Introduction
Gliomas are the most common malignant tumors of the
central nervous system, accounting for approximately 40% of all
brain tumors (1). Despite recent
advances including adjuvant chemotherapy, radiotherapy and
extensive tumor resection, the prognosis of glioma patients and the
5-year survival rate remain unfavorable (2). Thus, there is an urgent need to
identify the mechanisms involved in glioma progression and
metastasis to elucidate novel diagnostic and therapeutic targets
for this malignancy.
MicroRNAs (miRNAs), single-stranded RNAs with a
length of approximately 19–24 nucleotides, play significant roles
in a series of biological cellular processes such as cell
proliferation, migration, invasion and tumorigenesis (3). miR-342 serves a critical role in
numerous physiological and pathological processes. It has been
reported to be involved in the pathogenesis of many types of
cancers, such as gastric cancer (4), hepatocellular carcinoma (5) and non-small cell lung cancer
(6). Although these studies have
demonstrated the important role of miR-342 in cancer progression,
the modes of action of miR-342 in glioma have not been fully
characterized.
G-protein-coupled receptors are the largest protein
superfamily with more than 700 genes in the human genome (7). They play an important role in a
variety of biological processes (8). It has been demonstrated that GPRC5A,
one member of the GPCR family, is upregulated in many cancer
tissues and cell lines (9–11). Yet, the relationship between GPRC5A
and miR-342 remains unclear. We hypothesized that miR-342 directly
targets GPRC5A, and the present study was designed to explore this
hypothesis.
In the present study, the expression level of
miR-342 was measured to assist the investigation of its regulatory
roles in glioma. miR-342 was found to be markedly downregulated in
glioma tissues and cell lines, and to exert tumor-suppressing
functions in glioma. Moreover, miR-342 was found to regulate cell
proliferation by targeting GPRC5A.
Materials and methods
Clinical samples and cell culture
Human glioma cell lines U-87MG (ATCC HTB-14 (RRID:
CVCL_0022, glioblastoma of unknown origin), U251 (The Cell Bank of
Type Culture Collection of Chinese Academy of Sciences; cat. no.
TCHu 58), T98G [American Type Culture Collection (ATCC); cat. no.
CRL-1690] and SNB19 (ATCC; cat. no. CRL-2219) and normal human
astrocytes (NHAs; ScienCell Research Laboratories, Inc.; cat. no.
1820) were cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco™, 10564011) supplemented with 10% fetal bovine serum (FBS,
Gibco™, 10099141) (both from Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 IU/ml penicillin, and 100 µg/ml streptomycin
at 37°C under a 5% CO2 atmosphere. Glioma specimens were
collected from 39 patients following prior approval and written
informed consent. Ten normal brain tissue samples used as controls
were collected by donations from individuals who died in traffic
accidents. All clinical samples were collected and histologically
examined by pathologists from July 2016 to May 2018 at The Second
Affiliated Hospital of Harbin Medical University. The present study
was approved by The Ethics Committee of The Second Affiliated
Hospital of Harbin Medical University. Written informed consent was
obtained from all enrolled subjects.
Cell transfection
The miR-342 mimics, inhibitor and their
corresponding miRNA negative control (miR-NC) were chemically
synthesized by GenePharma (Shanghai, China) and transfected using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Overexpression or knockdown plasmids were transfected using
Lipofectamine 2000 following the manufacturer's instructions.
GPRC5A overexpression and
shRNA-mediated knockdown plasmids
The longest transcript human genes of GPRC5A
(NCBI Reference Sequence: NM_003979.3) were cloned into pcDNA 3.1
plasmids and then sequenced for validation. The siRNA duplexes
targeting GPRC5A were obtained from Invitrogen; Thermo Fisher
Scientific, Inc. In order to knock down GPRC5A expression in U87
cells, subconfluently cultured U87 cells were transfected with
GPRC5A shRNA, or negative control shRNA using the RNAiMAX
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. GPRC5A expression was
assessed following 3 days. The shRNAs were designed by Invitrogen
(Invitrogen; Thermo Fisher Scientific, Inc.) and cloned using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Stable population
transfection was obtained following selection with 1 µg/ml G418
(Amresco, LLC, Solon, OH, USA) for 2 weeks. All shRNA oligos were
obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Quantitative real-time PCR (qPCR)
Trizol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for total RNA extraction. Six
microliters of the extracted RNA was reverse transcribed using the
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan) according to the provider's protocol. Quantitative PCR
was performed using SYBR® Green Real-Time PCR Master Mix
(Takara) in the StepOnePlus Real-Time System (Applied Biosystems™
ABI Prism 7500 Fast; Thermo Fisher Scientific, Inc.). The sequences
of primers used were: GPRC5A forward 5′-CGCCACAAAGCAACGAA-3′ and
reverse primer 5′-ATAGAGCGTGTCCCCTGTCT-3′; GAPDH forward
5′-GAAAGCCTGCCGGTGACTAA-3′ and reverse primer
5′-GCATCACCCGGAGGAGAAAT-3′; U6 small nuclear RNA forward
5′-CTCGCTTCGGCGCACA-3′ and reverse primer:
5′-AACGCTTCACGAATTTGCGT-3′; miR-342 forward
5′-AGGTGAGGGGTGCTATCTGT-3′ and reverse primer
5′-GGGTGCGATTTCTGTGTGAG-3′. All the samples were amplified in
triplicate and each experiment was repeated three times. The
conditions for the real-time fluorescent quantitative PCR were: 1
cycle at 95°C for 5 min in the holding stage; 40 cycles at 95°C for
15 sec and 60°C for 60 sec in the cycling stage; 1 cycle at 95°C
for 15 sec, 60°C for 1 min and 95°C for 15 sec in the melt curve
stage. Thermal cycling and real-time detection were conducted using
the StepOnePlus Real-Time PCR Systems (ABI, Thermo Fisher
Scientific, Inc.). The quantities of each mRNA were calculated
using the comparative (2−ΔΔCq) method (12).
