Introduction
Formaldehyde (FA) is hematotoxic to humans and mice
(1), and has been classified as a
human leukemogen by the International Agency for Research on Cancer
(2) and the U.S. National
Toxicology Program (3); however,
the exact mechanism remains unclear. The bone marrow (BM) is the
site of blood cell generation from hematopoietic stem cells, as
well as the target site for the induction of leukemia (4). Numerous studies have investigated the
toxicity of FA; however, few investigations into the effects of FA
on the BM have been conducted.
Phosphatase and tensin homologue deleted on
chromosome 10 (PTEN) was the first phosphatase identified as a
tumor suppressor gene and has been considered as a negative
regulator of the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt) signaling pathway, which modulates the cell cycle, apoptosis
and differentiation (5,6). Additionally, PTEN is expressed at low
levels in leukemia cells, and can regulate the invasive ability and
angiogenesis of these cells (7).
Downregulated PTEN can lead to the abnormal expression of proteins
involved in the PI3K/Akt signaling pathway, and induce malignant
diseases of the blood (8,9). PI3K is the initiator of PI3K/Akt
signaling pathway, which regulates the proliferation and survival
of tumor cells, and serves an important role in the onset of
leukemia (10). The abnormal
activity of PI3K not only induces the malignant transformation of
cells, but is also associated with the migration and adhesion of
tumor cells, as well as the degradation of extracellular matrix
(11). Akt is the direct target
protein of PI3K, and can activate or inhibit numerous downstream
signaling molecules, including B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X (Bax), mammalian target of rapamycin (mTOR) and
Caspase-9 (12,13). At present, no studies into the
association between FA and the PTEN/PI3K/Akt signal transduction
pathway in bone marrow cells (BMCs) have been conducted. Therefore,
the aim of the present study was to determine whether FA could
induce the apoptosis of BMCs via the PTEN/PI3K/Akt signal
transduction pathway, so as to investigate the potential mechanism
underlying the progression of leukemia. Our results may provide an
experimental basis for future studies into the mechanisms of FA
toxicity and the prevention of leukemia.
Materials and methods
Reagents
Dulbecco's Modified Eagle's medium (DMEM) and fetal
bovine serum were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Tryptase and 100X penicillin/streptomycin
mixing liquid were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). FA (36.5–38% in water, formula
weight, 30.03), a Cell Counting Kit 8 (CCK-8) assay kit, ethidium
iodide and RNA enzymes were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). An apoptosis analysis kit was purchased
from BD Biosciences (San Jose, CA, USA). Antibodies against PTEN
(cat. no. sc-7974), PI3K (cat. no. sc-374534), Akt (cat. no.
sc-5298), Bcl-2 (cat. no. sc-7382), Bax (cat. no. sc-20067), and
Caspases-3 (cat. no. sc-56053) and −9 (cat. no. sc-73548) were
purchased from Santa Cruz Biotechnology, Inc. (Dallax, TX, USA).
All reagents were of the highest purity commercially available.
Animals and treatment
A total of 30BALB/c mice (specific-pathogen free,
male, 6–8-weeks-old, 18–20 g) were purchased from the Experimental
Animal Center of Chongqing Medical University (Chongqing, China,
license no. SCXK-Yu 2012-0001), and housed under standard
laboratory conditions (temperature: 20–25°C; relative humidity:
50–70%; 12 h light dark cycle). Food and water were provided ad
libitum. All animal experiments were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (14) and
were approved by the Ethics Committee of Jilin University
(Changchun, China).
Cell culture
Following sacrifice of the BALB/c mice, the femurs
were harvested and the surrounding tissues were removed. Then, an
incision was made on the greater trochanter and the samples were
washed twice with 0.01 mol/l PBS; 1-ml sterile syringes were used
to wash the BMCs. This process was repeated until the cells were
completely removed from the femur samples. Subsequently, BMCs were
filtered with a 200-mesh nylon filter to obtain a single bone
marrow cell suspension. The cells were washed with 0.01 mol/l PBS,
resuspended in DMEM and counted using a cell counter (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for subsequent analysis.
Cell viability assay
The cell viability assay was performed using a CCK-8
assay kit according to the manufacturer's protocols. BMCs were
seeded in 96-well plates with a density of 1×105
cells/ml, and exposed to various doses of FA (50, 100 and 200
µmol/l) for 12, 24 and 48 h at 37°C in an incubator with 5%
CO2; untreated cells served as the control (Ctrl) group.
Additionally, a blank group containing medium only with no cells
was also included. The cells (untreated and treated with FA) and
the blank group were then incubated with 10 µl of the CCK-8
solution for 1.5 h at 37°C in an incubator with 5% CO2.
Then, the number of viable cells in each well was counted by
measuring the absorbance at a wavelength of 450 nm with a
microplate reader (Thermo Fisher Scientific, Inc.).
Cell viability (%) = [A (experimental group) - A
(blank group)]/[A (Ctrl group) - A (blank group)] ×100%.
Cell cycle assay
Following treatment with various doses of FA (50,
100 and 200 µmol/l) for 24 h at 37°C in an incubator with 5%
CO2, BMCs were washed twice with 0.01 mol/l pre-cooled
PBS. Then, the cells were fixed overnight in pre-cooled 70% ethanol
at 4°C; 100 µl RNase (10 µg/ml) and 100 µl propidium iodide (5
µg/ml) were added to cells. The cells were incubated without light
for 30 min at 37°C. The cell cycle was measured with a flow
cytometer (FACSVantage SE; BD Biosciences) and data were analyzed
using Cell Quest software (version 5.1; BD Biosciences).
Cell cycle assay following PI3K
inhibitor treatment
The cells were divided into the Ctrl, FA, negative
control (NC; untreated cells) + LY294002 and FA + LY294002 groups;
10 µmol/l LY294002 (Santa Cruz Biotechnology, Inc.) was added into
the inhibitor treatment groups and 100 µmol/l FA was selected for
treatment. Then, the cells were incubated for 24 h at 37°C in an
incubator with 5% CO2. Analysis of the cell cycle was
conducted as aforementioned.
