Introduction
At present, acute myocardial infarction (AMI)
remains a major cause of mortality globally (1). Timely and effective reperfusion
significantly decreases mortality (2); however, reperfusion can result in
myocardial injury, inducing myocardial apoptosis and cardiac
contractile dysfunction (3).
Various studies have suggested that cardiac ischemia/reperfusion
(I/R) resulting in oxidative damage (4), inflammation (5) and cardiac dysfunction (6) leads to cardiac dysfunction and
arrhythmia. Thus, the pathogenic mechanisms underlying reperfusion
injury have received substantial attention.
MicroRNAs (miRNAs/miRs) are small noncoding RNAs
that are ~17–27 nucleotides in length, which can suppress gene
expression via translational repression and transcript cleavage
(7). miRNAs serve important roles
in regulating cardiac function, including heart-muscle contraction,
conducting electrical signals, heart morphogenesis and I/R injury
pathways (8). A number of miRNAs
have been identified as aberrantly expressed following cardiac I/R,
including miR-1 (9), miR-15
(10), miR-92a (11), miR-320 (12) and miR-494 (13); however, the underling mechanisms
remain unclear.
Recent studies reported that miR-199a-5p is a
regulator of cardiac remodeling, ventricular hypertrophy, status
epilepticus and heart failure (14,15).
Our recent study reported that atorvastatin attenuated the
apoptosis of I/R-induced cardiomyocytes by regulating the
miR-199a-5p-mediated expression of glycogen synthase kinase 3β
(GSK3β); however, our research also revealed that GSK3β was not a
target of miR-199a-5p, indicating that other molecules downstream
of miR-199a-5p regulated GSK3β signaling (16). Hypoxia-inducible factor-1α (HIF-1α)
is involved in the regulation of GSK3β signaling in H9c2 cells in
myocardial I/R injury (17);
however, the association between miR-199a-5p, HIF-1α and GSK3β
signaling and proapoptotic molecular mechanisms remain unknown.
The present study demonstrated that the upregulation
of miR-199a-5p reduced the viability of, and promoted lactate
dehydrogenase (LDH) release from rat cardiomyocyte H9c2 cells
during oxygen-glucose deprivation and reperfusion (OGD/R). It was
hypothesized that miR-199a-5p downregulation may protect H9c2
cardiomyocytes against I/R-induced mitochondrial permeability
transition pore (mPTP) opening. Furthermore, a regulatory role for
HIF-1α and GSK3β in mPTP opening was identified, potentially
contributing to OGD/R-induced cell apoptosis. Additionally, the
effects of miR-199a-5p downregulation on apoptosis, mPTP opening,
p-GSK3β signaling and HIF-1α expression were determined.
Materials and methods
Reagents
DMEM, FBS and antibiotics (penicillin and
streptomycin) were purchased from Gibco (Thermo Fisher Scientific,
Inc.). HIF-1α (cat. no. 20960-1-AP), GSK3β (cat. no. 22104-1-AP),
phosphorylated (p)-GSK3β (cat. no. 14850-1-AP), adenine nucleotide
transferase (ANT; cat. no. 17796-1-AP), cyclophilin D (Cyp-D; cat.
no. 12716-1-AP) and β-actin antibodies (cat. no. 60008-1-Ig) were
purchased from ProteinTech Group, Inc. Transfection of H9c2 cells
was performed using HiPerFect Transfection Reagent (Qiagen, Inc.).
miR-199a-5p mimic and inhibitor were obtained from Sigma-Aldrich
(Merck KGaA). SuperScript® VILO™ cDNA Synthesis and
SYBR-Green qPCR Super Mix UDG kits were obtained from Invitrogen
(Thermo Fisher Scientific, Inc.).
Patients and specimens
A total of 19 male patients with AMI (range: 42–69
age, 55.2±11.8 years) and 20 male patients with unstable angina as
control (range: 37–64 age, 51.4±12.9 years), who were admitted to
Emergency and Cardiology departments at the Second Hospital of
Hebei Medical University between May 2016 and August 2017. Clinical
and demographic characteristics of the subjects are presented in
Table I. Venous blood samples (5
ml) were centrifuged at ~1,000 × g for 30 min at room temperature,
and plasma was stored at −80°C prior to subsequent analysis.
Diagnostic criteria for patients with AMI were previously described
(10). Male healthy control
samples (n=23) were collected in the study. All subjects provided
written informed consent. The present study was approved by the
Research Ethics Committee of Second Hospital of Hebei Medical
University (Shijiazhuang, China).
 | Table I.Clinicopathological characteristics
of the 19 patients with AMI and 23 healthy subjects. |
Table I.
Clinicopathological characteristics
of the 19 patients with AMI and 23 healthy subjects.
|
Characteristics | AMI (n=19) | Control (n=23) | P-value |
|---|
| Age (years) | 55.2±11.8 | 51.4±12.9 | 0.326 |
| Hyperlipidemia, n
(%) | 3 (16) | 4 (17) | 0.890 |
| Hypertension, n
(%) | 10 (53) | 12 (52) | 0.627 |
| SBP (mmHg) | 131.1±27 | 121±19 | 0.241 |
| DBP (mmHg) | 86±22 | 81±17 | 0.423 |
| Fasting glucose
(mmol/l) | 6.14±2.11 | 5.31±1.79 | 0.183 |
| DM, n (%) | 4 (21) | 4 (17) | 0.764 |
| WBC
(×109/l) | 7.9±2.8 | 6.5±2.77 | 0.113 |
| Cr (µmol/l) | 91±47.2 | 67±44.3 | 0.100 |
| TG (mmol/l) | 1.44±0.62 | 1.32±0.81 | 0.590 |
| TC (mmol/l) | 4.23±0.41 | 4.11±0.91 | 0.575 |
| HDL C (mmol/l) | 1.03±0.71 | 1.17±0.54 | 0.485 |
| LDL C (mmol/l) | 2.57±0.9 | 2.39±0.51 | 0.445 |
| Current smoking, n
(%) | 13 (68) | 15 (65) | 0.826 |
H9c2 cells and OGD/R treatment
H9c2 cell (ATCC® CRL-1446™) were
purchased from the American Type Culture Collection (ATCC). The
cells were cultured at 37°C in a humidified incubator with 5%
CO2 and 95% air, and maintained in Dulbecco's modified
Eagles medium (DMEM) supplemented with 10% FBS. H9c2 cells were
treated with OGD/R or without pre-treatment as previously described
with minor modifications (16).
Briefly, cells were incubated in glucose-free DMEM and subsequently
placed in an anaerobic chamber (95% N2 and 5%
CO2) at 37°C for 6 h. Then glucose was added (final
concentration 4.5 mg/ml), and cells were incubated under normal
growth conditions (95% air and 5% CO2 for 18 h).
Cell transfection
Prior to transfection, the medium was replaced with
DMEM without serum and antibiotics. The miRNA mimic and inhibitor
sequences were as follows: miR-199a-5p mimic,
5′-ACAGUAGUCUGCACAUUGGUUA-3′; miR-199a-5p inhibitor,
5′-AGUCUCUCAUAUCUUCGGT-3′ and scrambled control (SC),
5′-UUGUACUACACAAAAGUACUG-3′. Following OGD/R treatment,
Lipofectamine® 2000 Transfection Reagent was mixed with
50 nM miR-199a-5p mimic, inhibitor, short interfering RNA (siRNA)
specific to HIF-1α (si-HIF-1α) or the siRNA negative control
(si-NC), and the mixture was added to the cells and incubated for 6
h. Complete medium was then used for culture for 24 h, following
which the cells were collected for subsequent experiments.
Transfection efficiency was observed and evaluated under the
microscope (>70% GFP-positive cells for si-RNA; data not shown)
or via quantitative PCR (qPCR) analysis.
siRNA obtained from Merck KGaA was used to knockdown
the expression of HIF-1α; si-NC was used as the control. siRNA
sequences were as follows: si-NC, 5′-GAGGCATACAGGGACAACACAGC-3′ and
si-HIF-1α, 5′-TTGAATCTGGGGGCATGGTAAAAG-3′. siRNA (5 µM) were
transfected using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) (18,19).
