Cardiovascular disease has become a major global
health issue which is attributed to multiple factors, including
diet, smoking, lifestyle and environmental change (1). The sharp increase in the diabetic
population has been proposed to be closely associated with the
rising prevalence of cardiovascular disease. According to
statistics, the number of diabetic patients worldwide has currently
increased to 1.8 billion, and has doubled since the year 2000. It
is predicted that the global diabetes population will increase from
285 million to 439 million between 2010 and 2030 (2). Compared to non-diabetic individuals
of the same age, the risk of heart failure in male and female
diabetic patients is increased 2-fold and 5-fold, respectively
(3).
In a recent review, the correlation between
hyperinsulinemia or insulin resistance, cardiac dysfunction and
hyperglycemia was discussed (7).
The authors also summarized the recent advances in the research on
DC, its pathogenesis and the underlying molecular mechanisms, and
the promising prophylactic and curative approaches (7). Here, we aimed to summarize the
current advances in DC with strong emphasis on the molecular
mechanisms.
The incidence of DC is not yet clear due to a lack
of large data of outcomes from different populations with diabetes
mellitus (DM). A higher prevalence rate (40 to 60%) of diastolic
dysfunction in type 2 DM (T2DM) patients has been reported in the
literature (8). Autopsies have
revealed that DC has a number of pathological features, including
cardiomyocyte hypertrophy, interstitial fibrosis,
periodic-acid-Schiff (PAS)-positive infiltration, thickening of the
basal membrane of coronary arterioles, and myocardium microvascular
lesions (9–11). The structural changes in DC are
characterized by a near-normal left ventricular end-diastolic
volume, increased left ventricular weight and thickness, cardiac
hypertrophy and fibrosis, and fat deposition (12,13).
The functional alterations include an impaired diastolic function
without obvious decreased systolic function, and a decreased
ventricular wall elasticity (14).
Currently, the diagnosis of early DC has not yet
made substantial progress, and the mainstream methods for DC
diagnosis include imaging methods and serum biomarkers. The
pathological changes in early DC include cardiac hypertrophy,
fibrosis and lipid deposition. Abnormal cardiac function is
characterized by early diastolic dysfunction and late systolic
dysfunction. The diagnostic criteria for DC refer to the method of
Tarquini et al (15), which
are mainly as follows: Presence of DM, exclusion of coronary artery
disease, valvular or congenital heart disease, exclusion of
hypertensive heart disease, exclusion of viral myocarditis by
endomyocardial biopsy and left ventricular ejection fraction (LVEF)
<50%, left ventricular end-diastolic volume index (LVEDVI)
>97 ml/m2. For distinguishing these pathological
features, the following methods can be employed.
In the early stages of DC, echocardiography can be
the prior method for assessing cardiac structural and functional
abnormalities in diabetic patients due to its inexpensive and
non-invasive advantages (16,17).
Tissue Doppler imaging (TDI) measures left ventricular
isovolumetric diastolic time, early mitral annular diastolic motion
velocity (Ve), late diastolic motion velocity (Va) and the Ve/Va
ratio (18). By comparing these
data, cardiac diastolic function images can be divided into 2
types: Restricted mitral flow pattern and unrestricted mitral flow
pattern. Unrestricted mitral flow patterns include normal, impaired
and false normalization (16–18).
This technique has high sensitivity and specificity, and is
currently considered to be a reliable method for evaluating left
ventricular diastolic function in patients with coronary heart
disease (19,20). Intravenous contrast
echocardiography is based on the elevated sonic reflectivity of
contrast agents; therefore, the contrast injection can aid in the
accurate monitoring of the endocardial borders, and the measuring
of ventricular size and ventricular motion. The complement of
intra-cardiac echocardiography (ICE) into 2D-echocardiography
provides an improved evaluation of left ventricular function
(21). A previous study
demonstrated that alterations in the contractility, systolic
function and microcirculation can be well observed via ICE in T1DM
murine models (22).
Speckle tracking echocardiography (STE) is a novel
imaging technique for achieving mechanical deformation of the
myocardia (23). It uses the
images acquired from echocardiography to quantify the inter-pixel
distance during the cardiac cycle. Therefore, morphological
alterations of myocardial deformation can be detected (23,24).
It has been demonstrated that the diabetic microangiopathy in T2DM
patients is closely associated with the myocardial deformation
through 3D-STE (25). In this
regard, ~24% of diabetic patients are diagnosed with systolic
functional impairment by 2D-STE, which cannot be detected through
conventional methods (26).
MRI is a highly sensitive tool that not only detects
abnormal wall motion and cardiac hypertrophy, but also provides
information concerning arrhythmias and cardiomyopathy. Rijzewijk
et al (27) believe that
contrast-enhanced MRI can be used to predict the progression of
ventricular arrhythmias, myocardial steatosis and heart failure in
patients with a history of ischemic heart disease. Cardiac MRI can
detect myocardial metabolic abnormalities through different
radioactive elements and positron emission tomography (PET)
(28).
Cardiac catheterization is currently the best
technique for assessing intracardiac hemodynamics and is considered
to be the gold standard for the diagnosis of diastolic cardiac
dysfunction, but it is not widely used due to its invasiveness
(29,30). Left and right ventricular
catheterization can be employed to assess left ventricular
end-diastolic pressure and mean pulmonary arterial wedge pressure,
respectively (29,30). The left ventricular end-diastolic
pressure >16 mmHg or a pulmonary capillary wedge pressure >12
mmHg can be used as criteria for the diagnosis of diastolic
dysfunction (31). Coronary
angiography is mainly used to determine coronary artery stenosis,
which tends to occur in late DC (32–34).
Previous studies have found that extracellular
matrix proteins are associated with persistent ventricular
remodeling (35,36). MMPs are endogenous proteolytic
enzymes that degrade type I and type III collagen during the
development of heart failure, thereby promoting myocardial
fibrosis; they can also affect the expression of microRNAs (miRNAs)
and cause myocardial contractile dysfunction (37–39).
During the process of myocardial fibrosis, serum MMPs (especially
MMP-9) are increased, while tissue inhibitory factors of MMPs are
decreased (40). Ban et al
(41) measured serum MMP-7 in
patients with DC via enzyme-linked immunosorbent assay (ELISA), and
found that MMP-7 was significantly higher in patients with
diastolic dysfunction than in patients with normal diastolic
functions.
Hcy is a type of sulfur-containing amino acid that
is an intermediate metabolite of methionine. It has been reported
that plasma Hcy levels are positively correlated with the risk of
cardiovascular events (42). For
every 5 µmol/l increase in plasma Hcy, the risk of ischemic heart
disease is increased by 32%, and for each 5 µmol/l decrease, the
risk is reduced by 16% (43).
Previous studies have shown that high Hcy (HHcy) and hyperglycemia
can both lead to DC by inducing oxidative stress and reducing the
level of peroxisome proliferator-activated receptor γ (PPAR-γ)
(44–46), but whether there is a synergistic
mechanism between them is not yet clear. Mishra et al
(44) found that in HHcy and
hyperglycemic murine models, the ventricular end-diastolic diameter
was increased, and PPAR-γ, tissue metalloproteinase-4, and
thioredoxin inhibitor levels were decreased, whereas those of MMP-9
were increased, suggesting that endogenous Hcy reduces the
expression of PPAR-γ and induces the decoupling of
endothelial-myocytes (EMs), resulting in diastolic dysfunction and
further progression of DC.
The serum procollagen type III aminopeptide (PIIINP)
level reflects the metabolism of type III collagen. A previous
study proposed that PIIINP is an important indicator of early left
ventricular dysfunction in obese patients with insulin resistance
(47). Epshteyn et al
(48) found that 96% of diabetic
patients with left ventricular dysfunction had increased levels of
brain natriuretic peptides (>90 pg/ml). Cardiac troponin is a
type of protein released from ischemic or inflammatory
disease-damaged cardiomyocytes. Russell et al (49) reported that the levels of troponin
T were elevated in the blood of neonates with congenital
cardiomyopathy or cardiac insufficiency in mothers with diabetes.
However, in adult DC patients, its clinical significance remains
uncertain.
Early diagnosis and intervention of DC can slow
disease progression, and provide an improved prognosis. Therefore,
a combination of multiple techniques for the diagnosis of DC is
recommended. For example, a combination of NT-proBNP quantification
and echocardiography has more reliable diagnostic value for DC
diagnosis than either technique alone (50). In addition, further identification
of novel biomarkers is needed, such as microRNAs and long
non-coding RNAs (lncRNAs).
Hyperglycemia plays a key role in the pathogenesis
of DC. Hyperglycemia has been shown to directly or indirectly
damage cardiomyocytes, fibroblasts and endothelial cells via the
accumulation of reactive oxygen species (ROS) (51). Hyperglycemia can increase the
production of ROS through the electron chain, which can induce
apoptosis of cardiomyocytes (52).
ROS activate polyADP-ribose polymerase (PARP), consequently causing
an increase in glycosylation and inhibition of glyceraldehyde
phosphate dehydrogenase (GAPDH), which transforms the glycolysis
process into a cascade of cardiomyocyte injury (53). This process involves increased
production of glycation end products, activation of hexosamine
pathways, and increased production of protein kinases (PKCs). High
levels of ROS, PARP, glycation end products, and aldose reductase
production induced by hyperglycemia can lead to cell apoptosis
(54,55).
In the diabetic state, increased hepatic fat
synthesis and fat lysis in adipocytes promote the formation of
circulating fatty acids and triglycerides (56,57).