Determination of cell apoptosis by
flow cytometry (FCM)
Following BMC exposure to different doses of FA (50,
100 and 200 µmol/l) for 24 h, cells were collected and centrifuged
at 800 × g for 5 min at room temperature; the cells were then
washed twice with 0.01 mol/l PBS, and centrifuged at 800 × g for 5
min at room temperature. A total of 1×105 cells were
collected, to which 5 µl 7-aminoactinomycin D dye solution was
added. The cells were incubated in the dark at room temperature for
15 min. Subsequently, 450 µl Binding Buffer (BD Biosciences) was
applied, followed by 1 µl Annexin V-phycoerythrin; cells were
incubated at room temperature for 15 min. Cell apoptosis was
measured by FCM (FACSVantage SE) and data were analyzed using Cell
Quest software.
Expression of PTEN, PI3K and Akt as
analyzed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
BMCs were divided into the Ctrl and various
FA-treatment groups (50, 100 and 200 µmol/l). Cells were treated
with FA for 24 h and the total RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.).
PrimeScript™ RT reagent (Takara Biotechnology, Co., Ltd., Dalian,
China) was used to reverse transcribe RNA samples into cDNA under
the following conditions: 25°C for 10 min, 42°C for 50 min and 85°C
for 5 min. Based on the sequence complementarity of the cDNA
template to the upstream and downstream primers of target genes,
cDNA was used for qPCR. The mRNA expression levels were determined
using an SYBR® Premix Ex Taq™ kit (Takara Biotechnology
Co., Ltd.) on an FTC-3000 system (Funglyn Bio Inc., Toronto,
Canada). qPCR was conducted as follows: 35 cycles of 94°C for 20
sec, 60°C for 30 sec and 72°C for 30 sec. The primers of PTEN, PI3K
and Akt employed in the present study were designed as follows:
PTEN, forward 5′-AAGACCATAACCCACCACAGC-3′, reverse,
5′-CCAGTCCGTCCCTTTCCAG-3′ (amplicon size: 124 bp); PI3K, forward
5′-AAGCCATTGAGAAGAAAGGACTG-3′, reverse,
5′-ATTTGGTAAGTCGGCGAGATAG-3′ (amplicon size: 176 bp); and Akt,
forward 5-TGT CTG CCC TGG ACT ACT TGC-3′ and reverse,
5′-GGCGTTCCGCAGAATGTC-3′ (amplicon size: 166 bp). β-actin served as
the internal reference (forward, 5′-GAGACCTTCAACACCCCAGC-3′ and
reverse, 5′-ATGTCACGCACGATTTCCC-3′; amplicon size: 263 bp). The
relative expression (2−∆∆Cq) (15) of PTEN, PI3K and Akt was calculated
as follows:
Relative expression = 2−∆∆Cq; DCq = Cq
PTEN/PI3K/Akt - Cqβ-actin.
Expression of PTEN, PI3K and Akt via
western blot analysis
BMCs were divided into the Ctrl and various
FA-treatment groups (50, 100 and 200 µmol/l). Following treatment
with FA for 24 h, the protein in BMCs was extracted using a Protein
Extraction kit (Beyotime Institute of Biotechnology), and the
protein level was determined using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Then, equal amounts of
protein (35 µg) were separated via 12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes via electroblotting. The
membranes were blocked with 5% non-fat dried milk in 1X TBST buffer
(pH 7.6; 2.42 g Tris base, 8.0 g NaCl, 1,000 ml ddH2O
and 0.5 ml Tween-20) for 1.5 h at room temperature. Subsequently,
the membranes were incubated with primary polyclonal antibodies, at
a dilution of 1:1,000 for β-actin (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.), and 1:500 for PTEN, PI3K and Akt for 1.5 h at
room temperature. Then, the membranes were washed three times with
TBST, and incubated for 1.5 h at room temperature with a
horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(IgG) secondary antibody (1:1,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.). The protein bands were visualized using an
enhanced chemiluminescence detection system (Tannon Science &
Technology, Co., Ltd., Shanghai, China), and an Image analysis
system (Labworks™ analysis software, Labworks LLC, Lehi, UT,
USA).
Expression of Bcl-2, Bax, and
Caspases-3 and −9 via immunohistochemistry
BMCs were collected and divided into the Ctrl and
various FA-treatment groups (50, 100 and 200 µmol/l). Following
treatment with FA for 24 h, the cells of each group were
conventionally smeared onto slides. Following fixation with
formalin buffer solution 10% for 15 min at room temperature, the
slides were air dried and then stored in a refrigerator at −20°C.
The slides were treated with 3% H2O2 for 15
min at room temperature, washed with 0.01 mol/l PBS three times,
and then blocked with 10 µl 10% non-immune mouse serum (Abbkine
Scientific Co., Ltd., Lake Bluff, IL, USA) for 15 min at room
temperature. Subsequently, slides were incubated with primary
antibodies against Bcl-2, Bax, Caspases-3 and −9 (all 1:100; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. After washing with PBS
three times, the slides were incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(1:1,000; cat. no. L3032-2; Signalway Antibody LLC, College Park,
MD, USA) for 15 min at room temperature. Following a further three
washes with PBS, 10 µl of Streptomyces antibiotic
protein-peroxidase solution (Beijing Zhongshan Golden Bridge
Biotechnology Co, Ltd.; OriGene Technologies, Inc., Beijing, China)
for 15 min at room temperature. The color reaction was performed
with 3,3-diaminobenzidine (Beyotime Institute of Biotechnology),
then slides were counterstained with hemotoxylin (Beyotime
Institute of Biotechnology) for 2 min at room temperature.
Subsequently, the slides were placed in 70% HCl-ethanol for 15 sec
and washed in water, and then in weak ammonia for 15 sec. Slides
were then dehydrated and mounted. Five random visual fields were
observed using an Olympus BX-50 light microscope (magnification,
×200; Olympus Corporation, Tokyo, Japan); 100 cells were selected
in each field. Cells with brown-yellow particles deposited on the
membrane or nucleus were counted as positive cells, and the
positive rate was calculated by the formula:
Positive staining (%) = Positive cells/cells
×100%.
