Introduction
Atopic dermatitis (AD) is a chronic inflammatory
disease of skin that is characterized by intense itching and
recurrent eczematous lesions (1).
The main pathological changes associated with AD are immunologic
disturbance and skin barrier disorders (2). Topical use of glucocorticoids or
calcineurin inhibitors are the predominant methods for the
management of AD, and systemic anti-inflammatory treatment with
glucocorticosteroids (short term), cyclosporine (in adults) or
azathioprine is used in some severe AD cases (3). Considering the important role of
anti-inflammatory therapy in the treatment of dermatitis, the
present study sought to evaluate the effectiveness of novel
anti-inflammatory agents in the treatment of AD.
Previous studies have demonstrated that highly
adverse conditions lead to the generation of oxidative stress in
skin tissue and that this phenomenon can stimulate the occurrence
of AD (4,5). The transcription factor nuclear
factor-E2-related factor 2 (Nrf2), which is involved in phase II
detoxification, inflammatory signaling, DNA repair and antioxidant
responses in cellular defense, is considered as a protective factor
against oxidative modification in keratinocytes, melanocytes and
fibroblasts, and improves skin barrier function and photoprotection
(4,6). Nrf2-deficient skin fibroblasts are
more susceptible to increased inflammation than normal skin
fibroblasts in the skin under UVA irradiation (7). Sulforaphane is a natural dietary
isothiocyanate extracted from cruciferous vegetables and it can
increase the antioxidative ability in tissues following focal
cerebral ischemia, brain inflammation, intracerebral hemorrhage and
many types of inflammation (8). A
recent study demonstrated that sulforaphane can stimulate the
expression of Nrf2 in human skin fibroblasts and decrease DNA
double-strand breaks after exposure to ionizing radiation; these
results suggest that sulforaphane could protect the skin from
ionizing radiation-induced injury by upregulating the expression of
Nrf2 (9). In addition, an
antioxidant gene, heme oxygenase-1 (HO-1), whose expression is
induced by increased Nrf2 expression, has been shown to be an
anti-inflammatory factor that protects the skin tissue against
oxidative stress (10).
The high expression of serum IgE in response to
exogenous and endogenous allergens in patients with AD is
associated with severe skin inflammation, and chronicity of AD and
indicates poor long-term prognosis for patients (11–13).
To illustrate the role IgE in AD, serum IgE autoantibodies have
been identified by immunostaining, which indicated that IgE is
expressed at a high level in the keratinocytes of patients with AD
(14). In a clinical study,
anti-IgE therapy demonstrated positive effects in controlling the
development of AD (15).
Eosinophils and mast cells also have an important role in the
prognosis of AD and there is some evidence that eosinophils can not
only activate the proinflammatory process but also participate in
tissue repair and the fibrotic processes of allergic inflammation
(16). Chemokines produced by mast
cells can also promote the development of AD, as demonstrated by
treatment of mast cells with dexamethasone and a calcineurin
inhibitor (FK506) (17).
In the present study, sulforaphane reduced
epithelial thickness, serum IgE level and infiltration of
eosinophils and mast cells in AD epithelial tissue and increased
the levels of Nrf2, phosphorylated (p-)Nrf2 and HO-1 and reduced
the levels of p-Janus kinase 1 (JAK1) and p-STAT3. This indicated
that sulforaphane can reduce the level of inflammation in the skin
of AD mice model and it may have a curative effect on patients with
AD.
Materials and methods
Animals
A total of 40 Female BALB/c mice, aged 6 weeks, were
purchased from Beijing HFK Bioscience Co., Ltd. They were housed
under specific pathogen-free conditions at a controlled temperature
of 20–25°C and 35–75% humidity with a 12-h light/dark cycle. The
animals were provided with sterile food and water ad
libitum. All animal care and experiments were performed in the
Experimental Animal Center in accordance with the national
guidelines and were approved by the animal care committee of
Shengjing Hospital of China Medical University (approval no.
2016PS001K).
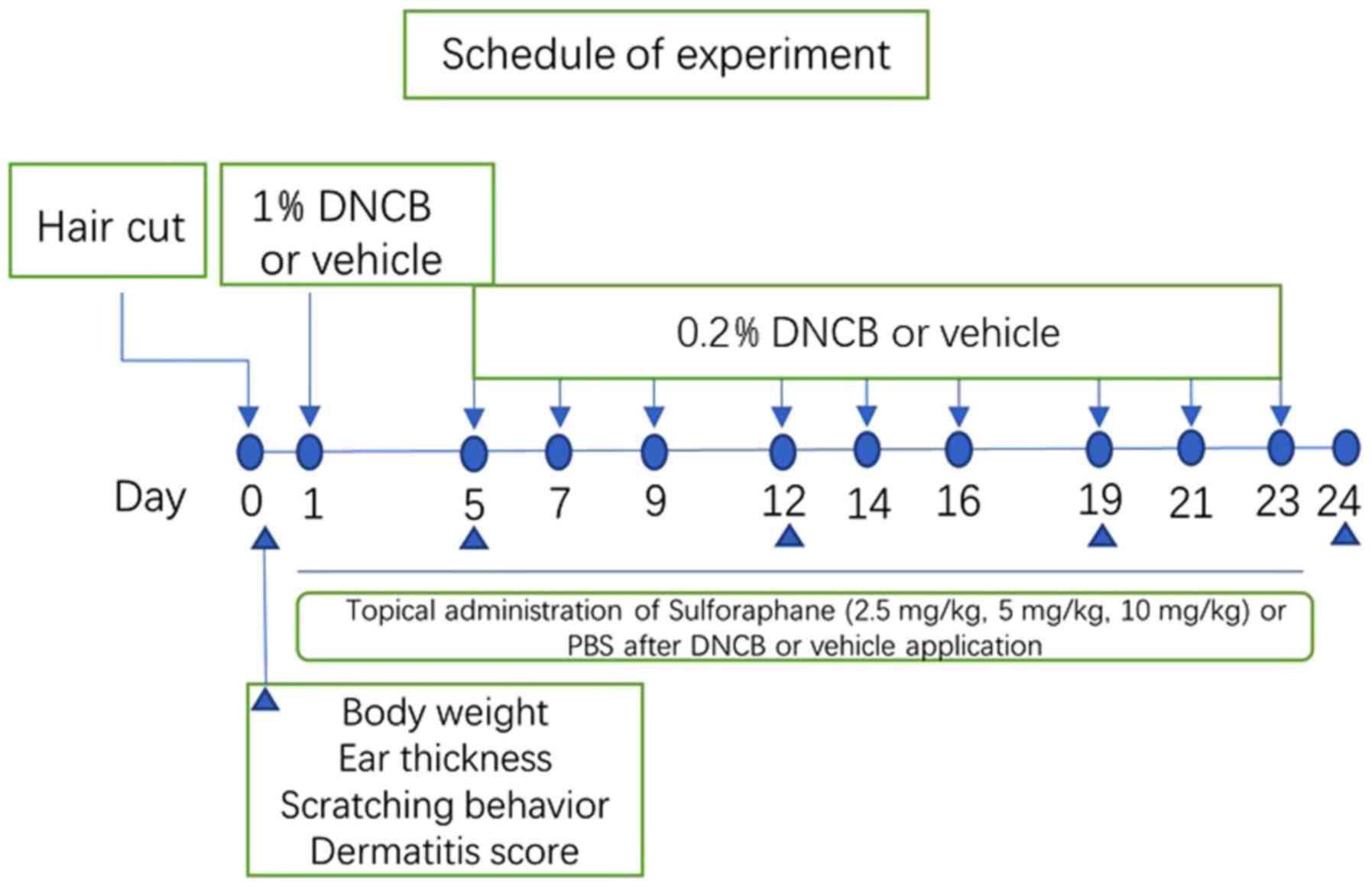
Induction of AD-like lesions and
sulforaphane administration
After a week of acclimation, the mice were divided
into 5 groups (n=8 per group): i) Vehicle, vehicle + phosphate
buffer saline (PBS); ii) AD, dinitrochlorobenzene (DNCB) + PBS;
iii) SFN2.5, DNCB + sulforaphane intraperitoneal (i.p.) injection
(2.5 mg/kg); iv) SFN5, DNCB + sulforaphane i.p. injection (5
mg/kg); and v) SFN10, DNCB + sulforaphane i.p. injection (10
mg/kg). The sulforaphane doses were selected and modified according
to previous studies (18–20).
