Introduction
Hepatitis B virus (HBV) infection is a global health
problem, particularly in Southeast Asia and Africa (1). An estimated ~260 million individuals
are chronic carriers, serving as the main reservoir for continued
HBV transmission (2–4). Almost 25% of carriers develop serious
liver diseases, including cirrhosis, chronic hepatitis and primary
hepatocellular carcinoma (5). It
is predicted that 500,000–1.2 million individuals succumb every
year from the chronic consequences of HBV infection (6); however, the prevalence of HBV
infection varies markedly between different countries. For example,
the incidence rate is 15–20% in Taiwanese adults, 9.8% in Egypt,
7.4% in Iran, 8.6% in Israel and 7.18% in China (7,8).
Numerous countries introduced the HBV vaccine into
national routine immunization schemes of infants during the 1990s
to eliminate HBV transmission and prevent HBV-associated chronic
liver disease. The prevalence of HBV infection subsequently
decreased in the following years, as the vaccination program was
universally implemented (9). The
universal HBV vaccination program for infants has been incorporated
into the national immunization scheme in China since 1992 (10). Between 1992 and 2006, the surface
antigen of HBV (HBsAg)-positive rate among young children in China
decreased from ~10% to 2.08% (11). High titers of HBV surface antibody
(anti-HBs) produced in response to the HBV vaccine were revealed to
be able to effectively protect humans from infection with HBV
(12). At present, HBV vaccination
is regarded as the most effective and economical method for the
prevention and control of HBV infection (11,13,14).
Anti-HBs levels ≥10 IU/l in response to HBV
vaccination are generally regarded as seroprotective, and are
considered to effectively prevent HBV infection (15); however, due to interindividual
differences, 5–10% of healthy immunocompetent subjects do not
elicit an antibody response (16),
notably increasing the possibility of HBV infection. A large number
of clinical data have revealed that males exhibit an increased risk
of non-responsiveness to the HBV vaccine (17–19).
In addition, males are more likely than females to be HBV carriers,
exhibit a notably increased mortality rate from hepatocellular
carcinoma associated with HBV infection (13,14,20),
and HBV infection has been observed to be sexually dimorphic in
humans (14). Consistent with
these differences are sex-specific differences in the antibody
response following vaccination; antibody responses are more easily
stimulated in females than in males (21). At present, however, the mechanism
underlying sex-based differences with respect to the anti-HBV
immune response remains unclear. The present study aimed to
determine the immunological differences in response to the HBV
vaccine between immunized female and male mice, providing a
potential theoretical framework for the prevention and control of
HBV infection.
Materials and methods
Mice
A total of 72 BALB/c mice (36 males and 36 females,
specific pathogen-free grade; aged 4–6 weeks; 13–15 g) were
purchased from Beijing Huafukang Biological Technology Co., Ltd.
and housed in an aseptic environment at the Animal Center of State
Key Laboratory of Biotherapy of Sichuan University for 2 weeks,
with adaptive feeding prior to the start of experiment. The animals
were fed with a standard laboratory chow and sterile water ad
libitum. They were maintained in a controlled environment at a
temperature of 20–25°C, relative humidity of 40–70%, artificially
illuminated with a 12:12-h light/dark cycle and air exchanges of
10–15 times/h. The experimental protocol was approved by the Ethics
Review Committee for Animal Experimentation of Chengdu Blood
Center. A total of 3 cohorts of 24 mice were used during the
present study; a preliminary cohort, and 2 experimental cohorts.
Data from the second cohort were presented for ELISpot assays,
whereas data for all other assays were generated from the third
cohort.
Vaccines and immunization
protocol
HBV vaccines were prepared by mixing 1 µg HBsAg
(cat. no. P4875; Abnova) with 25 µg alum (cat. no. 21645-51-2;
Brenntag Biosector A/S) in PBS in a total volume of 100 µl. BALB/c
mice were randomly divided into four groups: Male control; female
control; male treated and female treated. Treated groups (n=6) were
vaccinated 3 times intramuscularly with the HBV vaccine on days 0,
14 and 28. The mice in the control groups (n=6) were treated with
PBS. Blood samples were collected from the tail vein, with the
exception of week 7, when blood was collected via retro-orbital
sampling under 4% isoflurane to obtain sufficient blood (150–200
µl) to analyze total and subtype anti-HBs titers. Serum samples
were obtained via centrifugation at 4,000 × g for 10 min at 4°C
following incubation at 37°C for 2 h. Sera were analyzed using
ELISA for the presence of HBsAg-specific immunoglobulin G (IgG) at
weeks 1, 3, 5 and 7. Enzyme-linked immunosorbent spot (ELISpot)
assays were performed to determine the secreted levels of
interleukin-4 (IL-4)/interferon-γ (IFN-γ) at 1 week following the
third immunization. Immunological memory stimulated by the vaccine
was detected using flow cytometric analysis and ELISA at 1 week
following the booster immunization (week 31 immunization; schematic
of treatments in Fig. 1A).
