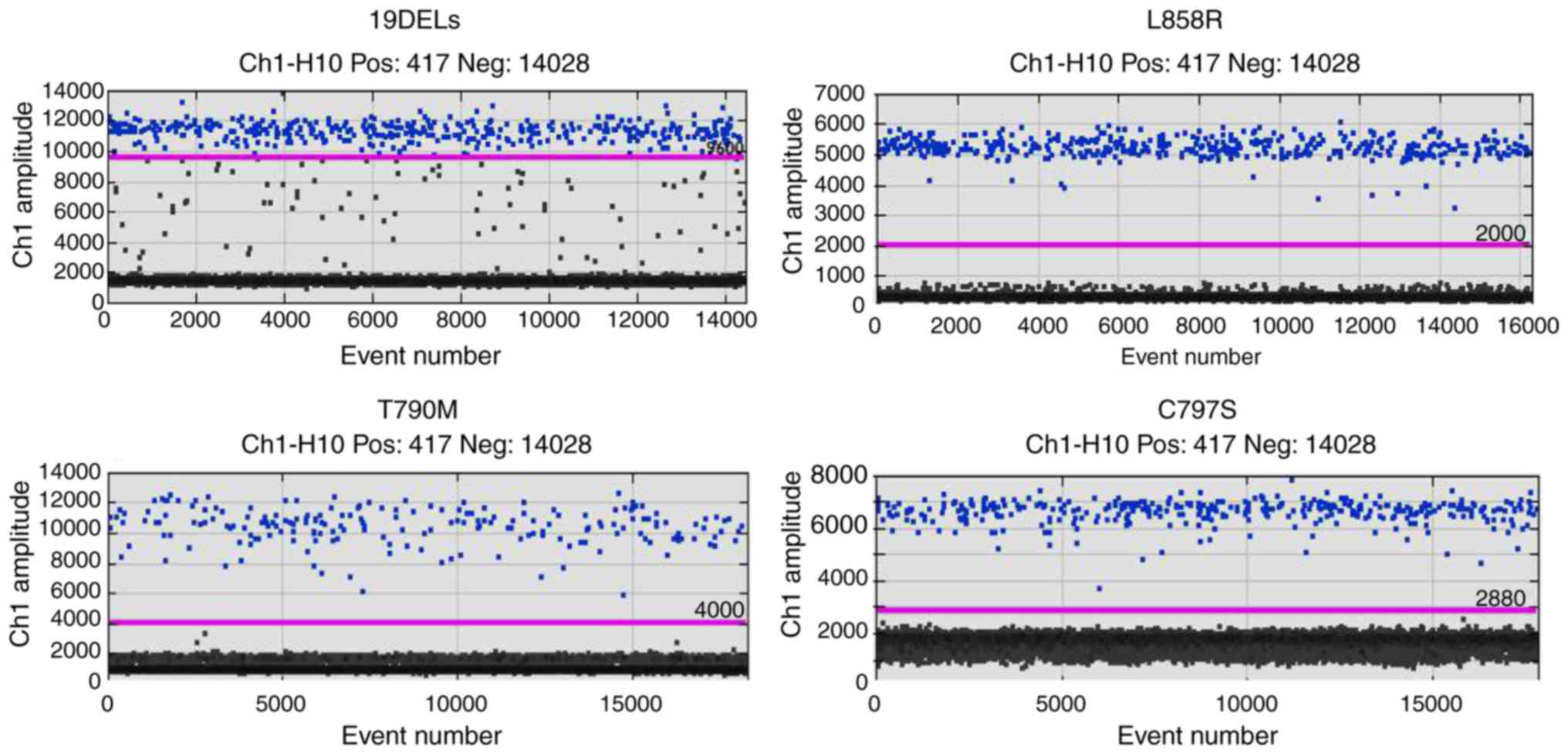
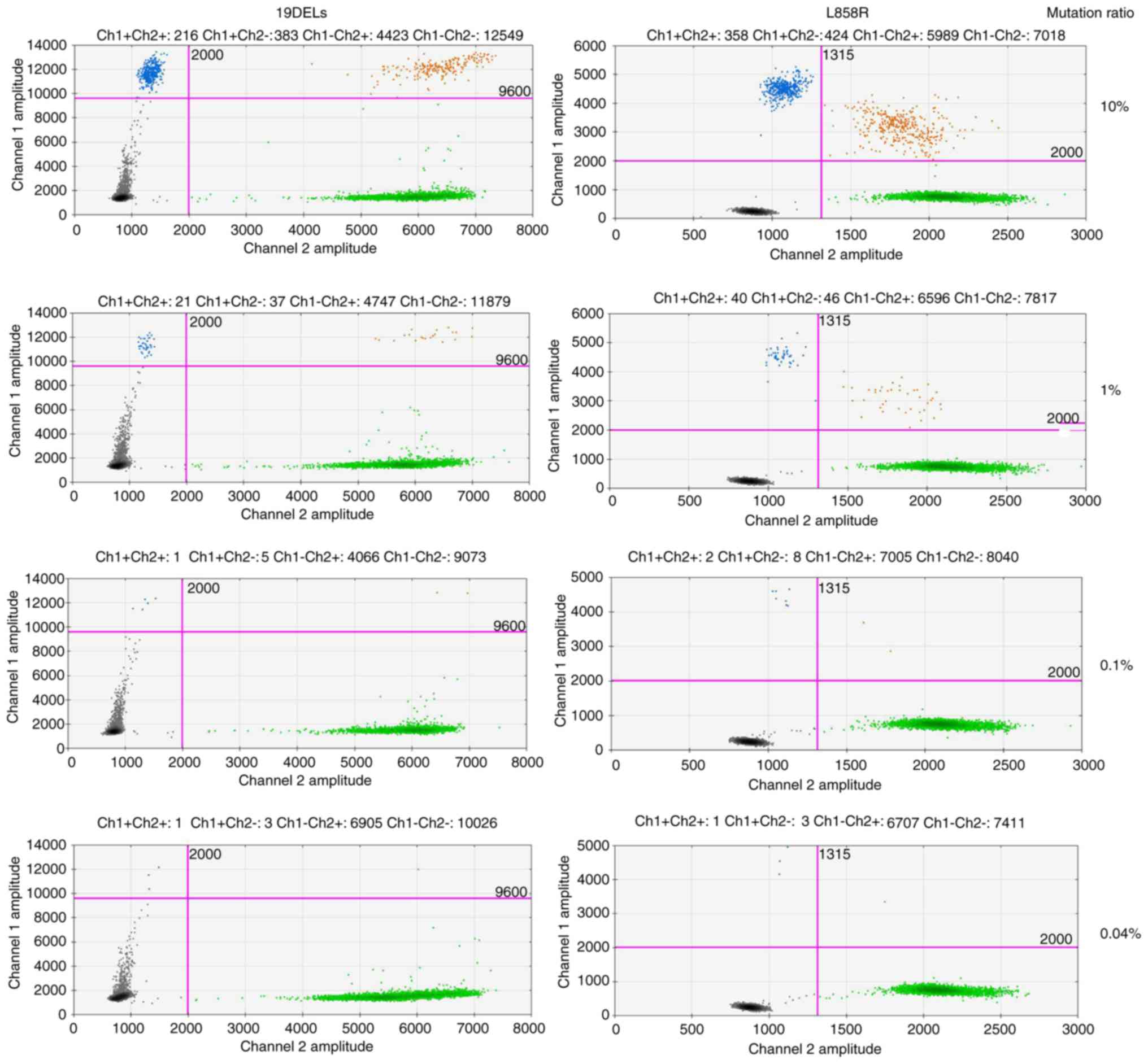
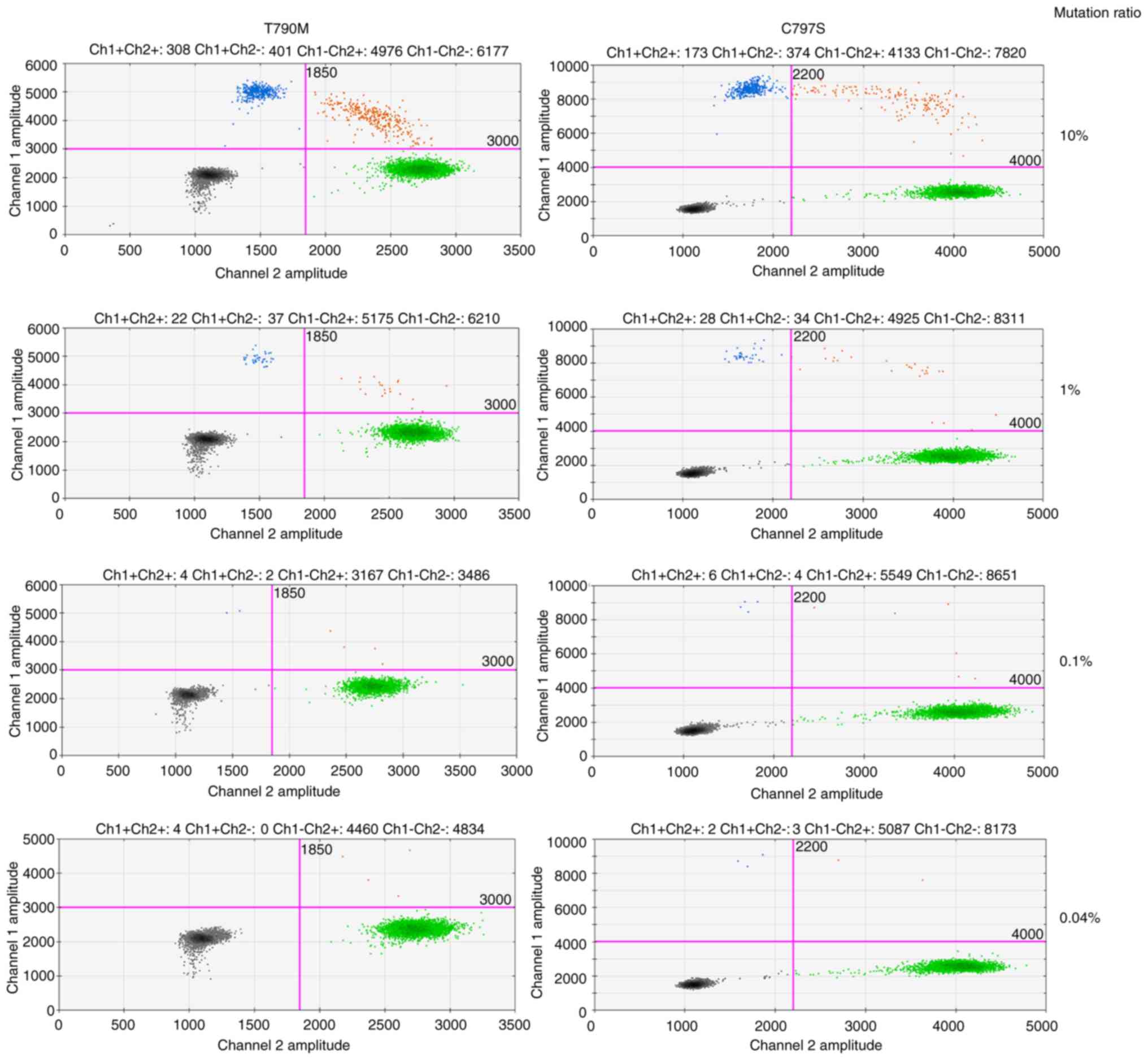
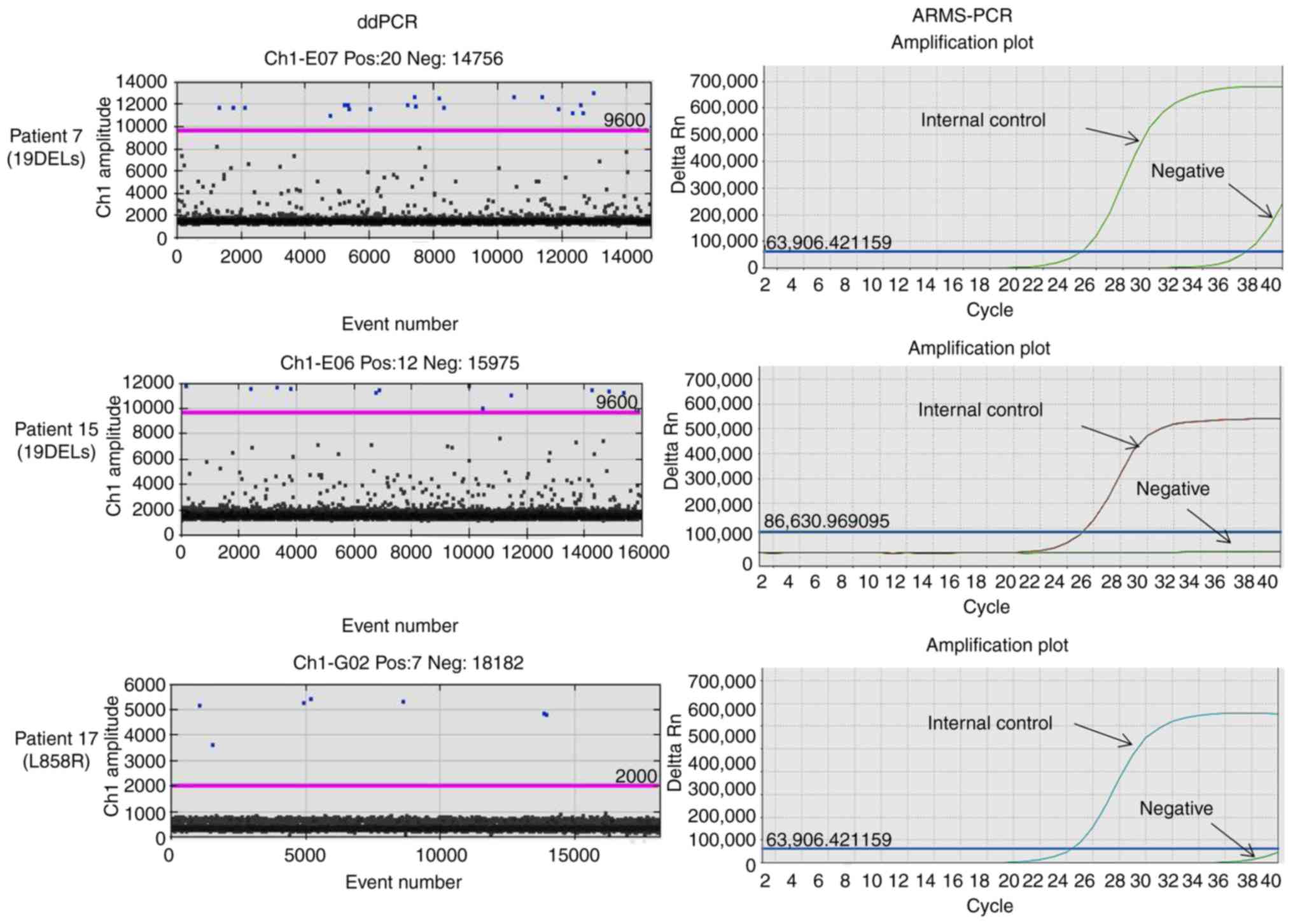
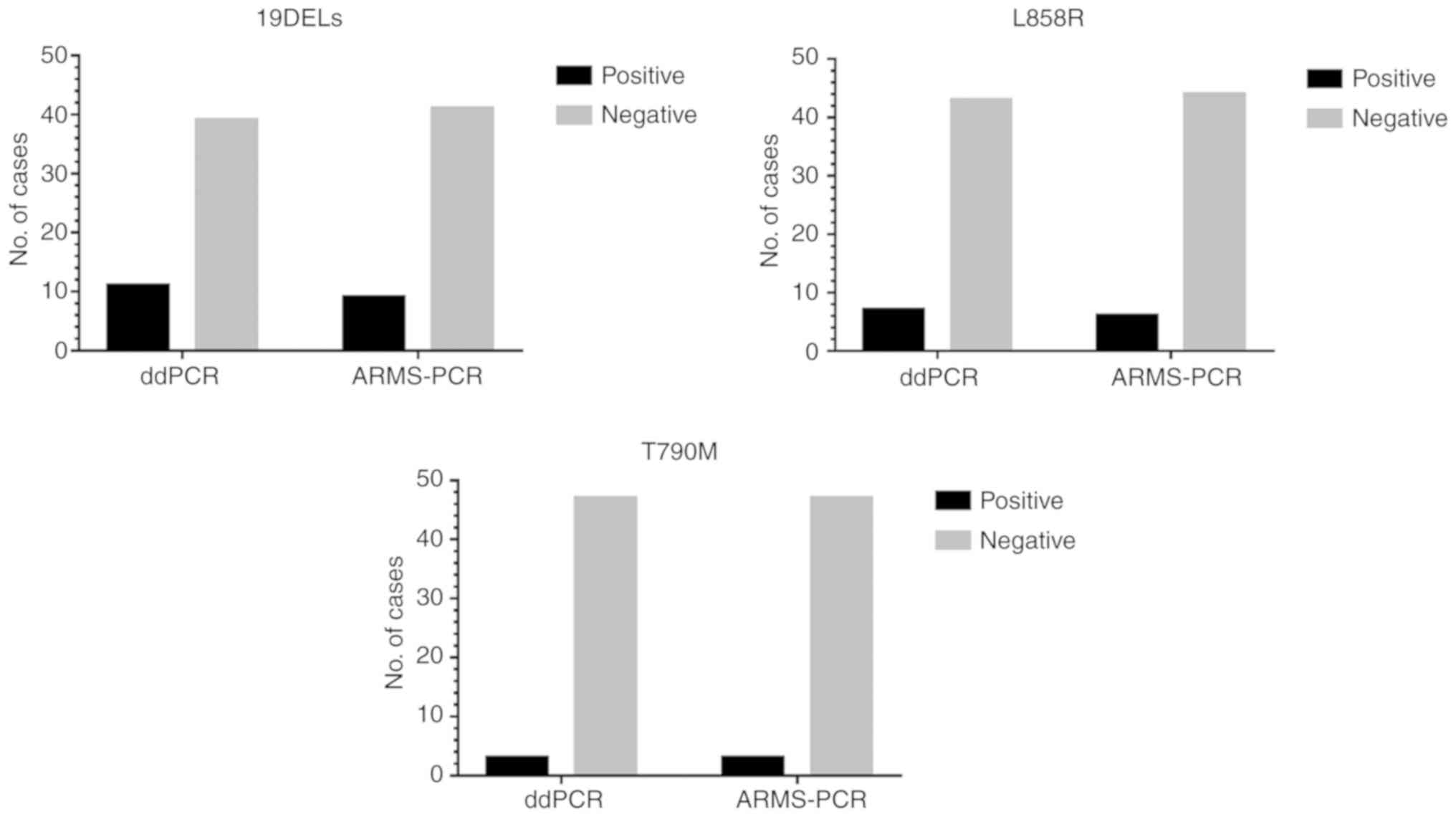
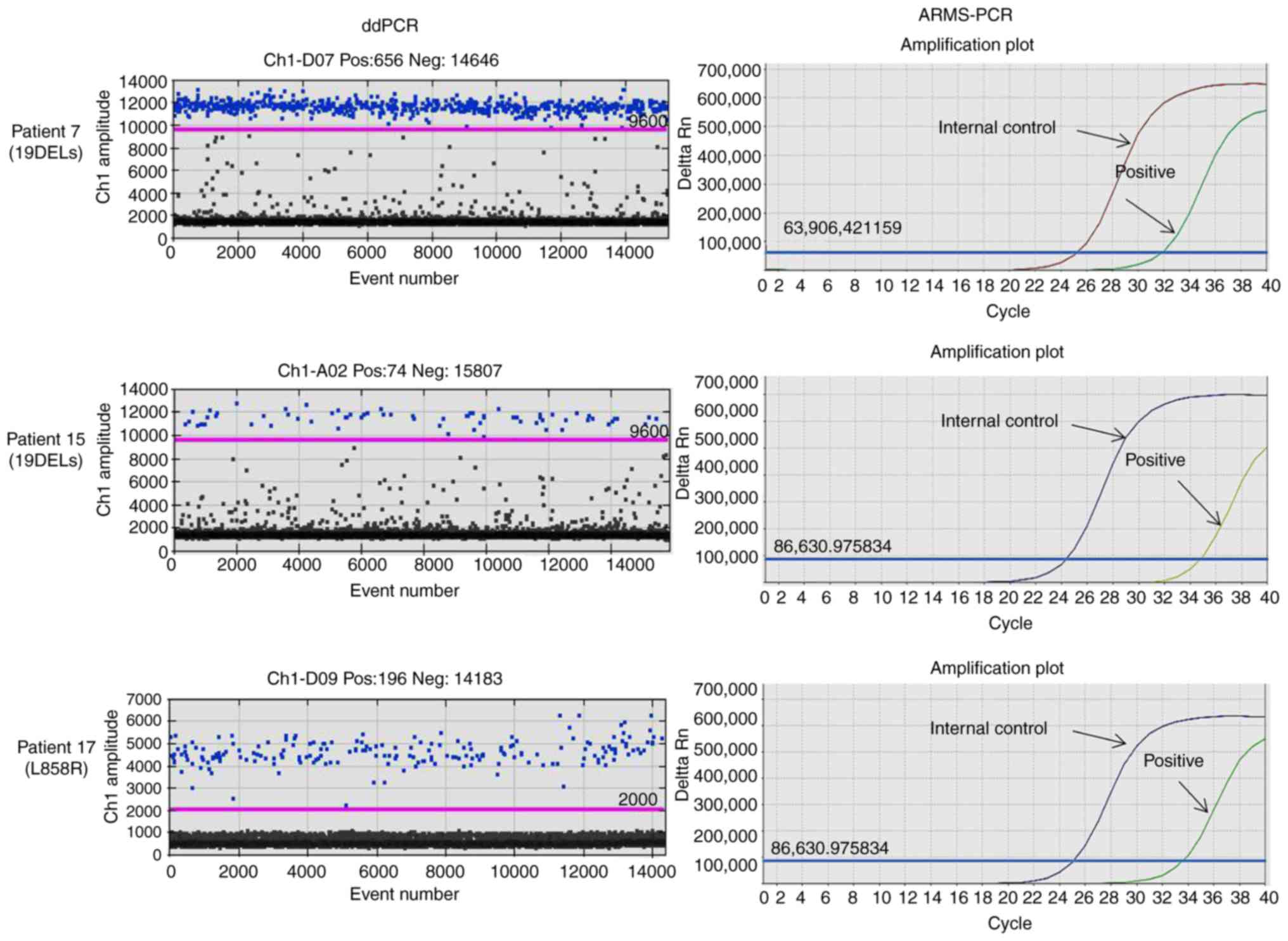
|
1
|
Chen W, Zheng R, Zeng H and Zhang S: The
incidence and mortality of major cancers in China, 2012. Chin J
Cancer. 35:732016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar M, Ernani V and Owonikoko TK:
Biomarkers and targeted systemic therapies in advanced non-small
cell lung cancer. Mol Aspects Med. 45:55–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer Society. Lung cancer
(non-small cell). 2016, . http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdfOctober
27–2016
|
|
4
|
Signorovitch J, Zhou Z, Ryan J, Anhorn R
and Chawla A: Budget impact analysis of comprehensive genomic
profiling in patients with advanced non-small cell lung cancer. J
Med Econ. 22:140–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Wang Q, Han ZG and Shan L:
Differences in epidermal growth factor receptor gene mutations and
relationship with clinicopathological features in NSCLC between
Uygur and Han ethnic groups. Asian Pac J Cancer Prev. 14:2879–2883.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shih AJ, Telesco SE and Radhakrishnan R:
Analysis of somatic mutations in cancer: Molecular mechanisms of
activation in the ErbB family of receptor tyrosine kinases.