The dorsal hair of mice was completely removed with
an electric razor before the day of administration (an area of ~4
cm2). On the first day, 150 µl 1% DNCB (Sigma-Aldrich;
Merck KGaA) dissolved in a mixture containing acetone and olive oil
(3:1 v/v), was dropped on the dorsal skin, and 20 µl 1% DNCB
solution was dropped in ears. On the 5th day, 150 or 20 µl 0.2%
DNCB solution dissolved in an acetone and olive oil mixture (3:1
v/v), respectively, were applied to the dorsal skin and ears three
times a week for 3 weeks (days 5–23). For the vehicle group, the
same dose of the mixture comprising acetone and olive oil (3:1 v/v)
was applied to the dorsal skin and ears of the mice.
Then, 1 h after each DNCB application, sulforaphane
at the doses of 2.5, 5 and 10 mg/kg i.p. or PBS i.p. was injected
(days 1–23), for a total of 10 times. When the experiments were
completed, the animals were anesthetized with 2% isoflurane, and
blood, dorsal dermal tissue and ear tissue were collected for
analysis. The experimental schedule is summarized in Fig. 1.
Evaluation of severity of
dermatitis
Dermatitis in each mouse was observed and the score
was recorded once a week according to the criteria described
previously (21). The severity of
dermatitis was assessed according to four symptoms: i)
Erythema/hemorrhage; ii) scar/dryness; iii) edema; and iv)
excoriation/erosion. The score of each clinical symptom ranged from
0 to 3 (none, 0; mild, 1; moderate, 2; and severe, 3). The total
dermatitis score (maximum score 12) was the sum of individual
scores. Additionally, ear thickness of the mice was measured and
recorded once a week by using a micrometer (Mitutoyo Kawasaki). On
day 24, the mice were anesthetized with 2% isoflurane before
sacrifice and the dorsal lesions were imaged using a digital camera
(Praktica Luxmedia16-Z21C; Pentacon GmbH).
Evaluation of scratching behavior and
ear thickness
To avoid statistical bias between groups, the number
of scratches in each mouse was recorded before administration. Mice
with high and low number of scratches were excluded from the
experiment, and the remaining mice were randomly divided into five
groups. Each mouse was observed for 10 min, and the number of
scratches was recorded and videotaped once a week. A scratching
event was defined as the mouse rubbing the dorsal skin and ears
with the hind paws. Moreover, when the continuous scratch time
exceeded 3 sec, it was recorded as two scratches and the scratching
was terminated by human intervention. In addition, mouse ear
thickness was measured and recorded once a week by using a
micrometer (Mitutoyo Kawasaki). All data measurements were
performed by a single investigator to avoid inter-observer
variation.
Histological analysis
To assess epidermal thickness and inflammatory cell
infiltration (i.e., eosinophils and mast cells), the dorsal skin
lesions of the mice were fixed in 10% paraformaldehyde for 24 h at
37°C on the last day, embedded in paraffin and 4-µm-thick paraffin
sections were made. Hematoxylin and eosin (H&E) staining and
toluidine blue staining were then performed for 30 sec each at 37°C
to identify epidermal thickness and inflammatory cells of each
group, respectively. The number of eosinophils and mast cells in
each section was obtained from five random views under ×400
magnification. Tissue sections were observed using an inverted
microscope (Y-TV55; Nikon Corporation) and the data were obtained
from five sections per mouse. Histopathological evaluation of all
skin sections was carried out in a blind manner.
Serum IgE measurements
Abdominal aortic blood of mice was collected on day
24. IgE levels were measured with an ELISA kit (cat. no. E01G0277;
Abcam) in accordance with the manufacturer's instructions.
cDNA synthesis and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA of mouse dorsal skin in each group was
extracted with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA extraction was performed according to the
manufacturer's protocol. cDNA was prepared from 1 µg RNA using
RevertAid Reverse Transcriptase (Thermo Fisher Scientific, Inc.)
and incubated for 4 h at 37°C and 5% CO2. RT-qPCR
analyses were performed on the 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Cycling conditions
were 3 min at 95°C followed by 60 cycles of 30 sec at 95°C, 30 sec
at 56°C and 30 sec at 72°C. The expression level of genes was
detected using the following primers: Interleukin (IL)-6,
5′-TTGCCTTCTTGGGAC-3′ (forward), 5′-TTGCCATTGCACAACTCTT-3′
(reverse); IL-1β, 5′-CTGTCGGACCCATATGAGC-3′ (forward),
5′-GCTCATGGAGAATATCACTTGTTG-3′ (reverse); tumor necrosis factor-α
(TNF-α), 5′-AAGCCTGTAGCCCACGTCGTA-3′ (forward),
5′-GGCACCACTAGTTGGTTGTCTTTG-3′ (reverse); mouse GAPDH,
5′-AAATGGTGAAGGTCGGTGTG-3′ (forward), 5′-TGAAGGGGTCGTTGATGG-3′
(reverse). All experiments were performed in duplicate. For
relative quantification analyses, a comparative 2−ΔΔCq
method was used (22), where the
median value of the vehicle group was used as the calibrator.
Western blot analysis
Western blotting was used to detect the protein
levels of Nrf2, p-Nrf2, HO-1, p-JAK1, JAK1, p-STAT3 and STAT3.
Dorsal skin tissues were processed by the protein extraction kit
and each sample in one group was homogenized in RIPA buffer
(BioLegend, Inc.) and then centrifuged for 30 min at 12,000 × g and
4°C to collect the supernatant. Then, eight protein samples from
each group were pooled together, separated by SDS-PAGE and then
transferred onto a PVDF membrane (EMD Millipore). The
quantification of protein concentration was performed by using
bichinchoninic acid protein assay kit (Pierce, Thermo Fisher
Scientific, Inc.): 20 µg protein from each sample was separated by
SDS-PAGE on a 10% gel and transferred onto a polyvinylidene
difluoride membrane and then blocked with 5% non-fat milk for 2 h
at 37°C. The membrane was incubated with monoclonal rabbit
anti-mouse Nrf2 (cat. no. 2772, 1:10,000 dilution), p-Nrf2 (cat.
no. 9524, 1:10,000 dilution), HO-1 (cat. no. 2882, 1:10,000
dilution), p-JAK1 (cat. no. 4970, 1:10,000 dilution), JAK1 (cat.
no. 8675, 1:10,000 dilution), p-STAT3 (cat. no. 4462, 1:10,000
dilution), STAT3 (cat. no. 6643, 1:10,000 dilution) and polyclonal
rabbit anti-mouse HO-1 (cat. no. 4480, 1:10,000 dilution, all from
Abcam), respectively, along with monoclonal rabbit anti-mouse
tubulin (cat. no. SC-2357, 1:10,000 dilution; ProteinTech Group,
Inc.) as an internal reference for 15 h at 4°C, followed by
incubation with Alexa Fluor 800-labeled goat anti-rabbit IgG
(Invitrogen; Thermo Fisher Scientific, Inc.) for 1.5 h at 37°C.
Images were acquired using an AI600 (BD Biosciences). Band
intensities (pixels/mm2) were obtained using Image Quant
5.2 software (Molecular Dynamics) after subtracting the background
intensities. The values of the sulforaphane-treated group values
were normalized to those of β-actin as normal control (NC).
Statistical analysis
GraphPad Prism software 7.0 (GraphPad Software,
Inc.) was used to analyze the statistical data, and the data were
expressed as mean ± SEM. For comparison of multiple groups data,
one-way or two-way ANOVA followed by Dunnett's post hoc test or
Tukey's honest significant difference (HSD) test to detect the
differences in these groups. All the experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sulforaphane has a protective effect
against DNCB-induced AD in mice
To study the therapeutic effects of sulforaphane on
skin lesions in AD mice, AD mice were treated with a concentration
gradient of sulforaphane (2.5, 5 and 10 mg/kg), with the different
groups termed SFN2.5, SFN5 and SFN10, respectively. The AD model
group exhibited severe dermatitis with erythema, scarring, edema
and erosion (Fig. 2A). The
dermatitis scores gradually increased throughout the 3-week
experimental period (Fig. 2B).
Skin condition was significantly improved in sulforaphane-treated
groups compared with those in the AD group (Fig. 2A and B). Moreover, sulforaphane
decreased the dermatitis score of DNCB-induced skin lesions on days
14 and 21 in a dose-dependent manner. Scratching behavior was the
most noticeable clinical feature of AD (Fig. 2A and B).