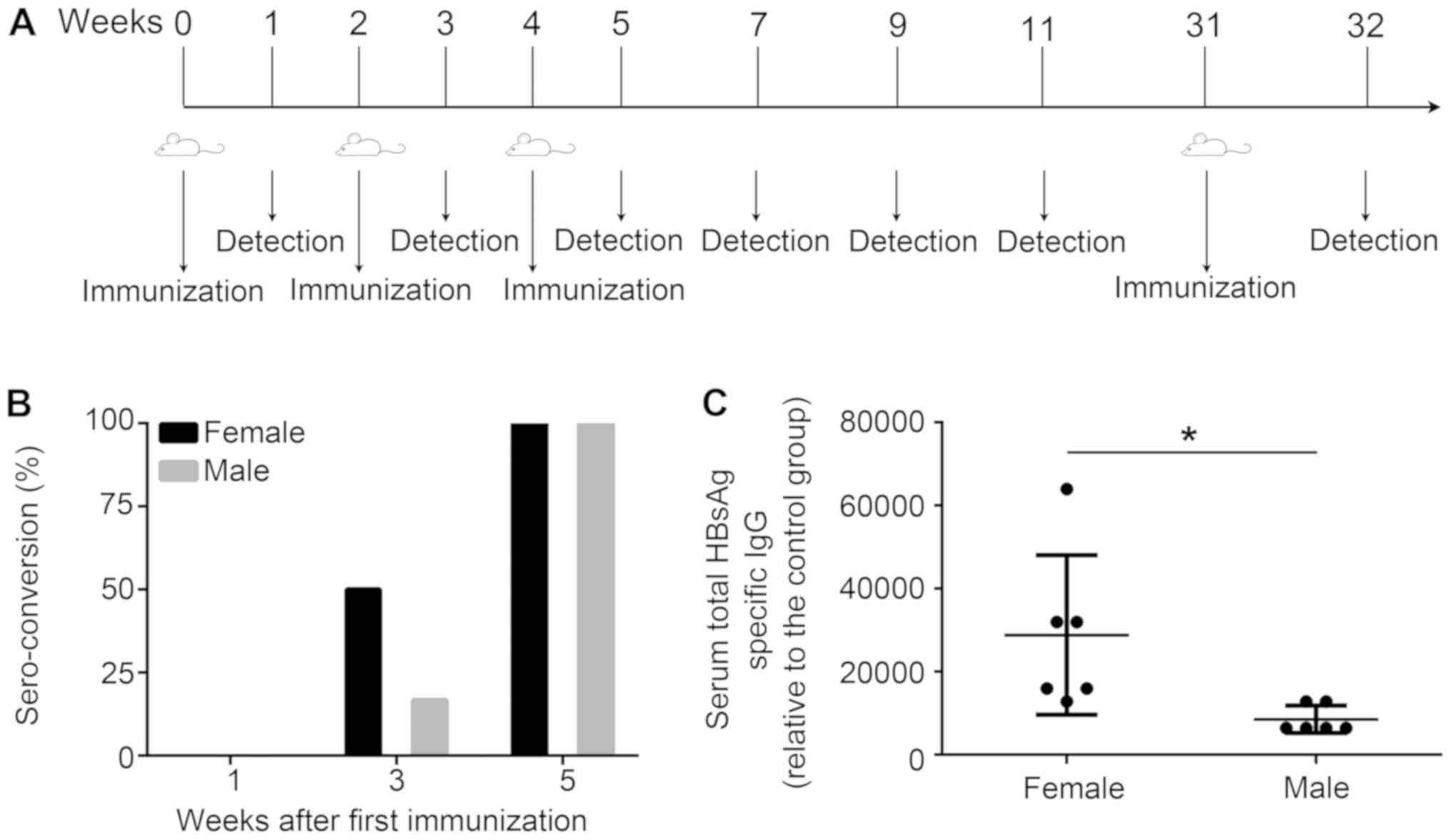 | Figure 1.Induction of HBV immunity in mice.
(A) Schematic of the HBV immunization protocol. Mice (n=6) were
immunized with HBsAg (1 µg) and alum (25 µg) at weeks 0, 2 and 4,
or treated with PBS as control. In addition, mice were immunized
with the same dose of vaccine at week 31. At 1 week following the
third immunization, mice (n=3) were sacrificed, and an
enzyme-linked immunosorbent spot assay was performed. Sera were
collected on weeks 1, 3, 5, 7, 9, 11 and 32, and the anti-HBs
titers were measured by endpoint-dilution ELISA. The immunological
memory stimulated by the vaccine was detected using flow cytometric
analysis and ELISA at one week following the booster injection. For
the measurement of antibodies, serum from the control groups was
analyzed and used as the negative control. The positive cut-off for
seroconversion was set as 2.1×[(OD450(negative control
serum)-OD450(blank control)]. (B) Percentage of
seroconversion for treated mice at weeks 0, 2 and 4. (C) Total
anti-HBs IgG titers measured one week following the third
immunization. Data are presented as the mean ± standard deviation.
*P<0.05 vs. male group. HBV, hepatitis B virus; anti-HBs,
hepatitis B surface antibody; OD, optical density; HBsAg, surface
antigen of HBV; IgG, immunoglobulin G. |
Measurement of antibodies
HBsAg solution (100 µl) diluted in carbonate buffer
to a concentration of 1 µg/ml was applied to Nunc™ MaxiSorp™
96-well flat-bottomed ELISA plates (BioLegend, Inc.) overnight at
4°C. Following washing with 5% (v/v) Tween-20 in PBS (pH 7.2;
PBST), the plates were blocked with 5% non-fat dry milk and 0.05%
Tween-20 in PBS for 1 h at 37°C. The plates were subsequently
incubated with 2-fold diluted serum from animals for 1 h at 37°C.
The initial dilution of serum for total IgG was set at 1:2,000,
whereas that of the antibody subtype was 1:100. Plates were washed
5 times with PBST and subsequently detected with horseradish
peroxidase-conjugated goat anti-mouse IgG (1:3,000 dilution; cat.
no. ZB-2305; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.; OriGene Technologies, Inc.), IgG1 (cat. no. 1070-05), IgG2a
(cat. no. 1080-05) or IgG2b (cat. no. 1090-05; all 1:1,000
dilution; SouthernBiotech) for 1 h at 37°C. Plates were developed
using Thermo Scientific™ Pierce™ 3,3′,5,5′-tetramethylbenzidine
substrate (cat. no. 34028; Pierce; Thermo Fisher Scientific, Inc.)
for the peroxidase color reaction, followed by detection of the
absorbance at 450 nm on an ELISA microplate reader.
Detection of seroconversion
Sera from weeks 1, 3 and 5 were diluted to 1:100
with 5% non-fat milk and 0.05% Tween-30 in PBS, and subsequently
analyzed for total anti-HBs IgG, following the aforementioned ELISA
protocol. The calculation formula for the cut-off value was as
follows: 2.1×[A450(negative
control)-A450(blank)]. When the value of
A450(negative control)-A450(blank) exceeded
the cutoff value, the animals were classified as being anti-HBs
seropositive. The seroconversion rate was determined as the
percentage of anti-HBs seropositive mice in total immunized
mice.
ELISpot assay
Splenocytes were isolated from immunized mice at 1
week following the final immunization as previously described
(22), and ELISpot assays were
performed using the mouse IFN-γ/IL-4 Dual-Color ELISpot kit (cat.
no. ELD5217; R&D Systems, Inc.). Briefly, splenocytes
(5×105 cells/well) were seeded in microplates that were
pre-coated with mouse IFN-γ-specific monoclonal antibody and
IL-4-specific polyclonal antibody. Subsequently, cells were
co-incubated with 5 µg/ml HBsAg or 1 µg/ml Concanavalin A (cat. no.
11028-71-0, Sigma-Aldrich; Merck KGaA) as a positive control at
37°C for 48 h. ELISA analysis was then conducted according to the
manufacturer's protocol. The spots were counted using an ELISpot
reader system (Dakewe Biotech Co., Ltd.).
