Introduction
Manganese, as a rich metal element in the earth, is
widely found in nature (1).
Manganese has been shown to be a necessary factor for various
enzymes in humans, including manganese superoxide dismutase and
pyruvate carboxylase, which are involved in protein synthesis,
neurotransmitter transmission and nervous system development.
Manganese enters the human body primarily through respiration.
Long-term and high-dose exposure to manganese can lead to various
types of damage to human organs. The brain is the major organ which
can be damaged by manganese exposure. In addition, manganese can
pass through the blood-brain barrier and accumulate in the brain
basal ganglia, striatum and substantia nigra (2,3). The
mechanisms of manganese-induced neurological damage have been
described to involve dopamine oxidative damage, oxygen free
radicals, excitotoxicity, functional imbalances in mitochondria and
iron metabolism disorders. However, the detailed molecular
mechanism remains to be fully elucidated (4). It has been shown that endoplasmic
reticulum stress (ERS) may be involved in the pathogenesis of
neurotoxicity caused by manganese poisoning. The endoplasmic
reticulum (ER) is a subcellular structure, which is involved in
protein synthesis, processing and transport in cells. The ER is key
in maintaining intracellular calcium stabilization (5). An absence of energy, calcium
homeostasis, and oxidative stress conditions destroy the normal ER
physiological function, resulting in the misfolding of proteins.
Subsequently, these adverse reactions can induce ERS and the
unfolding protein response (UPR).
Glucose regulated protein 78KD (GRP78) is an ER
chaperone protein. GRP78 has been shown to assist in the processing
and modification of new synthetic proteins. Therefore, GRP78 is
considered to be a landmark protein for detecting ERS (6). Under a non-stressed state, three
transmembrane receptors, including double-stranded RNA-dependent
protein kinase-like ER kinase (PERK), activation of transcription
factor 6 (ATF6) and type I transmembrane protein kinase (IRE-1),
can bind to GRP78 and maintain an inactive state of GRP78. When ERS
occurs, a large number of abnormal proteins are produced. These
abnormal protein molecules occupy the binding sites on GRP78, which
cause the dissociation of IRE-1, PERK and ATF6 from GRP78. In the
initial stage of ERS, the activation of chaperones promotes
degradation of the incorrect proteins and restores cell
homeostasis. However, if the stress exceeds the ability of the
cells to maintain their homeostasis, PERK, ATF6 and IRE-α activate
the apoptotic program to degenerate cells. Previous studies have
suggested that C/EBP homologous protein (CHOP), c-Jun N-terminal
kinase (JNK) and caspase-12 are key in ERS-activated apoptosis: i)
Activation of CHOP/growth arrest and DNA damage-inducible 153 gene
transcription through the PERK/eukaryotic translation initiation
factor 2α (eIF2α) pathway (7,8).
This signaling pathway can reduce anti-apoptotic gene B-cell
lymphoma 2 (Bcl-2) translation, and upregulate the expression of
pro-apoptotic factor Bcl-2-assocated X protein (Bax); this
accelerates cell apoptosis; ii) IRE1 can activate the JNK signaling
pathway, which downregulates the expression of Bcl-2. The low
expression of Bcl-2 upregulates the gene expression of
pro-apoptotic Bcl-2-interacting mediator of cell death and
mitochondrial cascade activation (9); iii) ERS can specifically activate
caspase-12, which is located in the ER. Caspase-12 can regulate the
expression of apoptotic promoters caspase-9 and caspase-3, which
ultimately leads to cell function impairment (10). ERS inhibitor 4-phenylbutyrate
(4-PBA), as a chaperone, can stabilize the protein structure and
transports large quantities of aggregated proteins in the ER.
Therefore, 4-PBA can reduce the ER burden and inhibit the
occurrence of ERS. It has been identified that 4-PBA can inhibit
ERS. For example, reducing blood glucose levels in patients with
diabetes (11), improving the
insulin secretion barrier (12),
alleviating the occurrence of diabetic nephropathy (13), reducing ischemia in liver cells
(14), and reducing nerve cell
apoptosis and hypoxic ischemic injury (15,16).
In the present study, a sub-acute manganese exposure
rat model was established by intraperitoneal injection of
manganese. Hematoxylin and eosin (H&E) staining, a TUNEL assay
and electron microscopy were used to evaluate the effect of
manganese on neurotoxicity. Western blot analysis was used to
determine whether ERS was involved in the neurotoxicity. In
addition, the potential value of 4-PBA in neuron protection was
probed. The results of the study provide a basis for the early
diagnosis and treatment of manganese affecting a population.
Materials and methods
Experimental animals and groups
The experiment was approved by the Committee on the
Ethics of Animal Experiments of the First Affiliated Hospital of
Guangxi Medical University [Nanning, China; permit no. 2015
(KY-E-67)]. A total of 60 healthy male Sprague-Dawley rats, aged
3–4 months old and weighing 200±20 g, were obtained from the Animal
Center of Guangxi Medical University (certificate of conformity no.
SCXK Gui 2014~0002). The animal room was maintained in clean and
sterile environment, with a relative humidity of 40–50%, and
22–25°C room temperature. The cage was paved with cleaning
accessories. The rats were free to move within the cage, which was
equipped with black holes to avoid danger. The experimental animals
were healthy, disease-free and had normal physiological functions.
All rats were housed in quiet conditions to avoid fright or panic.
The rats were provided with clean feed and free water. Following 3
days of adaptive feeding, the rats were randomized divided into
four subgroups using a random number table: i) Vehicle group
(n=15); ii) LoMag group (n=15); iii) HiMag group (n=15); and iv)
HiMag + PBA group (n=15). The animal model was constructed
according to previous studies (15,17).