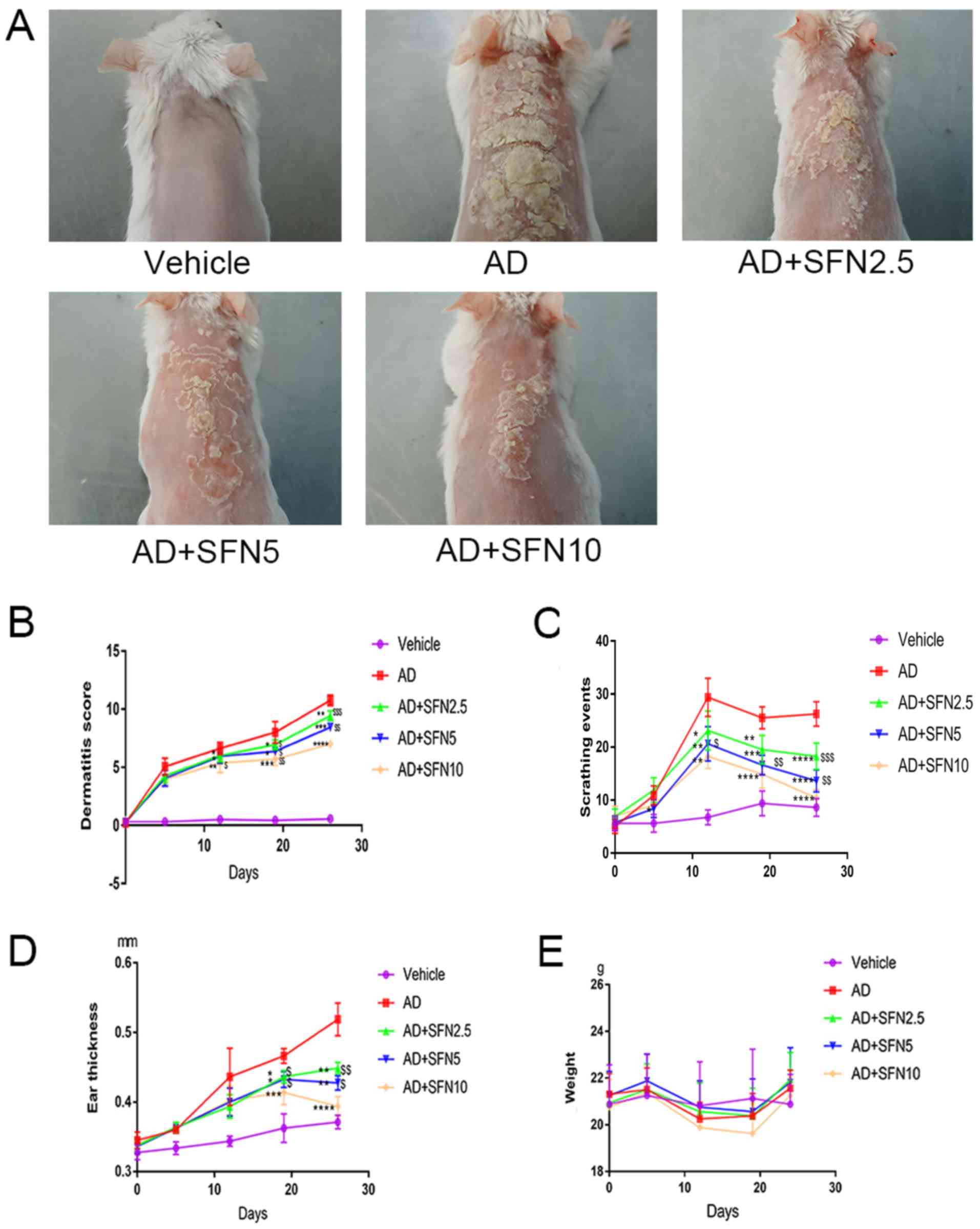 | Figure 2.Effects of SFN on atopic
dermatitis-like symptoms in BALB/c mice. (A) Images of skin lesions
from the groups were taken on the last day of the experiment (day
24). (B) Dermatitis scores were evaluated once a week for 5 weeks
(two-way ANOVA analysis followed by Dunnett's post hoc test). (C)
The number of scratching events was recorded for 10 min for each
mouse in a cage once a week (two-way ANOVA analysis followed by
Dunnett's post hoc test). (D) Ear thickness was measured once a
week for 5 weeks using a micrometer (two-way ANOVA analysis
followed by Dunnett's post hoc test). (E) Mouse body weight was
recorded once a week (two-way ANOVA followed by Tukey's honest
significant difference test). Results are expressed as the mean ±
SEM (n=8). *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. AD group. $P<0.05,
$$P<0.01 and $$$P<0.001 vs. AD + SFN10
group. DNCB, 2,4-dinitrochlorobenzene; SFN, sulforaphane; Vehicle,
vehicle + PBS-treated group; AD, DNCB + PBS-treated atopic
dermatitis group; AD + SFN2.5, DNCB + SFN (2.5 mg/kg i.p.)-treated
group; AD + SFN5, DNCB + SFN (5 mg/kg i.p.)-treated group; AD +
SFN10, DNCB + SFN (10 mg/kg i.p.)-treated group. |
The results of monitoring mouse scratching behavior
showed that scratching events increased rapidly in the DNCB-induced
AD group and were maintained at a high level during the 3-week
experimental period compared to those of the vehicle group
(Fig. 2C). However, after
treatment with sulforaphane, the number of scratching events was
rapidly decreased in the SFN2.5, SFN5, and SFN10 groups compared
with those of the AD group (Fig.
2C). Moreover, the SFN10 group had lower scratching events than
the SFN2.5 group (P<0.01) and the SFN5 group (P<0.001;
Fig. 2C). Further, SFN2.5, SFN5
and SFN10 groups also exhibited a significant reduction in
DNCB-induced ear thickness compared to that of the AD group
(two-way ANOVA analysis followed by Dunnett's post hoc test;
Fig. 2D). To determine the
toxicity of sulforaphane, changes in weight of each mouse were
recorded and found that sulforaphane had no effect on the
maintenance of body weight (two-way ANOVA analysis followed by
Tukey's HSD test; Fig. 2E) that
there was a significant difference in changes weight by different
concentration SFN treatment.
Previous studies have indicated that DNCB-induced
AD-like lesions have high levels of inflammatory cell infiltration,
leading to thickening of the skin (23). H&E staining revealed that the
epidermal thickness of the dorsal skin was attenuated by treatment
in the SFN2.5 SFN5, and SFN10 groups compared with the AD group
(one-way ANOVA analysis followed by Dunnett's post hoc test). The
epidermal thickness was significantly lower in the SFN10 group
compared with the SFN2.5 and SFN5 groups (P<0.01; Fig. 3A and B).
