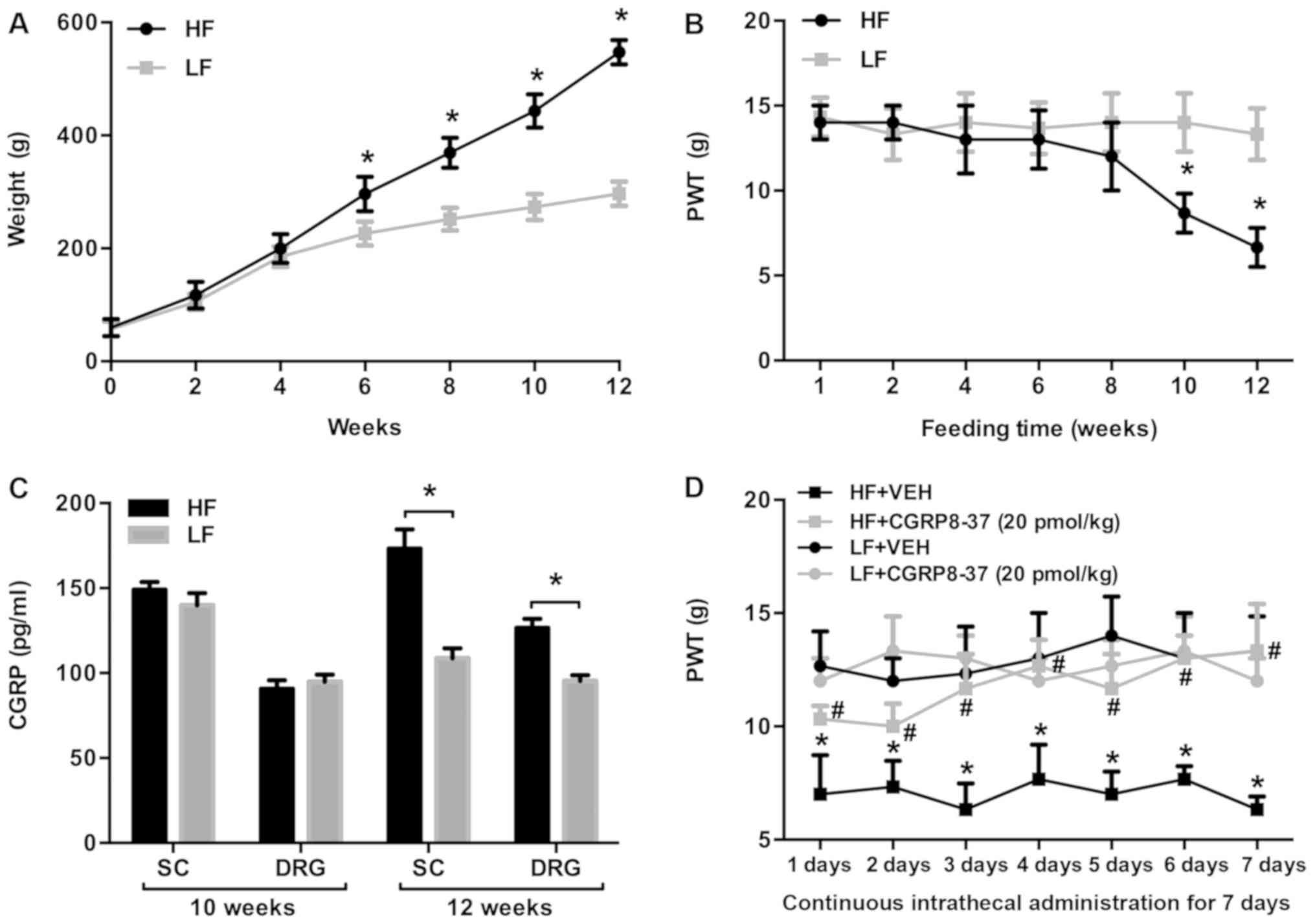
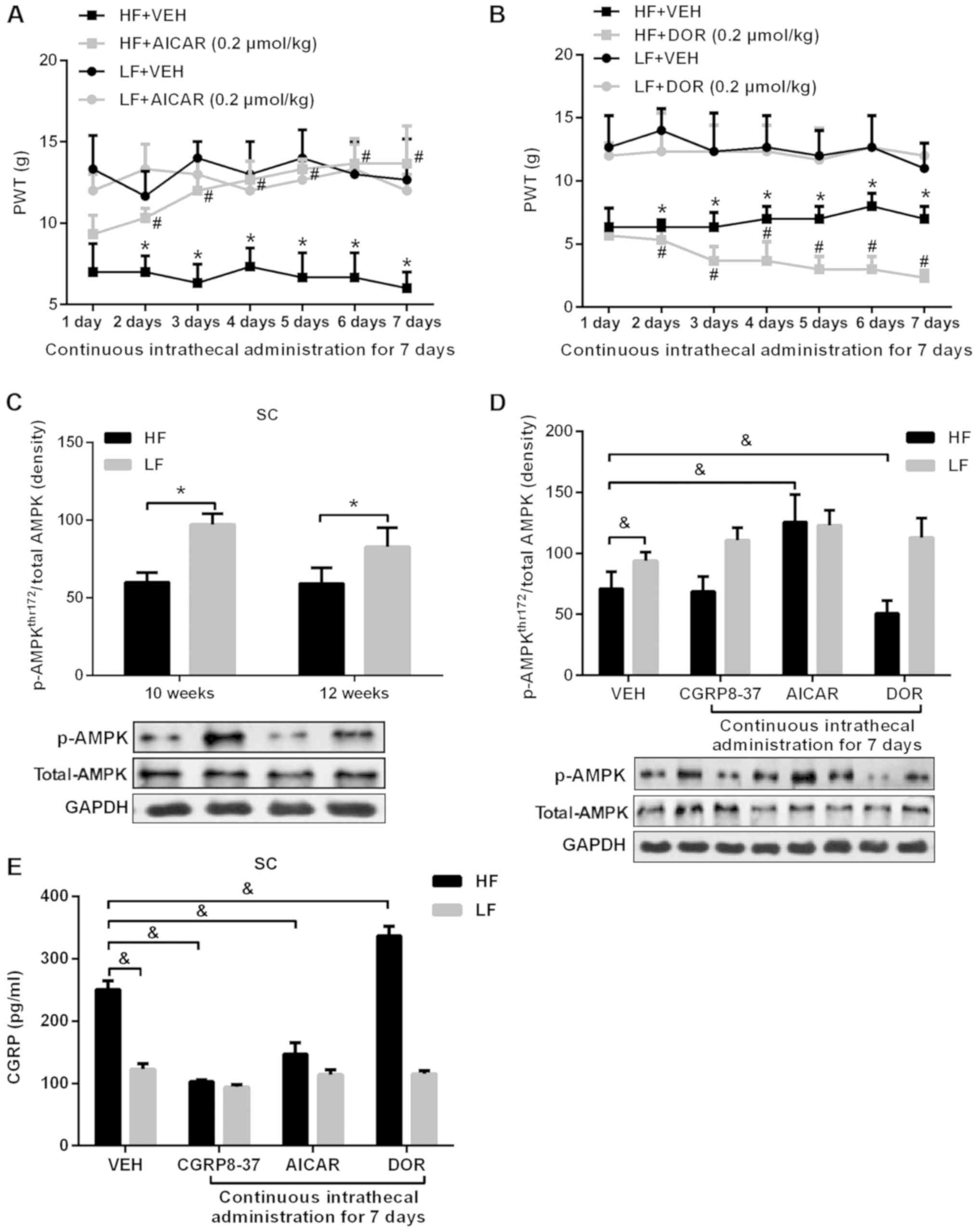
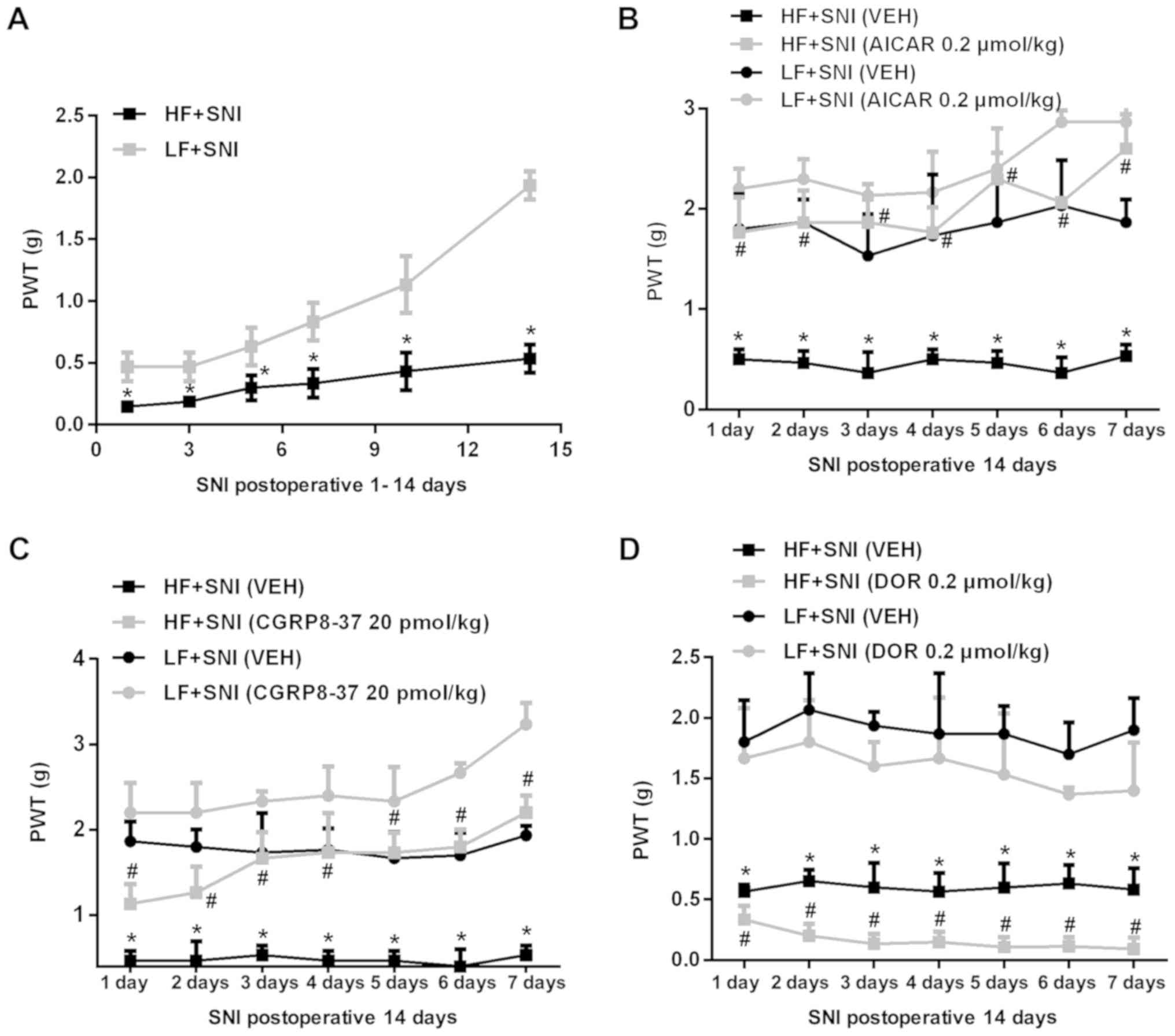
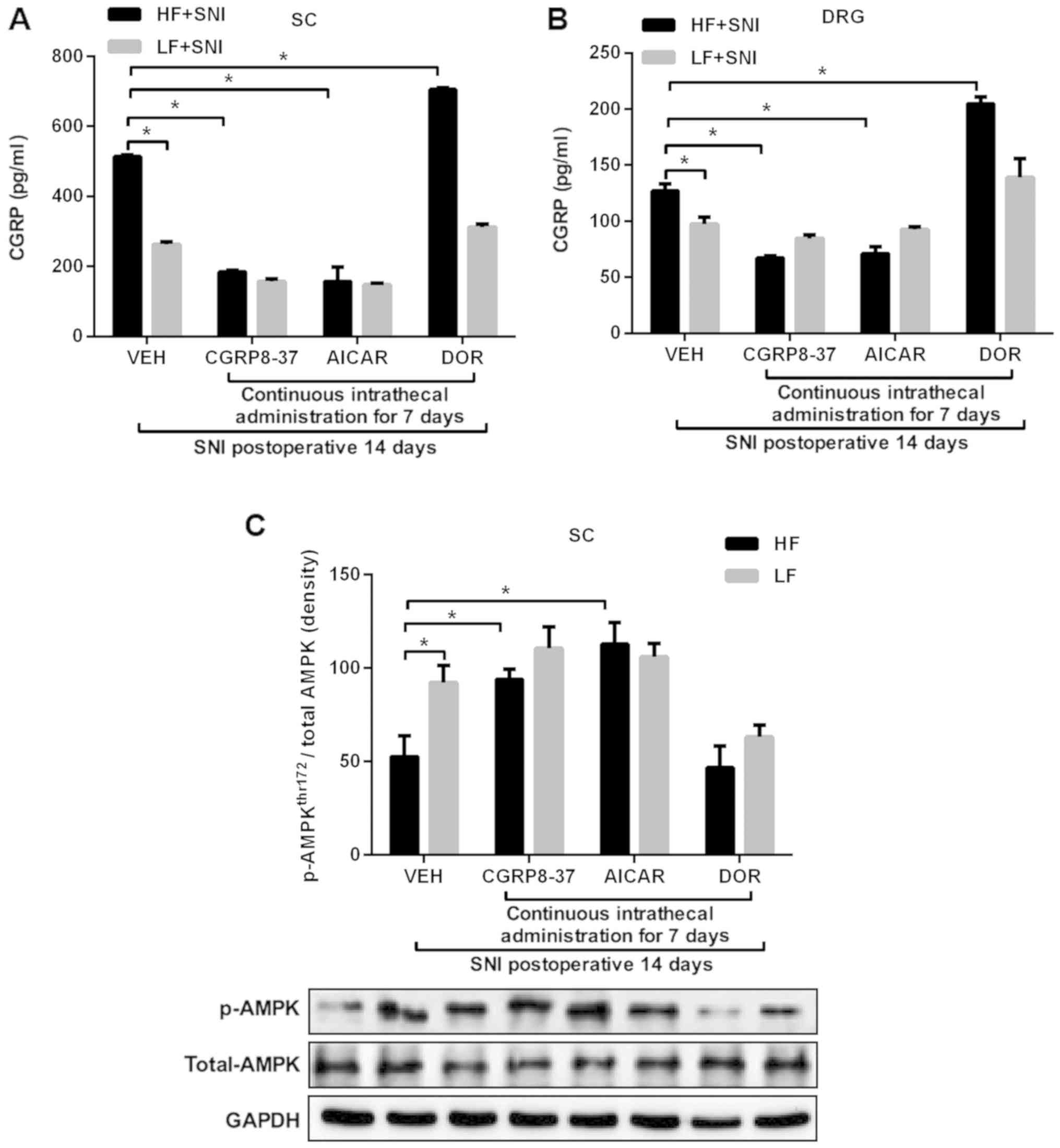
|
1
|
Schwartz MW, Seeley RJ, Zeltser LM,
Drewnowski A, Ravussin E, Redman LM and Leibel RL: Obesity
Pathogenesis: An endocrine society scientific statement. Endocr
Rev. 38:267–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roane DS and Porter JR: Nociception and
opioid-induced analgesia in lean (Fa/-) and obese (fa/fa) Zucker
rats. Physiol Behav. 38:215–218. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Croci T and Zarini E: Effect of the