Statistical analysis
Statistical analysis was performed using SPSS
software v24.0 (IBM Corp., Armonk, NY, USA). All experiments were
performed at least three times, and the data were expressed as the
mean ± standard deviation. Significant differences between groups
were determined by one-way analysis of variance followed by a
Tukey's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
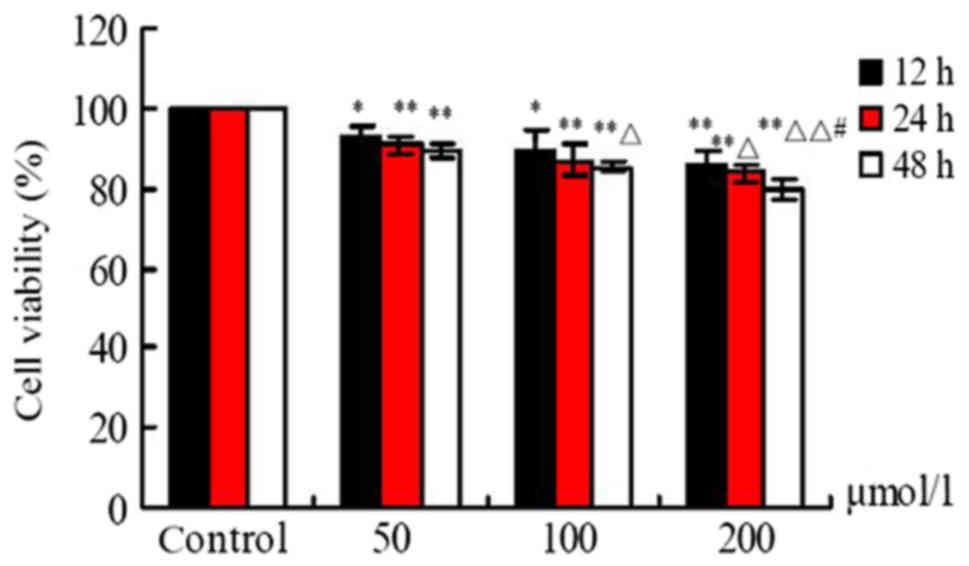
Effects of FA on cell viability
Following exposure to different doses of FA (50, 100
and 200 µmol/l) for 12, 24 and 48 h, cell viability was
significantly reduced in response to increasing concentrations of
FA compared with the Ctrl group. As presented in Fig. 1, FA suppressed the viability of
BMCs in a dose- and time-dependent manner; a significant difference
in cell viability was observed at 12, 24 and 48 h (P<0.05). In
addition, a significant difference between the 200 and 50 µmol/l FA
treatment groups at 24 and 48 h was reported (P<0.05), and
compared with the 100 µmol/l group at 48 h (P<0.01; Fig. 1).
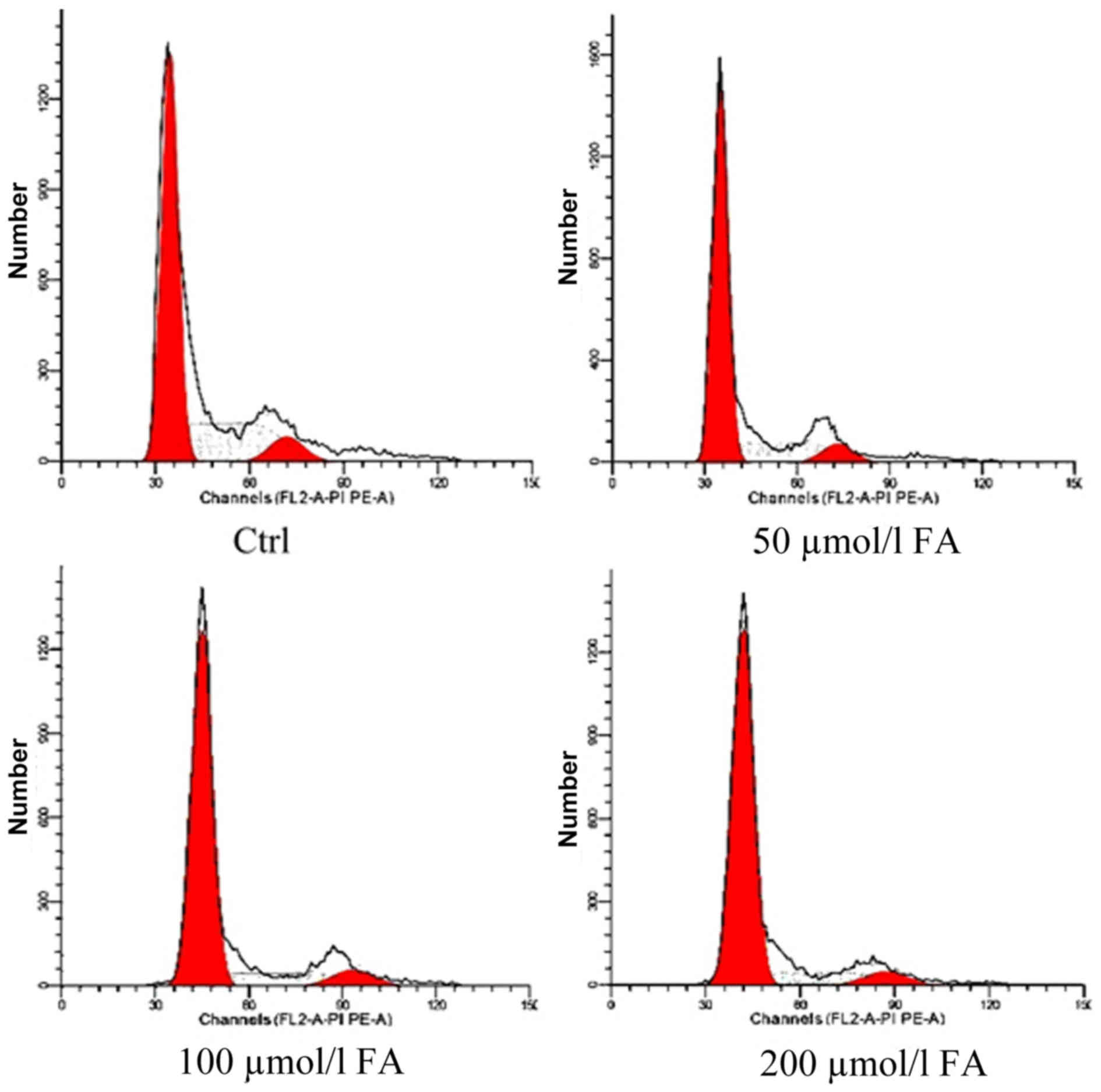
Effects of FA on the cell cycle
Following exposure to different doses of FA (50, 100
and 200 µmol/l) for 24 h, the proportion cells in
G0/G1-phase increased with FA treatment,
while the number of cells in S-phase was decreased. A significant
difference in the number of cells in G0/G1
and S-phase following FA treatment was detected compared with the
Ctrl group (P<0.01). Additionally, significant differences in
the proportion of cells in G0/G1 and S-phase
of the 100 and 200 µmol/l groups compared with the 50 µmol/l group
were observed (P<0.01). The number of cells in S-phase
significantly differed between the 200 and 100 µmol/l groups
(P<0.05). The number of G2/M-phase cells in each
group was markedly unaltered. These results demonstrated that FA
may induce cell cycle arrest at G0/G1 phase
in BMCs and alter cell proliferation to inhibit cell growth and
development (Table I; Fig. 2).
 | Table I.Effects of formaldehyde on the cell
cycle of bone marrow cells from mice. |
Table I.