Flow cytometric analysis
A total of 3 treated mice were randomly selected to
measure the percentage of memory T lymphocytes. Splenocytes were
isolated from the immunized mice at week 32 and adjusted to
2×106 cells/ml. Splenic lymphocyte-suspending liquid
(100 µl) was added into the flow tube and centrifuged at 1,000 × g
for 5 min at 4°C. The cells were blocked with 1 µl anti-mouse
cluster of differentiation (CD)16/CD32 mAb (cat. no. 553141) in PBS
for 5 min at 4°C. Then cells were stained with
peridinin-chlorophyll protein (perCP)-cyanine 5 (cy5)-anti-mouse
CD4 (cat. no. 550954), perCP-cy5-anti-mouse CD8 (cat. no. 561109),
phycoerythrin-anti-mouse CD44 (cat. no. 553134) and fluorescein
isothiocyanate-anti-mouse CD62L (cat. no. 561917; 1 µl of each
antibody) for 30 min at 4°C. All antibodies were purchased from BD
Pharmingen (BD Biosciences). Then, the cells were washed twice with
PBS. Stained cells were analyzed on a BD FACSCalibur™ flow
cytometer (BD Biosciences). Flow cytometric analysis was performed
using NovoExpress 1.2.1 software (ACEA Biosciences, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc.). All values were presented as
the mean ± standard deviation. The independent-samples t-test was
used for comparisons between treated groups. One-way ANOVA followed
by Tukey's post hoc test was used for comparisons between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Seroconversion following HBV
vaccination
To investigate the seroconversion rate of anti-HBs,
the prepared HBV vaccines were separately injected intramuscularly
into the hind legs of female or male mice at 0, 2 and 4 weeks, and
subsequently the anti-HBs titers were detected at 1, 3 and 5 weeks
(Fig. 1A). No changes in the
immune response were elicited by the HBV vaccine in any of the mice
at 1 week following the first immunization; however, 3 of the 6
female mice generated anti-HBs 1 week following the second
immunization (a seroconversion rate of 50%). Conversely, the
seroconversion rate of the treated male mice group at this time
point was 16.7% (Fig. 1B). A 100%
seroconversion rate was observed for the two treated groups
following the third immunization; however, treated female mice
produced a significantly increased total anti-HBs titer compared
with the treated male mice (Fig.
1C).
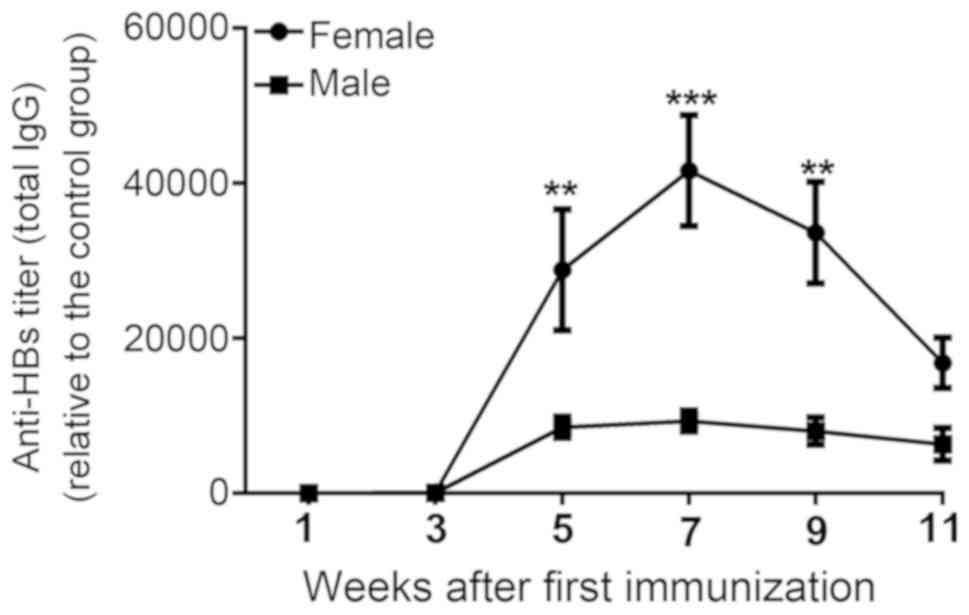
Detection of increased levels of
anti-HBs titer in treated female mice
It was previously reported that antibody responses
to HBV vaccines were typically elevated in females compared with in
males, particularly in young and elderly adult populations
(12). To determine the effects of
sex-specific differences on the efficacy of HBV vaccines, total
anti-HBs titers were evaluated at 1, 3, 5, 7, 9 and 11 weeks
following the first injection (Fig.
1A). The specific anti-HBs response was elicited in all
immunized mice from week 3 compared with the control group
(Fig. 2). In the treated female
group, anti-HBs levels peaked at week 7 with a subsequent gradual
reduction by weeks 9 and 11. Immune responses to the HBV vaccine
were markedly increased in the treated female group compared with
the treated males during the entire course of the experiment, and
were significantly increased at weeks 5, 7 and 9 (Fig. 2).
