Introduction
Myocarditis is an inflammatory disease of the
myocardium with a wide clinical spectrum, ranging from asymptomatic
to fulminant heart failure (1–3).
Myocarditis is underdiagnosed, with an estimated incidence of
0.46–0.72 per 100,000 and a prevalence to be approximately 22 per
100,000 population annually (4).
Autoimmunity and recent infection are risk factors for the
development of myocarditis. The main etiological agents are
viruses, with, the most commonly identified including adenovirus,
enterovirus (including coxsackie virus), parvovirus B-19 and human
herpesvirus (5). They mainly
affect younger patients (20–51 years), ~21% of whom develop dilated
cardiomyopathy (DCM), which is associated with a deteriorating
quality of life and physical inability to work (6).
Coxsackievirus and adenovirus receptor (CAR) is a
transmembrane 46-kDa protein of the Ig superfamily, located in the
complex binding between endothelial and epithelial cells (7,8). It
is a multifunctional molecule that interacts with proteins involved
in cellular communication, adhesion (adherent junctions), binding
to the cytoskeleton, regulation and signal transduction, and it has
recently been implicated in cardiac remodeling and atrioventricular
electrical conduction (7–9). CAR is a common receptor for
coxsackievirus and adenovirus, allowing viral binding and
endocytosis (10). High CAR mRNA
levels have been detected during the late embryonal and early
postnatal period (11). CAR
transcription is very low in the normal heart, but was found to be
overexpressed in patients with DCM and ischemic heart disease;
there were no differences regarding age, sex, or CAR mutations
between the study groups (12–14).
Regarding the performance of CAR in the presence of
myocarditis, most information is derived from animal studies
(13,15–17).
To the best of our knowledge, there are no studies on the
expression of CAR in patients with active myocarditis, and only a
few cases have been included in the final sample of protocols with
DCM (12–14,18).
It remains elusive which proteins are implicated in human
myocarditis. In animal models of myocarditis, a difference in the
expression of CAR has been documented, considering the time of
evolution of the disease. Ito et al (15), reported that CAR expression in rat
hearts was low or undetectable prior to disease onset, became
evident during the active phase of myocarditis, and decreased in
the chronic phase. Similarly, the expression of CAR was preceded by
several days of massive inflammatory cell infiltration, and was
induced by inflammatory mediators such as interferon γ, tumor
necrosis factor α, interleukin 1β and nitric oxide synthase
(19). Subsequently, CAR induced
stress-activated mitogen-activated protein kinase (MAPK) signaling,
which may contribute to the development of cardiac inflammation
unrelated to the viral infection per se. Consequently, the
expression of CAR may not only be associated with myocardial damage
in acute myocarditis; due to its role as a cell adhesion molecule
(7,20,21),
it may also be associated with the phase of healing or regeneration
of the damaged myocardium. It is possible that MAPK and NOD2
mediate CAR expression in viral myocarditis (22,23).
The treatment of myocarditis varies, depending on
the stage and severity of the patient's clinical profile.
Immunosuppressive treatment (usually steroids and azathioprine) has
long been used in myocarditis, with varying indications, often
based on evidence of myocarditis with inflammatory cell
infiltration and inflammatory response. In our hospital, clinical
criteria of high probability include de novo arrhythmia,
heart failure in a previously healthy patient, severe infectious
conditions manifesting prior to the onset of symptoms, low ejection
fraction of unknown etiology, and age <45 years, as well as the
actual presence of histological evidence of myocarditis. Those
patients may receive immunosuppressive treatment and are classified
as responders or non-responders, based on their response to such
treatment.
The aim of the present study was to assess CAR mRNA
levels in myocardial biopsies from patients with myocarditis, in
order to determine how these may contribute to the clinical
evolution of myocarditis.
Materials and methods
Study design and ethics statement
In this analytical cross-sectional study, CAR mRNA
levels were determined by examination of endomyocardial biopsy
specimens obtained from patients with myocarditis (17 responders
and 9 non-responders to immunosuppressive or conventional therapy;
16 with active and 10 with borderline histological myocarditis)
(2,24), and subjects without myocarditis.
The present study was approved by the National Research Scientific
and Ethics Committee of Instituto Mexicano del Seguro Social
(IMSS). The study protocol conformed to the principles outlined in
the Declaration of Helsinki. All the participants were informed on
the nature of the study and provided written consent.
Study population
The study included 36 patients (aged >18 years)
between January 2009 and May 2015. A total of 26 patients were
diagnosed with myocarditis by endomyocardial biopsy
(histopathological, immunological and immunohistochemical
criteria). A total of 10 subjects who underwent endomyocardial
biopsy (EMB) for on suspicion of myocarditis and in whom the
histopathological findings were not compatible with myocarditis, or
myocardial samples obtained by necropsies in which the
histopathological findings were considered as normal, comprised the
non-cardiac disease (NM group, without myocarditis also showed no
other signs of cardiovascular disease; the characteristics of this
group will be described in Results section). The samples obtained
from autopsies performed immediately after death and that were
formalin-fixed and embedded in paraffin for their preservation. The
patients were admitted to the Heart Failure Clinic, Cardiology
Hospital of the Centro Medico Nacional Siglo XXI (CMN-Siglo XXI),
IMSS, in Mexico City. The treatment method was at the discretion of
the treating physician.
Sample/data collection
The endomyocardial samples were collected from the
Department of Pathology of the Cardiology Hospital CMN-Siglo XXI;
IMSS. For all cases, formalin-fixed and paraffin-embedded samples
were available. An expert pathologist analyzed all the samples to
confirm the diagnosis.
Socio-demographic data and clinical information were
recorded at the time of inclusion. The collected information
included age, sex, clinical presentation (medical history,
symptoms, signs and non-invasive assessment) and histological
diagnosis. Other parameters included left ventricular ejection
fraction (LVEF), troponins, functional class, and response or lack
thereof to immunosuppressive therapy (evaluated based on clinical
and echocardiographic criteria) (24).
Histopathological examination
EMB was used for histopathological diagnosis by
optical and electron microscopy. Sections from EMB were stained
with hematoxylin and eosin (H&E). The gold standard for
diagnosis was EMB in conjunction with determination of
immunohistochemical markers, according to the 1995 World Health
Organization/International Society and Federation of Cardiology
classification of cardiomyopathies (2). The histological level of inflammation
in each lesion was graded according to the Dallas criteria
(25). The Dallas criteria
classify myocarditis as follows: i) Active myocarditis, defined as
an inflammatory infiltration of the myocardium with necrosis and/or
degeneration of adjacent myocytes, not typical of ischemic damage
associated with coronary artery disease. The infiltrating cells are
usually mononuclear, but may include neutrophils and occasional
eosinophils; and ii) borderline myocarditis, which is a term used
when the inflammatory infiltrate is small, or myocyte damage is not
apparent.
