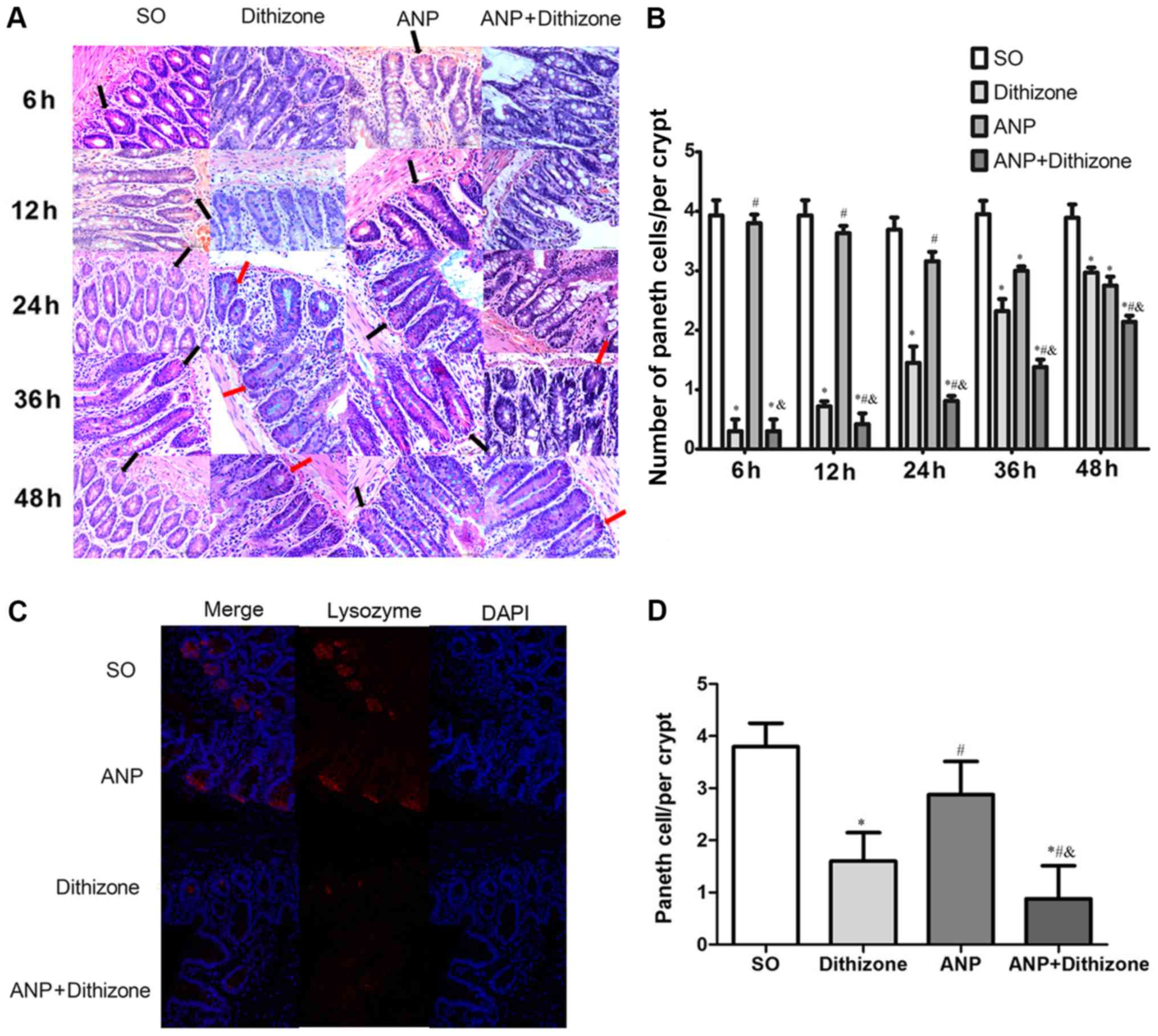
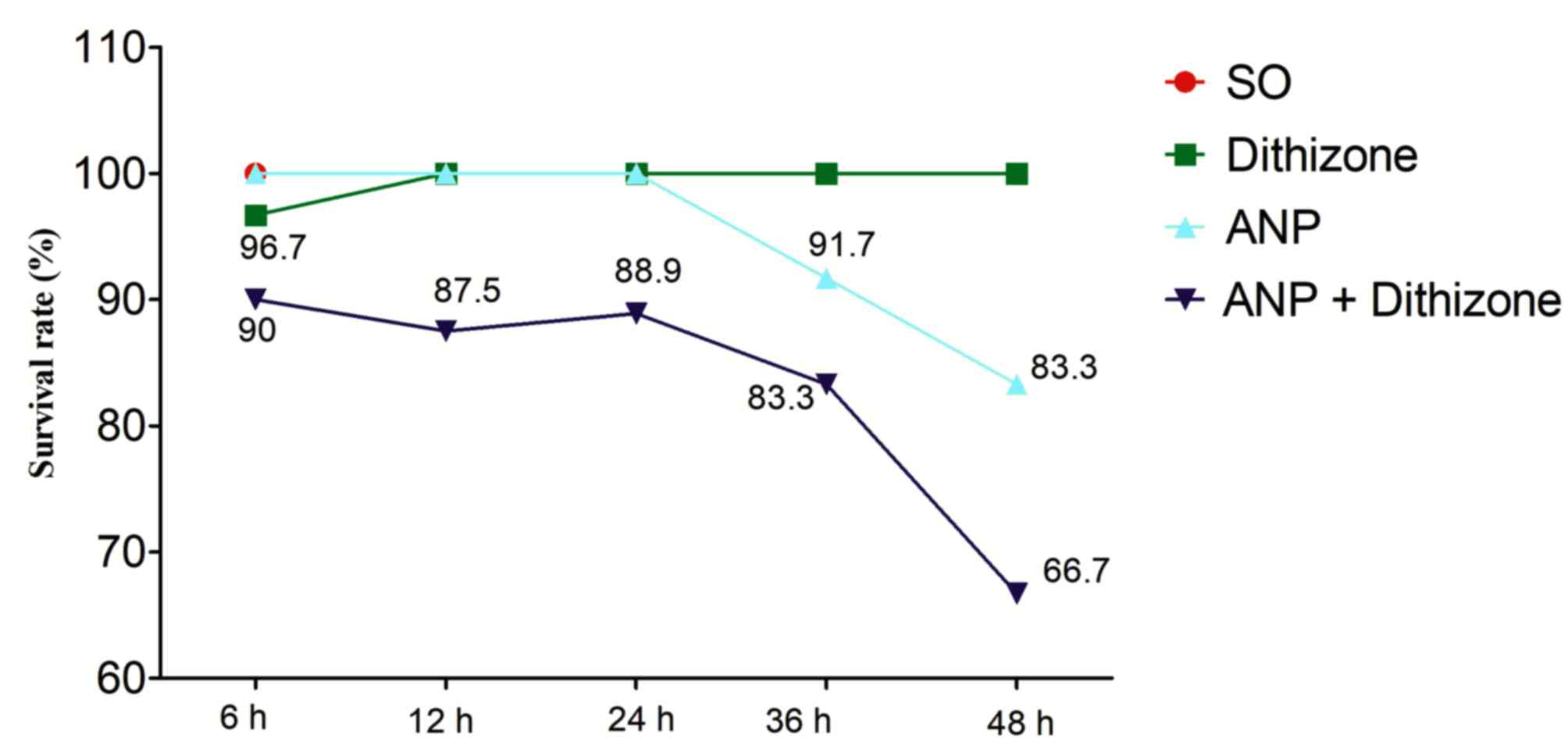
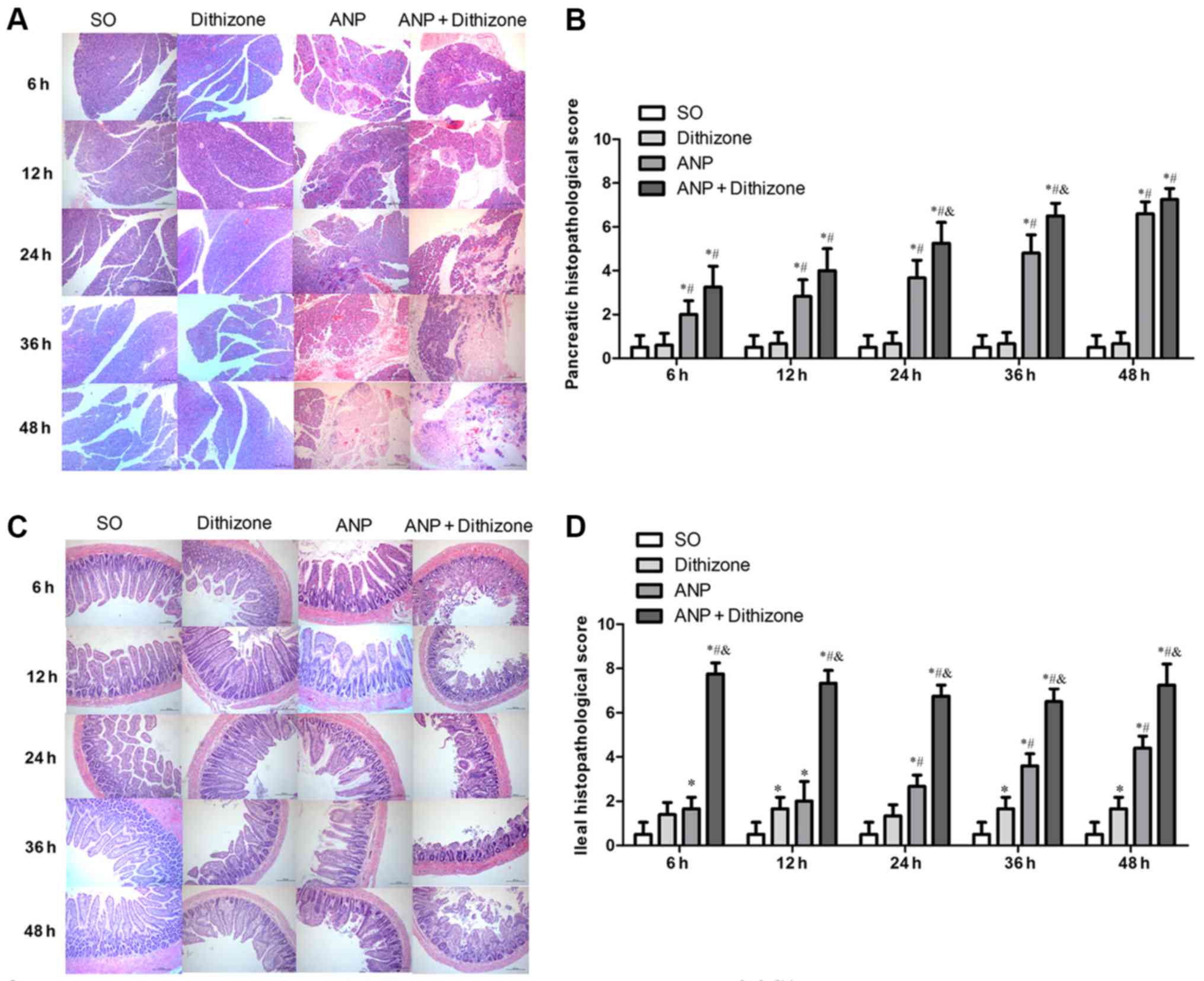
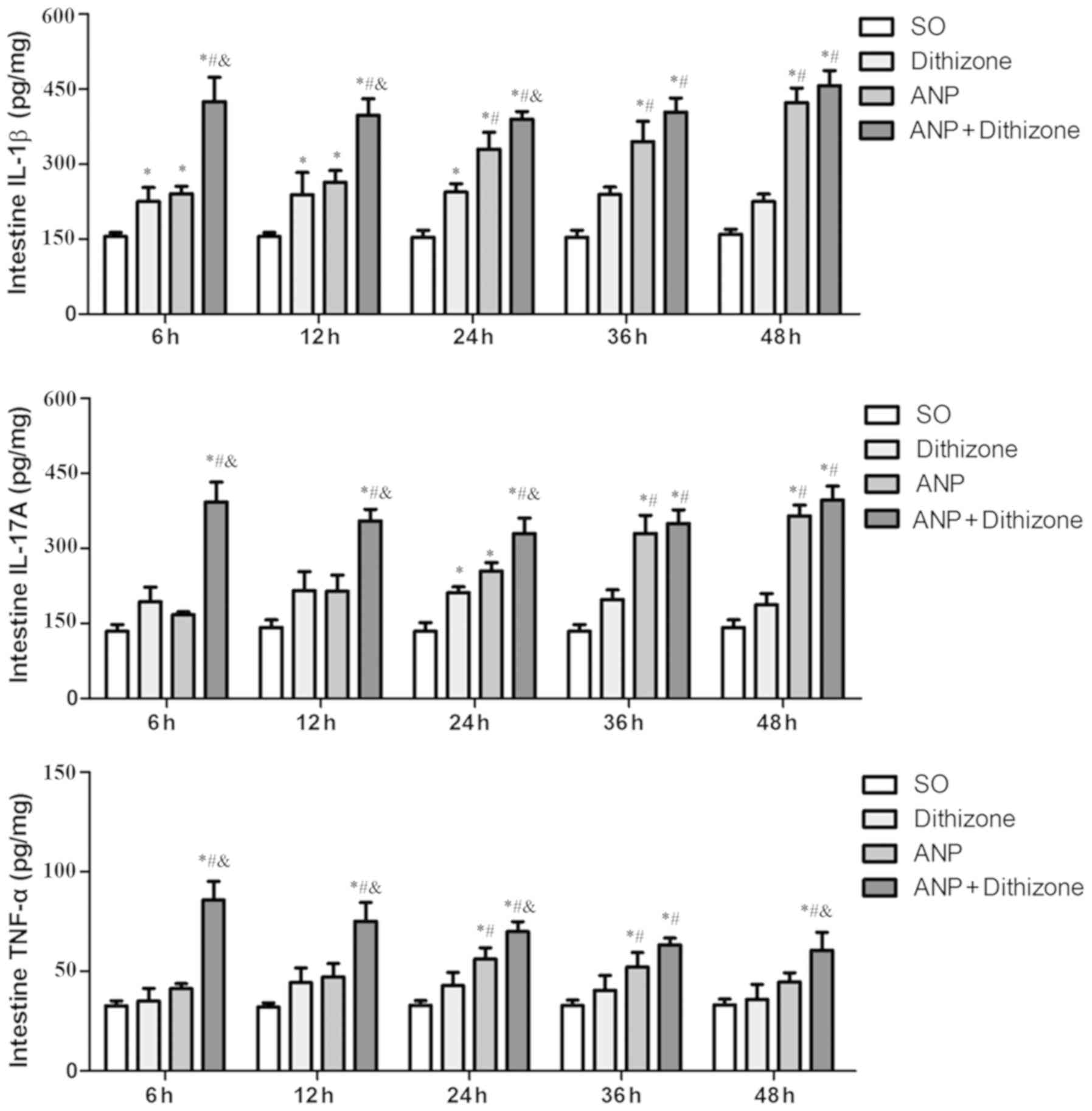
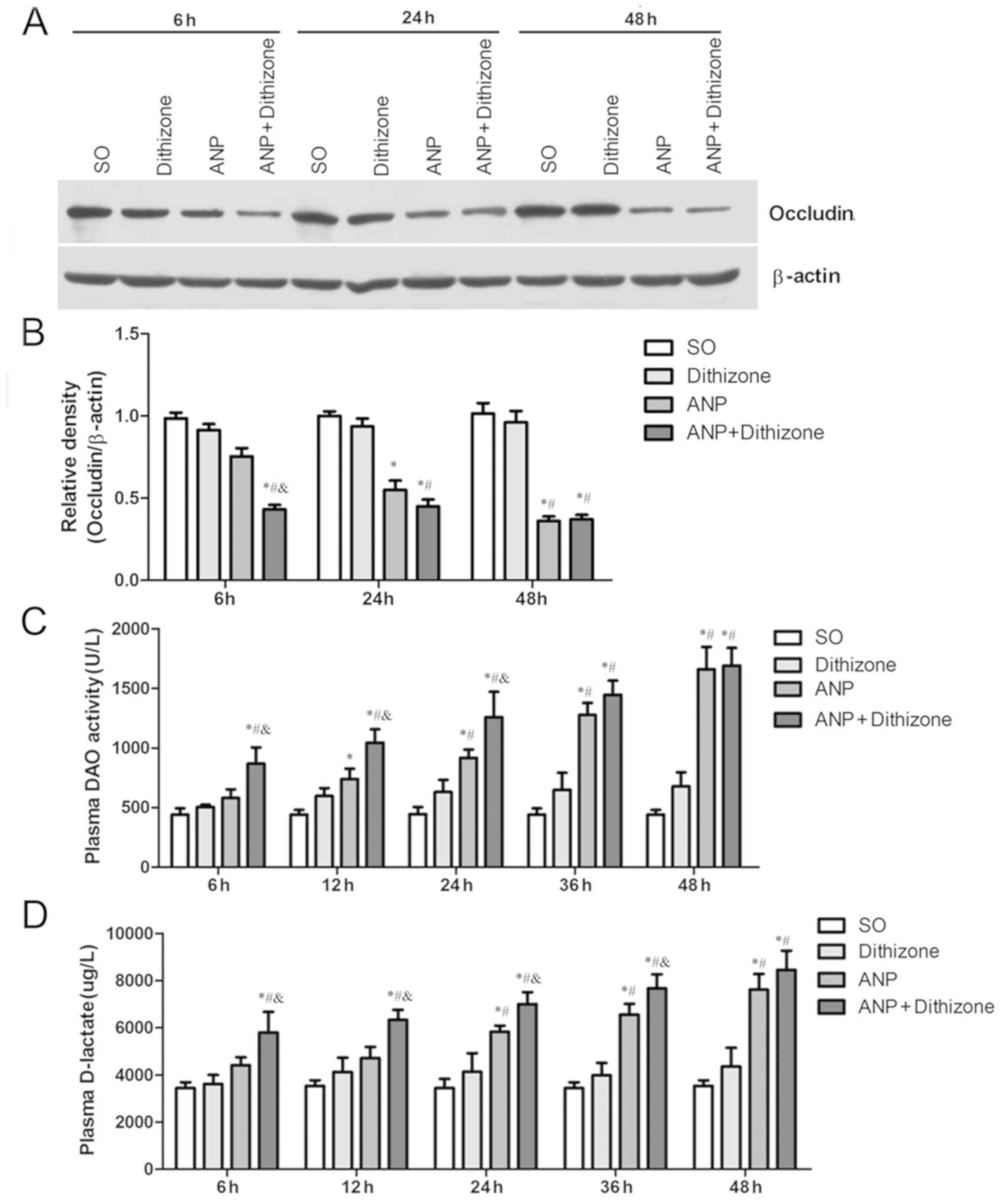