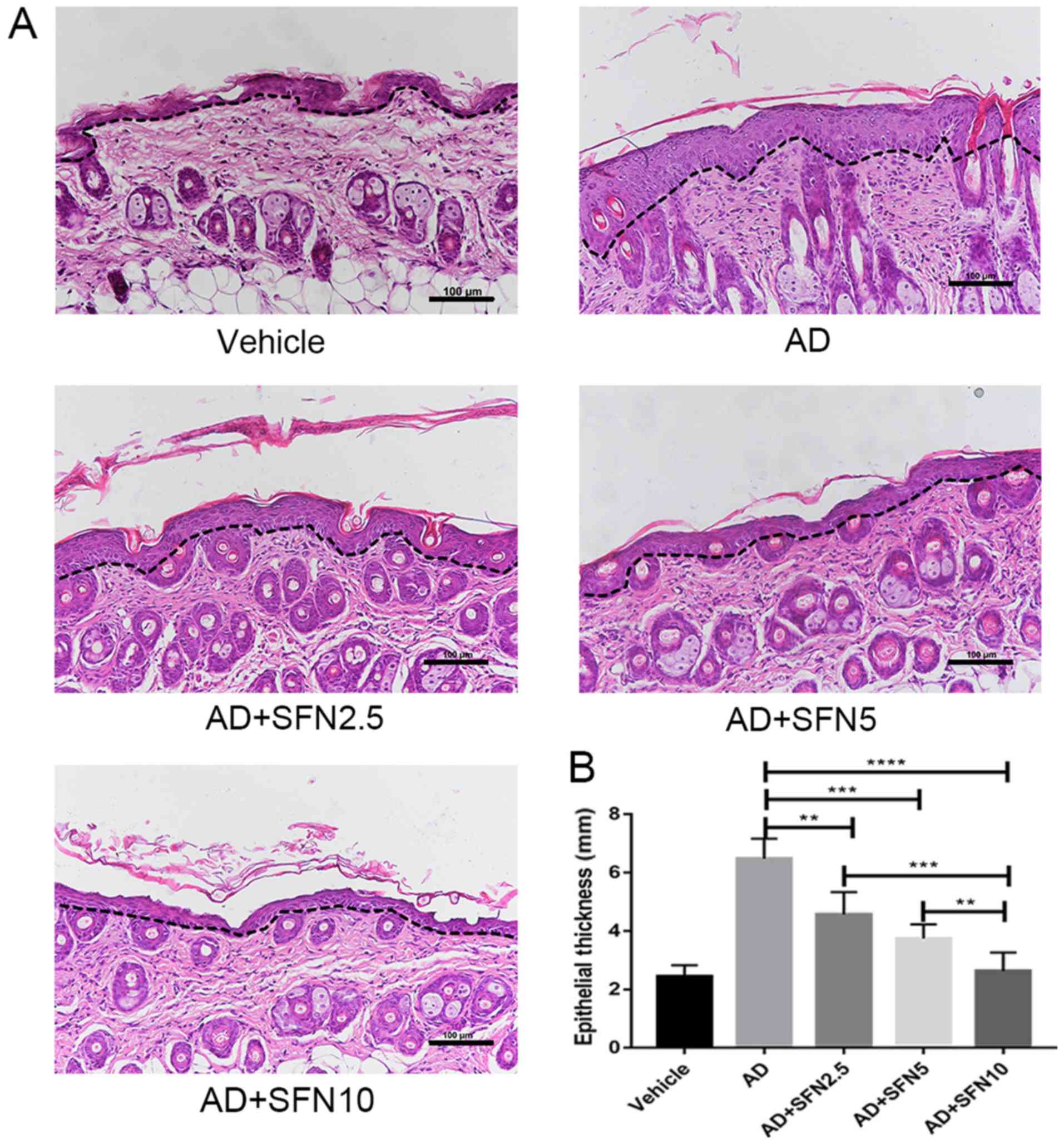 | Figure 3.Effects of SFN on dorsal skin
thickness in atopic dermatitis mouse skin lesions (5 fields per
animal). (A) Epidermal thickness was presented in hematoxylin and
eosin-stained sections (×20; scale bar, 100 µm). The black dotted
line indicates the boundary line between the epidermis and the
dermis. (B) Measurement of epidermal thickness. Results are
expressed as the mean ± SEM (n=8). **P<0.01, ***P<0.001 and
****P<0.0001. One-way ANOVA analysis followed by Dunnett's post
hoc test. DNCB, 2,4-dinitrochlorobenzene; SFN, sulforaphane;
Vehicle, vehicle + PBS-treated group; AD, DNCB + PBS-treated atopic
dermatitis group; AD + SFN2.5, DNCB + SFN (2.5 mg/kg i.p.)-treated
group; AD + SFN5, DNCB + SFN (5 mg/kg i.p.)-treated group; AD +
SFN10, DNCB + SFN (10 mg/kg i.p.)-treated group. |
Sulforaphane reduced eosinophil
accumulation, mast cell infiltration, and serum IgE levels in AD
mice
Eosinophil levels are elevated in most AD patients
and are closely associated with disease activity. In this study,
H&E staining of the drug-administered dorsal skin was performed
and eosinophil infiltration was observed under an optical
microscope for each group. The number of eosinophils in skin was
reduced in SFN2.5, SFN5, and SFN10 groups compared with the number
in the AD group (one-way ANOVA analysis followed by Dunnett's post
hoc test; Fig. 4).
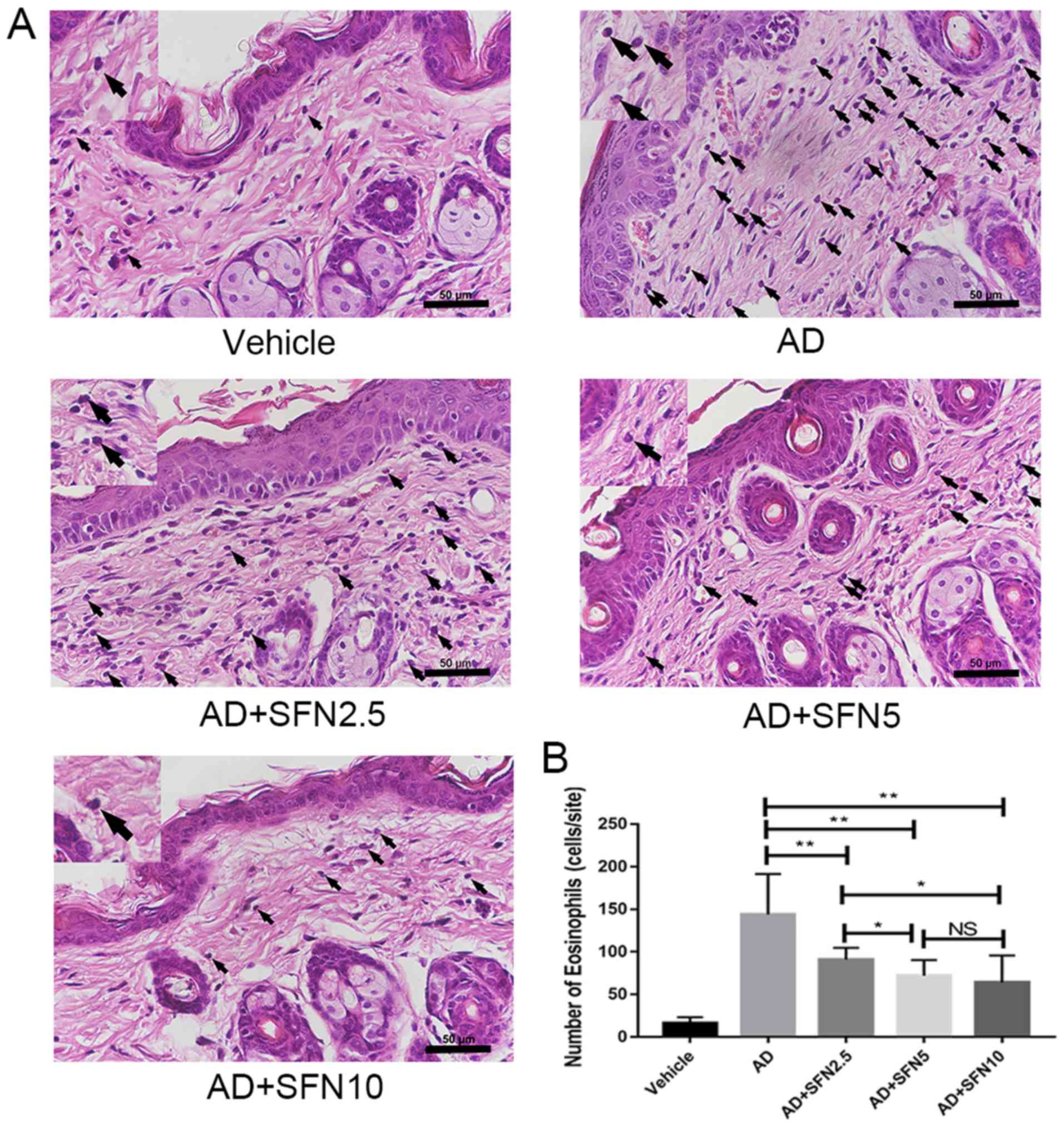 | Figure 4.Effects of SFN on eosinophil
accumulation in atopic dermatitis mouse skin lesions (5 fields per
animal). (A) Eosinophils were presented in hematoxylin and
eosin-stained sections (×40; scale bar, 50 µm), and the morphology
of eosinophils was clearly observed in the partially enlarged image
on the left upper corner. Black arrows indicate stained
eosinophils. (B) Average number of eosinophils in five sites chosen