Western blot analysis
Protein was isolated from the cells and tissues with
RIPA lysis buffer containing 1% protease inhibitor cocktails
(Pierce Biotechnology, Inc.; Thermo Fisher Scientific, Inc.). After
sample buffer was added to the proteins (each well, 30 µg per
sample), proteins were boiled at 95°C for 10 min. Then, the
proteins were separated using 10% polyacrylamide gel
electrophoresis. After electrophoresis, proteins were transferred
to polyvinylidene fluoride (PVDF) membranes with 100 V
transfer-molded voltage lasting for 45 to 70 min. After
determination of the protein concentration, primary antibodies for
western blotting were applied which included anti-GPRC5A (dilution,
1:1,000; PAB14597; Abnova, Taipei, Taiwan) and anti-GAPDH
(dilution, 1:2,000; ab8245; Abcam, Cambridge, UK). HRP-conjugated
IgG (1:5,000) antibody was used as the secondary antibody. After
which membranes were washed 3 times (5 min/time). Development was
completed with chemiluminescence reagents. GAPDH was used as an
internal reference. Bands were visualized with a Bio-Rad Gel Doc EZ
imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
specific bands were visualized with a chemiluminescence system
(Millipore), and then visualized with Quantity One software 4.6.2
(Bio-Rad Laboratories, Inc.).
Cell Counting Kit-8 (CCK-8) assay
Cells in the logarithmic growth phase were digested
with trypsin and seeded on 96-well plates at 100 µl of medium
containing 1×104 cells per well. Then we measured the
cell proliferation rate at 0, 24, 48, and 72 h after transfection.
Ten microliters of CCK-8 reagent was added into each well following
another 2-h incubation at 37°C. The absorbance value was determined
by using the XT-96DJ ELISA analyzer at a wavelength of 490 nm.
Luciferase reporter assay
Wild-type and mutated GPRC5A 3′-UTR containing the
putative binding site of miR-342 were synthesized and sequenced.
Cells were seeded in 24-well plates and transfected with reporter
vectors together with miR-342 mimics, miR-342 inhibitor or the
corresponding miR-NC. The firefly luciferase activity was measured
and normalized to Renilla signals at 48 h
post-transfection.
Tumorigenicity assay
In total, 16 BALB/c male nude mice (specific
pathogen-free grade; weight, 16–18 g; age, 4–6 weeks) were
purchased from the Laboratory Animal Center of Harbin Medical
University. Lentiviral-mediated stable GAPC5A, GAPC5A+miR-342,