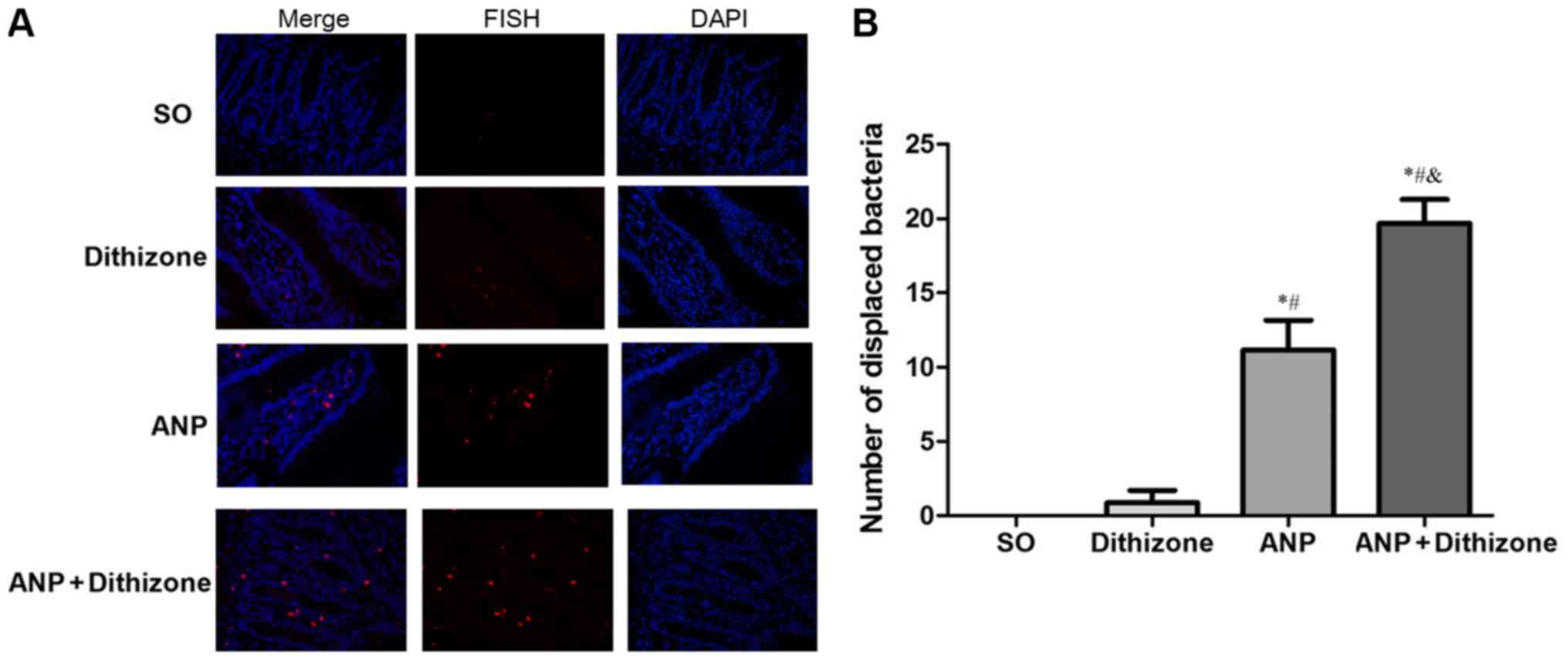
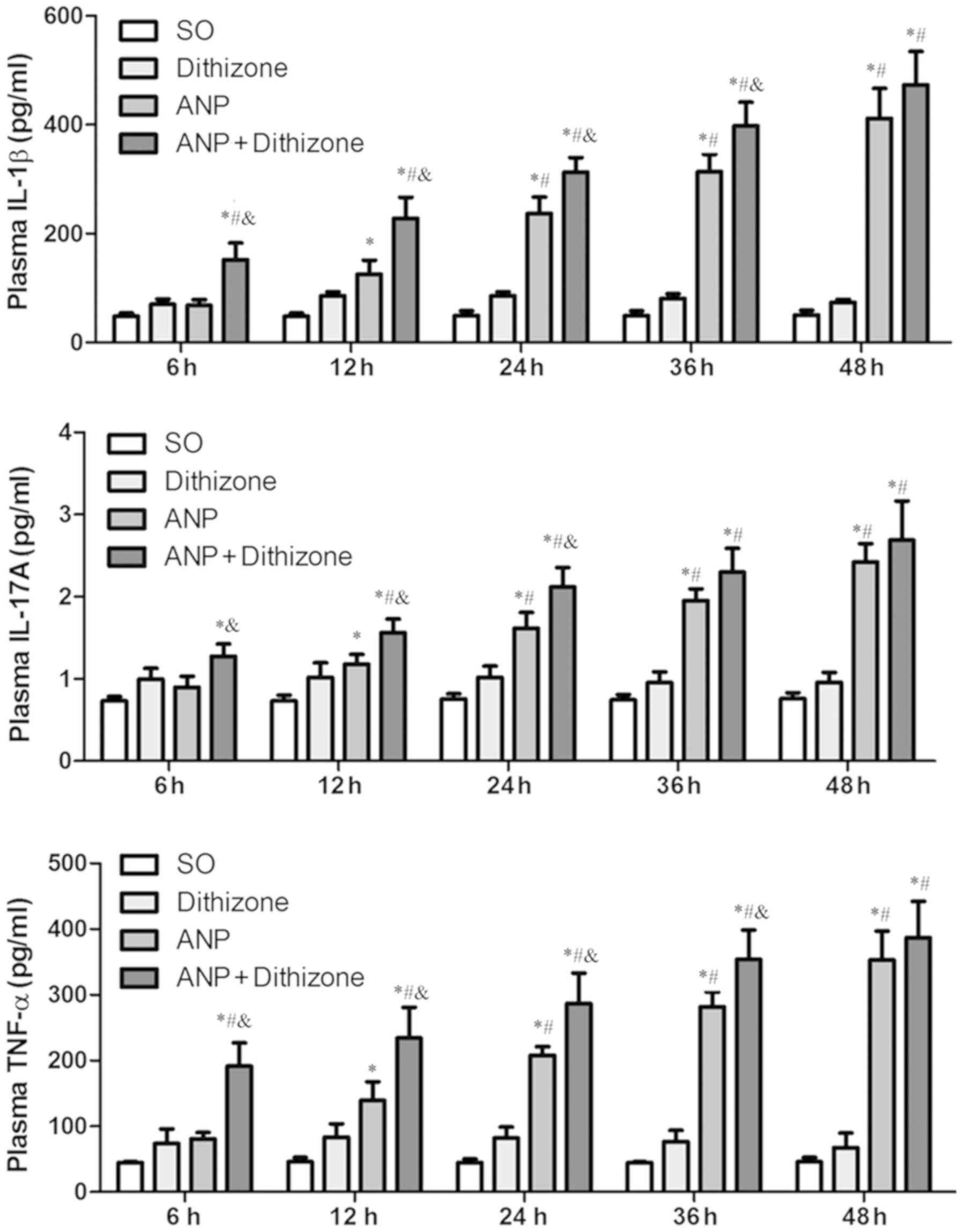
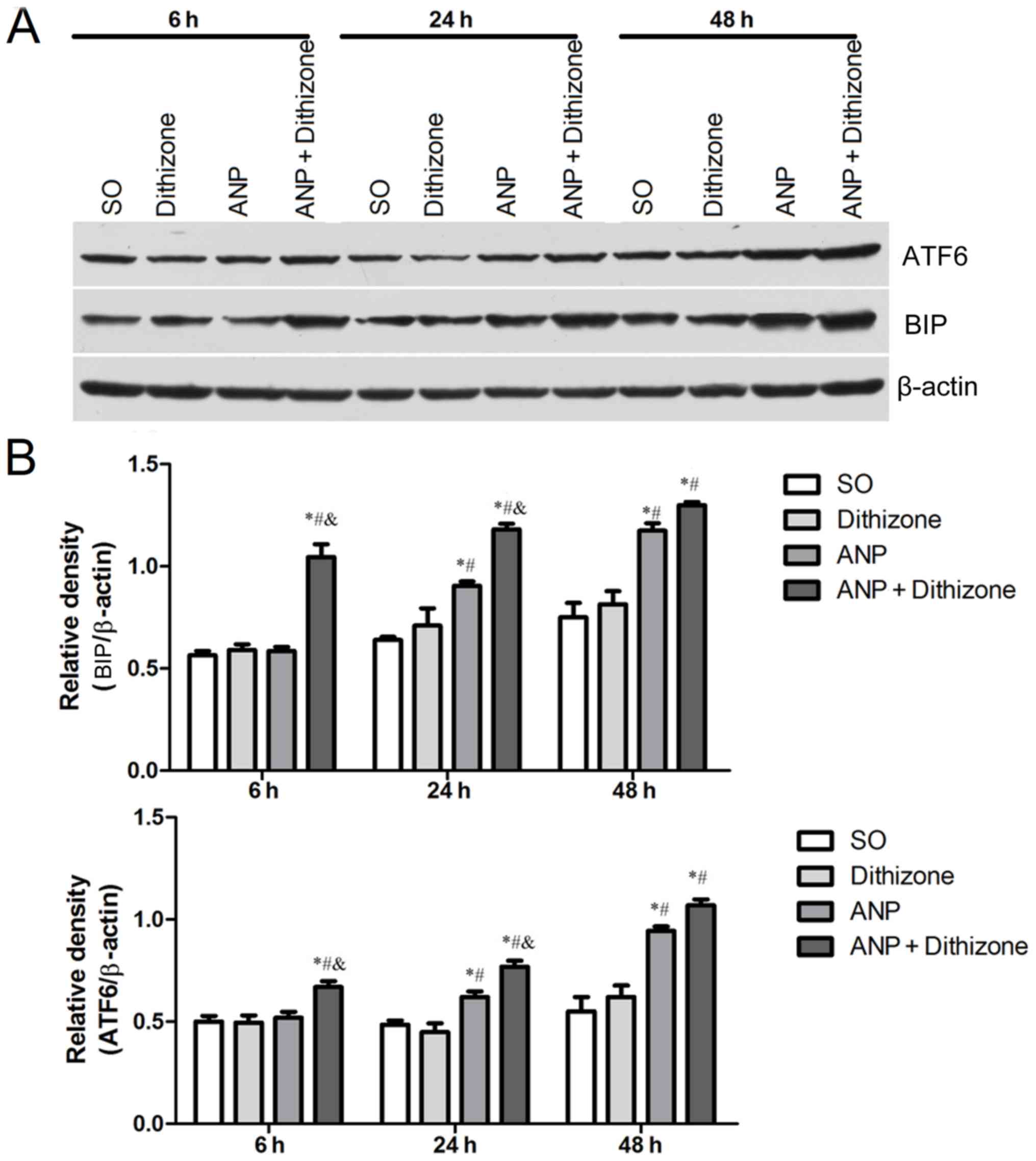
|
1
|
Reid GP, Williams EW, Francis DK and Lee
MG: Acute pancreatitis: A 7 year retrospective cohort study of the
epidemiology, aetiology and outcome from a tertiary hospital in
Jamaica. Ann Med Surg. 20:103–108. 2017. View Article : Google Scholar
|
|
2
|
Roberts SE, Morrisonrees S, John A,
Williams JG, Brown TH and Samuel DG: The incidence and aetiology of
acute pancreatitis across Europe. Pancreatology. 17:155–165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrov MS, Shanbhag S, Chakraborty M,
Phillips AR and Windsor JA: Organ failure and infection of
pancreatic necrosis as determinants of mortality in patients with
acute pancreatitis. Gastroenterology. 139:813–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sternby H, Bolado F, Canaval-Zuleta HJ,
Marra-López C, Hernando-Alonso AI, Del-Val-Antoñana A,
García-Rayado G, Rivera-Irigoin R, Grau-García FJ, Oms L, et al:
Determinants of severity in acute pancreatitis: A nation-wide
multicenter prospective cohort study. Ann Srrg. 18:2018.
