Introduction
Medullary thyroid carcinoma (MTC) arises from the
calcitonin-producing parafollicular C cells of the thyroid and was
first described by Hazard in 1959 (1). MTC comprises 5–10% of all primary
thyroid malignancies (2,3). MTC is mainly sporadic (termed SMTC)
and only 20–30% of cases are hereditary MTC (HMTC) (4–6).
Activating mutations in the RET proto-oncogene are responsible for
medullary thyroid carcinoma, and these mutations are believed to be
associated with a more persistent disease and a lower overall
survival (7–9). However, to date, the molecular
mechanisms of MTC carcinogenesis remain unclear.
MicroRNAs (miRNAs or miRs) are a large subgroup of
non-coding RNAs, 18–25 nucleotides in length, that are evolutionary
conserved. These molecules control post-transcriptional gene
expression through the inhibition of mRNA translation or induction
of its degradation (10) miRNAs
can act as oncogenes, or tumor suppressor genes and can be used as
diagnostic and predictive biomarkers, and even for the treatment of
diseases, including MTC (11,12).
Shabani et al reported that a high expression of hsa-miR-144
and hsa-miR-183miR-34a could be considered as biomarkers of MTC
(13). Furthermore, in a previous
study, hsa-miR-375 was upregulated and hsa-miR-9* was downregulated
in SMTC vs. HMTC, and the overexpression of hsa-miR-183 and
hsa-miR-375 in MTC predicted lateral lymph node metastases
(14).
Although advances have been made in understanding
the mechanisms of MTC, studies on these are limited and further
confirmation is required. This study aimed to identify biomarkers
by analyzing differentially expressed miRNAs (DEMs) and
differentially expressed genes (DEGs) between MTC and normal
thyroid tissues. Subsequently, Gene Ontology (GO) and pathway
enrichment analysis, protein-protein interaction (PPI) and
transcription factor (TF)-DEMs-genes networks were conducted to
identify key miRNAs and elucidate the potential molecular
mechanisms of MTC.
Materials and methods
Data source
The original datasets comparing the gene expression
profiles between MTC and normal thyroid tissue were downloaded from
the NCBI GEO databases. The accession numbers were GSE97070
(15), GSE40807 (16) and GSE27155 (17,18).
The microarray data of GSE97070 were based on GPL18402
(Agilent-046064 Unrestricted_Human_miRNA_V19.0_Microarray), and
included 8 MTC samples, 9 lymph node metastasis samples and 3
normal samples. GSE40807 was based on GPL18227 (Agilent-019118
Human miRNA Microarray 2.0 G4470B), which consisted of 40 MTC
samples and 40 normal samples. The platform of GSE27155 was GPL96,
[HG-U133A] Affymetrix Human Genome U133A Array, including 96
samples with 2 MTC and 4 normal samples. Platform and series matrix
file(s) were downloaded as TXT files.
Data pre-processing and DEM
analysis
A comparison between the 2 sample groups, MTC and
lymph node metastasis vs. normal thyroid tissues, was performed in
each GEO dataset to identify DEMs and DEGs. The R software package
was used to process the downloaded files and to convert and reject
the unqualified data, and the limma R package was then used to
identify DEMs and DEGs. Before we decided to conduct this study, we
carried out research on bioinformatics analysis, and found that the
‘false discovery rate (FDR) <0.05, |log2FC|>1’ was a common
criteria for screening DEMs or DEGs (19–21).
Thus, in this study, samples with the criteria mentioned above were
considered DEMs or DEGs. The TXT results were preserved for
subsequent analysis. Heat maps of DEMs and DEGs were generated
using FunRich 3.1.3 software.
Identification of miRNA targets
starBase (http://starbase.sysu.edu.cn/index.php) provides
certain miRNA-target regulatory association pairs, which are
verified by experiments and predicted by 7 programs, including
microT, miRanda, miRmap, PITA, RNA22, PicTar and TargetScan
(22). StarBase v3.0 identifies
>1.1 million miRNA-ncRNA, 2.5 million miRNA-mRNA, 2.1 million
RBP-RNA and 1.5 million RNA-RNA interactions from multi-dimensional
sequencing data. In this study, miRNA-target gene regulatory
association pairs were verified according to the following
standards: CLIP Data (low stringency), Degradome Data (low
stringency), Pan-cancer (1 cancer type) and Program Number (3
programs).