Following mixing of Lipofectamine 2000 with si-HIF-1α or si-NC, the
mixture was added to the cells prior to incubation for 6 h.
Complete medium was then used; following treatment with lithium
chloride (LiCl; 20 mM) for 24 h at 37°C, which the cells were
collected for subsequent experiments.
MTS assay
The viability of cells was determined using the
CellTiter 96® AQueous One Cell Proliferation
Assay kit (Promega Corporation). H9c2 cells (5×103/well)
were seeded in 96-well plates. Following the aforementioned
treatments and transfections, 20 µl per 100 µl reagent was added to
cells prior to incubation for a further 1 h. The absorbance at 490
nm was detected using a microplate reader.
LDH activity assay
The activity of LDH in the supernatant of treated
cells was detected using LDH-Cytotoxicity Assay Kit (cat. no.
K311-400; BioVision, Inc.).
Reverse transcription (RT)-qPCR
Total RNA from treated cells and plasma samples were
obtained using RNAiso Plus reagent (Takara Bio, Inc.) and
TRIzol® LS reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively. A total of 0.5 µg RNA from each
sample was reverse transcribed into cDNA using the
SuperScript® VILO™ cDNA Synthesis kit according to the
manufacturer's protocols. RT was conducted at 42°C for 60 min and
70°C for 5 min. An SYBR-Green qPCR Super Mix UDG kit (Takara Bio,
Inc.) was used for qPCR according to the manufacturer's protocol.
PCR was conducted as follows: 95°C for 2 min, then 40 cycles of
95°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec. The relative
expression was calculated using the 2−ΔΔCq formula and
normalized to U6 (20). All PCRs
were performed in triplicate. The primer sequences were as follows:
miR-199a-5p, forward, 5′-CCCAGTGTTCAGACTACCTGT-3′, reverse,
5′-GTGCGTGTCGTGGAG-3′; and U6, forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGGT-3′.
Western blot analysis
Western blotting was performed as previously
described (16). RIPA lysis buffer
containing PMSF (Beyotime Institute of Biotechnology) was used to
extract total proteins at 4°C for 30 min. The total protein
concentration was determined via a BCA assay. Protein (45 µg) was
separated via 12% SDS-PAGE and then transferred onto PVDF membranes
(EMD Millipore). Blocking for 1 h at room temperature with 5%
non-fat milk in 0.5% TBST (Boster Biological Technology) was
followed by incubation overnight at 4°C with the following primary
antibodies: Anti-HIF-1α (1:1,000); anti-GSK3β (1:1,000);
anti-p-GSK3β (1:1,000); anti-ANT (1:1,000); anti-Cyp-D (1:1,000)
and anti-β-actin (1:1,000). Membranes were washed in PBS with 0.1%
Tween-20 and incubated with horseradish peroxidase-conjugated goat
anti-mouse IgG and goat anti-rabbit IgG secondary antibodies
(1:3,000; cat. nos. ZDR-5307 and ZDR-5306, respectively; OriGene
Technologies, Inc. at room temperature for 2 h, according to the
manufacturer's protocols. The bonded proteins were visualized with
a chemiluminescence detection kit (Clarity Western ECL substrate;
Bio-Rad Laboratories, Inc.), and expression was quantified using
ImageQuant LAS400 (GE Healthcare Life Sciences).
TUNEL assay
The procedure was conducted using an in situ
Cell Death Detection kit (Roche Diagnostics) according to the
manufacturer's protocols. Nuclei were stained with DAPI (1:2,000;
37°C for 15 min). TUNEL-positive cells were analyzed as for the
immunofluorescent staining described below.
Measurement of mitochondrial membrane
potential (ΔΨm)
A MitoProbe™ JC-1 Assay kit (Thermo Fisher
Scientific, Inc.) was used to measure the ΔΨm according to the
manufacturer's protocols. Briefly, following reoxygenation for 6 h,
the cells were harvested. The cells in each group were then
resuspended in PBS containing 2 µmol/l JC-1 at 37°C for 30 min with
5% CO2. Following incubation, the cells were collected
and were analyzed using a flow cytometer (Elite Epics; Beckman
Coulter, Inc.) with excitation at 488 nm, and emission at 529 nm
(monomer form of JC-1, green) and at 590 nm (aggregate form of
JC-1, red). The ΔΨm of H9c2 cells in each group was calculated as
the fluorescent ratio of red to green. Signals were quantified
using ImageJ 2× software (National Institutes of Health).
Immunoprecipitation (IP)
IP was performed as previously described (21). In brief, 500 µg of total cellular
proteins from the various treatment groups was incubated with 1 µg
primary antibodies against ANT and p-GSK3β for 1 h at 4°C. The
mixture was incubated with 20 µl protein A/G PLUS-agarose slurry
(Santa Cruz Biotechnology, Inc.) at 4°C overnight. The pellets were
dissolved in 60 µl 1X electrophoresis sample buffer and boiled for
5 min. Samples (30 µl) were analyzed by western blotting as
aforementioned.
Cell immunofluorescence assay
Following removal of media, H9c2 cells were fixed
and permeated by 4% paraformaldehyde in PBS for 30 min at room
temperature, followed by blocking for 25 min at room temperature in
10% goat serum (Dako; Agilent Technologies, Inc.). The cells were
incubated with ANT anti-rabbit IgG (1:50) and p-GSK3β anti-rabbit
IgG (1:50) antibody at 4°C overnight. Samples were then washed with
PBS three times and incubated with Alexa Fluor®
594-conjugated anti-rabbit IgG (H+L; 1:150; cat. no. 8889, Cell
Signaling Technology, Inc.) for 1 h at room temperature. Nuclei
were counter-stained with 100 nM DAPI for 10 min at room
temperature. Images were acquired using a confocal microscope
(Leica SP5; Leica Microsystems GmbH) and digitized using LAS AF
Lite software (versions 2.1 and 2.0; Leica Microsystems GmbH).
Luciferase reporter assay
TargetScan (release 7.1; http://www.targetscan.org/vert_71/) was employed to
predict miR-199a-5p targets; HIF-1α was identified as a putative
target gene of miR-199a-5p. To construct luciferase reporter
vectors, the 3′-untranslated region (3′-UTR) sequence of HIF-1α
containing the predicted miR-199a-5p binding sites obtained from
rat genomic DNA (50 µg; cat. no. 55704; Celprogen, Inc.) was cloned
into a psiCHECK™-2 luciferase reporter vector (cat. no. C8021,
Promega) to generate HIF-1α (WT). The mutant (MUT) version of the
HIF-1α 3′-UTR lacking complementary with the binding sequence in
miR-199a-5p was constructed using a Quick Change Site-Directed
Mutagenesis kit [Stratagene; Agilent Technologies, Inc.; HIF-1α
(MUT)]. 293A cells (ATCC® CRL-1573™; ATCC) were cultured
at 37°C in a humidified incubator with 5% CO2 and 95%
air, and maintained in DMEM supplemented with 10% FBS and 1%
penicillin/streptomycin (100 U/ml; 100 mg/ml) in 24-well plates.
Cells were co-transfected with the constructed luciferase reporter
vectors (1–2 µg) and 50 mM miR-199a-5p or matched controls using
Lipofectamine 2000. At 48 h post-transfection, luciferase activity
was evaluated using a Dual-Luciferase Reporter Detection System
(Promega Corporation). The relative luciferase activity was
expressed as the ratio of firefly luciferase to Renilla
luciferase activity.
Statistical analyses
Statistical analyses using SPSS 13.0 software (SPSS,
Inc.) were performed using a two-tailed unpaired t-test for
comparing clinicopathological characteristics between patients with
AMI (n=19) and healthy subjects (n=23; Table I), or one-way analysis of variance
followed by Bonferroni post hoc tests for multiple comparisons.