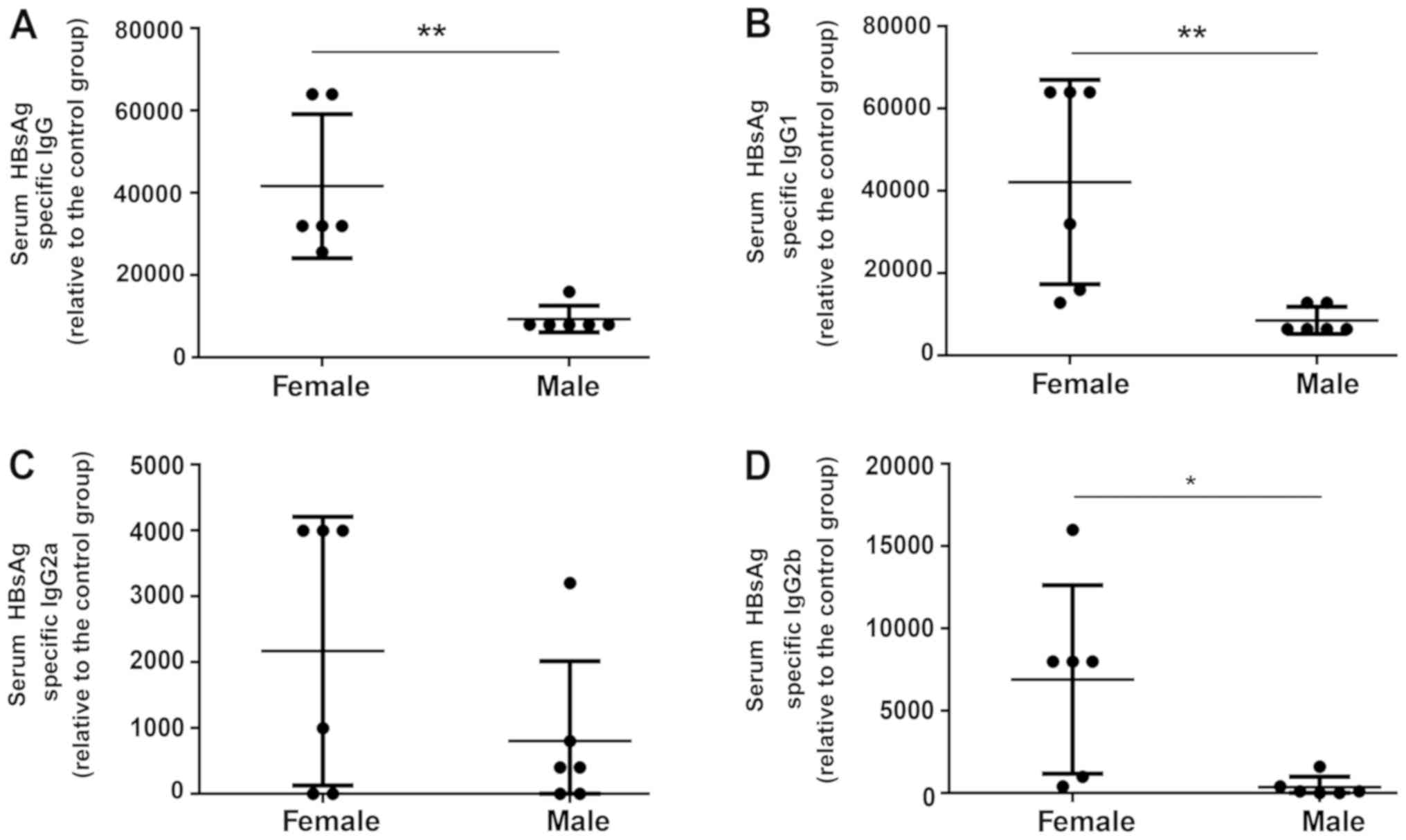
Detection of increased levels of
HBsAg-specific antibody subtypes in treated female mice
To further investigate the mechanisms of humoral
immunity elicited by HBV vaccines, the expression of various
subtypes of anti-HBs antibody were detected at week 7. Treated
female mice produced a significantly increased total anti-HBs titer
compared with the treated male mice at week 7 (Fig. 3A). Additionally, it was revealed
that the HBV vaccine led to a non-significant trend towards an
increased HBsAg-specific IgG2a titer in treated female mice
compared with treated male mice (Fig.
3C). Furthermore, serum IgG1 (7.5-fold) and IgG2b (32-fold)
levels were significantly increased in the treated female group
compared with the treated male group (Fig. 3B and D). Collectively, the results
indicated that compared with male mice, the HBV vaccine induced an
increased antibody response in female mice.
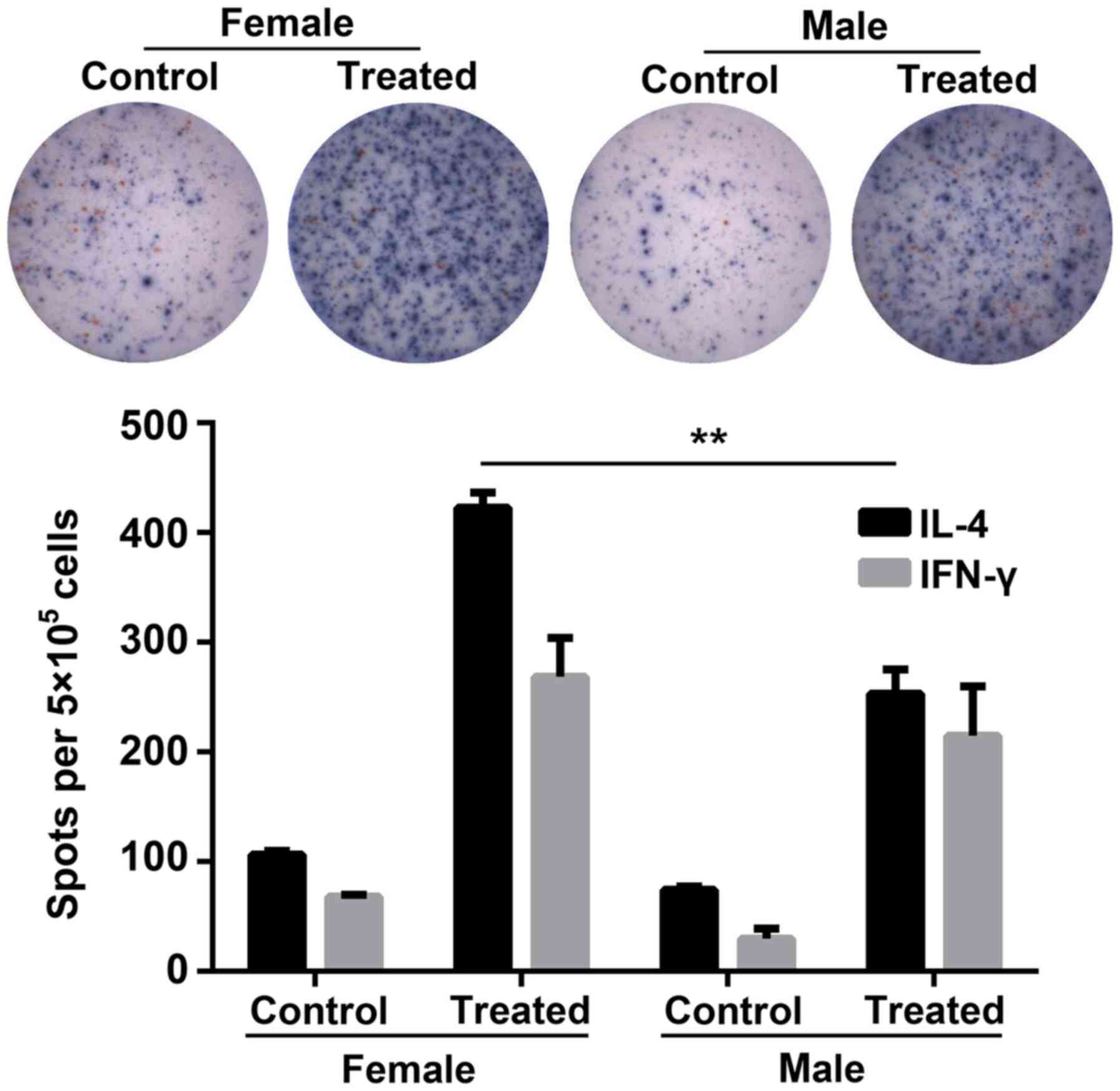
Cytotoxic T lymphocyte (CTL) responses
are increased in female mice compared with male mice
To further investigate the sex-based immunological
differences between the female and the male mice, the production of
IFN-γ and IL-4 by HBsAg-exposed splenocytes extracted from the
immunized mice was detected via ELISpot assays in vitro. The
number of IL-4 spot-forming cells (SFCs; blue; Fig. 4) was significantly increased in
female mice compared with the treated males. Furthermore, the
secretion of IFN-γ (red SFCs) was markedly increased in treated
female mice compared with treated males.
