Introduction
Type III Bartter syndrome (Type III BS; Online
Mendelian Inheritance in Man no. 607364), also termed classic
Bartter syndrome, is a rare autosomal recessive disorder induced by
pathogenic sequence variants in the chloride voltage_gated channel
Kb (CLCNKB) gene (1) that
affect salt reabsorption in the thick ascending loop of Henle. BS
was first reported in 1962 (2).
The prevalence of the disorder in Costa Rica, Kuwait and Sweden was
estimated to be 12 (3), 17
(4) and 1.2 individuals per
1,000,000 (5), respectively. The
prevalence of the disorder in the Chinese population is
unknown.
The CLCNKB gene encodes chloride channel KB
(ClC-KB), a member of the ClC chloride channel family that serves
as a component of ClC-K/Barttin ClC-K-type accessory β subunit
(BSND) heteromers and is involved in sodium reabsorption (6). Defects in the ClC-KB protein results
in the suppression of sodium reabsorption,
renin-angiotensin-aldosterone system (RAAS) dysfunction and
aldosterone secretion (1,7). The clinical manifestations of type
III BS are heterogeneous, including developmental retardation,
polyuria, polydipsia, cramps, dehydration, constipation, renal
dysfunction and in certain cases, sudden death (8); however, the mechanisms underlying the
phenotypic heterogeneity of the disorder remain unknown.
Plasma biomarkers and genetic mutations are used in
the diagnosis of type III BS. In addition, RAA levels in blood are
a biochemical diagnosis method (1,7).
Genetic testing has revealed ~100 sequence variants in the
CLCNKB gene (http://www.hgmd.cf.ac.uk/ac/index.php). p.A204T was
described as the founder mutation of Type III BS in Spain (9). A previous study observed that the
deletion of the CLCNKB gene is a frequent pathogenic
variation in the Chinese population (10).
Type III BS is the most common among the Bartter
syndrome group of kidney disorders globally; however, only a small
number of cases have been reported in mainland China (10), as prenatal diagnosis is not common
in the country. The present study characterized the clinical,
biochemical and mutation spectra of five Chinese patients with type
III BS.
Materials and methods
Patients
A total of 5 patients aged 8 months to 24 years (2
male children, 1 female child and 2 adult males) from 5 unrelated
Chinese families were firstly diagnosed with type III BS at the
Department of Pediatrics, Peking University First Hospital
(Beijing, China), and the Department of Nephrology, General
Hospital of Tianjin Medical University (Tianjin, China) between
January 2011 and October 2017. The clinical symptoms of type III BS
developed between the ages of 3 months and 5 years. The patients
were admitted with presentations of polydipsia, polyuria, low
weight, myasthenia, cramps and fatigue (Table I). The parents of the patients were
all healthy and nonconsanguineous. The mother of Patient 2 was
subsequently admitted at 20 weeks of pregnancy seeking genetic
counseling and prenatal screening for type III BS. Additionally, a
total of 100 male and 100 female patients were recruited between
December 2014 and January 2016 in The Peking University First
Hospital and General Hospital of Tianjin Medical University, were
recruited as normal controls for insulin-like growth factor-1
(IGF-1) analysis; the individuals were divided into four age groups
(1–3, 8–10, 18–21 and 22–24 years). The study was conducted in
accordance with the Code of Ethics of the World Medical Association
and the Declaration of Helsinki (11). The present study was approved by
the Institutional Review Board of the General Hospital of Tianjin
Medical University, Institute of Hematology and Blood Diseases
Hospital, and Beijing University First Hospital. Informed consent
for biochemical and genetic analysis was obtained from all patients
or parents of patients.
 | Table I.Clinical and laboratory data of five
Chinese patients with type III Bartter syndrome. |
Table I.
