Introduction
Currently, liver transplantation is a first-line
treatment for end-stage disease that provides long-term survival.
However, with increasing demand, the shortage of donor livers has
become a primary concern. Multiple strategies, including marginal
grafts [donation following circulatory death (DCD) and extended
criteria donor livers (ECD)], have been proposed to overcome the
organ shortage. As the demand for transplanted donor organs
continues to grow, hypothermic machine perfusion (HMP) has been
proposed to be a beneficial alternative to static cold storage
(SCS), particularly for marginal grafts.
HMP can be implemented to preserve kidney
transplantation grafts. It has been indicated that HMP exhibits
improved results compared with SCS. Advances have been also been
demonstrated in experimental and clinical liver studies. The
majority of studies have demonstrated that HMP could improve or
maintain a variety of post-ischemic hepatobiliary parameters,
sustain effective liver function and minimize liver damage
(1,2).
Different metabolites accumulate during circulation
on perfusion, despite the efforts to offset this by use of
counteracting substances in order to control the biochemical
environment (3,4). Previously, researchers have used
membrane dialyzers to filter these metabolites (5). The hypothesis that a filter organ can
improve perfusion blood biochemistry has been demonstrated by
adding kidney to ex vivo liver perfusion (6). Adding a filter organ to liver
circulation helps maintain a better physiological balance in the
multi-organ perfusion model, which overcomes the technical
challenges of extracorporeal perfusion of multiple organs and the
analysis of the response of two organs to the stress states
(7).
Although a number of studies (6,7) have
provided certain information regarding kidney liver (KL) perfusion,
no studies have implicated KL in HMP and the underlying mechanisms.
The present study hypothesized that KL perfusion is superior to
liver perfusion alone in protecting liver grafts from ischemic
injury. Therefore, in the present study an ex vivo KL
perfusion system was used and the initial results were presented to
verify the hypothesis and investigate the underlying
mechanisms.
Materials and methods
Animals
In the present study, 12 adult male Sprague Dawley
rats (Beijing Vital River Laboratory Animal Technology Co., Ltd.,
260–310 g) were used and given free access to water and standard
diets and were housed in a 12-h light/dark cycle with a temperature
maintained at 20–25°C and a relative humidity of 40–60%. All
experimental procedures in the present study were approved by the
Ethics Committee of the First Affiliated Hospital, College of
Medicine, Zhejiang University (2016–374) and were implemented in
accordance with the Animal Research: Reporting in vivo
experiments guidelines (www.nc3rs.org.uk/arrive-guidelines) and the AVMA
euthanasia guidelines 2013.
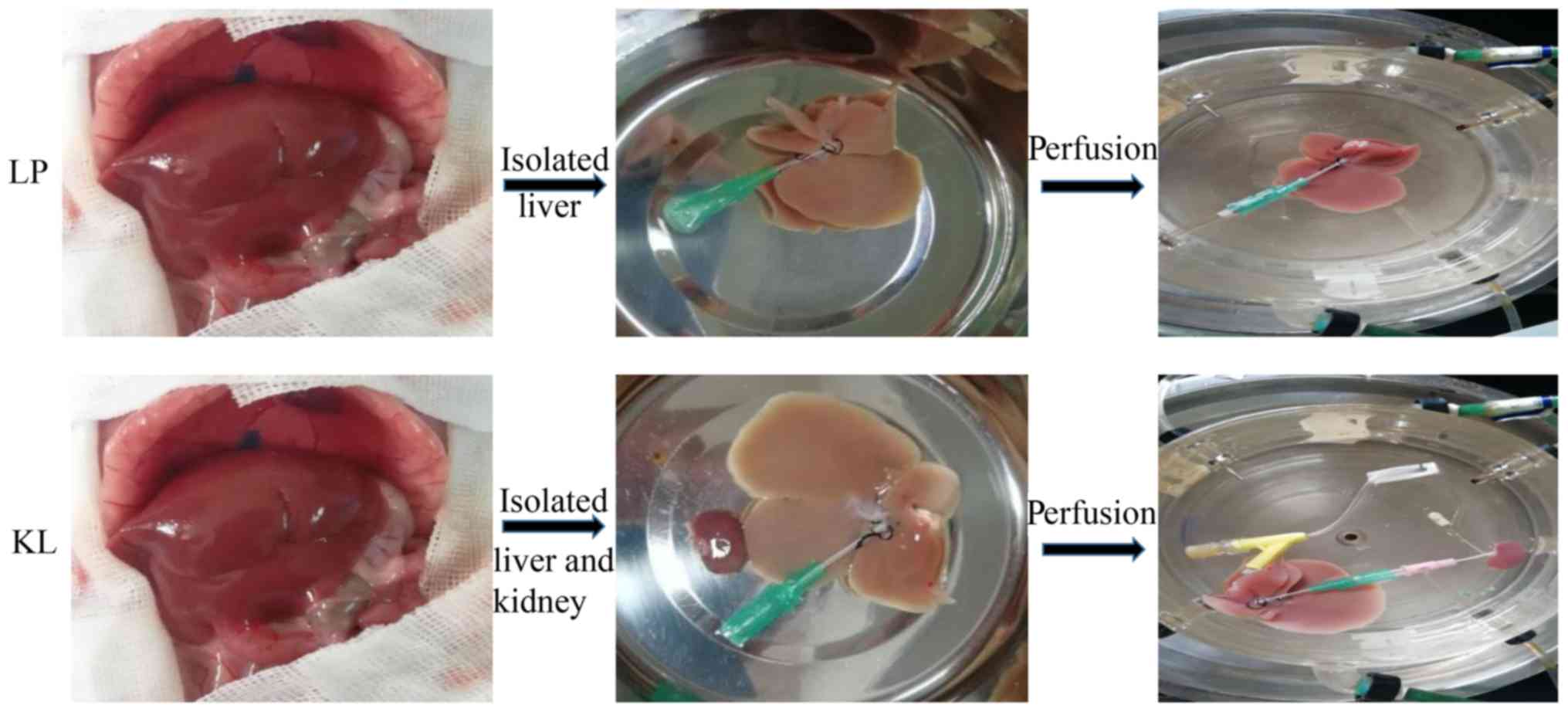
Experimental design
A total of 12 rats were randomly assigned into two
groups (n=6 for each group). In the KL group, kidneys and livers
were preserved by HMP with oxygenated
histidine-tryptophanketoglutarate solution perfusate (95%
O2 and 5% CO2) for hypothermic oxygenated
perfusion for 6 h. In the LP group, livers were preserved as
described in the KL group for 6 h (Fig. 1).