The rats in the four groups were injected intraperitoneally with
the same volume of normal saline. The rats in the LoMag group and
HiMag group were administered with an intraperitoneal injection of
6 and 15 mg/kg MnCl2 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), respectively. The rats in the HiMag + PBA
group were administered with an intraperitoneal injection of 200
mg/kg 4-PBA (Sigma-Aldrich; Merck KGaA) and, after 30 min, 15 mg/kg
MnCl2 was administered by intraperitoneal injection. The
injection volume of each solution was calculated using the
following formula: Injection volume=rat weight/(2 ml/kg). The rats
were injected daily between 6:00 and 7:00 pm and injected weekly
for 6 days. The health status and behavioral changes of the animals
were observed every day. In addition, the following criteria were
applied to determine when the animals should be sacrificed in
advance to avoid causing pain to the animal: Abdominal infection,
weight loss, loss of appetite and weakness. After 4 weeks of
continuous injection, five rats were randomly selected from each
group. The rats were anesthetized and decapitated 24 h following
the final Mn Cl2 injection. All rats were anesthetized
through intraperitoneal injection of 300 mg/kg body weight of 10%
(w/v) chloral hydrate solution (National Drug Approval no.
H37022673, Qingdao Yulong Algae Co., Ltd., Qingdao, China). The
anesthetized rats were then placed under a heating light to
maintain normal body temperature. Subsequently, the hearts and
brains were separated immediately following anesthesia. The heart
was perfused with 0.9% frozen saline (250 ml; Sigma-Aldrich; Merck
KGaA). The brain striatum was stored in a −80°C cryogenic
refrigerator for further analysis.
Striatal manganese content
determination
The same quantity of rat striatum tissue was weighed
accurately using an electronic balance (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and then transferred into 10-ml tubes.
Subsequently, 3 ml mixed acid (concentrated HNO3,
concentrated H2SO4 and concentrated HCI)
prepared at a ratio of 9: 1: 1 (Sigma-Aldrich; Merck KGaA) was
added into the 10-ml tube to digest tissues at 37°C overnight. The
following day, the solution was digested and clarified to near
dryness in an adjustable electric furnace. Ultra-pure water was
added to 5 ml. The Mn content was measured by ICP-AES with default
parameters (PerkinElmer, Inc., Waltham, MA, USA).
Sectioning
Five rats were randomly selected from each group.
Following anesthesia, thoracotomy was performed and the apical
catheter was inserted into the ascending aorta. The right atrial
appendage was cut off, and 250 ml of 0.9% frozen normal saline
(Sigma-Aldrich; Merck KGaA) was rapidly infused. Subsequently, 200
ml of 4% paraformaldehyde solution (Sigma-Aldrich; Merck KGaA) was
infused. The brain striatum was rapidly removed, and the tissue was
placed in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 8 h.
The specimens were diced and placed in 75, 85, 95, and 100%
gradient in ethanol (Sigma-Aldrich; Merck KGaA) for dehydration.
Xylene-soaked transparent processing was performed prior to wax
embedding. The slice thickness was ~5 µm. All slices were fixed at
60°C for 3 h.
H&E and TUNEL staining
The H&E staining kit (Beyotime Institute of
Biotechnology, Haimen, China) and TUNEL assay (Beyotime Institute
of Biotechnology) were used to detect neuronal apoptosis in the
striatum. H&E staining was performed with the following steps:
Xylene dewaxing for 15 min, xylene washed with gradient alcohol,
hematoxylin dye staining 15 min after distilled water washing for 5
min, hydrochloric acid color separation for 20 sec, distilled water
immersion for 10 min, 0.5% eosin soak 20 sec after washing, gland
packing sheet after natural drying, sample observation using a
light microscope (magnification, ×200 or ×400). The TUNEL method
was used to detect striatal neuron apoptosis. The detailed steps
were as follows: Conventional paraffin section, dewaxing, washing
in PBS for 5 min, 3% hydrogen peroxide incubation at 37°C for 20
min, washing in PBS for 5 min (repeat once), addition of marker
solution and incubation at 37°C in the dark for 2 h, washing in PBS
for 5 min, addition of POD reagent and incubation at 37°C for 30
min, washing in PBS, drying, DAB colorization, drying, placement in
a neutral gland plate, sample observation using a light microscope
(magnification, ×400). The neuronal apoptotic index (AI) was
calculated as follows: AI = nuclei of apoptotic cells/nuclei of
total cells × 100%.
Transmission electron microscopy
In the present study, transmission electron
microscopy (FEI; Thermo Fisher Scientific, Inc.) was used to
observe stromal cell structure changes and ER morphology. Five rats
in each group were randomly selected. The brain specimens were
rinsed with PBS (Sigma-Aldrich; Merck KGaA) and fixed with 1%
osmium tetroxide solution (Sigma-Aldrich; Merck KGaA).
Subsequently, the treated specimens were dehydrated with gradient
alcohol and treated with acetone and embedding agent overnight. The
embedded samples were sectioned (~65–80 nm in thickness). The
sections were stained with lead citrate for 15 min and stained with
50% ethanolic uranyl acetate (Sigma-Aldrich; Merck KGaA) for 15
min. The sample was observed under a transmission electron
microscope.
Western blot analysis
Western blot analysis was used to detect changes in
the expression of ERS-related proteins (GRP78, CHOP, JNK and
caspase-12). The tissue was transferred into a 1.5-ml EP tube. Eye
scissors were used to cut the samples into pieces on ice, and
pre-cooled cell lysate was added (mixture of PMSF and RIPA cell
lysate at a volume ratio of 1:100; Sigma-Aldrich; Merck KGaA) to
0.5 ml. The sample was ground evenly until no tissue residue was
present. The slurry was placed in a low-temperature high-speed
centrifuge (Eppendorf, Hamburg, Germany), and centrifuged at 9,500
× g at 4°C for 5 min. The supernatant was transferred into a new EP
tube and maintained at −80°C. The protein concentration was
quantified using the bicinchoninic acid assay method. Protein
concentration was detected at 562 nm using the
Varioskan™ LUX multimode reader (Thermo Fisher
Scientific, Inc.). The protein standard curve was calculated prior
to the calculation of the protein concentration in each sample.
Following boiling with SDS-PAGE sample buffer (Sigma-Aldrich; Merck
KGaA) for 5 min, 100 µg total protein was loaded in each lane.
Proteins were separated by 10% SDS-PAGE. The proteins were then
transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with western
blot blocking buffer (cat. no. T7132A; Takara Bio, Inc., Otsu,
Japan) for 1 h at room temperature, the membrane was incubated with
a 1:1,000 diluted primary antibody at 4°C overnight. The primary
antibodies used were the following: Rabbit polyclonal anti-mouse
GRP78 (cat. no. M00955), CHOP (cat. no. M00311), JNK (cat. no.
