Introduction
Chronic kidney disease (CKD) has become one of the
major diseases that threatens public health worldwide, and causes
an enormous amount of suffering and economic expense (1). The prevalence of CKD has reached
epidemic proportions, as it currently affects 10–13% of the
populations in Taiwan, Japan, China, Canada, India and the USA
(2). More than 100 million
individuals rely on dialysis to survive worldwide, and this number
increases at a mean annual rate of 8% (3). Moreover, increasing numbers of young
people suffer from CKD, with the youngest patient being <10
years of age (4). Therefore, there
is a critical and urgent need to reduce the frequency of kidney
injury and protect the kidneys from all types of kidney
disease.
Renal interstitial fibrosis (RIF) is a kidney
disorder characterized by dilated kidney tubules and interstitial
fibrosis (5). Studies have
indicated that the degree of RIF indicates the degree of kidney
damage, which makes RIF an important factor when establishing a
prognosis for patients with kidney diseases (6–8). RIF
is also an important factor for predicting CKD, which is currently
increasing in prevalence (9,10).
Therefore, to delay the development of CKD, it is of great
importance that we identify biomarkers for CKD, and also explore
methods for preventing and treating RIF. The occurrence and
development of RIF are influenced by numerous factors, including
renal tubular epithelial cell apoptosis, inflammation, cytokines,
oxidative stress, fibroblast proliferation and activation, the
production of vasoactive substances, and an imbalance between
extracellular matrix synthesis and degradation (11–14).
Due to the large number of pathologic mechanisms for RIF, we
speculate that additional unknown mechanisms and biomarkers for RIF
remain to be discovered. Renal fibrogenesis is related to multiple
cellular events and molecular mediators (15). Among the fibrogenic factors that
regulate the renal fibrotic process, transforming growth factor β
(TGF-β) has long been considered as a central mediator of the
fibrotic response (16). TGF-β
exerts its biological effects mainly via its downstream Smad
signaling molecules (17,18). The TGF-β/Smad3 signal is transduced
by type I and type II serine/threonine kinase receptors found in
the cell membrane (19). TGF-β1
binds to its type II receptor, and this complex then forms a dimer
with the type I receptor, resulting in phosphorylation and
activation of the type I receptor. The activated type I receptor
phosphorylates Smad2 and/or Smad3, which then heterodimerizes with
Smad4 and subsequently translocates into the nuclei, where it
regulates the transcription of TGF-β-target genes (20). Some adaptor proteins, including
Smad anchor for receptor activation (SARA), disabled-2 (DAB2),
ELF-spectrin and endofin have been reported to facilitate TGF-β
signaling by linking Smad2/Smad3 to the receptor complex (21). However, the pathogenic molecular
mechanisms involved in RIF are poorly understood.
Acupuncture is a traditional Chinese therapy with
thousands of years of history, and is now recognized and accepted
as a medical therapy worldwide (22). As a green complementary and
alternative therapy, acupuncture has demonstrated significant
effects on many diseases, especially on functional diseases
(23–25). However, the mechanism by which
acupuncture exerts its effects remains elusive. This limits our
progress in understanding acupuncture and how to optimize its
clinical effects. Therefore, clarifying the mechanism by which
acupuncture affects RIF would be valuable for further development
in the field of acupuncture therapy.
In the present study a rabbit model of CRF was
utilized to explore the renal protective effect of acupuncture and
the underlying mechanism for that effect. Our results provide a
theoretical basis for the clinical use of acupuncture in treating
CKD.
Materials and methods
Animal preparation
A total of 30 6-month-old healthy male adult New
Zealand White rabbits (body weight, 1.8–2.2 kg) were obtained from
the Experimental Animal Center of Yunnan College of Traditional
Chinese Medicine. On arrival, the rabbits were allowed to
acclimatize to their new environment for one week before any
procedure was performed. The rabbits were fed separately, and had
free access to 200 g of food and water in an undisturbed and clean
environment. The animals were housed in a room that had a 12-h
light/dark cycle, and was maintained at a temperature of 18–24°C
and a relative humidity ranging from 40 to 70%. All experiments
were performed in accordance with the recommendations provided in
Guidelines for the Care and Use of Laboratory Animals, and the
study protocol was approved by the Animal Research Committee of
Yunnan College of Traditional Chinese Medicine (Cumming, China).
Animals were anesthetized by pentobarbital (i.v., 40 mg/kg) and
sacrificed by exsanguination (blood volume, ~85 ml each).
Chronic renal failure (CRF) model
The CRF model was created by gavaging the rabbits
with adenine for 3 weeks, as previously described (26). Adenine (purity >98%; FW: 135.14)
was purchased from Guangzhou Weijia Technology Co., Ltd.
(Guangzhou, China). New Zealand White rabbits (n=6) in the control
group were gavaged with an equivalent amount of distilled water (10
ml/kg/day) for 21 days. The other New Zealand White rabbits (n=24)
were used to construct the CRF rabbit model. In brief, the rabbits
were gavaged with 25% adenine (150 mg/kg/day) dissolved in normal
saline for 21 consecutive days. After 21 days, their kidney
function, blood pressure and urinary protein levels were
measured.
Renal function analysis
Serum creatinine (SCr) and blood urea nitrogen (BUN)
were detected with an IDEXX Catalyst One Chemistry Analyzer (IDEXX
Laboratories, Inc., Westbrook ME, USA).
Experiments and acupuncture
procedure
The CRF rabbits were randomly assigned to a CRF
model group (n=6), a losartan potassium positive control group
(Posi, CRF model treated with losartan potassium; n=6), an
acupuncture group (Acup, CRF model administered acupuncture; n=6),
or an acupuncture+SB 431542 group (Acup+inhibitor, CRF model
administered acupuncture and SB 431542; n=6). Rabbits in the CRF
model group were fed a standard diet without drugs. CRF rabbits in
the Posi group were fed a standard diet and also received 2.33
mg/kg/day of losartan potassium (100 mg/tablet, produced by
Hangzhou MSD Pharmaceutical Co., Ltd., Hangzhou, China) by gavage,
once a day. CRF rabbits in the acupuncture group were fed a
standard diet and were stimulated by acupuncture treatment
(Shenshu, Mingmen and Pishu) for 30 min, once a day. CRF rabbits in
the Acup+inhibitor group were fed a standard diet and received SB
431542 (2.5 mg/kg/2 days; Tocris Bioscience, Bristol, UK) by
intraperitoneal injection. Acupuncture was administered with a
sterilized acupuncture needle (diameter: 0.25 mm, length: 40 mm;
TCM Supply Co., Ltd. Yokohama, Japan). During acupuncture,
sterilized acupuncture needles were gently inserted to a depth of 5
mm below the surface of acupuncture points at Shenshu, Mingmen and
Pishu. The treatments were continued for 36 days, and each animal's
diet, urine volume, mental state, hair color and weight were
monitored.