Organ retrieval and preservation
Rats were anesthetized with 10% chloral hydrate (200
mg/kg, Shanghai No. 1 Biochemical and Pharmaceutical Company,
Shanghai, China) and none of them exhibited signs of peritonitis.
The kidney and liver grafts were retrieved according to the
protocols described by Mahboub et al (8) and Kamada et al (9), respectively. After the kidney and
liver were isolated, the rats were euthanized by cervical
dislocation. The grafts were perfused through the dorsal penile
vein with cooled saline containing 25 U/ml heparin. In the KL
group, the renal artery and ureter were cannulated, connected to
the oxygenated perfusate (95% O2 and 5% CO2),
recirculated and connected to liver graft via the portal vein (PV;
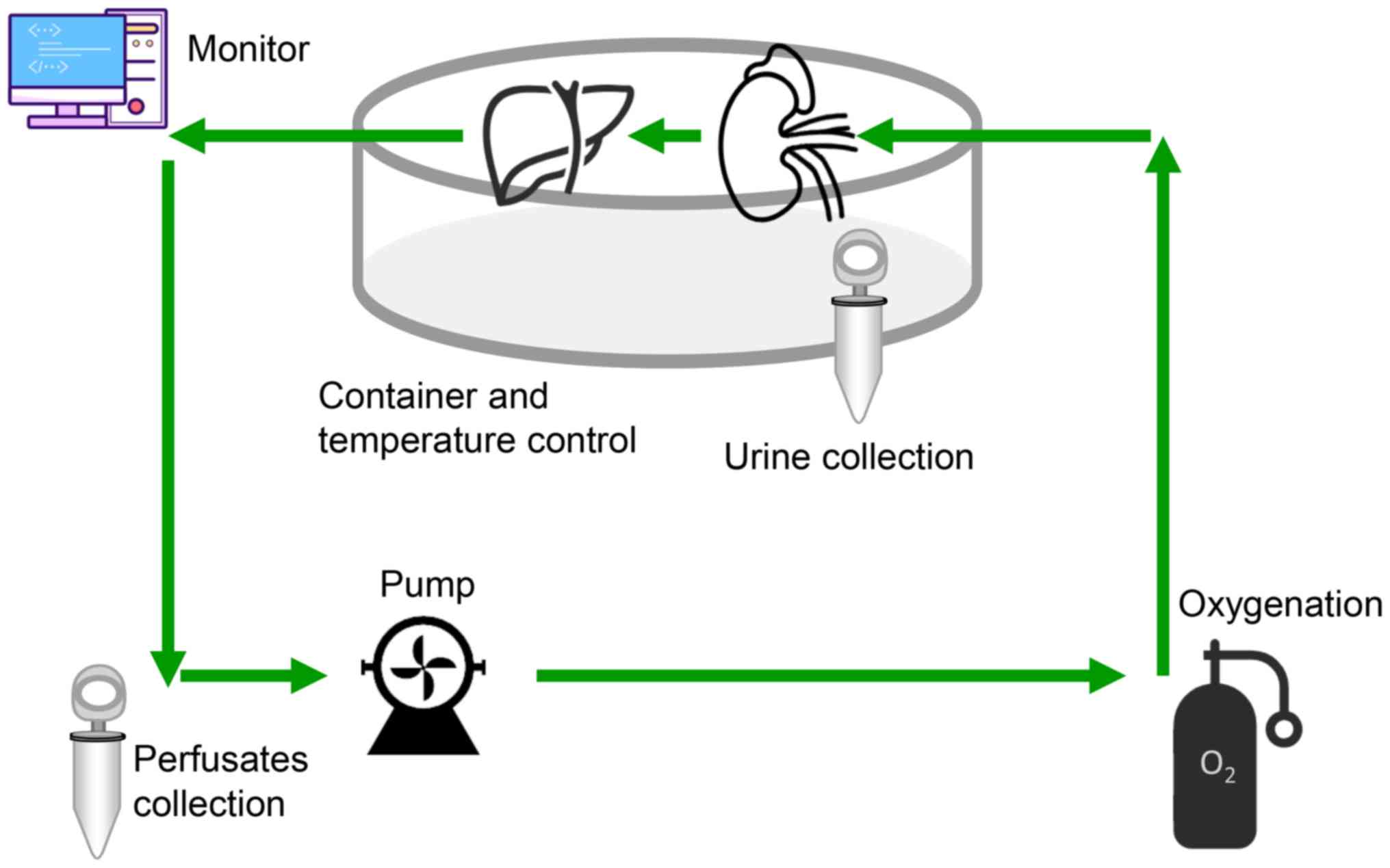
Fig. 2). HMP was performed using a
machine perfusion transporter that was constructed as described
previously (2), which included a
cryostat (DC-1015, Shanghai Bilon Instrument Co., Ltd., Shanghai,
China), a four-channel physiology recorder (BL-420S, Chengdu
Taimeng Software Co., Ltd., Chengdu, China) and a BT200-2J low flow
peristaltic pump (Xi'an Yima Opto-electrical Co., Ltd., Xi'an,
China). The perfusion velocity was set at 5 rpm (3.5 ml/min) and
the pressure of the PV was recorded using the computer.
Sample collection
At 0, 1, 3 and 6 h during the perfusion process, 2
ml perfusate samples were collected from the two groups for the
analysis of pH, K+, alanine aminotransferase (ALT),
aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and
adenosine triphosphate (ATP). Liver tissues were obtained at the
end of the preservation and fixed in 10% neutral formalin at room
temperature (18–25°C) for 24 h for immunohistochemical analyses.
The other liver tissues were stored at −80°C for further
experimental analysis.
Immunofluorescence examination and
liver function tests
Excised liver specimens were fixed in 4%
paraformaldehyde at room temperature (18–25°C) for 24 h prior to
being paraffin-embedded and sectioned (thickness, 3 µm). The
sections were deparaffinized, hydrated gradually and examined using
routine Ki67 immunofluorescence staining as previously described
(10). Perfusate samples were
collected for ALT, AST, LDH, BUN and CR analysis using the Hitachi
7600 automatic analyzer (Hitachi, Ltd., Tokyo, Japan).