miR-342 mimic cells and miR-NC cells were resuspended in Hank's
buffer and mixed with an equal volume of Matrigel (BD Biosciences)
at a concentration of 5×106 cells/ml. The cells were
subcutaneously injected into the flanks of nude mice (n=4 in each
group). Subsequently, the mice were maintained in a specific
pathogen-free grade laboratory, under the following conditions:
Controlled temperature, 23±2°C; humidity, 40–70%; 12-h light/dark
cycle) at the Laboratory Animal Center in our hospital with ad
libitum access to food and water for 4 weeks. The volume of
xenograft tumors was monitored every 3 days by measuring the length
and width (Volume=length × width × width/2). The animal study was
conducted in accordance with the Institutional Animal Care and Use
Committee (IACUC) guidelines, and was approved by the Experimental
Animal Ethics Committee of The Second Affiliated Hospital of Harbin
Medical University.
Statistical analysis
All values are expressed as mean ± SEM and were
analyzed by one-way analysis of variance (ANOVA) followed by
Tukey's post hoc test among groups using Statistical Product and
Service Solutions (SPSS) (version 17.0) (SPSS, Inc., Chicago, IL,
USA). Pearson's correlation analysis was performed to study the
correlation between the expression of miR-342 and GPRC5A in cancer
tissues. A P-value <0.05 was considered to indicate a
statistically significant difference between groups.
Results
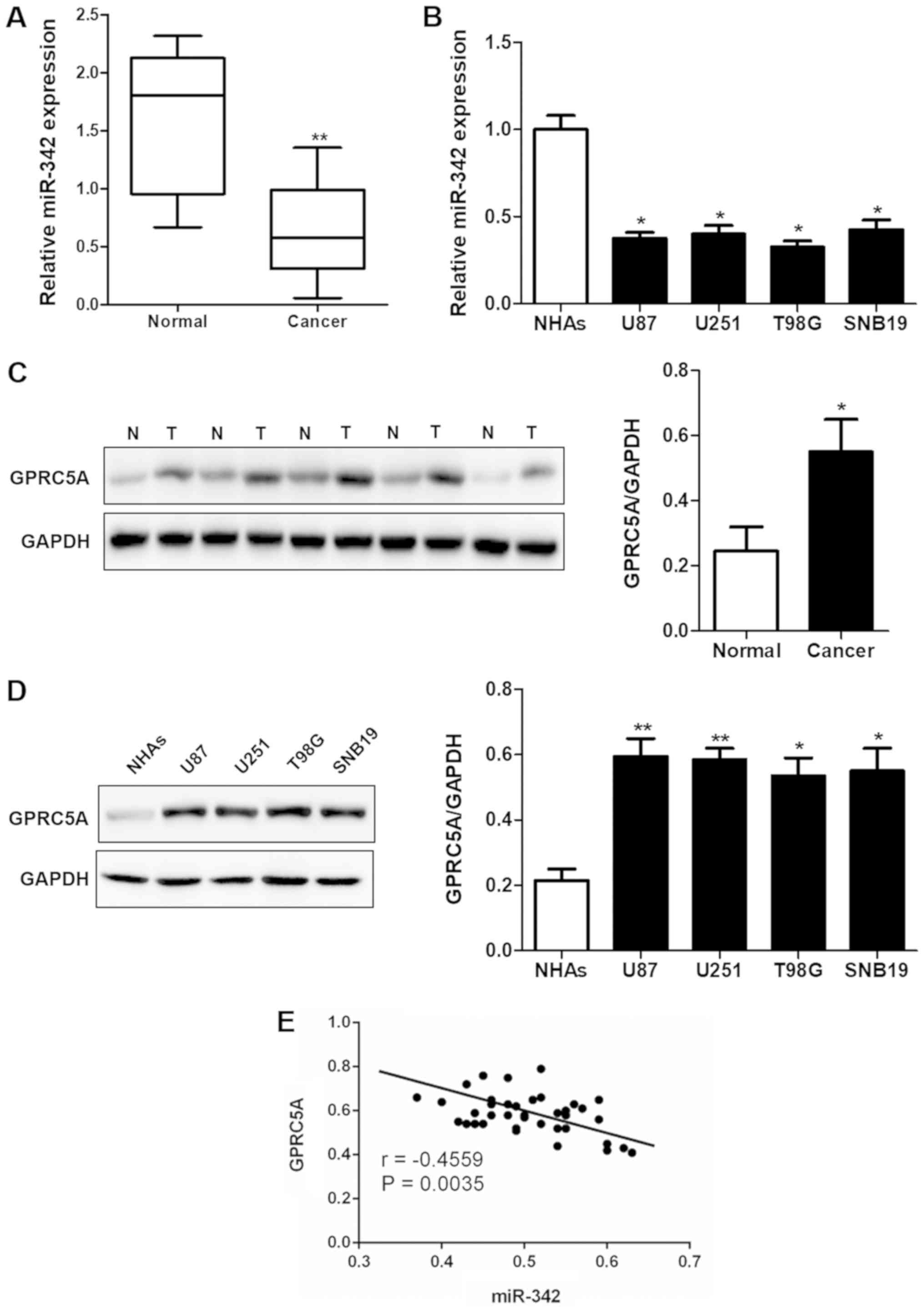
miR-342 is downregulated and GPRC5A is
upregulated in glioma tissues and cell lines
Downregulation of miR-342 was observed in the glioma
tissues (P<0.01; Fig. 1A) and
cell lines (P<0.05; Fig. 1B)
when compared with that noted in the normal human prostate tissues
and the normal human astrocytes (NHAs). Meanwhile, the western blot
results showed that the protein expression of GPRC5A was
significantly upregulated in human glioma tissues (Fig. 1C) and cell lines (Fig. 1D). According to the results of
RT-qPCR, GPRC5A expression in the U87 cell line was the highest,
therefore, we chose this cell line for further experiments. The
correlation analysis confirmed that the expression of miR-342 and
GPRC5A was significantly negatively correlated (r=−0.4559,
P=0.0035; Fig. 1E).
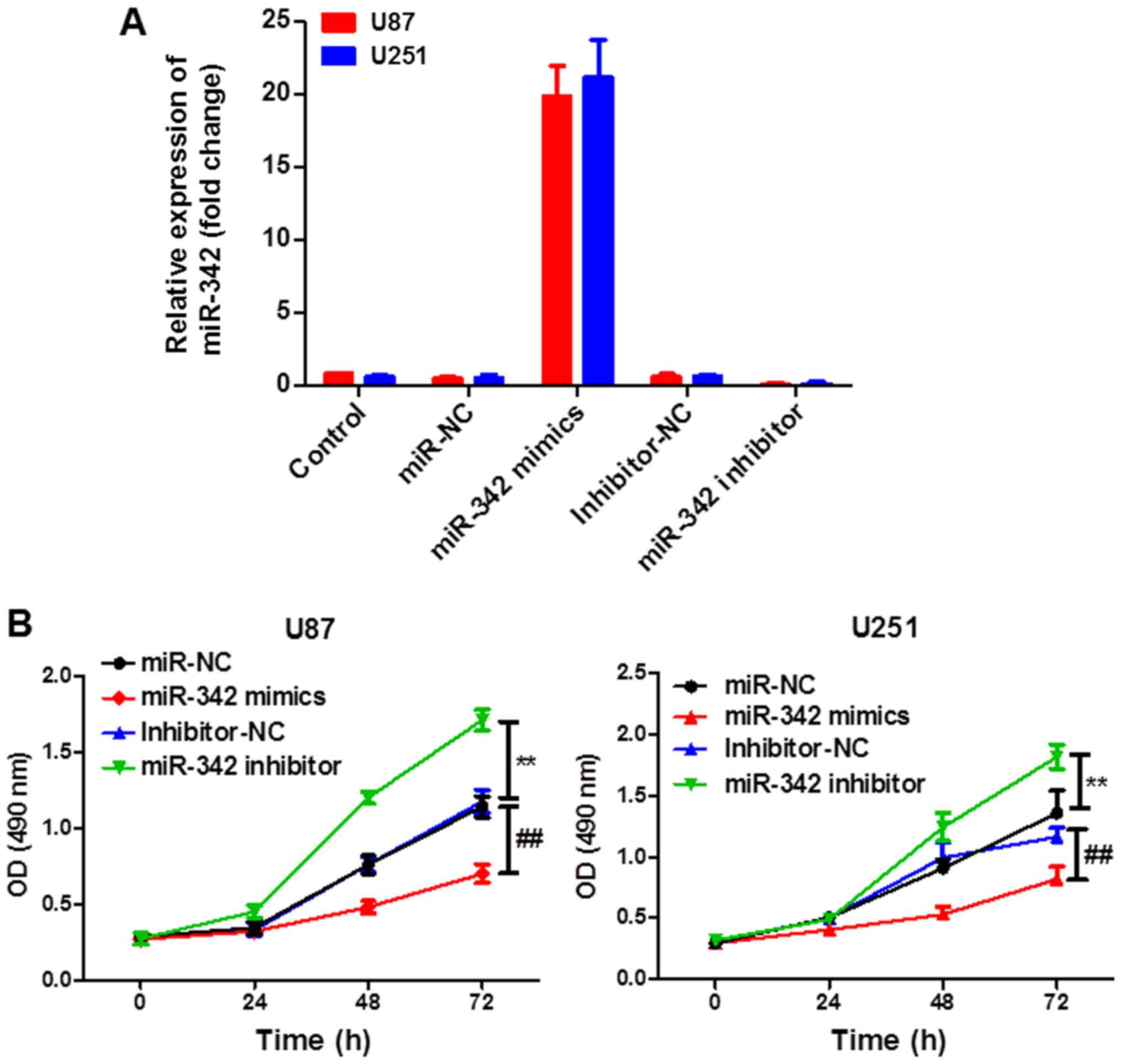
miR-342 inhibits the proliferation of
glioma cells
Next, we explored the potential role of miR-342 in