Data were presented as the mean ± standard deviation of at least
three experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
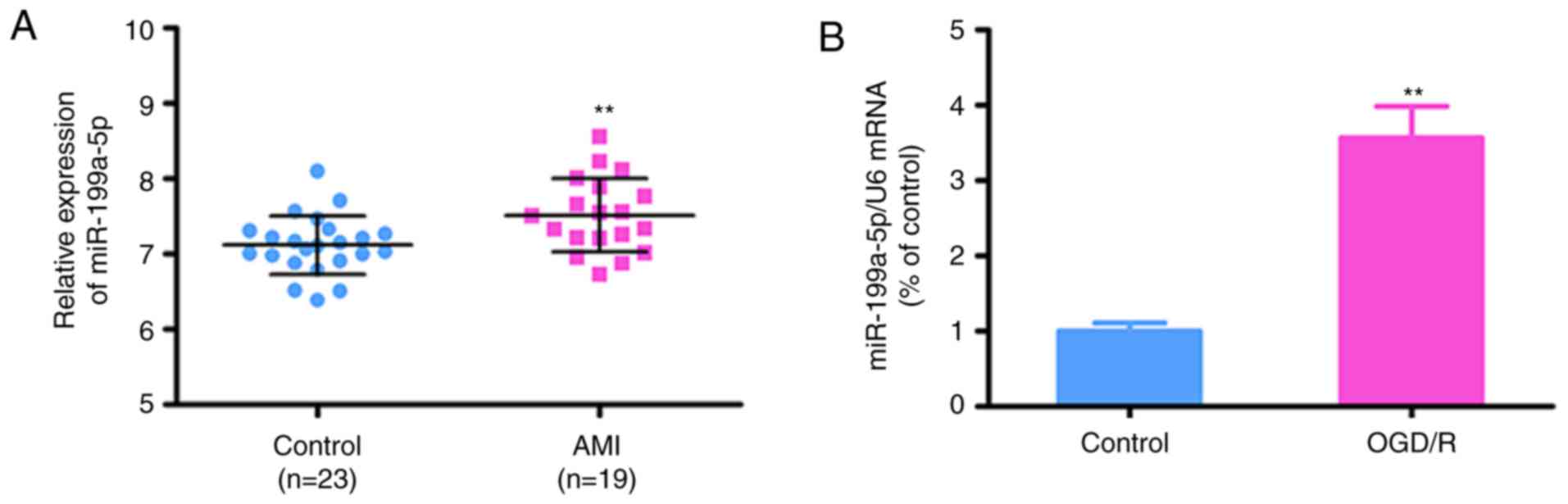
miR-199a-5p is upregulated in the
plasma of patients with AMI and OGD/R-treated H9c2 cells
The expression levels of miR-199a-5p were determined
in plasma obtained from male patients with AMI (n=19) and healthy
subjects (n=23) to investigate its potential roles in patients. The
clinicopathological data of patients and controls are presented in
Table I. There were no significant
differences between patients with AMI and the control group for any
of the reported variables, including age, hypertension,
hyperlipidemia, diabetes, LDL cholesterol and blood pressure
(P>0.05). Conversely, RT-qPCR analysis revealed that the plasma
levels of miR-199a-5p were significantly increased in patients with
AMI compared with in healthy controls (7.118±0.08 vs. 7.516±0.113;
P=0.007; Fig. 1A).
To further investigate whether the expression of
miR-199a-5p is associated with AMI, RT-qPCR analysis was conducted
in the OGD/R H9c2 model; it was revealed that the expression of
miR-199a-5p was upregulated in the OGD/R-induced H9c2 cells
compared with control cells (P<0.01; Fig. 1B). These findings suggested that
the expression of miR-199a-5p may serve important roles in
OGD/R-induced injury.
miR-199a-5p mimic promotes
OGD/R-induced cytotoxicity in H9c2 cells
To determine the effects of the miR-199a-5p
overexpression on OGD/R-induced injury, H9c2 cells were transfected
with miR-199a-5p mimic or SC. RT-qPCR analysis revealed that
transfection with miR-199a-5p mimic significantly increased its
expression in H9c2 cells following transfection for 24 h compared
with NC-transfected cells (P<0.05; Fig. 2A).
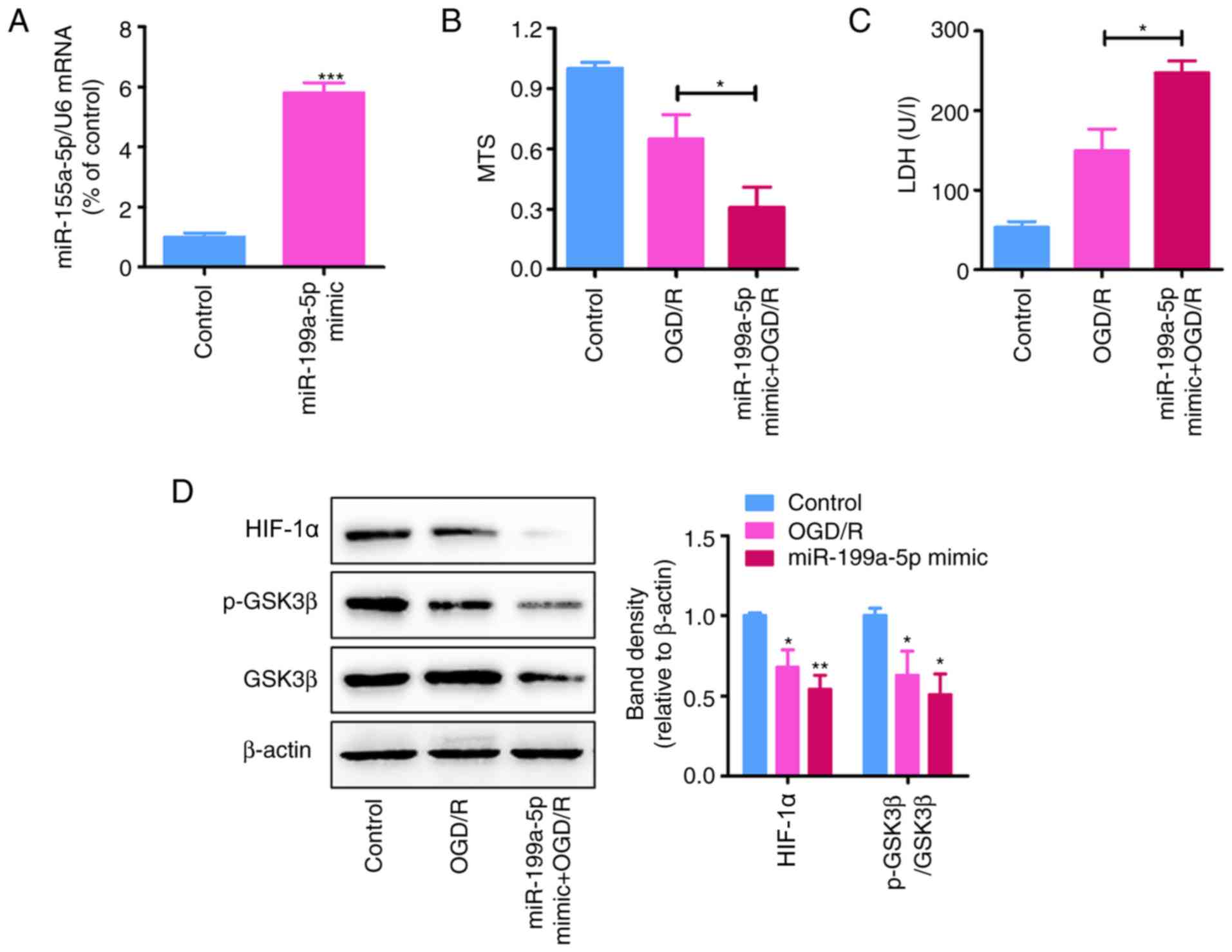 | Figure 2.miR-199a-5p overexpression increases
OGD/R-induced cytotoxicity in H9c2 cells. (A) miR-199a-5p
expression in H9c2 cells transfected with miR-199a-5p mimic or SC
(200 nM) under normoxia for 24 h, as determined by reverse
transcription-quantitative PCR analysis. ***P<0.001 vs. control.