Effects of formaldehyde on the cell
cycle of bone marrow cells from mice.
|
| Cell cycle |
|---|
|
|
|
|---|
| Group |
G0/G1 | S |
G2/M |
|---|
| Control | 60.43±1.66 | 31.47±1.10 | 8.10±0.62 |
| 50 µmol/l |
70.32±0.73a |
21.78±0.98a | 7.90±0.48 |
| 100 µmol/l |
76.86±1.45a,b |
16.27±0.25a,b | 6.87±1.48 |
| 200 µmol/l |
78.04±1.10a,b |
15.20±0.47a–c | 6.76±0.65 |
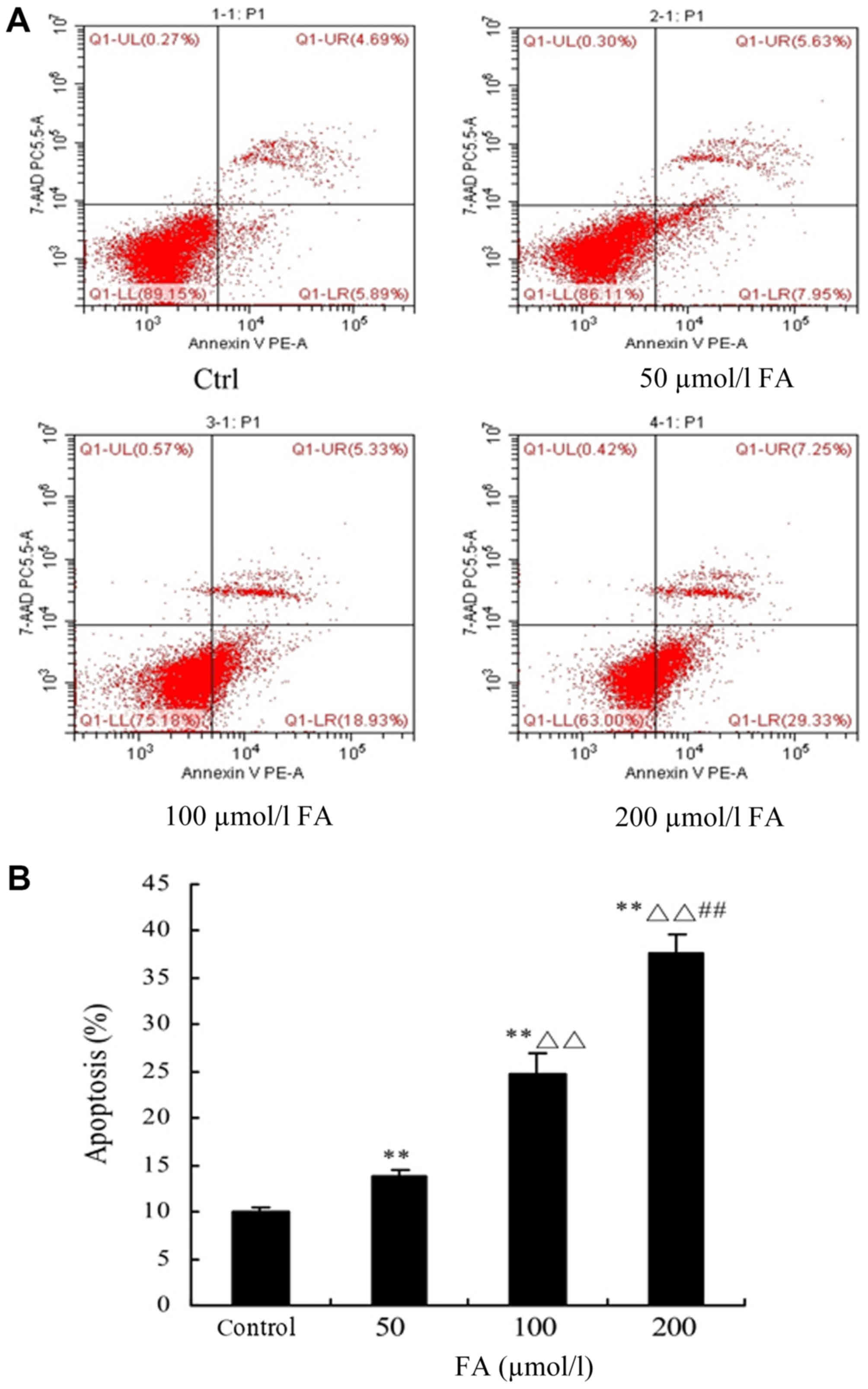
Determination of cell apoptosis by
FCM
Following treatment with different doses of FA (50,
100 and 200 µmol/l) for 24 h, FCM was conducted to detect
apoptosis. The results demonstrated that the percentage of
apoptotic cells was significantly increased with increasing
concentrations of FA; significant increases were reported with FA
treatment compared with the Ctrl group (P<0.01). Additionally,
significant differences in the 100 and 200 µmol/l groups compared
with the 50 µmol/l group were observed (P<0.01). Furthermore, a
significant difference between the 200 and 100 µmol/l groups was
reported (P<0.01; Fig. 3).
Determination of PTEN, PI3K and Akt
expression by RT-qPCR
Following treatment with different doses of FA (50,
100 and 200 µmol/l) for 24 h, the mRNA expression levels of PTEN
were notably suppressed with increasing concentrations of FA;
significant decreases in the 100 and 200 µmol/l groups were
observed compared with the Ctrl (P<0.05). Additionally,
significant differences following treatment with 200 µmol/l FA were
detected compared with 50 and 100 µmol/l FA (P<0.05). The mRNA
expression levels of PI3K were upregulated with increasing
concentrations of FA; significant increases in expression following
treatment with 100 and 200 µmol/l FA were reported compared with
the Ctrl (P<0.01). The mRNA expression levels of Akt were also
upregulated in response to increasing concentrations of FA;
significant increases between the FA treatment groups and the Ctrl
were observed (P<0.01). In addition, there were significant
differences in Akt expression following treatment with 100 and 200
µmol/l FA compared with 50 µmol/l FA (P<0.05; Fig. 4).