Histopathological analysis
The samples obtained from all groups were washed and
then fixed in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 30 min. Next, the tissues were dehydrated
in a gradient alcohol series, treated in xylene, imbedded with
paraffin, and cut into 4-µm sections. For the hematoxylin and eosin
(H&E) staining assay, the sections were stained with
hematoxylin for 5 min and then dehydrated with 70 and 90% ethanol;
after which, they were treated with eosin for 3 min. Each section
was then evaluated under a light microscope (CX41; Olympus
Corporation, Tokyo, Japan; magnification, ×200). For Masson's
trichrome staining, the sections were stained with reagents in a
Masson's Trichrome Stain kit (Sigma-Aldrich; Merck KGaA) according
to the manufacturer's instructions. Masson staining results were
quantified using Image-Pro Plus (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA). Briefly, integral optical density of
three areas-of-interest was assessed for staining
quantification.
Real-time quantitative PCR (qPCR)
assay
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA
from the tissue samples of all groups as described in the
manufacturer's instructions. RNA concentrations were determined by
measuring absorbance at 260/280 nm. cDNA was obtained by using a
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
Specific genes were amplified by using SYBR-Green PCR Master Mix
(Takala Biotechnology, Co., Ltd., Dalian, China) and an ABI 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). β-actin served as an endogenous housekeeping gene.
Amplification data were analyzed using the 2−ΔΔCq method
(27). The following primers were
used for amplification: TGF-β: 5′-CAAGTGGACATCAACGGGA-3′ (forward)
and 5′-GCAGAAGTTGGCGTGGTAG-3′ (reverse); Smad3:
5′-GAAGGATGAGGTTTGCGTGA-3′ (forward) and
5′-GGATGGAATGGCTGTAGTCGT-3′ (reverse); ILK:
5′-TCACCCAACCCTCATTACG-3′ (forward) and
5′-TCATCAATCATTACACTACGGCTAT-3′ (reverse); β-actin:
5′-CCAGGTCATCACCATCGG-3′ (forward) and 5′-TGTCCACGTCGCACTTCA-3′
(reverse).
Western blot assays
The total proteins were extracted from all groups of
tissue samples by using a radio-immunoprecipitation assay (RIPA)
buffer (Beyotime Institute of Biotechnology, Shanghai, China). The
concentration of total protein in each sample was measured by using
a protein reagent (Bio-Rad Laboratories, Hercules, CA, USA). Next,
aliquots of total protein (30 µg per sample) were separated by
electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels,
and the protein bands were transferred onto polyvinylidene fluoride
(PVDF) membranes (cat. no. IPVH00010; Millipore, Burlington, MA,
USA). The membranes were then blocked with 5% non-fat dry milk for
1 h, and subsequently incubated overnight at 4°C with specific
primary antibodies. After being washed, the membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:2,000, cat. no. ab97057; Abcam, Cambridge, UK) at room
temperature for 1.5 h. The immunoprecipitated proteins were
detected by using an enhanced chemiluminescence substrate kit that
was included in an enhanced chemiluminescence detection system
(Amersham Biosciences Inc., Piscataway, NJ, USA). The primary
antibodies were anti-TNF-α (dilution 1:1,000; cat. no. ab6671;
Abcam), anti-Smad3 (dilution 1:1,000; cat. no. ab84177; Abcam),
anti-ILK (dilution 1:1,000; cat. no. ab227154; Abcam), anti-TGF-β
(dilution 1:1,000; cat. no. ab92486; Abcam), anti-eNOS (dilution
1:1,000; cat. no. ab76198; Abcam) and anti-GAPDH (dilution 1:3,000,
cat. no. ab9485; Abcam).
Enzyme-linked immunosorbent assay
(ELISA)
Samples of blood from the right common carotid
artery of each rabbit were collected into anticoagulant tubes and
centrifuged. TGF-β, IL-8, TNF-α and IL-1β were measured by using
specific ELISA kits according to the manufacturer's instructions.
The ELISA kits used in the present study were for TGF-β (cat. no.
CSB-E06900Rb; Cusabio, Wuhan, China), IL-8 (cat. no. SB-E13942Rb;
Cusabio), TNF-α (cat. no. CSB-E06998Rb; Cusabio) and IL-1β (cat.
no. E-EL-RB0385c; Elabscience Biotechnology Co., Ltd., Wuhan,
China).
Immunohistochemistry (IHC) assays
The tissues in all groups were fixed in 4% formalin
(cat. no. SF100-20; Thermo Fisher Scientific, Inc.), embedded in
paraffin, and cut into 4-µm thick sections. The sections were then
deparaffinized, rehydrated and treated with 10 mM citrate buffer
for 5 min at 100°C; after which, they were incubated with 10% fetal
bovine serum (FBS) for 2 h at 37°C. Next, the sections were
incubated overnight with anti-TGF-β (dilution 1:25; cat. no.
ab92486; Abcam) at 4°C. After being washed with phosphate-buffered
saline (PBS), the sections were incubated with an anti-rabbit
secondary antibody (cat. no. ab150077; Abcam) for 2 h at room
temperature. The immunostained tissues were then photographed under
a light microscope (CX41; Olympus Corporation).
Statistical analysis
Each experiment was repeated at least 3 times, and
results represent the mean ± standard deviation (SD). Student's
t-test was used in data analysis between two groups. Statistical
analyses of multiple groups were performed using the one-way
analysis of variance (ANOVA) followed by post-hoc Tukey's test
through IBM SPSS Statistics for Windows (version 23.0 software; IBM
Corp, Armonk, NY, USA). P-values <0.05 were considered
statistically significant.
Results
General condition of the experimental
animals
In the present study, no rabbit in any group died,
and no abnormalities of feeding or activity were observed among
rabbits in the control group. However, some rabbits in the other
groups displayed loss of appetite, lethargy, slowed mobility and
weight loss. Rabbits in the model group displayed the most
significant symptoms.