M02608) caspase-12 (cat. no. A04700) and β-actin (cat. no. M01263).
All primary antibodies were purchased from Boster Biological
Technology (Pleasanton, CA, USA). Prior to detection with an ECL
chemiluminescence detection kit (Beyotime Institute of
Biotechnology), the proteins were incubated with the corresponding
secondary antibody (cat. no. BA1001; dilution, 1:5,000) for 1 h at
room temperature (Boster Biological Technology). The bands were
examined using an Odyssey Infrared Imaging system (LI-COR
Biosciences, Lincoln, NE, USA) scan; the resolution of the
instrument ranges between 21 and 339 µm, and images of the blots
and gels were captured at 169 µm. Quantification was performed on
single channels with the Odyssey CLx software (version 1.0.9;
LI-COR Biosciences) as previously described (18).
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Data for manganese content, neuron
apoptotic rate, protein expression and other data are expressed as
the mean ± standard deviation. One-way analysis of variance was
applied. If there were differences in the overall data, further
comparison of individual data was performed. The LSQ test was
applied if variance was the same. Tamhane's T2 method was used if
variance was not the same. P<0.05 was considered to indicate a
statistically significant difference.
Results
4-PBA has no effect on manganese
content in the rat striatum
In order to examine whether manganese exposure can
lead to accumulation in the rat striatum, the manganese content in
the rat striatum was examined. The results indicated that manganese
content in the LoMag group and the HiMag group was significantly
higher than that in the Vehicle group (P<0.01). Manganese
content in the HiMag + PBA group was similar to that in the HiMag
group (P>0.05). Manganese content in the HiMag + PBA group was
also significantly higher than that in the Vehicle group
(P<0.01) (Table I). The results
suggested that 4-PBA treatment did not reduce manganese content in
the rat striatum exposed to a high dose of MnCl2.
 | Table I.Manganese content in the rat striatum
of different groups. |
Table I.
Manganese content in the rat striatum
of different groups.
| Group | Rats (n) | Manganese content
(µg/g) |
P-valuea |
|---|
| Vehicle | 5 | 0.43±0.08 | 1.00000 |
| LoMag | 5 | 1.02±0.11 | 0.06000 |
| HiMag | 5 | 1.98±0.67 | 0.00007 |
| HiMag+PBA | 5 |
1.93±0.62b | 0.00098 |
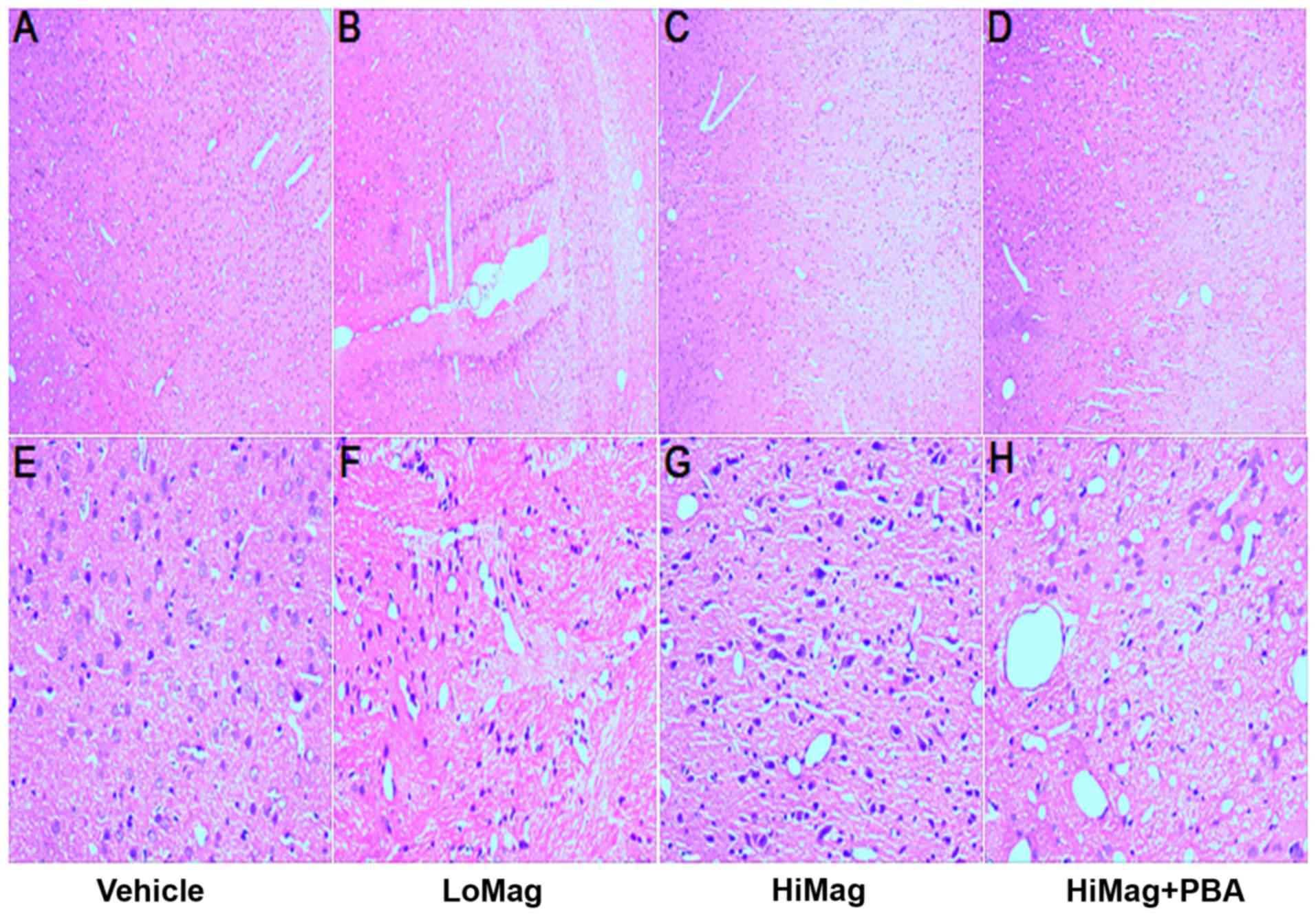
4-PBA alleviates neuronal tissue
damage caused by manganese exposure
In order to investigate whether manganese exposure
causes damage to striatal neurons, H&E staining analysis was
performed (Fig. 1). In the Vehicle
group, striatal neurons exhibited typical normal cellular features,
including a clear, closely arranged structure, the nucleus in the
middle of the cells, loose chromatin, nuclear membrane integrity
and a clear nucleolus. However, neurons exhibited different
morphologies in the manganese exposure groups, including disordered
arrangement, ambiguous structure, a significantly swollen
cytoplasm, volatile degeneration, eosinophilic formation, nuclear
pyknosis and necrosis, and nuclear fragmentation or absence. In
addition, the degree of damage was consistent with the dose of
MnCl2. A high MnCl2 dose was closely
associated with a higher level of damage. However, the damage was
reduced by adding 4-PBA.