Clinical and laboratory data of five
Chinese patients with type III Bartter syndrome.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Normal range |
|---|
| Gender | Male | Female | Male | Male | Male |
|
| Age of onset | 3 m | 5 m | 6 m | 5 y | 3 y |
|
| Age of
diagnosis | 8 m | 3 y | 8 y | 24 y | 18 y |
|
| Present age | 3 y | 9 y 11 m | 9 y 9 m | 24 y | 19 y |
|
| Symptoms and
signs |
|
|
|
|
|
|
| Polydipsia | − | + | + | + | + |
|
| Polyuria | − | + | + | + | + |
|
| Myasthenia | + | − | − | − |
|
|
| Developmental
retardation | + | + | + | + | + |
|
| Cramps | − | + | − | + | − |
|
| Fatigue | − | − | − | + | + |
|
| Orthostatic
hypotension | − | − | − | + | + |
|
| Positive family
history | − | − | − | − | − |
|
| Laboratory
findings |
|
|
|
|
|
|
| pH (blood gas
analysis) | 7.647↑ | 7.48↑ | 7.75↑ | 7.55↑ | 7.71↑ | 7.34–7.45 |
| Serum
Cl− (mmol/l) | 89↓ | 93↓ | 98 | 96↓ | 85↓ | 98–106 |
| Serum
Na+ (mmol/l) | 132 ↓ | 142 | 139 | 125↓ | 137 | 135–145 |
| Serum K+
(mmol/l) | 1.76↓ | 2.3↓ | 2.7↓ | 2.4↓ | 2.5↓ | 3.5–5.5 |
| Serum
Mg2+ (mmol/l) | 0.87 | 0.65↓ | 0.97 | 0.89 | 0.77↓ | 0.8–1.2 |
| CO2
combining power (mmol/l) | 33.8↑ | 43.1↑ | 29.3↑ | 26.6 | 32.1↑ | 22–28 |
| Renin [ng/ml/h
(recumbent)] | 6.57↑ | 2.53↑ | 4.31↑ | 1.48↑ | 3.65↑ | 0.05–0.79 |
| Aldosterone [ng/l
(recumbent)] | 204.3↑ | 104.6 | 165.43↑ | 135.69 | 216.5↑ | 12.0–157.5 |
| GH peak (ng/ml) of
GH | 11.40 | 2.14↓ | 5.21↓ | 10.37 | 13.84 | >10 ng/ml
in |
| excitation
test | (60 min) | (90 min) | (60 min) | (60 min) | (60 min) | children |
|
|
|
|
|
|
| >5 ng/ml in |
|
|
|
|
|
|
| adults |
| IGF-1 (ng/ml) | 24-51↓ | 27↓ | 19↓ | 74↓ | 143 | 78–242 (1–3
y); |
|
|
|
|
|
|
| 87–353 (8–10
y); |
|
|
|
|
|
|
| 122–379 (18–21
y); |
|
|
|
|
|
|
| 105–342 (22–24
y) |
| Outcome | Healthy | Healthy | Low | Hypovolemia, | Low |
|
|
|
|
| weight | low weight | weight |
|
Routine tests and evaluation of IGF-1
levels
A total of 4 ml peripheral venous blood was
centrifuged for 5 min at 1,000 × g and 20°C, and laboratory tests
for liver and renal function, electrolytes, glucose, ammonia,
creatine kinase (UniCel DxC600; Beckman Coulter, Inc.) were
performed. In total, 2 ml peripheral venous blood was centrifuged
for 5 min at 1,000 × g and 4°C, and renin and aldosterone (LIAISON
XL; DiaSorin, Inc.) levels were measured. Then, 2 ml arterial blood
was collected from the radial artery, and blood gas (Cobas b 123;
Roche Diagnostics) test was performed according to the
manufacturer's protocol. Additionally, 2 ml peripheral venous blood
from each patient and healthy controls was centrifuged for 15 min
at 1,800 × g and 4°C, and immediately stored at −80°C. Levels of
IGF-1 were measured using an ELISA kit (cat. no. DG100; R&D
Systems, Inc.). Moreover, 1.5 ml peripheral venous blood was
collected at 0, 30, 60, 90 and 120 min after GH excitation for the
GH excitation test [L-dopa stimulation test was performed in
children, and insulin tolerance test (ITT) in adults]. The samples
were centrifuged for 10 min at 1,000 × g and 4°C, GH levels were
measured using a DXI800 (Beckman Coulter, Inc.) and the GH peak was
calculated.
Prenatal diagnosis
The mother of Patient 2 opted to undergo prenatal
diagnosis of type III BS. This was the second pregnancy of the
31-year-old female. Amniocentesis was performed at 20 weeks
gestational age. Amniotic fluid was collected by transabdominal
amniocentesis under ultrasound guidance. The amniotic fluid was
immediately analyzed.
CLCNKB gene analysis
Genomic DNA was extracted from peripheral blood
lymphocytes from patients, their parents, and amniotic fluid cells
using the TIANamp blood DNA kit (Tiangen Biotech Co., Ltd.,
Beijing, China). Whole exome sequencing was performed using
SureSelectXT Human All Exon V6 (Agilent Technologies, Inc., Santa
Clara, CA, USA); exons and flanking intronic regions of the
variants detected by whole exome sequencing were amplified via PCR
and then sequenced, as previously described (12). The results were compared with the
reference sequence of CLCNKB (NM_000085) deposited in the
UCSC genome (genome.ucsc.edu/). Sequencing data
were compared with an integrated set of variants (hgmd.cf.ac.uk), genotypes and haplotypes from the
1,000 Genomes Project (1000genomes.org) to identify mutations.
Prediction of the effects of the
sequence variants in the CLCNKB gene and conservation analysis
Numerous sequence alignments were performed to
verify the degree of sequence conservation of p.L656 of the ClC-KB
protein. The PolyPhen-2 (Polymorphism Phenotyping; version 2;
genetics.bwh.harvard.edu/pph/) and
MutationTaster (MutationTaster; version 2; mutationtaster.org/) programs were used to predict the
effects of missense alterations on protein function. Similarly,
numerous sequence alignments were obtained using the Basic Local
Alignment Search Tool (BLAST+ version 2.7.1; release date, October
23, 2017; blast.ncbi.nlm.nih.gov/Blast.cgi).