All diagnoses and classifications were reviewed by
hospital expert pathologists who were blinded to the patients'
clinical data. According to the above mentioned criteria, 16
patients were diagnosed with active myocarditis and 10 with
borderline myocarditis.
Deparaffinization and digestion of
tissues
For RNA extraction, the samples were obtained by
deparaffinization of formalin-fixed paraffin-embedded samples. For
each paraffin-embedded sample, 5 sections of 10 microns were
obtained. Between each microtome cut, the cutting blade was wiped
with ethanol to remove ribonuclease (RNase). Excess paraffin around
the section was removed with a razor knife. The first two sections
were removed due to the risk of the presence of RNases. The
sections were immediately placed in a microtube and 1 ml xylene was
added, followed by incubating twice for 3 min at 42°C with
stirring, centrifuging at 18,407 g between each incubation for 5
min and removing the supernatant. The tissue was washed with 1 ml
of absolute ethanol three times for 3 min each time at room
temperature with stirring, with centrifugation at maximum speed
21,130 g each time. The residual ethanol was allowed to evaporate
until the tissue was completely dry. A total of 350 µl tissue
digestion buffer [proteinase K treatment with 500 mg/ml mM Tris HCl
(pH 8), 10 mM EDTA, 1% SDS] was added per heart sample and
incubated at 42°C for ~24 h with stirring, until the heart tissue
had been lysed and digested (26).
Total RNA extraction and RNA
purification
Total RNA was extracted from degraded heart tissues
by adaptation of the hot phenol method (27). Briefly, after the lysed sample was
obtained, 500 µl of phenol acid preheated to 65°C was added,
vortexed and incubated at 65°C for 5 min. The samples were
centrifuged at 18,407 g for 5 min at room temperature. The aqueous
phase was transferred to a clean microtube with 1 ml of cold
absolute ethanol and incubated at −70°C for at least 30 min. The
RNA was pelleted by centrifugation at 18,407 g for 10 min at 4°C.
Pellets were washed by adding 1 ml 70% cold ethanol and centrifuged
at 13,523 g for 2 min at 4°C to precipitation. The pellets were
dried in the Centrifugal Vacuum Concentrator 5301 (Eppendorf). The
pellets were resuspended in 30 µl ultrapure DEPC-treated water
(Invitrogen; Thermo Fisher Scientific, Inc.). DNA was removed with
the Turbo DNA-Free kit (AM1907; Ambion; Thermo Fisher Scientific,
Inc.). The amount and quality of RNA were evaluated by measuring
the optical density (OD) at 260/280 ratios using a Nanodrop
spectrophotometer (ND-100, Nanodrop, Thermo Fisher Scientific,
Inc.). The RNA quality was assessed using a bleach gel with 2%
agarose, as previously described (28).
CAR expression by reverse
transcription quantitative PCR (RT-qPCR) analysis
The quantification of CAR transcription from the
samples was performed by RT-qPCR using LightCycler® 480
SYBR Green I Master (4707516001; Roche). cDNA was synthesized with
100 ng of each RNA sample, 0.22 µg/µl random hexamer primers and 2
U/µl Reverse transcriptase of Moloney Murine Leukemia Virus
(M-MuLV-RT; Thermo Fisher Scientific, Inc.). The cDNA generated was
used for qPCR using a LightCycler 480 thermocycler (Roche) and the
following primer pairs: For the human CAR gene,
5′-GCCCACTTCATGGTTAGCAG-3′ and 5′-TACGGCTCTTTGGAGGTGGC-3′ (13). For the housekeeping β-globin gene,
5′-ACACAACTGTGTTCACTAGC-3′ and 5′-TGGTCTCCTTAAACCTGTCTTG-3′; and
for the β-actin gene, 5′-TCGTGCGTGACATTAAGGAG and
5′-TTGCCAATGGTGATGACCTG 3′. The PCR reactions were carried out in a
total volume of 10 µl, containing 1.5 µl molecular grade sterile
water included in the commercial kit, 0.5 µl of each specific
primer pair corresponding to a concentration of 20 µM, 2.5 µl cDNA
to the appropriate dilution, and 5.0 µl of the mixture of 2X Master
SYBR Green I, which contains FastStart Taq DNA polymerase, reaction
buffer, dNTP mixture, fluorochrome SYBR Green I and
MgCl2. Each sample was assessed in triplicate. The qPCR
analysis was performed using the following optimized assay
conditions: Denaturation at 95°C for 10 min, followed by
amplification repeated for 45 cycles at 95°C for 10 sec,
quantification at 58°C for 20 sec and extension at 72°C for 30 sec
with single-measurement fluorescence. A melting curve analysis was
run at 95°C for 10 sec, 65°C for 1 min with continuous measurement
of fluorescence at 97°C, and finally a cooling step at 40°C for 10
sec. Analysis of the melting curve after each run was performed to
confirm the specificity of the primers. The mRNA levels of CAR were
calculated from the relative quantification of CAR and the level of
reference genes determined for each sample (29). For mRNA level quantification, the
ΔΔCq method was used (30). Genes
coding for β-globin and/or β-actin were used as reference for
normalization.
Statistical analysis
Differences among ≥3 groups were compared by one-way
analysis of variance followed by post-hoc Scheffe's test, or by the
Kruskal-Wallis test followed by Mann-Whitney U test for
non-normally distributed variables. For comparison of CAR
expression, the latter tests were used, as they do not assume a
normal distribution. A two-tailed P<0.05 was considered to
indicate statistically significant differences. Bivariate analysis
was used for categorical variables with Pearson Chi-squared or
Fisher's exact test for small samples.
Results
Subjects
A total of 36 patients were included in the
cross-sectional study, among whom 10 patients did not meet the
criteria for myocarditis and non-cardiac disease (NCD) and were
considered as the control group without myocarditis (no
myocarditis; NM group). The NM group mainly comprised samples
obtained from autopsies preserved over a period of 5 years; the
median patients age was 38.3±13.8 years, the sex ratio was 1:1, and
the main diagnosis was hemorrhagic stroke (50%) and thrombophilia.
