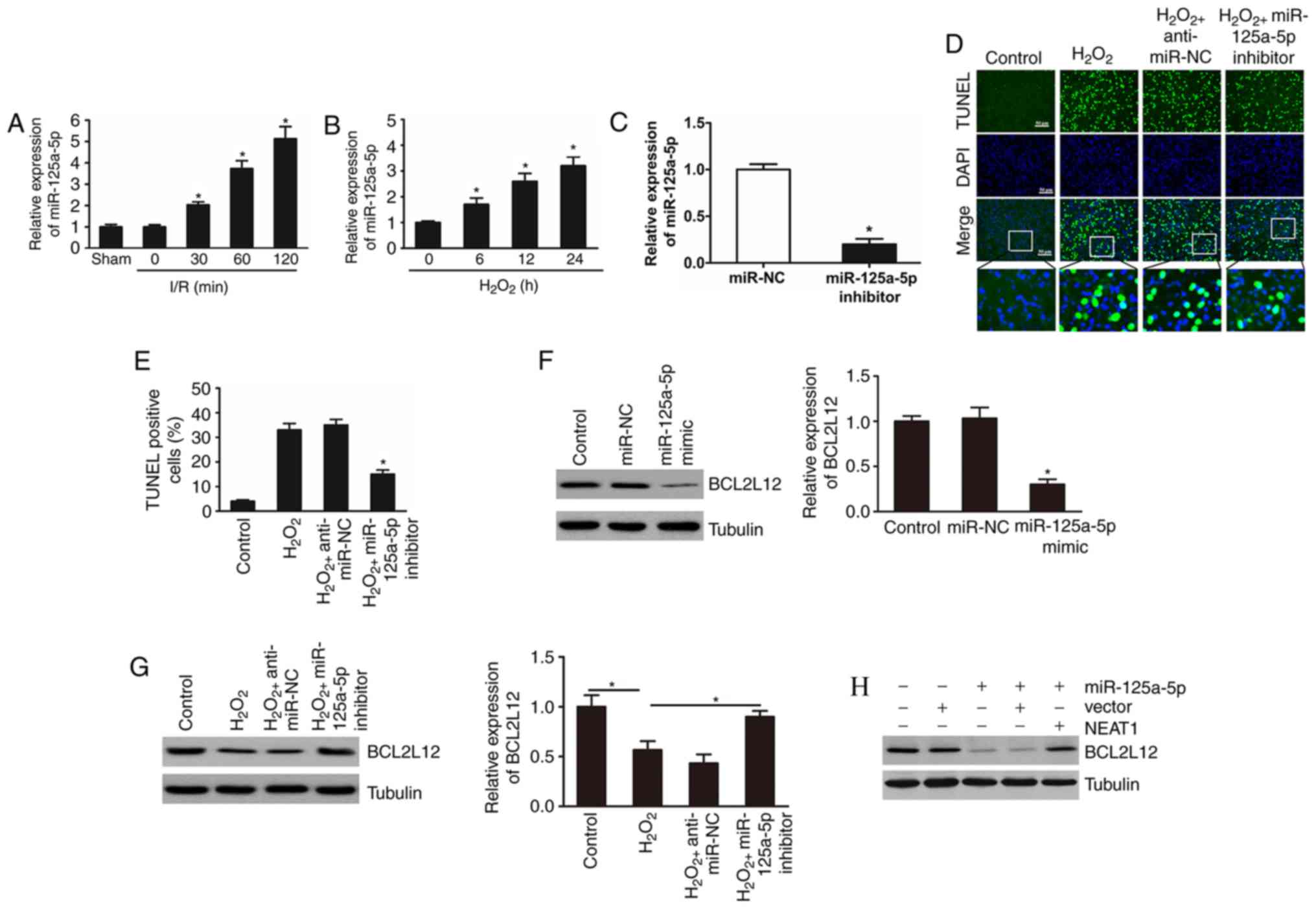 | Figure 4.NEAT1 regulates cardiomyocyte
apoptosis through miR-125a-5p and BCL2L12. (A) Mice were subjected
to I/R injury and the expression of miR-125a-5p was determined
after the indicated periods of time (n=6 mice for each time-point).