Single-nucleotide polymorphism (SNP)
array analysis
Microdeletion screening was performed using an SNP
array platform (Affymetrix GCS 3000Dx v.2; Affymetrix; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with a GeneChip
(Affymetrix CytoScan HD/750k; Affymetrix; Thermo Fisher Scientific,
Inc.). Array data were then analyzed using the GenomeStudio version
2010.1, KaryoStudio version 1.2 (standard settings; Illumina, Inc.,
San Diego, CA, USA) and Nexus Copy Number 5.0 (BioDiscovery, Inc.,
El Segundo, CA, USA) (13).
Results
Clinical data and laboratory
examinations
Table I presents
the clinical data of patients and the results of the laboratory
assays. With the exception of Patient 1, all patients were born at
term. All patients were from nonconsanguineous families. The
pediatric patients were admitted for polydipsia, polyuria and
developmental retardation, whereas the adult patients first
presented with fatigue, polydipsia and polyuria, and subsequently
exhibited cramps. Following diagnosis of type III BS, the patients
were treated with potassium supplements and indometacin. When
gastrorrhagia was detected in Patient 4, indometacin treatment was
suspended. The GH excitation test results identified two patients
with GH deficiency and partial GH deficiency, whereas the others
were normal (in children, GH response peak <5 ng/ml indicated GH
deficiency; 5–10 ng/ml indicated partial GH deficiency; >10
ng/ml indicated normal GH levels. In adults, GH response peak <5
ng/ml indicated GH deficiency; >5 ng/ml indicated normal GH
levels. The patients with defects in GH levels received recombinant
human somatropin injection treatment. The development and symptoms
of type III BS improved in all patients following treatment;
however, low weight was observed in Patients 3, 4 and 5, and
postural hypotension and reduced exercise tolerance were observed
in the adult patients.
Prolonged hypokalemia was detected in Patient 1
following the development of a severe cold. The patient, a male
aged 3 years, had been delivered prematurely, and thus, presented
with extremely low weight and a history of hypokalemia from the age
of 3 months. He came to our hospital when he was 8 months old, his
height (65.5 cm) and body weight (6.5 kg) were below average upon
presentation; however, chronic diseases or a family history of
sudden death were not reported. Biochemical analysis of the serum
of the patient revealed low levels of chloride, sodium, potassium
and IGF-1 (24 ng/ml), elevated blood gas pH, and high levels of
CO2 combining power, renin and aldosterone. GH
excitation test was not performed because of the age and the
conditions of the patient. The patient, who was diagnosed with type
III BS at the age of 8 months, returned to normal development
following potassium and indometacin treatment. However, when he was
3 years old, decreased IGF-1 (51 ng/ml) with normal GH excitation
test were found.
Patients 2 and 3, a female aged 9 years and 11
months, and a male aged 9 years and 9 months, presented with a
similar medical history. They were admitted to the hospital with
growth retardation, Patient 2 at 3 years of age and Patient 3 at 8
years. Polydipsia and polyuria were initially detected, followed by
electrolytic metabolic disturbance and RAAS dysfunction. Delayed
bone age and low levels of IGF-1 were detected in the two patients.
Patient 2 showed partial GH deficiency and Patient 3 exhibited GH
deficiency. Patient 2 and 3 received an injection of human
recombinant GH, potassium supplementation and indometacin
treatment. The two patients subsequently returned to school;
however, Patient 3 continued to exhibit low weight (23 kg), whereas
Patient 2 met the weight criteria for normal development.
Patients 4 and 5, 24- and 19-year-old males,
respectively, were admitted separately but shared a similar medical
history. Initial medical complaints included fatigue, low weight,
polydipsia, polyuria, discontinuous cramps and reduced exercise
tolerance. Polydipsia and polyuria were first observed in Patient 4
at the age of 13 years, whereas low weight and muscle weakness were
first detected in Patient 5 at the age of 7 years. Postural
hypotension was identified in the two patients following admission
at 24 and 18 years of age, respectively. Patient 4 was 164 cm tall
and weighed 47 kg, whereas Patient 5 was 174 cm tall and weighed 57
kg. Results from the GH excitation test (insulin tolerance test) of
both patients were normal. Patient 4 underwent plasma transfusion
monthly for hypovolemia. After 3 months of treatment, the two
patients experienced relief from polydipsia, polyuria and
discontinuous cramps; however, gastrorrhagia was detected in
Patient 4, following which indometacin treatment was suspended.
Laboratory examinations
All five patients exhibited markedly reduced levels
of serum potassium and chloride, increased levels of renin and
metabolic alkalosis compared with healthy controls (Table I). Additionally, increased levels
of aldosterone, and decreased serum levels of magnesium and sodium
were observed in certain patients. Compared with the healthy
controls, notably reduced IGF-1 levels were observed in 4 patients
(Patient 1–4), while Patient 1, 4 and 5 had normal serum levels of
GH (Table I).
