Introduction
Legg-Calvé-Perthes disease (LCPD) is a pediatric
form of femoral head osteonecrosis that mainly affects children
between the ages of 2–12 years; LCPD is characterized by painful
synovitis in the knees, avascular necrosis of the femoral head, and
deformities of the femoral head and acetabulum (1–3). The
disease typically leads to deformities, physical dysfunction and
lifelong disability in children (4,5). At
present, the main therapy for LCPD is surgical treatment; however,
long-term (1.5–2 months) use of a brace is required to improve the
state of the hip, resulting in a certain degree of limited hip
joint activity (6,7). In addition, due to the incomplete
development of the nervous system and low tolerance to pain,
children often experience strong reactions towards treatment,
resulting in poor cooperation, which may affect postoperative
recovery (8). Therefore, high
quality care is particularly important. Nurses should be familiar
with the aforementioned characteristics of children, and take
appropriate measures to prevent complications and promote
postoperative rehabilitation.
Various studies have indicated that the pathogenesis
of LCPD is due to the uncoupling of bone metabolism (9,10);
however, the exact pathophysiology of this disease remains unknown.
Recent studies demonstrated increased levels of pro-apoptotic
factors in patients with LCPD, suggesting that apoptotic processes
may promote the development of LCPD (9,11);
however, further investigation is required to determine the
potential molecular mechanisms of apoptosis in LCPD.
MicroRNAs (miRNAs/miRs) are small noncoding RNAs
(19–25 nucleotides) that can regulate the expression of numerous
target genes via binding to their 3′-untranslated regions (3′-UTRs)
(12,13). miRNAs regulate a variety of
cellular functions, including proliferation, apoptosis,
differentiation and metastasis (14,15).
The aberrant expression of miRNAs can lead to cellular and tissue
disorders. It has been reported that miRNAs serve important roles
in chondrogenesis and LCPD. Luo et al (16) revealed that miR-206 promoted cell
apoptosis in LCPD via downregulation of SRY-box 9. Furthermore, it
was demonstrated that overexpression of miR-214 in vitro and
in vivo negatively regulated chondrocyte differentiation,
possibly by targeting activating transcription factor 4 (ATF4)
(17). Additionally, Wang et
al (18) revealed that miR-214
functions as a tumor suppressor in cervical cancer by inhibiting
cell proliferation and invasion, and promoting apoptosis.
Collectively, these studies suggest that miR-214 may serve
important roles in regulating cell growth and apoptosis, and that
apoptotic processes may be involved in the pathogenesis of LCPD
(9,11).
To the best of our knowledge, the role of miR-214 in
LCPD has not been investigated. Therefore, the present study aimed
to determine whether miR-214 may be involved in the development and
progression of LCPD via the regulation of apoptosis. It was
demonstrated that miR-214 was downregulated in cartilage, serum and
chondrocytes from patients with LCPD, whereas B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax) expression was upregulated.
Furthermore, it was revealed that Bax was a target gene of miR-214;
this miRNA increased the viability of the TC28 human chondrocyte
cell line and inhibited apoptosis via downregulation of Bax. The
results indicated that miR-214 may function as a predictive
biomarker and potential therapeutic target for the treatment of
LCPD.
Materials and methods
Clinical samples
All patients signed informed consent forms prior to
the study, and the study received approval from the Institutional
Ethics Committee of Nanjing Children's Hospital Affiliated to
Nanjing Medical University (Nanjing, China). Human femoral head
cartilage tissue was isolated from patients with LCPD (n=20, <14
years old, male 12, female 8) and healthy volunteers (n=20, <14
years old, 12 male, 8 female) from December 2015 to October 2017.
LCPD was diagnosed on the basis of ultrasonographic examination and
magnetic resonance imaging. Patients with other diseases, such as
primary osteoarthritis, ankylosing spondylitis, systemic lupus
erythematosus and inflammatory diseases, were excluded. Peripheral
venous blood samples were drawn from all patients in the morning
prior to surgery, collected into two 4.5-ml Vacutainer sodium
citrate anticoagulant tubes (BD Biosciences, Franklin Lakes, NJ,
USA) and stored at −80°C until use. Peripheral venous blood samples
were centrifuged at 1,000 × g for 10 min at 4°C to obtain
serum.
Chondrocyte isolation and culture
Chondrocytes were isolated from LCPD and healthy
control femoral head cartilage tissues via collagenase digestion of
cartilage, and cultured in monolayer as described previously
(19). Cells of the first passage
were used in the experiments of the study. The human cartilage cell
line TC28 (immortalized human primary juvenile costal chondrocytes)
was purchased from the American Type Culture Collection (Manassas,
VA, USA). The two cell lines were propagated as monolayers
maintained in Dulbecco's Modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
at 37°C in a humidified atmosphere of 5% CO2.
TC28 cells were treated with 0.01 mmol dexamethasone
(DEX) (cat. no. kf1419; Guangzhou Kafen Biological Technology Co.,
Ltd., Guangzhou, China) at 37°C for 2 h to establish an in
vitro model of LCPD. Cells without any treatment were used as
control. Then, the levels of miR-214 and Bax expression were
evaluated to characterize the effects of DEX on TC28 cells.
Plasmids, oligonucleotides and
transfection
miR-214 mimic (5′CCUGACAAUUAGUAUUU-3′) and mimic
control (5′-ACAGGUAGCUGAACACUGGGUU-3′) were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequence of Bax
was inserted into pcDNA3.1 (Invitrogen; Thermo Fisher Scientific,
Inc.); empty vector (control-plasmid) was used as the control. TC28
cells were seeded in 6-well plates (1×106 cells/well),
cultured for 24 h and transfected with 100 nM miR-214 mimic, 100 nM
mimic control, 1 µg control-plasmid, 1 µg Bax-plasmid or 100 nM
miR-214 mimic + 1 µg Bax-plasmid using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols. The transfection efficiency was
determined 48 h later.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from serum, cartilage tissue
or cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific); 1 µg of total RNA was reverse transcribed to
cDNA using a PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocols. Reaction
conditions for reverse transcription were: 50°C for 5 min and 80°C
for 2 min. An miRNA-specific TaqMan MiRNA Assay kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for the
detection of miRNA expression according to the manufacturer's
protocols. SYBR Premix Ex Taq (Takara Bio, Inc.) was used to
analyze Bax mRNA expression. U6 small nuclear RNA (U6) and GAPDH
expression were used as an internal control for miR-214 and Bax,
respectively. The sequences of primers were as follows: miR-214,
forward 5′-AGCATAATACAGCAGGCACAGAC-3′, reverse,
5′-AAAGGTTGTTCTCCACTCTCTCAC-3′; Bax, forward
5′-GGCCCACCAGCTCTGAGCAGA-3′, reverse, 5′-GCCACGTGGGCGTCCCAAAGT-5′;
GAPDH, forward 5′-TGAACGGGAAGCTCACTGG-3′, reverse,
3′-TCCACCACCCTGTTGCTGTA-5′; and U6, forward
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AAATATGGAACGCTTCACGA-3′.