*P<0.05 vs. sham. (B) Cardiomyocytes were treated with 100 mM
H2O2 and miR-125a-5p levels were detected by
reverse transcription-quantitative polymerase chain reaction. Three
independent experiments were performed, and the data are shown as
the mean ± standard deviation. *P<0.05 vs. 0 h. (C) miR-125a-5p
expression level was decreased in cardiomyocytes transfected with
miR-125a-5p inhibitor. *P<0.05 vs. miR-NC. (D) TUNEL assay was
used to identify apoptotic cardiomyocytes, and (E) the percentage
of apoptotic cardiomyocytes was quantitatively determined. Three
independent experiments were performed, and the data are shown as
the mean ± standard deviation. *P<0.05 vs. control group. Bar,
50 µm. (F) miR-125a-5p mimics or NC were used to transfect
cardiomyocytes. The expression of BCL2L12 was detected by
immunoblot analysis. Three independent experiments were performed,
and the data are shown as the mean ± standard deviation. *P<0.05
vs. control group. (G) BCL2L12 levels in different groups were
detected by immunoblot analysis. Three independent experiments were
performed, and the data are shown as the mean ± standard deviation.
*P<0.05 vs. control group. (H and I) Cardiomyocytes were
transfected with miR-125a-5p mimics, NEAT1, and BCL2L12 levels were
determined by immunoblot analysis. Three independent experiments
were performed, and the data are shown as the mean ± standard
deviation. *P<0.05 vs. control group. (J and K) BCL2L12
expression was analysed by immunoblot in the different groups.
Three independent experiments were performed, and the data are
shown as the mean ± standard deviation. *P<0.05 vs. control
group. (L) The percentage of apoptotic cardiomyocytes was
quantitatively determined from (M) TUNEL analysis. Three
independent experiments were performed, and the data are shown as
the mean standard deviation. *P<0.05 vs. control group. Bar, 50
µm. miR, microRNA; I/R, ischemia/reperfusion; NC, negative control;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; BCL2L12, B-cell lymphoma-2-like 12; NEAT1, nuclear
paraspeckle assembly transcript 1. |
A previous study has demonstrated that miR-125a-5p
induces apoptosis in colon cancer by targeting BCL2L12 (21). To explore whether miR-125a-5p is
responsible for the regulation of BCL2L12 in cardiomyocyte
apoptosis induced by H2O2, the expression
levels of BCL2L12 following overexpression or silencing of
miR-125a-5p in the cultured cardiomyocytes was examined. The
results indicated that ectopic overexpression of miR-125a-5p in
cardiomyocytes led to a reduction in BCL2L12 levels (Fig. 4F). In addition, knockdown of
miR-125a-5p abolished the decrease in BCL2L12 levels in
cardiomyocytes following H2O2 treatment
(Fig. 4G).
Subsequently, the association between NEAT1,
miR-125a-5p and BCL2L12 in cardiomyocytes was explored. As
presented in Fig. 4H and I,
overexpression of NEAT1 attenuated the inhibitory effect of
miR-125a-5p on BCL2L12 levels. Notably, overexpression of
miR-125a-5p abolished the effect of NEAT1 on BCL2L12 expression and
apoptosis under H2O2 treatment (Fig. 4J-M). Overall, these results suggest
that NEAT1 impairs the miR-125a-5p/BCL2L12 interaction in
cardiomyocytes to inhibit H2O2-induced
apoptosis.
Discussion
Cardiomyocyte apoptosis is an important contributor
to the development of myocardial infarction and heart failure
(22). Although evidence has
indicated the upregulation of lncRNA NEAT1 in numerous cancer types
(23,24), its role in heart diseases has
remained elusive. The present study indicated that ectopic
expression of NEAT1 inhibited apoptosis of cultured cardiomyocytes
subjected to H2O2 treatment. The molecular
mechanisms by which NEAT1 regulates
H2O2-induced cardiomyocyte apoptosis were
then investigated, revealing that NEAT1 functions as a ceRNA to
upregulate BCL2L12 expression by sponging miR-125a-5p, which
consequently leads to cardiomyocyte apoptosis.