ATP and portal vessel resistance
(VR)
Portal VR was equal to portal vein pressure/velocity
(ml/min). ATP expression was measured using an ATP kit (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocols.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) assay
Identification of hepatocyte apoptosis was performed
using the TUNEL assay (Roche Diagnostics, Basel, Switzerland) as
previously described (11).
Briefly, the tissue sections were fixed with paraformaldehyde (4%
in PBS, pH 7.4, freshly prepared) for 20 min at 15–25°C. Then they
were washed for 30 min with PBS. The slides were incubated in
Permeabilization solution (0.1% Triton X-100 in 0.1% sodium
citrate, freshly prepared) for 2 min on ice (4°C). The slides were
rinsed twice with PBS. Then 50 µl TUNEL reaction mixture (45 µl
TUNEL Label with 5 µl TUNEL Enzyme) was added to the sample. The
section was incubated for 60 min at 37°C in a humidified chamber in
the dark. The slide was then rinsed 3 times with PBS. DAPI (0.5–10
µg/ml) was added and the slide incubated for 10 min at room
temperature in a humidified chamber in the dark. The slide was then
rinsed 3 times with PBS. Apoptotic hepatocytes were examined using
a fluorescence microscope at a magnification, ×200.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was derived from liver homogenates using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
qPCR was performed using a SYBR Green PCR kit (Takara Bio, Inc.,
Otsu, Japan). The gene expression levels of Caspase 3, B-cell
lymphoma-2-associated X (Bax), P21 and P27 were quantified using a
7900 Fast Real-Time PCR instrument. The following primers were
used: Caspase 3, forward, 5′-CGGTATTGAGACAGACAGTGGA-3′ and reverse,
5′-CGCAAAGTGACTGGATGAAC-3′; Bax, forward,
5′-TTTGCTACAGGGTTTCATCCA-3′ and reverse,
5′-TGTCCAGTTCATCGCCAAT3-3′; P21, forward
5′-AAAGTATGCCGTCGTCTGTTC-3′ and reverse,
5′-AAGTCAAAGTTCCACCGTTCTC-3′; P27, forward,
5′-ACGGAGCAGAACCCAACT-3′ and reverse, 5′-ACCATTAGCGTGTCCAGG-3′; and
β-actin, forward, 5′-ACGGTCAGGTCATCACTATCG-3′ and reverse,
5′-GAGGTCTTTACGGATGTCAACG-3′. Gene expression was quantified using
the following conditions: 1 cycle of 95°C for 30 sec; 40 cycles of
95°C for 5 sec; and 1 cycle of 60°C for 30 sec. The method of
quantification was performed as previously described (12).
IL-6, hepatocyte growth factor (HGF)
and tumor necrosis factor-α (TNF-α)
Liver lysates were quantified. Protein
concentrations of IL-6, HGF and TNF-α were determined using the
IL-6 (R6000B), HGF (MHG00) and TNF-α (DY510) ELISA assay kits
(R&D systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Western blot analysis
Liver tissues were homogenized in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Following centrifugation (at 15,000 × g for 15 min
at 4°C), protein concentrations were quantified using a
bicinchoninic acid (BCA) protein assay kit (Thermo Fisher
Scientific, Inc.). Proteins (25 µg) were separated by 10% gel
electrophoresis and transferred onto nitrocellulose membranes.
Following blocking with skimmed milk (232100; BD Biosciences,
Franklin Lakes, NJ, USA) at room temperature (18–25°C) for 2 h,
membranes were incubated with primary antibodies at 4°C overnight
under shaking conditions. The membranes were incubated with cleaved
caspase-3 (1:2,000; 9661; Cell Signaling Technology, Inc., Danvers,
MA, USA), caspase-3 (1:1,000; 9662; Cell Signaling Technology,
Inc.), Bcl-2 (1:500; ab32124; Abcam, Cambridge, MA, USA), Bax
(1:500; ab32503, Abcam), phosphorylated (p)-stat3 (1:2,000; 9145;
Cell Signaling Technology, Inc.), total (t)-Stat3 (1:1,000; 9139;
Cell Signaling Technology, Inc.) and β-actin (1:1,000; 4970; Cell
Signaling Technology, Inc.) primary antibodies. Following this, the
blots were washed in TBS-T and incubated with appropriate
horseradish peroxidase (HRP)-linked anti-mouse (1:4,000; ab6728;
Abcam) or anti-rabbit (1:4,000; ab6721; Abcam) secondary antibodies
for 1 h at room temperature. Then these bands were visualized by
chemiluminescence using an enhanced chemiluminescence kit (Pierce;
Thermo Fisher Scientific, Inc.). Image J (National Institutes of
Health, Bethesda, MD, USA; version 1.48) was used for semi
quantitative analysis.
Statistical analysis
Statistical analysis of the data was performed using
SPSS version 20.0 (IBM Corp., Armonk, NY, USA). A total of 6
animals each group were used for the experimental and experimental
repeats 3 times for each animal. The data were represented as the
mean ± standard error of the mean. Data was analyzed using the
one-way analysis of variance followed by the least squares
difference or Dunnett's test for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of KL on pH and
K+
Overall pH values of the KL group were significantly
different from the LP group and more stable (P<0.05). The
difference resulted from a drop in the LP group at 6 h of perfusion
compared with pre-perfusion levels and the KL group. Such a drop
was not identified in the KL group, with no modifications during
the perfusion (Fig. 3A). The same
trend was indicated regarding K+ values (Fig. 3B).