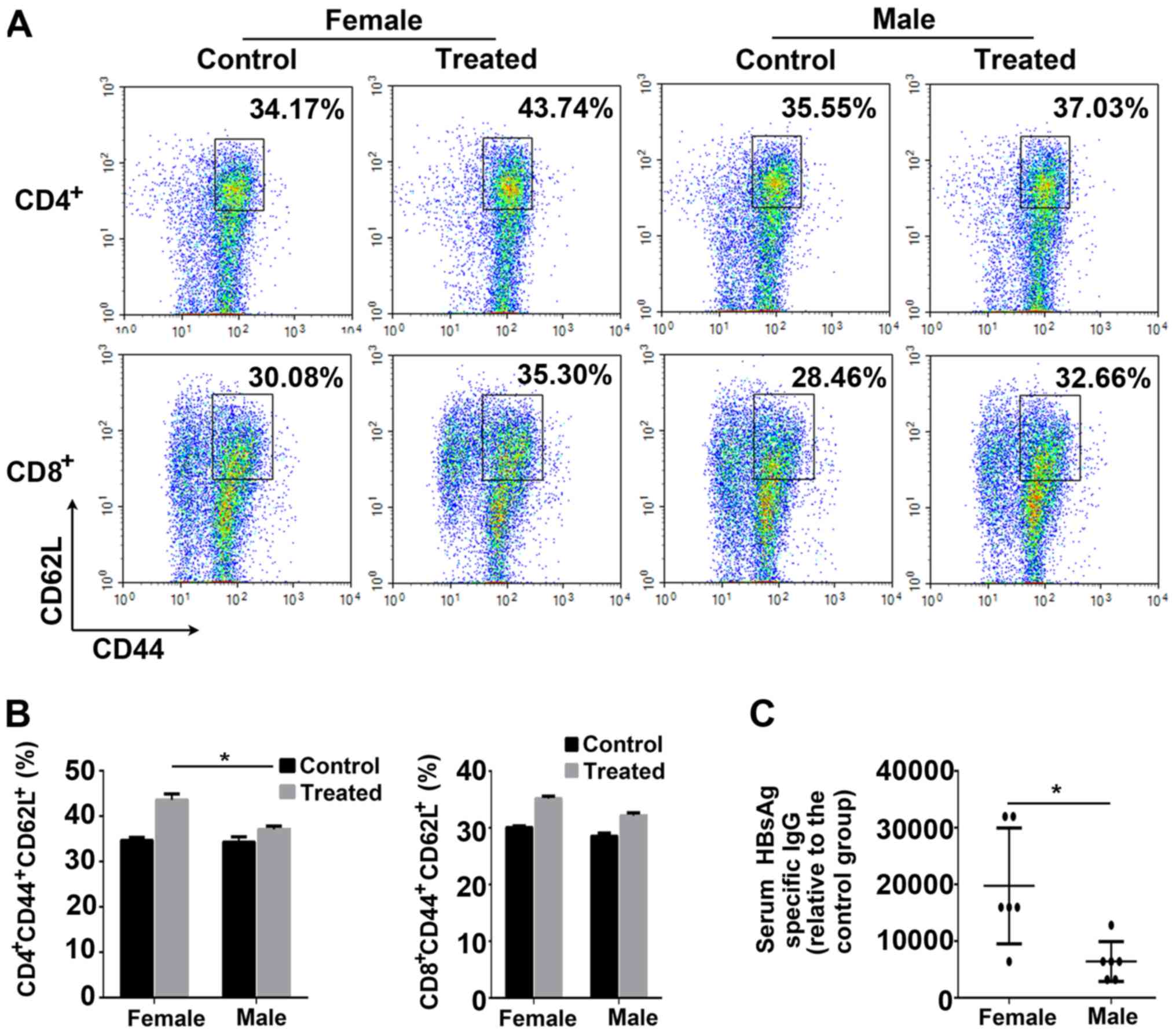
Female mice exhibit heightened immune
memory compared with male mice
Memory lymphocytes capable of rapid proliferation or
production of specific antibodies upon encountering HBV antigens
serve an important role in suppressing HBV re-infection (23,24).
To determine the extent of long-term protection against HBV,
treated mice were injected with an HBV vaccine booster at week 31,
and anti-HBs levels were detected one week later. A total of 3
treated mice were randomly selected to measure the percentage of
memory T lymphocytes. As presented in Fig. 5A and B, treated female mice
exhibited a significantly increased percentage of
CD4+CD44+CD62L+ memory T cells
compared with treated male mice. In addition, the anti-HBs titers
in the treated female mice were significantly increased compared
with the male mice (Fig. 5C).
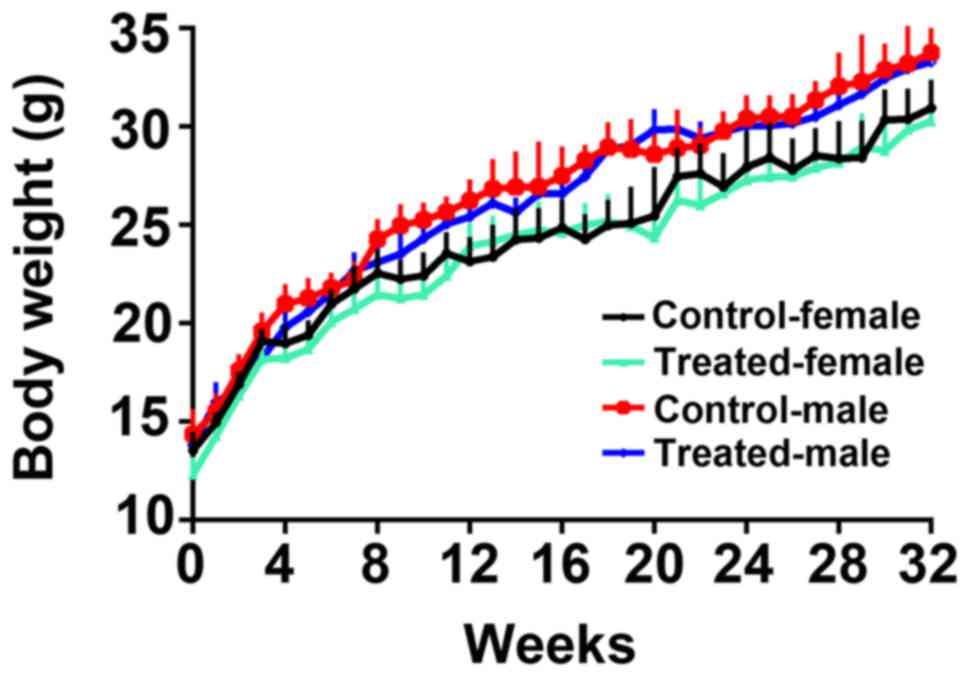
Body weight
Age-matched Balb/c mice were randomly assigned to
the aforementioned four treatment groups. Body weight was measured
every week. Female and male treated mice exhibited normal body
weights. There were no significant differences in body weight
between the control and treated animals for either gender (Fig. 6).