(B) MTS and (C) LDH assays of control and OGD/R-treated H9c2 cells
transfected with miR-199a-5p mimic or control (n=3). *P<0.05.
(D) Expression of HIF-1α, GSK3β and p-GSK3β (Ser9) in OGD/R-treated
H9c2 cells transfected with miR-199a-5p mimic or control, as
determined by western blotting. Protein expression levels of HIF-1α
and the relative p-GSK3β/GSK3β protein ratio were quantified using
ImageJ software and normalized to β-actin. *P<0.05, **P<0.01
vs. SC. Data are presented as the mean ± standard deviation. Each
experiment was repeated three times. miR-199a-5p, microRNA-199a-5p;
SC/control, scrambled control; OGD/R, oxygen-glucose deprivation
and reperfusion; LDH, lactate dehydrogenase; HIF-1α,
hypoxia-inducible factor-1α; p-, phosphorylated; GSK3β, glycogen
synthase kinase 3β. |
MTS and LDH assays revealed that miR-199a-5p mimic
significantly decreased the viability of (P<0.01), and promoted
LDH leakage from (P<0.05) OGD/R-treated H9c2 cells compared with
the SC (Fig. 2B and C).
Additionally, western blot analysis demonstrated that the
upregulation of miR-199a-5p significantly inhibited the expression
of HIF-1α and the p-GSK3β/GSK3β protein ratio in OGD/R-treated H9c2
cells (P<0.01; Fig. 2D).
Knockdown of miR-199a-5p attenuates
cytotoxicity in OGD/R-treated H9c2 cells
To further determine the role of miR-199a-5p in
OGD/R-treated H9c2 cells and investigate whether miR-199a-5p
knockdown may alleviate the OGD/R-induced myocardial cytotoxicity,
OGD/R-induced H9c2 cells were transfected with miR-199a-5p
inhibitor or SC. Following transfection with inhibitor for 24 h,
the expression of miR-199a-5p was significantly reduced compared
with SC (P<0.001; Fig. 3A). The
effects of miR-199a-5p downregulation on the viability,
cytotoxicity, apoptosis, and ΔΨm of OGD/R-treated cells was
subsequently investigated. As demonstrated using an MTS assay, the
OGD/R-induced decrease in H9c2 cell viability was significantly
rescued following transfection with miR-199a-5p inhibitor for 24 h,
compared with OGD/R-treated H9c2 cells (Fig. 3B). Similarly, compared with the
OGD/R-treated group, miR-199a-5p inhibitor significantly suppressed
the OGD/R-induced leakage of LDH from H9c2 cells (P<0.05;
Fig. 3C). Furthermore, TUNEL
staining revealed that miR-199a-5p inhibitor significantly
decreased the number of apoptotic H9c2 cells compared with the
OGD/R-treated group (P<0.01; Fig.
3D and E). JC-1 was used to detect alterations in the ΔΨm,
which indirectly reflects the degree of mitochondrial permeability
transition pore (mPTP) opening. Compared with the OGD/R-treated
group, transfection with the miR-199a-5p inhibitor significantly
rescued OGD/R-induced ΔΨm depolarization in H9c2 cells (Fig. 3F and G). In addition, western blot
analysis revealed that knockdown of miR-199a-5p in OGD/R-induced
H9c2 cells significantly rescued the expression of HIF-1α and the
phosphorylation of GSK3β (P<0.01; Fig. 3H). Overall, these findings
indicated that miR-199a-5p promoted OGD/R-induced apoptosis, LDH
leakage and alterations in the ΔΨm in H9c2 cells, potentially via
regulation of the expression of HIF-1α and p-GSK3β.
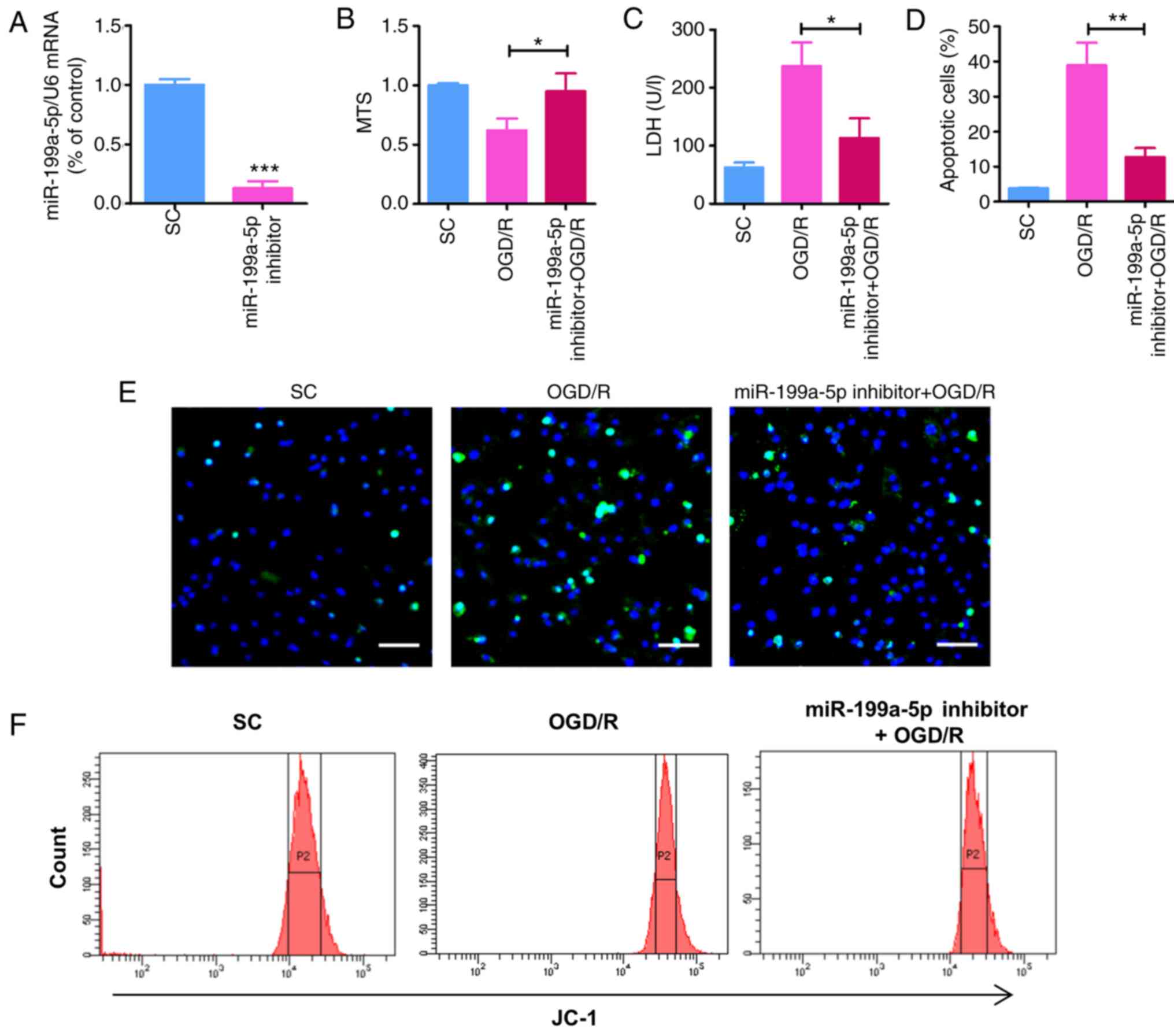 | Figure 3.miR-199a-5p downregulation reduces
OGD/R-induced cytotoxicity and increases the expression of HIF-1α
and p-GSK3β in H9c2 cells. (A) Expression of miR-199a-5p in H9c2
cells transfected with miR-199a-5p inhibitor or SC (200 nM) for 24
h, as determined by reverse transcription-quantitative PCR
analysis. ***P<0.001 vs. SC. (B) MTS and (C) LDH assays of H9c2
cells treated with OGD/R and transfected with miR-199a-5p inhibitor
or SC. *P<0.05. (D) Percentage of apoptosis H9c2 cells following
OGD/R treatment, as determined by a TUNEL assay. **P<0.01. (E)
Images of TUNEL staining for the detection of apoptosis of
OGD/R-injured cardiomyocytes. Scale bar, 50 µm. (F) Flow cytometric
analysis of the ΔΨm in H9c2 cells. (G) Analysis of the ΔΨm
determined by JC-1 detection. *P<0.05 vs.OGD/R. (H) Western
blotting and quantification of HIF-1α expression and the relative
p-GSK3β/GSK3β protein ratio. **P<0.01 vs. SC. (I) Predicted
binding sites between miR-199a-5p and HIF-1α mRNA via complementary
base-pairs. Luciferase reporter plasmids containing WT or MUT
3′-UTRs of HIF-1α were constructed. (J) Luciferase reporter assays
were performed to detect the luciferase activity in 293A cells
following co-transfection with miR-199a-5p or miR-NC, and HIF-1α
(WT) or HIF-1α (MUT) reporter plasmids. **P<0.01 vs. miR-NC.