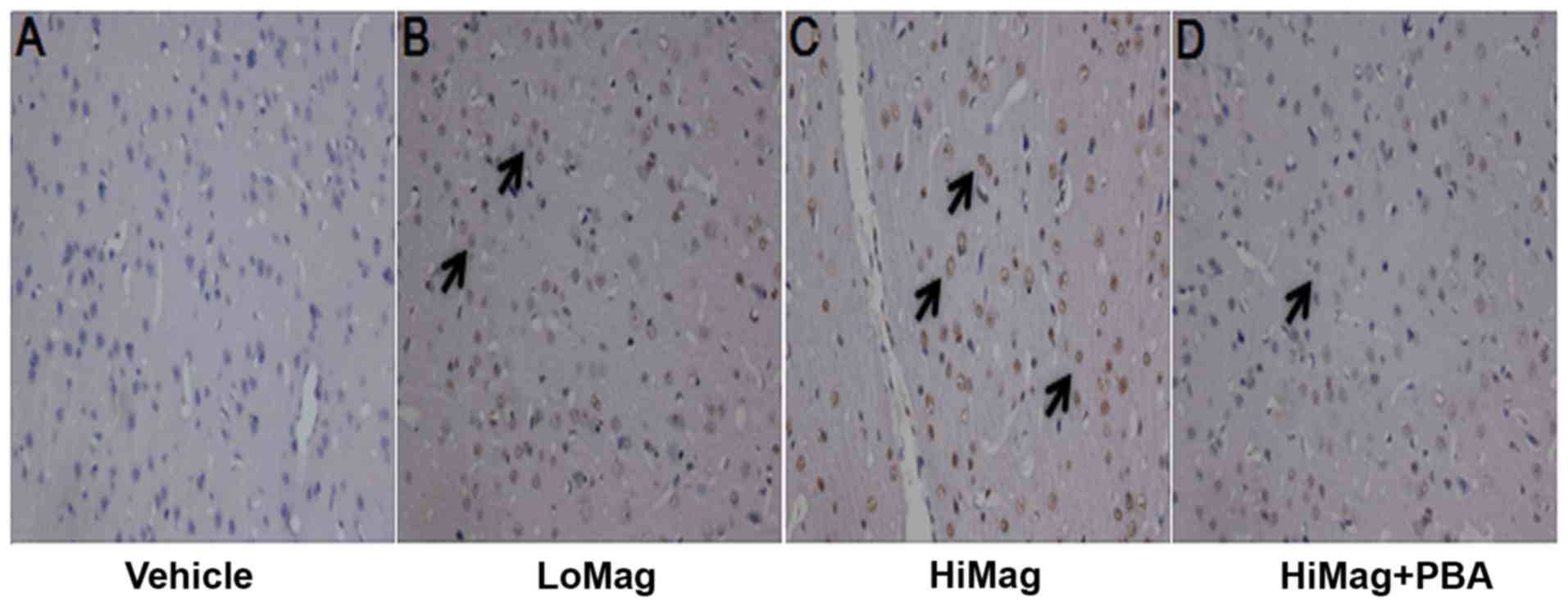
4-PBA reduces the number of apoptotic
cells
In order to examine whether manganese exposure
increases striatal neurons apoptosis, a TUNEL assay was performed
to examine the striatum neuron apoptotic rates (Fig. 2 and Table II). TUNEL-positive cells were
stained brown-yellow. In the Vehicle group, a small number of
positive cells was observed (Fig.
2). However, the numbers of apoptotic cells in the manganese
exposure groups were significantly higher than that in the Vehicle
group (Table II). 4-PBA
significantly reduced the number of positive cells (P<0.001;
Table II). However, the positive
cell number in the HiMag + PBA group remained higher than that in
the Vehicle group (P<0.01).
 | Table II.Apoptotic rate of rat striatal
neurons in different treatment groups. |
Table II.
Apoptotic rate of rat striatal
neurons in different treatment groups.