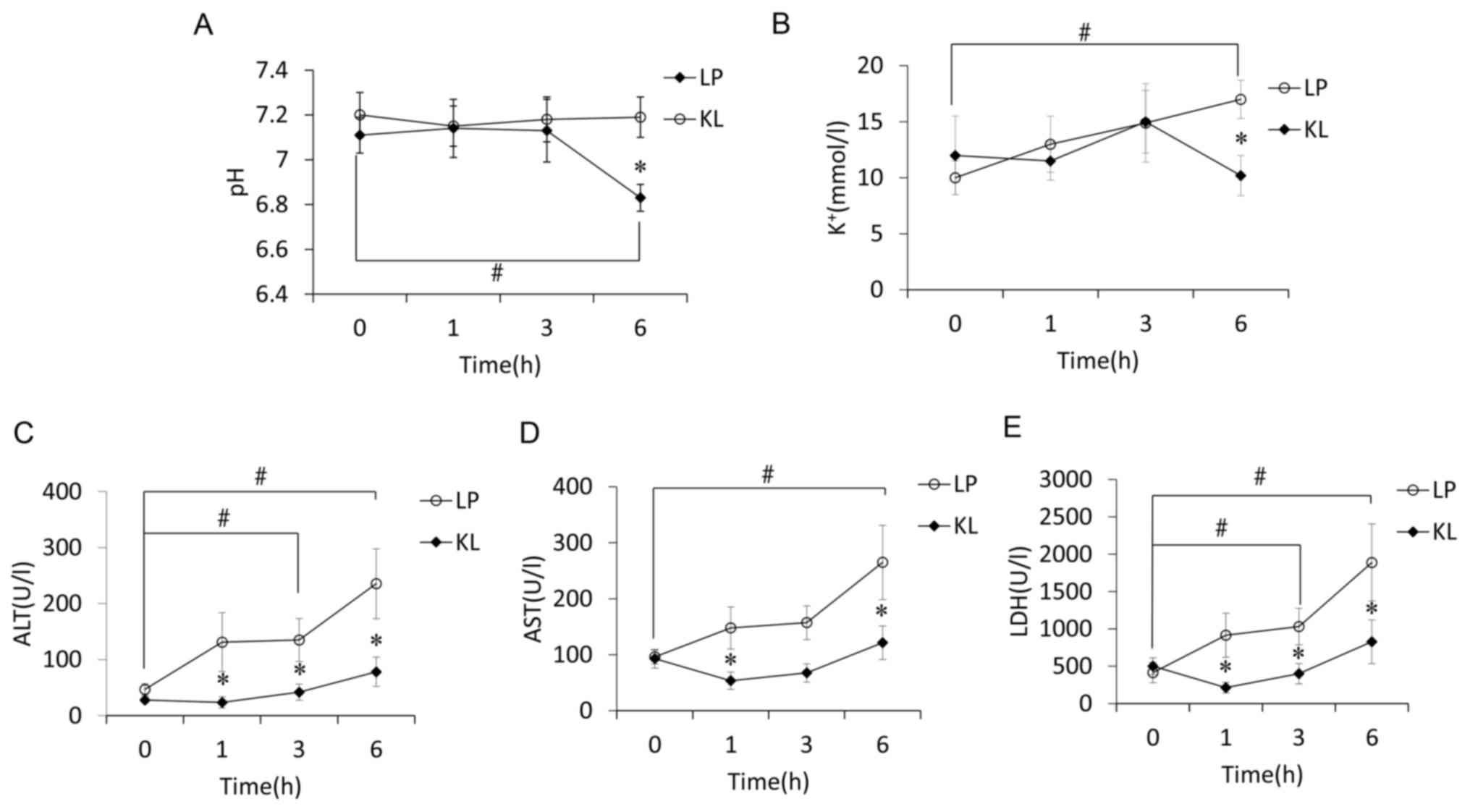 | Figure 3.pH, K+ and liver function
tests are performed over time. The level of (A) pH, (B)
K+, (C) ALT, (D) AST and (E) LDH in KL and LP groups
over time. Circles or boxes represented the mean values, whereas
bars indicated the mean ± standard error of mean. *P<0.05 vs. LP
group at the same time point; #P<0.05 vs.
pre-perfusion levels (0 h). LP, liver perfusion; KL, kidney liver
perfusion; LDH, lactate dehydrogenase; AST, aspartate
aminotransferase; ALT, alanine transaminase. |
Effects of KL on liver function
ALT levels increased progressively over time in the
LP group. Levels were significantly different at 1, 3 and 6 h
compared with pre-perfusion levels and with the KL group at the
same time points (P<0.05; Fig.
3C). AST levels also increased progressively over time in the
LP group. The levels were significantly different at 6 h compared
with pre-perfusion levels and with the KL group at 1 and 6 h
(P<0.05; Fig. 3D). LDH
exhibited the same trend as ALT levels (Fig. 3E).
Effects of KL on VR and ATP
The mean portal pressure of the LP and KL groups was
5 and 4.1 mmHg, respectively (Fig.
4A). Portal VR levels between the two groups was indicated in
Fig. 4B. The KL group exhibited a
significantly lower VR compared with the LP group (P<0.05). ATP
levels between the two groups are presented in Fig. 4C and the higher ATP level was
indicated in the KL group compared with the LP group
(P<0.05).