RT-qPCR data were analyzed with an ABI 7900 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Amplification
conditions for qPCR were as follows: 5 min at 95°C, followed by 35
cycles at 95°C for 15 sec, 40 sec at 55°C, and 72 °C for 1 min. The
relative expression levels were calculated using the
2−ΔΔCq method (20).
Dual-luciferase reporter assay
The bioinformatics tool miRBase (http://www.mirbase.org) was used to predict the
potential targets of miR-214. The results indicated that Bax was a
potential target of miR-214. To confirm this prediction, a
dual-luciferase reporter assay was performed. A wild type (WT-Bax)
and mutant (MUT-Bax) 3′-untranslated regions of Bax were cloned
into a pmiR-RB-Report™ dual luciferase reporter gene plasmid vector
(Guangzhou RiboBio Co., Ltd., Guangzhou, China). Then, the WT-Bax
or MUT-Bax vectors, the miR-214 mimic or mimic control, and the
pRL-TK Renilla luciferase reporter (Promega Corporation,
Madison, WI, USA) were co-transfected into TC28 cells using
Lipofectamine® 2000. TC28 cells were collected 48 h
following transfection and luciferase activity was analyzed using
dual-luciferase assay system (Promega Corporation, Madison, WI,
USA). Firefly luciferase activity was normalized to that of
Renilla.
Western blot assay
Proteins from cells, serum and tissues were
extracted using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) with
Protease/Phosphatase Inhibitor Cocktail (Cell Signaling Technology,
Inc., Danvers, MA, USA). Protein concentrations were determined
using a bicinchoninic acid assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of protein samples (30 µg/lane)
were separated via 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Merck KGaA, Darmstadt, Germany). The
membranes were blocked in 5% non-fat dry milk at room temperature
for 1 h and incubated with primary antibodies overnight at 4°C. The
primary antibodies used were: Anti-Bax (1:2,000; ab32503, Abcam,
Cambridge, UK), anti-Bcl-2 (1:2,000; ab196495, Abcam) and
anti-GAPDH (1:5,000; ab9485, Abcam). Membranes were then incubated
with a goat anti-rabbit immunoglobulin G conjugated with
horseradish peroxidase (cat. no. ab7090; 1:2,000; Abcam) at room
temperature for 3 h. All bands were visualized using enhanced
chemiluminescence western blotting detection kits (Merck KGaA).
ImageJ 1.38X software (National Institutes of Health, Bethesda, MD,
USA) was used to quantify protein expression.
MTT assay
Cell viability was determined by an MTT assay.
Following treatment with DEX and transfection with miR-214 mimic,
mimic-control, or miR-214 mimic + Bax-plasmid for 48 h, TC28 cells
(1×104 cells/well) were seeded in 96-well plates and
cultured for 24 h. Subsequently, 20 µl MTT solution (0.5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well and the plates
were further incubated at 37°C for 4 h. The medium in each well was
discarded, and 150 µl dimethyl sulfoxide was added for 30 min. The
absorbance at 570 nm was measured using a FLUOstar®
Omega Microplate Reader (BMG Labtech GmbH, Ortenberg, Germany).
Flow cytometry analysis
An Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection kit [cat. no.
70-AP101-100; MultiSciences (Lianke) Biotech, Co., Ltd., Hangzhou,
China] was used to evaluate the apoptotic rate of TC28 cells.
Following 48 h since transfection, TC28 cells were collected,
washed with PBS and suspended with 5 µl Annexin V-FITC and 5 µl PI
for 30 min in the dark at room temperature. A flow cytometer was
used to analyze cell apoptosis. And the cell apoptotic rate (early
apoptosis and late apoptosis in the right quadrant) was determined
using FlowJo software version 7.6.1 (FlowJo LLC, Ashland, OR,
USA).
Statistical analysis
Each experiment was repeated three times.