randomly was counted at ×400 magnification. The enlargement part of
each groups was enlarged by 2.5 times. Results are expressed as the
mean ± SEM (n=8). *P<0.05 and **P<0.01. One-way ANOVA
analysis followed by Dunnett's post hoc test. DNCB,
2,4-dinitrochlorobenzene; SFN, sulforaphane; Vehicle, vehicle +
PBS-treated group; AD, DNCB + PBS-treated group; AD + SFN2.5, DNCB
+ SFN (2.5 mg/kg i.p.)-treated group; AD + SFN5, DNCB + SFN (5
mg/kg i.p.)-treated group; AD + SFN10, DNCB + SFN (10 mg/kg
i.p.)-treated group; NS, no significance. |
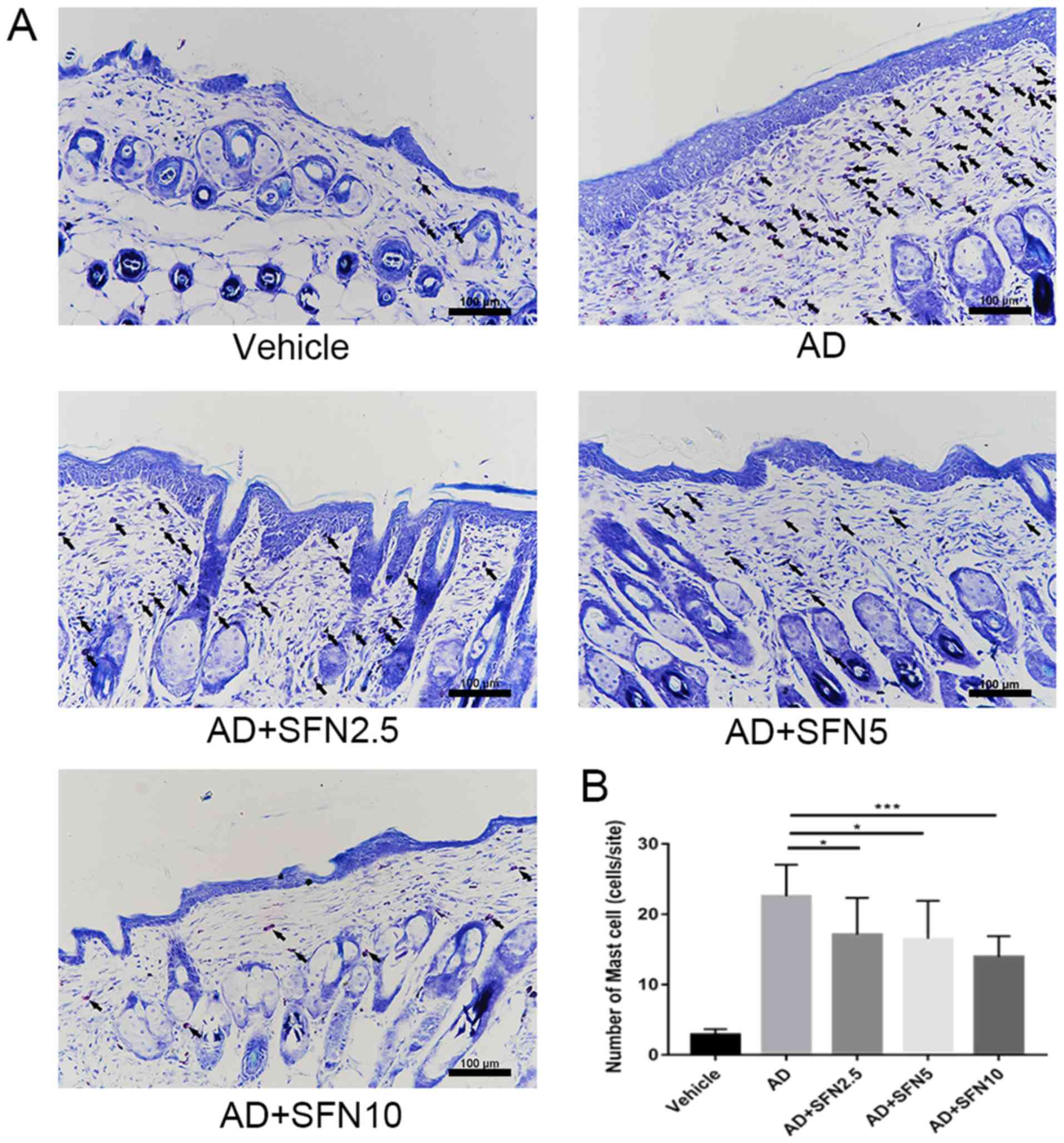
Increased serum IgE levels and mast cell
infiltration in skin tissue are the main features of AD (24). Toluidine blue staining of the
dorsal skin lesions was performed and the infiltration of mast
cells in the dermis was observed under the optical microscope
(Fig. 5A). A large number of mast
cells appeared in the dermis of the AD group. The number of mast
cells decreased significantly after sulforaphane treatment, and the
effect was significantly increased with the increasing dose of in
the SFN2.5, SFN5 and SFN10 groups compared with the AD group
(one-way ANOVA analysis followed by Dunnett's post hoc test;
Fig. 5B). The effect of
sulforaphane on total serum IgE levels was also investigated. The
results showed that serum IgE levels were significantly elevated in
the AD group; however, increased serum IgE levels were
significantly attenuated in the SFN2.5, SFN5 and SFN10 groups
compared with those in the AD group, while the SFN10 group had
lower levels of serum IgE than the SFN2.5 group (P<0.05) and the
SFN5 group (P<0.01; Fig.
6A).
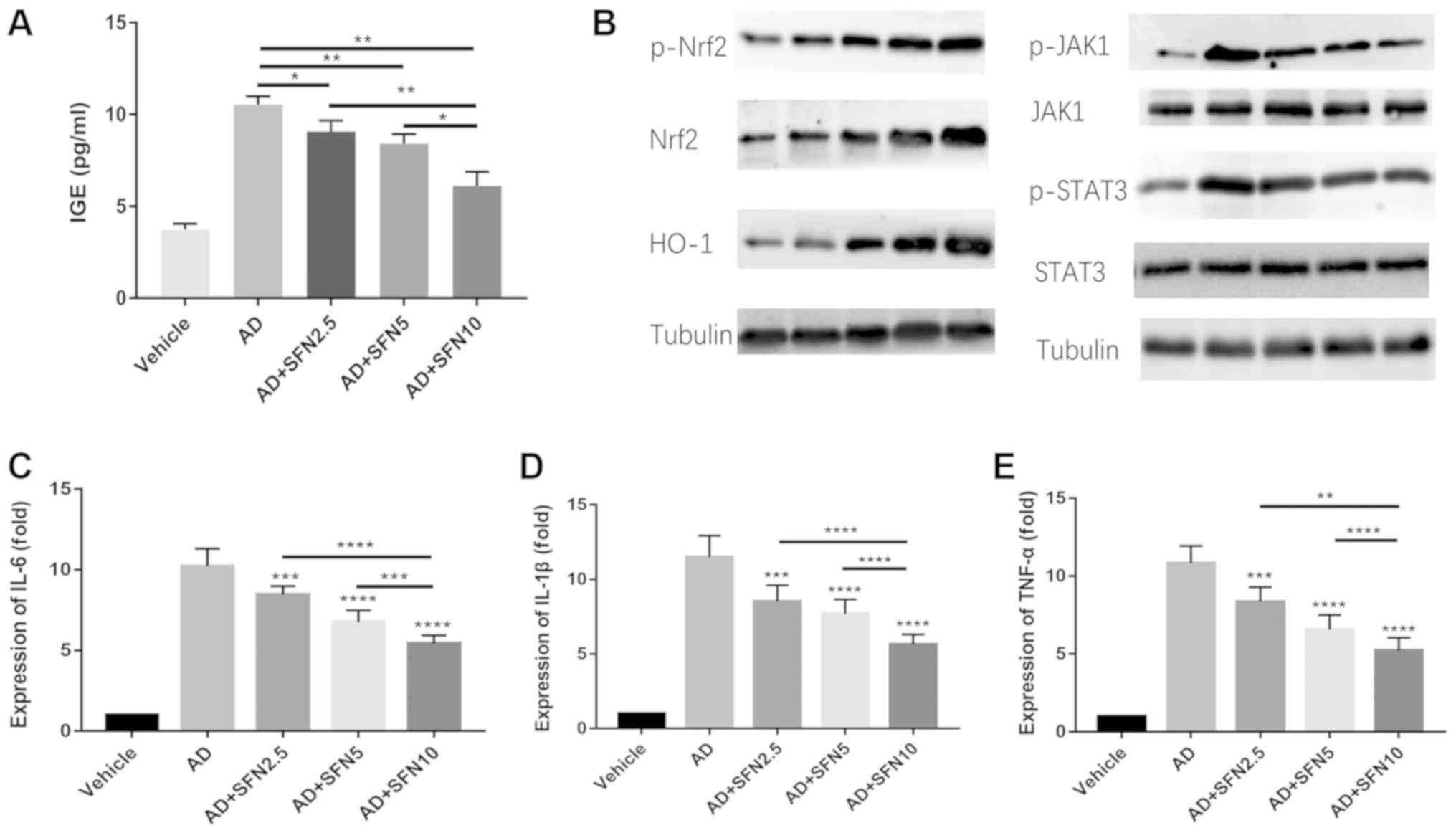 | Figure 6.(A) Serum IgE levels were measured by
ELISA. (B) Western blot revealed protein level changes in the
expression of Nrf2, p-Nrf2, HO-1, p-JAK1, JAK1, p-STAT3 and STAT3.