Molecular analysis
In addition to eight previously reported variants
identified in the patients (c.1967T>C, c.595G>T, c.908A>C,
c.1004T>C, c.1312C>T, c.1334_1335delCT, c.1718C>A and
whole CLCNKB gene mutation), the novel sequence variant
c.1967T>C (p.L656P) was found (Table II). Genetic variants were also
detected in the parents of patients. The whole gene deletion
regions were 1p36.13 (16,367,268-16,383,421) del and 1p36.13
(16,367,268-16,382,989 (Fig. 1).
Previous studies usually described whole gene deletions without
listing the specific regions that were deleted; thus, it remains
unclear whether the observed deletions have been reported prior to
the present study. Sanger sequencing was performed to analyze the
c.1967T>C mutation, and a ‘false positive’ homozygous variant
(Fig. S1) was identified, as it
was confirmed to be heterozygous by SNP array test. This mutation
was not detected in the 1,000 Genomes Project database.
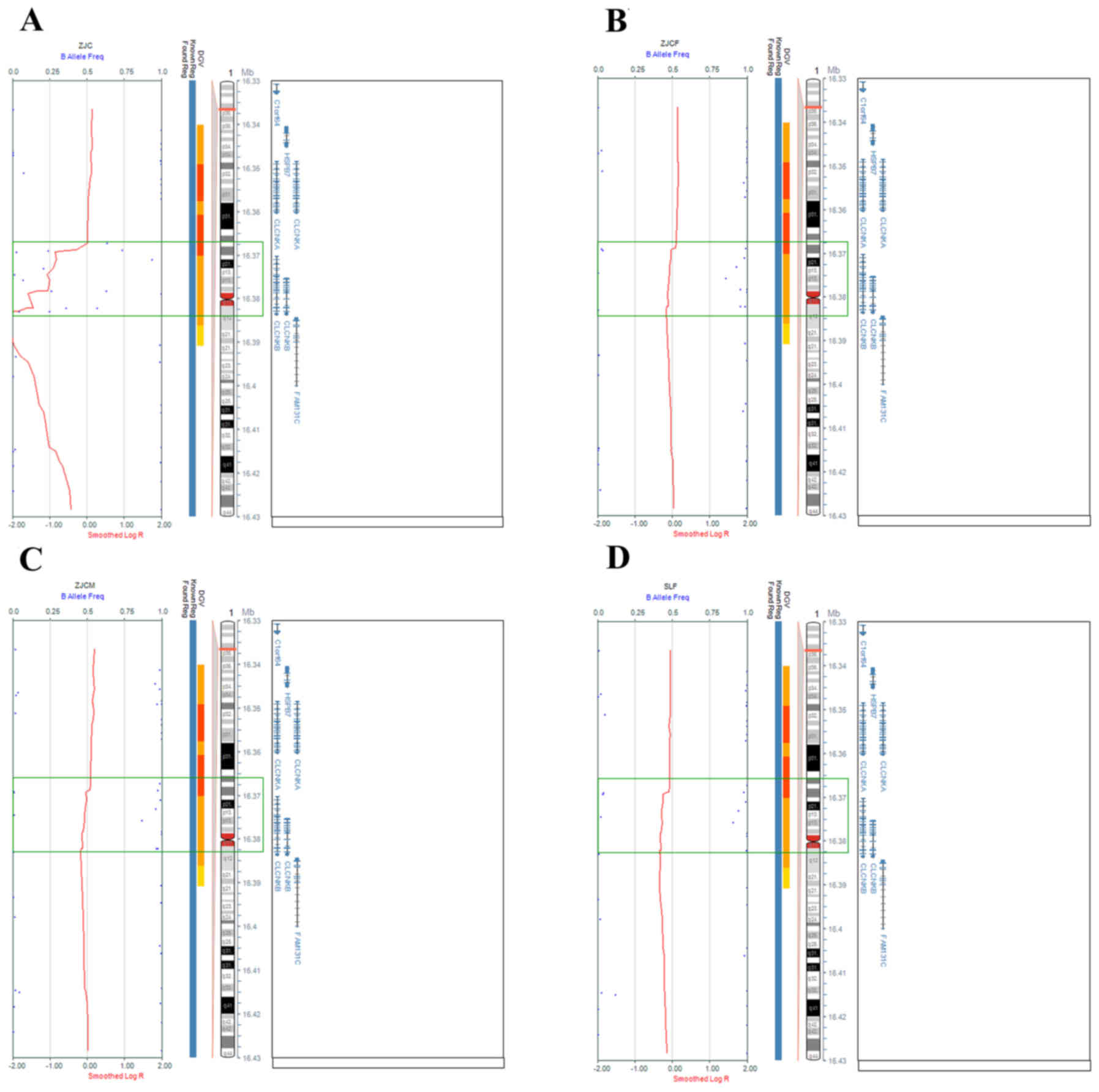 | Figure 1.SNP array analysis of Family 1 and
Patient 2. Scattered blue points regions and green rectangles
indicate deleted regions. Homozygous and heterozygous 16.15 kb
microdeletions (16,367,268-16,383,421 in chromosome 1p36.13) were
detected in (A) Patient 1, and the (B) father and (C) mother,
comprising the whole CLCNKB gene. (D) Heterozygous 15.72 kb
microdeletion (from 16,367,268-16,382,989 in chromosome 1p36.13)
detected in Patient 2, comprising the whole CLCNKB gene. |
 | Table II.Mutations detected in the
CLCNKB gene among five Chinese patients with type III
Bartter syndrome. |
Table II.