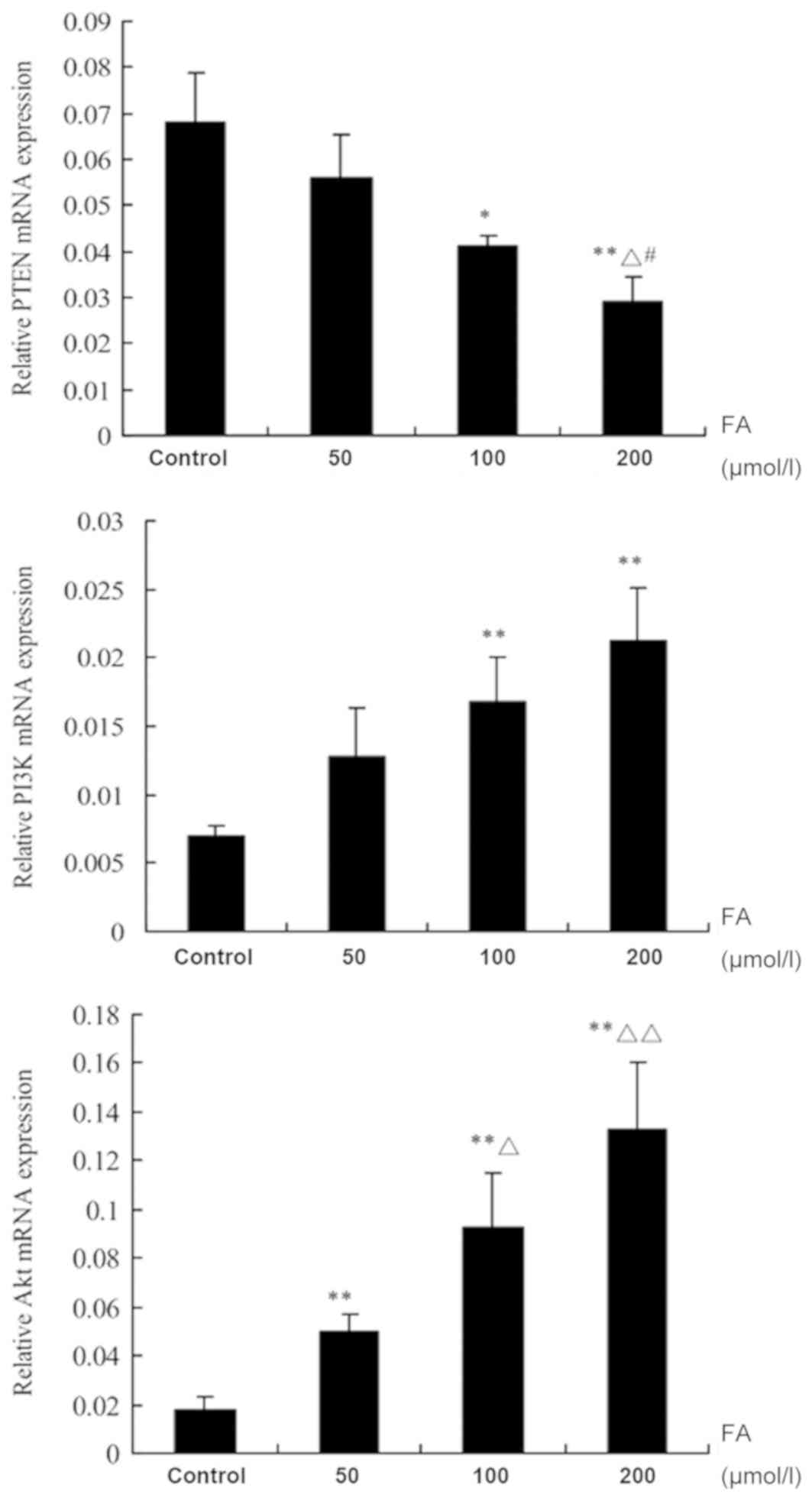 | Figure 4.Effects of FA on the mRNA expression
of PTEN, PI3K and Akt in bone marrow cells. Data are presented as
the mean ± standard deviation. *P<0.05, **P<0.01, vs. control
group; ΔP<0.05, ΔΔP<0.01, vs. 50 µmol/l
FA group; #P<0.05, vs. 100 µmol/l FA group. Akt,
protein kinase B; FA, formaldehyde; PI3K, phosphoinositide
3-kinase; PTEN, phosphatase and tensin homologue deleted on
chromosome 10. |
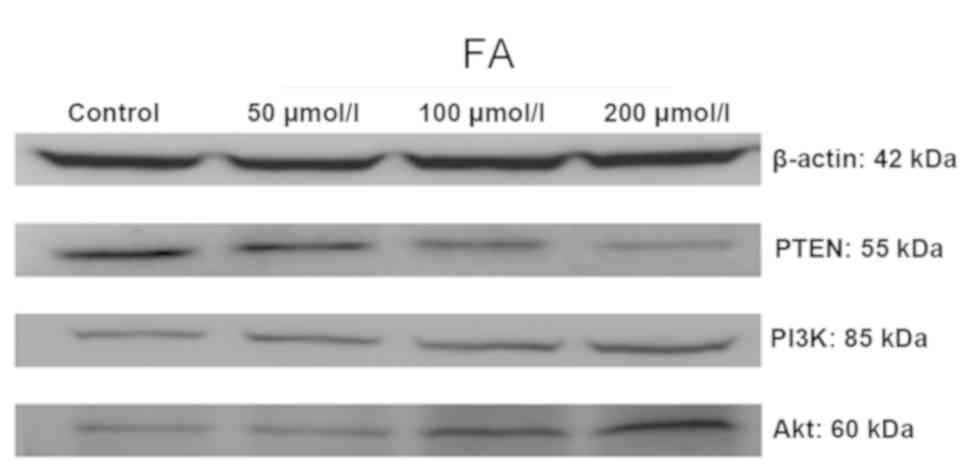
Determination of PTEN, PI3K and Akt
protein expression by western blot analysis
As presented in Fig.
5, following treatment with different doses of FA (50, 100 and
200 µmol/l) for 24 h, the expression levels of PTEN protein were
decreased, whereas the protein expression levels of PI3K and Akt
were upregulated with increasing concentrations of FA.