Tubulin was used as an internal reference. (C) Reverse
transcription-quantitative PCR assay revealed mRNA level changes in
the expression of (C) IL-6, (D) IL-1β and (E) TNF-α. Results are
expressed as the mean ± SEM (n=8). *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. One-way ANOVA analysis followed
by Dunnett's post hoc test. DNCB, 2,4-dinitrochlorobenzene; SFN,
sulforaphane; Vehicle, vehicle + PBS-treated group; AD, DNCB +
PBS-treated group; AD + SFN2.5, DNCB + SFN (2.5 mg/kg i.p.)-treated
group; AD+SFN5, DNCB + SFN (5 mg/kg i.p.)-treated group; AD +
SFN10, DNCB + SFN (10 mg/kg i.p.)-treated group. |
Sulforaphane increases the expression
of Nrf2 and HO-1, and downregulated the JAK2/STAT3 pathway and the
levels of IL-6, IL-1β and TNF-α in AD mice
To explicate antioxidant and anti-inflammatory
effects of sulforaphane in AD mice, western blot assays were
performed to detect the expression of Nrf2, p-Nrf2, HO-1, p-JAK1,
JAK1, p-STAT3 and STAT3 in full-thickness dorsal skin. The results
showed that p-Nrf2, Nrf2 and HO-1 were slightly elevated in the AD
group compared with that in the vehicle group. However, the levels
of p-Nrf2, Nrf, and HO-1 in the SFN2.5, SFN5, and SFN10 groups were
significantly higher than those in the AD group; furthermore, the
SFN10 group exhibited higher levels of Nrf2 and HO-1 expression
than the SFN2.5 and SFN5 groups (Fig.
6B). By contrast, the mRNA expression levels of IL-6, IL-1β and
TNF-α and the phosphorylation of JAK2/STAT3 were downregulated in
the SFN2.5, SFN5 and SFN10 groups compared with those in the AD
group (Fig. 6B-E).
Discussion
AD is a chronic disease characterized by skin
barrier dysfunction, IgE-mediated hypersensitivity and alterations
in cell-mediated immune responses (23). Previous studies have suggested that
oxidative stress, which can lead to skin barrier dysfunction, has a
central role in the occurrence and development of AD, and that this
is related to poor prognosis of AD (5,24).
In the present study, sulforaphane treatment alleviated the
inflammation in AD mice, reduced the accumulation of eosinophils
and mast cells in the epithelial tissue and reduced the expression
levels of serum IgE. Furthermore, the expression levels of Nrf2 and
HO-1 were upregulated in the epithelial tissue of AD mice following
sulforaphane treatment.
Oxidative stress in turn can directly damage
epithelial cells and induce eosinophil and mast cell accumulation,
together with secretion of proinflammatory cytokines, which can
cause dermal inflammation, which leads to itching and scratching.
The dermal inflammation, and subsequent itching and scratching, in
turn increase the release of proinflammatory cytokines; thus, these
factors form a positive loop that can exaggerate AD illness
(5). Sulforaphane, a natural
isothiocyanate extracted from cruciferous vegetables such as
broccoli, has a variety of potential abilities for the treatment of
solid cancers, cardiovascular diseases, neurodegenerative diseases
and diabetes (25). One of the
major molecular mechanisms of action of sulforaphane is the
activation of the Nrf2-Kelch-like ECH-associated protein 1 pathway,
which is involved in the response to damage caused by oxidant
compounds (25). Nrf2, a
transcription factor that regulates genes encoding antioxidant and
detoxifying molecules, can ameliorate oxidative stress and
inflammation in chronic kidney disease, cardiovascular disease and
Alzheimer's disease in humans (26–28).
Furthermore, Nrf2 is associated with epidermal barrier function to
protect against oxidant damage (29). Choi et al (30) demonstrated that Platycodon
grandiflorum root-derived saponins can improve AD-like lesions
in mice by activating Nrf2/ARE. However, whether sulforaphane has
similar therapeutic effects in an AD mouse model remains to be
elucidated.
The present study used 2.5, 5 and 10 mg/kg
sulforaphane to treat AD mice. The concentrations of sulforaphane
were in accordance with other studies on sulforaphane treatment
(18,19). As no studies on SFN in AD have been
identified, the present study explored the appropriate therapeutic
concentration of SFN in AD, so that it can be used for further
research. The comparison of studies on sulforaphane and clinically
positive control drugs is also a matter for further study. Previous
studies examined the use of SFN in UVB-induced skin treatment
(31,32) and demonstrated that SFN has the
potential to treat skin disease induced by immune-inflammation
responses. The present study demonstrated that sulforaphane
upregulated the expression levels of p-Nrf2 and Nrf2, and its
downstream antioxidant molecule HO-1 in the epithelial cells of
DNCB-induced AD mice, and downregulated the expression levels of
IL-6, IL-1β and TNF-α, and the phosphorylation of JAK2/STAT3. A
previous study demonstrated that Nrf2 knockout mice require higher
levels of inflammatory stimulation to initiate contact dermatitis
compared with normal mice and that the presence of Nrf2 in
keratinocytes limits inflammation (33). In contact dermatitis, Nrf2 improves
the condition of patients and the activation of Nrf2 is required
for the activation of the ARE reporter gene (34). Nrf2 is associated with epidermal
barrier function for protection against oxidant damage (29). In UVA irradiation-related study,
sulforaphane was considered as an anti-oxidative stress-associated
agent to treat photoaging in BALB/c mice and the activation of Nrf2
and reduction of MMP-1 induced by sulforaphane were observed
(32). These studies suggested
that Nrf2 has the ability to control epidermal inflammation and
this conclusion is consistent with the experimental results of the
present study. It is therefore concluded that sulforaphane exerted
a therapeutic effect in the AD mouse model by the activation of the
Nrf2/HO-1 axis. The present study also found that the
phosphorylation of JAK2/STAT3 and the expression levels of IL-6,
IL-1β and TNF-α were reduced in the SFN-treated group compared with
the AD group. Welsch et al (35) reported that JAK/STAT signaling
serves an important role in inflammatory skin diseases and that
JAK/STAT inhibitors should presumably have many applications in
dermatology. Jin et al (36) demonstrated that the JAK1/JAK2
inhibitor momelotinib inhibits the inflammatory response in
DNCB-induced AD mice. Accordingly, the results of the present study
indicated that SFN not only upregulated the Nrf2/HO-1 pathway, but
also downregulated the JAK2/STAT3 pathway associated with
inflammation. Abe and Tanaka (37)
demonstrated that Nrf2 can attenuate the expression of IL-6 and
IL-1β, which decreased the macrophage inflammatory response. The
study of Chu et al (38),
demonstrated that the expression levels of IL-6, IL-1β and TNF-α
are significantly inhibited by SFN in a rheumatoid arthritis model.
These results demonstrate that Nrf2 can regulate the downstream
cytokines, including IL-6, IL-1β and TNF-α, to influence the
progression of inflammation in AD.
However, there are some limitations to the present
study. In a previous study, Roy et al (39) demonstrated that the expression of
NF-κB and mitogen-activated protein kinases (MAPKs), as
inflammatory mediators, are associated with the progression of AD.