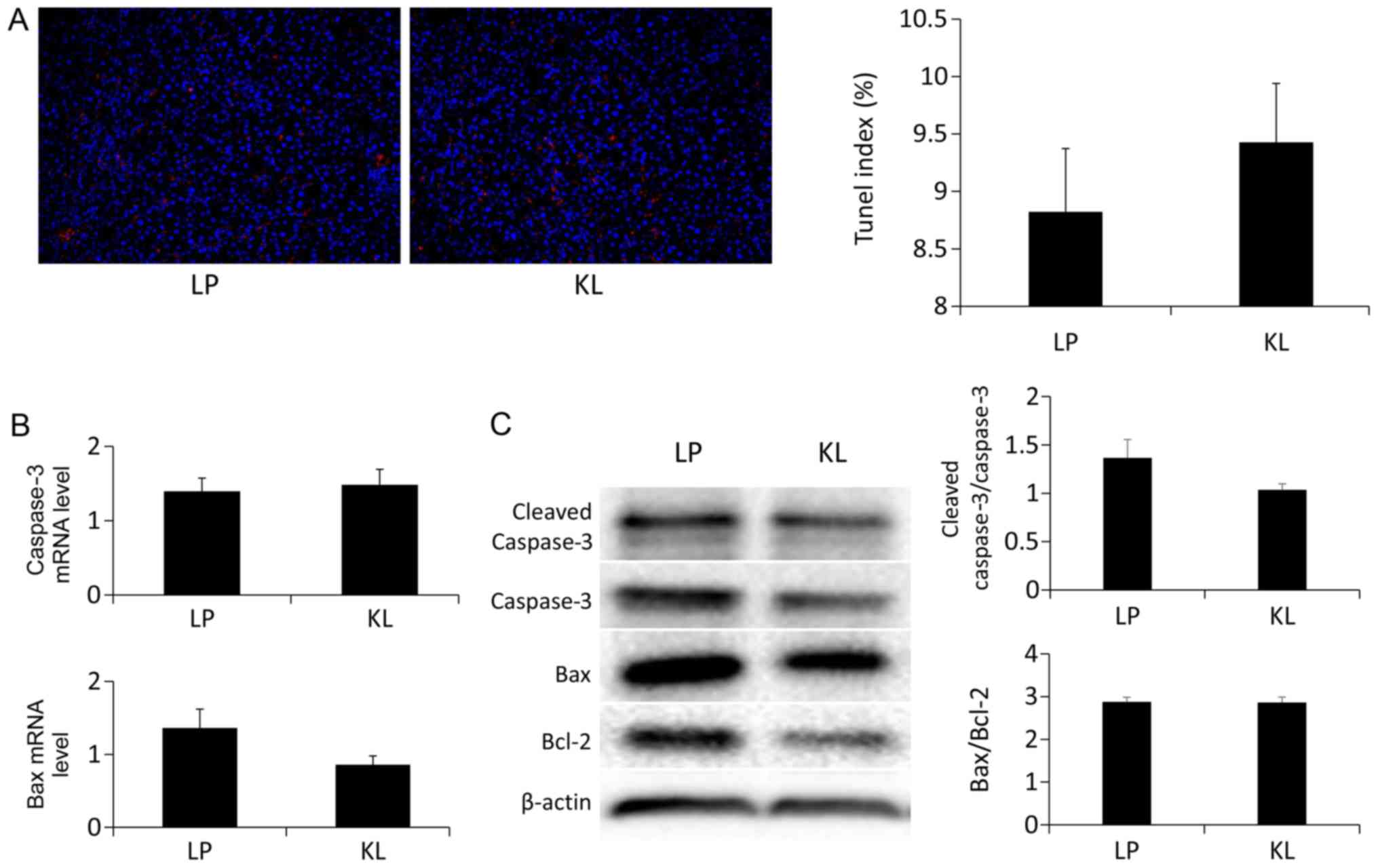
Effects of KL on hepatocyte apoptosis
and proliferation
No significant difference was indicated in the
number of the TUNEL+ hepatocytes in the KL and LP groups
(Fig. 5A). Similarly, the same
trend was identified in the mRNA (Fig.
5B) and protein expression (Fig.
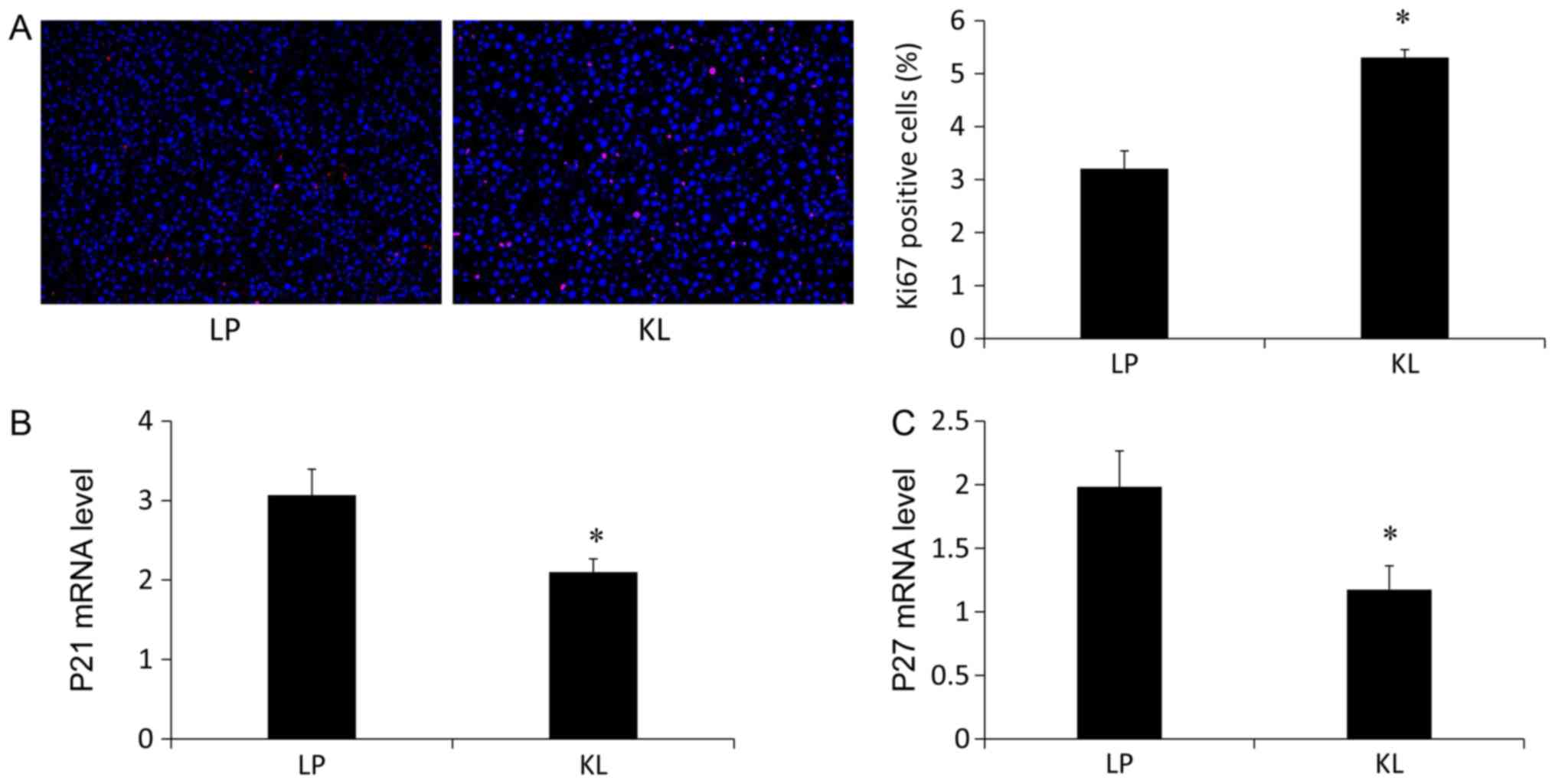
5C) levels of apoptosis-associated markers (13). Notably, the percentage of
Ki67+ hepatocytes was increased in the KL group compared
with the LP group (Fig. 6A). The
mRNA expression levels of P21 (Fig.