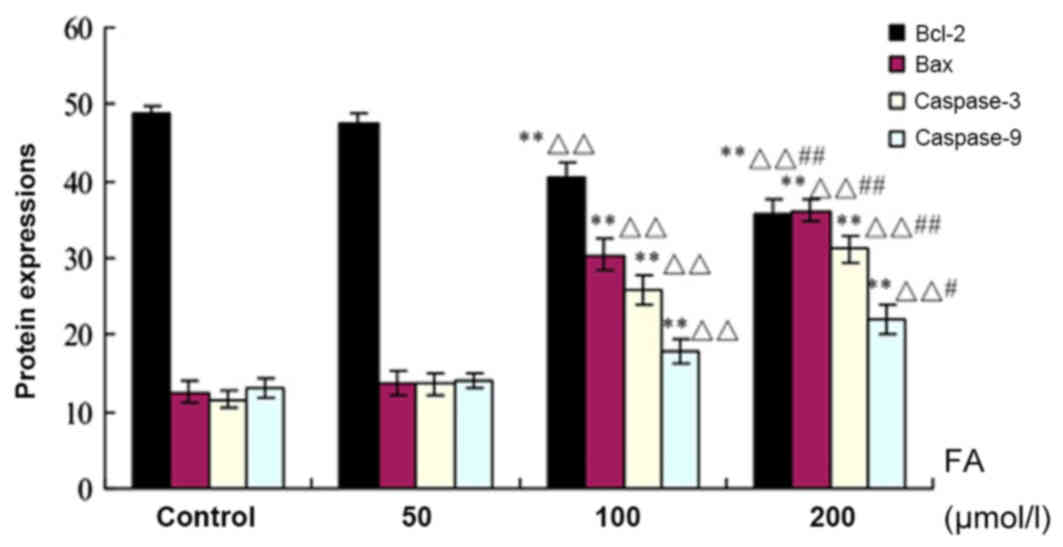
Bcl-2, Bax, and Caspases-3 and −9
protein expression as determined by immunohistochemistry
As presented in Fig.
6, the expression levels of Bax, and Caspases-3 and −9 protein
were upregulated with increasing concentrations of FA, while the
expression of Bcl-2 was decreased. There were significant increases
in the expression levels of the aforementioned proteins in response
to 100 and 200 µmol/l FA compared with the Ctrl (P<0.01). In
addition, significant increases in the 100 and 200 µmol/l FA groups
were reported compared with the 50 µmol/l FA group (P<0.01); a
significant difference between the 200 and 100 µmol/l FA groups was
also observed (P<0.05).
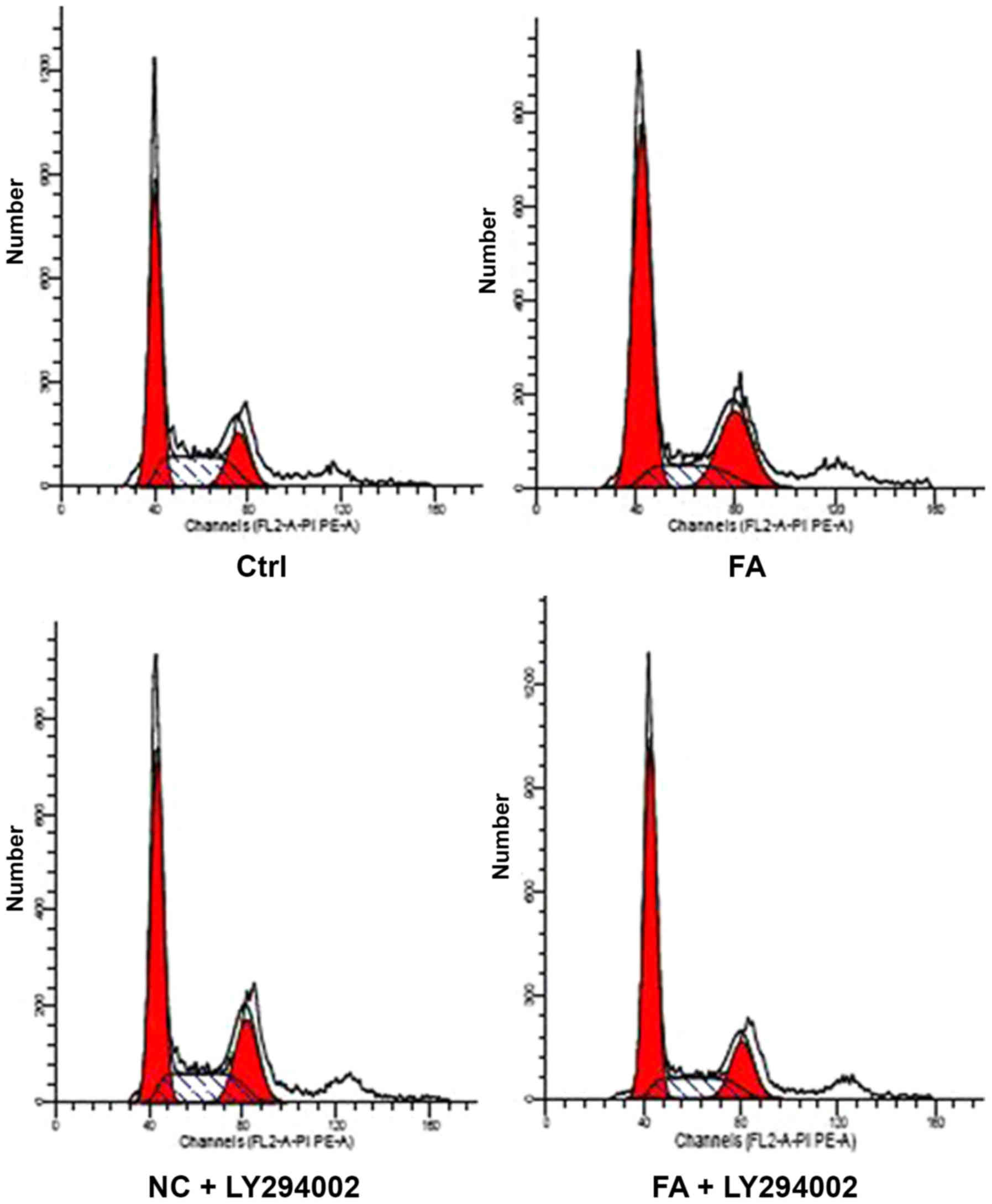
Determination of the cell cycle by FCM
following treatment with LY294002
The BMCs were divided into Ctrl, FA, NC + LY294002
and FA + LY294002 groups. After 24 h of FA (100 µmol/l) treatment,
the proportion of cells in S-phase in the FA, NC + LY294002 and FA
+ LY294002 groups decreased compared with the Ctrl group
(P<0.01), whereas the number of cells in
G0/G1 in the FA and FA + LY294002 groups was
significantly increased (P<0.01); however, there was no notable
alteration in the number of cells in G0/G1 in
the NC + LY294002 group compared with the Ctrl. Additionally, the
proportion of cells in G2/M-phase in the NC + LY294002
and FA groups was also significantly increased compared with the
Ctrl group (P<0.01), whereas there was no difference between the
FA + LY294002 and Ctrl groups. Furthermore, compared with the NC +
LY294002 group, significant differences were observed in the FA and
FA + LY294002 groups in the number of cells in
G0/G1 and S-phase (P<0.01), whereas the
number of cells in G2/M-phase increased in the FA group
(P<0.05) and decreased in the FA + LY294002 group (P<0.01).