The transportation of fatty acids into cardiomyocytes requires the
intermediation of insulin, hyperinsulinemia and hyperlipidemia can
increase the fatty acid transportation, and thus induce
lipotoxicity in cardiomyocytes (58). The following three mechanisms are
included in lipotoxicity: i) Increased production of ROS [increased
levels of oxidized fatty acids can increase the mitochondrial
membrane potential and thus increase production of ROS (55,59)]; ii) increased production of
ceramide [ceramide is a sphingomyelin that induces apoptosis of
cardiomyocytes by inhibiting the mitochondrial respiratory chain
(59)]; and iii) effects on
myocardial contractile function. Elevated fatty acid levels in
cardiomyocytes can cause the opening of potassium channels,
resulting in shortening of the action potential duration and
opening of L-type calcium channels, ultimately affecting the
calcium storage of calcium pump of sarcoplasmic reticulum and
impairing myocardial contractile function (60,61).
Excessive fatty acid uptake and metabolism not only cause the
accumulation of intermediate metabolites of fatty acids, increase
the oxygen demand and uncoupling of mitochondria, but also increase
the production of ROS, reduce the synthesis of ATP, cause
mitochondrial dysfunction, and increase apoptosis (62). Therefore, hyperlipidemia plays a
core role in the pathogenesis of DC.
Insulin resistance and subsequent hyperinsulinemia
are typical pathophysiological characteristics of diabetes
mellitus. In the normal heart, 2/3 of the energy provided for
myocardial contraction is derived from fatty acid oxidation, and
the remaining 1/3 is derived from glucose and lactic acid
metabolism (63). Glucose
utilization is significantly limited in patients with
hyperinsulinemia or insulin resistance, therefore myocardial energy
metabolism is more dependent on fatty acid oxidation (64). Moreover, hyperinsulinemia induces
cardiomyocyte hypertrophy through a variety of mechanisms. Multiple
epigenetic and genetic changes caused by hyperinsulinemia lead to
the dysregulation of extracellular and intracellular protein
expression, especially extracellular matrix proteins, leading to
cardiomyocyte hypertrophy and myocardial fibrosis (65).
A balanced regulation of calcium metabolism in
cardiomyocytes guarantees normal cardiac contractility (66). Oxidative stress, aggregation of
long-chain acetylcarnitine, and changes in lipid membrane
components can affect myocardial calcium homeostasis (66–68).
Recent reports have shown that calcium pump ATPase,
sodium-potassium ATPase and sodium-calcium exchange, and changes in
ryanodine receptor function are involved in the pathogenesis of DC
(69,70).
The mechanism of the renin-angiotensin-aldosterone
system (RAAS) in the development of diabetes to heart failure has
long been known (71,72). Research has confirmed that
upregulation of the RAAS system in diabetic patients is closely
related to cardiac hypertrophy and fibrosis (71–75).
Angiotensin directly acts on cardiomyocytes and cardiac fibroblasts
via angiotensin receptor-1, causing cardiac hypertrophy and
fibrosis (76).
The main characteristic of DC is the disorder of
energy metabolism, which is caused by glucose toxicity,
lipotoxicity and the dysregulation of mitochondrial function
(12). P53 and cytochrome C
oxidase 2 (SCO2) are important regulators of the mitochondrial
respiratory chain, which play an essential role in the pathogenesis
of DC (12,77–80).
In a diabetic condition, the production of ROS activates the
P53/SCO2 signaling system in cardiomyocytes, thereby increasing the
oxygen consumption of mitochondria, and causing excessive
production of ROS and lipid deposition, which induce cardiac
function insufficiency (81).
In order to maintain the efficient function of the
myocardium, the β-adrenergic receptors of cardiomyocytes of
diabetic patients are activated, promoting the synthesis of
nicotinamide adenine dinucleotide phosphate oxidase and ROS; this
pathway mediates myocardial reformation (82–84).
The significant factors involved in the development of myocardial
reformation and heart failure are p38 mitogen-activated protein
kinase and heat shock protein-27, which are both phosphorylated
during the process (85).
In a diabetic condition, a series of signaling
pathways are activated and involved in cardiomyocyte hypertrophy
and interstitial fibrosis. For example, in the case of acute
hyperglycemia, mannoses bind to coagulins and the
coagulin-complements, which is an important mechanism leading to
vascular dysfunction and cardiomyopathy (86,87).
The level of serum transforming growth factor-β1 (TGF-β1) is also
significantly reduced (88).
Thrombospondin-1 activates the potential TGF-β1 complex in DC,
stimulating cardiomyocytes to secrete collagen fibers III (89). The nuclear factor κB (NF-κB)
pathway has also been found to promote the pathogenesis and
progression of DC in diabetic animal models. This signaling pathway
is involved in oxidative stress, inflammation, endothelial
dysfunction, cardiac hypertrophy and fibrosis (90). Cardiomyocytes contain numerous
messenger systems to regulate their normal physiological functions,
and these messenger systems produce synergistic or antagonistic
effects. Diabetes almost simultaneously causes lesions in these
messenger systems, making the condition more complex. The signaling
pathways involved in the pathogenesis of DC are described
below.
An antioxidant response element (ARE) is a promoter
sequence located at the 5′end of the protective gene such as
superoxide dismutase (SOD) or glutathione S-transferase (GST), and
is a specific DNA-promoter-binding sequence that can be activated
by a variety of oxidative and electrophilic compounds, thereby
activating phase II detoxification enzymes and antioxidant enzyme
gene expression, and protecting normal tissues (91). NF-E2 related factor 2 (Nrf2) is a
transcriptional factor that regulates the transcriptional activity
of its gene by binding to the 5′ end ARE sequence of its own gene
(92). It is believed that Nrf2
plays a key role in cellular oxidative injury protection and the
regulation of cell sensitivity to stress. Nrf2 upregulates the
expression of antioxidant genes; thus, activation of Nrf2 is
beneficial for cells to resist oxidative stress (93). Yoh et al (94) found that hyperglycemia and
oxidative stress can accelerate renal injury, and Nrf2 is an
important factor in preventing the occurrence of diabetic
complications. He et al (95) found that hyperglycemia damages
cardiomyocytes through the ROS system and induces DC. Nrf2-depleted
rats can quickly develop DC and exhibit increased levels of
glucose-induced apoptosis at lower concentrations, therefore, Nrf2
has been suggested to be a key regulator of ARE's anti-oxidative
stress and can prevent the progression of DC.
Hemeoxygenase 1 (HO-1), or heat shock protein 32
(HSP32), is a stress protein induced by a variety of factors, and
oxidative stress can activate its expression. HO-1 induction is
considered to be a common marker of oxidative stress (96). Shi et al (97) showed that activation and
intra-nuclear expression of Nrf2 protein can promote the expression
of anti-oxidative protein HO-1, which can significantly reduce
renal oxidative stress injury in type 1 diabetic mice. The
overexpression of HO-1 can protect the myocardia from
ischemia/reperfusion injury, and bilirubin produced by HO-1 can
protect cardiac function under an ischemic condition (98–102). Therefore, HO-1 is an important
antioxidant protease of the Nrf2-ARE pathway. The Nrf2-ARE pathway
is a key approach for the body to resist oxidative stress injury.
Oxidative stress is an important mechanism of the pathogenesis of
DC. Resisting oxidative stress and scavenging ROS are potent
strategies for the treatment of DC and have been demonstrated to be
an effective anti-oxidative stress enzyme system in vivo,
and also an important cardioprotective factor for the prevention
and treatment of DC. Activation of Nrf2 is a key step in cellular
antioxidative stress. It is important to elucidate whether HO-1
exerts cardioprotective effects against DC by activating the
Nrf2-ARE pathway (98).
TGF-β1 and its downstream Smad proteins are involved
in the pathological process of myocardial interstitial fibrosis and
cardiac hypertrophy (103). In
normal hearts, the levels of TGF-β1, type I and type III collagen
are extremely low, while diabetic hearts are stimulated by multiple
factors such as hyperglycemia, local renin-angiotensin system
(RAS), and oxidative stress inflammation. Therefore, the expression
of TGF-β1 is upregulated and eventually leads to the
differentiation of cardiac fibroblasts into myofibroblasts, which
also induces the over-synthesis of collagen, fibronectin and
proteoglycans (104). The process
of myocardial fibrosis is accompanied by upregulation of TGF-β1 and
its downstream Smad2 and Smad3 proteins (105–111). In vascular smooth muscle cells,
TGF-β1 promotes the phosphorylation of Smad2 and Smad3 to form a
Smad4 trimer (112). This complex
translocates into the nucleus and binds to Smad-associated DNA
sequences to promote the transcription of fibrogenic factors such
as fibronectin and type I collagen; TGF-β1-mediated gene expression
in human fibroblasts depends on the presence of Smad3 (105–112). In Smad3 gene-deficient mice,
myocardial fibrosis can be reduced by 60%, indicating that Smad3 is
essential for the development of myocardial fibrosis (113). At present, myocardial fibrosis
still lacks effective treatment methods. In-depth study of the role
of TGF-β1/Smad signal transduction pathways in diabetic myocardial
fibrosis is expected to provide new and effective therapies for the
treatment of DC.
The NF-κB signaling pathway mainly mediates
inflammatory responses. It has been shown that NF-κB can activate
the expression of inflammatory factors such as interleukin (IL)-1,
tumor necrosis factor (TNF) and interferon (IFN), which in turn can
promote activation of NF-κB bypass (114). The activation of the NF-κB
signaling pathway is closely related to the occurrence of
cardiovascular disease in diabetes, and is an important
intermediate link in various signaling pathways in the pathogenesis
of diabetic vascular complications (115). Excessive glucose in diabetic
patients causes non-enzymatic glycation, producing advanced
glycation end products (AGEs) that bind to receptors of AGEs and
release large amounts of ROS to activate NF-κB translocation into
the nucleus, which in turn initiates transcription of inflammatory
factors such as TNF-α, ultimately leading to damage of
cardiovascular endothelial cells and proliferation of smooth muscle
cells, subsequently promoting cardiovascular disease in diabetes
(116–127).