Cases with a history of treatment with immunosuppressants or
immunomodulatory drugs, history of ischemic heart disease, systemic
viral infections, autoimmune diseases, congenital or acquired
immunodeficiencies and cancer were excluded. Also, histologically,
the myocardium was considered normal, without any evidence of
ischemia, myocarditis or other cardiac disease that would interfere
with our analysis. The remaining patients (n=26) were diagnosed
with myocarditis by histopathological and clinical criteria; 16
patients had active myocarditis and 10 had borderline myocarditis
according to the Dallas criteria (Table I).
 | Table I.General characteristic of the study
population according to diagnosis with myocarditis by
histopathological examination and clinical criteria. |
Table I.
General characteristic of the study
population according to diagnosis with myocarditis by
histopathological examination and clinical criteria.
| Variable | Active
myocarditis | Borderline
myocarditis | P-value |
|---|
| n (%) | 16 (61.5) | 10 (38.5) |
|
| Age, years (Mean ±
SD) | 35.4±13.9 | 36.9±13.7 | 0.792a |
| Sex, male/female
ratio | 8/8=1.0 | 9/1=9 | 0.088b |
| Cardiovascular risk
factors |
|
|
|
|
Diabetes mellitus, n (%) | 0 (0) | 1(10) | 0.385b |
|
Systemic hypertension, n
(%) | 0 (0) | 3 (30) | 0.046b,d |
|
Dyslipidemia, n (%) | 1 (6.3) | 4 (40) | 0.055b |
|
Smoking, n (%) | 6 (37.5) | 5 (50) | 0.689c |
| Body
mass index (kg/m2), median (range) | 26.7
(18.8–39.4) | 27.7
(23.4–33.96) | 0.562a |
| Background of
recent infection |
|
|
|
|
Airways, n (%) | 7 (43.8) | 7 (70) | 0.248b |
|
Gastrointestinal, n (%) | 2 (12.5) | 0 (0) | 0.508b |
Active and borderline myocarditis
The group of patients with active myocarditis
comprised 8 men and 8 women, with a mean age of 35.4±13.9 years,
while the borderline myocarditis group included 9 men and 1 woman,
with a mean age of 36.9±13.7 years. No difference in age was
observed between the NM group and patients with active myocarditis
or those with borderline myocarditis. The main characteristics at
the time of diagnosis according to histopathological examination
(active and borderline myocarditis) are summarized in Table II.
 | Table II.Clinical characteristics at the time
of diagnosis according to histopathological examination. |
Table II.
Clinical characteristics at the time
of diagnosis according to histopathological examination.
| Variable | Active n=16 | Borderline
n=10 | P-value |
|---|
| Clinical
symptoms |
|
|
|
|
Dyspnea, n (%) | 8 (50) | 7 (70) | 0.428b |
| Chest
pain, n (%) | 10 (62.5) | 6 (60) | 0.609b |
|
Palpitations, n (%) | 6 (37.5) | 1 (10) | 0.190b |
|
Syncope, n (%) | 5 (31.3) | 2 (20) | 0.668b |
| Functional class
(NYHA) |
|
|
|
|
CF-I–II, n (%) | 13 (81.3) | 9 (90) | 0.496b |
| CF
III–IV, n (%) | 3 (18.8) | 1 (10) | 0.496b |
| Physical findings
upon exploration |
|
|
|
| Cardiac
frequency (bpm) median (range) | 90 (30–130) | 67 (40–88) | 0.015a,d |
|
Hypotension, n (%) | 0 | 2 (20) | 0.138b |
| Rales,
n (%) | 4 (25) | 3 (30) | 0.562b |
| Laboratory
findings |
|
|
|
| Maximum
troponin, median (range) (ng/ml) | 2.4 (0.01–30) | 0.05
(0.1–13.3) | 0.238a |
| Total
CPK maximum, median (range) (U/l) | 595 (56–2,444) | 185.5
(63–1,690) | 0.350a |
| Total
CPK-MB maximum, median (range) (Ul) | 46.5 (1–301) | 22 (15.4–138) | 0.433a |
| Electrocardiograph
findings |
|
|
|
|
Prolonged QRS (>120 m sec),
n (%) | 6 (37.5) | 4 (40) | 0.609b |
| AV
blockage, n (%) | 4 (25) | 2 (20) | 0.580b |
|
Tachyarrhythmia, n (%) | 8 (50) | 2 (20) | 0.218b |
| Echocardiograph
findings |
|
|
|
| LVEF
(%) median (range) | 35 (13–57) | 60 (31–70) | 0.005a,e |
| LVEF
<45%, n (%) | 8 (50) | 5 (50) | 0.656b |
| Right
ventricular dysfunction, n (%) | 4 (25) | 3 (33) | 0.673b |
|
Ventricular dysfunction, n
(%) | 9 (56) | 5 (50) | 0.756c |
| PASP
(mmHg) median (range) | 30.5 (17–46) | 37.5 (25–50) | 0.053a |
| TAPSE
(mm) median (range) | 18 (11–27) | 21,5 (19–26) | 0.017a,d |
No differences in clinical presentation (dyspnea,
chest pain, palpitations, syncope), functional class, findings on
physical examination, laboratory and electrocardiography findings
and echocardiography findings were observed between the two groups.
Significant differences between active and borderline myocarditis
were only found in terms of cardiac frequency (P=0.015), LVEF
(P=0.005) and tricuspid annular plane systolic excursion (TAPSE)
(P=0.01; Table II).