glioma. The transfection efficiency was determined according to the
level of miR-342 using RT-qPCR. As shown in Fig. 2A, a significantly increased
expression of miR-342 was achieved after miR-342 mimic transfection
and a significantly decreased expression of miR-342 was achieved
after miR-342 inhibitor transfection. Moreover, upregulation of
miR-342 resulted in greater suppression of cell proliferation than
the control (mimics NC), whereas downregulation of miR-342 promoted
cell proliferation (Fig. 2B) as
determined using the CCK-8 assay. These results indicated that
miR-342 inhibited the proliferation of U87 and U251 cells and
downregulation of miR-342 promoted the proliferation of cells.
GPRC5A is a direct target of miR-342
in glioma
In order to determine the mechanism of miR-342 in
cell proliferation, G-protein coupled receptor family C group 5
member A (GPRC5A) was found to be a putative target of miR-342
(Fig. 3A). At the protein and mRNA
levels, overexpression or knockdown of miR-342 resulted in a
significant decrease or increase in the expression level of GPRC5A,
respectively (Fig. 3B and C). In
addition, luciferase reporter assays were performed to ascertain
whether miR-342 targets GPRC5A by binding to its 3′UTR. The results
from the luciferase reporter assay indicated that upregulated
expression of miR-342 significantly inhibited the activity of the
reporter gene, whereas miR-342 inhibitor significantly increased it
(Fig. 3D). The results indicate
that GPRC5A is a direct target of miR-342.
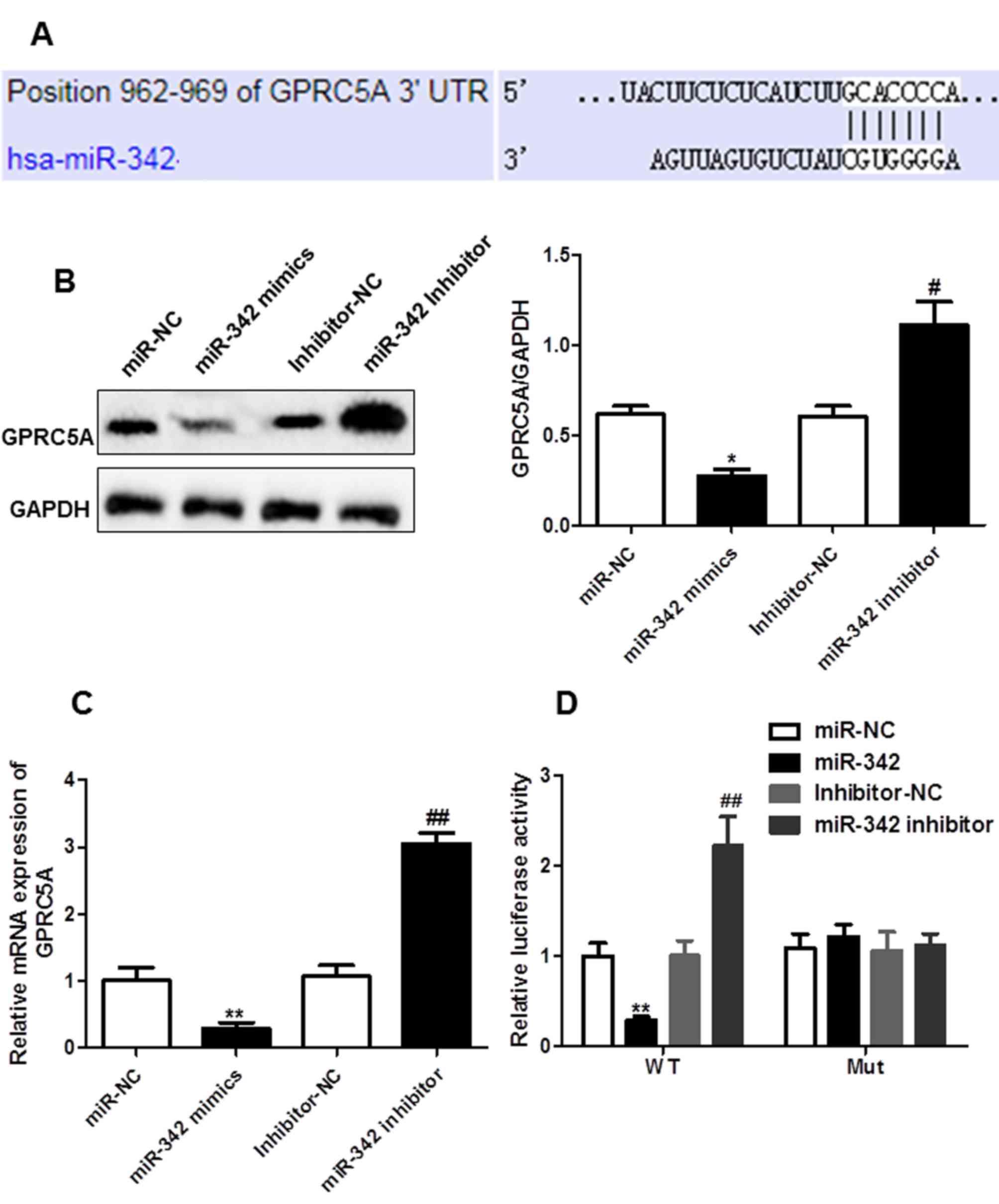 | Figure 3.miR-342 directly targets GPRC5A in U87
cells. (A) Sequence complementary pairings of miR-342 with GPRC5A
wild-type (WT) and mutant (Mut) 3′UTR are shown. (B) Protein levels
of GPRC5A in U87 cells transfection with miR-NC, miR-342 mimics,
inhibitor-NC or miR342-inhibitor were determined by western blot
analysis. Representative images of western blot were shown, bands
were quantitated by densitometry and normalized against GAPDH. (C)
mRNA levels of GPRC5A in U87 cells transfected with miR-NC, miR-342
mimics, inhibitor-NC or miR342-inhibitor were determined by reverse
transcription-quantitative PCR. (D) Luciferase activities were
determined in U87 cells 48 h after co-transfection with miR-NC,
miR-342 mimics, inhibitor-NC or miR342-inhibitor and juciferase
reporter vector containing WT or mutants of GPRC5A 3′UTR.