At present, it is believed that the pathogenesis of
DC is related to inflammation. Feng et al (128) studied myocardial
ischemia-reperfusion injury in rats and found that knocking out the
MyD88 gene reduced the myocardial infarction size and improved
cardiac function compared to wild-type mice. Furthermore, less
inflammatory cell infiltration and lower expression of monocyte
chemoattractant protein-1 (MCP-1) and intercellular adhesion
molecule-1 (ICAM-1) were found in MyD88-deficient mice, suggesting
that the knockdown of MyD88 attenuates the NF-κB activation during
ischemia-reperfusion.
Protein kinase C (PKC) is a family of
serine/threonine kinases that are widely present in cells. Most of
the neurotransmitters, hormones and growth factors can activate PKC
through activating phospholipase C, which produces a lipid-derived
secondary messenger (such as diacylglycerol) that phosphorylates
the serine/threonine residue of the target protein (129). Previous studies have found that
excessive activation of PKC can lead to cardiac hypertrophy and
fibrosis, suggesting that PKC inhibitors may prevent and delay
cardiac hypertrophy and myocardial fibrosis (130,131). Hyperglycemia increases the de
novo synthesis of diacylglycerols in cardiomyocytes, vascular
smooth muscle cells and endothelial cells, which in turn activates
PKC. In addition, Ca2+, angiotensin, endothelin,
vascular endothelial growth factor (VEGF) and osmotic factors can
activate PKC. The possible mechanism is that the above factors bind
to the cell membrane receptors, and diacylglycerol is activated by
membrane phospholipids to activate PKC (130,131). Similarly, the PKC inhibitory
therapy should be an essential part of DC treatment. PKC inhibitors
have been initially used in the clinical treatment of diabetic
vascular complications, but their long-term efficacy and side
effects still need to be further observed (132–135).
Several PPAR isoforms, including PPAR-α, PPAR-β/δ,
and PPAR-γ, have been shown to be expressed in cardiomyocytes and
act as key regulators for glucose and lipid metabolism, and
energetic homeostasis. Interestingly, they are also involved in
other cellular events such as inflammation and oxidative stress
(136). PPAR-α is relatively
highly expressed in the heart, and its activation influences
cellular free fatty acid (FFA) uptake and mitochondrial FFA
oxidation (137). PPAR-α
regulates the assembly and transport of lipoproteins, and regulates
the defenses of both oxidant and anti-oxidant. Previous research
has shown that overexpression of PPAR-α results in decreased
sarcoplasmic reticulum Ca2+ uptake, contractile
dysfunction, left ventricular hypertrophy and increased B-type
natriuretic peptide (138).
Conversely, depletion of PPAR-α prevents fasting-induced FFA
metabolic gene expression and enhances glucose metabolism (139). With the progression of DC,
long-term exposure to increased FFAs was found to result in reduced
PPAR-α expression, which was shown to further injure the cardiac
function by inhibiting FFA oxidation and increasing intracellular
lipid accumulation in rodent cardiomyocytes (140,141). However, clinical studies have
suggested that PPAR-α is not significantly overexpressed in the
cardiomyocytes of patients with type 2 diabetes mellitus (142,143). The activation of PPAR-α and the
following FFA oxidation in cardiomyocytes under DC situation may
act as a compensatory mechanism for substrate supply. Furthermore,
a reduction in PPAR-α in advanced disease may have maladaptive
consequences in terms of cardiac metabolism, including
glucotoxicity and functional cardiac abnormalities. The role of
PPAR-α in the progression of cardiac function decrease in DC has
not been fully understood and warrants further investigation.
Similarly, PPAR-β/δ isoforms are also abundantly expressed in
cardiomyocytes and can regulate FFA metabolism (144). Enhanced PPAR-β/δ signaling
promotes FFA metabolism and vice versa. Additionally, PPAR-γ
provides anti-hypertrophic and anti-inflammatory effects in
cardiocytes (145). PPAR-γ
agonists promote insulin sensitivity and enhance cardiomyocyte
glucose intake (136). Therefore,
PPAR-γ may help maintain glucose and FFA metabolism, and can
protect cardiac function.
MAPK overactivation has been reported to contribute
to the pathogenesis of DC and cardiac dysfunction. Erk1/2, p38
MAPK, and JNKs are 3 essential MAPK members that regulate
cardiomyocyte growth, hypertrophy and remodeling (146–150). Increased phosphorylation of
Erk1/2 and p38 MAPK was observed in streptozotocin-induced DC
models (151). Previous research
has demonstrated that obesity- or insulin resistance-induced heart
failure is associated with the overactivation of S6 kinase 1 and
Erk1/2 signaling (152,153). JNK can be activated under the
conditions of oxidative stress and inflammation (154). In turn, enhanced JNK signaling in
the diabetic heart contributes to oxidative stress, endoplasmic
reticulum stress and interstitial fibrosis (4,154).
In contrast, inhibition of JNK phosphorylation by a curcumin analog
was found to prevent high glucose-induced inflammation and
apoptosis in diabetic hearts (154,155). In addition to these observations,
JNK may play an important role in cardiomyocyte apoptosis (155). As such, JNK activation has been
showed to cause increased cardiomyocyte apoptosis as early as at
day 3 and 7 in a type 1 diabetic rodent model (155). Collectively, activation of both
MAPK and JNK signaling seems to significantly contribute to the
development of DC.
AMPK is a serine/threonine kinase that can detect
cellular energy status and regulate energy homeostasis (156). Activation of AMPK is involved in
multiple cellular processes such as autophagy (157). Activation of AMPK has been shown
to inhibit mTOR, which is a protein kinase that regulates autophagy
(158). A previous study showed
that high glucose inhibited autophagic activity and the AMPK
pathway in a DC cell model, thereby stimulating the interaction of
beclin 1 (BECN1) and the anti-apoptotic protein BCL2 (159). Activation of AMPK results in
phosphorylation of MAPK8, which in turn drives BCL2 phosphorylation
and dissociates from the BECN1-BCL2 complex (15). Thus, AMPK restores cardiomyocyte
autophagy through BCL2, preventing cardiomyocyte apoptosis.
Dissociation of BCL2 from BECN1 via activation of MAPK8-BCL2
signaling may restore autophagy by driving AMPK activation and is
important for the prevention of DC processes (160).
DC is associated with the dysregulated expression of
miRNAs, short single-stranded noncoding RNAs of ~20 nucleotides in
length (161). Importantly,
miRNAs bind to the 3′-untranslated region (3′UTR) of the mRNAs of
target genes. Therefore miRNAs can participate in the regulation of
multiple events in the pathogenesis and progression of DC, such as
mitochondrial function, Ca2+ handling, ROS production,
apoptosis, autophagy and fibrosis (162,163). miR-15a, miR-21, miR-24, miR-29,
miR-30d, miR-103, miR-126, miR-146a, miR-150, miR-191, miR-223,
miR-320, miR-375 and miR-486 have all been reported to be
overexpressed in type 2 diabetic patients (163–168). miR-103, miR-107, miR-143 and
miR-181 play essential roles in the regulation of cellular insulin
sensitivity and glucose metabolism (163,169). High expression levels of miR-454,
miR-500, miR-142-3p/5p and miR-1246 have been found in the
circulating blood of patients with cardiac diastolic dysfunction
(170). Other microRNAs, such as
miR-113a, miR-133a and miR-150, have been reported to be related to
cardiomyocyte hypertrophy and interstitial fibrosis (171).
At present, the therapeutic strategies for DC are
still based on drug treatment. The principles include primarily
acting against the pathogenesis of DC, and delaying the development
of heart failure (172).
Controlling blood sugar is fundamental for effectively reducing the
cardiovascular morbidity of diabetes. Diet control and regular
exercise are necessary for disease treatment. Clinical studies have
found that the use of hypoglycemic agents to control blood glucose
in patients with early stages of myocardial dysfunction can
effectively delay the progression of cardiomyopathy in diabetic
patients (173). Studies have
confirmed that the use of metformin in diabetic patients can
effectively reduce the mortality (174).
Most diabetic patients have hyperlipidemia in the
early stage of the disease. Clinical studies have shown that
statins and fibrates can effectively reduce the blood lipid levels
and the mortality of cardiovascular disease in diabetic patients
(175,176). A large amount of ROS produced by
oxidative stress can directly damage myocardial cells and vascular
endothelial cells, causing myocardial dysfunction (177). A variety of antioxidant drugs
such as vitamin E and angiotensin-converting enzyme inhibitors
(ACEIs) can reduce the levels of ROS in the blood and therefore
protect the myocardium (178).
For DC patients with symptoms of heart failure, beta blockers have
been shown to have a significant effect on improving ventricular
function (179). Sharma and
McNeill (180) tested the
inhibitory effect of metoprolol on DC in animal models and found
that metoprolol reduced fatty acid oxidation and improved
ventricular function. Increased levels of angiotensin 2 in
myocardial tissue, leading to increased aldosterone secretion,
enhanced the content of ROS and led to myocardial cell damage and
apoptosis (181).
In recent years, physical therapy has also been
gradually applied to the treatment of diabetic complications.
Studies have shown that hyperbaric oxygen therapy can reduce the
fasting blood glucose by at least 20% in patients with type 2
diabetes, and reduce insulin resistance in obese diabetic patients
(182). On the other hand,
hyperbaric oxygen was found to reduce myocardial cell damage in DC
rats (183). These studies
indicate that hyperbaric oxygen therapy has considerable promise in
the treatment of cardiovascular disease and DC.
In addition, stem cell therapy is the future of
disease treatment, and it has been proven to be effective in
treating a variety of cardiovascular diseases. Although stem cell
therapy has not been applied to clinical DC treatment to date,
in vivo and in vitro research suggests that bone
marrow mesenchymal stem cells can effectively promote the growth
and differentiation of cardiomyocytes in DC rats and significantly
improve cardiac function (184,185). Neel and Singla (186) found that induced pluripotent stem
cells can effectively inhibit cardiomyocyte apoptosis and fibrosis
in DC rats, and significantly improve the cardiac function of model
animals. The protective effects of stem cells on the cardiovascular
system in the DC model indicate that it has broad prospects in the
treatment of DC.