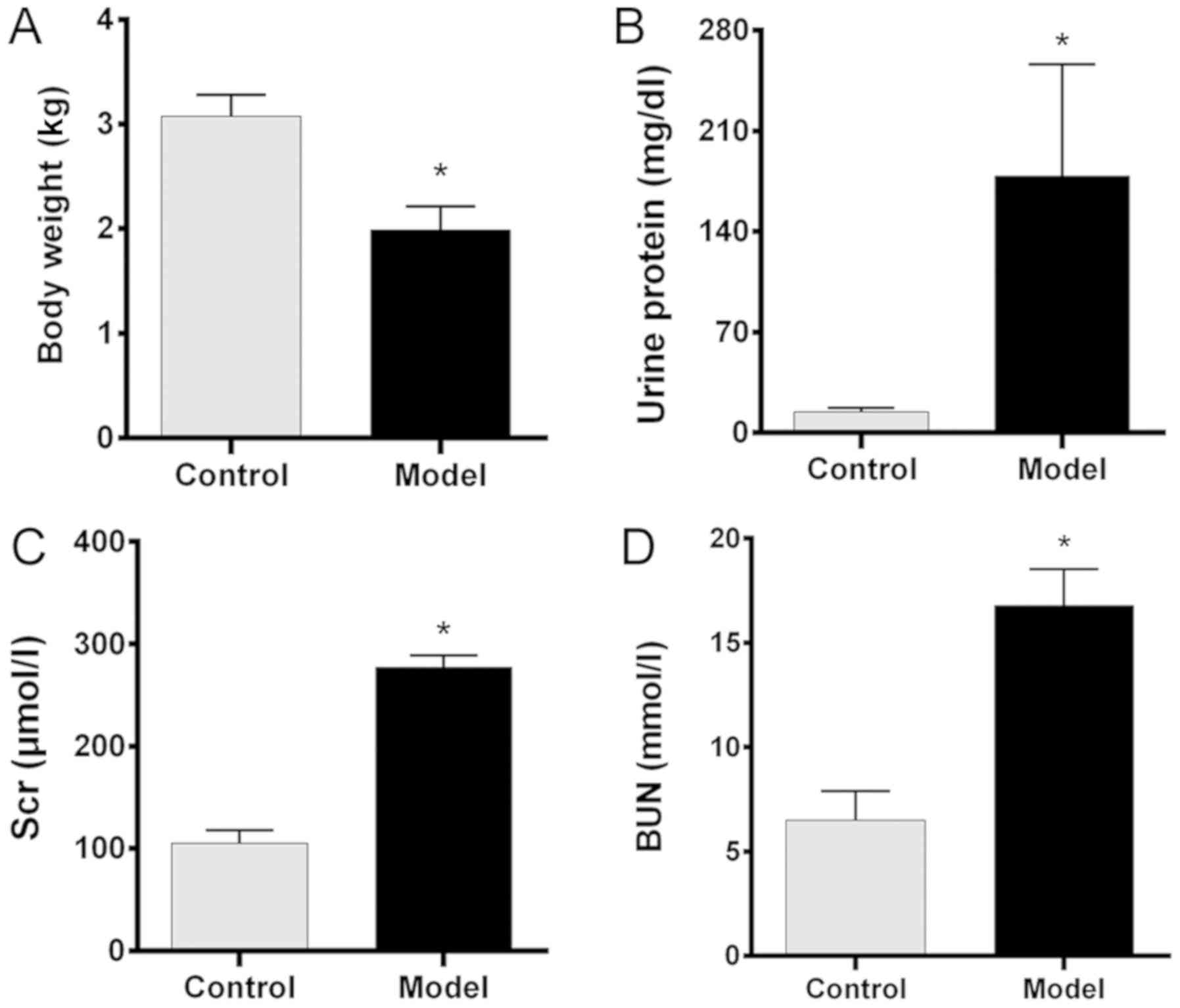
Comparison of renal function in the
control and CRF model groups
Our chronic renal failure (CRF) model was created by
gavaging rabbits with adenine for 3 weeks. In this study, the base
line body weights, urine protein, SCr and BUN levels of all the
rabbits were similar prior to establishment of the CRF model. After
treatment, there were no significant changes in body weight, urine
protein, SCr and BUN levels of rabbits in the control group when
compared with the pre-treatment values. While the mean body weight
in the model group after treatment was significantly lower than
that in the control group (P<0.05; Fig. 1A), the urine protein levels in the
model group were significantly higher than those in the control
group (P<0.05; Fig. 1B).
Furthermore, the SCr and BUN levels in the model group were also
significantly higher than those in the control group (both
P<0.05; Fig. 1C and D,
respectively). These findings indicated that the CRF model had been
successfully created.
Acupuncture attenuates kidney
fibrogenesis and pathological changes in the CRF model rabbits
To explore how acupuncture affects RIF, H&E and
Masson's trichrome staining were performed to evaluate
morphological features of the renal tissues. As shown in Fig. 2A, the sizes of the glomeruli in the
model group were dramatically decreased, and the glomeruli
displayed features of patchy tubulointerstitial injury, such as a
fuzzy boundary. However, these features were absent in the Posi
control group, and both the losartan potassium and acupuncture
groups had glomeruli of significantly increased sizes. Acupuncture
was able to attenuate these symptoms. As shown in Fig. 2B, interstitial fibrosis was
observed more often in the model group than in the control group,
and the symptoms were attenuated in the animals treated with
losartan or acupuncture. Additionally, we found that the
fibrogenesis was even further attenuated by TGF-β inhibitor (SB
431542) administration without significant difference (Fig. 2B).
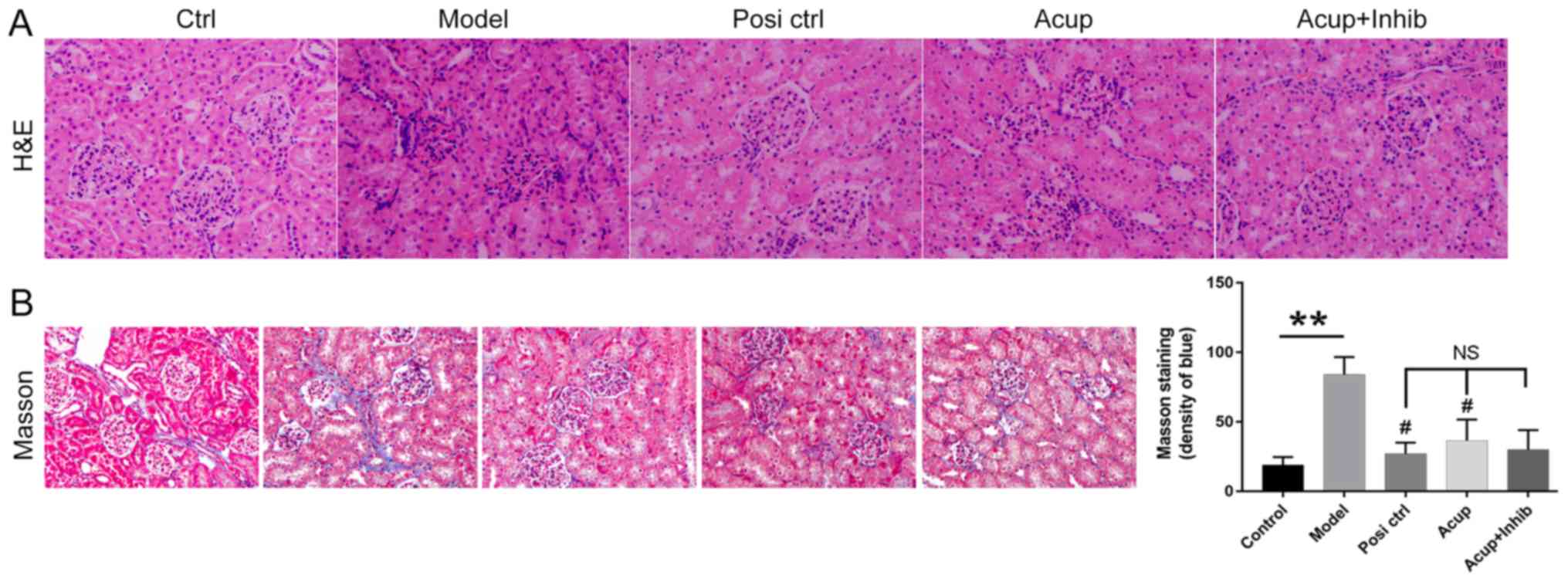 | Figure 2.Acupuncture attenuates pathological
characteristics and fibrogenesis in the kidney. The pathological
characteristics of kidney tissues were examined by (A) H&E and
(B) Masson trichrome staining assays. Original magnification, ×200;
Severe fibrosis (blue color). **P<0.01 vs. Control, #P<0.05
vs. Model. NS, not significant; H&E, hematoxylin and eosin.