6B) and P27 (Fig. 6C) were
also measured, which were significantly reduced in the KL group
compared with the LP group (P<0.05).
Effects of KL on hepatocyte
proliferation signaling
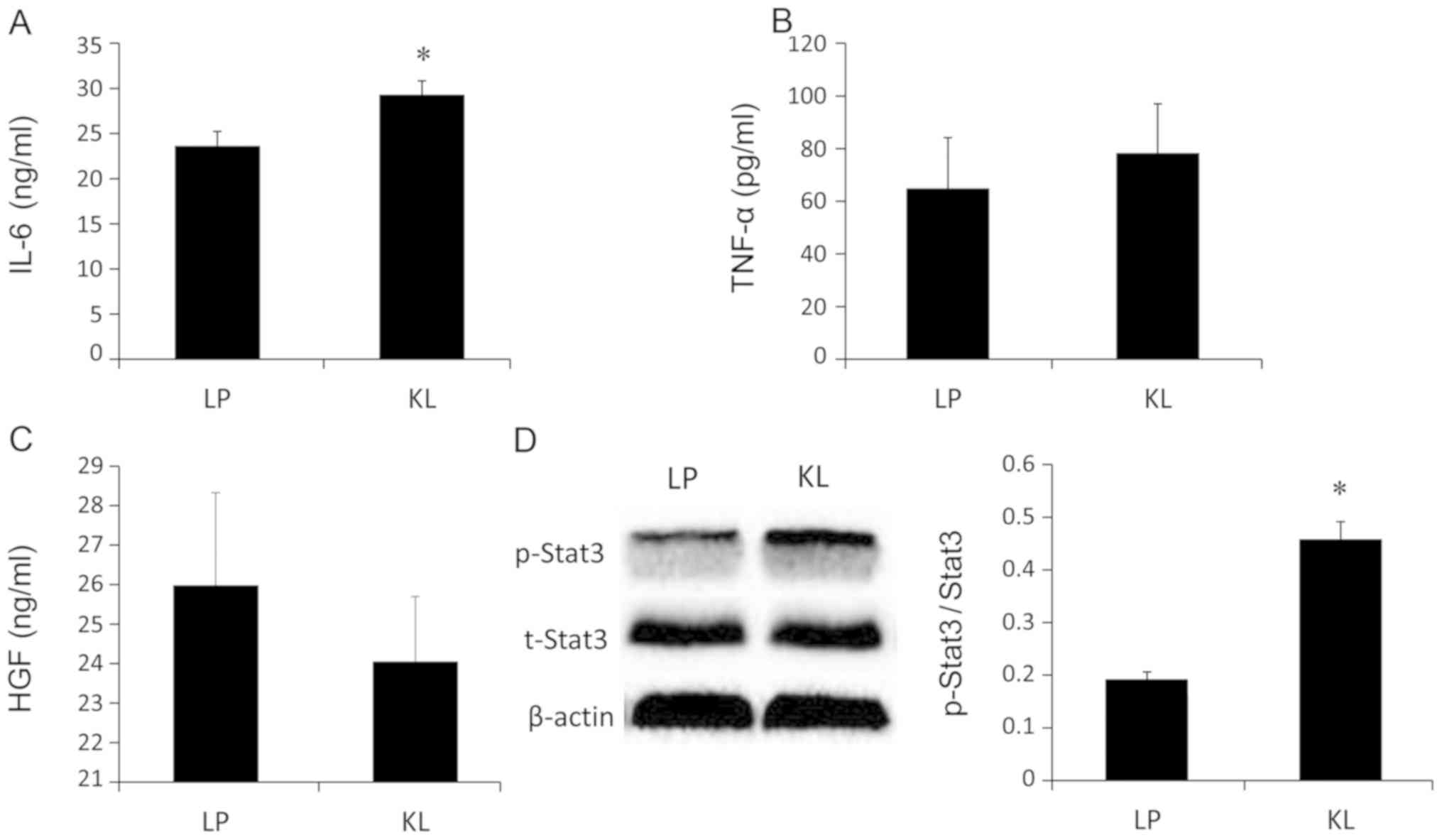
IL-6, HGF and TNF-α levels were examined in liver
tissue lysates. The IL-6 levels were significantly increased in the
KL group compared with the LP group (P<0.05; Fig. 7A). However, no significant changes
in the HGF and TNF-α levels were indicated between the KL and LP
groups (Fig. 7B and C). Similarly,
the same trend was identified in the protein levels of
p-Stat3/t-Stat3 (Fig. 7D).
Furthermore, the present results indicated that there was no damage
to the kidneys of the KL group compared with the LP group (Fig. 8).
Discussion
In the present study, overall pH and K+
values of the KL group were different compared with the LP group
and more stable. ALT, AST and LDH levels increased progressively
over time in the LP group and were different at different time
points compared with pre-perfusion levels and the KL group. The
results indicated that KL group was superior to the LP group. KL
reduced portal VR and was associated with lower ATP consumption
compared with the LP group. The results also suggested the lack of
involvement of hepatocyte apoptosis. In addition, for the first
time to the best of our knowledge liver proliferation was
identified to be upregulated in KL compared with LP ex
vivo.
Over the past decade, the number of liver
transplants has been relatively stagnant. Notably, the requirement
for surgery has expanded; however, there are few liver grafts
available. Therefore, there is an urgent need to identify an
effective way to increase the viability and usability of marginal
grafts (DCD and ECD livers). MP has been proposed to be a
beneficial alternative to SCS, particularly for marginal
grafts.