A total of 17 (65.4%) of the 26 patients
histologically diagnosed with myocarditis by endomyocardial biopsy
exhibited a satisfactory response to therapy (Table III), confirmed by
echocardiographic variables before and after medical treatment, and
according to the definition of such groups by Frustaci et al
(24). A total of 11 (64.7%) of
the responder patients had active myocarditis and 6 (35.3%)
patients had borderline myocarditis. Regarding analysis of the
treatment used with relation to the type of myocarditis, the
frequency was higher (P=0.02; Pearson Chi square) for the use of
oral steroids in responders compared with non-responders in
subjects with active myocarditis; there was no significant
difference in the use of steroids between responders and
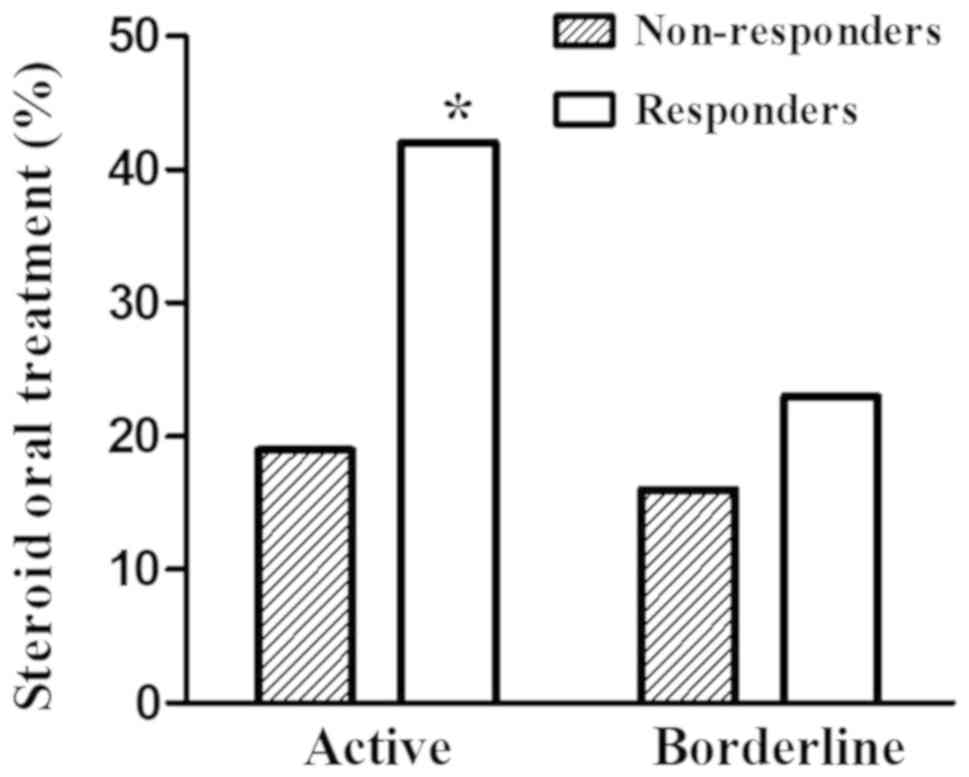
non-responders in borderline myocarditis (Fig. 1). Significant differences in
cardiac frequency (P=0.01), tachyarrhythmia (P=0.046), LVEF
(P=0.004) and TAPSE (P=0.016) were found at the time of diagnosis
between responders and non-responders (Table IV). The responders exhibited a
higher (P=0.038) level of ventricular dysfunction (LVEF <45%
and/or right ventricular dysfunction).
 | Table III.General description of the study
population according the treatment response. |
Table III.
General description of the study
population according the treatment response.
| Variable | Responders | Non-responders |
P-valuea |
|---|
| n (%) | 17 (65.4) | 9 (34.6) |
|
| Age, years (Mean ±
SD) | 36.4±14.3 | 35.2±13.2 | 0.850a |
| Sex, male/female
ratio | 12/5=2.4 | 5/4=1.25 | 0.528b |
| Myocarditis |
|
|
|
| Active,
n (%) | 11 (64.7) | 5 (55.6) | 0.692b |
|
Borderline, n (%) | 6 (35.3) | 4 (44.4) | 0.692b |
| Treatment |
|
|
|
|
Immunosuppressive, n (%) | 14 (82.4) | 8 (88.9) | 0.569b |
|
Conventional, n (%) | 3 (17.6) | 1 (11.1) | 0.569b |
| Cardiovascular risk
factors |
|
|
|
|
Diabetes mellitus, n (%) | 1 (5.9) | 0 (0) | 0.654b |
|
Systemic hypertension, n
(%) | 2 (11.8) | 1 (11.1) | 0.732b |
|
Dyslipidemia, n (%) | 5 (29.4) | 0 (0) | 0.129b |
|
Smoking, n (%) | 7 (41.2) | 4 (44.4) | 0.598b |
| Body
mass index (kg/m2), median (range) | 26.5
(18.8–39.4) | 27.7 (22.4–29) | 0.957a |
| Antecedent of
recent infection |
|
|
|
|
Airways, n (%) | 10 (58.8) | 4 (44.4) | 0.683b |
|
Gastrointestinal, n (%) | 2 (11.8) | 0 (0) | 0.529b |
 | Table IV.Clinical characteristics at the time
of diagnosis according the treatment response. |
Table IV.
Clinical characteristics at the time
of diagnosis according the treatment response.
| Variable | Responders
n=17 | Non-responders
n=9 | P-value |
|---|
| Clinical
symptoms |
|
|
|
|
Dyspnea, n (%) | 12 (70.6) | 3 (33.3) | 0.103b |
| Chest
pain, n (%) | 12 (70.6) | 4 (44.4) | 0.234b |
|
Palpitations, n (%) | 3 (17.6) | 4 (44.4) | 0.188b |
|
Syncope, n (%) | 3 (17.6) | 4 (44.4) | 0.188b |
| Functional class
(NYHA) |
|
|
|
|
CF-I–II, n (%) | 15 (88.2) | 7 (77.8) | 0.591b |
| CF
III–IV, n (%) | 2 (11.8) | 2 (22.2) | 0.591b |
| Physical findings
upon exploration |
|
|
|
| Cardiac
frequency (bpm) median (range) | 90 (30–130) | 67 (40–88) | 0.010a,d |
|
Hypotension, n (%) | 2 (11.8) | 0 | 0.529b |
| Rales,
n (%) | 6 (35.3) | 1 (11.1) | 0.357b |
| Laboratory |
|
|
|
| Maximum
troponin, median (range) (ng/ml) | 7.9 (0.01–30) | 3.3
(0.01–13.3) | 0.26a |
| Total
CPK maximum, median (range) (U/l) | 806 (56–2,444) | 438 (63–1,690) | 0.38a |
| Total
CPK-MB maximum, median (range) (U/l) | 72 (1–301) | 43 (15–138) | 0.445a |
| Electrocardiograph
findings |
|
|
|
|
Prolonged QRS (>120 msec),
n (%) | 6 (35.3) | 4 (44.4) | 0.692b |
| AV
blockage, n (%) | 4 (23.5) | 2 (22.2) | 0.580b |
|
Tachyarrhythmia, n (%) | 4 (23.5) | 6 (66.7) | 0.046b,c |
| Echocardiograph
findings |
|
|
|
| LVEF
(%) median (range) | 35 (13–57) | 60 (31–70) | 0.004a,d |
| LVEF
<45%, n (%) | 11 (64.7) | 2 (22.2) | 0.097b |
| Right
ventricular dysfunction, n (%) | 7 (41.2) | 0 | 0.057b |
|
Ventricular dysfunction, n
(%) | 12 (70.6) | 2 (22.2) | 0.038b,c |
| PASP
(mmHg) median (range) | 34 (17–50) | 27 (25–40) | 0.181a |
| TAPSE
(mm) median (range) | 18 (11–27) | 21.5 (19–26) | 0.016a,c |
The median follow-up time was 12 months (range, 2–72
months), which was mainly determined by the time the diagnosis was
made. The responder patients had significantly lower initial LVEF
and initial pulmonary artery systolic pressure (P=0.017; Table V), whereas the non-responders
exhibited deterioration of the initial LVEF (median decrease of
10%; range, 1–20%).