It was also observed that the proportion of cells in S- and
G2/M-phase significantly varied in FA+ LY294002 group
compared with the FA group (P<0.01); however, there was no
change in G0/G1-phase (Table II; Fig. 7). The results suggested that FA may
alter cell proliferation and induce
G0/G1-phase arrest. After using a PI3K
inhibitor to block the PTEN/PI3K/Akt signaling pathway, it was
observed that the proportion of cells in S-phase increased compared
with FA group, whereas that in G2/M-phase decreased,
indicating that DNA synthesis in cells was increased, and that the
cell cycle was arrested in S-phase.
 | Table II.Effects of PI3K inhibitor on the cell
cycle of bone marrow cells from mice. |
Table II.
Effects of PI3K inhibitor on the cell
cycle of bone marrow cells from mice.
|
| Cell cycle |
|---|
|
|
|
|---|
| Group |
G0/G1 | S |
G2/M |
|---|
| Control | 52.26±0.68 | 30.12±0.9 | 17.62±0.26 |
| FA |
59.35±0.8a |
15.88±0.31a |
24.77±0.78a |
| NC + LY294002 |
51.90±0.92c |
25.34±1.31a,c |
22.76±0.59a,b |
| FA + LY294002 |
60.99±1.30a,d |
20.53±0.6a,c,d |
18.48±1.26c,d |
Discussion
PTEN, as a tumor-suppressing gene, is widely
expressed in numerous tissues and organs of the human body
(16). It can regulate apoptosis,
participate in oncogenesis, and serve an important role in inducing
cell cycle arrest, cell adhesion, migration and differentiation
(17,18). It has been reported that, in the
hematopoietic cells of mice with PTEN mutation or null expression,
the abundance of myeloid cells and T lymphocytes increased, which
was accompanied with enlarged lymph nodes of the liver and spleen;
the onset of myeloid or lymphoid leukemia was then detected
(19). In addition, reduced
function of PTEN was associated with a marked increase in the
levels of phosphatidylinositol (3,4,5)-triphosphate and the activation of the
Akt signaling pathways, contributing to tumorigenesis (20). It is well reported that the
regulation of Akt activity is mainly dependent upon PI3K activity.
Therefore, Akt as an important downstream target protein of PI3K,
and can effectively participate in mediating the cell cycle,
apoptosis, and the occurrence of cancer by activating or inhibiting
a variety of downstream target proteins (21). To further determine the underlying
mechanisms of FA toxicity, the expression levels of PTEN, PI3K and
Akt were analyzed by RT-qPCR and western blotting in the present
study. The results revealed that FA could decrease the expression
of PTEN, while upregulating that of PI3K and Akt. This is
consistent with the findings of the aforementioned reports.
Therefore, the PTEN/PI3K/Akt signal transduction pathway may be
involved in the process of BM toxicity induced by FA and serve a
role in the onset of leukemia.
The cell cycle serves an important role in
maintaining the growth and development of cells; however, the
regulation of this biological process is complex (22). Disruption of the regulatory
mechanism can lead to uncontrolled cell growth and suppressed cell
differentiation, which can induce apoptosis and tumorigenesis
(23). In the present study,
following BMC treatment with different doses of FA for 24 h, cell
viability was analyzed. The results suggested that FA could
suppress cell viability with increasing concentrations of FA;
significant differences between the FA and the Ctrl groups were
observed. Furthermore, the cell cycle and apoptosis were
investigated. After 24 h of treatment with FA, the proportion in
BMCs in G0/G1-phase was increased, while the
number of S-phase cells was decreased. Additionally, with the
increasing concentrations of FA, the rate of apoptosis
increased.
The PTEN/PI3K/Akt signal transduction pathway is
involved in various processes, the regulation of the cell cycle.
Akt can activate mTOR to promote cell cycle progression from
G0/G1 phase to S-phase; Akt is an important
regulator of cell growth and proliferation (24,25).
Therefore, in the present study, 10 µmol/l PI3K inhibitor
(LY294002) was applied to suppress the PTEN/PI3K/Akt signal
transduction pathway and 100 µmol/l FA was employed. Then,
alterations in the cell cycle were investigated. The results
demonstrated that FA could increase the proportion of cells in
G0/G1-phase, whereas the number of cells in
S-phase was decreased. Additionally, significant differences were
also observed in the PI3K inhibitor group compared with FA group,
with the results suggesting that LY294002 promoted DNA synthesis in
cells and induced S-phase arrest. This suggested that the
PTEN/PI3K/Akt signal transduction pathway served an important role
in the cell cycle induced by FA, in which regulation of this
process may affect apoptosis.
The occurrence of apoptosis is associated with the
regulation of numerous genes in cells. It was reported that the
members of the Bcl-2/Bax protein family are important downstream
targets of the PI3K/Akt signal transduction pathway, and served a
critical role in the onset of apoptosis (26,27).
Therefore, in the present study, immunohistochemistry was conducted
to examine the protein expression of Bcl-2, Bax, and Caspases-3 and
−9. The results demonstrated that the expression levels of Bcl-2
were reduced with increasing concentrations of FA, while the
expression of Bax, and Caspases-3 and −9 protein were upregulated.
This suggested that FA could induce cell apoptosis via the
mitochondrial apoptosis pathway, and this process may be associated
with the PTEN/PI3K/Akt signal transduction pathway.