Discussion
Infection with HBV, an acute and chronic
communicable disease, has developed into a major global health
problem (7,8,25).
At present, vaccination is regarded as the most effective
prevention measure against HBV infection; however, ~5–10% of
healthy immunocompetent subjects are non-responsive (26). Previous studies demonstrated that
the male gender was associated with increased incidence of
non-response to HBV vaccination (19,25,27),
whereas others indicated that there was no sex-based difference in
the immune response to the HBV vaccine between males and females
(28,29). To clarify the biological influences
of sex on the immune response to the HBV vaccine, the present study
aimed to investigate the sexual dimorphism in the immune response
to HBV vaccination in mice.
In the present study, sex-specific differences in
antibody responses to the HBV vaccine were reported. Female mice
exhibited an increased seroconversion rate in the early
immunization stages, and sustained increased humoral immunity and
cellular immunity compared with male mice. In addition, female mice
developed a more effective immune memory that could rapidly induce
high levels of anti-HBs or activate cellular immunity to clear HBV
infection. Consistent with these results, sex-specific differences
in the humoral immune response were previously observed with other
vaccines, such as influenza, hepatitis B and meningococcal A
(27). It was reported that
females mount more frequent and stronger humoral and cell-mediated
immune responses to bacterial and viral vaccines compared with
males (30). According to certain
studies (31–35), in addition to gender, the
immunogenicity of the HBV vaccine was associated with age and body
mass index; old age and excessive weight reduced the immune
response to HBV vaccination. In the present study, mice of the same
age were randomly divided into groups and body weight was measured
every one week. There were no significant differences in body
weight between the groups during the study period.
The results of the present study revealed that ~50%
of the female mice were seropositive for anti-HBs antibody
following two injections, compared with 16.7% of the male mice.
Furthermore, the female mice developed more sustained,
longer-lasting and significantly increased levels of anti-HBs
following the second dose of HBV vaccine. The IgG2a:IgG1 antibody
level ratio is frequently used to evaluate the type of T helper
cell (Th) response, with a value >1 indicating a predominantly
Th1 response, and a value <1 indicating a predominantly Th2
response (36–38). To evaluate the type of immune
response, the levels of total IgG1, IgG2a, and IgG2b were
evaluated. The production of IgG1 and IgG2b antibodies in the
female group was significantly increased compared with the male
group, whereas no significant difference was observed between the
two groups regarding IgG2a levels; however, this may be a result of
the small sample size of the study (n=6), as relative IgG2a levels
were notably increased in the female group compared with the male
group. These findings suggested that the immune response evoked by
HBV vaccine in the two treated groups was Th2-type.
The mechanisms underlying sex differences associated
with antibody responses to vaccines remain unclear. It has been
reported that an observed relative increase in CD4+ T
cell numbers in females compared with males may partially explain
the more vigorous antibody response in females (39,40).
In addition, increased levels of T cell activation and
proliferation were previously detected in females compared with
males when cultured peripheral blood mononuclear cells were
stimulated in vitro (41,42).
HBV-specific CTLs and CD4+ T lymphocytes serve important
roles in determining the outcome of HBV infection (43). Viral replication usually activates
the CD8+ CTL response, which contributes towards the
elimination of infected cells and reduced viral loads (44). Accompanying the development of the
CTL response, CD4+ T cells proliferate and produce
antiviral cytokines to regulate the immune response, preventing
persistent infection by sustaining the CD8+ CTL function
(45) or by providing signals for
the induction of CD8+ T-cell memory (46–49).
To characterize the functional differences between the immune
responses induced by HBV vaccination in mice of different sex, the
production of the antiviral cytokines, IL-4 and IFN-γ, by
HBsAg-exposed splenocytes from immunized mice was analyzed by an
ELISpot assay. IFN-γ, predominantly secreted by HBsAg-specific
CD8+ T cells, but also by HBsAg-specific Th1
CD4+ T cells, is required for the elimination of HBV
infection (50,51). IL-4 produced by CD4+ Th2
cells contributes to B-cell development and the promotion of
humoral responses; IL-4 was revealed to suppress the expression and
replication of HBV in a hepatocellular carcinoma cell line
(52). In the present study, the
number of IL-4-secreting T cells was significantly increased in
females compared with in males; however, the number of SFCs
secreting IFN-γ in females was not significantly different compared
with in males.
HBV vaccination induces the production of a high
titer of antibodies and an effective CTL response with the ability
to eliminate HBV in immunized subjects (53); however, HBV-specific T-cell
responses have a tendency to decline, even beyond the point of
detection, in the years following vaccination (54). Therefore, the induction of
immunological memory is required for achieving long-term protection
(54). To characterize whether
there were sexual differences in immune memory, in the present
study, memory T cells were detected via flow cytometric analysis.
The percentage of CD4+CD44+CD62L+
memory T cells was significantly increased in treated female mice
compared with treated male mice; however, the percentage of
CD8+CD44+CD62L+ memory T cells was
not significantly different between the two genders. Sex-dependent
differences in immunological memory have also been reported in
response to other vaccines (55).
The anti-HBs titers in the treated female mice were increased
compared with those in the treated male mice at one week following
the booster injection. A limitation of the present study is that
blood samples were not collected during week 31, meaning that
alterations in antibody titers prior to and following the booster
could not be determined.
In conclusion, the present study may improve
understanding of sexual dimorphism in the immune responses to HBV
vaccine. Increasing evidence has indicated that biological sex
influences the immune responses to vaccination and infection;
however, the biological mechanisms underpinning such differences
are yet to be determined (30,56,57).
Among the most well-established mechanisms for differences in
immunity is the sex-hormone milieu. Therefore, further studies into
the regulatory effects of sex hormones on the immune system are
required.