In addition, the decline of MAPKs and NF-κB alleviates AD symptoms
(40). Pastore et al
(41) reported that activation of
c-JUN/c-FOS pathway promotes inflammation in AD. However, there are
no studies, to the best of our knowledge, which report that the
expression of c-JUN/c-FOS, NF-κB and MAPKs are associated with SFN
treatment in AD. Further studies are required to verify the
inflammation mediator function in the SFN treatment of AD and that
was a limitation of the present study. The results demonstrated a
new approach to treat dermatitis by activating skin protective
molecules that can enhance the skin barrier and the role of this
mechanism in specific dermatitis needs to be identified in future
experiments.
In patients with AD, mast cell numbers are increased
in skin lesions (40). Although
the function and status of mast cells in AD is not clear, there is
a potential mechanism by which mast cells contribute to AD
progression, whereby mast cells release cytokines such as IL-17 and
IL-22 that induce epithelial inflammation and allergic response
(42). In addition, mast cells can
intensify scratching damage by releasing pruritogenic substances
(43). This further disrupts the
skin barrier and exacerbates the disease. In previous studies, the
Nrf2-HO-1 pathway was found to mediate anti-allergic actions in
rodent mast cells and HO-1 was shown to control inflammation by
stabilizing mast cells (43–45).
In the present study, AD mice that underwent sulforaphane treatment
demonstrated a decline in mast cells in skin in the SFN2.5, SFN5
and SFN10 groups compared with the AD group. Combined with the
results of previous studies, these results indicate that
sulforaphane can control inflammation by inhibiting mast cell
infiltration through the Nrf2-HO-1 pathway.
A consensus has been reached that elevated serum IgE
levels in patients with AD are directly associated with poor
prognosis and more severe development of AD (11). The anti-IgE antibody omalizumab was
used to treat severe AD along with extracorporeal immunoadsorption
and the combination therapy resulted in an improvement in AD during
the treatment period (46). In a
recent study, 6-shogaol, an active compound present in ginger, was
found to activate the reactive oxygen species (ROS)/MAPKs/Nrf2
anti-inflammatory pathway to alleviate AD-like skin lesions by
inhibiting the development of DNCB-induced AD-like skin lesions and
scratching behavior, and reducing the expression of IgE and ROS
generation (47). The present
study identified that serum IgE levels in the AD group were >2
times higher compared with the vehicle group and that there was a
significant decrease in IgE levels in the SFN2.5, SFN5 and SFN10
groups compared with the AD group. It was also identified that as
the drug concentration increased gradually, the serum IgE levels in
the SFN2.5, SFN5 and SFN10 groups decreased compared with the AD
group. Eosinophil infiltration is characteristic of patients with
AD and it is associated with disease severity (48). Eosinophils are involved in T helper
2 (Th2)-immune response in AD and Th2/Th22-dominant allergic
responses, which are considered as the major molecular mechanism in
AD. In addition, Th2 cytokines induce oxidative stress and severe
inflammatory response, and lead to the aggravation of AD (49). Infiltration of eosinophils
amplifies Th2-immune responses and Th2 cells begin secreting IL-4,
IL-5 and IL-13, which augments skin inflammation (43,50).
A recent study (49) reported
notable findings about AD; dupilumab, an anti-IL-4-receptor-α
monoclonal antibody, can block signaling of IL-4 and IL-13, which
are type 2/Th2 cytokines implicated in numerous allergic diseases,
including AD. In this 1-year, randomized, double-blinded,
placebo-controlled, phase 3 trial, signs and symptoms of patients
with AD were alleviated by dupilumab with acceptable safety. It can
be concluded that the reduction of type 2/Th2 will be a key factor
for the treatment of AD and that the accumulation of eosinophils in
skin could be considered as a therapeutic effect indicator, as the
accumulation of eosinophils is suppressed by sulforaphane treatment
in AD mice. In the present study, AD mice that underwent
sulforaphane treatment (SFN2.5, SFN5 and SFN10 groups) also
demonstrated a decrease in eosinophil infiltration in skin compared
with the AD group. The results of the present study demonstrated
that sulforaphane can downregulate the level of IgE in DNCB-induced
AD mice, alleviate the edema and itching, and reduce the
infiltration of eosinophils and mast cells; thus, it could be
considered as a potential agent to treat AD.
In conclusion, the results of the present study
demonstrated that sulforaphane alleviated AD symptoms in
DNCB-induced AD mice, potentially through the activation of the
Nrf2/HO-1 pathway and the suppression of JAK1/STAT3 signaling, and
that sulforaphane may be a potential therapeutic option for
treating patients with AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific
Research Key Project of Educational Department in Liaoning Province
of China in 2017 (grant no. 2017225026) and Science and Technology
Project of Shenyang (grant no. 17-230-9-25). The funders had no
role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WW induced AD-mice by DNCB, executed sulforaphane
administration performed H&E and toluidine blue staining for
measurement of epidermal thickness, eosinophils and mast cells of
each group, and respectively and analyzed these data. GP evaluated
the severity of dermatitis, scratching behavior and ear thickness
of each group and analyzed these data. FY measured serum IgE
expression of each group by Elisa and analyzed these data. YZ
measured mRNA level of IL-6, IL-1β and TNF-α of each group and
analyzed these data. ZM performed western blot analysis for
Nrf2/HO-1 and JAK1/STAT3 pathway in each group and analyzed these
data. XH was a major contributor to the study design and data
interpretation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study procedures were approved by the ethics
committee of Shengjing Hospital of China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werfel T, Schwerk N, Hansen G and Kapp A:
The diagnosis and graded therapy of atopic dermatitis. Dtsch
Arztebl Int. 111:509–520. 2014.PubMed/NCBI
|
|
4
|
Gęgotek A and Skrzydlewska E: The role of
transcription factor Nrf2 in skin cells metabolism. Arch Dermatol
Res. 307:385–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji H and Li XK: Oxidative stress in atopic
dermatitis. Oxid Med Cell Longev. 2016:27214692016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rojo de la Vega M, Krajisnik A, Zhang DD
and Wondrak GT: Targeting NRF2 for improved skin barrier function
and photoprotection: Focus on the achiote-derived apocarotenoid
bixin. Nutrients. 9:E13712017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gruber F, Ornelas CM, Karner S, Narzt MS,
Nagelreiter IM, Gschwandtner M, Bochkov V and Tschachler E: Nrf2
deficiency causes lipid oxidation, inflammation and matrix-protease
expression in DHA supplemented and UVA irradiated skin fibroblasts.
Free Radic Biol Med. 88:439–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guerrero-Beltrán CE, Calderón-Oliver M,
Pedraza-Chaverri J and Chirino YI: Protective effect of
sulforaphane against oxidative stress: Recent advances. Exp Toxicol
Pathol. 64:503–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathew ST, Bergström P and Hammarsten O:
Repeated Nrf2 stimulation using sulforaphane protects fibroblasts
from ionizing radiation. Toxicol Appl Pharmacol. 276:188–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar KJ, Yang HL, Tsai YC, Hung PC, Chang
SH, Lo HW, Shen PC, Chen SC, Wang HM, Wang SY, et al: Lucidone
protects human skin keratinocytes against free radical-induced
oxidative damage and inflammation through the up-regulation of
HO-1/Nrf2 antioxidant genes and down-regulation of NF-κB signaling
pathway. Food Chem Toxicol. 59:55–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furue M, Chiba T, Tsuji G, Ulzii D,
Kido-Nakahara M, Nakahara T and Kadono T: Atopic dermatitis: Immune
deviation, barrier dysfunction, IgE autoreactivity and new
therapies. Allergol Int. 66:398–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiiski V, Karlsson O, Remitz A and Reitamo
S: High serum total IgE predicts poor long-term outcome in atopic
dermatitis. Acta Derm Venereol. 95:943–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lucae S, Schmid-Grendelmeier P, Wüthrich
B, Kraft D, Valenta R and Linhart B: IgE responses to exogenous and
endogenous allergens in atopic dermatitis patients under long-term
systemic cyclosporine A treatment. Allergy. 71:115–118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altrichter S, Kriehuber E, Moser J,
Valenta R, Kopp T and Stingl G: Serum IgE autoantibodies target
keratinocytes in patients with atopic dermatitis. J Invest
Dermatol. 128:2232–2239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu FT, Goodarzi H and Chen HY: IgE, mast
cells, and eosinophils in atopic dermatitis. Clin Rev Allergy
Immunol. 41:298–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elovic A, Wong DT, Weller PF, Matossian K
and Galli SJ: Expression of transforming growth factors-alpha and
beta 1 messenger RNA and product by eosinophils in nasal polyps. J
Allergy Clin Immunol. 93:864–869. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato A, Chustz RT, Ogasawara T, Kulka M,
Saito H, Schleimer RP and Matsumoto K: Dexamethasone and FK506
inhibit expression of distinct subsets of chemokines in human mast
cells. J Immunol. 182:7233–7243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma
M, Han M, Wu J, Ushida Y, Suganuma H and Hashimoto K: Prophylactic
effects of sulforaphane on depression-like behavior and dendritic
changes in mice after inflammation. J Nutr Biochem. 39:134–144.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan B, Ma Z, Shi S, Hu Y, Ma T, Rong G and
Yang J: Sulforaphane prevents bleomycin-induced pulmonary fibrosis
in mice by inhibiting oxidative stress via nuclear factor erythroid
2-related factor-2 activation. Mol Med Rep. 15:4005–4014. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connor JC, Lawson MA, André C, Moreau M,
Lestage J, Castanon N, Kelley KW and Dantzer R:
Lipopolysaccharide-induced depressive-like behavior is mediated by
indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry.