Although the current treatments have largely
improved the short-term prognosis of patients with diabetic
cardiomyopathy, there are still no convincing or effective
prevention strategies to avoid repeated and prolonged
rehospitalization. The main challenges remain in the complexity of
the pathogenesis of DC and the inefficiency of diagnostic
approaches. Future studies need to focus on the improvement in
diagnosis by coupling different diagnostic approaches. Advances in
next generation sequencing and imaging technologies may help
overcome these challenges.
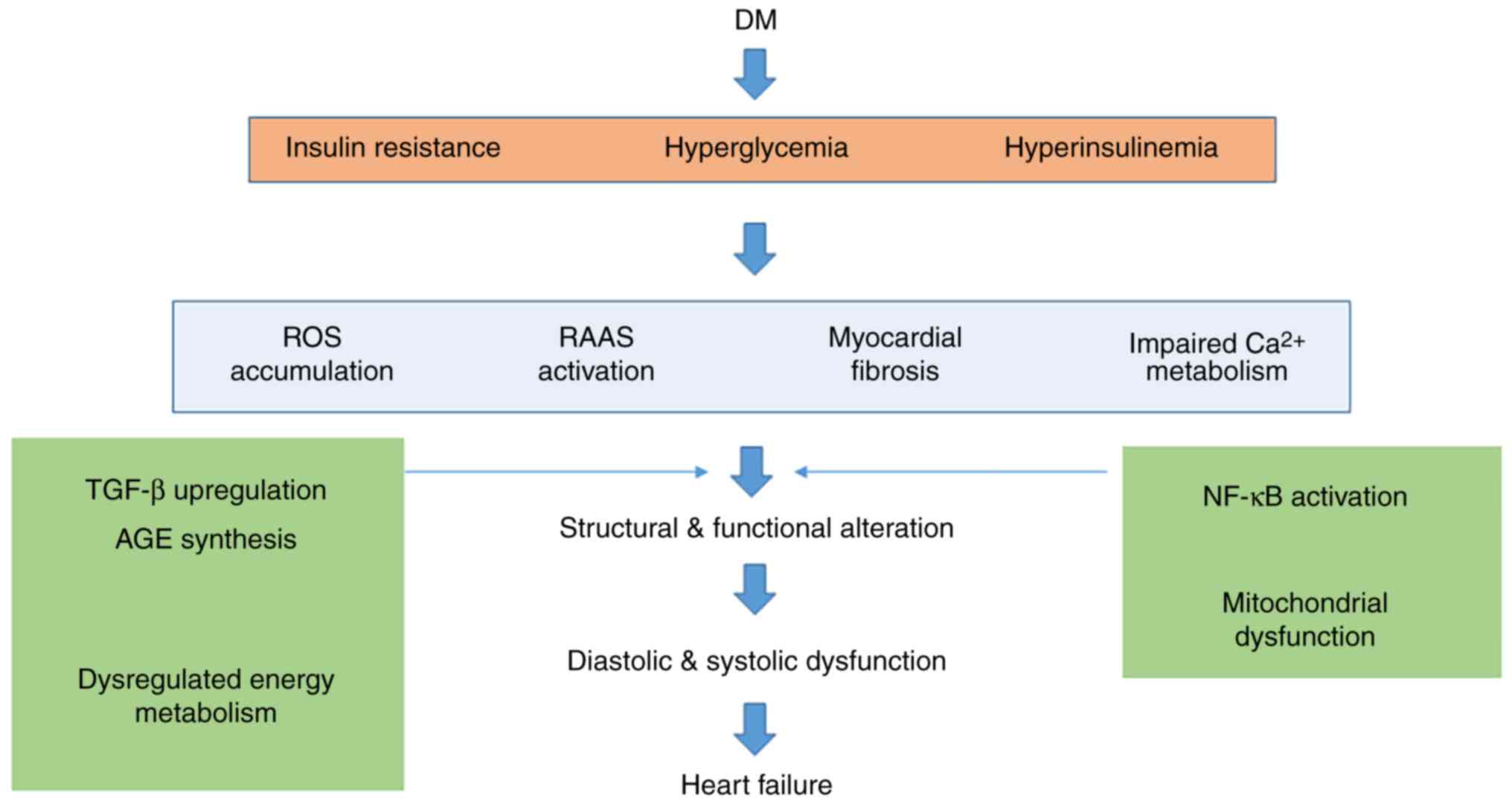
DC is a major complication of diabetes that requires
further elucidation. DC is closely correlated with cardiac
mortality and morbidity. Echocardiography is currently the most
commonly used diagnostic method for DC diagnosis. Novel modalities
including MRI, PET and multiple serum/plasma biomarkers are
emerging. However, the present methods have their limits, and
therefore a combination of multiple methods should be recommended
for the diagnosis of DC. The pathophysiology and pathogenesis of DC
have not been fully elucidated. The presently proposed hypothesis
(Fig. 1) includes
hyperglycemia-associated metabolic and oxidative stress,
lipotoxicity and hyperinsulinemia, and the treatment of DC is still
based on drug therapy. The principles are mainly aimed at the
pathogenesis of DC, and delay of the development of heart failure.
Controlling blood sugar is fundamental for effectively reducing the
cardiovascular morbidity of diabetes. Diet control and regular
exercise are necessary for disease treatment. New therapeutic
approaches covering cell-based or gene-based therapies are
currently being investigated. Further research is required for
understanding the mechanisms involved in the development of DC to
enhance the discovery of clinically effective targets for
preventing this condition and its progression to heart failure. We
apologize to those authors for whom we did not cite their valuable
works due to the large number of papers to be cited.
Not applicable.
No funding was received.
Not applicable.
LS, GW and XG participated in the study design. GW,
MY, TZ, SZ and GH participated in the preparation of the
manuscript. LS, TZ and GW wrote and edited the manuscript. GW and
XG revised the manuscript and supervised the study. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lloyd-Jones D, Adams RJ, Brown TM,
Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K,
Gillespie C, et al: Executive summary: Heart disease and stroke
statistics-2010 update: A report from the American heart
association. Circulation. 121:948–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle JP, Thompson TJ, Gregg EW, Barker LE
and Williamson DF: Projection of the year 2050 burden of diabetes
in the US adult population: Dynamic modeling of incidence,
mortality, and prediabetes prevalence. Popul Health Metr. 8:292010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Regensteiner JG, Golden S, Huebschmann AG,
Barrett-Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N,
Reckelhoff JF, et al: Sex differences in the cardiovascular
consequences of diabetes mellitus: A scientific statement from the
American heart association. Circulation. 132:2424–2447. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Authors/Task Force Members, ; Rydén L,
Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C,
Escaned J, Hammes HP, et al: ESC Guidelines on diabetes,
pre-diabetes, and cardiovascular diseases developed in
collaboration with the EASD: The Task Force on diabetes,
pre-diabetes, and cardiovascular diseases of the European Society
of Cardiology (ESC) and developed in collaboration with the
European Association for the Study of Diabetes (EASD). Eur Heart J.
34:3035–3087. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American college of cardiology
foundation/American heart association task force on practice
guidelines. J Am Coll Cardiol. 62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia G, Whaley-Connell A and Sowers JR:
Diabetic cardiomyopathy: A hyperglycaemia- and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Redfield MM, Jacobsen SJ, Burnett JC Jr,
Mahoney DW, Bailey KR and Rodeheffer RJ: Burden of systolic and
diastolic ventricular dysfunction in the community: Appreciating
the scope of the heart failure epidemic. JAMA. 289:194–202. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miki T, Yuda S, Kouzu H and Miura T:
Diabetic cardiomyopathy: Pathophysiology and clinical features.
Heart Fail Rev. 18:149–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Song Y, Wang Q, Kralik PM and
Epstein PN: Causes and characteristics of diabetic cardiomyopathy.
Rev Diabet Stud. 3:108–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pappachan JM, Varughese GI, Sriraman R and
Arunagirinathan G: Diabetic cardiomyopathy: Pathophysiology,
diagnostic evaluation and management. World J Diabetes. 4:177–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghosh N and Katare R: Molecular mechanism
of diabetic cardiomyopathy and modulation of microRNA function by
synthetic oligonucleotides. Cardiovasc Diabetol. 17:432018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varma U, Koutsifeli P, Benson VL, Mellor
KM and Delbridge LMD: Molecular mechanisms of cardiac pathology in
diabetes-Experimental insights. Biochim Biophys Acta Mol Basis Dis.
1864:1949–1959. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang Y, Wang S, Huang J, Cai L and Keller
BB: Right ventricular dysfunction and remodeling in diabetic
cardiomyopathy. Am J Physiol Heart Circ Physiol. 316:H113–H122.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarquini R, Pala L, Brancati S, De Cosmo
S, Mazzoccoli G and Rotella CM: Clinical approach to diabetic
cardiomyopathy: A review of human studies. Curr Med Chem.
25:1510–1524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao S, Ho D, Vatner DE and Vatner SF:
Echocardiography in mice. Curr Protoc Mouse Biol. 1:71–83.
2011.PubMed/NCBI
|
|
17
|
Qian C, Gong L, Yang Z, Chen W, Chen Y, Xu
Z, Wu B, Tang C, Gao F and Zeng W: Diastolic dysfunction in
spontaneous type 2 diabetes rhesus monkeys: A study using
echocardiography and magnetic resonance imaging. BMC Cardiovasc
Disord. 15:592015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caglar Acar O, Epcacan S, Uner A, Ece I
and Dogan M: Evaluation of left and right ventricular functions
using conventional and tissue Doppler echocardiography in children
with type 1 diabetes mellitus. J Pediatr Endocrinol Metab.