Cancers. 3:1195–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawson JP, Berger MB, Lin CC, Schlessinger
J, Lemmon MA and Ferguson KM: Epidermal growth factor receptor
dimerization and activation require ligand-induced conformational
changes in the dimer interface. Mol Cell Biol. 25:7734–7742. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo M and Fu LW: Redundant kinase
activation and resistance of EGFR-tyrosine kinase inhibitors. Am J
Cancer Res. 4:608–628. 2014.PubMed/NCBI
|
|
9
|
Weiss GA, Rossi MR, Khushalani NI, Lo K,
Gibbs JF, Bharthuar A, Cowell JK and Iyer R: Evaluation of
phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and
epidermal growth factor receptor (EGFR) gene mutations in
pancreaticobiliary adenocarcinoma. J Gastroint Oncol. 4:20–29.
2013.
|
|
10
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: Role in clinical
response to EGFR tyrosine kinase inhibitors. Oncogene. 28 (Suppl
1):S24–S31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Normanno N, Luca AD, Bianco C, Strizzi L,
Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma C, Wei S and Song Y: T790M and acquired
resistance of EGFR TKI: A literature review of clinical reports. J
Thorac Dis. 3:10–18. 2011.PubMed/NCBI
|
|
13
|
Köhler J and Schuler M: Afatinib,
erlotinib and gefitinib in the first-line therapy of EGFR
mutation-positive lung adenocarcinoma: A review. Onkologie.
36:510–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih JY, Gow CH and Yang PC: EGFR mutation
conferring primary resistance to gefitinib in non-small-cell lung
cancer. N Engl J Med. 353:207–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:225–235.
2005. View Article : Google Scholar
|
|
16
|
Kosaka T, Yamaki E, Mogi A and Kuwano H:
Mechanisms of resistance to EGFR TKIs and development of a new
generation of drugs in non-small-cell lung cancer. J Biomed
Biotechnol. 2011:1652142011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nat Med.
21:560–562. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Xu X, Kim H, Jin Y, Sun P, Kim JE
and Chung JH: Comparison of direct sequencing, PNA clamping-real
time polymerase chain reaction, and pyrosequencing methods for the
detection of EGFR mutations in non-small cell lung carcinoma and
the correlation with clinical responses to EGFR tyrosine kinase
inhibitor treatment. Korean J Pathol. 47:52–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang T, Zhuge J and Zhang WW: Sensitive
detection of BRAF V600E mutation by amplification refractory
mutation system (ARMS)-PCR. Biomark Res. 1:32013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Liu B, Li XY, Li JJ, Qin HF, Tang
CH, Guo WF, Hu HX, Li S, Chen CJ, et al: A comparison of ARMS and
direct sequencing for EGFR mutation analysis and tyrosine kinase
inhibitors treatment prediction in body fluid samples of
non-small-cell lung cancer patients. J Exp Clin Cancer Res.
30:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hindson BJ, Ness KD, Masquelier DA,
Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY,
Hiddessen AL, Legler TC, et al: High-throughput droplet digital PCR
system for absolute quantitation of DNA copy number. Anal Chem.
83:8604–8610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JD, Yang HI, Iloeje UH, You SL, Lu
SN, Wang LY, Su J, Sun CA, Liaw YF and Chen CJ; Risk Evaluation of
Viral Load Elevation and Associated Liver Disease/Cancer in HBV
(REVEAL-HBV) Study Group, : Carriers of inactive hepatitis B virus
are still at risk for hepatocellular carcinoma and liver-related
death. Gastroenterology. 138:1747–1754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morisset D, Štebih D, Milavec M, Gruden K
and Žel J: Quantitative analysis of food and feed samples with
droplet digital PCR. PLoS One. 8:e625832013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miotke L, Lau BT, Rumma RT and Ji HP: High
sensitivity detection and quantitation of DNA copy number and
single nucleotide variants with single color droplet digital PCR.