Data are presented as the mean ± standard deviation of three
independent experiments. miR-199a-5p, microRNA-199a-5p; SC,
scrambled control; OGD/R, oxygen-glucose deprivation and
reperfusion; LDH, lactate dehydrogenase; ΔΨm, mitochondrial
membrane potential; HIF-1α, hypoxia-inducible factor-1α; p-,
phosphorylated; GSK3β, glycogen synthase kinase 3β; UTR,
untranslated region; WT, wild-type; MUT, mutant; miR-NC, miR
negative control. |
Knockdown of miR-199a-5p attenuates
OGD/R-induced cytotoxicity in H9c2 cells by regulating the
HIF-1α-GSK3β pathway
HIF-1α was reported to be a putative target of
miR-199a-5p (17,18), and the p-GSK3β signaling serves as
an effector of cardioprotection (22); therefore, whether p-GSK3β is an
effector of HIF-1α was investigated. To determine the molecular
mechanisms underlying the role of miR-199a-5p as a proapoptotic
factor in the OGD/R model, TargetScan was used to predict the
potential targets of miR-199a-5p. HIF-1α was predicted to be a
candidate target gene of miR-199a-5p, with complementary binding
sites of miR-199a-5p identified in the 3′-UTR of HIF-1α (Fig. 3I). To validate the prediction of
direct binding between miR-199a-5p and HIF-1α, a luciferase
reporter assay was performed. As presented in Fig. 3J, co-transfection with miR-199a-5p
and HIF-1α (WT) significantly reduced the luciferase activity
(P<0.01), whereas co-transfection with miR-199a-5p and HIF-1α
(mut) did not significantly alter luciferase activity in 293A
cells. To determine the effects of HIF-1α on p-GSK3β signaling in
H9c2 cells under OGD/R, the expression of HIF-1α was inhibited via
transfection of H9c2 cells with siRNA-HIF-1α, which significantly
reduced the expression of HIF-1α and p-GSK3β compared with si-NC
(P<0.05; Fig. 4A and B).
Furthermore, the reduced apoptosis (P<0.01; Fig. 4C and F) and ΔΨm depolarization
(Fig. 4D and G) following
miR-199a-5p knockdown in OGD/R-induced H9c2 cells was significantly
attenuated by the downregulation of HIF-1α expression (Fig. 4B) or the inhibition of p-GSK3β
expression via treatment with LiCl (a specific inhibitor of
p-GSK3β; P<0.01; Fig. 4E).
Collectively, these results suggested that miR-199a-5p mediates
OGD/R-induced cytotoxicity in H9c2 cells, partially by suppressing
the expression of HIF-1α and the downstream phosphorylation of
GSK3β.
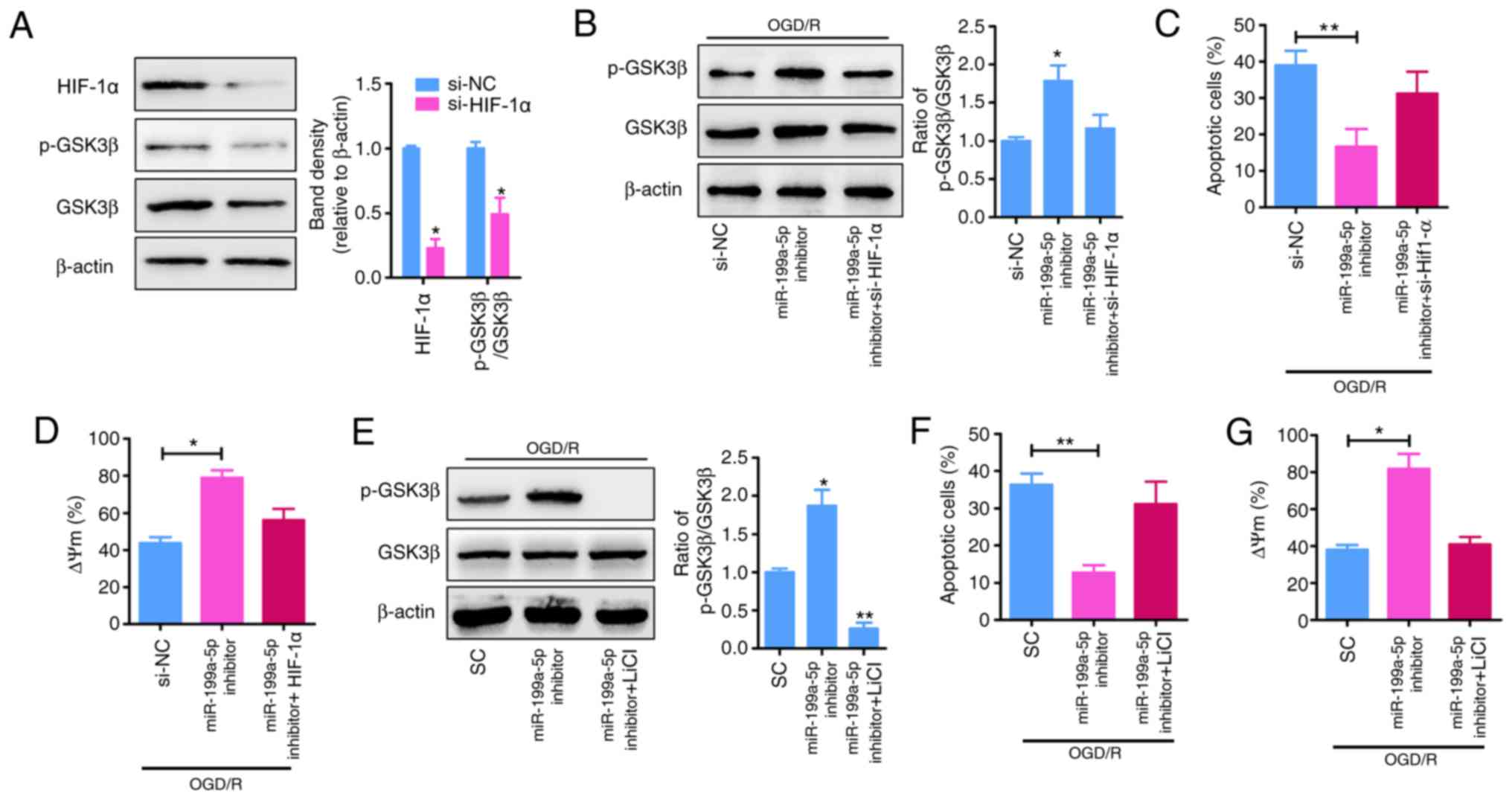 | Figure 4.miR-199a-5p inhibitor mediates
protection against OGD/R-induced injury by upregulating the
expression of HIF-1α and p-GSK3β. (A) Analysis of HIF-1α levels and
the relative p-GSK3β/GSK3β protein ratio in H9c2 cells transfected
with si-NC or si-HIF-1α. *P<0.05 vs. si-NC. (B) H9c2 cells were
treated with OGD/R and transfected with miR-199a-5p inhibitor in
the presence or absence of si-HIF-1α. The cell lysates were
analyzed by western blotting for GSK3β and p-GSK3β; expression was
quantified by ImageJ software and normalized to β-actin. *P<0.05
vs. si-NC. (C) Apoptosis and (D) ΔΨm were analyzed by
TUNEL and flow cytometry analyses, respectively. *P<0.05,
**P<0.01. (E) OGD/R-treated H9c2 cells were transfected with
miR-199a-5p inhibitor in the presence or absence of LiCl. The cell
lysates were analyzed by western blotting for GSK3β and p-GSK3β;
expression was quantified by ImageJ software and normalized to
β-actin. *P<0.05, **P<0.01 vs. SC. (F) Apoptosis and (G) ΔΨm
were analyzed by TUNEL and flow cytometry analyses, respectively.