GO and kyoto encyclopedia of genes and
genomes (KEGG) pathway enrichment analyses of target genes
GO is a common method for annotating genes, gene
products and sequences to underlying biological phenomena; KEGG is
an integrated database resource for the biological interpretation
of genome sequences and other high-throughput data (23). Metascape (http://metascape.org) is an online program that aims
to develop a set of reliable, productive and intuitive tools that
help biomedical research community to analysis gene/protein lists
and make better data-driven decisions (24). In this study, Metascape was used to
perform GO and KEGG pathway analysis on target genes of DEMs. In
addition, GO terms consisted of 3 aspects: Biological process (BP),
cellular component (CC) and molecular function (MF).
PPI network construction and analysis
of modules
NetworkAnalyst (https://www.networkanalyst.ca/) is a series of
web-based tools for statistical meta-analysis, visual data mining
and data integration, through the rapid generation of biological
networks. It supports the meta-analysis of gene lists, and data
integration is achieved through robust statistical procedures and
subsequently visually examined within PPI networks (25,26).
In this study, STRING was selected as a PPI database. To access
more objective and reliable results, this study restricted the
sources requiring experimental evidence and the cut-off score was
set at a high confidence (900). Nodes with more degrees were
considered as hub genes and may serve as core proteins or key
candidates with important physiological regulatory functions. The
pathway enrichment analysis of genes in the modules was performed,
and P<0.05 was considered to indicate a statistically