 | Table V.Echocardiographic behavior of
patients in relation to response to treatment. |
Table V.
Echocardiographic behavior of
patients in relation to response to treatment.
| Variable | Responders | Non-responders |
P-valuea |
|---|
| LVEF (%) |
|
|
|
|
Initial | 35 (13–57) | 60 (31–70) | 0.004a,c |
|
Follow-up | 60 (26–75) | 42 (24–66) | 0.155a |
| Diastolic VI
diameter (mm) |
|
|
|
|
Initial | 54 (40–72) | 48 (43–77) | 0.646a |
|
Follow-up | 48 (33–58) | 51(41–77) | 0.513a |
| Systolic VI
diameter (mm) |
|
|
|
|
Initial | 40 (30–61) | 32 (28–65) | 0.145a |
|
Follow-up | 32 (20–51) | 39 (20–57) | 0.281a |
| PASP (mmHg) |
|
|
|
|
Initial | 34 (17–50) | 27 (25–40) | 0.168a |
|
Follow-up | 29 (22–43) | 35 (25–47) | 0.065a |
| TAPSE (mm) |
|
|
|
|
Initial | 18 (11–27) | 21 (16–26) | 0.016a,b |
|
Follow-up | 21(17–27) | 20 (18–24) | 0.669a |
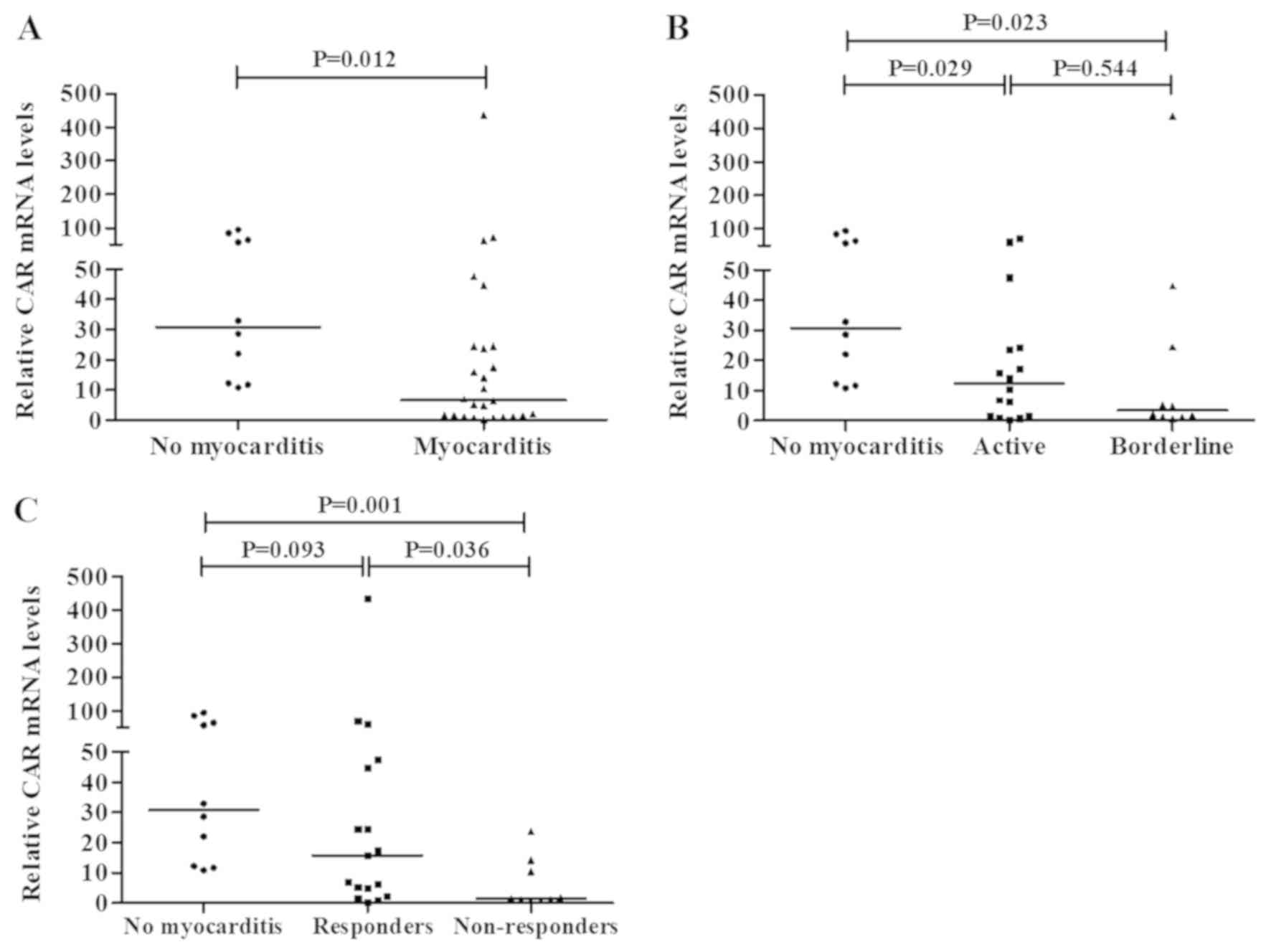
CAR expression in myocarditis
The expression levels of CAR were significantly
lower in patients with myocarditis (P=0.012) compared with those in
the NM group (Fig. 2A). CAR
expression was significantly lower in patients with borderline
myocarditis [P=0.023; median, 3.5 (range, 0.70–435.5)] and active
myocarditis [P=0.029; median, 12.3 (range, 0.26–71.3); Mann-Whitney
U test)] compared with that in the NM control group [median, 30.7
(range, 10.8–94.6)] (Fig. 2B).