Statistical analysis was performed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). Data are presented as the mean ± standard
deviation of at least three independent experiments. Differences
between groups were analyzed by Student's t-tests or one-way
analysis of variance with Tukey's post hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
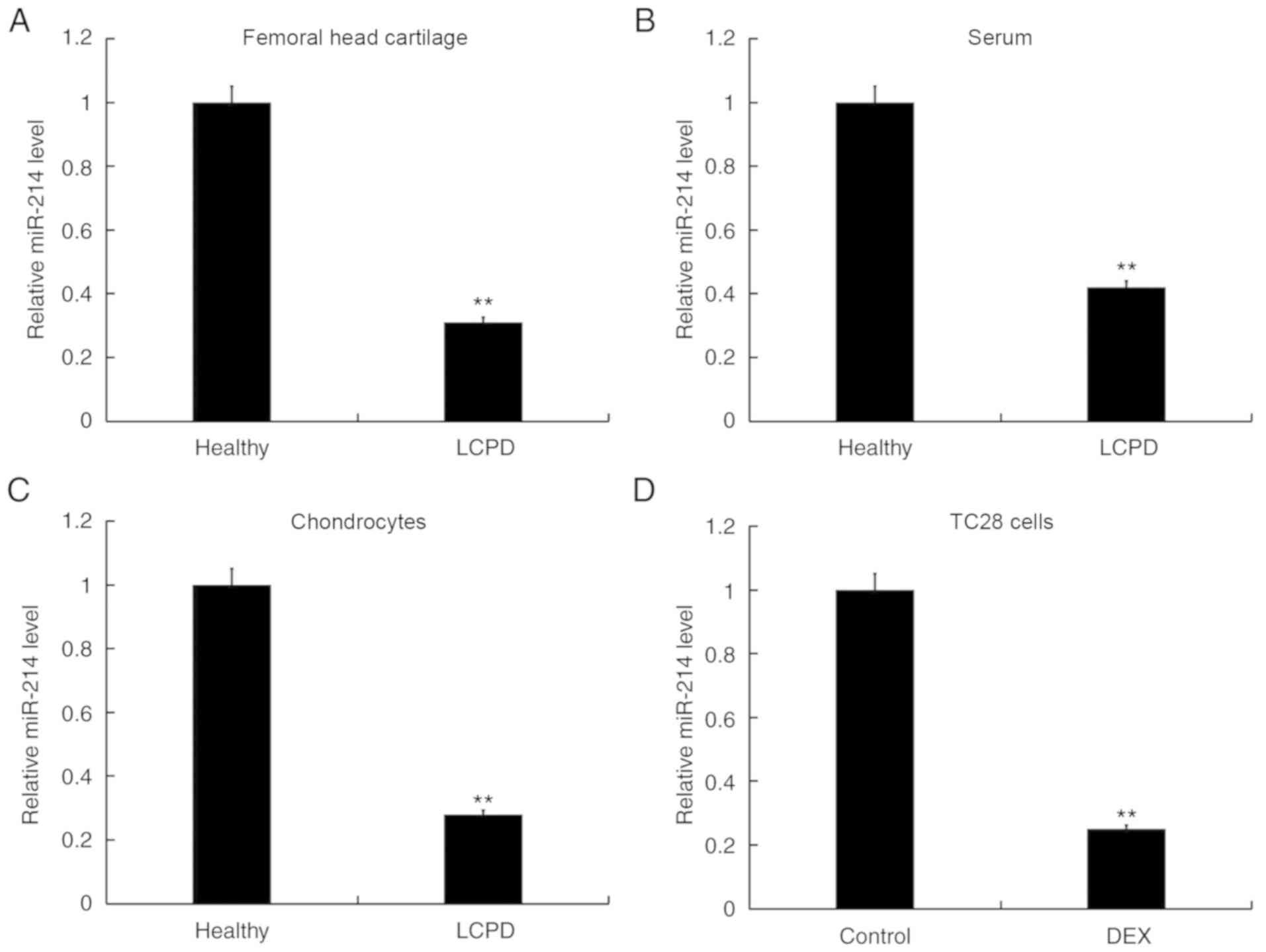
Expression of miR-214 is decreased in
human LCPD cartilage, serum and chondrocytes
To determine miR-214 expression in human LCPD
cartilage, serum and chondrocytes, samples were collected from 20
patients with LCPD and 20 healthy individuals. As presented in
Fig. 1A and B, compared with the
healthy group, miR-214 expression was significantly downregulated
in femoral head cartilage and serum samples from patients with LCPD
compared with the healthy controls. In addition, human primary
chondrocytes were isolated from femoral head cartilage tissues from
patients with LCPD and healthy controls. The results of RT-qPCR
revealed that miR-214 expression was significantly downregulated in
human primary chondrocytes from patients with LCPD compared with in
healthy controls (Fig. 1C).
Furthermore, the expression levels of miR-214 were significantly
downregulated in DEX-treated TC28 cells compared with untreated
control cells (Fig. 1D).
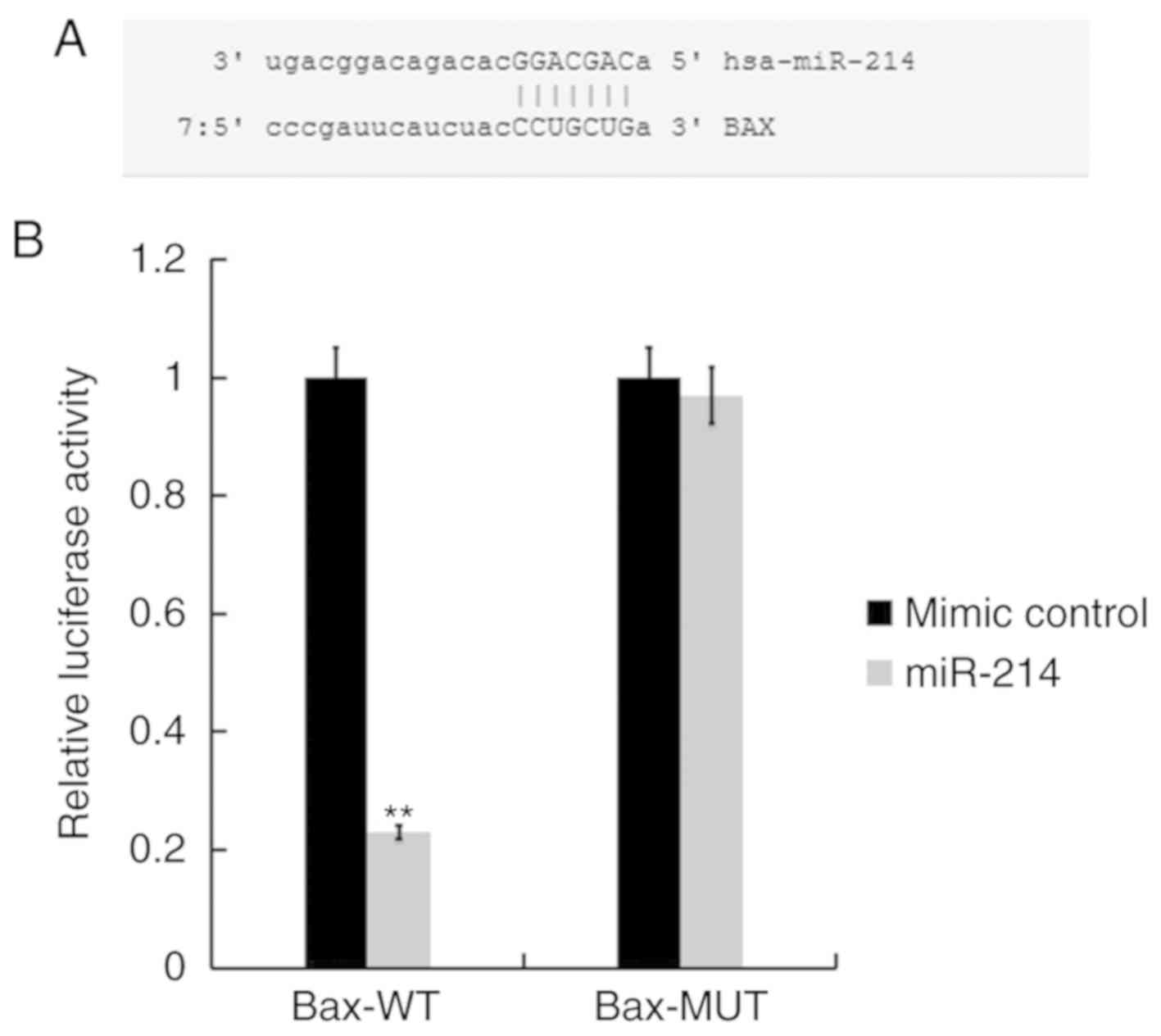
Bax is a direct target of miR-214
The bioinformatics tool miRBase (http://www.mirbase.org) was used to predict the
potential targets of miR-214. Bioinformatics analyses indicated
that Bax was a potential target of miR-214 (Fig. 2A). Subsequently, to determine
whether miR-214 directly modulates Bax expression via interactions
with potential binding sites, a luciferase reporter assay was
performed using TC28 cells transfected with vectors harboring the
WT or MUT 3′-UTR of Bax, in the presence or absence of the miR-214
mimic or mimic control. As presented in Fig. 2B, compared with co-transfection
with Bax-WT and mimic control, the luciferase activity was
significantly decreased following co-transfection with Bax-WT and
miR-214 mimic, while Bax-MUT did not. The results indicated that
Bax was a target of miR-214.