significant difference.
TF-DEMs-target gene and DEGs
regulatory network construction
TransmiR (http://www.cuilab.cn/transmir) is a database for
TF-miR regulations, through which regulatory associations between
TFs and miRNAs can be identified. To date, TransmiR v2.0 contains
3,730 literature-curated TF-miRNA regulations from 1,349
publications and 1,785,998 TF-miRNA regulations derived from
ChIP-seq evidence (27,28). This study inputted the overlapped
DEMs into the database to examine the regulatory association pairs
between TFs and DEMs. The inclusion criteria of evidence were
supported by high-throughput experiments from literature.
Based on the data this study obtained,
TF-DEMs-target gene and DEGs regulatory network was constructed and
visualized by Cytoscape 3.6.1 software to show the overlapped TFs,
DEMs, target genes and DEGs. Therefore, these TFs, DEGs, DEMs and
target genes may play a potential role in the pathogenesis and
treatment of MTC.
Results
Microarray data information and
identification of DEMs and DEGs
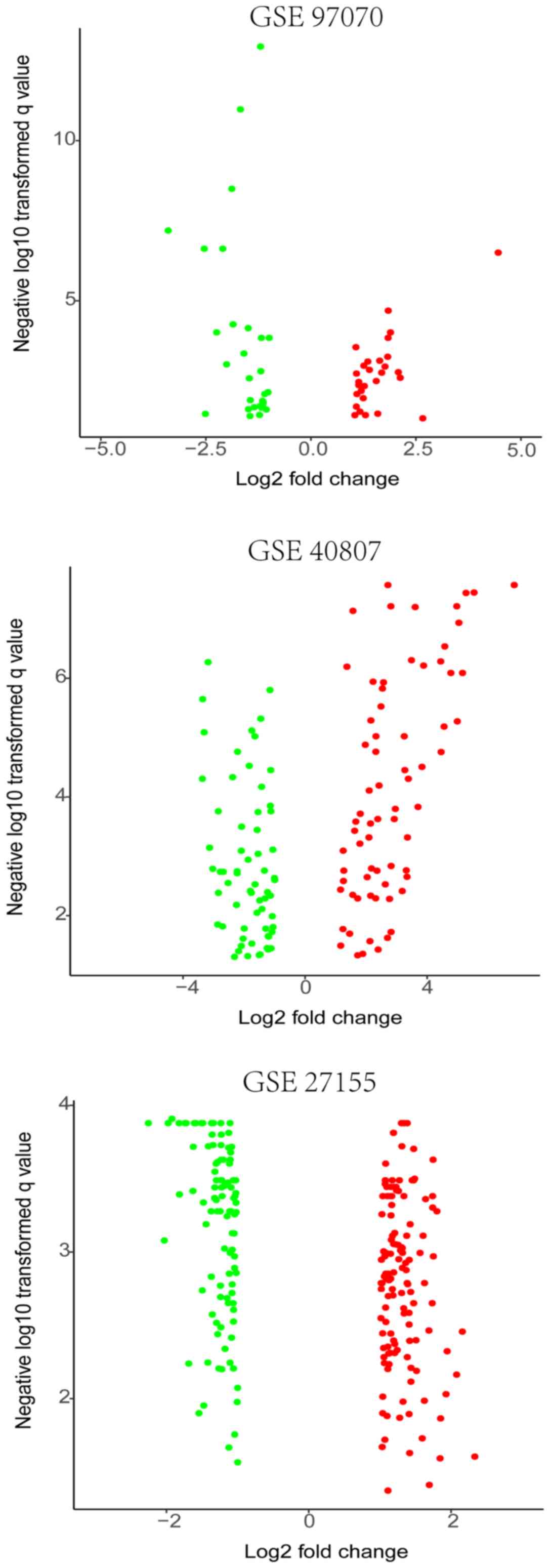
The MTC expression microarray datasets GSE97070,
GSE40807 and GSE27155 were standardized. The datasets were
subsequently screened using the limma package (FDR<0.05,
|log2FC|>1); 57 DEMs were obtained in GSE97070. Among these, 29
upregulated and 28 downregulated DEMs were identified. Overall, 134
DEMs were screened from the GSE40807 dataset, including 70
upregulated genes and 64 downregulated genes. Among these,
hsa-miR-375, hsa-miR-127-3p and hsa-miR-429 were significantly
upregulated in both the GSE97070 and GSE40807 datasets, whereas the
expression of hsa-miR-199b-5p and hsa-miR-199a-3p was