| Group | Rats (n) | Results |
P-valuea |
|---|
| Vehicle | 5 | 0.03±0.02 | 1.00000 |
| LoMag | 5 | 0.16±0.01 | <0.00001 |
| HiMag | 5 | 0.28±0.03 | <0.00001 |
| HiMag+PBA | 5 |
0.14±0.02b | <0.00001 |
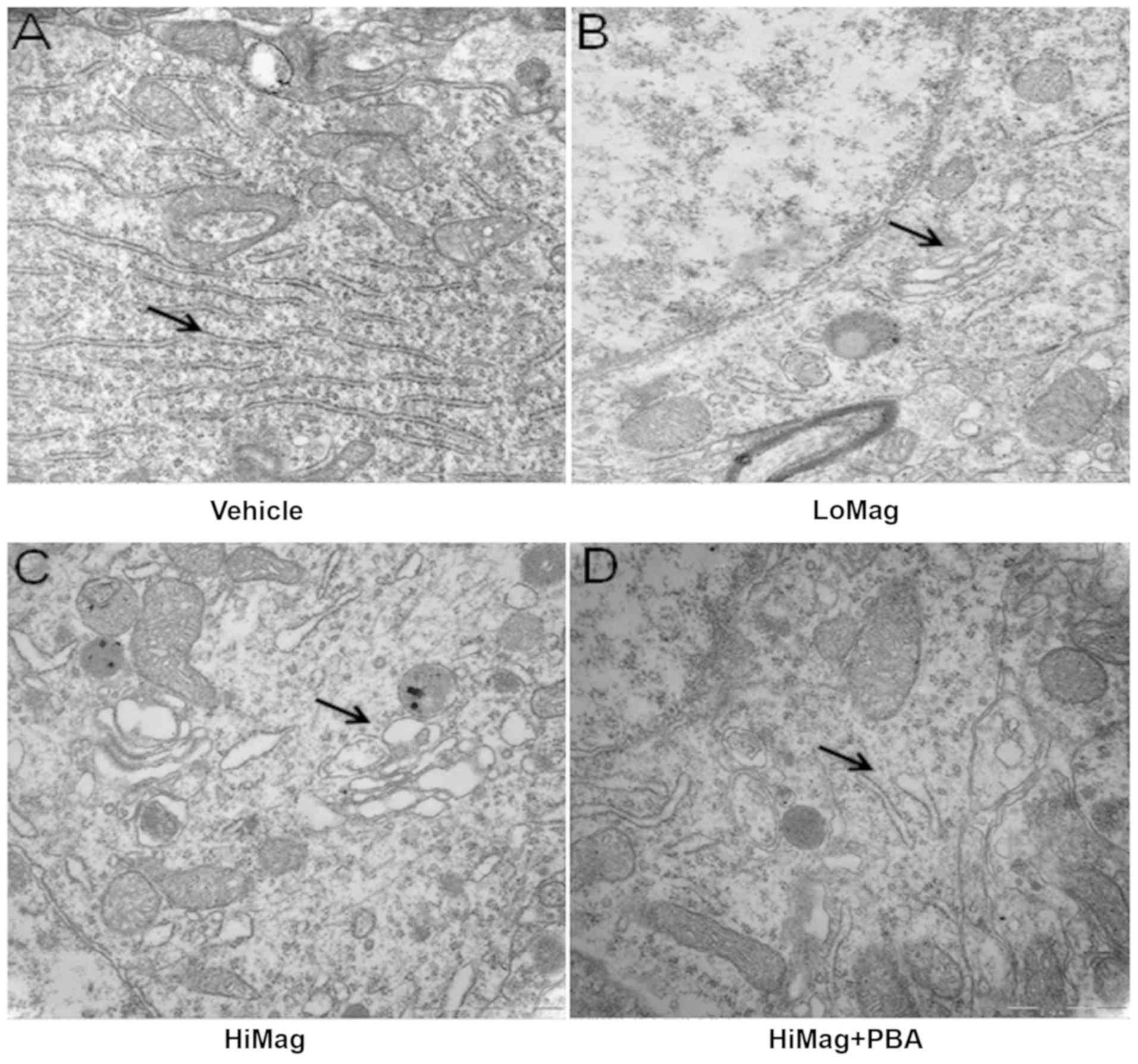
4-PBA helps to maintain normal cell
ultrastructure
Electron microscopy revealed the ultrastructure
changes in the differently treated groups (Fig. 3). In the Vehicle group, rat
striatal neurons maintained a normal morphology, including loose
nuclear chromatin, a clear nucleolus, and intact mitochondria, ER
and other organelle structures. However, in the manganese exposure
groups, cell morphology was significantly different. For example,
nuclear chromatin condensation, swelling of ER, vacuolar formation,
and ER and cell structure damage were observed. Following 4-PBA
treatment, the damage effects were relieved (complete cell
structure, and reduced degranulation and ER swelling).
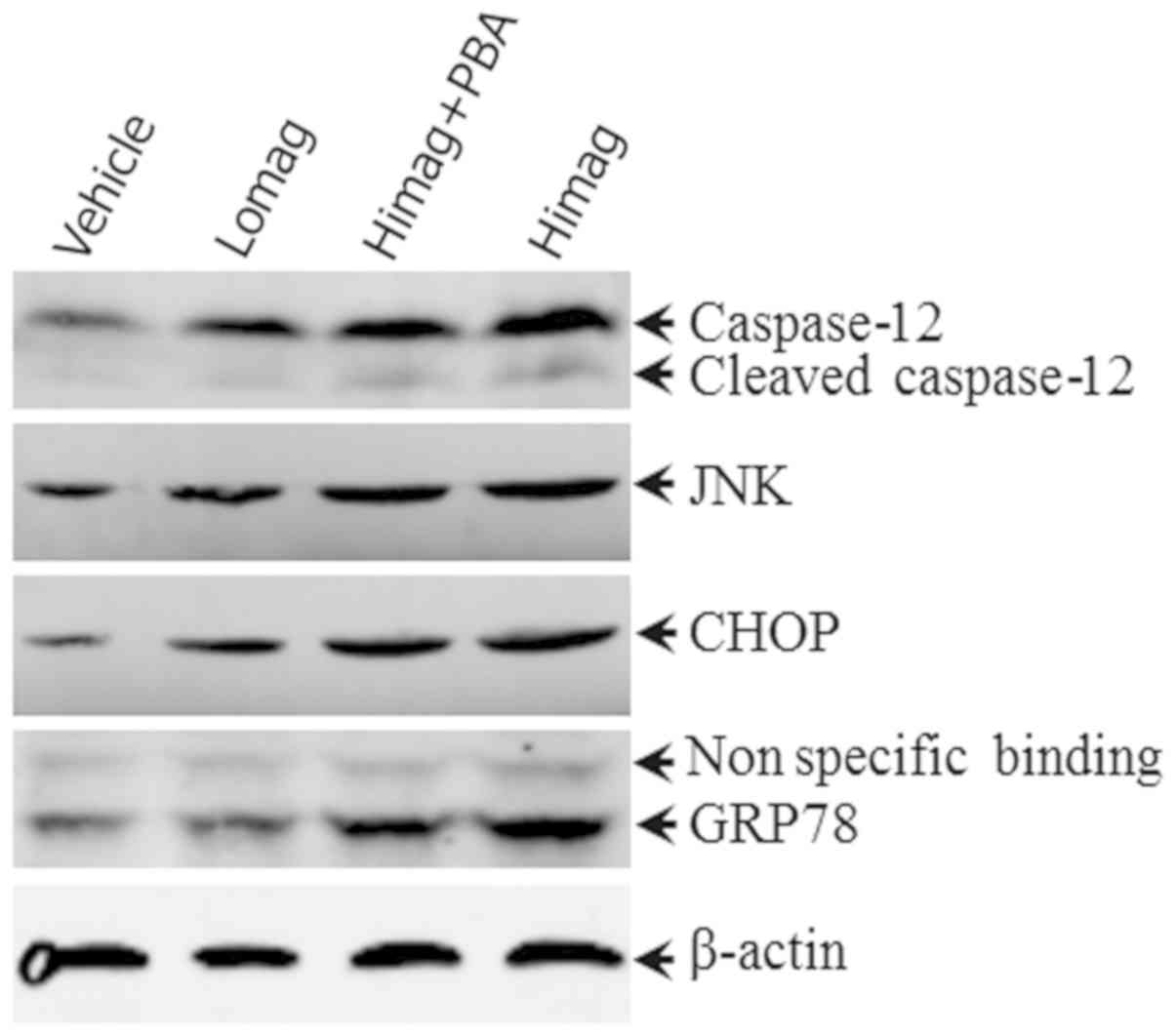
4-PBA treatment reduces ERS-related
protein expression
In order to identify the molecular changes between
differently treated groups, western blot analysis was performed to
determine the expression levels of GRP78, CHOP, JNK and caspase-12
(Figs. 4 and 5). The results suggested that the protein
expression levels of GRP78, CHOP, JNK and caspase-12 in the LoMag
and HiMag groups were significantly higher than those in the
Vehicle group (P<0.05; Figs. 4
and 5). The protein expression
levels of GRP78, CHOP, JNK and caspase-12 in the HiMag + PBA group
were significantly lower than those in the HiMag group (P<0.05).