*P<0.05, **P<0.01. Data are presented as the mean ± standard
deviation of four independent experiments. HIF-1α,
hypoxia-inducible factor-1α; p-, phosphorylated; GSK3β, glycogen
synthase kinase 3β; si-, short interfering RNA; NC, negative
control; OGD/R, oxygen-glucose deprivation and reperfusion;
miR-199a-5p, microRNA-199a-5p; ΔΨm, mitochondrial membrane
potential; SC, scrambled control. |
Downregulation of miR-199a-5p
contributes to the binding of p-GSK3β to ANT in the mPTP
The opening of the mPTP induces apoptotic cell death
during I/R (23). To determine the
effects of the downregulation of miR-199a-5p on the opening of the
mPTP, the OGD/R-induced formation of protein complexes between two
main mPTP components, ANT and Cyp-D was detected via IP assays. It
was demonstrated that miR-199a-5p downregulation notably decreased
the formation of ANT-Cyp-D complexes (Fig. 5A, lane 2 vs. 3), whereas the
interaction between ANT and Cyp-D was restored when OGD/R-treated
cells were co-transfected with si-HIF-1α and miR-199a-5p inhibitor
(Fig. 5A, lane 3 vs. 4).
Furthermore, the binding of ANT and p-GSK3β was analyzed by an IP
assay using anti-p-GSK3β antibodies. As presented in Fig. 5B, miR-199a-5p downregulation
increased the binding of p-GSK3β to ANT, indicating that
miR-199a-5p inhibitor-induced interactions between p-GSK3β and ANT
inhibited OGD/R-induced interactions between ANT and Cyp-D
(Fig. 5B, lane 2 vs. 3).
Similarly, OGD/R-induced H9c2 cells were treated with miR-199a-5p
inhibitor and LiCl, which also notably promoted the interaction
between Cyp-D and ANT (Fig. 5C,
lane 3 vs. 4). The binding of p-GSK3β to the mitochondrial membrane
was further characterized via immunofluorescence staining. In OGD/R
+ miR-199a-5p inhibitor-treated H9c2 cells, p-GSK3β (red) and ANT
(green) were localized predominately in the mitochondria (yellow;
Fig. 5D), whereas LiCl treatment
notably decreased the binding of p-GSK3β to ANT in the mPTP. These
findings indicated that the effect of miR-199a-5p knockdown on the
cytotoxicity of OGD/R-induced H9c2 cells is mediated by
upregulating the expression of HIF-1α and p-GSK3β, promoting the
interaction between p-GSK3β and ANT, which, in turn, reduces the
interaction between ANT and Cyp-D, and subsequently contributing to
the suppression of mPTP opening.
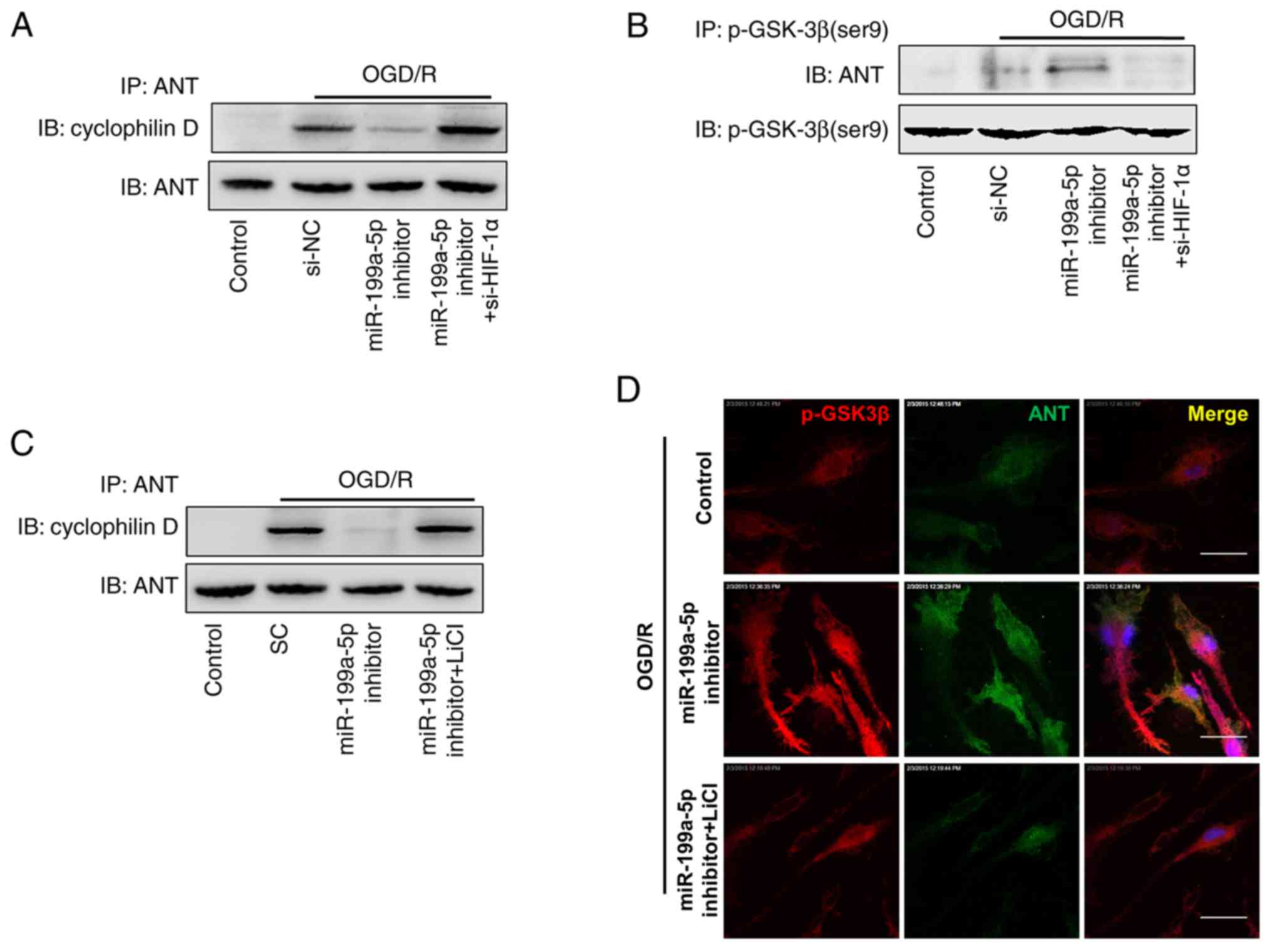 | Figure 5.Interactions between p-GSK3β and ANT
following miR-199a-5p downregulation inhibit the formation of the
ANT-Cyp-D complex. H9c2 cells were transfected with si-NC,
miR-199a-5p inhibitor, or co-transfected with miR-199a-5p inhibitor
and si-HIF-1 under OGD/R conditions. Detection of ANT
coimmunoprecipitated with (A) Cyp-D or (B) p-GSK3β by western
blotting. (C) H9c2 cells were transfected with SC, or miR-199a-5p
inhibitor in the presence or absence of LiCl treatment under OGD/R
conditions. Detection of ANT coimmunoprecipitated with Cyp-D by
western blotting. (D) Colocalization of ANT with p-GSK3β in treated
H9c2 cells was analyzed by confocal fluorescence microscopy. Nuclei
were stained with DAPI. Scale bar, 10 µm. IP, immunopreciptation;
ANT, adenine nucleotide transferase; OGD/R, oxygen-glucose
deprivation and reperfusion; Control, untreated H9c2 cells; si-,
short interfering RNA; NC, negative control; miR-199a-5p,
microRNA-199a-5p; HIF-1α, hypoxia-inducible factor-1α; p-,
phosphorylated; GSK3β, glycogen synthase kinase 3β; SC, scrambled
control; Cyp-D, cyclophilin D. |
Discussion
Myocardial I/R injury is an inevitable consequence
of the treatment of ischemic heart disease. Thus, the protection of
I/R-exposed cardiac tissue is a major therapeutic challenge in
modern cardiology (24). The
present study detected the plasma levels of miR-199a-5p in patients
with AMI and in an OGD/R-induced H9c2 cell model, in addition to
the effects of miR-199a-5p on OGD/R-induced cytotoxicity in H9c2
cells. OGD/R-treated H9c2 cells were transfected with miR-199a-5p
mimic or inhibitor to overexpress or knockdown miR-199a-5p, as
previously described (16). It was
observed that the upregulation of miR-199a-5p inhibited cell
survival and promoted LDH leakage from OGD/R-induced H9c2 cells,
whereas miR-199a-5p knockdown promoted the viability, and inhibited
the apoptosis and alterations in the ΔΨm of OGD/R-treated H9c2
cells, potentially by increasing the expression of HIF-1α and
p-GSK3β, and promoting the interaction between p-GSK3β and ANT,
thereby suppressing the OGD/R-induced interaction of ANT with Cyp-D
and opening of the mPTP.
miRNAs are involved in cardiac pathological
processes, including cardiac hypertrophy (25), arrhythmogenesis (26) and heart failure (27). Increasing evidence indicates that
miRNAs may be potential therapeutic targets against myocardial I/R.
Reportedly, miR-199a-3p is upregulated in injured mouse kidneys
following renal I/R (28).
Additionally, miRNA-199a overexpression decreased sirtuin 1 (Sirt1)
levels, whereas silencing miRNA-199a using antisense
oligonucleotides or by hypoxic preconditioning increased Sirt1