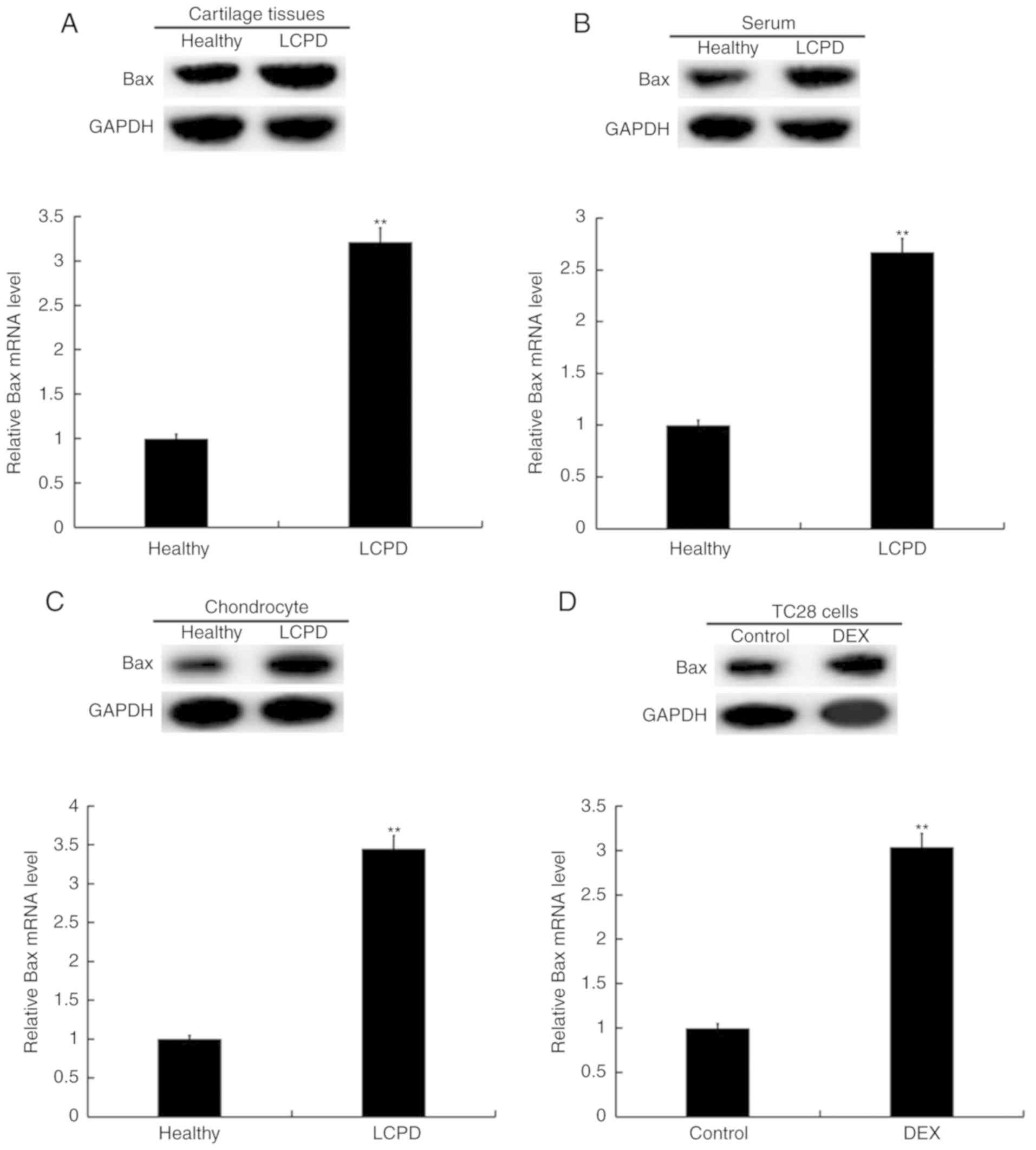
Expression of Bax is increased in
human LCPD cartilage, serum and chondrocytes
The expression of Bax was determined in human LCPD
cartilage, serum and chondrocytes. As presented in Fig. 3A-C, compared with the healthy
control group, the expression level of Bax mRNA was significantly
upregulated in femoral head cartilage, serum and primary
chondrocytes of patients with LCPD. Meanwhile, compared with the
healthy control group, the levels of Bax protein appear to be
higher in the femoral head cartilage, serum and primary
chondrocytes of patients with LCPD. Furthermore, TC28 cells treated
with DEX exhibited significantly elevated Bax mRNA expression
compared with untreated control cells and seemingly higher Bax
protein levels as well (Fig.
3D).