Groups: Ctrl, control group, Model, CRF model group; Posi ctrl, a
losartan potassium positive control group; Acup, CRF model
administered acupuncture; Acup+Inhib, CRF model administered
acupuncture and SB 431542. |
Acupuncture downregulates TNF-α, Smad3, ILK and
TGF-β expression, and upregulates eNOS in the CRF model via
the TGF-β/Smad pathway. To explore the mechanism by
which acupuncture affects RIF, New Zealand White rabbits were
assigned to a control group, a CRF model group, a losartan
potassium (Posi) group, an acupuncture (Acup) group, and an
acupuncture+SB 431542 (Acup+inhibitor) group, respectively. As
shown in Fig. 3A and B, the mRNA
(Fig. 3A) and proteins (Fig. 3B) expression levels of TNF-α,
Smad3, ILK and TGF-β expression were significantly upregulated in
the model group when compared with the control group; TNF-α, Smad3,
ILK and TGF-β expression levels were markedly downregulated in the
Posi group and Acup group when compared with the model group; TNF-α
and Smad3 expression were significantly decreased in the
Acup+inhibitor group when compared with the Acup group (P<0.05
or P<0.01), while the levels of ILK and TGF-β expression did not
significantly change. In addition, we also found that eNOS
expression was markedly downregulated in the model group when
compared with the control group; eNOS expression was dramatically
upregulated in the Posi group and Acup group when compared with the
model group; and eNOS expression was significantly increased in the
Acup+inhibitor group when compared with the Acup group (Fig. 3A and B).
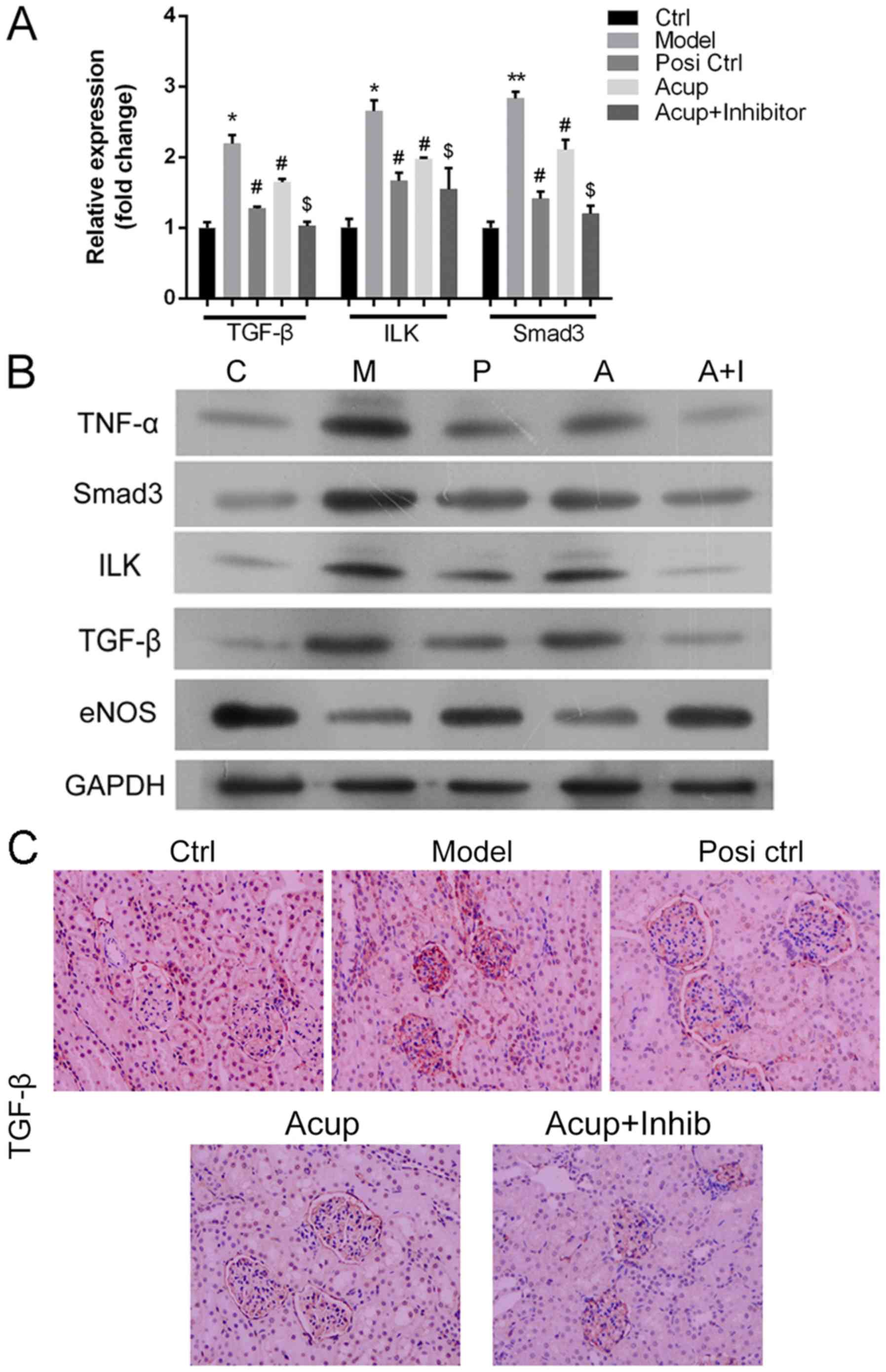 | Figure 3.Acupuncture downregulates TNF-α,
Smad3, ILK and TGF-β expression and upregulates eNOS in the CRF
model via the TGF-β/Smad pathway. (A) qRT-PCR assays were performed
to measure the expression of mRNA for TGF-β, ILK and Smad3 in all
groups. *P<0.05, **P<0.01 vs. the control group; #P<0.05
vs. the model group; $P<0.05 vs. the Acup group. (B) Western
blot assays were used to analyze the levels of TNF-α, Smad3, ILK,
TGF-β and eNOS protein expression in all groups. GAPDH served as an
internal reference. (C) TGF-β expression was detected by an IHC
assay. All H&E, Masson trichrome and IHC assays (Original
magnification, ×200). Positive signal (blue color). TNF-α, tumor
necrosis factor-α; ILK, integrin-linked kinase; TGF-β, transforming
growth factor β; eNOS, endothelial nitric oxide synthase; IHC,
immunohistochemistry; H&E, hematoxylin and eosin. Groups: Ctrl
or C, control group, Model or M, CRF model group; Posi ctrl or P, a
losartan potassium positive control group; Acup or A, CRF model
administered acupuncture; Acup+Inhib or A+I, CRF model administered
acupuncture and SB 431542. |
TGF-β expression was detected by an IHC assay, and
the results showed that TGF-β was rarely found in the control
group, but was highly expressed in renal tissues (mainly in injured
tubulointerstitium and renal tubular epithelial cells). It was also
found that TGF-β expression was markedly reduced in the Posi and
Acup groups relative to its expression in the model group.