The CAR mRNA levels according to treatment response
are shown in Fig. 2C [improvement
assessed by clinical and echocardiographic criteria, as defined by
Frustaci et al (24)].
Responder patients exhibited higher transcription of CAR (P=0.036,
Mann-Whitney U test) [median, 15.9 (range, 0.14–435)] compared with
non-responders [median, 1.5 (range, 0.7–23.8)]. The CAR mRNA levels
were significantly lower in non-responder patients with myocarditis
(P=0.001) compared with those in the NM control group. This
difference was estimated by relative expression of the housekeeping
genes β-actin and β-globin (data not shown).
Discussion
The present study involved NM patients and patients
with myocarditis. In line with the findings reported by Hufnagel
et al (31), we observed
that the main clinical manifestations in symptomatic patients
included chest pain, dyspnea, palpitations and syncope, in that
order of frequency. In the literature, a history of recent
infection is reported as one of the main predisposing factors for
the development of myocarditis. The presence of this factor (mainly
airway infections) was documented in over half of the population of
the present study.
Myocarditis can be classified considering several
characteristics, which emphasize, among others, causal agent,
histological findings (Dallas criteria) (32), time of evolution and
clinicopathological background (33). However, histological classification
remains the gold standard for its diagnosis. In the present study,
no statistically significant differences were observed between the
two groups (active and borderline) in terms of age, cardiovascular
risk factors and predisposing factors. Regarding the initial
clinical presentation, patients with active myocarditis presented
with higher heart rate, as well as lower LVEF and TAPSE, compared
with borderline myocarditis, in contrast to the study of Angelini
et al (34), who reported a
higher incidence of left bundle branch block in patients with
borderline myocarditis, as well as higher left ventricular
end-diastolic volume and lower ventricular mass/volume ratio.
However, despite the clinical differences, the presence of
myocardial necrosis is not considered an indicator of unfavorable
prognosis, as might be expected; the histological finding of active
or borderline myocarditis does not affect the evolution and
severity of the disease, or the response to treatment.
In animal models of myocarditis, a differential
expression was observed in each of the stages of this pathology
according with the evolution time. Ito et al (15) reported that the expression of CAR
in rat hearts was low or undetectable prior to disease onset,
became evident during the active phase of myocarditis and decreased
in the chronic phase. The CAR transcriptional level was found to be
lower in patients with myocarditis compared with that in controls,
and it was lower in patients with borderline compared with those
with active myocarditis. These results are in agreement with Kaur
et al (12), who evaluated
9 cases of myocarditis, without finding a statistically significant
association between CAR positivity and active myocarditis in any of
the groups.
The study of myocarditis in humans is limited by
ethical issues, therefore it was necessary to revert to autopsies
in order to establish reference points. The use of myocardial
tissue from autopsies as control samples for experimental studies
in myocarditis has been carried out by several international
researchers. Tatrai et al (14), in a period from 2005 to 2008, used
10 controls to assess the expression of CAR mRNA and mutations of
the CAR gene, the myocardial tissue was obtained from individuals
who died suddenly of accident or suicide. Also, Kaur et al
(12) determined the expression of
CAR in myocardial tissue, the study was performed on autopsied
myocardial tissues preserved over a period of 10 years,
formalin-fixed, collected from 26 myocarditis/DCM patients and 20
cases each of NCD and cardiac disease other than DCM were included
as control groups (12).
Of note, the focus of the present study was the
behavior of CAR considering in terms of response to treatment. To
the best of our knowledge, this is the first study to document
higher transcription of this molecule in patients with adequate
response to treatment, despite the fact that these patients
exhibited greater ventricular dysfunction at the time of
diagnosis.
The treatment of myocarditis remains debated upon,
since the use of immunosuppression and/or immunomodulation is not
universally accepted, and there is lack of evidence-based
guidelines in favor of such therapeutic options. However, there is
currently no method that predicts whether a patient will respond to
that treatment. The present study provided molecular evidence of an
increased level of CAR transcription in patients responding to
therapy (immunosuppressive or conventional) compared with
non-responders. In addition, response to treatment among patients
with active myocarditis was associated with a greater frequency of
oral steroid use compared with non-responders. Moreover, the
responder patients had a higher incidence of ventricular
dysfunction and other abnormal clinical variables. These results
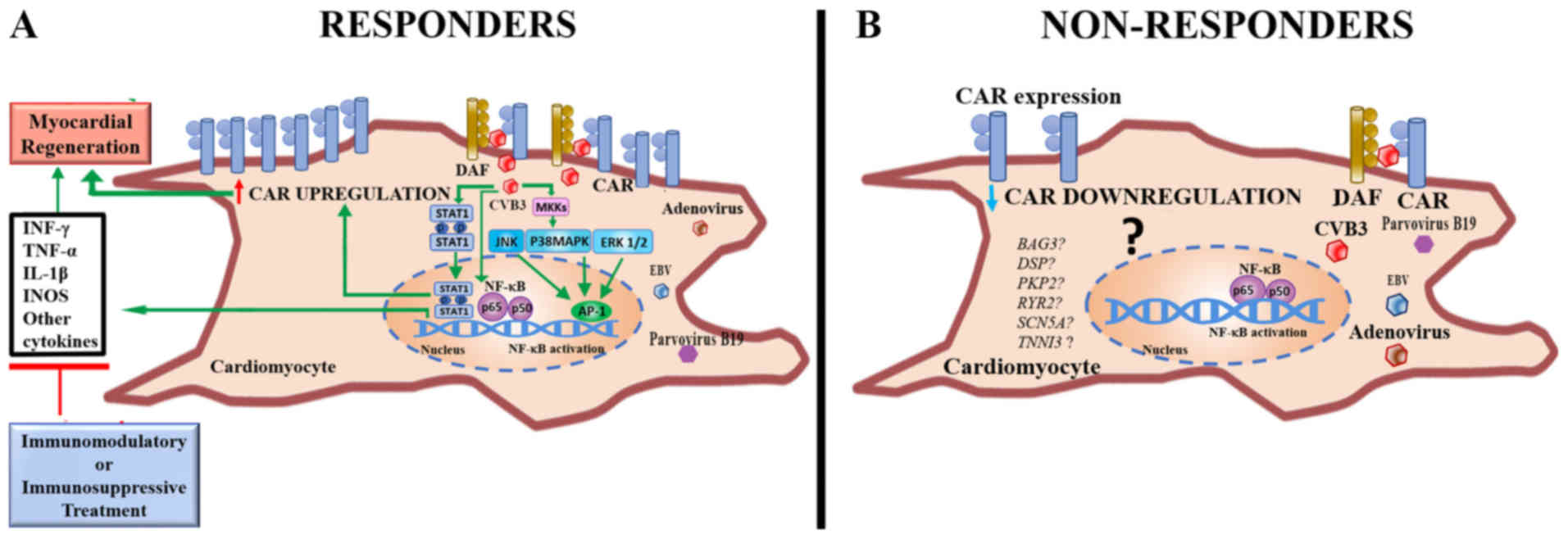
support the hypothesis of Ito et al (15) and Noutsias et al (35) regarding the participation of CAR in
the regeneration of damaged myocardial tissue. However, a
pro-inflammatory effect mediated by CAR, where the use of
immunomodulatory therapy may be important, cannot be excluded
[Fig. 3; modified from Ghigo et
al (36)].