|
|
5
|
Ammori BJ: Role of the gut in the course
of severe acute pancreatitis. Pancreas. 26:122–129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Li W, Wang X, Li J and Yu W: Early
gut mucosal dysfunction in patients with acute pancreatitis.
Pancreas. 36:192–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YL, Zheng YJ, Zhang ZP, Su JY, Lei
RQ, Tang YQ and Zhang SD: Effects of gut barrier dysfunction and
NF-kappaB activation on aggravating mechanism of severe acute
pancreatitis. J Dig Dis. 10:30–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasuda T, Takeyama Y, Ueda T, Shinzeki M,
Kishi S, Sawa H, Nakajima T and Kuroda Y: Protective effect of
caspase inhibitor on intestinal integrity in experimental severe
acute pancreatitis. J Surg Res. 138:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda T, Takeyama Y, Ueda T, Shinzeki M,
Sawa H, Nakajima T and Kuroda Y: Breakdown of intestinal mucosa via
accelerated apoptosis increases intestinal permeability in
experimental severe acute pancreatitis. J Surg Res. 135:18–26.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong Y, Cai D, Cai W, Geng S, Chen L and
Han T: Protective effect of galactooligosaccharide-supplemented
enteral nutrition on intestinal barrier function in rats with
severe acute pancreatitis. Clin Nutr. 28:575–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu F, Feng W, Chen Z and Huang H: Effect
of mesenchymal stem cells on small intestinal injury in a rat model
of acute necrotizing pancreatitis. Stem Cell Res Ther. 8:122017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XP, Jie Z, Song QL and Chen HQ:
Mechanism of acute pancreatitis complicated with injury of
intestinal mucosa barrier. J Zhejiang Univ Sci B. 8:888–895. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee C, Minich A, Li B, Miyake H, Seo S and
Pierro A: Influence of stress factors on intestinal epithelial
injury and regeneration. Pediatr Surg Int. 34:155–160. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuda M, Mizutani T, Mochizuki W,
Matsumoto T, Nozaki K, Sakamaki Y, Ichinose S, Okada Y, Tanaka T,
Watanabe M and Nakamura T: Small intestinal stem cell identity is
maintained with functional Paneth cells in heterotopically grafted
epithelium onto the colon. Genes Dev. 28:1752–1757. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato T, van Es JH, Snippert HJ, Stange DE,
Vries RG, van den Born M Barker N, Shroyer NF, van de Wetering M
and Clevers H: Paneth cells constitute the niche for Lgr5 stem
cells in intestinal crypts. Nature. 469:415–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nalapareddy K, Nattamai KJ, Kumar RS,
Karns R, Wikenheiserbrokamp KA, Sampson LL, Mahe MM, Sundaram N,
Yacyshyn MB, Yacyshyn B, et al: Canonical wnt signaling ameliorates
aging of intestinal stem cells. Cell Rep. 18:2608–2621. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodríguez-colman MJ, Schewe M, Meerlo M,
Stigter E, Gerrits J, Prasraves M, Sacchetti A, Hornsveld M, Oost
KC, Snippert HJ, et al: Interplay between metabolic identities in
the intestinal crypt supports stem cell function. Nature.
543:424–427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cazorla SI, Maldonadogaldeano C, Weill R,
De JP and Perdigón G: Oral administration of probiotics increases
paneth cells and intestinal antimicrobial activity. Front
Microbiol. 9:7362018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holly MK and Smith JG: Paneth cells during
viral infection and pathogenesis. Viruses. 10(pii): E2252018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gassler N: Paneth cells in intestinal
physiology and pathophysiology. World J Gastrointest Pathophysiol.
8:150–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Huang C, Wang J, Zhou H, Lu Y, Lou
L, Zheng J, Tian L, Wang X, Cao Z and Zeng Y: Dysbiosis of
intestinal microbiota and decrease in paneth cell antimicrobial
peptide level during acute necrotizing pancreatitis in rats. Plos
One. 12:e01765832017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Chen J, Wang J, Zhou H, Lu Y, Lou
L, Zheng J, Tian L, Wang X, Cao Z and Zeng Y: Dysbiosis of
intestinal microbiota and decreased antimicrobial peptide level in
paneth cells during hypertriglyceridemia-related acute necrotizing
pancreatitis in rats. Front Microbiol. 8:7762017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawada M, Takahashi K, Sawada S and
Midorikawa O: Selective killing of Paneth cells by intravenous
administration of dithizone in rats. Int J Exp Pathol. 72:407–421.