Furthermore, TGF-β expression was also dramatically reduced in the
Acup+inhibitor group when compared with its expression in the Acup
group (Fig. 3C).
Acupuncture decreases TGF-β, IL-8,
TNF-α and IL-1β expression in the CRF model rabbits via the
TGF-β/Smad pathway
ELISA assays were performed to measure differences
in TGF-β, IL-8, TNF-α and IL-1β concentrations in blood serum. The
results revealed that the concentrations of TGF-β, IL-8, TNF-α and
IL-1β in the model group were significantly increased when compared
with those in the control group. Furthermore, the concentrations of
TGF-β, IL-8, TNF-α and IL-1β in the Posi and Acup groups were
significantly decreased relative to those in the model group.
Finally, the concentrations of TGF-β, IL-8, TNF-α and IL-1β in the
Acup+inhibitor group were significantly decreased relative to those
in the Acup group (P<0.05 or P<0.01; Fig. 4).
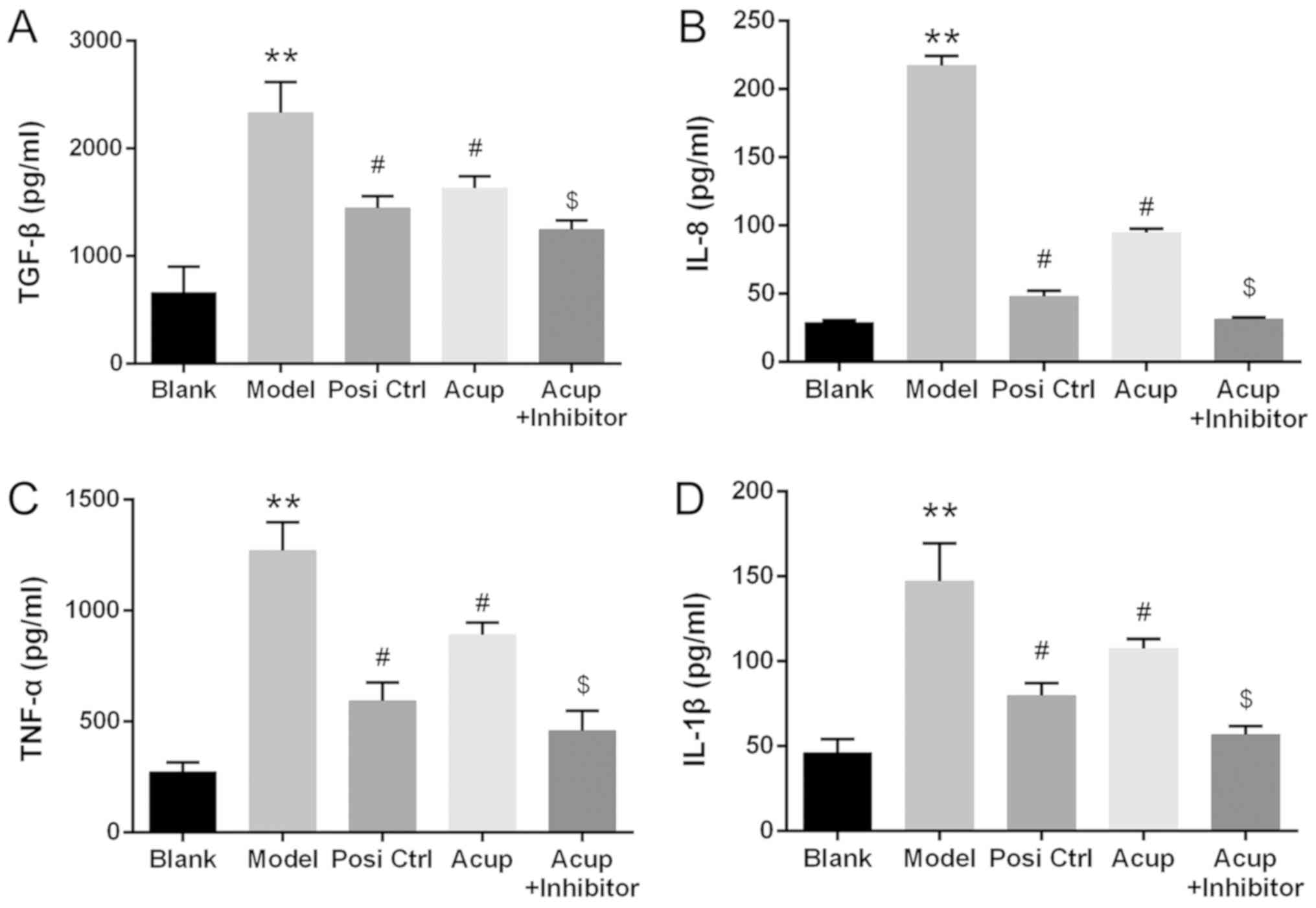 | Figure 4.Acupuncture decreases TGF-β, IL-8,
TNF-α and IL-1β expression in the CRF model via the TGF-β/Smad
pathway. The concentrations of (A) TGF-β, (B) IL-8, (C) TNF-α and
(D) IL-1β in serum samples of the mice were measured by ELISA
assays in all groups. **P<0.01 vs. the control group, #P<0.05
vs. the model group, $P<0.05 vs. the Acup group. TGF-β,
transforming growth factor β; IL-8, interleukin 8; TNF-α, tumor
necrosis factor-α; IL-1β, interleukin-1β; CRF, chronic renal
failure. Groups: Blank, control group, Model, CRF model group; Posi
Ctrl, a losartan potassium positive control group; Acup, CRF model
administered acupuncture; Acup+Inhibitor, CRF model administered
acupuncture and SB 431542. |
Discussion
Renal interstitial fibrosis (RIF) is the main
pathological change that occurs in various types of chronic kidney
disease (CKD) and inevitably progresses to end-stage renal disease
(ESRD) (28). The extent of RIF is
correlated with the progression of renal disease (29). Therefore, it is important to
alleviate any renal injury and its associated RIF. The essence of
renal insufficiency is the associated decrease in the glomerular
filtration rate (GFR) (30). SCr
and BUN levels are good indicators of GFR, and are widely used to
evaluate renal function (31). In
this study, the rabbits in the model group were gavaged with
adenine for 3 weeks, and their body weight was significantly
decreased. Moreover, the urine protein, and SCr and BUN levels in
the model group were significantly higher than those in the control
group. Therefore, it was demonstrated that the renal function of
rabbits in the model group had been damaged, and the CRF model had
been successfully constructed.
Acupuncture has been used as a complementary or
alternative treatment for various diseases. However, to the best of
our knowledge, there have been few reports of clinical trials that
used acupuncture to treat CKD. Research has proven that stimulation
of Zusanli (ST36) by electro-acupuncture (EA) can inhibit a
reduction of endothelial nitric oxide synthase in the blood, reduce
renal artery pressure, and protect kidney function (32). Furthermore, EA exerts an
anti-inflammatory effect by reducing the levels of tumor necrosis
factor-α (TNF-α) (33). Research
has shown that periorbital acupuncture reduced the severity of a
chemically induced renal injury in animals (34). More importantly, acupoint massage
acts as a non-invasive technique that can boost energy levels, and
improve an individual's health and comfort. For example, fatigue
can be improved by massaging Yanglingchuan (GB34), Sanyinjiao (SP6)
and Zusanli (35); sleep
disturbances can be treated by Hand Shenmen (HT7) and Ear Shenmen
(MATF1) (36); acupuncture or
acupressure on Hegu (LI4) can relieve pain and exert
anti-inflammatory effects (37);
acupuncture on Taixi (KI3) can relieve renal disease, cognitive
disorders and impotence (38). In
this study, CRF rabbits were stimulated by acupuncture on Shenshu,
Ming Men and Pishu, and also by Zusanli acupoint and Guanyuan
point; after which, the effect on RIF was examined.