downregulated. In addition, 235 DEGs were screened from the
GSE27155 dataset, including 135 upregulated genes and 100
downregulated genes (Fig. 1 and
Table SI). Heatmaps were
generated based on the expression levels, where each column
represented a biological sample and each row in the heat map
represented a DEM or DEG. The color indicated the relative
expression levels of miRNA in tissue specimens (Figs. S1-S3).
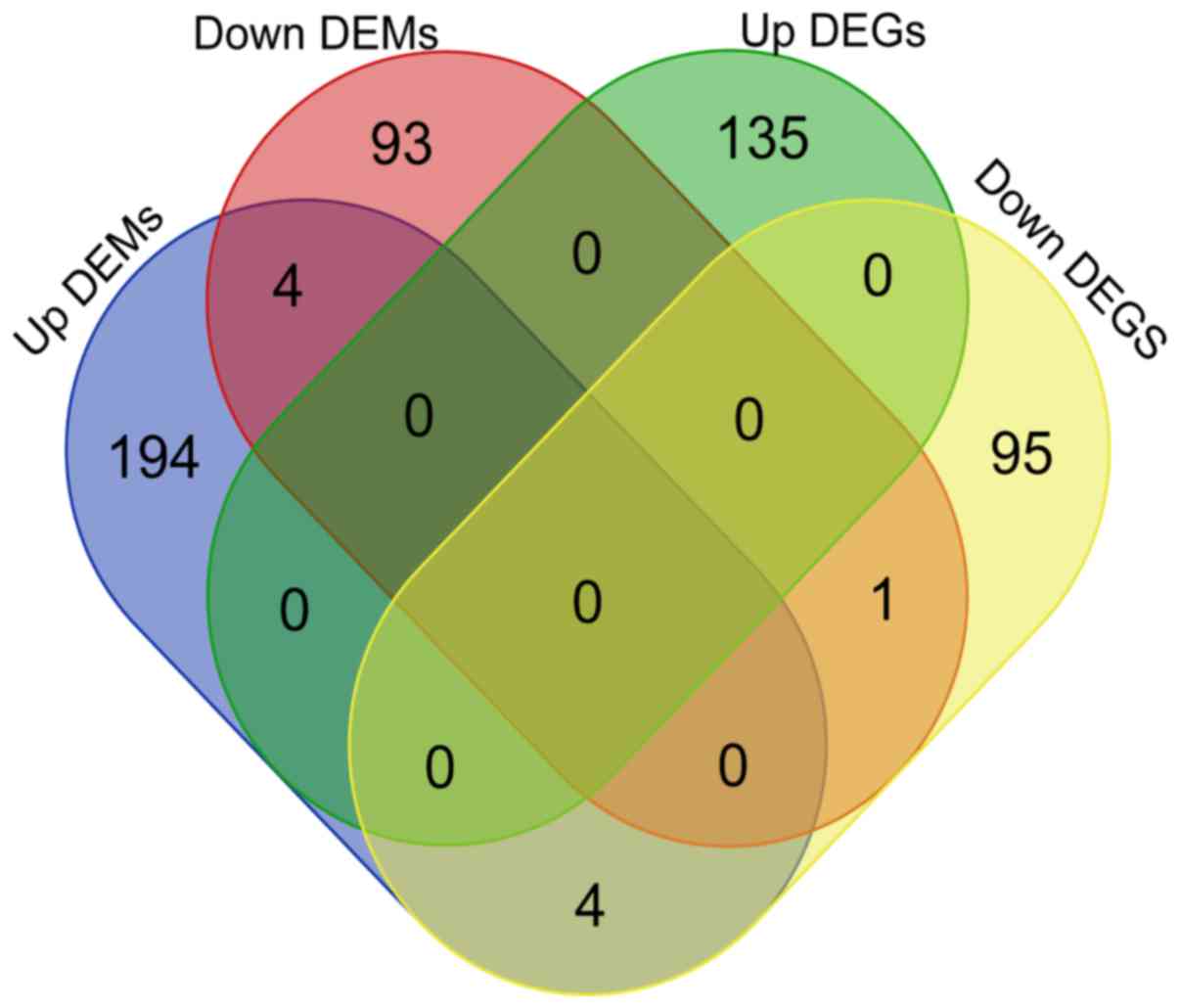
Target genes of DEMs
The target genes of DEMs were identified according
to the standards described above. For the 5 commonly altered
miRNAs, a total of 300 target genes were obtained, including 202
genes of upregulated DEMs and 98 genes of downregulated DEMs
(Table SII).
SNTB2 and MED13 were predicted as the potential
targets of hsa-miR-375 and hsa-miR-429. A total of 4 genes were the
potential targets of hsa-miR-429 and hsa-miR-199a-3p, including
SCD, CFL2, IREB2 and DTNA. In addition, 5 genes, including CITED2,
DUSP1, FHL1, LDHB and C1orf115, of 100 downregulated DEGs were
potentially targeted by DEMs (Figs.
2 and 3).
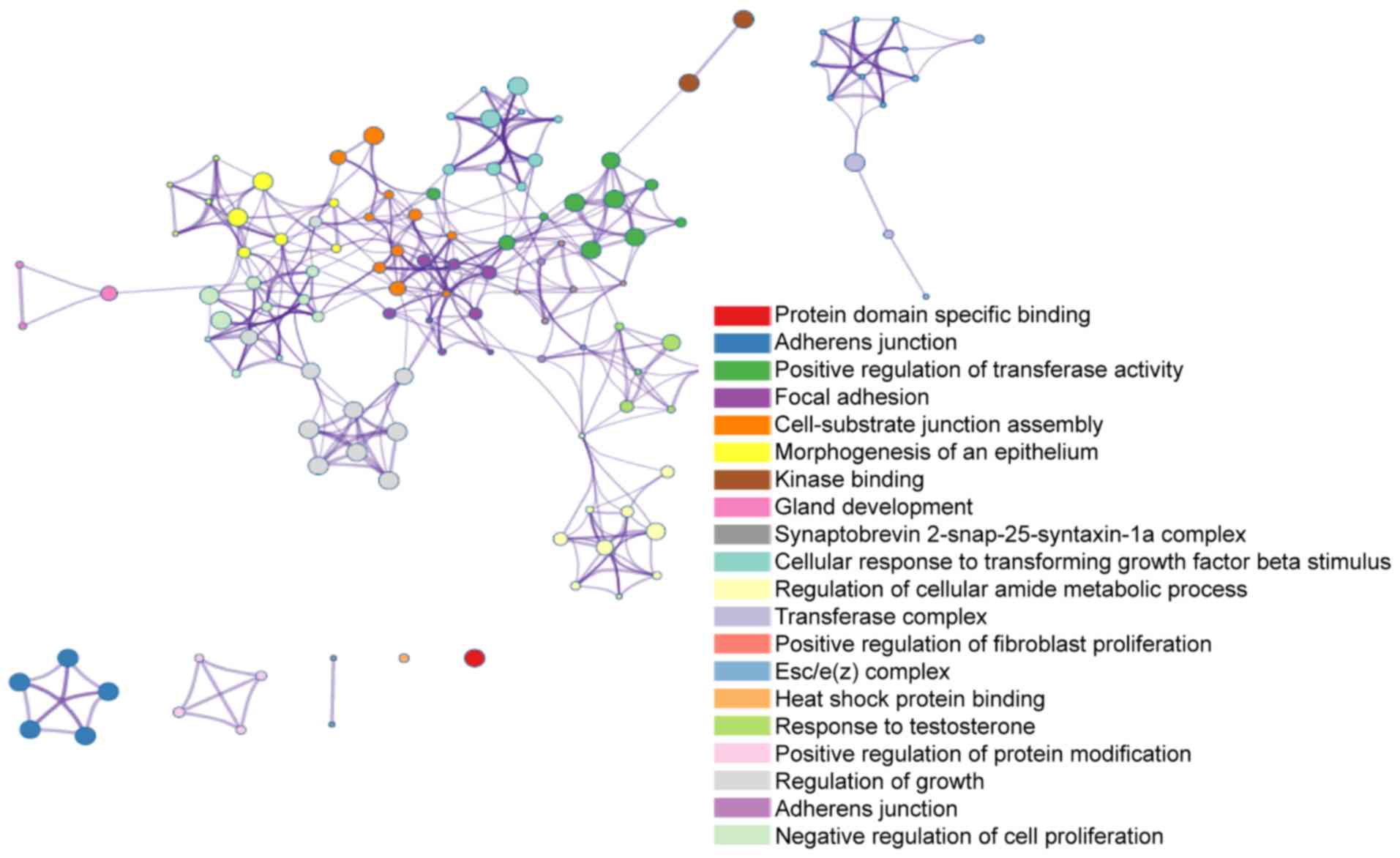
Significant functions and pathway
enrichment analysis
Metascape was used to analyze the downstream target
genes of the five common DEMs. For each given gene list, a pathway
and process enrichment analysis was carried out with the following
ontology sources: KEGG pathway, GO BP, GO CC and GO MF. All genes
in the genome were used as the enrichment background. Terms with a
P-value <0.01, a minimum count of 3, and an enrichment factor
>1.5 were collected and grouped into clusters based on their
membership similarities. The enrichment factor is the ratio between