expression (19). A previous study
suggested that inhibition of the mPTP opening may be a novel target
for cardioprotective drugs against myocardial infarction (23). Based on this finding, it was
investigated as to r whether miR-199a-5p exhibited cardioprotective
effects via regulation of mPTP opening. It was demonstrated that
miR-199a-5p inhibitor effectively suppressed cell apoptosis and LDH
leakage, and rescued cell viability and the ΔΨm
following OGD/R. Additionally, it was observed that miR-199a-5p
inhibitor significantly upregulated the expression of HIF-1α and
p-GSK3β. The inhibition of p-GSK3β modulated the opening of the
mPTP, contributing to cardiac injury during myocardial I/R
(29).
The present study determined that miR-199a-5p
knockdown regulated HIF-1α activation-mediated cardioprotection in
OGD/R-injured H9c2 cells. Of note, the knockdown of HIF-1α reversed
the protective effects of the miR-199a-5p inhibitor on
OGD/R-injured H9c2 cells via by decreasing p-GSK3β expression
(Fig. 4B). The activation of GSK3β
via dephosphorylation during prolonged ischemia has been reported
to be protective; however, the inactivation of GSK3β via
phosphorylation during the reperfusion phase may also be protective
(30). The protein stability of
HIF-1α and the phosphorylation of GSK3β are cardioprotective
against I/R (31). The
stabilization of HIF-1α in the murine heart may inhibit mPTP
opening following I/R (32). In
addition, the phosphorylation of GSK3β inhibited mPTP opening
following the I/R injury of cardiomyocytes (33). Of note, in non-neuronal cells,
increased serine phosphorylation and subsequent inactivation of
GSK3β is associated with increased HIF-1α expression (34). Furthermore, GSK3β phosphorylation
is associated with HIF-1α expression in H9c2 cells (35).
Juhaszova et al (36) first reported that the activity of
GSK3β sets a threshold for mPTP opening in cardiomyocytes, and that
the threshold for the reactive oxygen species-induced opening of
the mPTP is increased by the inhibitory phosphorylation of GSK3β.
The molecular mechanisms via which the inactivation of GSK3β (or
its phosphorylation at Ser9) increases the threshold for mPTP
opening remains unclear; however, it appears that the suppression
of ANT-Cyp-D interactions via the binding of p-GSK3β to ANT
contributes to the elevation of the threshold (33,36,37).
The present findings suggested that the knockdown of miR-199a-5p
reduced the interaction between ANT and Cyp-D, indicating that
inactivation of GSK3β via its phosphorylation at Ser9 dismantles
the mPTP complex. It has been reported that pharmacological
inhibitors of GSK3β limit the infarct size when administered prior
to I/R injury (38), suggesting
fine-tuned mechanisms via which the altered phosphorylation GSK3
serves complex roles in mPTP opening in cardiomyocytes.
Collectively, the present findings suggested that
the upregulation of miR-199a-5p expression contributed to I/R
injury in cardiomyocytes. Knockdown of miR-199a-5p protected
against I/R-induced cardiomyocyte apoptosis by targeting the
HIF-1α-GSK3β-mPTP axis, which may be a potential target for
therapeutic intervention in patients with AMI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
the Second Hospital of Hebei Medical University (grant no.
2h201804).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DWL, YNZ, HJH, PQZ and WC conducted the experiments
and data collection, and interpreted the data. DWL was involved in
the design and coordination of experiments, the acquisition and
analysis of data, and drafting the manuscript. WC made substantial
contributions to the design, analysis and interpretation of
data.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Second Hospital of Hebei Medical University.
Experimental procedures were implemented in according to the
guidelines and regulations of Hebei Medical University. Patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hausenloy DJ and Yellon DM: Targeting
myocardial reperfusion injury-the search continues. N Engl J Med.
373:1073–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pagliaro P, Moro F, Tullio F, Perrelli MG
and Penna C: Cardioprotective pathways during reperfusion: Focus on
redox signaling and other modalities of cell signaling. Antioxid
Redox Signal. 14:833–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen
X, Cai Y, Li L, Wang WE and Zeng C: Mitochondrial DNA oxidative
damage contributes to cardiomyocyte Ischemia/Reperfusion-injury in
rats: Cardioprotective role of lycopene. J Cell Physiol.
230:2128–2141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaczorowski DJ, Nakao A, McCurry KR and
Billiar TR: Toll-like receptors and myocardial
ischemia/reperfusion, inflammation, and injury. Curr Cardiol Rev.
5:196–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad A, Stone GW, Holmes DR and Gersh B:
Reperfusion injury, microvascular dysfunction, and
cardioprotection: The ‘dark side’ of reperfusion. Circulation.