Mutations detected in the
CLCNKB gene among five Chinese patients with type III
Bartter syndrome.
| Patient | Mutation at
nucleotide level | Mutation type | Mutation at protein
level | PolyPhen-2.0
prediction and score | MutationTaster
prediction and score | Conservation | Frequency | (Refs.) |
|---|
| 1 | 1p36.13
16,367,268-16, 383,421 deletion | Homozygous | N/A | N/A | N/A | N/A | 2/10 | The present
study |
| 2 | 1p36.13
16,367,268-16, 382,989 deletion | Heterozygous | N/A | N/A | N/A | N/A | 1/10 | The present
study |
|
| c.1967T>C | Heterozygous | p.L656P | Probably damaging
(0.99) | Disease causing
(0.99) | Conserved in
mammals | 1/10 | The present
study |
| 3 | 1334_1335delCT | Heterozygous | S445Ffs*5 | N/A | Disease Causing
(0.99) | Yes | 1/10 | (30) |
|
| 595G>T | Heterozygous | E199Ter | N/A | Disease Causing
(0.99) | Yes | 1/10 | (31) |
| 4 | 1312C>T | Heterozygous | R438C | Probably damaging
(0.999) | Disease causing
(0.99) | Yes | 1/10 | (32) |
|
| 908A>C | Heterozygous | Q303P | Probably damaging
(0.987) | Disease causing
(0.99) | Conserved in
mammals | 1/10 | (33) |
| 5 | 1004T>C | Heterozygous | L335P | Probably damaging
(0.999) | Disease causing
(0.99) | Conserved in
mammals | 1/10 | (34) |
|
| 1718C>A | Heterozygous | S573Y | Probably damaging
(0.999) | Disease causing
(0.99) | Conserved in
mammals | 1/10 | (35) |
Sequence alignment of the CLCNKB gene
revealed that the L656 amino acid is highly conserved in humans and
other mammals (Table III),
including Pan troglodytes, Mus musculus, Takifugu rubripes
and Danio rerio, but not in Drosophila melanogaster
and Xenopus tropicalis. The mutation detected in the present
study was predicted to be ‘probably damaging’ and ‘disease
causing’, according to PolyPhen-2.0 and MutationTaster programs,
respectively.
 | Table III.Amino acid alignment of p.L656
residue of CLC-KB. |
Table III.
Amino acid alignment of p.L656
residue of CLC-KB.
| Organisms | ClC-KB
sequences |
|---|
| Homo
sapiens |
LLNLHSLFVTS |
| Pan
troglodytes |
LLNLHSLFVTS |
| Mus
musculus |
LLTLQALFVTS |
| Takifugu
rubripes |
LVGAKTLFVAD |
| Danio
rerio | ITGEQRLF I T
E |
| Drosophila
melanogaster |
MVGINHAFYVT |
| Xenopus
tropicalis |
LLGLNRAYVTK |
Prenatal diagnosis
Only one heterozygous deletion was detected from
amniocytes harvested from the mother of patient 2, indicating that
the fetus was not affected by type III BS, due to the fact that the
fetus did not share the same mutation of the proband. Normal serum
electrolytic and RAAS activity level analyses were performed
following delivery. At 9 months of age, the infant exhibited normal
psychomotor development.
Discussion
In the present study, five cases of type III BS were
reported in patients from mainland China, including symptoms,
clinical findings, treatment and response. As only a small number
of cases of type III BS have been reported in this population
(10), the present findings may
aid the evaluation of future cases in the Chinese population.
Furthermore, two patients (Patient 1 and 4) with type III BS
exhibited decreased serum levels of IGF-1 and normal serum GH
levels. A novel mutation c.1967T>C and whole gene deletion were
reported in patients with early-onset type III BS in the present
study.
The CLCNKB gene encodes the voltage-gated
chloride channel ClC-KB, which is located on chromosome lp36
(14). ClC-KB, ClC-KA and Barttin
are involved in chloride absorption, and are primarily expressed in
the renal thick ascending limb, distal convoluted tubules,
connecting tubules and collecting tubes. Enhanced distal convoluted
tubule sodium chloride reabsorption eventually leads to the loss of
potassium in urine and increased hydrogen ion secretion (1). Additionally, the exchange of
bicarbonate for chloride ions decreases the chloride absorption
defect; therefore, bicarbonate retention and hypokalemia leads to
metabolic alkalosis (1).