cannabinoid CB1 receptor antagonist rimonabant on nociceptive
responses and adjuvant-induced arthritis in obese and lean rats. Br
J Pharmacol. 150:559–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iannitti T, Graham A and Dolan S:
Increased central and peripheral inflammation and inflammatory
hyperalgesia in Zucker rat model of leptin receptor deficiency and
genetic obesity. Exp Physiol. 97:1236–1245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Zhang Q, Zhao L, Li D, Fu Z and
Liang L: Down-regulation of PPARα in the spinal cord contributes to
augmented peripheral inflammation and inflammatory hyperalgesia in
diet-induced obese rats. Neuroscience. 278:165–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Song C, Li H, Hou J and Li D:
Ursolic acid prevents augmented peripheral inflammation and
inflammatory hyperalgesia in high-fat diet-induced obese rats by
restoring downregulated spinal PPARα. Mol Med Rep. 13:5309–5316.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hozumi J, Sumitani M, Matsubayashi Y, Abe
H, Oshima Y, Chikuda H, Takeshita K and Yamada Y: Relationship
between neuropathic pain and obesity. Pain Res Manag.
2016:24879242016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim M and Tian R: Targeting AMPK for
cardiac protection: Opportunities and challenges. J Mol Cell
Cardiol. 51:548–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kialka M, Doroszewska K, Janeczko M and
Milewicz T: Metformin-new potential medicine in pain treatment?
Przegl Lek. 74:81–83. 2017.(In Polish). PubMed/NCBI
|
|
10
|
Price TJ, Das V and Dussor G: Adenosine
Monophosphate-activated Protein Kinase (AMPK) Activators For the
Prevention, Treatment and Potential Reversal of Pathological Pain.