However, the expression levels of the four proteins in the HiMag +
PBA group remained higher than those in the Vehicle group
(P<0.05). These results indicated that 4-PBA effectively reduced
ERS-related protein expression. However, 4-PBA did not fully
eliminate the damage reduced by manganese exposure.
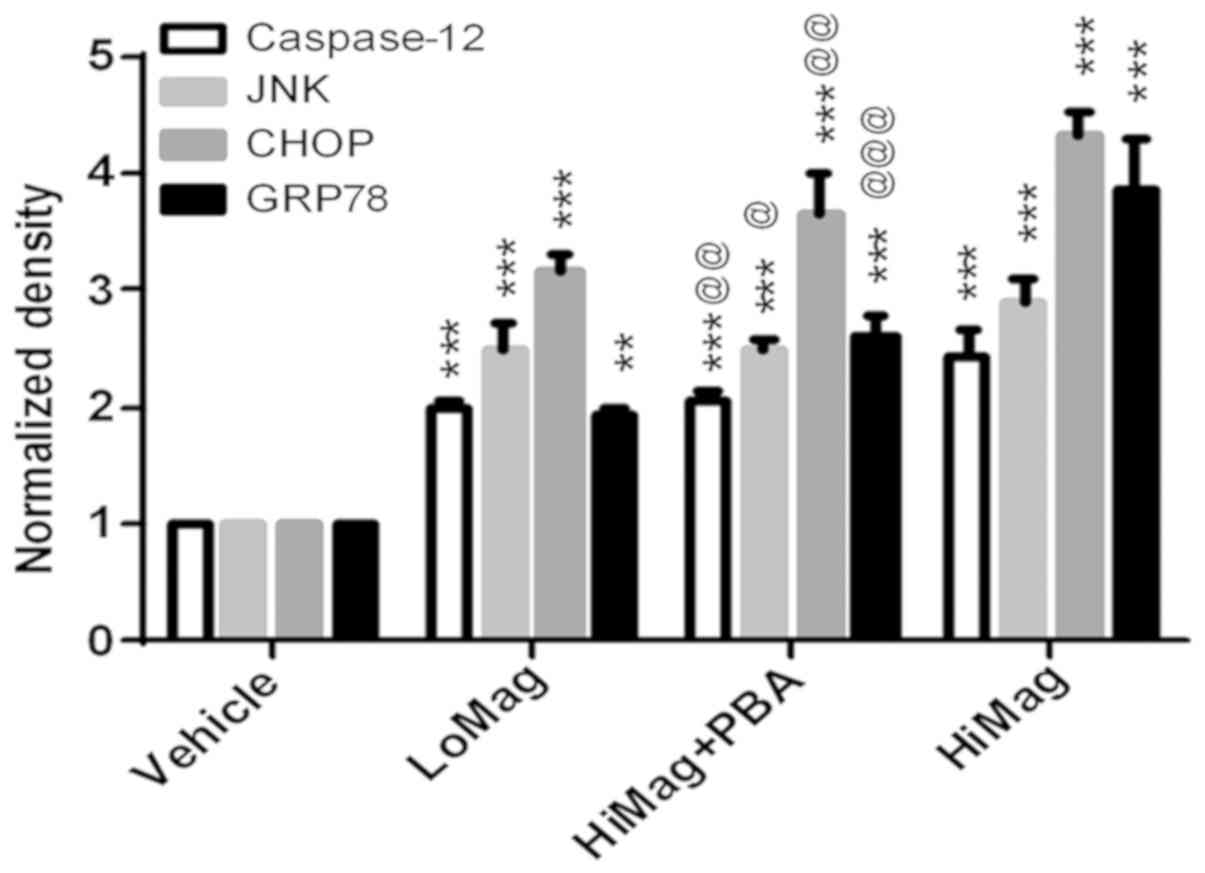 | Figure 5.Quantitative protein expressions
analysis of caspase-12, JNK, CHOP and GRP78 in the rat striatum.