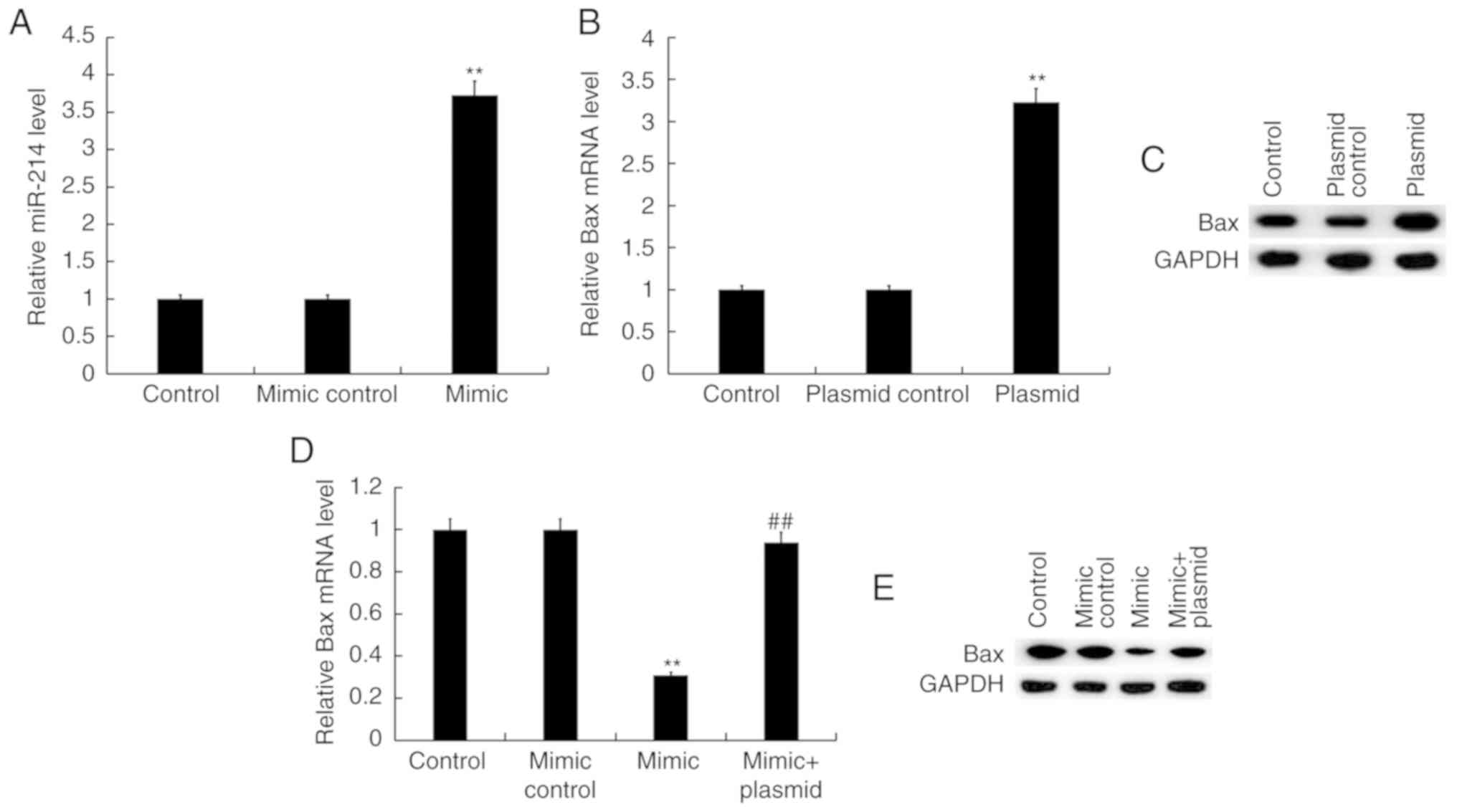
miR-214 negatively regulates Bax
expression in TC28 cells
To further investigate the effects of miR-214 on Bax
expression, TC28 cells were transfected with mimic control, miR-214
mimic, control-plasmid, Bax-plasmid, or miR-214 mimic + Bax-plasmid
for 48 h. As presented in Fig. 4A,
miR-214 mimic significantly increased the expression levels of
miR-214 in TC28 cells compared with the control group (Fig. 4A). In addition, TC28 cells
transfected with the Bax-plasmid exhibited significantly increased
mRNA expression of Bax (Fig. 4B)
and seemingly higher Bax protein levels as well (Fig. 4C). Compared with the mimic control
group, transfection with miR-214 mimic significantly decreased the
mRNA expression of Bax in TC28 cells, which was reversed by Bax
overexpression (Fig. 4D). The
results of western blot assay suggested that miR-214 mimic
decreased the protein levels of Bax in TC28 cells, which may have
been reversed by Bax overexpression (Fig. 4E).