Curr Drug Targets. 17:908–920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asiedu MN, Dussor G and Price TJ:
Targeting AMPK for the alleviation of pathological pain. Exp Suppl.
107:257–285. 2016.PubMed/NCBI
|
|
12
|
Yang YJ, Hu L, Xia YP, Jiang CY, Miao C,
Yang CQ, Yuan M and Wang L: Resveratrol suppresses glial activation
and alleviates trigeminal neuralgia via activation of AMPK. J
Neuroinflammation. 13:842016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fei W, Tian DR, Tso P and Han JS:
Diet-induced obese rats exhibit impaired LKB1-AMPK signaling in
hypothalamus and adipose tissue. Peptides. 35:23–30. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song T, Lv LY, Xu J, Tian ZY, Cui WY, Wang
QS, Qu G and Shi XM: Diet-induced obesity suppresses sevoflurane
preconditioning against myocardial ischemia-reperfusion injury:
Role of AMP-activated protein kinase pathway. Exp Biol Med
(Maywood). 236:1427–1436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Decosterd I and Woolf CJ: Spared nerve
injury: An animal model of persistent peripheral neuropathic pain.
Pain. 87:149–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Storkson RV, Kjorsvik A, Tjolsen A and
Hole K: Lumbar catheterization of the spinal subarachnoid space in
the rat. J Neurosci Methods. 65:167–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanai Y, Nakazato E, Fujiuchi A, Hara T
and Imai A: Involvement of an increased spinal TRPV1 sensitization
through its up-regulation in mechanical allodynia of CCI rats.
Neuropharmacology. 49:977–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossi HL, Luu AK, DeVilbiss JL and Recober
A: Obesity increases nociceptive activation of the trigeminal
system. Eur J Pain. 17:649–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baron R, Binder A and Wasner G:
Neuropathic pain: Diagnosis, pathophysiological mechanisms, and
treatment. Lancet Neurol. 9:807–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gavini CK, Bookout AL, Bonomo R, Gautron
L, Lee S and Mansuy-Aubert V: Liver X receptors protect dorsal root
ganglia from obesity-induced endoplasmic reticulum stress and
mechanical allodynia. Cell Rep. 25:271–277 e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Price TJ and Dussor G: AMPK: An emerging
target for modification of injury-induced pain plasticity. Neurosci
Lett 557 Pt A. 9–18. 2013. View Article : Google Scholar
|
|
23
|
Melemedjian OK, Asiedu MN, Tillu DV,
Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow
T, et al: Targeting adenosine monophosphate-activated protein
kinase (AMPK) in preclinical models reveals a potential mechanism
for the treatment of neuropathic pain. Mol Pain. 7:702011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Melemedjian OK, Tillu DV, Asiedu MN,
Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S and Price TJ: BDNF
regulates atypical PKC at spinal synapses to initiate and maintain
a centralized chronic pain state. Mol Pain. 9:122013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maixner DW, Yan X, Gao M, Yadav R and Weng
HR: Adenosine monophosphate-activated protein kinase regulates
interleukin-1beta expression and glial glutamate transporter
function in rodents with neuropathic pain. Anesthesiology.
122:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren H, Jin H, Jia Z, Ji N and Luo F:
Pulsed Radiofrequency Applied to the Sciatic Nerve Improves
Neuropathic Pain by Down-regulating The Expression of Calcitonin
Gene-related Peptide in the Dorsal Root Ganglion. Int J Med Sci.
15:153–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pezet S and McMahon SB: Neurotrophins:
mediators and modulators of pain. Annu Rev Neurosci. 29:507–538.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SE and Kim JH: Involvement of
substance P and calcitonin gene-related peptide in development and
maintenance of neuropathic pain from spinal nerve injury model of
rat. Neurosci Res. 58:245–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun RQ, Lawand NB and Willis WD: The role
of calcitonin gene-related peptide (CGRP) in the generation and
maintenance of mechanical allodynia and hyperalgesia in rats after
intradermal injection of capsaicin. Pain. 104:201–208. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu P, Bembrick AL, Keay KA and McLachlan
EM: Immune cell involvement in dorsal root ganglia and spinal cord
after chronic constriction or transection of the rat sciatic nerve.
Brain Behav Immun. 21:599–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vincent HK and Taylor AG: Biomarkers and
potential mechanisms of obesity-induced oxidant stress in humans.