Proteins expression levels in the rat striatum were examined. The
rat brains were collected from the LoMag group, HiMag group and
HiMag + PBA group. **P<0.01 and ***P<0.001, compared with
vehicle; @P<0.05, @@P<0.01 and
@@@P<0.001, compared with
HiMag. JNK, c-Jun N-terminal kinase; CHOP, C/EBP homologous
protein; GRP78, glucose-regulated protein 78KD; LoMag, 6 mg/kg
MnCl2; HiMag, 15 mg/kg MnCl2; PBA,
4-phenylbutyrate. |
Discussion
The neurotoxicity mechanisms caused by manganese
exposure include excitotoxicity, mitochondrial injury (19–21),
ERS, oxygen free radical damage (22), thiol damage, dopamine
auto-oxidation and iron metabolism (23). Wang et al (17) revealed that manganese can cause
swelling of ER cell apoptosis and cell necrosis in rat neurons;
whereas protein expression levels of ATF-6α, GRP78, PERK, CHOP,
caspase-12 were upregulated and the expression of Bcl-2 was
downregulated. Xu et al (24) reported that manganese can induce
ERS-induced apoptosis through PERK and IRE1 in the brain culture
model. Seo et al (25)
suggested that manganese exposure may damage cells via ERS
responses and UPR responses. In addition, manganese can cause
apoptosis through ER and mitochondrial dysfunction (26). ERS has dual functions for external
stimuli. During initial stimulation, ERS is involved in adaptive
regulation. The induction of ERS can reduce the harmful substances
caused by renal damage in an in vitro cell culture model
(27). Increased expression of
GRP78 can reduce the apoptosis induced by ischemia (28). The upregulation of GRP78 can
suppress the effects of harmful factors against hippocampal neurons
(29). However, excessive or
prolonged stress stimulation activates apoptotic signaling
pathway-related proteins, including CHOP, JNK and caspase-12. This
pathway leads to the removal of cells and structures that cannot be
repaired. In addition, islet β-cell apoptosis induced by diabetes
hyperglycemia, myocardial ischemia-reperfusion injury, nephron
injury and renal failure are all associated with ERS (30–32).
In the present study, a sub-acute manganese exposure
rat model was established by intraperitoneal injection with
different doses of MnCl2 solution. The content of
manganese in the striatum was detected. Compared with the Vehicle
group, manganese levels in the LoMag group and HiMag groups were
increased, which indicated that manganese can accumulate in the
nervous system. H&E staining revealed that striatal neurons in
the MnCl2-exposed groups showed pyknosis, cell swelling
and other structural damage. The TUNEL assay suggested that the
number of apoptotic cells in the manganese-exposed groups was
significantly increased. Electron microscopy revealed that the ER
structure of the striatum was altered in the manganese-exposed
groups. Swelling and degranulation appeared in the ER, and the
degree of destruction in the ER was increased in a dose-dependent
manner. Western blot analysis suggested that the protein expression
levels of GRP78, CHOP, JNK and caspase-12 were also higher than
those in the Vehicle group. These results indicated that
MnCl2 exposure damaged the neurons of the striatum, ER
structure and mitochondria. These adverse reactions promoted the
expression of stress-related proteins, which led to the occurrence
of apoptosis. ERS may be an important pathogenic mechanism for
neurotoxicity caused by manganese exposure in rats.
The ERS inhibitor 4-PBA has been reported to assist
abnormal proteins to regain the correct molecular structure in the
ER (33,34). A previous study showed that 4-PBA
can effectively inhibit the activity of eIF-2 and PERK (11). 4-PBA has a protective role against
ERS; therefore, 4-PBA reduced neuronal apoptosis in a cerebral
ischemic injury mouse model (15).
In addition, Mizukami et al (16) suggested that 4-PBA can inhibit ERS
activation and improve motor impairment in rabbits. Currently,
there is no relevant literature on the role of 4-PBA in inhibiting
the ERS induced by manganese exposure. In the present study, 4-PBA
did not reduce manganese content in the striatum of
manganese-exposed rats. However, the results of H&E staining
and TUNEL assay revealed that 4-PBA improved the changes of cell
structure and apoptotic cell number, which were caused by manganese
exposure. The electron microscopy results suggested that 4-PBA
reduced ER structure changes (swelling, vacuolization and
degranulation). In addition, 4-PBA altered the protein expression
levels of GRP78, CHOP, JNK and caspase-12 in the striatal neurons
of rats exposed to a high dose of manganese. These experimental
results suggested that 4-PBA reduced neuronal damage and apoptosis
by inhibiting ERS.
In conclusion, the results of the present study
demonstrated that ERS is key in striatal neuron apoptosis following
sub-acute manganese exposure. 4-PBA improved multiple indicators;
for example, 4-PBA reduced the expression of apoptosis-related
proteins. These results provide a reliable reference for the early
detection and treatment of manganese poisoning. However, there were
limitations in the present study; for example, the experiment did
not set different time points for manganese exposure. Therefore,
further experiments with additional manganese dose treatments are
required to identify ERS changes at different time points and
different doses of manganese, to elaborate on these findings.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81460181) and the
Natural Science Foundation of Guangxi, China (grant no. Junior
program 2017JJB140225z).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CW, GY and JW guaranteed the integrity of the
present study, the clinical studies, data analysis and statistical
analysis; JW and GY conceived and designed the study and the
manuscript review; CW, RM and JW completed the study design; CW,
GY, RM and JW revised the manuscript for important intellectual
content; CW, GY and JW performed the literature research; CW, RM,
YH, GY and TL performed the experimental studies and data
acquisition; CW, YH and TL were involved in manuscript preparation;
CW, GY and JW edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Committee on
the Ethics of Animal Experiments of the First Affiliated Hospital
of Guangxi Medical University [permit no. 2015 (KY-E-67)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berger MM and Shenkin A: Vitamins and
trace elements: Practical aspects of supplementation. Nutrition.
22:952–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nutt DJ, Baldwin DS, Clayton AH, Elgie R,
Lecrubier Y, Montejo AL, Papakostas GI, Souery D, Trivedi MH and
Tylee A: Consensus statement and research needs: The role of
dopamine and norepinephrine in depression and antidepressant
treatment. J Clin Psychiatry. 67 (Suppl 6):S46–S49. 2006.
|
|
3
|
Moussavi S, Chatterji S, Verdes E, Tandon
A, Patel V and Ustun B: Depression, chronic diseases, and
decrements in health: Results from the world health surveys.
Lancet. 370:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burton NC and Guilarte TR: Manganese
neurotoxicity: Lessons learned from longitudinal studies in
nonhuman primates. Environ Health Perspect. 117:325–332. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sundar Rajan S, Srinivasan V,
Balasubramanyam M and Tatu U: Endoplasmic reticulum (ER) stress
& diabetes. Indian J Med Res. 125:411–424. 2007.PubMed/NCBI
|
|
6
|
Mu YP, Ogawa T and Kawada N: Reversibility
of fibrosis, inflammation, and endoplasmic reticulum stress in the
liver of rats fed a methionine-choline-deficient diet. Lab Invest.