*P<0.05, **P<0.01 vs. miR-NC; #P<0.05,
##P<0.01 vs. Inhibitor-NC. GPRC5A, G-protein coupled
receptor family C group 5 member A. |
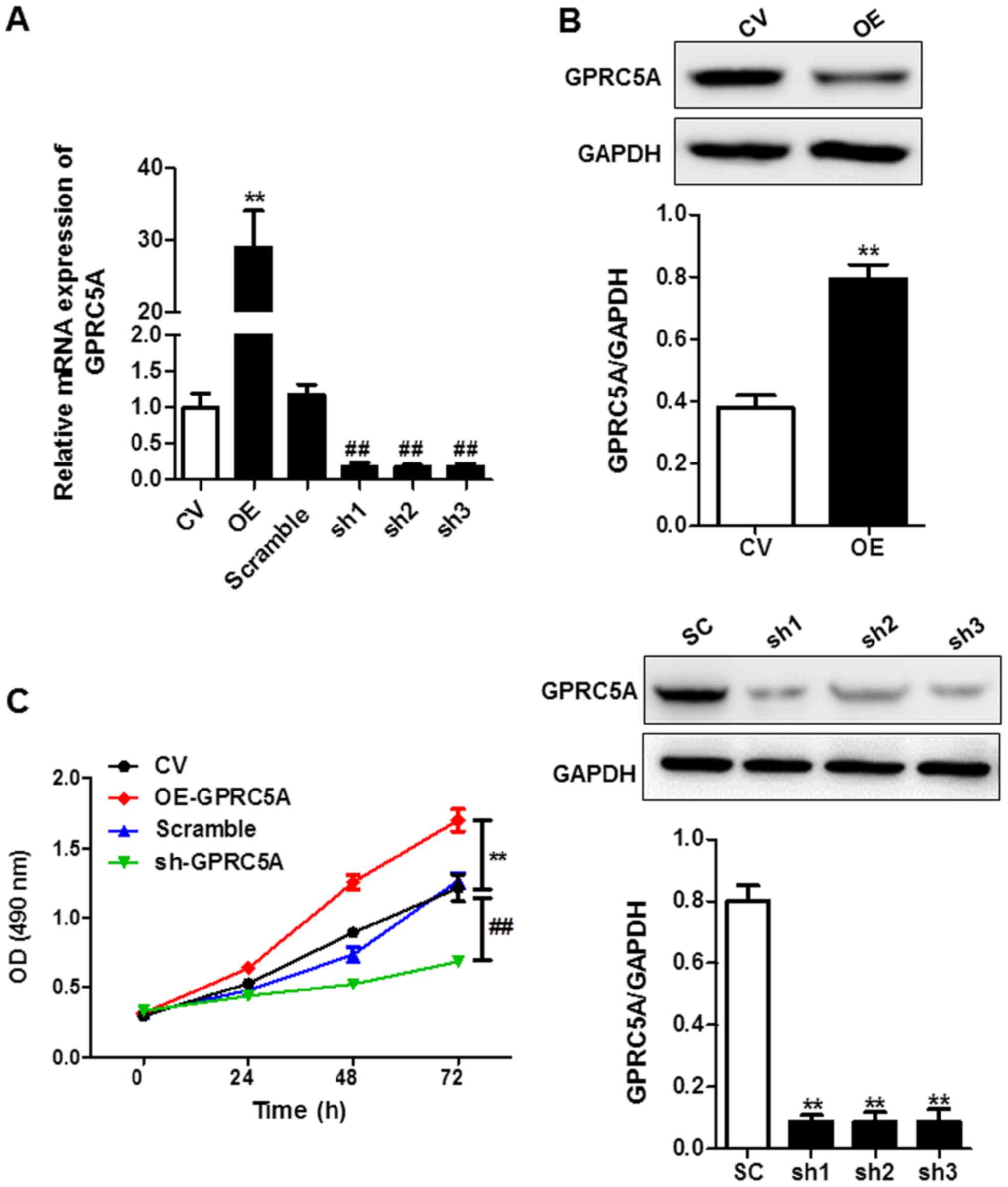
GPRC5A promotes cell proliferation of
U87
In order to investigate the cellular function of
GPRC5A, the expression level of GPRC5A in U87 cells was manipulated
by transfection with an overexpression (OE) or shRNA-mediated
knockdown plasmids. The mRNA and protein levels of GPRC5A were
determined in the transfected cells for which the expression levels
of GPRC5A were markedly increased in the presence of overexpression
plasmids or decreased in the absence of plasmids or silenced by
shRNA. The inhibitory effect of each shRNA (sh1, sh2, sh3) was not
significantly different while sh2 had the highest inhibition rate
of GPRC5A, thus this shRNA was selected for further experiments
(Fig. 4A and B). Moreover, CCK-8
assay results showed that overexpression of GPRC5A significantly
promoted cell growth, while knockdown of GPRC5A suppressed it
(Fig. 4C). The observation
indicated GPRC5A expression associated with cell proliferation
in vitro.