120:2105–2112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laina A, Gatsiou A, Georgiopoulos G,
Stamatelopoulos K and Stellos K: RNA therapeutics in cardiovascular
precision medicine. Front Physiol. 9:9532018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H and Fan GC: Role of microRNAs in the
reperfused myocardium towards post-infarct remodelling. Cardiovasc
Res. 94:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bian B, Yu XF, Wang GQ and Teng TM: Role
of miRNA-1 in regulating connexin 43 in ischemia-reperfusion heart
injury: A rat model. Cardiovasc Pathol. 27:37–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Zhang L, Su T, Li H, Huang Q, Wu
D, Yang C and Han Z: Kinetics of plasma microRNA-499 expression in
acute myocardial infarction. J Thorac Dis. 7:890–896.
2015.PubMed/NCBI
|
|
11
|
Zhang XH, Zheng B, Han M, Miao SB and Wen
JK: Synthetic retinoid Am80 inhibits interaction of KLF5 with RAR
alpha through inducing KLF5 dephosphorylation mediated by the
PI3K/Akt signaling in vascular smooth muscle cells. FEBS Lett.
583:1231–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song CL, Liu B, Diao HY, Shi YF, Zhang JC,
Li YX, Liu N, Yu YP, Wang G, Wang JP and Li Q: Down-regulation of
microRNA-320 suppresses cardiomyocyte apoptosis and protects
against myocardial ischemia and reperfusion injury by targeting
IGF-1. Oncotarget. 7:39740–39757. 2016.PubMed/NCBI
|
|
13
|
Su S, Luo, Liu X, Liu J, Peng F, Fang C
and Li B: miR-494 up-regulates the PI3K/Akt pathway via targetting
PTEN and attenuates hepatic ischemia/reperfusion injury in a rat
model. Biosci Rep. 37(pii): BSR201707982017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liu G, Zhang H and Wang J:
MiRNA-199a-5p influences pulmonary artery hypertension via
downregulating Smad3. Biochem Biophys Res Commun. 473:859–866.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Li Z, Zhang Y, Wang G, Wei M, Hu
Y, Ma S, Jiang Y, Che N, Wang X, et al: Targeting of
microRNA-199a-5p protects against pilocarpine-induced status
epilepticus and seizure damage via SIRT1-p53 cascade. Epilepsia.
57:706–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zuo Y, Wang Y, Hu H and Cui W:
Atorvastatin protects myocardium against ischemia-reperfusion
injury through inhibiting miR-199a-5p. Cell Physiol Biochem.
39:1021–1030. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schnitzer SE, Schmid T, Zhou J, Eisenbrand
G and Brüne B: Inhibition of GSK3beta by indirubins restores
HIF-1alpha accumulation under prolonged periods of hypoxia/anoxia.
FEBS Lett. 579:529–533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Lei S, Long J, Liu X and Wu Q:
MicroRNA-199a-5p inhibits tumor proliferation in melanoma by
mediating hif-1α. Mol Med Rep. 13:5241–5249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR-199a derepresses hypoxia-inducible
factor-1alpha and Sirtuin 1 and recapitulates hypoxia
preconditioning in cardiac myocytes. Circ Res. 104:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishihara M, Miura T, Miki T, Tanno M,
Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y and Shimamoto K:
Modulation of the mitochondrial permeability transition pore
complex in GSK-3β-mediated myocardial protection. J Mol Cell
Cardiol. 43:564–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Cai P, Cheng Z, Zhang Z and Fang
J: Pharmacological postconditioning with atorvastatin calcium
attenuates myocardial ischemia/reperfusion injury in diabetic rats
by phosphorylating GSK3β. Exp Ther Med. 14:25–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gyulkhandanyan AV, Mutlu A, Freedman J and
Leytin V: Mitochondrial permeability transition pore
(MPTP)-dependent and -independent pathways of mitochondrial
membrane depolarization, cell shrinkage and microparticle formation
during platelet apoptosis. Br J Haematol. 169:142–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivaraman V and Yellon DM: Pharmacologic
therapy that simulates conditioning for cardiac
ischemic/reperfusion injury. J Cardiovasc Pharmacol Ther. 19:83–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heggermont WA, Papageorgiou AP, Quaegebeur
A, Deckx S, Carai P, Verhesen W, Eelen G, Schoors S, van Leeuwen R,
Alekseev S, et al: Inhibition of MicroRNA-146a and overexpression
of its target dihydrolipoyl succinyltransferase protect against
pressure overload-induced cardiac hypertrophy and dysfunction.
Circulation. 136:747–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danielson LS, Park DS, Rotllan N,
Chamorro-Jorganes A, Guijarro MV, Fernandez-Hernando C, Fishman GI,
Phoon CK and Hernando E: Cardiovascular dysregulation of miR-17-92
causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis.
FASEB J. 27:1460–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verjans R, Peters T, Beaumont FJ, van
Leeuwen R, van Herwaarden T, Verhesen W, Munts C, Bijnen M, Henkens
M, Diez J, et al: MicroRNA-221/222 family counteracts myocardial
fibrosis in pressure overload-induced heart failure. Hypertension.
71:280–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Godwin JG, Ge X, Stephan K, Jurisch A,
Tullius SG and Iacomini J: Identification of a microRNA signature
of renal ischemia reperfusion injury. Proc Natl Acad Sci USA.
107:14339–14344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomez L, Paillard M, Thibault H, Derumeaux
G and Ovize M: Inhibition of GSK3beta by postconditioning is
required to prevent opening of the mitochondrial permeability
transition pore during reperfusion. Circulation. 117:2761–2768.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhai P, Sciarretta S, Galeotti J, Volpe M
and Sadoshima J: Differential roles of GSK-3β during myocardial
ischemia and ischemia/reperfusion. Circ Res. 109:502–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen T, Wong R, Wang G, Gucek M,
Steenbergen C and Murphy E: Acute inhibition of GSK causes
mitochondrial remodeling. Am J Physiol Heart Circ Physiol.
302:H2439–H2445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Ke X, Fu W, Gao X, Zhang H, Wang W,
Ma N, Zhao M, Hao X and Zhang Z: Inhibition of hypoxia-induced
retinal angiogenesis by specnuezhenide, an effective constituent of
ligustrum lucidum Ait., through suppression of the HIF-1α/VEGF
signaling pathway. Molecules. 21(pii): E17562016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Zhang F, Zhao G, Cheng Y, Wu T, Wu
B and Zhang YE: Mitochondrial PKC-ε deficiency promotes
I/R-mediated myocardial injury via GSK3β-dependent mitochondrial
permeability transition pore opening. J Cell Mol Med. 2:2009–2021.
2017. View Article : Google Scholar
|
|
34
|
Flügel D, Görlach A and Kietzmann T:
GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7
and USP28-dependent degradation of HIF-1α. Blood. 119:1292–1301.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang X, Zuo L, Lv Y, Chen C, Yang Y, Xin
H, Li Y and Qian Y: Asiatic acid attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β/HIF-1α signaling in rat
H9c2 cardiomyocytes. Molecules. 21:E12482016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Juhaszova M, Wang S, Zorov DB, Nuss HB,
Gleichmann M, Mattson MP and Sollott SJ: The identity and
regulation of the mitochondrial permeability transition pore: Where
the known meets the unknown. Ann NY Acad Sci. 1123:197–212. 2010.
View Article : Google Scholar
|
|
37
|
Zhu H, Ding Y, Xu X, Li M, Fang Y, Gao B,
Mao H, Tong G, Zhou L and Huang J: Prostaglandin E1 protects
coronary microvascular function via the glycogen synthase kinase
3β-mitochondrial permeability transition pore pathway in rat hearts
subjected to sodium laurate-induced coronary microembolization. Am
J Transl Res. 9:2520–2534. 2017.PubMed/NCBI
|
|
38
|
Obame FN, Plin-Mercier C, Assaly R, Zini
R, Dubois-Randé JL, Berdeaux A and Morin D: Cardioprotective effect
of morphine and a blocker of glycogen synthase kinase 3 beta,
SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)- 1H-
pyrrole-2,5-dione], via inhibition of the mitochondrial
permeability transition pore. J Pharmacol Exp Ther. 326:252–258.
2008. View Article : Google Scholar : PubMed/NCBI
|