Emerging evidence has revealed that certain lncRNAs
are crucial factors in the development of various diseases
(25–27). Recent studies have indicated that
lncRNAs also contribute to the progression of various types of
heart disease (28–30). For instance, lncRNA FTX transcript
XIST regulator was reported to suppress cell apoptosis via
targeting miR-29b-1-5p and BCL2L12 in cardiomyocytes (16). Zhou et al (29) indicated that lncRNA myocardial
infarction associated transcript functions as a ceRNA to upregulate
death associated protein kinase 2 by sponging miR-22-3p in diabetic
cardiomyopathy. In the present study, the expression of lncRNA
NEAT1 was significantly downregulated in the myocardium of a mouse
model of I/R-induced myocardial injury; however, these data are not
in accordance with the recent data from the work of Ma et al
(31), which NEAT1 was markedly
increased rats following I/R. The distinction could be explained by
the potential differential expression of lncRNAs in ischemic rat
hearts compared with ischemic mice hearts. Additionally, the
previous study was investigating high glucose damage, whereas
hypoxia injury was the focus of the present study. Furthermore,
NEAT1 was observed to be significantly downregulated in
H2O2-treated cardiomyocytes in the current
study, and ectopic overexpression of NEAT1 inhibited
H2O2-induced cardiomyocyte apoptosis in
vitro. However, further in vivo investigations are
required to verify whether NEAT1 suppresses cardiomyocyte apoptosis
in heart tissue.
Regulatory interactions exist between lncRNAs and
miRNAs (10,32). LncRNAs may function as ceRNAs
and/or molecular sponges to modulate the effects of miRNA. For
instance, knockdown of lncRNA X inactive specific transcript has
been reported to exert tumor-suppressive functions in human
glioblastoma stem cells by increasing miR-152 levels (33). Based on this known regulatory
mechanism and the present results, it may be hypothesized that
NEAT1 acts as a ceRNA in cardiomyocytes. In support of this notion,
the present results validated the direct binding ability
miR-125a-5p to NEAT1, predicted by bioinformatics analysis, and
confirmed in luciferase reporter and RIP assays. Further functional
assays demonstrated that NEAT1 increased the levels of the
miR-125a-5p target gene BCL2L12 by competitively ‘sponging’
miR-125a-5p in cardiomyocytes, which may be a crucial molecular
mechanism involved in the regulation of
H2O2-induced cardiomyocyte apoptosis.
In conclusion, the present study indicated that
lncRNA NEAT1 is downregulated in I/R-injured mouse hearts and may
function as a ceRNA to upregulate BCL2L12 expression by sponging
miR-125a-5p, consequently contributing to cardiomyocyte apoptosis,
which is involved in the pathogenesis of ischemic heart
disease.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81470505) and the
Province natural science fund of Guangdong (grant no.
2014A030313001).
Availability of data and materials
Not applicable.
Authors' contributions
HY designed and revised the manuscript. QZ
contributed to the analysis and interpretation of data, and wrote
the manuscript. HL, LL and DC performed the experiments. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were performed according to the
protocol approved by the Animal Care Committee of Guangdong
Cardiovascular Research Institute (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hausenloy DJ and Yellon DM: Targeting
myocardial reperfusion injury-the search continues. N Engl J Med.
373:1073–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Writing Group Members, ; Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update: A report from the american heart
association. Circulation. 133:e38–e360. 2016.PubMed/NCBI
|
|
3
|
Kajstura J, Cheng W, Reiss K, Clark WA,
Sonnenblick EH, Krajewski S, Reed JC, Olivetti G and Anversa P:
Apoptotic and necrotic myocyte cell deaths are independent
contributing variables of infarct size in rats. Lab Invest.
74:86–107. 1996.PubMed/NCBI
|
|
4
|
Haunstetter A and Izumo S: Toward
antiapoptosis as a new treatment modality. Circ Res. 86:371–376.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sallam T, Sandhu J and Tontonoz P: Long
noncoding RNA discovery in cardiovascular disease: Decoding form to
function. Circ Res. 122:155–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ginger MR, Shore AN, Contreras A, Rijnkels
M, Miller J, Gonzalez-Rimbau MF and Rosen JM: A noncoding RNA is a
potential marker of cell fate during mammary gland development.
Proc Natl Acad Sci USA. 103:5781–5786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saha P, Verma S, Pathak RU and Mishra RK:
Long noncoding RNAs in mammalian development and diseases. Adv Exp
Med Biol. 1008:155–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Juan L, Wang G, Radovich M, Schneider BP,
Clare SE, Wang Y and Liu Y: Potential roles of microRNAs in
regulating long intergenic noncoding RNAs. BMC Med Genomics. 6
(Suppl 1):S72013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi L, Liu F, Zhang F, Zhang S, Lv L, Bi Y
and Yu Y: lncRNA NEAT1 competes against let-7a to contribute to
non-small cell lung cancer proliferation and metastasis. Biomed
Pharmacother. 103:1507–1515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. BMC
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tu J, Zhao Z, Xu M, Lu X, Chang L and Ji
J: NEAT1 upregulates TGF-β1 to induce hepatocellular carcinoma
progression by sponging hsa-mir-139-5p. J Cell Physiol.