Acknowledgements
We thank Professor Li Yang (State Key Laboratory of
Biotherapy and Cancer Center, Sichuan University) for her
assistance with experiments.
Funding
This work was supported by the Medical Research
Program of Sichuan Medical Association (grant no. S16018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT and BL made significant contributions to the
design of the study, supervised the research and finalized the
manuscript. ML, YZ and XC performed the majority of the in
vitro studies, and all of the in vivo experiments. ML
drafted the manuscript. CL and XZ contributed to the analysis and
interpretation of data. XF, WL, HL, YD and LS contributed to the
design and coordination of experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Ethics
Review Committee for Animal Experimentation of Chengdu Blood
Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lavanchy D: Worldwide epidemiology of HBV
infection, disease burden, and vaccine prevention. J Clin Virol. 34
(Suppl 1):S1–S3. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Hara GA, McNaughton AL, Maponga T,
Jooste P, Ocama P, Chilengi R, Mokaya J, Liyayi MI, Wachira T,
Gikungi DM, et al: Hepatitis B virus infection as a neglected
tropical disease. PLoS Neglect Trop Dis. 11:e00058422017.
View Article : Google Scholar
|
|
3
|
Ott JJ, Stevens GA, Groeger J and Wiersma
ST: Global epidemiology of hepatitis B virus infection: New
estimates of age-specific HBsAg seroprevalence and endemicity.
Vaccine. 30:2212–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Invernizzi F, Viganò M, Grossi G and
Lampertico P: The prognosis and management of inactive HBV
carriers. Liver Int. 36 (Suppl 1):S100–S104. 2016. View Article : Google Scholar
|
|
5
|
Schweitzer A, Horn J, Mikolajczyk RT,
Krause G and Ott JJ: Estimations of worldwide prevalence of chronic
hepatitis B virus infection: A systematic review of data published
between 1965 and 2013. Lancet. 386:1546–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment, and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su FH, Cheng SH, Li CY, Chen JD, Hsiao CY,
Chien CC, Yang YC, Hung HH and Chu FY: Hepatitis B seroprevalence
and anamnestic response amongst Taiwanese young adults with full
vaccination in infancy, 20 years subsequent to national hepatitis B
vaccination. Vaccine. 25:8085–8090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melhem NM, Rahhal N, Charide R, Kreidieh K
and El-Khatib R: Human immunodeficiency virus and viral hepatitis
among high-risk groups: Understanding the knowledge gap in the
Middle East and North Africa Region. World J Hepatol. 7:2619–2630.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Namgyal P: Impact of hepatitis B
immunization, Europe and worldwide. J Hepatol. 39 (Suppl
1):S77–S82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Zhong Y and Guo L: Strategies to
prevent hepatitis B virus infection in China: Immunization,
screening, and standard medical practices. Biosci Trends. 7:7–12.
2013.PubMed/NCBI
|
|
11
|
Huang YT, Jen CL, Yang HI, Lee MH, Su J,
Lu SN, Iloeje UH and Chen CJ: Lifetime risk and sex difference of
hepatocellular carcinoma among patients with chronic hepatitis B
and C. J Clin Oncol. 29:3643–3650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawyer LA: Antibodies for the prevention
and treatment of viral diseases. Antiviral Res. 47:57–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka J, Kumagai J, Katayama K, Komiya Y,
Mizui M, Yamanaka R, Suzuki K, Miyakawa Y and Yoshizawa H: Sex- and
age-specific carriers of hepatitis B and C viruses in Japan
estimated by the prevalence in the 3,485,648 first-time blood
donors during 1995–2000. Intervirology. 47:32–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blumberg BS: The curiosities of hepatitis
B virus: Prevention, sex ratio, and demography. Proc Am Thorac Soc.
3:14–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James L, Fong CW, Foong BH, Wee MK, Chow
A, Shum E and Chew SK: Hepatitis B seroprevalence study 1999.
Singapore Med J. 42:420–424. 2001.PubMed/NCBI
|
|
16
|
Zuckerman JN: Protective efficacy,
immunotherapeutic potential, and safety of hepatitis B vaccines. J
Med Virol. 78:169–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vermeiren AP, Hoebe CJ and Dukers-Muijrers
NH: High non-responsiveness of males and the elderly to standard
hepatitis B vaccination among a large cohort of healthy employees.
J Clin Virol. 58:262–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yen YH, Chen CH, Wang JH, Lee CM,
Changchien CS and Lu SN: Study of hepatitis B (HB) vaccine
non-responsiveness among health care workers from an endemic area
(Taiwan). Liver Int. 25:1162–1168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang S, Tian G, Cui Y, Ding C, Deng M, Yu
C, Xu K, Ren J, Yao J, Li Y, et al: Factors influencing immunologic
response to hepatitis B vaccine in adults. Sci Rep. 6:272512016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klein SL and Flanagan KL: Sex differences
in immune responses. Nat Rev Immunol. 16:626–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Camoglio L, Te Velde AA, de Boer A, ten
Kate FJ, Kopf M and van Deventer SJ: Hapten-induced colitis
associated with maintained Th1 and inflammatory responses in
IFN-gamma receptor-deficient mice. Eur J Immunol. 30:1486–1495.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banatvala J, Van Damme P and Oehen S:
Lifelong protection against hepatitis B: The role of vaccine
immunogenicity in immune memory. Vaccine. 19:877–885. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu CL, Liu P, Chen T, Ni Z, Lu LL, Huang
F, Lu J, Sun Z and Qu C: Presence of immune memory and immunity to
hepatitis B virus in adults after neonatal hepatitis B vaccination.
Vaccine. 29:7835–7841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karatekin G, Kilinç M, Gulcan Öksuz B and
Iğde M: Hepatitis B seroprevalence in children and women and the
impact of the hepatitis B vaccination program in the Black Sea
Region of Turkey. J Infect Dev Ctries. 7:960–965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mast EE, Margolis HS, Fiore AE, Brink EW,
Goldstein ST, Wang SA, Moyer LA, Bell BP and Alter MJ; Advisory
Committee on Immunization Practices (ACIP), : A comprehensive
immunization strategy to eliminate transmission of hepatitis B
virus infection in the United States: Recommendations of the
Advisory Committee on Immunization Practices (ACIP) part 1:
Immunization of infants, children, and adolescents. MMWR Recomm
Rep. 54:1–31. 2005.PubMed/NCBI
|
|
27
|
Cook IF: Sexual dimorphism of humoral
immunity with human vaccines. Vaccine. 26:3551–3555. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rezaei M, Nooripoor S, Ghorbani R,
Ramezanshams F, Mamishi S and Mahmoudi S: Seroprotection after
hepatitis B vaccination in children aged 1 to 15 years in central
province of Iran, Semnan. J Prev Med Hyg. 55:1–3. 2014.PubMed/NCBI
|
|
29
|
Al-Shamahy HA, Hanash SH, Rabbad IA,
Al-Madhaji NM and Naser SM: Hepatitis B vaccine coverage and the
immune response in Children under ten years old in Sana'a, Yemen.