29:885–891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danzmann LC, Bodanese LC, Köhler I and
Torres MR: Left atrioventricular remodeling in the assessment of
the left ventricle diastolic function in patients with heart
failure: A review of the currently studied echocardiographic
variables. Cardiovasc Ultrasound. 6:562008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu CM, Sanderson JE, Marwick TH and Oh JK:
Tissue Doppler imaging a new prognosticator for cardiovascular
diseases. J Am Coll Cardiol. 49:1903–1914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malm S, Frigstad S, Sagberg E, Larsson H
and Skjaerpe T: Accurate and reproducible measurement of left
ventricular volume and ejection fraction by contrast
echocardiography: A comparison with magnetic resonance imaging. J
Am Coll Cardiol. 44:1030–1035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cosyns B, Droogmans S, Hernot S,
Degaillier C, Garbar C, Weytjens C, Roosens B, Schoors D, Lahoutte
T, Franken PR and Van Camp G: Effect of streptozotocin-induced
diabetes on myocardial blood flow reserve assessed by myocardial
contrast echocardiography in rats. Cardiovasc Diabetol. 7:262008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Xu JZ, Chen X, Xu TY, Zhang W, Li
Y and Wang JG: Left atrial myocardial dysfunction in patients with
primary aldosteronism as assessed by speckle-tracking
echocardiography. J hypertension. May 30–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
24
|
Cameli M, Mondillo S, Galderisi M, Mandoli
GE, Ballo P, Nistri S, Capo V, D'Ascenzi F, D'Andrea A, Esposito R,
et al: Speckle tracking echocardiography: A practical guide. G Ital
Cardiol (Rome). 18:253–269. 2017.(In Italian). PubMed/NCBI
|
|
25
|
Enomoto M, Ishizu T, Seo Y, Kameda Y,
Suzuki H, Shimano H, Kawakami Y and Aonuma K: Myocardial
dysfunction identified by three-dimensional speckle tracking
echocardiography in type 2 diabetes patients relates to
complications of microangiopathy. J Cardiol. 68:282–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang ZY, Schull-Meade R, Leano R, Mottram
PM, Prins JB and Marwick TH: Screening for heart disease in
diabetic subjects. Am Heart J. 149:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rijzewijk LJ, van der Meer RW, Smit JW,
Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A and Lamb HJ:
Myocardial steatosis is an independent predictor of diastolic
dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol.
52:1793–1799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwong RY and Korlakunta H: Diagnostic and
prognostic value of cardiac magnetic resonance imaging in assessing
myocardial viability. Top Magn Reson Imaging. 19:15–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sorrell VL and Reeves WC: Noninvasive
right and left heart catheterization: Taking the echo lab beyond an
image-only laboratory. Echocardiography. 18:31–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grady RM, Eisenberg PR and Bridges ND:
Rational approach to use of heparin during cardiac catheterization
in children. J Am Coll Cardiol. 25:725–729. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kindermann M: How to diagnose diastolic
heart failure: A consensus statement on the diagnosis of heart
failure with normal left ventricular ejection fraction by the heart
failure and echocardiography associations of the European society
of cardiology. Eur Heart J. 28:2686–2687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang FB, Guo WL, Sheng M, Sun L, Ding YY,
Xu QQ, Xu MG and Lv HT: Diagnostic accuracy of coronary angiography
using 64-slice computed tomography in coronary artery disease.
Saudi Med J. 36:1156–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dharampal AS, Rossi A and de Feyter PJ:
Computed tomography-coronary angiography in the detection of
coronary artery disease. J Cardiovasc Med (Hagerstown). 12:554–561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiangping S, Zhe Z, Wei W, Yunhu S, Jie H,
Hongyue W, Hong Z and Shengshou H: Assessment of coronary artery
stenosis by coronary angiography: A head-to-head comparison with
pathological coronary artery anatomy. Circ Cardiovasc Interv.
6:262–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dinh W, Bansemir L, Füth R, Nickl W,
Stasch JP, Coll-Barroso M, Lapp H, Bufe A, Wolfertz J, Scheffold T
and Lankisch M: Increased levels of laminin and collagen type VI
may reflect early remodelling in patients with acute myocardial
infarction. Acta Cardiol. 64:329–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Souza A, Howarth FC, Yanni J, Dobryznski
H, Boyett MR, Adeghate E, Bidasee KR and Singh J: Left ventricle
structural remodelling in the prediabetic Goto-Kakizaki rat. Exp
Physiol. 96:875–888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen LC, Shibu MA, Liu CJ, Han CK, Ju DT,
Chen PY, Viswanadha VP, Lai CH, Kuo WW and Huang CY: ERK1/2
mediates the lipopolysaccharide-induced upregulation of FGF-2, uPA,
MMP-2, MMP-9 and cellular migration in cardiac fibroblasts. Chem
Biol Interact. 306:62–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
DeLeon-Pennell KY, Meschiari CA, Jung M
and Lindsey ML: Matrix metalloproteinases in myocardial infarction
and heart failure. Prog Mol Biol Transl Sci. 147:75–100. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Radosinska J, Barancik M and Vrbjar N:
Heart failure and role of circulating MMP-2 and MMP-9. Panminerva
Med. 59:241–253. 2017.PubMed/NCBI
|
|
40
|
Tanaka K, Essick EE, Doros G, Tanriverdi
K, Connors LH, Seldin DC and Sam F: Circulating matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
cardiac amyloidosis. J Am Heart Assoc. 2:e0058682013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ban CR, Twigg SM, Franjic B, Brooks BA,
Celermajer D, Yue DK and McLennan SV: Serum MMP-7 is increased in
diabetic renal disease and diabetic diastolic dysfunction. Diabetes
Res Clin Pract. 87:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeh JK, Chen CC, Hsieh MJ, Tsai ML, Yang
CH, Chen DY, Chang SH, Wang CY, Lee CH and Hsieh IC: Impact of
homocysteine level on long-term cardiovascular outcomes in patients
after coronary artery stenting. J Atheroscler Thromb. 24:696–705.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shargorodsky M, Boaz M, Pasternak S, Hanah
R, Matas Z, Fux A, Beigel Y and Mashavi M: Serum homocysteine,
folate, vitamin B12 levels and arterial stiffness in diabetic
patients: Which of them is really important in atherogenesis?
Diabetes Metab Res Rev. 25:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mishra PK, Tyagi N, Sen U, Joshua IG and
Tyagi SC: Synergism in hyperhomocysteinemia and diabetes: Role of
PPAR gamma and tempol. Cardiovasc Diabetol. 9:492010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chavali V, Tyagi SC and Mishra PK:
Predictors and prevention of diabetic cardiomyopathy. Diabetes
Metab Syndr Obes. 6:151–160. 2013.PubMed/NCBI
|
|
46
|
Hayden MR and Tyagi SC: Homocysteine and
reactive oxygen species in metabolic syndrome, type 2 diabetes
mellitus, and atheroscleropathy: The pleiotropic effects of folate
supplementation. Nutr J. 3:42004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Quilliot D, Alla F, Böhme P, Bruntz JF,
Hammadi M, Dousset B, Ziegler O and Zannad F: Myocardial collagen
turnover in normotensive obese patients: Relation to insulin
resistance. Int J Obes (Lond). 29:1321–1328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Epshteyn V, Morrison K, Krishnaswamy P,
Kazanegra R, Clopton P, Mudaliar S, Edelman S, Henry R and Maisel
A: Utility of B-type natriuretic peptide (BNP) as a screen for left
ventricular dysfunction in patients with diabetes. Diabetes Care.
26:2081–2087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Russell NE, Higgins MF, Amaruso M, Foley M
and McAuliffe FM: Troponin T and pro-B-type natriuretic Peptide in
fetuses of type 1 diabetic mothers. Diabetes Care. 32:2050–2055.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Betti I, Castelli G, Barchielli A, Beligni
C, Boscherini V, De Luca L, Messeri G, Gheorghiade M, Maisel A and
Zuppiroli A: The role of N-terminal PRO-brain natriuretic peptide
and echocardiography for screening asymptomatic left ventricular
dysfunction in a population at high risk for heart failure. The
PROBE-HF study. J Card Fail. 15:377–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chiu AP, Bierende D, Lal N, Wang F, Wan A,
Vlodavsky I, Hussein B and Rodrigues B: Dual effects of
hyperglycemia on endothelial cells and cardiomyocytes to enhance
coronary LPL activity. Am J Physiol Heart Circ Physiol.
314:H82–h94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Poornima IG, Parikh P and Shannon RP:
Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ
Res. 98:596–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Du X, Matsumura T, Edelstein D, Rossetti
L, Zsengellér Z, Szabó C and Brownlee M: Inhibition of GAPDH
activity by poly(ADP-ribose) polymerase activates three major
pathways of hyperglycemic damage in endothelial cells. J Clin
Invest. 112:1049–1057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
Mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nishikawa T, Edelstein D, Du XL, Yamagishi
S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP,
et al: Normalizing mitochondrial superoxide production blocks three
pathways of hyperglycaemic damage. Nature. 404:787–790. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mavrogiannaki AN and Migdalis IN:
Nonalcoholic Fatty liver disease, diabetes mellitus and
cardiovascular disease: Newer data. Int J Endocrinol.
2013:4506392013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mracek T, Gao D, Tzanavari T, Bao Y, Xiao
X, Stocker C, Trayhurn P and Bing C: Downregulation of
zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese
ob/ob mice and by tumour necrosis factor-alpha in adipocytes. J
Endocrinol. 204:165–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wende AR and Abel ED: Lipotoxicity in the
heart. Biochim Biophys Acta. 1801:311–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
van de Weijer T, Schrauwen-Hinderling VB
and Schrauwen P: Lipotoxicity in type 2 diabetic cardiomyopathy.
Cardiovasc Res. 92:10–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Raeis V, Philip-Couderc P, Roatti A, Habre
W, Sierra J, Kalangos A, Beghetti M and Baertschi AJ: Central
venous hypoxemia is a determinant of human atrial ATP-sensitive
potassium channel expression: Evidence for a novel
hypoxia-inducible factor 1alpha-Forkhead box class O signaling
pathway. Hypertension. 55:1186–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ouwens DM, Diamant M, Fodor M, Habets DDJ,
Pelsers MMAL, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R,
Rietdijk A, et al: Cardiac contractile dysfunction in
insulin-resistant rats fed a high-fat diet is associated with
elevated CD36-mediated fatty acid uptake and esterification.