14:511–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng G, Mu Z, Cui L, Liu P, Wang Y, Wu W
and Han X: Anti-IL-33 antibody has a therapeutic effect in an
atopic dermatitis murine model induced by 2,
4-dinitrochlorobenzene. Inflammation. 41:154–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
David Boothe W, Tarbox JA and Tarbox MB:
Atopic dermatitis: Pathophysiology. Adv Exp Med Biol. 1027:21–37.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Briganti S and Picardo M: Antioxidant
activity, lipid peroxidation and skin diseases. What's new. J Eur
Acad Dermatol Venereol. 17:663–669. 2010. View Article : Google Scholar
|
|
25
|
Yang L, Palliyaguru DL and Kensler TW:
Frugal chemoprevention: Targeting Nrf2 with foods rich in
sulforaphane. Semin Oncol. 43:146–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruiz S, Pergola PE, Zager RA and Vaziri
ND: Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lawal AO: Air particulate matter induced
oxidative stress and inflammation in cardiovascular disease and
atherosclerosis: The role of Nrf2 and AhR-mediated pathways.
Toxicol Lett. 270:88–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prasad KN: Simultaneous activation of Nrf2
and elevation of antioxidant compounds for reducing oxidative
stress and chronic inflammation in human Alzheimer's disease. Mech
Ageing Dev. 153:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schäfer M, Farwanah H, Willrodt AH,
Huebner AJ, Sandhoff K, Roop D, Hohl D, Bloch W and Werner S: Nrf2
links epidermal barrier function with antioxidant defense. EMBO Mol
Med. 4:364–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi JH, Jin SW, Han EH, Park BH, Kim HG,
Khanal T, Hwang YP, Do MT, Lee HS, Chung YC, et al: Platycodon
grandiflorum root-derived saponins attenuate atopic dermatitis-like
skin lesions via suppression of NF-κB and STAT1 and activation of
Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 21:1053–1061.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saw CL, Huang MT, Liu Y, Khor TO, Conney
AH and Kong AN: Impact of Nrf2 on UVB-induced skin
inflammation/photoprotection and photoprotective effect of
sulforaphane. Mol Carcinog. 50:479–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaiprasongsuk A, Lohakul J, Soontrapa K,
Sampattavanich S, Akarasereenont P and Panich U: Activation of Nrf2
reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling
cascades: The photoprotective effects of sulforaphane and
hispidulin. J Pharmacol Exp Ther. 360:388–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Migdal C, Botton J, El Ali Z, Azoury ME,
Guldemann J, Giménez-Arnau E, Lepoittevin JP, Kerdine-Römer S and
Pallardy M: Reactivity of chemical sensitizers toward amino acids
in cellulo plays a role in the activation of the Nrf2-ARE pathway
in human monocyte dendritic cells and the THP-1 cell line. Toxicol
Sci. 133:259–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Emter R, van der Veen JW, Adamson G,
Ezendam J, van Loveren H and Natsch A: Gene expression changes
induced by skin sensitizers in the KeratinoSens™ cell line:
Discriminating Nrf2-dependent and Nrf2-independent events. Toxicol
In Vitro. 27:2225–2232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Welsch K, Holstein J, Laurence A and
Ghoreschi K: Targeting JAK/STAT signalling in inflammatory skin
diseases with small molecule inhibitors. Eur J Immunol.
47:1096–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin W, Huang W, Chen L, Jin M, Wang Q, Gao
Z and Jin Z: Topical application of JAK1/JAK2 inhibitor momelotinib
exhibits significant anti-inflammatory responses in DNCB-induced
atopic dermatitis model mice. Int J Mol Sci. 19:E39732018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abe Y and Tanaka N: The Hedgehog signaling
networks in lung cancer: The mechanisms and roles in tumor
progression and implications for cancer therapy. Biomed Res Int.
2016:79692862016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chu J, Wang X, Bi H, Li L, Ren M and Wang
J: Dihydromyricetin relieves rheumatoid arthritis symptoms and
suppresses expression of pro-inflammatory cytokines via the
activation of Nrf2 pathway in rheumatoid arthritis model. Int
Immunopharmacol. 59:174–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roy R, Dagher A, Butterfield C and Moses
MA: ADAM12 is a novel regulator of tumor angiogenesis via STAT3
signaling. Mol Cancer Res. 15:1608–1622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi JK, Jang YH, Lee S, Lee SR, Choi YA,
Jin M, Choi JH, Park JH, Park PH, Choi H, et al: Chrysin attenuates
atopic dermatitis by suppressing inflammation of keratinocytes.
Food Chem Toxicol. 110:142–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pastore S, Giustizieri ML, Mascia F,
Giannetti A, Kaushansky K and Girolomoni G: Dysregulated activation
of activator protein 1 in keratinocytes of atopic dermatitis
patients with enhanced expression of granulocyte/macrophage-colony
stimulating factor. J Invest Dermatol. 115:1134–1143. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mashiko S, Bouguermouh S, Rubio M, Baba N,
Bissonnette R and Sarfati M: Human mast cells are major IL-22
producers in patients with psoriasis and atopic dermatitis. J
Allergy Clin Immunol. 136:351–359.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mu Z, Zhao Y, Liu X, Chang C and Zhang J:
Molecular biology of atopic dermatitis. Clin Rev Allergy Immunol.
47:193–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsushima M, Takagi K, Ogawa M, Hirose E,
Ota Y, Abe F, Baba K, Hasegawa T, Hasegawa Y and Kawabe T: Heme
oxygenase-1 mediates the anti-allergic actions of quercetin in
rodent mast cells. Inflammation Res. 58:705–715. 2009. View Article : Google Scholar
|
|
45
|
Ma YY, Yang MQ, Wang CF, Ding J and Li JY:
Inhibiting mast cell degranulation by HO-1 affects dendritic cell
maturation in vitro. Inflamm Res. 63:527–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zink A, Gensbaur A, Zirbs M, Seifert F,
Suarez IL, Mourantchanian V, Weidinger S, Mempel M, Ring J and
Ollert M: Targeting IgE in severe atopic dermatitis with a
combination of immunoadsorption and omalizumab. Acta Derm Venereol.
96:72–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park G, Oh DS, Lee MG, Lee CE and Kim YU:
6-Shogaol, an active compound of ginger, alleviates allergic
dermatitis-like skin lesions via cytokine inhibition by activating
the Nrf2 pathway. Toxicol Appl Pharmacol. 310:51–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kiehl P, Falkenberg K, Vogelbruch M and
Kapp A: Tissue eosinophilia in acute and chronic atopic dermatitis:
A morphometric approach using quantitative image analysis of
immunostaining. Br J Dermatol. 145:720–729. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ajith Y, Dimri U, Gopalakrishnan A,
Madhesh E, Jhambh R, Joshi V and Devi G: Th1/Th2 immune responses
and oxidative stress in caprine flea allergy dermatitis. Parasite
Immunol. 39:2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Werfel T, Allam JP, Biedermann T, Eyerich
K, Gilles S, Guttman-Yassky E, Hoetzenecker W, Knol E, Simon HU,
Wollenberg A, et al: Cellular and molecular immunologic mechanisms
in patients with atopic dermatitis. J Allergy Clin Immunol.
138:336–349. 2016. View Article : Google Scholar : PubMed/NCBI
|