Renal tubular salt wasting results in the loss of
water from the body. Physiologically, salt absorption in the loop
of Henle results in the generation of concentrated urine.
Therefore, urinary concentration and dilution defects occur in
patients with type III BS (15).
Defective sodium chloride reabsorption leads to an abnormal
electrochemical gradient, which leads to additional calcium and
magnesium reabsorption defects (1). Furthermore, hypokalemia and elevated
levels of prostaglandin E2 (PGE2) aggravate the condition, and
eventually lead to polydipsia, polyuria and hypovolemia, and
low-to-normal blood pressure under the activated RAAS (16). The decrease in blood volume
activates the RAAS and subsequently induces hyperaldosteronism,
secondary juxtaglomerular apparatus hyperplasia and increases in
the levels of renin (16).
Aldosterone accelerates injury via hemodynamic alterations,
cytokine expression and renal fibrosis promotion (17).
Patients with type III BS are typically asymptomatic
or mildly symptomatic for 2 years after birth (1), which is why certain patients were not
diagnosed until late adolescence or early adulthood. The disease
presents with highly variable phenotypes, including polyuria,
polydipsia, nocturia, halophilic tendencies, dehydration, vomiting,
constipation and muscle weakness (1,5).
Electrolyte and RAAS disorders are frequently reported, including
hypokalemic metabolic alkalosis, normal or slightly increased
urinary calcium with decreased serum magnesium, high PGE2
production, and increased renin and aldosterone with normal blood
pressure (1). Similarly,
nephrocalcinosis is frequently observed in patients with BS due to
excessive loss of calcium in urine. The patients included in the
present study were admitted due to hypokalemia, metabolic alkalosis
and secondary hyperaldosteronism. The majority of the patients
exhibited good prognosis; however, Patient 4 had certain abnormal
phenotypes, including low weight, hypovolemia and orthostatic
hypotension.
IGF-1, which is synthesized by the liver, is an
important regulator of the proliferation, differentiation and
apoptosis of cells. It serves an important role in physiological
development and the pathology of various diseases and disorders,
including neuroinflammation (18),
heart failure (19) and
hepatocellular carcinoma (20). To
the best of our knowledge, although combined GH and IGF-1
deficiency were found in type III BS patients, which may be
associated to hypokalemia (21),
decreased serum IGF-1 with normal GH levels in type III BS have not
been previously reported. Flyvbjerg et al (22) revealed that low potassium-induced
reductions in serum IGF-1 levels and growth defects in young rats.
Furthermore, angiotensin II has been hypothesized to activate
nicotinamide adenine dinucleotide phosphate oxidase expression and
promote reactive oxygen species-induced downregulation of IGF-1
(23). Additionally, Zaika et
al (24) reported that IGF-1
stimulated epithelial sodium channel-mediated sodium transport in
principal cells and ClC-K2 channels in intercalated cells, thus
facilitating cooperative sodium and chloride reabsorption in the
cortical collecting duct. IGF-1 has been observed to be an
effective treatment for muscle weakness in a mouse model of spinal
and bulbar muscular atrophy (25).
A deficiency in IGF-1 may contribute to muscle weakness and
developmental delay in type III BS patients. Therefore, it was
hypothesized that low serum potassium content and high levels of
angiotensin II were involved in growth retardation and decreased
reabsorption function via reductions in the serum levels of
IGF-1.
It has been reported that CLCNKB gene
deletion is frequently observed in Chinese patients with type III
BS (~32%) (10). However, genetic
testing results are frequently misinterpreted by Sanger sequencing
or next-generation sequencing due to undetectable deletions. In the
present study, the homozygous c.1967T>C mutation was first
observed in Patient 2 and SNP array analysis subsequently revealed
a heterozygous 15.721-kb deletion, suggesting that the homozygous
mutation (c.1967T>C) identified was a false positive. The
deleted regions comprised a region between one point shared among
all patients and different termination positions, causing the
deletion of chr1 16,367,268-16,383,421 in Patient 1, and chr1
16,367,268-16,382,989 in Patient 2.
The CLCNKB gene deletion appeared to induce
the greatest effect on early-onset patients in the present study;
however, the symptoms did not notably vary from patients possessing
missense mutations. In addition, whole-gene deletions have been
reported in adult-onset patients (26); therefore, the genotype-phenotype
association in Chinese patients remains unclear. Patients in an
African American cohort possessing a homozygous CLCNKB gene
deletion exhibited partial correction of hypokalemia and suboptimal
growth even following therapy (27). CLCNKB mutations located in
Barttin-binding sites, dimer interfaces and selectivity filters
frequently induce severe functional consequences, as ClC-KB and
ClC-KA require interactions with BSND to function optimally
(28).
Patients with type III BS require lifelong potassium
supplementation. Cyclo-oxygenase inhibitors, such as indomethacin
(12), are also frequently
prescribed to suppress plasma renin activity. Early diagnosis and
treatment are important for the improvement of hypokalemia,
hypercalciuria, developmental retardation and renal dysfunction. It
was previously reported that, even with treatment, certain patients
with type III BS exhibited pathological proteinuria and kidney
dysfunction following a median follow-up of 14 years (29). In the present study, all patients
exhibited reduced blood potassium chloride levels, however,
following potassium and indometacin treatment, electrolyte
disorders were markedly corrected and development notably improved,
particularly in children.