Diabetologia. 50:1938–1948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ritchie RH, Love JE, Huynh K, Bernardo BC,
Henstridge DC, Kiriazis H, Tham YK, Sapra G, Qin C, Cemerlang N, et
al: Enhanced phosphoinositide 3-kinase(p110α) activity prevents
diabetes-induced cardiomyopathy and superoxide generation in a
mouse model of diabetes. Diabetologia. 55:3369–3381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Durgan DJ, Moore MW, Ha NP, Egbejimi O,
Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, et
al: Circadian rhythms in myocardial metabolism and contractile
function: Influence of workload and oleate. Am J Physiol Heart Circ
Physiol. 293:H2385–H2393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Harmancey R, Lam TN, Lubrano GM, Guthrie
PH, Vela D and Taegtmeyer H: Insulin resistance improves metabolic
and contractile efficiency in stressed rat heart. FASEB J.
26:3118–3126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Feng B, Chen S, Chiu J, George B and
Chakrabarti S: Regulation of cardiomyocyte hypertrophy in diabetes
at the transcriptional level. Am J Physiol Endocrinol Metab.
294:E1119–E1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yusuf J, Khan MU, Cheema Y, Bhattacharya
SK and Weber KT: Disturbances in calcium metabolism and
cardiomyocyte necrosis. Prog Cardiovasc Dis. 55:77–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shamoo AE and Ambudkar IS: Regulation of
calcium transport in cardiac cells. Can J Physiol Pharmacol.
62:9–22. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Barry WH and Bridge JH: Intracellular
calcium homeostasis in cardiac myocytes. Circulation. 87:1806–1815.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bigi MAB, Faramarzi H, Gaeini AA, Ravasi
AA, Izadi MR, Delfan M and Izadi E: Upregulation of ryanodine
receptor calcium channels (RyR2) in rats with induced diabetes
after 4 weeks of high intensity interval training. Int Cardiovasc
Res J. 10:1–5. 2016. View Article : Google Scholar
|
|
70
|
Uthman L, Baartscheer A, Schumacher CA,
Fiolet JWT, Kuschma MC, Hollmann MW, Coronel R, Weber NC and
Zuurbier CJ: Direct cardiac actions of sodium glucose cotransporter
2 inhibitors target pathogenic mechanisms underlying heart failure
in diabetic patients. Front Physiol. 9:15752018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ghazi L and Drawz P: Advances in
understanding the renin-angiotensin-aldosterone system (RAAS) in
blood pressure control and recent pivotal trials of RAAS blockade
in heart failure and diabetic nephropathy. F1000Res. 6(pii): F1000
Faculty Rev. –1297. 2017.PubMed/NCBI
|
|
72
|
Underwood PC and Adler GK: The renin
angiotensin aldosterone system and insulin resistance in humans.
Curr Hypertens Rep. 15:59–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pacurari M, Kafoury R, Tchounwou PB and
Ndebele K: The Renin-Angiotensin-aldosterone system in vascular
inflammation and remodeling. Int J Inflam. 2014:6893602014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lim HS, MacFadyen RJ and Lip GY: Diabetes
mellitus, the renin-angiotensin-aldosterone system, and the heart.
Arch Intern Med. 164:1737–1748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cooper ME: The role of the
renin-angiotensin-aldosterone system in diabetes and its vascular
complications. Am J Hypertens. 17:16S–20S.A2-A4. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ohkura SI, Usui S, Takashima SI, Takuwa N,
Yoshioka K, Okamoto Y, Inagaki Y, Sugimoto N, Kitano T, Takamura M,
et al: Augmented sphingosine 1 phosphate receptor-1 signaling in
cardiac fibroblasts induces cardiac hypertrophy and fibrosis
through angiotensin II and interleukin-6. PLoS One.
12:e01823292017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Al Hroob AM, Abukhalil MH, Hussein OE and
Mahmoud AM: Pathophysiological mechanisms of diabetic
cardiomyopathy and the therapeutic potential of
epigallocatechin-3-gallate. Biomed Pharmacother. 109:2155–2172.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Marwick TH, Ritchie R, Shaw JE and Kaye D:
Implications of underlying mechanisms for the recognition and
management of diabetic cardiomyopathy. J Am Coll Cardiol.
71:339–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Westermeier F, Riquelme JA, Pavez M,
Garrido V, Díaz A, Verdejo HE, Castro PF, García L and Lavandero S:
New molecular insights of insulin in diabetic cardiomyopathy. Front
Physiol. 7:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nakamura H, Matoba S, Iwai-Kanai E, Kimata
M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y,
et al: p53 promotes cardiac dysfunction in diabetic mellitus caused
by excessive mitochondrial respiration-mediated reactive oxygen
species generation and lipid accumulation. Circ Heart Fail.
5:106–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Limas C and Limas CJ: Reduced number of
beta-adrenergic receptors in the myocardium of spontaneously
hypertensive rats. Biochem Biophys Res Commun. 83:710–714. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lorenz K, Rosner MR, Brand T and Schmitt
JP: Raf kinase inhibitor protein: Lessons of a better way for
β-adrenergic receptor activation in the heart. J Physiol.
595:4073–4087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wallukat G: The beta-adrenergic receptors.
Herz. 27:683–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie
RH, Sim K, Gao XM, Drummond G, Sarwar M, Zhang YY, et al:
Myocardial oxidative stress contributes to transgenic
β₂-adrenoceptor activation-induced cardiomyopathy and heart
failure. Br J Pharmacol. 162:1012–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Day RT, Cavaglieri Rde C, Tabatabaimir H,
Mantravadi V, Lee MJ, Barnes JL, Kasinath BS and Feliers D: Acute
hyperglycemia rapidly stimulates VEGF mRNA translation in the
kidney. Role of angiotensin type 2 receptor (AT2). Cell Signal.
22:1849–1857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ali MO: Pulmonary complications in
diabetes mellitus. Mymensingh Med J. 23:603–605. 2014.PubMed/NCBI
|
|
88
|
Pavlov VI, La Bonte LR, Baldwin WM,
Markiewski MM, Lambris JD and Stahl GL: Absence of mannose-binding
lectin prevents hyperglycemic cardiovascular complications. Am J
Pathol. 180:104–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tang M, Zhou F, Zhang W, Guo Z, Shang Y,
Lu H, Lu R, Zhang Y, Chen Y and Zhong M: The role of
thrombospondin-1-mediated TGF-β1 on collagen type III synthesis
induced by high glucose. Mol Cell Biochem. 346:49–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim Biophys Acta Mol
Basis Dis. 1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee JM, Calkins MJ, Chan K, Kan YW and
Johnson JA: Identification of the NF-E2-related factor-2-dependent
genes conferring protection against oxidative stress in primary
cortical astrocytes using oligonucleotide microarray analysis. J
Biol Chem. 278:12029–12038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kwak MK, Itoh K, Yamamoto M and Kensler
TW: Enhanced expression of the transcription factor Nrf2 by cancer
chemopreventive agents: Role of antioxidant response element-like
sequences in the nrf2 promoter. Mol Cell Biol. 22:2883–2892. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mercado N, Thimmulappa R, Thomas CM,
Fenwick PS, Chana KK, Donnelly LE, Biswal S, Ito K and Barnes PJ:
Decreased histone deacetylase 2 impairs Nrf2 activation by
oxidative stress. Biochem Biophys Res Commun. 406:292–298. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yoh K, Hirayama A, Ishizaki K, Yamada A,
Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H,
et al: Hyperglycemia induces oxidative and nitrosative stress and
increases renal functional impairment in Nrf2-deficient mice. Genes
Cells. 13:1159–1170. 2008.PubMed/NCBI
|
|
95
|
He X, Kan H, Cai L and Ma Q: Nrf2 is
critical in defense against high glucose-induced oxidative damage
in cardiomyocytes. J Mol Cell Cardiol. 46:47–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cosso L, Maineri EP, Traverso N, Rosatto
N, Pronzato MA, Cottalasso D, Marinari UM and Odetti P: Induction
of heme oxygenase 1 in liver of spontaneously diabetic rats. Free
Radic Res. 34:189–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shi S, Lei S, Tang C, Wang K and Xia Z:
Melatonin attenuates acute kidney ischemia/reperfusion injury in
diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling
pathway. Biosci Rep. 39(pii): BSR201816142019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ye Z, Guo Q, Xia P, Wang N, Wang E and
Yuan Y: Sevoflurane postconditioning involves an up-regulation of
HIF-1α and HO-1 expression via PI3K/Akt pathway in a rat model of
focal cerebral ischemia. Brain Res. 1463:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tsuchihashi S, Fondevila C and
Kupiec-Weglinski JW: Heme oxygenase system in ischemia and
reperfusion injury. Ann Transplant. 9:84–87. 2004.PubMed/NCBI
|
|
100
|
Mallick IH, Winslet MC and Seifalian AM:
Pyrrolidine dithiocarbamate protects the small bowel from warm
ischaemia/reperfusion injury of the intestine: The role of haem
oxygenase. Clin Sci (Lond). 111:373–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen YH, Yet SF and Perrella MA: Role of
heme oxygenase-1 in the regulation of blood pressure and cardiac
function. Exp Biol Med (Maywood). 228:447–453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bai CH, Chen JR, Chiu HC, Chou CC, Chau LY
and Pan WH: Shorter GT repeat polymorphism in the heme oxygenase-1
gene promoter has protective effect on ischemic stroke in
dyslipidemia patients. J Biomed Sci. 17:122010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
García R, Nistal JF, Merino D, Price NL,
Fernández-Hernando C, Beaumont J, González A, Hurlé MA and Villar
AV: p-SMAD2/3 and DICER promote pre-miR-21 processing during
pressure overload-associated myocardial remodeling. Biochim Biophys
Acta. 1852:1520–1530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Niu L, Cui X, Qi Y, Xie D, Wu Q, Chen X,
Ge J and Liu Z: Involvement of TGF-β1/Smad3 signaling in carbon
tetrachloride-induced acute liver injury in mice. PLoS One.