It is generally known that losartan potassium, an
angiotensin II (Ang II) receptor antagonist, can disrupt the
angiotensin system, and thereby reduce proteinuria and delay
chronic renal dysfunction (39).
In this study, losartan potassium was used as a positive control
substance.
The development of RIF occurs in 4 phases:
Modulation of cytokine expression; inflammatory cell infiltration;
cell proliferation and epithelial-mesenchymal transition (EMT); and
the ectopic accumulation of extracellular matrix (ECM) (40–42).
During the development of RIF, EMT is defined as the process by
which renal epithelial cells and fibroblasts become altered to form
myofibroblasts (43). An abnormal
accumulation of ECM is the main cause of RIF (44). In addition, fibroblasts, one of the
main intrinsic cells in the renal interstitium, are the main cells
that secrete ECM during the development of RIF (45,46).
It has been demonstrated that the properties of fibroblasts become
altered during the progression of RIF; these alterations include
phenotypic changes, an abundant expression of α-SMA, increased
fibroblast proliferation, and an accumulation of interstitial ECM
components such as type I collagen and fibronectin (46).
The TGF-β/Smad pathway plays a critical role in
renal fibrosis and also inflammation. Researchers have suggested
the TGF-β/Smad pathway as a potential therapeutic target for
treating chronic kidney diseases (47). TGF-β plays a critical role in the
RIF process by promoting expression of key components of the ECM
(5,48). TGF-β can promote the synthesis of
adhesive proteins, collagen, and the proteoglycans found in
extracellular matrix; it can also attenuate the decrease in
protease synthesis, prevent decomposition of newly synthesized ECM,
disrupt the balance between ECM synthesis and degradation, and
accelerate the development of RIF (48,49).
TGF-β promotes fibrosis mainly via a signal transmitted by the
downstream Smad family; related studies have shown that TGF-β/Smad
signaling pathways play a key role in the pathogenesis of RIF
(50,51). In addition, TGF-β can activate the
mitogen activated protein kinase (MAPK) signal transduction
pathway, which includes c-Jun N-terminal kinase (JNK),
extracellular signal-regulated kinase (ERK), p38, and tumor
necrosis factor-α (TNF-α) (52).
Integrins can interact with various cytoplasmic
proteins including integrin-linked kinase (ILK), which transmits
signals from the extracellular matrix to cells. ILK serves as a
scaffolding protein, and helps to regulate functions involved in
tissue regeneration, such as cell survival, actin cytoskeleton
dynamics and cell migration (53).
A previous study demonstrated that ILK expression is required for
TGF-β induction (54).
Endothelial nitric oxide synthase (eNOS) is a key
enzyme involved in NO synthesis, which helps to regulate vascular
physiology (55). Studies have
confirmed that eNOS plays key roles in cell proliferation and
migration (56). Previous research
has shown that TGF-β partially regulates eNOS expression by
promoting the binding of a nuclear factor to the eNOS promoter
region (57). Another study
revealed that TGF-β regulates eNOS which can be explained by the
direct interaction between the eNOS promoter region and Smad2
(58). Moreover, the inflammation
process can also be mediated by eNOS signaling (59).
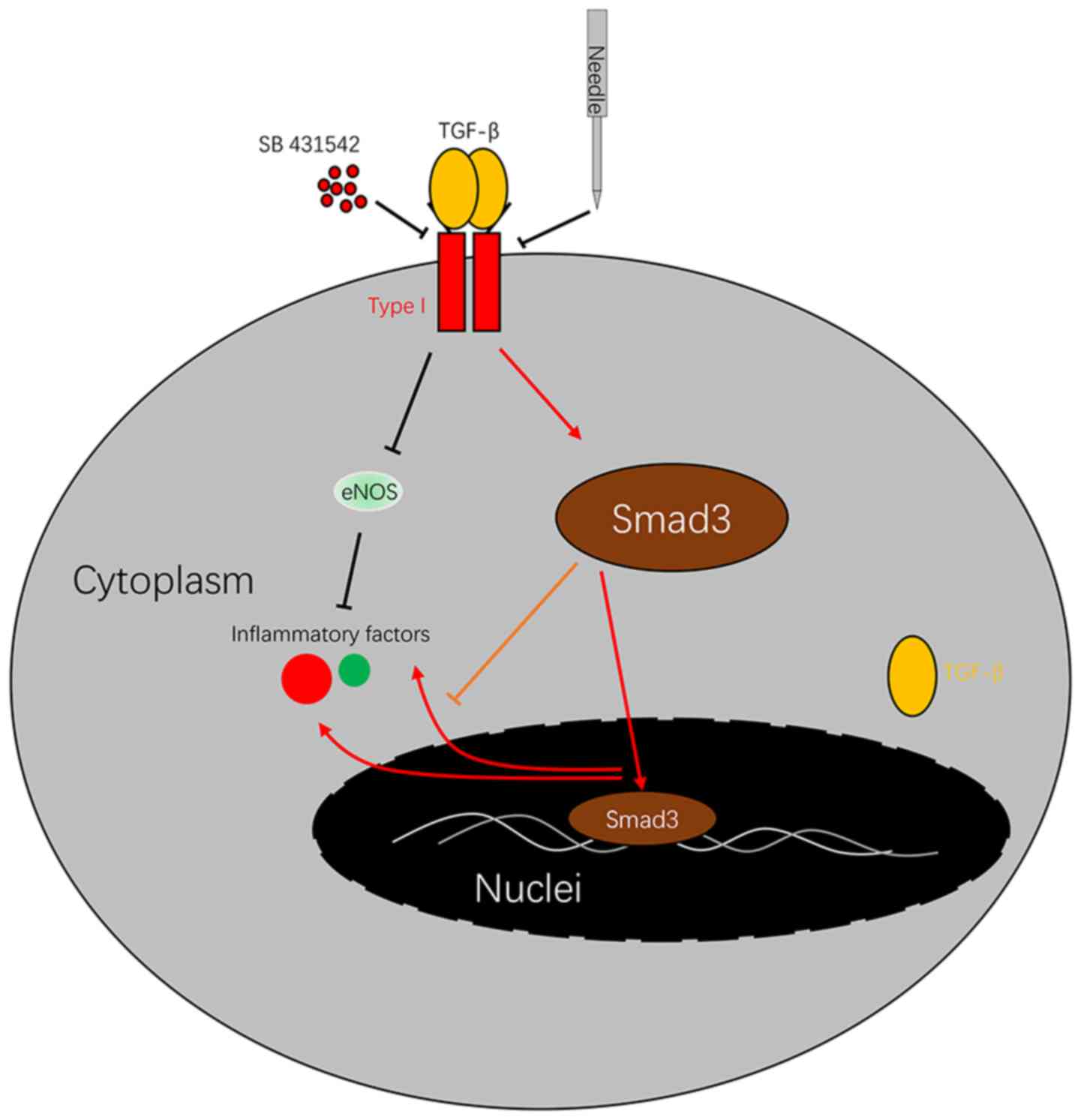
In the present study, it was demonstrated that
acupuncture downregulated TNF-α, Smad3, ILK and TGF-β expression,
and upregulated eNOS expression in a CRF model by affecting the
TGF-β/Smad pathway. SB 431542, a TGF-β inhibitor can block TGF-β
and attenuate the release of inflammatory factors (Fig. 5). In addition, it was also shown
that acupuncture decreased the levels of cytokines (IL-8, TNF-α and
IL-1β) in a CRF model via the TGF-β/Smad pathway, suggesting that
acupuncture can be used as an anti-inflammatory therapy.