miR-214 targets Bax to regulate
chondrocyte viability and apoptosis
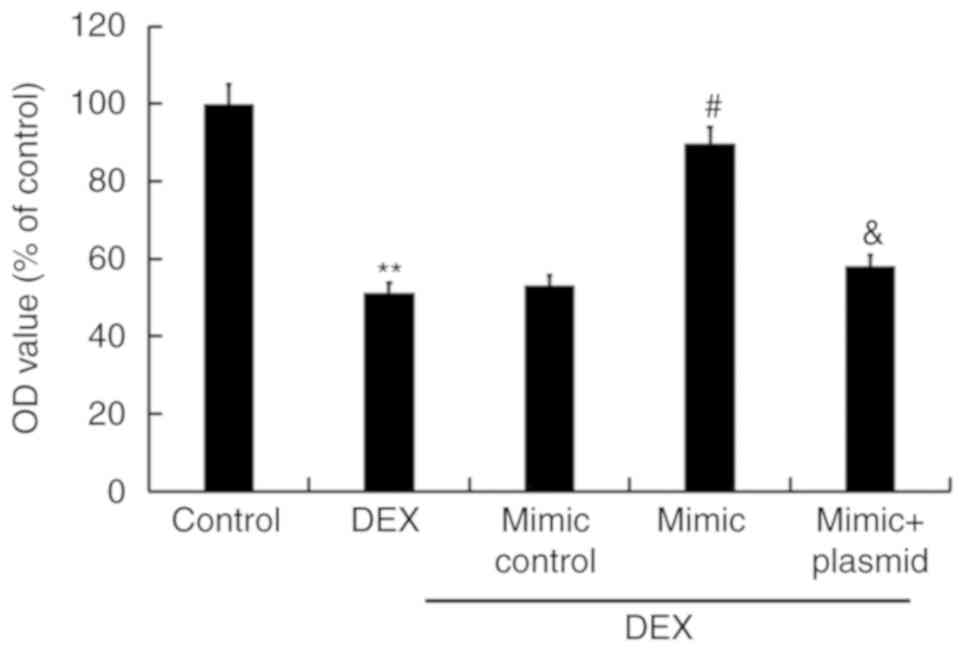
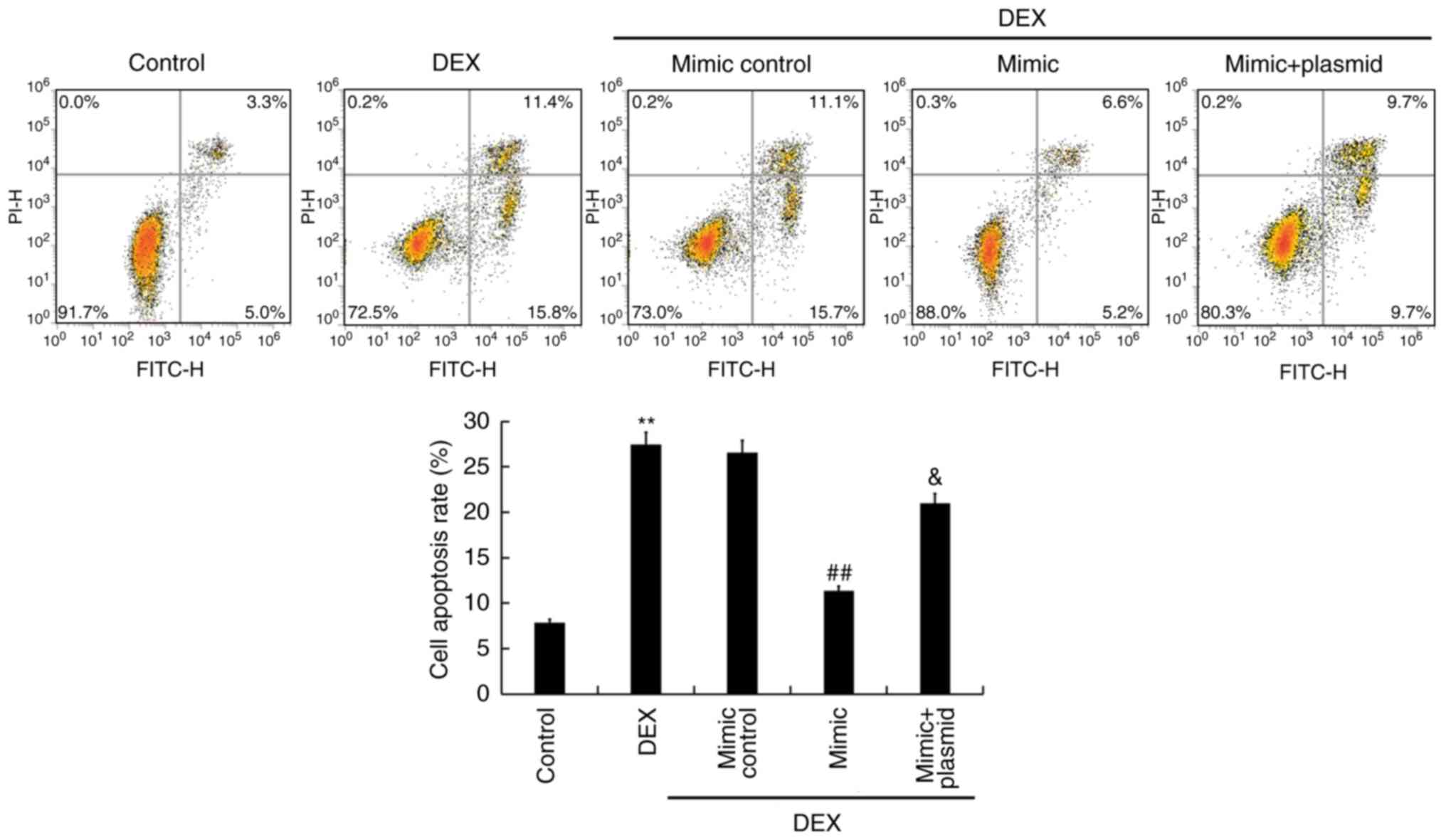
MTT assays were performed to detect the viability of
TC28 cells, and flow cytometry was conducted to detect TC28 cell
apoptosis. As presented in Figs. 5
and 6, compared with the control
group, DEX treatment significantly reduced viability of TC28 cells
and promoted apoptosis. Conversely, overexpression of miR-214
significantly increased cell viability and inhibited cell apoptosis
compared with DEX treatment alone; these effects were reversed by
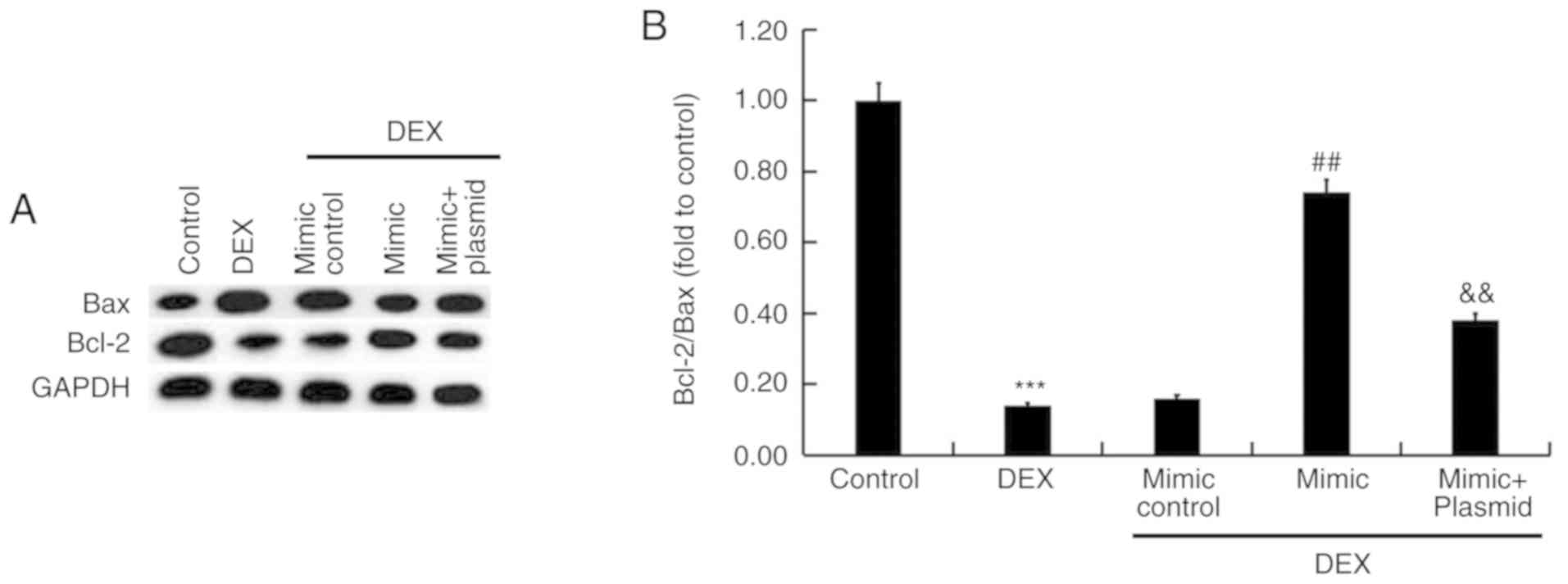
Bax overexpression. Additionally, the protein expression of Bax and
Bcl-2 was determined, and the Bcl-2/Bax ratio was calculated. As
presented in Fig. 7, compared with
the control, the protein expression of Bax increased following DEX
treatment, while that of Bcl-2 decreased and the Bcl-2/Bax ratio
was reduced. These effects were inhibited by miR-214 overexpression
and promoted by Bax overexpression.
Discussion
LCPD is an idiopathic osteonecrosis of the immature
femoral head, in which the supply of blood in the capital femoral
epiphysis is interrupted, resulting in osteonecrosis and cartilage
necrosis, leading to gradual malformation of the femoral head and
subsequent degenerative osteoarthritis (21,22).
Recently, miRNAs were identified as important regulators of
numerous diseases, including cancer, autoimmune diseases,
inflammation and infertility (23). For example, miR-214 inhibits
cervical cancer cell proliferation and invasion, and facilitates
apoptosis via regulating the expression of mechanistic target of
rapamycin (18). A recent study
demonstrated that miR-214 suppresses the osteogenic differentiation
of human periodontal ligament stem cells by targeting ATF4
(24). In addition, overexpression
of miR-214 exerts a negative role in chondrogenesis by affecting
chondrocyte differentiation (17).
In the present study, it was revealed that miR-214 expression was
significantly decreased in patients with LCPD compared with in
healthy controls. Additionally, treatment of TC28 cells with 0.01
mmol DEX significantly decreased the expression of miR-214.
Collectively, the results indicated that miR-214 was downregulated
in patients with LCPD and DEX-treated TC28 cells, suggesting that
miR-214 may serve an important role in the development of LCPD.
Calder et al (25) reported that the processes leading
to the death of femoral head cells in patients with femoral head
necrosis involves an increased rate of apoptosis rather than bone
cell necrosis alone. Furthermore, Zhang et al (26) revealed that chondrocyte apoptosis
in the femoral head is induced by glucocorticoids in broilers.
Additionally, miR-206 contributes to the progression of
steroid-induced avascular necrosis of the femoral head by inducing
osteoblast apoptosis via the suppression of programmed cell death 4
(27). A notable finding of the
present study was that Bax was a direct target gene of miR-214. Bax