Anal Chem. 86:2618–2624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beck J, Bierau S, Balzer S, Andag R,
Kanzow P, Schmitz J, Gaedcke J, Moerer O, Slotta JE, Walson P, et
al: Digital droplet PCR for rapid quantification of donor DNA in
the circulation of transplant recipients as a potential universal
biomarker of graft injury. Clin Chem. 59:1732–1741. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heredia NJ, Belgrader P, Wang S, Koehler
R, Regan J, Cosman AM, Saxonov S, Hindson B, Tanner SC, Brown AS
and Karlin-Neumann G: Droplet Digital™ PCR quantitation of HER2
expression in FFPE breast cancer samples. Methods. 59:S20–S23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Xu Y, Zhong W, Zhao J, Chen M,
Zhang L, Li L and Wang M: Total DNA input is a crucial determinant
of the sensitivity of plasma cell-free DNA EGFR mutation detection
using droplet digital PCR. Oncotarget. 8:5861–5873. 2017.PubMed/NCBI
|
|
30
|
Ahn MJ, Lee JY, Lim SH, Lee MY, Kim H, Kim
HK, Byeon S, Ham JS, Yali B, Xiumin W, et al: Dynamic serial
monitoring of EGFR mutations in plasma DNA samples in EGFR mutant
NSCLC patients treated with EGFR TKI. J Clin Oncol. 33:80782015.
View Article : Google Scholar
|
|
31
|
Watanabe M, Kawaguchi T, Isa S, Ando M,
Tamiya A, Kubo A, Saka H, Takeo S, Adachi H, Tagawa T, et al:
Ultra-Sensitive detection of the pretreatment EGFR T790M mutation
in non-small cell lung cancer patients with an EGFR-activating
mutation using droplet digital PCR. Clin Cancer Res. 21:3552–3560.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo SL, Chen XZ, Xiao YZ, Zhu SQ, Gong YQ
and Yang JP: Use of the duplex TaqMan MGB probe for simultaneous
detection of Perkinsus and Bonamia in marine shellfish. J Appl
Oceanography. 33:284–289. 2014.
|
|
33
|
Chang S, Chen W and Yang J: Another
formula for calculating the gene change rate in real-time RT-PCR.
Mol Biol Rep. 36:2165–2168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grabe T, Lategahn J and Rauh D: C797S
Resistance: The undruggable EGFR mutation in non-small cell lung
cancer? ACS Med Chem Lett. 9:779–782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao C, Yuan JQ, Yang ZY, Fu XH, Wu XY and
Tang JL: Blood as a substitute for tumor tissue in detecting EGFR
mutations for guiding EGFR TKIs treatment of nonsmall cell lung
cancer: A systematic review and meta-analysis. Medicine
(Baltimore). 94:e7752015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gonzalez de Castro D, Angulo B, Gomez B,
Mair D, Martinez R, Suarez-Gauthier A, Shieh F, Velez M, Brophy VH,
Lawrence HJ and Lopez-Rios F: A comparison of three methods for
detecting KRAS mutations in formalin-fixed colorectal cancer
specimens. Br J Cancer. 107:345–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SS, Choi HJ, Kim JJ, Kim MS, Lee IS,
Byun B, Jia L, Oh MR, Moon Y, Park S, et al: Droplet digital
PCR-based EGFR mutation detection with an internal quality control
index to determine the quality of DNA. Sci Rep. 8:5432018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Y, Guo Z, Liu Y, Zheng X, Yang G and
Zheng G: A novel ARMS-based assay for the quantification of EGFR
mutations in patients with lung adenocarcinoma. Oncol Lett.
15:2905–2912. 2018.PubMed/NCBI
|