90:245–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishitoh H, Matsuzawa A, Tobiume K,
Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A and Ichijo H: ASK1
is essential for endoplasmic reticulum stress-induced neuronal cell
death triggered by expanded polyglutamine repeats. Genes Dev.
16:1345–1355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi
F, Katayama T and Tohyama M: Activation of caspase-12, an
endoplastic reticulum (ER) resident caspase, through tumor necrosis
factor receptor-associated factor 2-dependent mechanism in response
to the ER stress. J Biol Chem. 276:13935–13940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SE, Lee YJ, Jang HJ, Lee KW, Kim YS,
Jun HS, Kang SS, Chun J and Kang Y: A chemical chaperone 4-PBA
ameliorates palmitate-induced inhibition of glucose-stimulated
insulin secretion (GSIS). Arch Biochem Biophys. 475:109–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo ZF, Feng B, Mu J, Qi W, Zeng W, Guo
YH, Pang Q, Ye ZL, Liu L and Yuan FH: Effects of 4-phenylbutyric
acid on the process and development of diabetic nephropathy induced
in rats by streptozotocin: Regulation of endoplasmic reticulum
stress-oxidative activation. Toxicol Appl Pharmacol. 246:49–57.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vilatoba M, Eckstein C, Bilbao G, Smyth
CA, Jenkins S, Thompson JA, Eckhoff DE and Contreras JL: Sodium
4-phenylbutyrate protects against liver ischemia reperfusion injury
by inhibition of endoplasmic reticulum-stress mediated apoptosis.
Surgery. 138:342–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi X, Hosoi T, Okuma Y, Kaneko M and
Nomura Y: Sodium 4-phenylbutyrate protects against cerebral
ischemic injury. Mol Pharmacol. 66:899–908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizukami T, Orihashi K, Herlambang B,
Takahashi S, Hamaishi M, Okada K and Sueda T: Sodium
4-phenylbutyrate protects against spinal cord ischemia by
inhibition of endoplasmic reticulum stress. J Vasc Surg.
52:1580–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Li X, Yang D, Zhang H, Zhao P, Fu
J, Yao B and Zhou Z: ER stress and ER stress-mediated apoptosis are
involved in manganese-induced neurotoxicity in the rat striatum
in vivo. Neurotoxicology. 48:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva DN, Coelho J, Frazílio Fde O,
Odashiro AN, Carvalho Pde T, Pontes ER, Vargas AF, Rosseto M and
Silva AB: End-to-side nerve repair using fibrin glue in rats. Acta
Cir Bras. 25:158–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Fu J and Zhou Z: Changes in the
brain mitochondrial proteome of male Sprague-Dawley rats treated
with manganese chloride. Toxicol Appl Pharmacol. 202:13–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dobson AW, Erikson KM and Aschner M:
Manganese neurotoxicity. Ann N Y Acad Sci. 1012:115–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Zhou Z and Fu J: Effect of
manganese chloride exposure on liver and brain mitochondria
function in rats. Environ Res. 93:149–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Fu J and Zhou Z: In vitro
effect of manganese chloride exposure on reactive oxygen species
generation and respiratory chain complexes activities of
mitochondria isolated from rat brain. Toxicol In Vitro. 18:71–77.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pang L, Wang J, Huang W and Guo S: A study
of divalent metal transporter 1 and ferroportin 1 in brain of rats
with manganese-induced parkinsonism. Zhonghua Lao Dong Wei Sheng
Zhi Ye Bing Za Zhi. 33:250–254. 2015.(In Chinese). PubMed/NCBI
|
|
24
|
Xu B, Shan M, Wang F, Deng Y, Liu W, Feng
S, Yang TY and Xu ZF: Endoplasmic reticulum stress signaling
involvement in manganese-induced nerve cell damage in organotypic
brain slice cultures. Toxicol Lett. 222:239–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo YA, Li Y and Wessling-Resnick M: Iron
depletion increases manganese uptake and potentiates apoptosis
through ER stress. Neurotoxicology. 38:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon H, Kim DS, Lee GH, Kim KW, Kim HR and
Chae HJ: Apoptosis induced by manganese on neuronal SK-N-MC Cell
Line: Endoplasmic reticulum (ER) stress and mitochondria
dysfunction. Environ Health Toxicol. 26:e20110172011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peyrou M and Cribb AE: Effect of
endoplasmic reticulum stress preconditioning on cytotoxicity of
clinically relevant nephrotoxins in renal cell lines. Toxicol In
Vitro. 21:878–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Zhao H, Min L, Zhang C, Liu P and
Luo Y: Effects of 2-Deoxyglucose on ischemic brain injuries in
rats. Int J Neurosci. 124:666–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Z, Luo H, Fu W and Mattson MP: The
endoplasmic reticulum stress-responsive protein GRP78 protects
neurons against excitotoxicity and apoptosis: Suppression of
oxidative stress and stabilization of calcium homeostasis. Exp
Neurol. 155:302–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oyadomari S, Araki E and Mori M:
Endoplasmic reticulum stress-mediated apoptosis in pancreatic
beta-cells. Apoptosis. 7:335–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DS, Kwon DY, Kim MS, Kim HK, Lee YC,
Park SJ, Yoo WH, Chae SW, Chung MJ, Kim HR and Chae HJ: The
involvement of endoplasmic reticulum stress in flavonoid-induced
protection on cardiac cell death caused by ischaemia/reperfusion. J
Pharm Pharmacol. 62:197–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajo A, Khoshnoodi J, Takenaka H,
Hagiwara E, Watanabe T, Kawakami H, Kurayama R, Sekine Y, Bessho F,
Takahashi S, et al: Mizoribine corrects defective nephrin
biogenesis by restoring intracellular energy balance. J Am Soc
Nephrol. 18:2554–2564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malo A, Krüger B, Göke B and Kubisch CH:
4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin
activation, and acinar cell apoptosis while increasing secretion in
rat pancreatic acini. Pancreas. 42:92–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mimori S, Okuma Y, Kaneko M, Kawada K,
Hosoi T, Ozawa K, Nomura Y and Hamana H: Protective effects of
4-phenylbutyrate derivatives on the neuronal cell death and
endoplasmic reticulum stress. Biol Pharm Bull. 35:84–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|