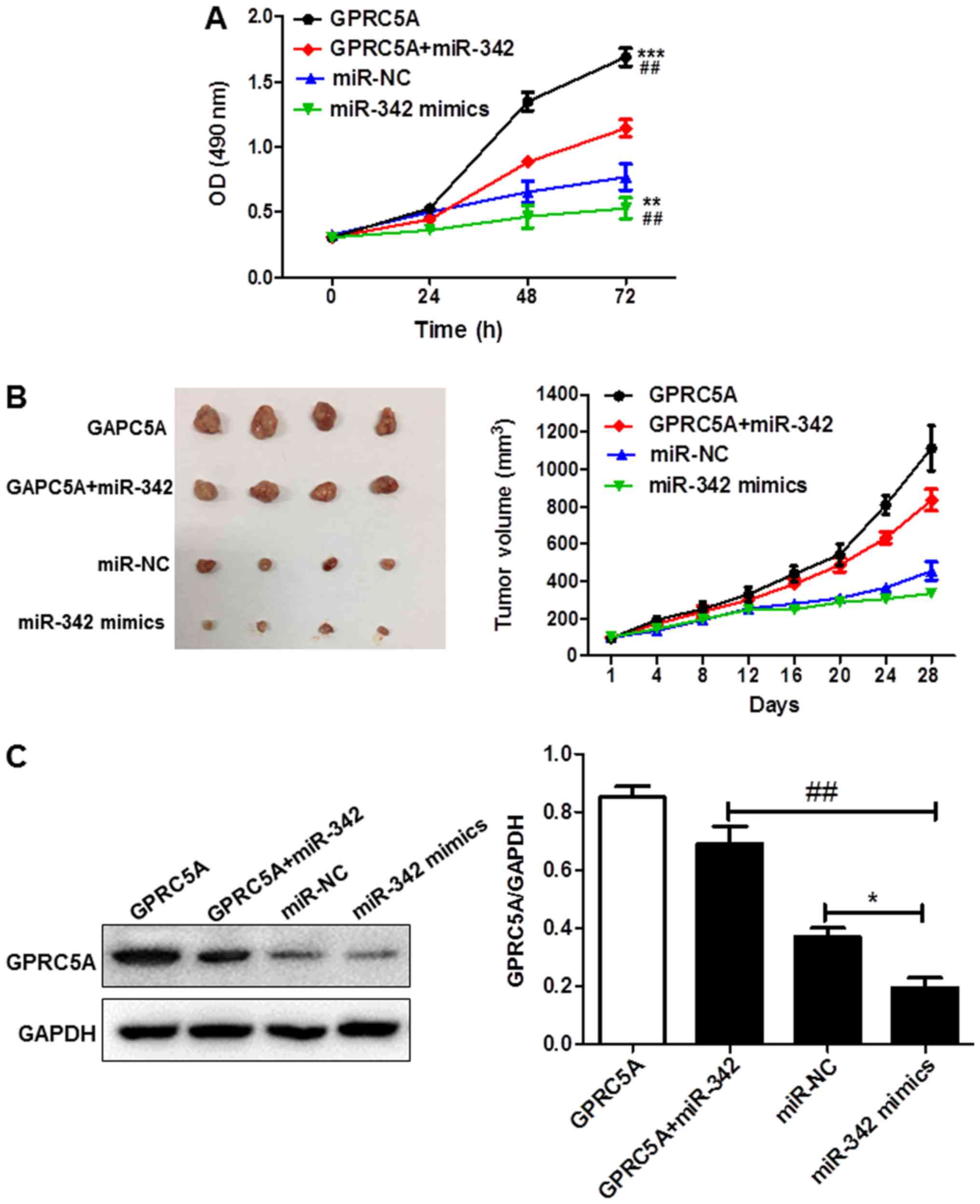
Restoration of GPRC5A reverses the
effects of miR-342 in vitro and in vivo
Based on the above results, it was proposed that
miR-342 suppresses cell proliferation via up-regulation of GPRC5A.
Considering the low expression level of miR-342, rescue experiments
were performed by co-transfecting the miR-342 mimics with or
without GPRC5A followed by determination of the cell proliferation
of U87 cells. Growth curves obtained by CCK-8 assay showed that
upregulation of the expression of miR-342 alone significantly
inhibited cell growth whereas overexpression of GPRC5A alone
significantly promoted cell growth; co-expression of GPRC5A with
miR-342 abrogated the inhibitory effects of miR-342 mimics on the
cell proliferation (Fig. 5A).
Based on the in vitro data, the effect of miR-342 and GPRC5A
on tumor growth was further evaluated in vivo. Lentivirus of
miR-342 and GPRC5A were either used to infect cells alone or
simultaneously. We found that tumor xenografts derived from cells
infected with miR-342 alone were significantly smaller than the
negative control while those infected with GPRC5A showed an
opposite trend; co-infection of GPRC5A with miR-342 abrogated the
inhibitory effects of miR-342 (Fig.