In conclusion, a novel mutation associated with type
III BS was reported in a Chinese patient cohort. Additionally, an
IGF-1 deficiency was observed in patients. The identification of a
frequent mutation (whole CLCNKB gene deletion) in the
Chinese population may aid improvements in the genetic diagnosis of
the disorder in the future. A large-scale study in China is
required to investigate the mutation spectra of disease-causing
genes associated with type III BS in the Chinese population.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The National Natural Science Foundation of China
(grant no. 81471097), The National Key Research and Development
Program of China (grant no. 2017YFC1001700), and the Tianjin
Research Program of Application Foundation and Advanced Technology
(grant no. 15JCYBJC26200) supported the present study.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and DL designed the present study. YL and CW
collected and analyzed the samples, and performed the experiments.
JG analyzed the follow-up data. YY revised and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Code of Ethics of the World Medical Association and the
Declaration of Helsinki. It was approved by the Institutional
Review Board of the General Hospital of Tianjin Medical University,
Institute of Hematology and Blood Diseases Hospital, and Beijing
University First Hospital. Informed consent was obtained from all
patients or parents of patients.
Patient consent for publication
All parents or legal guardians of the patients
signed written informed consent forms.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cunha TDS and Heilberg IP: Bartter
syndrome: Causes, diagnosis, and treatment. Int J Nephrol Renovasc
Dis. 11:291–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartter FC, Pronove P, Gill JR Jr and
Maccardle RC: Hyperplasia of the juxtaglomerular complex with
hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J
Med. 33:811–828. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madrigal G, Saborio P, Mora F, Rincon G
and Guay-Woodford LM: Bartter syndrome in Costa Rica: A description
of 20 cases. Pediatr Nephrol. 11:296–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdel-al YK, Badawi MH, Yaeesh SA, Habib
YQ, al-Khuffash FA, al-Ghanim MM and al-Najidi AK: Bartter's
syndrome in Arabic children: Review of 13 cases. Pediatr Int.
41:299–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudin A: Bartter's syndrome. A review of
28 patients followed for 10 years. Acta Med Scand. 224:165–171.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Estévez R and Jentsch TJ: CLC chloride
channels: Correlating structure with function. Curr Opin Struct
Biol. 12:531–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fulchiero R and Seo-Mayer P: Bartter
syndrome and gitelman syndrome. Pediatr Clin North Am. 66:121–134.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopez HU, Haverfield E and Chung WK: Whole
Exome sequencing reveals CLCNKB mutations in a case of sudden
unexpected infant death. Pediatr Dev Pathol. 18:324–326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriguez-Soriano J, Vallo A, Pérez de
Nanclares G, Bilbao JR and Castaño L: A founder mutation in the
CLCNKB gene causes Bartter syndrome type III in Spain. Pediatr
Nephrol. 20:891–896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han Y, Lin Y, Sun Q, Wang S, Gao Y and
Shao L: Mutation spectrum of Chinese patients with Bartter
syndrome. Oncotarget. 8:101614–101622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Medical Association: World medical
association declaration of Helsinki. Ethical principles for medical
research involving human subjects. Bull World Health Organ.
79:373–374. 2001.PubMed/NCBI
|
|
12
|
Xiumin W, Zheng S, Meichun X, Junfen F and
Li L: A Chinese Girl with Bartter syndrome type III due to a novel
mutation and/or single nucleotide polymorphisms (SNPs) in CLCNKB
Gene. Iran J Pediatr. 23:89–94. 2013.PubMed/NCBI
|
|
13
|
Srebniak M, Boter M, Oudesluijs G, Joosten
M, Govaerts L, Van Opstal D and Galjaard RJ: Application of SNP
array for rapid prenatal diagnosis: Implementation, genetic
counselling and diagnostic flow. Eur J Hum Genet. 19:1230–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito-Ohara F, Uchida S, Takeuchi Y,
Sasaki S, Hayashi A, Marumo F and Ikeuchi T: Assignment of the
genes encoding the human chloride channels, CLCNKA and CLCNKB, to
1p36 and of CLCN3 to 4q32-q33 by in situ hybridization. Genomics.
36:372–374. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee BH, Cho HY, Lee H, Han KH, Kang HG, Ha
IS, Lee JH, Park YS, Shin JI, Lee DY, et al: Genetic basis of
Bartter syndrome in Korea. Nephrol Dial Transplant. 27:1516–1521.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friis UG, Stubbe J, Uhrenholt TR,
Svenningsen P, Nüsing RM, Skøtt O and Jensen BL: Prostaglandin E2
EP2 and EP4 receptor activation mediates cAMP-dependent
hyperpolarization and exocytosis of renin in juxtaglomerular cells.