Several studies have indicated that the use of HMP
is associated with improved liver function and minimized liver
damage (1,2). However, due to the absence of
homeostatic organs, the persistence of the urea cycle and the high
insulin resistance to glycogenolytic leads to a steady increase in
circulating urea and glucose levels, which contributes to the
steady rise of osmotic pressure in hepatic cells (14). The more important metabolites,
hydrogen ions and electrolytes, also tend to accumulate, which
leads to acid-base and electrolyte balance alterations during
circulation on perfusion, despite the efforts to offset these
effects with the use of counteracting substances (including
bicarbonate) to control the biochemical environment, which
unfortunately leads to further deviations from normal physiology
(15). Previous studies have
demonstrated that the kidneys synchronously participate with the
liver as a natural filter of waste that accumulates during
perfusion, purging the circuit and avoiding the accumulation of
counteracting substances (6,7). In
order to improve acid-base and electrolyte balance alterations that
occur during perfusion, and possibly affect organ survival and
function, a second organ-kidney with a homeostatic function was
added to the circulation. Compared with previous studies, the pH
value and K+ levels of the KL group were identified to
be more linear and the levels were more similar over time compared
with the LP group, which suggested more stable circulation
conditions within the KL group. The liver lacks any organs that
control homeostatic pH (kidneys or lungs) during MP and therefore
complex acid-base alterations have not yet been compensated
(16). In the KL group, a
functional kidney ensures that excess HCO3−
is excreted into the urine. In the present study, this was
suggested by the flatter appearance of the pH curve compared with
the LP group. Similarly, the levels of K+ in the KL
group were maintained at a greater stability compared with the
separate liver circuits. However, it remains uncertain whether a
more physiological environment can translate into a
better-preserved organ.
Based on the effects that were observed on the pH
value and K+ levels during MP, whether KL-induced
physiological milieu participated in the protection against liver
damage induced by ischemic injury was next examined. He et
al (17) demonstrated improved
hepatocyte and biliary epithelial preservation by liver-kidney
normothermic machine perfusion (NMP) compared with liver NMP alone
using hematoxylin and eosin, and TUNEL staining. In addition, liver
function tests during liver preservation following reperfusion or
transplantation revealed that improved liver function was observed
in liver-kidney NMP compared with liver NMP alone (18). In agreement with this study, it was
demonstrated that ALT, AST and LDH levels increased progressively
over time in the LP group and were different at different time
points compared with pre-perfusion levels and the KL group.
Furthermore, it was also indicated that KL was superior to LP
regarding VR and ATP levels. Lack of ATP and direct inhibition at
low temperatures can impair the function of
Na+-K+ pumps, which are key components
involved in preventing cell swelling. The restored ATP content has
been indicated to be a good indicator of mitochondrial respiratory
function and is associated with the reduction of oxidative stress.
Furthermore, the poor recovery of ATP is associated with poor liver
function (18). In the present
study, KL reduced VR efficiently, which was associated with lower
ATP consumption compared with LP. This may be due to its
physiological milieu.
It was speculated that the KL-induced physiological
milieu may lower liver apoptosis compared with the LP group. A
TUNEL assay was used to confirm the extent of apoptosis of various
cell types by detecting late events, in which major DNA
fragmentation occurred (19). The
results of the present study demonstrated that no significant
difference was identified in the percentage of the
TUNEL+ cells. Notably, apoptosis is triggered by the
pro-apoptotic protein Bax, which is transported from the cytoplasm
to the mitochondria and is induced by cytochrome c. This promotes
the activation of procaspase-9 and −3 (20).
The caspase family serves an important role in
apoptosis. These factors are associated with identifying and
determining apoptosis in cells. Caspase-3 is particularly important
because it is involved in intrinsic and extrinsic apoptotic
pathways (21). In the present
study, the mRNA and expression levels of Bax and caspase-3 were
measured, which are commonly used targets for detecting apoptosis.
The results of the present study confirmed no significant
difference was indicated.
Since apoptosis could not explain the protective
effect of KL, KL-regulated hepatocyte proliferation response and
cell cycle regulators was assessed. Liver regeneration was
evaluated using Ki67 staining. The number of Ki67+
hepatocytes was increased in the KL group compared with the LP
group. Furthermore, the expression levels of P21 and P27, which are
known cell cycle inhibitors were measured. The mRNA expression
levels of P21 and P27 were reduced in the KL group compared with
the LP group. The results indicated that KL induced regeneration,
not apoptosis and exhibited a better protective effect against
liver injury compared with LP.
TNF-α and IL-6 are the major cytokines that can
trigger normally quiescent hepatocytes to enter cell cycle arrest
due to liver injury (22). HGF,
which is synthesized in response to IL-6 in the liver, is also a
key factor for liver growth and function (23). Examination of cytokine production
in the liver revealed that the IL-6 levels under combined perfusion
were raised compared with liver perfusion alone. However, no
significant changes of HGF and TNF-α were observed between the two
groups. Notably, IL-6 stimulates compensatory hepatocyte
proliferation by activating Stat3, which serves a key role in
hepatocyte proliferation during liver regenerative responses
(24). Accordingly, the protein
expression levels of Stat3 were measured. The present results
revealed the same trend as indicated for the IL-6 levels. These
results suggested that KL induced liver regeneration by
upregulating the IL-6/Stat3 signaling pathway.
There are several limitations including the lack of
a control group-SCS, which was demonstrated in the authors'
previous study that HMP is superior to SCS in maintaining the
architecture and function of liver grafts (2), and the lack of blood coagulation and
bilirubin measurements to assess liver function. Further future
experiments validating the participation of the proposed signaling
pathway is also needed. Nevertheless, this study provides clear
clues for future studies.
In conclusion, different metabolites tend to
accumulate during circulation on perfusion, despite the efforts to
offset by use of counteracting substances to control the
biochemical environment. Previously, researchers used the membrane
dialyzers to filter these metabolites. Then the hypothesis that a
filter organ can better improve perfusion blood biochemistry has
been identified by adding a kidney to the ex vivo liver
perfusion. Adding this filter organ to the liver circulation helps
maintain a better physiological balance in the novel multi-organ
perfusion model, which overcomes the technical challenges of
extracorporeal perfusion of multiple organs and the analysis of the
response of two organs to the stress states. To the best of our
knowledge this study revealed for the first time that combined KL
HMP provided a more proactive repair capability by maintaining
liver regeneration via upregulation of the IL-6/Stat3 signaling
pathway. Accordingly, this KL HMP model could be a potentially
important strategy for biochemical reconditioning of the grafts and
may be potentially applicable in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81421062 and
81470891); the Science and Technology Bureau of Zhejiang Province,
China (grant no. 2016C33145); the 863 National High Technology
Research and Development Program of China for young scientist
(grant no. 2015AA020923); the Public Technology Research Projects
(grant no. LGF18C100001); and the China Postdoctoral Science
Foundation (grant no. 2017M610374).