In conclusion, a rabbit model of CRF was utilized to
demonstrate that acupuncture can regulate TGF-β-related pathways
such as TNF-α, Smad3, ILK and eNOS. Moreover, acupuncture decreased
the levels of inflammation-associated cytokines, and attenuated RIF
via the TGF-β/Smad pathway. These findings suggest that acupuncture
can effectively reduce the typical kidney features of CRF, and
promote the recovery of renal function by inhibiting the TGF-β/Smad
pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation (grant no. 81660815).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZZ and PDH completed the experimental design. ZZ,
PDH, and YWJ performed most of the experiments with assistance from
YZ. ZZ and PDH collected the data, and YZ and MSZ completed the
data analysis. ZZ drafted the manuscript and MSZ revised it. All
authors approved the manuscript before submission.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the recommendations provided in Guidelines for the Care and Use of
Laboratory Animals, and the study protocol was approved by the
Animal Research Committee of Yunnan College of Traditional Chinese
Medicine (Cumming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alsahli M and Gerich JE: Hypoglycemia,
chronic kidney disease, and diabetes mellitus. Mayo Clin Proc.
89:1564–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: Global dimension and perspectives. Lancet. 382:260–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levey AS and Coresh J: Chronic kidney
disease. Lancet. 379:165–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hill NR, Fatoba ST, Oke JL, Hirst JA,
O'Callaghan CA, Lasserson DS and Hobbs FD: Global prevalence of
chronic kidney disease-a systematic review and meta-analysis. PLoS
One. 11:e01587652016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garber SL, Mirochnik Y, Brecklin CS,
Unemori EN, Singh AK, Slobodskoy L, Grove BH, Arruda JA and Dunea
G: Relaxin decreases renal interstitial fibrosis and slows
progression of renal disease. Kidney Int. 59:876–882. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lovisa S, LeBleu VS, Tampe B, Sugimoto H,
Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC,
Pentcheva-Hoang T, et al: Epithelial-to-mesenchymal transition
induces cell cycle arrest and parenchymal damage in renal fibrosis.
Nat Med. 21:998–1009. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie XS, Zuo C, Zhang ZY, Liu HC, Feng SG,
Zhang CL, Yuan W and Fan JM: Investigate the effects of compound
radix notoginseng on renal interstitial fibrosis and
kidney-targeting treatment. Sichuan Da Xue Xue Bao Yi Xue Ban.
43:28–33. 2012.(In Chinese). PubMed/NCBI
|
|
9
|
Naito Y, Fujii A, Sawada H, Oboshi M,
Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Hirotani S and Masuyama
T: Association between renal iron accumulation and renal
interstitial fibrosis in a rat model of chronic kidney disease.
Hypertens Res. 38:463–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menn-Josephy H, Lee CS, Nolin A, Christov
M, Rybin DV, Weinberg JM, Henderson J, Bonegio R and Havasi A:
Renal interstitial fibrosis: An imperfect predictor of kidney
disease progression in some patient cohorts. Am J Nephrol.
44:289–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lovisa S, Zeisberg M and Kalluri R:
Partial epithelial-to-mesenchymal transition and other new
mechanisms of kidney fibrosis. Trends Endocrinol Metab. 27:681–695.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He W, Dai C, Li Y, Zeng G, Monga SP and
Liu Y: Wnt/beta-catenin signaling promotes renal interstitial
fibrosis. J Am Soc Nephrol. 20:765–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omori H, Kawada N, Inoue K, Ueda Y,
Yamamoto R, Matsui I, Kaimori J, Takabatake Y, Moriyama T, Isaka Y
and Rakugi H: Use of xanthine oxidase inhibitor febuxostat inhibits
renal interstitial inflammation and fibrosis in unilateral ureteral
obstructive nephropathy. Clin Exp Nephrol. 16:549–556. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Irita J, Okura T, Jotoku M, Nagao T,
Enomoto D, Kurata M, Desilva VR, Miyoshi K, Matsui Y, Uede T, et
al: Osteopontin deficiency protects against aldosterone-induced
inflammation, oxidative stress, and interstitial fibrosis in the
kidney. Am J Physiol Renal Physiol. 301:F833–F844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tampe B and Zeisberg M: Evidence for the
involvement of epigenetics in the progression of renal
fibrogenesis. Nephrol Dial Transplant. 29 (Suppl 1):i1–i8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng XM, Tang PM, Li J and Lan HY:
TGF-β/Smad signaling in renal fibrosis. Front Physiol. 6:822015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macias MJ, Martin-Malpartida P and
Massagué J: Structural determinants of Smad function in TGF-beta
signaling. Trends Biochem Sci. 40:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwangbo C, Tae N, Lee S, Kim O, Park OK,
Kim J, Kwon SH and Lee JH: Syntenin regulates TGF-β1-induced Smad
activation and the epithelial-to-mesenchymal transition by
inhibiting caveolin-mediated TGF-β type I receptor internalization.
Oncogene. 35:389–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang JS, Liu C and Derynck R: New
regulatory mechanisms of TGF-beta receptor function. Trends Cell
Biol. 19:385–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhuang Y, Xing JJ, Li J, Zeng BY and Liang
FR: History of acupuncture research. Int Rev Neurobiol. 111:1–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lan L, Zeng F, Liu GJ, Ying L, Wu X, Liu M
and Liang FR: Acupuncture for functional dyspepsia. Cochrane
Database Syst Rev. CD0084872014.PubMed/NCBI
|
|
24
|
Haddad NE and Palesh O: Acupuncture in the
treatment of cancer-related psychological symptoms. Integr Cancer
Ther. 13:371–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amezaga Urruela M and Suarez-Almazor ME:
Acupuncture in the treatment of rheumatic diseases. Curr Rheumatol
Rep. 14:589–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Li M, Li X, Zhang M, Zhao Y, Ren W,
Cheng J and Wang X: Hyperbaric oxygen inhibits venous neointimal
hyperplasia following arteriovenous fistulization. Int J Mol Med.