11:e01560902016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li TT, Si GM and Chen FC: Effects of
Shenqi Jiedu Decoction on expressions of transforming growth
factor-beta1, smad2 and smad3 in renal tissues of rats with chronic
renal failure induced by adenine. Zhong Xi Yi Jie He Xue Bao.
8:263–268. 2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nelson EF, Huang CW, Ewel JM, Chang AA and
Yuan C: Halofuginone down-regulates Smad3 expression and inhibits
the TGFbeta-induced expression of fibrotic markers in human corneal
fibroblasts. Mol Vis. 18:479–487. 2012.PubMed/NCBI
|
|
109
|
Dong C, Mi R, Jin G, Zhou Y, Zhang J and
Liu F: Identification of the proliferative effect of Smad2 and 3 in
the TGF β2/Smad signaling pathway using RNA interference in a
glioma cell line. Mol Med Rep. 12:1824–1828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Piek E, Wen JJ, Heyer J, Escalante-Alcalde
D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP
and Roberts AB: Functional characterization of transforming growth
factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J
Biol Chem. 276:19945–19953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Feng XH, Liang YY, Liang M, Zhai W and Lin
X: Direct interaction of c-Myc with Smad2 and Smad3 to inhibit
TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B). Mol
Cell. 9:133–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li P, Oparil S, Novak L, Cao X, Shi W,
Lucas J and Chen YF: ANP signaling inhibits TGF-beta-induced Smad2
and Smad3 nuclear translocation and extracellular matrix expression
in rat pulmonary arterial smooth muscle cells. J Appl Physiol
(1985). 102:390–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Divakaran V, Adrogue J, Ishiyama M, Entman
ML, Haudek S, Sivasubramanian N and Mann DL: Adaptive and
maladptive effects of SMAD3 signaling in the adult heart after
hemodynamic pressure overloading. Circ Heart Fail. 2:633–642. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Van der Heiden K, Cuhlmann S, Luong le A,
Zakkar M and Evans PC: Role of nuclear factor kappaB in
cardiovascular health and disease. Clin Sci (Lond). 118:593–605.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hink U, Tsilimingas N, Wendt M and Munzel
T: Mechanisms underlying endothelial dysfunction in diabetes
mellitus: Therapeutic implications. Treat Endocrinol. 2:293–304.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sugiyama S, Miyata T, Ueda Y, Tanaka H,
Maeda K, Kawashima S, Van Ypersele de Strihou C and Kurokawa K:
Plasma levels of pentosidine in diabetic patients: An advanced
glycation end product. J Am Soc Nephrol. 9:1681–1688.
1998.PubMed/NCBI
|
|
117
|
Kanauchi M, Tsujimoto N and Hashimoto T:
Advanced glycation end products in nondiabetic patients with
coronary artery disease. Diabetes Care. 24:1620–1623. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hussain F, Sheikh MA, Maan MA and Jamil A:
Advanced glycation end products (AGEs) in diabetic patients with
systemic lupus erythematosus. Int J Agric Biol. 14:440–444.
2012.
|
|
119
|
Choi EY, Kwon HM, Ahn CW, Lee GT, Joung B,
Hong BK, Yoon YW, Kim D, Byun KH, Kang TS, et al: Serum levels of
advanced glycation end products are associated with in-stent
restenosis in diabetic patients. Yonsei Med J. 46:78–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Khalifah RG, Todd P, Booth AA, Yang SX,
Mott JD and Hudson BG: Kinetics of nonenzymatic glycation of
ribonuclease A leading to advanced glycation end products.
Paradoxical inhibition by ribose leads to facile isolation of
protein intermediate for rapid post-Amadori studies. Biochemistry.
35:4645–4654. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Vlassara H: Advanced glycation
end-products and atherosclerosis. Ann Med. 28:419–426. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Peppa M and Vlassara H: Advanced glycation
end products and diabetic complications: A general overview.
Hormones (Athens). 4:28–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nożyński J, Zakliczyński M, Konecka-Mrowka
D, Zielinska T, Zakliczynska H, Nikiel B, Mlynarczyk-Liszka J,
Mrowka A, Zembala-Nozynska E, Pijet M, et al: Advanced glycation
end product accumulation in the cardiomyocytes of heart failure
patients with and without diabetes. Ann Transplant. 17:53–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Grzebyk E, Knapik-Kordecka M and Piwowar
A: Advanced glycation end-products and cathepsin cysteine protease
in type 2 diabetic patients. Pol Arch Med Wewn. 123:364–370.
2013.PubMed/NCBI
|
|
125
|
Kuwajima S: Immunochemical determination
of advanced glycation end products in erythrocyte
peripheral-membrane proteins from diabetic patients. Hokkaido Igaku
Zasshi. 68:695–704. 1993.(In Japanese). PubMed/NCBI
|
|
126
|
Low H, Hoang A, Forbes J, Thomas M, Lyons
JG, Nestel P, Bach LA and Sviridov D: Advanced glycation
end-products (AGEs) and functionality of reverse cholesterol
transport in patients with type 2 diabetes and in mouse models.
Diabetologia. 55:2513–2521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Singh VP, Bali A, Singh N and Jaggi AS:
Advanced glycation end products and diabetic complications. Korean
J Physiol Pharmacol. 18:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Feng Y, Zhao H, Xu X, Buys ES, Raher MJ,
Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U and Chao W:
Innate immune adaptor MyD88 mediates neutrophil recruitment and
myocardial injury after ischemia-reperfusion in mice. Am J Physiol
Heart Circ Physiol. 295:H1311–H1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Weber NC, Toma O, Wolter JI, Obal D,
Müllenheim J, Preckel B and Schlack W: The noble gas xenon induces
pharmacological preconditioning in the rat heart in vivo via
induction of PKC-epsilon and p38 MAPK. Br J Pharmacol. 144:123–132.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Das Evcimen N and King GL: The role of
protein kinase C activation and the vascular complications of
diabetes. Pharmacol Res. 55:498–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Koya D and King GL: Protein kinase C
activation and the development of diabetic complications. Diabetes.
47:859–866. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Nogueira-Machado JA and Bosco AA: PKC
inhibition and diabetic complications. Recent Patents End Metab
Immune Drug Discov. 2:72–78. 2008. View Article : Google Scholar
|
|
133
|
Ishii H, Koya D and King GL: Protein
kinase C activation and its role in the development of vascular
complications in diabetes mellitus. J Mol Med (Berl). 76:21–31.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shen GX: Selective protein kinase C
inhibitors and their applications. Curr Drug Targets Cardiovasc
Haematol Disord. 3:301–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bursell SE and King GL: Can protein kinase
C inhibition and vitamin E prevent the development of diabetic
vascular complications? Diabetes Res Clin Pract. 45:169–182. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat
Rev Endocrinol. 12:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lee TI, Kao YH, Chen YC, Huang JH, Hsiao
FC and Chen YJ: Peroxisome proliferator-activated receptors
modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes
Res Clin Pract. 100:330–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Finck BN, Lehman JJ, Leone TC, Welch MJ,
Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD and
Kelly DP: The cardiac phenotype induced by PPARalpha overexpression
mimics that caused by diabetes mellitus. J Clin Invest.
109:121–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Leone TC, Weinheimer CJ and Kelly DP: A
critical role for the peroxisome proliferator-activated receptor
alpha (PPARalpha) in the cellular fasting response: The
PPARalpha-null mouse as a model of fatty acid oxidation disorders.
Proc Natl Acad Sci USA. 96:7473–7478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Pedraza N, Rosell M, Villarroya J,
Iglesias R, Gonzalez FJ, Solanes G and Villarroya F: Developmental
and tissue-specific involvement of peroxisome
proliferator-activated receptor-alpha in the control of mouse
uncoupling protein-3 gene expression. Endocrinology. 147:4695–4704.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhou YT, Grayburn P, Karim A, Shimabukuro
M, Higa M, Baetens D, Orci L and Unger RH: Lipotoxic heart disease
in obese rats: Implications for human obesity. Proc Natl Acad Sci
USA. 97:1784–1789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Anderson EJ, Kypson AP, Rodriguez E,
Anderson CA, Lehr EJ and Neufer PD: Substrate-specific derangements
in mitochondrial metabolism and redox balance in the atrium of the
type 2 diabetic human heart. J Am Coll Cardiol. 54:1891–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Razeghi P, Young ME, Cockrill TC, Frazier
OH and Taegtmeyer H: Downregulation of myocardial myocyte enhancer
factor 2C and myocyte enhancer factor 2C-regulated gene expression
in diabetic patients with nonischemic heart failure. Circulation.
106:407–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cheng L, Ding G, Qin Q, Huang Y, Lewis W,
He N, Evans RM, Schneider MD, Brako FA, Xiao Y, et al:
Cardiomyocyte-restricted peroxisome proliferator-activated
receptor-delta deletion perturbs myocardial fatty acid oxidation
and leads to cardiomyopathy. Nat Med. 10:1245–1250. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Scirpo R, Fiorotto R, Villani A, Fabris L
and Strazzabosco M: Stimulation of Nuclear Receptor PPAR-γ Limits
NF-kB-dependent inflammation in cystic fibrosis biliary epithelium.