Sultan Qaboos Univ Med J. 11:77–82. 2011.PubMed/NCBI
|
|
30
|
Klein SL, Jedlicka A and Pekosz A: The Xs
and Y of immune responses to viral vaccines. Lancet Infect Dis.
10:338–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tohme RA, Awosika-Olumo D, Nielsen C,
Khuwaja S, Scott J, Xing J, Drobeniuc J, Hu DJ, Turner C, Wafeeg T,
et al: Evaluation of hepatitis B vaccine immunogenicity among older
adults during an outbreak response in assisted living facilities.
Vaccine. 29:9316–9320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bender TJ, Sharapov U, Utah O, Xing J, Hu
D, Rybczynska J, Drobeniuc J, Kamili S, Spradling PR and Moorman
AC: Hepatitis B vaccine immunogenicity among adults vaccinated
during an outbreak response in an assisted living
facility-Virginia, 2010. Vaccine. 32:852–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeeshan M, Jabeen K, Ali AN, Ali AW,
Farooqui SZ, Mehraj V and Zafar A: Evaluation of immune response to
Hepatitis B vaccine in health care workers at a tertiary care
hospital in Pakistan: An observational prospective study. BMC
Infect Dis. 7:1202007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ingardia CJ, Kelley L, Steinfeld JD and
Wax JR: Hepatitis B vaccination in pregnancy: Factors influencing
efficacy. Obstet Gynecol. 93:983–986. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sangfelt P, Uhnoo I, Reichard O and
Weiland O: A low-dose intradermal hepatitis B vaccine programme in
health-care workers and students is highly effective and cost
saving: A retrospective follow-up survey in the clinical setting.
Scand J Gastroenterol. 43:465–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Collins JT and Dunnick WA: Germline
transcripts of the murine immunoglobulin gamma 2a gene: Structure
and induction by IFN-gamma. Int Immunol. 5:885–891. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cher DJ and Mosmann TR: Two types of
murine helper T cell clone. II. Delayed-type hypersensitivity is
mediated by TH1 clones. J Immunol. 138:3688–3694. 1987.PubMed/NCBI
|
|
38
|
Carcaboso AM, Hernandez RM, Igartua M,
Rosas JE, Patarroyo ME and Pedraz JL: Potent, long lasting systemic
antibody levels and mixed Th1/Th2 immune response after nasal
immunization with malaria antigen loaded PLGA microparticles.
Vaccine. 22:1423–1432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bede P, Elamin M, Byrne S and Hardiman O:
Sexual dimorphism in ALS: Exploring gender-specific neuroimaging
signatures. Amyotroph Lateral Scler Frontotemporal Degener.
15:235–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lisse IM, Aaby P, Whittle H, Jensen H,
Engelmann M and Christensen LB: T-lymphocyte subsets in West
African children: Impact of age, sex, and season. J Pediatr.
130:77–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sankaran-Walters S, Macal M, Grishina I,
Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller
MK, et al: Sex differences matter in the gut: Effect on mucosal
immune activation and inflammation. Biol Sex Differ. 4:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdullah M, Chai PS, Chong MY, Tohit ER,
Ramasamy R, Pei CP and Vidyadaran S: Gender effect on in vitro
lymphocyte subset levels of healthy individuals. Cell Immunol.
272:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kägi D, Ledermann B, Bürki K, Zinkernagel
RM and Hengartner H: Molecular mechanisms of lymphocyte-mediated
cytotoxicity and their role in immunological protection and
pathogenesis in vivo. Annu Rev Immunol. 14:207–232. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brooks DG, Teyton L, Oldstone MB and
McGavern DB: Intrinsic functional dysregulation of CD4 T cells
occurs rapidly following persistent viral infection. J Virol.
79:10514–10527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Janssen EM, Lemmens EE, Wolfe T, Christen
U, von Herrath MG and Schoenberger SP: CD4+ T cells are
required for secondary expansion and memory in CD8+ T
lymphocytes. Nature. 421:852–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shedlock DJ and Shen H: Requirement for
CD4 T cell help in generating functional CD8 T cell memory.
Science. 300:337–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee BW, Yap HK, Chew FT, Quah TC,
Prabhakaran K, Chan GS, Wong SC and Seah CC: Age- and sex-related
changes in lymphocyte subpopulations of healthy Asian subjects:
From birth to adulthood. Cytometry. 26:8–15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Uppal SS, Verma S and Dhot PS: Normal
values of CD4 and CD8 lymphocyte subsets in healthy indian adults
and the effects of sex, age, ethnicity, and smoking. Cytometry B
Clin Cytom. 52:32–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Webster GJ, Reignat S, Maini MK, Whalley
SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, et
al: Incubation phase of acute hepatitis B in man: Dynamic of
cellular immune mechanisms. Hepatology. 32:1117–1124. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mosmann TR and Coffman RL: Heterogeneity
of cytokine secretion patterns and functions of helper T cells. Adv
Immunol. 46:111–147. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin SJ, Shu PY, Chang C, Ng AK and Hu CP:
IL-4 suppresses the expression and the replication of hepatitis B
virus in the hepatocellular carcinoma cell line Hep3B. J Immunol.
171:4708–4716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang MH, You SL, Chen CJ, Liu CJ, Lai MW,
Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, et al: Long-term effects of
hepatitis B immunization of infants in preventing liver cancer.
Gastroenterology. 151:472–480.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shepard CW, Simard EP, Finelli L, Fiore AE
and Bell BP: Hepatitis B virus infection: Epidemiology and
vaccination. Epidemiol Rev. 28:112–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Voysey M, Barker CI, Snape MD, Kelly DF,
Trück J and Pollard AJ: Sex-dependent immune responses to infant
vaccination: An individual participant data meta-analysis of
antibody and memory B cells. Vaccine. 34:1657–1664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Furman D, Hejblum BP, Simon N, Jojic V,
Dekker CL, Thiébaut R, Tibshirani RJ and Davis MM: Systems analysis
of sex differences reveals an immunosuppressive role for
testosterone in the response to influenza vaccination. Proc Natl
Acad Sci USA. 111:869–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jørgensen TN: Sex disparities in the
immune response. Cell Immunol. 294:61–62. 2015. View Article : Google Scholar : PubMed/NCBI
|