In conclusion, the results of the present study
indicated the induction of the PTEN/PI3K/Akt signal transduction
pathway in BMCs. FA could suppress cell viability, induce apoptosis
and lead to cell cycle arrest. These effects may be associated with
the inhibition of the PTEN/PI3K/Akt signal transduction pathway
induced by FA. Furthermore, this pathway may be an underlying
mechanism of FA-induced leukemia; however, further investigation is
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81502839) and Jilin
Provincial Education Department in 13th Five-Year Planning (grant
no. JJKH20180237KJ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY conceived the study, collected and analyzed the
data, and drafted the manuscript. CW, XS, SL, YZ, LF, YY, YH and JS
performed the experiments, and contributed to collecting and
analyzing the data, and drafting the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jilin University (Changchun, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei C, Wen H, Yuan L, McHale CM, Li H,
Wang K, Yuan J, Yang X and Zhang L: Formaldehyde induces toxicity
in mouse bone marrow and hematopoietic stem/progenitor cells and
enhances benzene-induced adverse effects. Arch Toxicol. 91:921–933.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
IARC (International agency for Research on
Cancer), . A review of human carcinogens: Chemical agents and
related occupations: Formaldehyde. Monographs on the Evaluation of
Carcinogenic Risks to Humans 100F. 401–435. 2012.
|
|
3
|
NTP (National Toxicology Program), .
Report on carcinogens, 12th edition. National Toxicology Program.
195–205. 2011.
|
|
4
|
Renström J, Kröger M, Peschel C and
Oostendorp RA: How the niche regulates hematopoietic stem cells.
Chem Biol Interact. 184:7–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim G, Ouzounova M, Quraishi AA, Davis A,
Tawakkol N, Clouthier SG, Malik F, Paulson AK, D'Angelo RC, Korkaya
S, et al: SOCS3-mediated regulation of inflammatory cytokines in
PTEN and p53 inactivated triple negative breast cancer model.
Oncogene. 34:671–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santanam U, Banach-Petrosky W, Abate-Shen
C, Shen MM, White E and DiPaola RS: Atg7 cooperates with Pten loss
to drive prostate cancer tumor growth. Genes Dev. 30:399–407. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendes RD, Canté-Barrett K, Pieters R and
Meijerink JP: The relevance of PTEN-AKT in relation to
NOTCH1-directed treatment strategies in T-cell acute lymphoblastic
leukemia. Haematologica. 101:1010–1017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tesio M, Oser GM, Baccelli I, Blanco-Bose
W, Wu H, Göthert JR, Kogan SC and Trumpp A: Pten loss in the bone
marrow leads to G-CSF-mediated HSC mobilization. J Exp Med.
210:2337–2349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choorapoikayil S, Kers R, Herbomel P,
Kissa K and den Hertog J: Pivotal role of Pten in the balance
between proliferation and differentiation of hematopoietic stem
cells in zebrafish. Blood. 123:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fransecky L, Mochmann LH and Baldus CD:
Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell
Ther. 3:22015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faes S and Dormond O: PI3K and AKT:
Unfaithful partners in cancer. Int J Mol Sci. 16:21138–21152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Follo MY, Manzoli L, Poli A, McCubrey JA
and Cocco L: PLC and PI3K/Akt/mTOR signalling in disease and
cancer. Adv Bio Regul. 57:10–16. 2015. View Article : Google Scholar
|
|
13
|
Ferenc P, Solár P, Kleban J, Mikes J and
Fedorocko P: Down-regulation of Bcl-2 and Akt induced by
combination of photoactivated hypericin and genistein in human
breast cancer cells. J Photochem Photobiol B. 98:25–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
NIH (National Institutes of Health USA), .
Guide for the care and use of laboratory animals (8th edition).
Washington (DC): National Academies Press (US); 2011
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Xie W, Xie C, Huang C, Zhu J, Liang
Z, Deng F, Zhu M, Zhu W, Wu R, et al: Curcumin modulates
miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7
breast cancer cell proliferation. Phytother Res. 28:1553–1560.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nuciforo PG, Aura C, Holmes E, Prudkin L,
Jimenez J, Martinez P, Ameels H, de la Peña L, Ellis C, Eidtmann H,
et al: Benefit to neoadjuvant anti-human epidermal growth factor
receptor 2 (HER2)-targeted therapies in HER2-positive primary
breast cancer is independent of phosphatase and tensin homolog
deleted from chromosome 10 (PTEN) status. Ann Oncol. 26:1494–1500.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krohn A, Freudenthaler F, Harasimowicz S,
Kluth M, Fuchs S, Burkhardt L, Stahl P, C Tsourlakis M, Bauer M,
Tennstedt P, et al: Heterogeneity and chronology of PTEN deletion
and ERG fusion in prostate cancer. Mod Pathol. 27:1612–1620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu G, Chai J, Ma L, Duan H and Zhang H:
Downregulated microRNA-32 expression induced by high glucose
inhibits cell cycle progression via PTEN upregulation and Akt
inactivation in bone marrow-derived mesenchymal stem cells. Biochem
Biophys Res Commun. 433:526–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye X, Ji Z, Wei C, McHale CM, Ding S,
Thomas R, Yang X and Zhang L: Inhaled formaldehyde induces
DNA-protein crosslinks and oxidative stress in bone marrow and
other distant organs of exposed mice. Environ Mol Mutagen.
54:705–718. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Whitfield ML, Sherlock G, Saldanha AJ,
Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM,
Brown PO and Botstein D: Identification of genes periodically
expressed in the human cell cycle and their expression in tumors.
Mol Biol Cell. 13:1977–2000. 2002. View Article : Google Scholar PubMed/NCBI
|
|
23
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jing X, Cheng W, Wang S, Li P and He L:
Resveratrol induces cell cycle arrest in human gastric cancer
MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway.
Oncol Rep. 35:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng L, Brown J and Eng C: PTEN induces
apoptosis and cell cycle arrest through
phosphoinositol-3-kinase/Akt-dependent and -independent pathways.
Hum Mol Genet. 10:237–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahmani M, Aust MM, Attkisson E, Williams
DC Jr, Ferreira-Gonzalez A and Grant S: Dual inhibition of Bcl-2
and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in
human myeloid leukemia cells through a GSK3- and Bim-dependent
mechanism. Cancer Res. 73:1340–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vachhani P, Bose P, Rahmani M and Grant S:
Rational combination of dual PI3K/mTOR blockade and Bcl-2/-xL
inhibition in AML. Physiol Genomics. 46:448–456. 2014. View Article : Google Scholar : PubMed/NCBI
|