Am J Physiol Renal Physiol. 289:F989–F997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Zhang J, Zhang JQ and Ramires FJ:
Local angiotensin II and transforming growth factor-beta1 in renal
fibrosis of rats. Hypertension. 35:1078–1084. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Labandeira-Garcia JL, Costa-Besada MA,
Labandeira CM, Villar-Cheda B and Rodríguez-Perez AI: Insulin-Like
Growth Factor-1 and Neuroinflammation. Front Aging Neurosci.
9:3652017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marra AM, Bobbio E, D'Assante R, Salzano
A, Arcopinto M, Bossone E and Cittadini A: Growth Hormone as
Biomarker in Heart Failure. Heart Fail Clin. 14:65–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Li YC, Deng M, Jiang HY, Guo LH,
Zhou WJ and Ruan B: Serum insulin-like growth factor-1 and its
binding protein 3 as prognostic factors for the incidence,
progression, and outcome of hepatocellular carcinoma: A systematic
review and meta-analysis. Oncotarget. 8:81098–81108.
2017.PubMed/NCBI
|
|
21
|
Buyukcelik M, Keskin M, Kilic BD, Kor Y
and Balat A: Bartter syndrome and growth hormone deficiency: Three
cases. Pediatr Nephrol. 27:2145–2148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flyvbjerg A, Dørup I, Everts ME and Orskov
H: Evidence that potassium deficiency induces growth retardation
through reduced circulating levels of growth hormone and
insulin-like growth factor I. Metabolism. 40:769–775. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kackstein K, Teren A, Matsumoto Y, Mangner
N, Möbius-Winkler S, Linke A, Schuler G, Punkt K and Adams V:
Impact of angiotensin II on skeletal muscle metabolism and function
in mice: Contribution of IGF-1, Sirtuin-1 and PGC-1α. Acta
Histochem. 115:363–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zaika O, Tomilin V, Mamenko M, Bhalla V
and Pochynyuk O: New perspective of ClC-Kb/2 Cl-channel physiology
in the distal renal tubule. Am J Physiol Renal Physiol.
310:F923–F930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinaldi C, Bott LC, Chen KL, Harmison GG,
Katsuno M, Sobue G, Pennuto M and Fischbeck KH: Insulinlike growth
factor (IGF)-1 administration ameliorates disease manifestations in
a mouse model of spinal and bulbar muscular atrophy. Mol Med.
18:1261–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cha EJ, Hwang WM, Yun SR and Park MH: An
adult case of Bartter syndrome type III presenting with
proteinuria. J Pathol Transl Med. 50:160–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schurman SJ, Perlman SA, Sutphen R, Campos
A, Garin EH, Cruz DN and Shoemaker LR: Genotype/phenotype
observations in African Americans with Bartter syndrome. J Pediatr.
139:105–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng CJ, Lo YF, Chen JC, Huang CL and Lin
SH: Functional severity of CLCNKB mutations correlates with
phenotypes in patients with classic Bartter's syndrome. J Physiol.
595:5573–5586. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bettinelli A, Borsa N, Bellantuono R,
Syrén ML, Calabrese R, Edefonti A, Komninos J, Santostefano M,
Beccaria L, Pela I, et al: Patients with biallelic mutations in the
chloride channel gene CLCNKB: Long-term management and outcome. Am
J Kidney Dis. 49:91–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nozu K, Fu XJ, Nakanishi K, Yoshikawa N,
Kaito H, Kanda K, Krol RP, Miyashita R, Kamitsuji H, Kanda S, et
al: Molecular analysis of patients with type III Bartter syndrome:
Picking up large heterozygous deletions with semiquantitative PCR.
Pediatr Res. 62:364–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JW, Lee J, Heo NJ, Cheong HI and Han
JS: Mutations in SLC12A3 and CLCNKB and their correlation with
clinical phenotype in patients with Gitelman and Gitelman-like
syndrome. J Korean Med Sci. 31:47–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simon DB, Bindra RS, Mansfield TA,
Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay
H, Bakkaloglu A, et al: Mutations in the chloride channel gene,
CLCNKB, cause Bartter's syndrome type III. Nat Genet. 17:171–178.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urbanová M, Reiterová J, Stěkrova J,
Lněnická P and Ryšavá R: DNA analysis of renal electrolyte
transporter genes among patients suffering from Bartter and
Gitelman syndromes: Summary of mutation screening. Folia Biol
(Praha). 57:65–73. 2011.PubMed/NCBI
|
|
34
|
Chiang WF and Lin SH, Chan JS and Lin SH:
Hypokalemic paralysis in a middle-aged female with classic Bartter
syndrome. Clin Nephrol. 81:146–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konrad M, Vollmer M, Lemmink HH, van den
Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschênes G,
Antignac C, et al: Mutations in the chloride channel gene CLCNKB as
a cause of classic Bartter syndrome. J Am Soc Nephrol.
11:1449–1459. 2000.PubMed/NCBI
|