is a pro-apoptotic member of the Bcl-2 family of proteins, and
serves an important role in the mitochondrial apoptotic pathway;
Bax migrates to the mitochondrial membrane during apoptosis
(28). As a downstream target gene
of p53, Bax is required for p53-dependent apoptosis in certain
systems (29). It has been
reported that p53 can directly activate Bax without active
transcription (30). Pagliara
et al (31) recently
reported that, independent of p53 status, activated p21 can induce
Bax translocation to the mitochondria, which in turn, increases the
mitochondrial membrane permeability, leading to cytochrome c
release and caspase pathway activation. Of note, significantly
increased levels of Bax expression were reported in patients with
LCPD, along with a significantly elevated Bax/Bcl-2 ratio (9). Consistent with Srzentić et al
(9), the results of the present
study revealed that the levels of Bax protein and mRNA expression
in the cartilage, serum and chondrocytes of LCPD patients were
significantly enhanced compared with the healthy control. DEX
treatment also significantly increased Bax expression in TC28
cells. Then, to investigate the association between miR-214 and
Bax, TC28 cells were transfected with miR-214 mimic. The results
revealed that overexpression of miR-214 significantly decreased the
levels of Bax expression. Furthermore, it was demonstrated that DEX
treatment significantly decreased TC28 cell viability, promoted
apoptosis and reduced the Bcl-2/Bax ratio, whereas miR-214 mimic
exhibited opposing effects. Additionally, the effects of miR-214
upregulation on TC28 cells were eliminated by Bax overexpression.
Collectively, the results suggested that miR-214 and Bax
dysregulation may be involved in LCPD.
In conclusion, the present study revealed that
miR-214 was downregulated and Bax was upregulated in the cartilage,
serum and chondrocytes of patients with LCPD, and DEX-treated TC28
cells. miR-214 promoted chondrocyte viability and decreased
apoptosis via downregulation of Bax. The present study indicated
that miR-214 may function as a reliable biomarker and potential
therapeutic target in the treatment of LCPD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ, XQ and YL contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. WL, XS and YT contributed to statistical
analysis and literature search. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All patients signed informed consent forms prior to
the study, and the study received approval from the Institutional
Ethics Committee of the Children's Hospital Affiliated to Nanjing
Medical University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ponseti IV, Maynard JA, Weinstein SL,
Ippolito EG and Pous JG: Legg-Calvé-Perthes disease. Histochemical
and ultrastructural observations of the epiphyseal cartilage and
physis. J Bone Joint Surg Am. 65:797–807. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoenecker PL, Stone JW and Capelli AM:
Legg-Perthes disease in children under 6 years old. Orthop Rev.