The evidence of a higher transcriptional level
higher transcription of CAR in patients with response to treatment
compared with non-responders is of great value, since the
expression of this molecule may be a predictive factor in the
evolution of the disease and may determine the ability to respond
to treatment. Such an observation may also provide physicians with
valuable information to facilitate decisions on whether to initiate
immunomodulatory pharmacological management, as suggested by
Frustaci et al (24).
The sample size of the present study was small, but
myocarditis is underdiagnosed, and the results are in line with
those of other authors. Another limitation of the present study was
that it was impossible to analyze viral persistence in EMB,
although this was not a vital part of the study's objective.
In summary, CAR transcription was found to below in
the endomyocardial tissue of patients with myocarditis, and it was
lower in cases of borderline myocarditis. The CAR mRNA levels were
significantly higher in patients responding to therapy versus
non-responders. Patients not responding to therapy may present with
fewer clinical manifestations, lower CAR transcription levels, and
their outcome is less favorable. Thus, it is necessary to
investigate differentially the pathophysiological mechanisms
involved in such patients. The levels of CAR mRNA may not only be a
prognostic factor, but also a tool to guide the pharmacological
management of the patient with myocarditis.
Acknowledgements
The authors would like to thank Dr Armando Mansilla
Olivares (Laboratorio de Patología Molecular, Unidad de
Investigación Biomolecular, UMAE Hospital de Cardiología del Centro
Médico Nacional Siglo-XXI, Instituto Mexicano del Seguro Social,
Mexico City, Mexico) and Dr Javier Torres (Unidad de Investigación
Médica en Enfermedades Infecciosas y Parasitarias, UMAE, Hospital
de Pediatría ‘Dr Silvestre Frenk Freund’, Centro Médico Nacional
Siglo-XXI, Instituto Mexicano del Seguro Social, Mexico City,
Mexico) for their invaluable support. They also thank Ms. Erika
Cristina de la Peña Cárdenas, designer, for her support with
Fig. 3 and Ms. Susan Drier Jonas,
for her assistance with the manuscript. The authors would also like
to acknowledge the participation of Dr Gustavo Eduardo Garcia
Becerril (Clínica de Insuficiencia Cardiaca, UMAE Hospital de
Cardiología del Centro Médico Nacional Siglo-XXI, Instituto
Mexicano del Seguro Social, Mexico City, Mexico).
Funding
The present study was supported by grant
FIS/IMSS/PROT/G15/1466 (to M.G.C.-M.) from the Fondo de
Investigación en Salud (FIS)-IMSS, México.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MGCM and GEGB conceived and designed the
experiments. MGCM, AECM, MADLC, MAA, AJGC and CMB performed the
experiments. GEGB, AECM and MHGG were invovled in the selection and
evaluation of cases. CAFG and LMGJ reviewed the histology data.
MGCM, LAMR, AECM and MHGG analyzed the data. MGCM, AECM and MHGG
wrote the paper.
Ethics approval and consent to
participate
The present study was approved by the National
Research Scientific and Ethics Committee of Instituto Mexicano del
Seguro Social (number 17CI09015006 COFEPRIS, Mexico). The study
protocol conformed to the principles outlined in the Declaration of
Helsinki. All the participants were informed on the nature of the
study and provided written consent.
Patient consent for publication
Patients provided informed consent prior to
participation in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corsten MF, Schroen B and Heymans S:
Inflammation in viral myocarditis: Friend or foe? Trends Mol Med.
18:426–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson P, McKenna W, Bristow M, Maisch
B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I,
et al: Report of the 1995 World Health Organization/International
Society and Federation of Cardiology Task Force on the definition
and classification of cardiomyopathies. Circulation. 93:841–842.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Ambrosio A, Patti G, Manzoli A, Sinagra
G, Di Lenarda A, Silvestri F and Di Sciascio G: The fate of acute
myocarditis between spontaneous improvement and evolution to
dilated cardiomyopathy: A review. Heart. 85:499–504. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Global Burden of Disease Study 2013
Collaborators, . Global, regional, and national incidence,
prevalence, and years lived with disability for 301 acute and
chronic diseases and injuries in 188 countries, 1990–2013: A
systematic analysis for the Global Burden of Disease Study 2013.
Lancet. 386:743–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andréoletti L, Lévêque N, Boulagnon C,
Brasselet C and Fornes P: Viral causes of human myocarditis. Arch
Cardiovasc Dis. 102:559–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blauwet LA and Cooper LT: Myocarditis.