5B). The expression level of GPRC5A in tumor xenografts was
also assessed. The expression level of GPRC5A was much lower in the
miR-342 mimic group and higher in the GPRC5A overexpression or
co-infected groups, compared with their counterparts in the
negative control (NC) group (Fig.
5C). The in vitro and in vivo observations
suggest that miR-342 targets the 3′UTR of GPRC5A directly and
inhibits U87 cell proliferation via GPRC5A up-regulation.
Discussion
Gliomas are the most common primary brain tumors,
which show an extremely high proliferation and invasive capacity
(13,14). Therefore, effective diagnostic and
therapeutic strategies for glioma are urgently needed. Recently,
studies have confirmed that miRNAs play important roles in
tumorigenesis and development, and are involved in the regulation
of tumor growth, apoptosis, differentiation, invasion,
angiogenesis, and metastasis (15,16).
In addition, miRNAs can also act as an oncogenes or
tumor-suppressor genes, and their role in the development of
gliomas acts on the mechanisms of glioma (17). Downregulation of miR-342 has been
found in a number of cancer types, such as breast cancer (18), prostate cancer (19) and nasopharyngeal carcinoma
(20). Thus, it has been proposed
that miR-342 could be used as a diagnostic and prognostic
biomarker. In the present study, a significantly downregulated
miR-342 level was observed in glioma tissues and cell lines and the
increase in the expression level of miR-342 was found to suppress
the proliferation of glioma cells in vitro, suggesting that
miR-342 functions as an anti-oncogene.
G-protein-coupled receptors are the largest protein
superfamily with more than 700 genes in the human genome (7) playing an important role in a variety
of biological processes (8). This
protein superfamily also acts as drug targets in many different
diseases and more than 40% of FDA (Food and Drug
Administration)-approved drugs target GPCRs (G protein-coupled
receptors) or GPCR-associated pathways (21). GPCRs also play an integral role in
regulating and activating cancer-associated signaling pathways;
therefore, they may be used as biomarkers for the early diagnosis
of various types of cancer (22).
Further investigation of the pharmacological potential of GPCRs and
their downstream regulators is required in order to develop
therapies that can efficiently target cell signaling pathways in
cancer (23,24).
GPRC5A, also termed RAI3 (retinoic acid-induced
protein 3), located on chromosome 12p13-p12.3, has been found to
play significant roles in various biological processes, such as
cell proliferation and the cell cycle. However, the influences of
GPRC5A on different cancers vary. GPRC5A was reported to be
strongly expressed in the lung (25) and is regarded as a tumor suppressor
in lung cancer as well as in head and neck squamous cell carcinoma
(26). However, there are many
studies that have reported that high expression of GPRC5A is
correlated with a worse survival rate in colon, breast and gastric
cancer (27). Liu and colleagues
found high expression of GPRC5A in pancreatic cancer and it was
found to suppress the activity of phosphorylated GSK-3β at Ser9
(26). Moreover, Zhou and
Rigoutsos reported that miR-103a-3p also targets the 5′UTR of
GPRC5A and reduced GPRC5A protein expression in pancreatic cells.
It also may indirectly regulate many cell processes, such as DNA
repair, metabolism and the cell cycle (28). In the present study, we confirmed
that GPRC5A promoted the proliferation of U87 cells and confirmed
that GPRC5A is a direct target of miR-342. Furthermore, the
tumor-suppressive effect of miR-342 was reduced by enforced
expression of GPRC5A in vivo and in vitro. These
results suggest that miR-342 functions as an anti-oncogene via
multiple gene targeting, such as on GPRC5A.
In conclusion, the study availed a better
understanding of the function of miR-342 and GPRC5A in glioma. We
confirmed the downregulated level of miR-342 in glioma and revealed
the role of miR-342 in glioma cell proliferation and invasion. Our
data indicated the suppressive role of miR-342 in glioma
development and miR-342 may serve as a potential therapeutic target
in glioma. However, our results were based on one single cell line,
U87, and thus additional cell lines are needed to be included in
further research. Meanwhile, although the role of miR-342 in
cellular invasiveness and cancer progression is clear, its
mechanisms remain to be investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2017 China
Postdoctoral Science Foundation's 62nd Batch of Funded Projects
(2017M621787).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JW, YY, YC and XT interpreted and analyzed the data,
and drafted the manuscript. JW analyzed the data. XT designed the
study. All authors interpreted the results and wrote the
manuscript.
Ethics approval and consent to
participate
The animal study was conducted in accordance with
the Institutional Animal Care and Use Committee (IACUC) guidelines,
and was approved by the Experimental Animal Ethics Committee of The
Second Affiliated Hospital of Harbin Medical University. The
present study was approved by the Ethics Committee of The Second
Affiliated Hospital of Harbin Medical University. Written informed
consent was obtained from all enrolled subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Gu X, Gong H, Shen L and Gu Q:
MicroRNA-129-5p inhibits human glioma cell proliferation and
induces cell cycle arrest by directly targeting DNMT3A. Am J Transl
Res. 10:2834–2847. 2018.PubMed/NCBI
|
|
2
|
Ji ZG, Jiang HT and Zhang PS: FOXK1
promotes cell growth through activating wnt/β-catenin pathway and
emerges as a novel target of miR-137 in glioma. Am J Transl Res.
10:1784–1792. 2018.PubMed/NCBI
|
|
3
|
Gao Y, Lin L, Li T, Yang J and Wei Y: The
role of miRNA-223 in cancer: Function, diagnosis and therapy. Gene.
616:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang F, Liang S, Liu X, Han L, Wang J and
Du Q: LINC00460 modulates KDM2A to promote cell proliferation and
migration by targeting miR-342-3p in gastric cancer. Onco Targets
Ther. 11:6383–6394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu W, Kang L, Han J, Wang Y, Shen C, Yan
Z, Tai Y and Zhao C: miR-342-3p suppresses hepatocellular carcinoma
proliferation through inhibition of IGF-1R-mediated Warburg effect.