Dig Liver Dis. 47:e43–e44. 2015. View Article : Google Scholar
|
|
146
|
Ng DC, Long CS and Bogoyevitch MA: A role
for the extracellular signal-regulated kinase and p38
mitogen-activated protein kinases in Interleukin-1beta-stimulated
delayed signal tranducer and activator of transcription3
activation, atrial natriuretic factor expression and cardiac
myocyte morphology. J Biol Chem. 276:29490–29498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lei Y, Xu Q, Zeng B, Zhang W, Zhen Y, Zhai
Y, Cheng F, Mei W, Zheng D, Feng J, et al: Angiotensin-(1–7)
protects cardiomyocytes against high glucose-induced injuries
through inhibiting reactive oxygen species-activated leptin-p38
mitogen-activated protein kinase/extracellular signal-regulated
protein kinase 1/2 pathways but not leptin-c-Jun N-terminal kinase
pathway in vitro. J Diabetes Investig. 8:434–445. 2016. View Article : Google Scholar
|
|
148
|
Bo B, Tang J, Liu H, Chen J, Li Y and Song
W: Apelin-13 induces ERK1/2 but not p38 MAPK activation through
coupling of the human apelin receptor to the Gi2 pathway. Acta
Biochim Biophys Sin (Shanghai). 40:311–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kiribayashi K, Masaki T, Naito T, Ogawa T,
Ito T, Yorioka N and Kohno N: Angiotensin II induces fibronectin
expression in human peritoneal mesothelial cells via ERK1/2 and p38
MAPK. Kidney Int. 67:1126–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Blanc A, Pandey NR and Srivastava AK:
Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by
H2O2 in vascular smooth muscle cells: Potential involvement in
vascular disease (review). Int J Mol Med. 11:229–234.
2003.PubMed/NCBI
|
|
151
|
Strniskova M, Barancik M, Neckar J and
Ravingerova T: Mitogen-activated protein kinases in the acute
diabetic myocardium. Mol Cell Biochem. 249:59–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jia G, Habibi J, DeMarco VG,
Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL,
Zhu Y, Meininger GA, et al: Endothelial mineralocorticoid receptor
deletion prevents diet-induced cardiac diastolic dysfunction in
females. Hypertension. 66:1159–1167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Rouf R, MacFarlane EG, Takimoto E,
Chaudhary R, Nagpal V, Rainer PP, Bindman JG, Gerber EE, Bedja D,
Schiefer C, et al: Nonmyocyte ERK1/2 signaling contributes to
load-induced cardiomyopathy in Marfan mice. JCI Insight. 2(pii):
915882017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang Y, Zhou S, Sun W, McClung K, Pan Y,
Liang G, Tan Y, Zhao Y, Liu Q, Sun J and Cai L: Inhibition of JNK
by novel curcumin analog C66 prevents diabetic cardiomyopathy with
a preservation of cardiac metallothionein expression. Am J Physiol
Endocrinol Metab. 306:E1239–E1247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Gurusamy N, Watanabe K, Ma M, Zhang S,
Muslin AJ, Kodama M and Aizawa Y: Dominant negative 14-3-3 promotes
cardiomyocyte apoptosis in early stage of type I diabetes mellitus
through activation of JNK. Biochem Biophys Res Commun. 320:773–780.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shaw RJ: LKB1 and AMP-activated protein
kinase control of mTOR signalling and growth. Acta Physiol (Oxf).
196:65–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Xu K, Liu XF, Ke ZQ, Yao Q, Guo S and Liu
C: Resveratrol modulates apoptosis and autophagy induced by high
glucose and palmitate in cardiac cells. Cell Physiol Biochem.
46:2031–2040. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Jweied EE, Mckinney RD, Walker LA, Brodsky
I, Geha AS, Massad MG, Buttrick PM and de Tombe PP: Depressed
cardiac myofilament function in human diabetes mellitus. Am J
Physiol Heart Circ Physiol. 289:H2478–H2483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Khullar M, Cheema BS and Raut SK: Emerging
evidence of epigenetic modifications in vascular complication of
diabetes. Front Endocrinol (Lausanne). 8:2372017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Barwari T, Joshi A and Mayr M: MicroRNAs
in cardiovascular disease. J Am Coll Cardiol. 68:2577–2584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zampetaki A, Kiechl S, Drozdov I, Willeit
P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E,
et al: Plasma microRNA profiling reveals loss of endothelial
miR-126 and other microRNAs in type 2 diabetes. Circ Res.
107:810–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Chen Q, Qiu F, Zhou K, Matlock HG,
Takahashi Y, Rajala RVS, Yang Y, Moran E and Ma JX: Pathogenic Role
of microRNA-21 in Diabetic Retinopathy Through Downregulation of
PPARα. Diabetes. 66:1671–1682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rawal S, Munasinghe PE, Nagesh PT, Lew
JKS, Jones GT, Williams MJA, Davis P, Bunton D, Galvin IF, Manning
P, et al: Down-regulation of miR-15a/b accelerates fibrotic
remodelling in the Type 2 diabetic human and mouse heart. Clin Sci
(Lond). 131:847–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Assmann TS, Recamonde-Mendoza M, De Souza
BM and Crispim D: MicroRNA expression profiles and type 1 diabetes
mellitus: Systematic review and bioinformatic analysis. Endocr
Connect. 6:773–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Demirsoy IH, Ertural DY, Balci S, Çınkır
Ü, Sezer K, Tamer L and Aras N: Profiles of circulating MiRNAs
following metformin treatment in patients with type 2 diabetes. J
Med Biochem. 37:499–506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Prabu P, Rome S, Sathishkumar C, Gastebois
C, Meugnier E, Mohan V and Balasubramanyam M: MicroRNAs from
urinary extracellular vesicles are non-invasive early biomarkers of
diabetic nephropathy in type 2 diabetes patients with the ‘Asian
Indian phenotype’. Diabetes Metab. 45:276–285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Trajkovski M, Hausser J, Soutschek J, Bhat
B, Akin A, Zavolan M, Heim MH and Stoffel M: MicroRNAs 103 and 107
regulate insulin sensitivity. Nature. 474:649–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Nair N, Kumar S, Gongora E and Gupta S:
Circulating miRNA as novel markers for diastolic dysfunction. Mol
Cell Biochem. 376:33–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Liu X and Liu S: Role of microRNAs in the
pathogenesis of diabetic cardiomyopathy. Biomed Rep. 6:140–145.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lee WS and Kim J: Diabetic cardiomyopathy:
Where we are and where we are going. Korean J Intern Med.
32:404–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Cholesterol Treatment Trialists' (CTT)
Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes
J, Peto R, Armitage J and Baigent C: Efficacy of
cholesterol-lowering therapy in 18,686 people with diabetes in 14
randomised trials of statins: A meta-analysis. Lancet. 371:117–125.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Cosmi F and Cosmi D: Hypoglycemic therapy
in heart disease patients with type 2 diabetes mellitus. G Ital
Cardiol (Rome). 11:460–466. 2010.(In Italian). PubMed/NCBI
|
|
175
|
Anabtawi A, Moriarty PM and Miles JM:
Pharmacologic treatment of dyslipidemia in diabetes: A case for
therapies in addition to statins. Curr Cardiol Rep. 19:622017.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Brea A, Millán J, Ascaso JF, Blasco M,
Díaz A, Hernández-Mijares A, Mantilla T, Pedro-Botet JC and Pintó
X: Fibrates in primary prevention of cardiovascular disease.
Comments on the results of a systematic review of the Cochrane
Collaboration. Clin Investig Arterioscler. 30:188–192. 2018.(In
English, Spanish). PubMed/NCBI
|
|
177
|
Thomas CM, Yong QC, Seqqat R, Chandel N,
Feldman DL, Baker KM and Kumar R: Direct renin inhibition prevents
cardiac dysfunction in a diabetic mouse model: Comparison with an
angiotensin receptor antagonist and angiotensin-converting enzyme
inhibitor. Clin Sci (Lond). 124:529–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Machackova J, Liu X, Lukas A and Dhalla
NS: Renin-angiotensin blockade attenuates cardiac myofibrillar
remodelling in chronic diabetes. Mol Cell Biochem. 261:271–278.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Bangalore S, Messerli FH, Kostis JB and
Pepine CJ: Cardiovascular protection using beta-blockers: A
critical review of the evidence. J Am Coll Cardiol. 50:563–572.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sharma V and McNeill JH: Parallel effects
of β-adrenoceptor blockade on cardiac function and fatty acid
oxidation in the diabetic heart: Confronting the maze. World J
Cardiol. 3:281–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Mohamad HE, Askar ME and Hafez MM:
Management of cardiac fibrosis in diabetic rats; the role of
peroxisome proliferator activated receptor gamma (PPAR-gamma) and
calcium channel blockers (CCBs). Diabetol Metab Syndr. 3:42011.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ellestad MH: Hyperbaric oxygen: Its
application in cardiology: A historical perspective and personal
journey. Cardiol Rev. 17:280–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Karadurmus N, Sahin M, Tasci C, Naharci I,
Ozturk C, Ilbasmis S, Dulkadir Z, Sen A and Saglam K: Potential
benefits of hyperbaric oxygen therapy on atherosclerosis and
glycaemic control in patients with diabetic foot. Endokrynol Pol.
61:275–279. 2010.PubMed/NCBI
|
|
184
|
Katare R, Caporali A, Zentilin L, Avolio
E, Sala-Newby G, Oikawa A, Cesselli D, Beltrami AP, Giacca M,
Emanueli C and Madeddu P: Intravenous gene therapy with PIM-1 via a
cardiotropic viral vector halts the progression of diabetic
cardiomyopathy through promotion of prosurvival signaling. Circ
Res. 108:1238–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Cheng Y, Guo S, Liu G, Feng Y, Yan B, Yu
J, Feng K and Li Z: Transplantation of bone marrow-derived
endothelial progenitor cells attenuates myocardial interstitial
fibrosis and cardiac dysfunction in streptozotocin-induced diabetic
rats. Int J Mol Med. 30:870–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Neel S and Singla DK: Induced pluripotent
stem (iPS) cells inhibit apoptosis and fibrosis in
streptozotocin-induced diabetic rats. Mol Pharm. 8:2350–2357. 2011.
View Article : Google Scholar : PubMed/NCBI
|