1991.PubMed/NCBI
|
|
24
|
Zhang C, Sherman MP, Prince LS, Bader D,
Weitkamp JH, Slaughter JC and Mcelroy SJ: Paneth cell ablation in
the presence of Klebsiella pneumoniae induces necrotizing
enterocolitis (NEC)-like injury in the small intestine of immature
mice. Dis Model Mech. 5:522–532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XP, Ye Q, Jiang XG, Ma ML, Zhu FB,
Zhang RP and Cheng QH: Preparation method of an ideal model of
multiple organ injury of rat with severe acute pancreatitis. World
J Gastroenterol. 13:4566–4573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiu CJ, Scott HJ and Gurd FN: Intestinal
mucosal lesion in low-flow states. II. The protective effect of
intraluminal glucose as energy substrate. Arch Surg. 101:484–488.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Xia Y, Zhu G, Yan J, Tan C, Deng
B, Deng J, Yin Y and Ren W: Glutamine supplementation improves
intestinal cell proliferation and stem cell differentiation in
weanling mice. Food Nutr Res. 62:2018. View Article : Google Scholar
|
|
29
|
Park SW, Chen SW, Kib M, Brown KM, Kolls
JK, D'Agati VD and Lee TH: Cytokines induce small intestine and
liver injury after renal ischemia or nephrectomy. Lab Invest.
91:63–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinez Rodriguez NR, Eloi MD, Huynh A,
Dominguez T, Lam AH, Carcamo-Molina D, Naser Z, Desharnais R,
Salzman NH and Porter E: Expansion of paneth cell population in
response to enteric Salmonella enterica serovar typhimurium
infection. Infect Immun. 80:266–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef
M and Ma T: Occludin regulates macromolecule flux across the
intestinal epithelial tight junction barrier. Am J Physiol
Gastrointest Liver Physiol. 300:G1054–G1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luk GD, Bayless TM and Baylin SB: Diamine
oxidase (histaminase). A circulating marker for rat intestinal
mucosal maturation and integrity. J Clin Invest. 66:66–70. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray MJ, Barbose JJ and Cobb CF: Serum
D(−)-lactate levels as a predictor of acute intestinal ischemia in
a rat model. J Surg Res. 54:507–509. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohashi W, Kimura S, Iwanaga T, Furusawa Y,
Irié T, Izumi H, Watanabe T, Hijikata A, Hara T, Ohara O, et al:
Zinc transporter SLC39A7/ZIP7 promotes intestinal epithelial
self-renewal by resolving ER stress. Plos Genetics.
12:e10063492016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo K and Cao SS: Endoplasmic reticulum
stress in intestinal epithelial cell function and inflammatory
bowel disease. Gastroenterol Res Pract. 2015:3287912015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grootjans J, Hodin CM, de Haan JJ, Derikx
JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA and
Lenaerts K: Level of activation of the unfolded protein response
correlates with paneth cell apoptosis in human small intestine
exposed to ischemia/reperfusion. Gastroenterology. 140:529–539.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
White JR, Gong H, Pope B, Schlievert P and
Mcelroy SJ: Paneth-cell-disruption-induced necrotizing
enterocolitis in mice requires live bacteria and occurs
independently of TLR4 signaling. Dis Model Mech. 10:727–736. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chotikatum S, Naim HY and El-Najjar N:
Inflammation induced ER stress affects absorptive intestinal
epithelial cells function and integrity. Int Immunopharmacol.
55:336–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang
Y, Tso P, Wu G and Wu Z: Intestinal epithelial cell endoplasmic
reticulum stress and inflammatory bowel disease pathogenesis: An
update review. Front Immunol. 8:12712017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao SS: Endoplasmic reticulum stress and
unfolded protein response in inflammatory bowel disease. Inflamm
Bowel Dis. 21:636–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng J Wu J, Chen J, Liu J, Lu Y, Huang
C, Hu G, Wang X and Zeng Y: Therapeutic effects of quercetin on
early inflammation in hypertriglyceridemia-related acute
pancreatitis and its mechanism. Pancreatology. 16:200–210. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu J, Hu G, Lu Y, Zheng J, Jing C, Wang X
and Yue Z: Palmitic acid aggravates inflammation of pancreatic
acinar cells by enhancing unfolded protein response induced
CCAAT-enhancer-binding protein β–CCAAT-enhancer-binding protein α
activation. Int J Biochem Cell Biol. 79:181–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Riba A, Olier M, Lacroixlamandé S, Lencina
C, Bacquié V, Harkat C, Gillet M, Baron M, Sommer C, Mallet V, et
al: Paneth cell defects induce microbiota dysbiosis in Mice and
promote visceral hypersensitivity. Gastroenterology. 153:1594–1606.
2017. View Article : Google Scholar : PubMed/NCBI
|