Onco Targets Ther. 11:1643–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue X, Fei X, Hou W, Zhang Y, Liu L and Hu
R: miR-342-3p suppresses cell proliferation and migration by
targeting AGR2 in non-small cell lung cancer. Cancer Lett.
412:170–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkatakrishnan AJ, Deupi X, Lebon G, Tate
CG, Schertler GF and Babu MM: Molecular signatures of
G-protein-coupled receptors. Nature. 494:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solinski HJ, Gudermann T and Breit A:
Pharmacology and signaling of MAS-related G protein-coupled
receptors. Pharmacol Rev. 66:570–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin E, Wang W, Fang M, Wang W, Xie R, Zhou
H, Ye J, Xu R and Ma S: Clinical significance of reduced GPRC5A
expression in surgically resected non-small cell lung cancer. Oncol
Lett. 17:502–507. 2019.PubMed/NCBI
|
|
10
|
Gu C, Zhou N, Wang Z, Li G, Kou Y, Yu S,
Feng Y, Chen L, Yang J and Tian F: circGprc5a promoted bladder
oncogenesis and metastasis through Gprc5a-targeting peptide. Mol
Ther Nucleic Acids. 13:633–641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klaschik K, Hauke J, Neidhardt G, Tränkle
C, Surowy HM, Heilmann-Heimbach S, Rappl G, Mangold E, Arnold N,
Niederacher D, et al: The GPRC5A frameshift variant c.183del is not
associated with increased breast cancer risk in BRCA1 mutation
carriers. Int J Cancer. 144:1761–1763. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jing L, Li H, Zhang T, Lu J and Zhong L:
MicroRNA-4530 suppresses cell proliferation and induces apoptosis
by targeting RASA1 in human umbilical vein endothelial cells. Mol
Med Rep. 19:3393–3402. 2019.PubMed/NCBI
|
|
13
|
Zhu Y, Zhao H, Rao M and Xu S:
MicroRNA-365 inhibits proliferation, migration and invasion of
glioma by targeting PIK3R3. Oncol Rep. 37:2185–2192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Tang C, Na M, Ma W, Jiang Z, Gu Y,
Ma G, Ge H, Shen H and Lin Z: miR-422a inhibits glioma
proliferation and invasion by targeting IGF1 and IGF1R. Oncol Res.
25:187–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhi T, Jiang K, Xu X, Yu T, Wu W, Nie E,
Zhou X, Jin X, Zhang J, Wang Y and Liu N: MicroRNA-520d-5p inhibits
human glioma cell proliferation and induces cell cycle arrest by
directly targeting PTTG1. Am J Transl Res. 9:4872–4887.
2017.PubMed/NCBI
|
|
16
|
Gu G, Wang L, Zhang J, Wang H, Tan T and
Zhang G: MicroRNA-384 inhibits proliferation migration and invasion
of glioma by targeting at CDC42. Onco Targets Ther. 11:4075–4085.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Yu J, Liu J, Yang X, Lou M, Liu J,
Feng F, Ji P and Wang L: MicroRNA-302a targets GAB2 to suppress
cell proliferation, migration and invasion of glioma. Oncol Rep.
37:1159–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero-Cordoba SL, Rodriguez-Cuevas S,
Bautista-Pina V, Maffuz-Aziz A, D'Ippolito E, Cosentino G, Baroni
S, Iorio MV and Hidalgo-Miranda A: Loss of function of miR-342-3p
results in MCT1 over-expression and contributes to oncogenic
metabolic reprogramming in triple negative breast cancer. Sci Rep.
8:122522018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu K, Mu X, Kolibaba H, Yin Q, Liu C,
Liang X and Lu J: Metadherin is an apoptotic modulator in prostate
cancer through miR-342-3p regulation. Saudi J Biol Sci. 25:975–981.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Li W, Zhang R and Liu Y:
MicroRNA-342 inhibits cell proliferation and invasion in
nasopharyngeal carcinoma by directly targeting ZEB1. Oncol Lett.
16:1298–1304. 2018.PubMed/NCBI
|
|
21
|
Gentry PR, Sexton PM and Christopoulos A:
Novel allosteric modulators of G protein-coupled receptors. J Biol
Chem. 290:19478–19488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scholz N, Gehring J, Guan C, Ljaschenko D,
Fischer R, Lakshmanan V, Kittel RJ and Langenhan T: The adhesion
GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep.
11:866–874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferré S, Casadó V, Devi LA, Filizola M,
Jockers R, Lohse MJ, Milligan G, Pin JP and Guitart X: G
protein-coupled receptor oligomerization revisited: functional and
pharmacological perspectives. Pharmacol Rev. 66:413–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumari P, Ghosh E and Shukla AK: Emerging
approaches to GPCR ligand screening for drug discovery. Trends Mol
Med. 21:687–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kadara H, Fujimoto J, Men T, Ye X, Lotan
D, Lee JS and Lotan R: A Gprc5a tumor suppressor loss of expression
signature is conserved, prevalent, and associated with survival in
human lung adenocarcinomas. Neoplasia. 12:499–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Ye D, Wang T, Guo W, Song H, Liao
Y, Xu D, Zhu H, Zhang Z and Deng J: Repression of GPRC5A is
associated with activated STAT3, which contributes to tumor
progression of head and neck squamous cell carcinoma. Cancer Cell
Int. 17:342017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou H and Rigoutsos I: The emerging roles
of GPRC5A in diseases. Oncoscience. 1:765–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou H and Rigoutsos I: MiR-103a-3p
targets the 5′UTR of GPRC5A in pancreatic cells. RNA. 20:1431–1439.
2014. View Article : Google Scholar : PubMed/NCBI
|