Int J Obes. 30:400–418. 2006. View Article : Google Scholar
|
|
32
|
Natoli R, Fernando N, Dahlenburg T, Jiao
H, Aggio-Bruce R, Barnett NL, Chao de la Barca JM, Tcherkez G,
Reynier P, Fang J, Chu-Tan JA, et al: Obesity-induced metabolic
disturbance drives oxidative stress and complement activation in
the retinal environment. Mol Vis. 24:201–217. 2018.PubMed/NCBI
|
|
33
|
Dandona P, Mohanty P, Ghanim H, Aljada A,
Browne R, Hamouda W, Prabhala A, Afzal A and Garg R: The
suppressive effect of dietary restriction and weight loss in the
obese on the generation of reactive oxygen species by leukocytes,
lipid peroxidation, and protein carbonylation. J Clin Endocrinol
Metab. 86:355–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kiasalari Z, Rahmani T, Mahmoudi N,
Baluchnejadmojarad T and Roghani M: Diosgenin ameliorates
development of neuropathic pain in diabetic rats: Involvement of
oxidative stress and inflammation. Biomed Pharmacothe. 86:654–661.
2017. View Article : Google Scholar
|
|
35
|
Siotto M, Aprile I, Simonelli I, Pazzaglia
C, Ventriglia M, Santoro M and Padua L: An exploratory study of
BDNF and oxidative stress marker alterations in subacute and
chronic stroke patients affected by neuropathic pain. J Neural
Transm (Vienna). 124:1557–1566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao B, Pan Y, Wang Z, Tan Y and Song X:
Intrathecal administration of tempol reduces chronic constriction
injury-induced neuropathic pain in rats by increasing SOD activity
and inhibiting NGF expression. Cell Mol Neurobiol. 36:893–906.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SG, Wu HM, Lee CG, Oh CS, Chung SW and
Kim SG: Binge alcohol intake after hypergravity stress sustainably
decreases AMPK and transcription factors necessary for hepatocyte
survival. Alcohol Clin Exp Res. 41:76–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi L, Zhang T, Zhou Y, Zeng X, Ran L,
Zhang Q, Zhu J and Mi M: Dihydromyricetin improves skeletal muscle
insulin sensitivity by inducing autophagy via the AMPK-PGC-1α-Sirt3
signaling pathway. Endocrine. 50:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reyes C, Leyland KM, Peat G, Cooper C,
Arden NK and Prieto-Alhambra D: Ass-ociation between overweight and
obesity and risk of clinically diagnosed knee, hip, and hand
osteoarthritis: A population-based cohort study. Arthritis
Rheumatol. 68:1869–1875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fischer K, PrzepieraBędzak H, Sawicki M,
Walecka A, Brzosko I and Brzosko M: Serum Interleukin-23 in polish
patients with systemic lupus erythematosus: Association with lupus
nephritis, obesity, and peripheral vascular disease. Mediators
Inflamm. 2017:94014322017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dario AB, Ferreira ML, Refshauge KM, Lima
TS, Ordoñana JR and Ferreira PH: The relationship between obesity,
low back pain, and lumbar disc degeneration when genetics and the
environment are considered: A systematic review of twin studies.
Spine J. 15:1106–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gallagher HC, Gallagher RM, Butler M,
Buggy DJ and Henman MC: Venlafaxine for neuropathic pain in adults.
Cochrane Database Syst Rev. 8:CD0110912015.
|
|
43
|
Hozumi J, Sumitani M, Matsubayashi Y, Abe
H, Oshima Y, Chikuda H, Takeshita K and Yamada Y: Relationship
between neuropathic pain and obesity. Pain Res Manag.
2016:24879242016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bruun JM, Stallknecht B, Helge JW and
Richelsen B: Interleukin-18 in plasma and adipose tissue: Effects
of obesity, insulin resistance and weght loss. EurJ Endocrinl.
157:465–471. 2007. View Article : Google Scholar
|
|
45
|
Wisse BE: The inflammatory syndrome: The
role of adipose tissue cytokines in metabolic disorders linked to
obesity. J Am Soc Nephrol. 15:2792–2800. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barisione M, Carlini F, Gradaschi R,
Camerini G and Adami GF: Body weight at developmental age in
siblings born to mothers before and after surgically induced weight
loss. Surg Obes Relat Dis. 8:387–391. 2012. View Article : Google Scholar : PubMed/NCBI
|