233:8578–8587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Qiu J, Chen B, Lin Y, Chen Y, Xie
G, Qiu J, Tong H and Jiang D: Long non-coding RNA NEAT1 plays an
important role in sepsis-induced acute kidney injury by targeting
miR-204 and modulating the NF-kappaB pathway. Int Immunopharmacol.
59:252–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long B, Li N, Xu XX, Li XX, Xu XJ, Guo D,
Zhang D, Wu ZH and Zhang SY: Long noncoding RNA FTX regulates
cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2.
Biochem Biophys Res Commun. 495:312–318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsybouleva N, Zhang L, Chen S, Patel R,
Lutucuta S, Nemoto S, DeFreitas G, Entman M, Carabello BA, Roberts
R and Marian AJ: Aldosterone, through novel signaling proteins, is
a fundamental molecular bridge between the genetic defect and the
cardiac phenotype of hypertrophic cardiomyopathy. Circulation.
109:1284–1291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu
Y, Feng Y, Liu H, Fei B, Mao Y, et al: LncRNA-UCA1 enhances cell
proliferation and 5-fluorouracil resistance in colorectal cancer by
inhibiting miR-204-5p. Sci Re. 6:238922016.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Hu J, Pan H, Luo D, Huang M and Xu
W: CSN5 Promotes hepatocellular carcinoma progression by SCARA5
inhibition through suppressing β-catenin ubiquitination. Dig Dis
Sci. 63:155–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong Z, Liu N, Lin L, Guo X, Yang D and
Zhang Q: miR-125a-5p inhibits cell proliferation and induces
apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1.
Biomed Pharmacother. 75:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Narula J, Pandey P, Arbustini E, Haider N,
Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A,
Dec GW, et al: Apoptosis in heart failure: Release of cytochrome c
from mitochondria and activation of caspase-3 in human
cardiomyopathy. Proc Natl Acad Sci USA. 96:8144–8149. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Wang Y, Fu X and Lu Z: Long
non-coding RNA NEAT1 promoted ovarian cancer cells' metastasis
through regulation of miR-382-3p/ROCK1 axial. Cancer Sci.
109:2188–2198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Zhou Y, Sun AJ and Xue JL: NEAT1
contributes to breast cancer progression through modulating miR-448
and ZEB1. J Cell Physiol. 233:8558–8566. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Fang L, Pu Q, Bu H, Zhu P, Chen Z,
Yu M, Li X, Weiland T, Bansal A, et al: MEG3-4 is a miRNA decoy
that regulates IL-1β abundance to initiate and then limit
inflammation to prevent sepsis during lung infection. Sci Signal.
11(pii): eaao23872018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye J, Wang C, Wang D and Yuan H: LncRBA
GSA5, up-regulated by ox-LDL, aggravates inflammatory response and
MMP expression in THP-1 macrophages by acting like a sponge for
miR-221. Exp Cell Res. 369:348–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaynab M, Fatima M, Abbas S, Umair M,
Sharif Y and Raza MA: Long non-coding RNAs as molecular players in
plant defense against pathogens. Microb Pathog. 121:277–282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun. 5:35962014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Zhang W, Jin M, Chen J, Xu W and
Kong X: lncRNA MIAT functions as a competing endogenous RNA to
upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy.
Cell Death Dis. 8:e29292017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dangwal S, Schimmel K, Foinquinos A, Xiao
K and Thum T: Noncoding RNAs in heart failure. Handb Exp Pharmacol.
243:423–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma M, Hui J, Zhang QY, Zhu Y, He Y and Liu
XJ: Long non-coding RNA nuclear-enriched abundant transcript 1
inhibition blunts myocardial ischemia reperfusion injury via
autophagic flux arrest and apoptosis in streptozotocin-induced
diabetic rats. Atherosclerosis. 277:113–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su X, Xing J, Wang Z, Chen L, Cui M and
Jiang B: microRNAs and ceRNAs: RNA networks in pathogenesis of
cancer. Chin J Cancer Res. 25:235–239. 2013.PubMed/NCBI
|
|
33
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|