22:201–208. 1993.PubMed/NCBI
|
|
3
|
Oda J, Hirano T, Iwasaki K and Majima R:
Vascular occlusion and cartilage disorders in osteonecrosis of the
femoral head in rats. Int Orthop. 20:185–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hailer YD, Haag AC and Nilsson O:
Legg-Calvé-perthes disease: Quality of life, physical activity, and
behavior pattern. J Pediatr Orthop. 34:514–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leroux J, Abu Amara S and Lechevallier J:
Legg-Calvé-Perthes disease. Orthop Traumatol Surg Res.
104:S107–S112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KS, Cho KJ, Yang HY, Eshnazarov KE
and Yoon TR: Long-term results of modified salter innominate
osteotomy for Legg-Calvé-Perthes disease. Clin Orthop Surg.
9:397–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosello O, Solla F, Oborocianu I, Chau E,
ElHayek T, Clement JL and Rampal V: Advanced containment methods
for Legg-Calvé-Perthes disease: Triple pelvic osteotomy versus
Chiari osteotomy. Hip Int. 28:297–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Powell MK: Dealing with a casted
Legg-Calvé-Perthes diseases child. ONA J. 6:495–497.
1979.PubMed/NCBI
|
|
9
|
Srzentić S, Nikčević G, Spasovski D,
Baščarević Z, Živković Z, Terzic-Šupić Z, Matanović D, Djordjević
V, Pavlović S and Spasovski V: Predictive genetic markers of
coagulation, inflammation and apoptosis in Perthes disease-Serbian
experience. Eur J Pediatr. 174:1085–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koob TJ, Pringle D, Gedbaw E, Meredith J,
Berrios R and Kim HK: Biomechanical properties of bone and
cartilage in growing femoral head following ischemic osteonecrosis.
J Orthop Res. 25:750–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Yuan Z, Pei X and Ma R: In vivo
and in vitro characteristic of HIF-1alpha and relative genes in
ischemic femoral head necrosis. Int J Clin Exp Pathol. 8:7210–7216.
2015.PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J, Yao F, Xiao Z, Sun Y and Ma L:
MicroRNAs and metastasis: Small RNAs play big roles. Cancer
Metastasis Rev. 37:5–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujii T, Shimada K, Nakai T and Ohbayashi
C: MicroRNAs in smoking-related carcinogenesis: Biomarkers,
functions, and therapy. J Clin Med. 7:E982018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo J, Han J, Li Y and Liu Y:
Downregulated SOX9 mediated by miR-206 promoted cell apoptosis in
Legg-Calvé-Perthes disease. Oncol Lett. 15:1319–1324.
2018.PubMed/NCBI
|
|
17
|
Roberto VP, Gavaia P, Nunes MJ, Rodrigues
E, Cancela ML and Tiago DM: Evidences for a new role of miR-214 in
chondrogenesis. Sci Rep. 8:37042018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Tan WH, Liu W, Jin YX, Dong DD,
Zhao XJ and Liu Q: Effects of miR-214 on cervical cancer cell
proliferation, apoptosis and invasion via modulating PI3K/AKT/mTOR
signal pathway. Eur Rev Med Pharmacol Sci. 22:1891–1898.
2018.PubMed/NCBI
|
|
19
|
Yang H, Wu D, Li H, Chen N and Shang Y:
Downregulation of microRNA-448 inhibits IL-1β-induced cartilage
degradation in human chondrocytes via upregulation of matrilin-3.
Cell Mol Biol Lett. 23:72018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vandermeer JS, Kamiya N, Aya-ay J, Garces
A, Browne R and Kim HK: Local administration of ibandronate and
bone morphogenetic protein-2 after ischemic osteonecrosis of the
immature femoral head: A combined therapy that stimulates bone
formation and decreases femoral head deformity. J Bone Joint Surg
Am. 93:905–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loder RT and Skopelja EN: The epidemiology
and demographics of legg-calve-perthes' disease. ISRN Orthop.
2011:5043932011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Long H, Wang X, Chen Y, Wang L, Zhao M and
Lu Q: Dysregulation of microRNAs in autoimmune diseases:
Pathogenesis, biomarkers and potential therapeutic targets. Cancer
Lett. 428:90–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao S, Zhao W, Ou Q, Liang L, Lin X and
Wang Y: MicroRNA-214 suppresses osteogenic differentiation of human
periodontal ligament stem cells by targeting ATF4. Stem Cells Int.
2017:30286472017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calder JD, Buttery L, Revell PA, Pearse M
and Polak JM: Apoptosis-a significant cause of bone cell death in
osteonecrosis of the femoral head. J Bone Joint Surg Br.
86:1209–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Shi CY, Zhou ZL and Hou JF: Bone
characteristics, histopathology, and chondrocyte apoptosis in
femoral head necrosis induced by glucocorticoid in broilers. Poult
Sci. 96:1609–1614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Jin A and Yan D: MicroRNA206
contributes to the progression of steroid-induced avascular
necrosis of the femoral head by inducing osteoblast apoptosis by
suppressing programmed cell death 4. Mol Med Rep. 17:801–808.
2018.PubMed/NCBI
|
|
28
|
Li Z, Meng J, Xu TJ, Qin XY and Zhou XD:
Sodium selenite induces apoptosis in colon cancer cells via
Bax-dependent mitochondrial pathway. Eur Rev Med Pharmacol Sci.
17:2166–2171. 2013.PubMed/NCBI
|
|
29
|
Moll UM, Wolff S, Speidel D and Deppert W:
Transcription-independent pro-apoptotic functions of p53. Curr Opin
Cell Biol. 17:631–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Speidel D: Transcription-independent p53
apoptosis: An alternative route to death. Trends Cell Biol.
20:14–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pagliara V, Saide A, Mitidieri E,
d'Emmanuele di Villa Bianca R, Sorrentino R, Russo G and Russo A:
5-FU targets rpL3 to induce mitochondrial apoptosis via
cystathionine-β-synthase in colon cancer cells lacking p53.
Oncotarget. 7:50333–50348. 2016. View Article : Google Scholar : PubMed/NCBI
|