39:1299–1306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chawla LS, Amdur RL, Amodeo S, Kimmel PL
and Palant CE: The severity of acute kidney injury predicts
progression to chronic kidney disease. Kidney Int. 79:1361–1369.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muller GA, Markovic-Lipkovski J and
Rodemann HP: The progression of renal diseases: On the pathogenesis
of renal interstitial fibrosis. Klin Wochenschr. 69:576–586. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mian AN and Schwartz GJ: Measurement and
estimation of glomerular filtration rate in children. Adv Chronic
Kidney Dis. 24:348–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hall JA, Yerramilli M, Obare E, Yerramilli
M, Yu S and Jewell DE: Comparison of serum concentrations of
symmetric dimethylarginine and creatinine as kidney function
biomarkers in healthy geriatric cats fed reduced protein foods
enriched with fish oil, L-carnitine, and medium-chain
triglycerides. Vet J. 202:588–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DD, Pica AM, Durán RG and Durán WN:
Acupuncture reduces experimental renovascular hypertension through
mechanisms involving nitric oxide synthases. Microcirculation.
13:577–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang CL, Tsai PS, Wang TY, Yan LP, Xu HZ
and Huang CJ: Acupuncture stimulation of ST36 (Zusanli) attenuates
acute renal but not hepatic injury in lipopolysaccharide-stimulated
rats. Anesth Analg. 104:646–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Song KH, You MJ, Son DS, Cho SW and
Kim DH: The effect of oculo-acupuncture on recovery from ethylene
glycol-induced acute renal injury in dogs. Am J Chin Med.
35:241–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsay SL: Acupressure and fatigue in
patients with end-stage renal disease-a randomized controlled
trial. Int J Nurs Stud. 41:99–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bender BG, Leung SB and Leung DY:
Actigraphy assessment of sleep disturbance in patients with atopic
dermatitis: An objective life quality measure. J Allergy Clin
Immunol. 111:598–602. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang SP, Yip TP and Li QS: Acupuncture
treatment for plantar fasciitis: A randomized controlled trial with
six months follow-up. Evid Based Complement Alternat Med.
2011:1541082011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen S, Xu M, Li H, Liang J, Yin L, Liu X,
Jia X, Zhu F, Wang D, Shi X and Zhao L: Acupuncture at the Taixi
(KI3) acupoint activates cerebral neurons in elderly patients with
mild cognitive impairment. Neural Regen Res. 9:1163–1168. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li P, Chen YZ, Lin HL, Ni ZH, Zhan YL,
Wang R, Yang HT, Fang JA, Wang NS, Li WG, et al: Abelmoschus
manihot-a traditional Chinese medicine versus losartan potassium
for treating IgA nephropathy: Study protocol for a randomized
controlled trial. Trials. 18:1702017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao Q, Harris DC and Wang Y: Macrophages
in kidney injury, inflammation, and fibrosis. Physiology
(Bethesda). 30:183–194. 2015.PubMed/NCBI
|
|
41
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
Inflammatory processes in renal fibrosis. Nat Rev Nephrol.
10:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eddy AA: Overview of the cellular and
molecular basis of kidney fibrosis. Kidney Int Suppl (2011). 4:2–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grande MT, Sánchez-Laorden B, López-Blau
C, De Frutos CA, Boutet A, Arévalo M, Rowe RG, Weiss SJ,
López-Novoa JM and Nieto MA: Snail1-induced partial
epithelial-to-mesenchymal transition drives renal fibrosis in mice
and can be targeted to reverse established disease. Nat Med.
21:989–997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Genovese F, Manresa AA, Leeming DJ,
Karsdal MA and Boor P: The extracellular matrix in the kidney: A
source of novel non-invasive biomarkers of kidney fibrosis?
Fibrogenesis Tissue Repair. 7:42014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Urban ML, Manenti L and Vaglio A:
Fibrosis-a common pathway to organ injury and failure. N Engl J
Med. 373:95–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rockey DC, Bell PD and Hill JA: Fibrosis-a
common pathway to organ injury and failure. N Engl J Med.
373:962015.PubMed/NCBI
|
|
47
|
Lan HY and Chung AC: TGF-β/Smad signaling
in kidney disease. Semin Nephrol. 32:236–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neelisetty S, Alford C, Reynolds K,
Woodbury L, Nlandu-Khodo S, Yang H, Fogo AB, Hao CM, Harris RC,
Zent R and Gewin L: Renal fibrosis is not reduced by blocking
transforming growth factor-β signaling in matrix-producing
interstitial cells. Kidney Int. 88:503–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shen N, Lin H, Wu T, Wang D, Wang W, Xie
H, Zhang J and Feng Z: Inhibition of TGF-β1-receptor
posttranslational core fucosylation attenuates rat renal
interstitial fibrosis. Kidney Int. 84:64–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li J, Zhang Z, Wang D, Wang Y, Li Y and Wu
G: TGF-beta 1/Smads signaling stimulates renal interstitial
fibrosis in experimental AAN. J Recept Signal Transduct Res.
29:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ning XH, Ge XF, Cui Y and An HX:
Ulinastatin inhibits unilateral ureteral obstruction-induced renal
interstitial fibrosis in rats via transforming growth factor β
(TGF-β)/Smad signalling pathways. Int Immunopharmacol. 15:406–413.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He T, Bai X, Yang L, Fan L, Li Y, Su L,
Gao J, Han S and Hu D: Loureirin B inhibits hypertrophic scar
formation via inhibition of the TGF-β1-ERK/JNK pathway. Cell
Physiol Biochem. 37:666–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wickström SA, Lange A, Montanez E and
Fässler R: The ILK/PINCH/parvin complex: The kinase is dead, long
live the pseudokinase! EMBO J. 29:281–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vi L, de Lasa C, DiGuglielmo GM and
Dagnino L: Integrin-linked kinase is required for TGF-β1 induction
of dermal myofibroblast differentiation. J Invest Dermatol.
131:586–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Su Y: Regulation of endothelial nitric
oxide synthase activity by protein-protein interaction. Curr Pharm
Des. 20:3514–3520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
García C, Nuñez-Anita RE, Thebault S,
Arredondo Zamarripa D, Jeziorsky MC, Martínez de la Escalera G and
Clapp C: Requirement of phosphorylatable endothelial nitric oxide
synthase at Ser-1177 for vasoinhibin-mediated inhibition of
endothelial cell migration and proliferation in vitro. Endocrine.
45:263–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Inoue N, Venema RC, Sayegh HS, Ohara Y,
Murphy TJ and Harrison DG: Molecular regulation of the bovine
endothelial cell nitric oxide synthase by transforming growth
factor-beta 1. Arterioscler Thromb Vasc Biol. 15:1255–1261. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saura M, Zaragoza C, Cao W, Bao C,
Rodríguez-Puyol M, Rodríguez-Puyol D and Lowenstein CJ: Smad2
mediates transforming growth factor-beta induction of endothelial
nitric oxide synthase expression. Circ Res. 91:806–813. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Paterniti I, Esposito E, Mazzon E,
Bramanti P and Cuzzocrea S: Evidence for the role of
PI(3)-kinase-AKT-eNOS signalling pathway in secondary inflammatory
process after spinal cord compression injury in mice. Eur J
Neurosci. 33:1411–1420. 2011. View Article : Google Scholar : PubMed/NCBI
|