Prog Cardiovasc Dis. 52:274–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coyne C and Bergelson J: CAR: A virus
receptor within the tight junction. Adv Drug Deliv Rev. 57:869–882.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen CJ, Shieh JT, Pickles RJ, Okegawa T,
Hsieh JT and Bergelson JM: The coxsackievirus and adenovirus
receptor is a transmembrane component of the tight junction. Proc
Natl Acad Sci USA. 98:15191–15196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Excoffon KJ, Avenarius MR, Hansen MR,
Kimberling WJ, Najmabadi H, Smith RJ and Zabner J: The
Coxsackievirus and Adenovirus Receptor: A new adhesion protein in
cochlear development. Hear Res. 215:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergelson JM, Cunningham JA, Droguett G,
Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL and
Finberg RW: Isolation of a common receptor for Coxsackie B viruses
and adenoviruses 2 and 5. Science. 275:1320–1323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dorner AA, Wegmann F, Butz S,
Wolburg-Buchholz K, Wolburg H, Mack A, Nasdala I, August B,
Westermann J, Rathjen FG and Vestweber D: Coxsackievirus-adenovirus
receptor (CAR) is essential for early embryonic cardiac
development. J Cell Sci. 118:3509–3521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaur T, Mishra B, Saikia UN, Sharma M,
Bahl A and Ratho RK: Expression of coxsackievirus and adenovirus
receptor and its cellular localization in myocardial tissues of
dilated cardiomyopathy. Exp Clin Cardiol. 17:183–186.
2012.PubMed/NCBI
|
|
13
|
Ruppert V, Meyer T, Pankuweit S,
Jonsdottir T and Maisch B: Activation of STAT1 transcription factor
precedes up-regulation of coxsackievirus-adenovirus receptor during
viral myocarditis. Cardiovasc Pathol. 17:81–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatrai E, Bedi K, Kovalszky I, Hartyanszky
I, Laszik A, Acsady G, Sotonyi P and Hubay M: No mutation but high
mRNA expression of Coxsackie-Adenovirus Receptor was observed in
both dilated and ischemic cardiomyopathy. Forensic Sci Int.
212:47–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito M, Kodama M, Masuko M, Yamaura M, Fuse
K, Uesugi Y, Hirono S, Okura Y, Kato K, Hotta Y, et al: Expression
of coxsackievirus and adenovirus receptor in hearts of rats with
experimental autoimmune myocarditis. Circ Res. 86:275–280. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Kofahi M, Omura S, Tsunoda I, Sato F,
Becker F, Gavins FNE, Woolard MD, Pattillo C, Zawieja D, Muthuchamy
M, et al: IL-1β reduces cardiac lymphatic muscle contraction via
COX-2 and PGE2 induction: Potential role in myocarditis.
Biomed Pharmacother. 107:1591–1600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bacmeister L, Schwarzl M, Warnke S,
Stoffers B, Blankenberg S, Westermann D and Lindner D: Inflammation
and fibrosis in murine models of heart failure. Basic Res Cardiol.
114:192019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Su X, Yu Y and Liu Y: Correlation
between virus persistent infection and cardic function in patients
with dilated cardiomyopathy. J Zhejiang Univ Sci B. 14:749–753.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuen S, Smith J, Caruso L, Balan M and
Opavsky MA: The coxsackie-adenovirus receptor induces an
inflammatory cardiomyopathy independent of viral infection. J Mol
Cell Cardiol. 50:826–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu P, Aitken K, Kong YY, Opavsky MA,
Martino T, Dawood F, Wen WH, Kozieradzki I, Bachmaier K, Straus D,
et al: The tyrosine kinase p56lck is essential in coxsackievirus
B3-mediated heart disease. Nat Med. 6:429–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu PP and Opavsky MA: Viral myocarditis:
Receptors that bridge the cardiovascular with the immune system?
Circ Res. 86:253–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu L, Li C, Wang Z, Xu H and An X:
Effects of the MAPK pathway and the expression of CAR in a murine
model of viral myocarditis. Exp Ther Med. 13:230–234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tschöpe C, Müller I, Xia Y, Savvatis K,
Pappritz K, Pinkert S, Lassner D, Heimesaat MM, Spillmann F, Miteva
K, et al: NOD2 (Nucleotide-Binding Oligomerization Domain 2) is a
major pathogenic mediator of coxsackievirus B3-induced myocarditis.
Circ Heart Fail. 10:2017. View Article : Google Scholar
|
|
24
|
Frustaci A, Chimenti C, Calabrese F,
Pieroni M, Thiene G and Maseri A: Immunosuppressive therapy for
active lymphocytic myocarditis: Virological and immunologic profile
of responders versus nonresponders. Circulation. 107:857–863. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aretz HT: Myocarditis: The Dallas
criteria. Hum Pathol. 18:619–624. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ponce-Castañeda MV, García-Chéquer AJ,
Eguía Aguilar P, Abundes-Ramírez MA, Hernández-Angeles A,
Nieto-Martínez K, Gómez-Laguna L, Sadowinski-Pine S and
Cabrera-Muñoz Mde L: Detection of common chromosomal translocations
in small round blue cell pediatric tumors. Arch Med Res.
45:143–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jahn CE, Charkowski AO and Willis DK:
Evaluation of isolation methods and RNA integrity for bacterial RNA
quantitation. J Microbiol Methods. 75:318–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aranda PS, LaJoie DM and Jorcyk CL: Bleach
gel: A simple agarose gel for analyzing RNA quality.
Electrophoresis. 33:366–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hufnagel G, Pankuweit S, Richter A,
Schönian U and Maisch B: The European study of epidemiology and
treatment of cardiac inflammatory diseases (ESETCID). First
epidemiological results. Herz. 25:279–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aretz HT, Billingham ME, Edwards WD,
Factor SM, Fallon JT, Fenoglio JJ Jr, Olsen EG and Schoen FJ:
Myocarditis. A histopathologic definition and classification. Am J
Cardiovasc Pathol. 1:3–14. 1987.PubMed/NCBI
|
|
33
|
Lieberman EB, Hutchins GM, Herskowitz A,
Rose NR and Baughman KL: Clinicopathologic description of
myocarditis. J Am Coll Cardiol. 18:1617–1626. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Angelini A, Crosato M, Boffa GM, Calabrese
F, Calzolari V, Chioin R, Daliento L and Thiene G: Active versus
borderline myocarditis: Clinicopathological correlates and
prognostic implications. Heart. 87:210–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noutsias M, Fechner H, de Jonge H, Wang X,
Dekkers D, Houtsmuller AB, Pauschinger M, Bergelson J, Warraich R,
Yacoub M, et al: Human coxsackie-adenovirus receptor is colocalized
with integrins alpha(v)beta(3) and alpha(v)beta(5) on the
cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy:
Implications for cardiotropic viral infections. Circulation.
104:275–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghigo A, Franco I, Morello F and Hirsch E:
Myocyte signalling in leucocyte recruitment to the heart.
Cardiovasc Res. 102:270–280. 2014. View Article : Google Scholar : PubMed/NCBI
|