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
JL, JJ, NH, LZ and SZ contributed in the protocol
design; experimental design and implementation; manuscript
drafting; critical revisions of manuscript final approval of
manuscript. LJ, HY, HL and HX contributed to the conception;
critical revisions of manuscript; funding securement; and final
approval of manuscript. NH, YP and HX contributed in the data
acquisition; data analysis and statistics; data interpretation;
final approval of manuscript; and funding securement.
Ethics approval and consent to
participate
All experimental procedures in the present study
were approved by the Ethics Committee of The First Affiliated
Hospital, College of Medicine, Zhejiang University (2016–374) and
were implemented in accordance with the Animal Research: Reporting
In Vivo Experiments guidelines (http://www.nc3rs.org/ARRIVE) and the AVMA euthanasia
guidelines 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests and all authors confirm its accuracy.
References
|
1
|
Schlegel A and Dutkowski P: Role of
hypothermic machine perfusion in liver transplantation. Transpl
Int. 28:677–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jia JJ, Zhang J, Li JH, Chen XD, Jiang L,
Zhou YF, He N, Xie HY, Zhou L and Zheng SS: Influence of perfusate
on liver viability during hypothermic machine perfusion. World J
Gastroenterol. 21:8848–8857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hosgood SA, Bagul A and Nicholson ML:
Minimising cold ischaemic injury in an experimental model of kidney
transplantation. Eur J Clin Invest. 41:233–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newsome PN, Henderson NC, Nelson LJ, Dabos
C, Filippi C, Bellamy C, Howie F, Clutton RE, King T, Lee A, et al:
Development of an invasively monitored porcine model of
acetaminophen-induced acute liver failure. BMC Gastroenterol.
10:342010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wyss M and Kaddurah-Daouk R: Creatine and
creatinine metabolism. Physiol Rev. 80:1107–1213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung WY, Gravante G, Al-Leswas D, Arshad
A, Sorge R, Watson CC, Pollard C, Metcalfe MS and Dennison AR: The
development of a multiorgan ex vivo perfused model: Results with
the porcine liver-kidney circuit over 24 h. Artif Organs.
37:457–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung WY, Gravante G, Al-Leswas D, Alzaraa
A, Sorge R, Ong SL, Pollard C, Lloyd DM, Metcalfe MS and Dennison
AR: The autologous normothermic ex vivo perfused porcine
liver-kidney model: Improving the circuit's biochemical and
acid-base environment. Am J Surg. 204:518–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahboub P, Ottens P, Seelen M, t Hart N,
Van Goor H, Ploeg R, Martins PN and Leuvenink H: Gradual rewarming
with gradual increase in pressure during machine perfusion after
cold static preservation reduces kidney ischemia reperfusion
injury. PLoS One. 10:e01438592015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
10
|
Lai SS, Zhao DD, Cao P, Lu K, Luo OY, Chen
WB, Liu J, Jiang EZ, Yu ZH, Lee G, et al: PP2Acα positively
regulates the termination of liver regeneration in mice through the
AKT/GSK3β/Cyclin D1 pathway. J Hepatol. 64:352–360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NFKB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, Jiang H and Shi Y: Upregulation of
heme oxygenase-1 expression by curcumin conferring protection from
hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblasts. Cell
Biosci. 7:202017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reiling J, Lockwood DS, Simpson AH,
Campbell CM, Bridle KR, Santrampurwala N, Britton LJ, Crawford DH,
Dejong CH and Fawcett J: Urea production during normothermic
machine perfusion: Price of success? Liver Transpl. 21:700–703.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung WY, Gravante G, Eltweri A, Sorge R,
Ong SL, Pollard C, Metcalfe M and Dennison A: The ‘kidney-liver’
multiorgan ex vivo perfused model improves the circuit's
biochemical milieu during perfusion compared to the ‘liver-kidney’
counterpart. J Artif Organs. 18:151–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gravante G, Ong SL, Metcalfe MS, Sorge R,
Fox AJ, Lloyd DM, Maddern GJ and Dennison AR: Changes in acid-base
balance during electrolytic ablation in an ex vivo perfused liver
model. Am J Surg. 204:666–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He X, Ji F, Zhang Z, Tang Y, Yang L, Huang
S, Li W, Su Q, Xiong W, Zhu Z, et al: Combined liver-kidney
perfusion enhances protective effects of normothermic perfusion on
liver grafts from donation after cardiac death. Liver Transpl.
24:67–79. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Nassar A, Farias K, Buccini L,
Baldwin W, Mangino M, Bennett A, O'Rourke C, Okamoto T, Uso TD, et
al: Sanguineous normothermic machine perfusion improves
hemodynamics and biliary epithelial regeneration in donation after
cardiac death porcine livers. Liver Transpl. 20:987–999. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boncompagni E, Gini E, Ferrigno A,
Milanesi G, Gringeri E, Barni S, Cillo U, Vairetti M and Freitas I:
Decreased apoptosis in fatty livers submitted to subnormothermic
machine-perfusion respect to cold storage. Eur J Histochem.
55:e402011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan JJ, Zhang XD, Sun W, Qi L, Wu JC and
Qin ZH: DRAM1 regulates apoptosis through increasing protein levels
and lysosomal localization of BAX. Cell Death Dis. 6:e16242015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Zhang J, Gao Y, Liu S, Koh K, Zhu
X and Yin Y: Sensitive cell apoptosis assay based on caspase-3
activity detection with graphene oxide-assisted electrochemical
signal amplification. Biosens Bioelectron. 68:777–782. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Luque J, Caballero-Diaz D,
Martinez-Palacián A, Roncero C, Moreno-Càceres J, García-Bravo M,
Grueso E, Fernández A, Crosas-Molist E, García-Álvaro M, et al:
Dissecting the role of epidermal growth factor receptor catalytic
activity during liver regeneration and hepatocarcinogenesis.
Hepatology. 63:604–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai SS, Zhao DD, Cao P, Lu K, Luo OY, Chen
WB, Liu J, Jiang EZ, Yu ZH, Lee G, et al: PP2Acα positively
regulates the termination of liver regeneration in mice through the
AKT/GSK3β/Cyclin D1 pathway. J Hepatol. 64:352–360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Shao M, Wu Y, Yan C, Jiang S, Liu
J, Dai J, Yang L, Li J, Jia W, et al: Role for the endoplasmic
reticulum stress sensor IRE1α in liver regenerative responses. J
Hepatol. 62:590–598. 2015. View Article : Google Scholar : PubMed/NCBI
|