the observed counts and the counts expected by chance. For GO term
enrichment analysis, the target genes of upregulated DEMs were
mainly enriched in adherens junction, anchoring junction and
cell-substrate junction assembly, while the target genes of
downregulated DEMs were mainly enriched in non-canonical Wnt
signaling pathway, heat shock protein binding and regulation of
small molecule metabolic process. KEGG analysis revealed that the
target genes of upregulated DEMs were mostly enriched in focal
adhesion, regulation of actin cytoskeleton and adherens junction.
The target genes of downregulated DEMs were enriched in RNA
transport, the lysosome and mRNA surveillance pathway (Table I and Fig. 4).
 | Table I.Results of top 10 GO terms and top 3
KEGG pathway analysis. |
Table I.
Results of top 10 GO terms and top 3
KEGG pathway analysis.
| Category | Term | Description | Gene counts | LogP |
|---|
| Target genes of
upregulated DEMs |
|
|
|
|
| GO:0005912 | CC | Adherens
junction | 20 | −7.110 |
| GO:0070161 | CC | Anchoring
junction | 20 | −6.902 |
| GO:0007044 | CC | Cell-substrate
junction assembly | 9 | −6.880 |
| GO:0051347 | BP | Positive regulation
of transferase activity | 21 | −6.251 |
| GO:0005925 | CC | Focal adhesion | 16 | −6.123 |
| GO:0005924 | CC | Cell-substrate
adherens junction | 16 | −6.080 |
| GO:0030055 | CC | Cell-substrate
junction | 16 | −6.024 |
| GO:0019900 | MF | Kinase binding | 22 | −5.909 |
| GO:0034329 | BP | Cell junction
assembly | 12 | −5.872 |
| GO:0019904 | MF | Protein domain
specific binding | 21 | −5.842 |
| hsa04510 | KEGG | Focal adhesion | 12 | −6.581 |
| hsa04810 | KEGG | Regulation of actin
cytoskeleton | 10 | −4.637 |
| hsa04520 | KEGG | Adherens
junction | 6 | −4.333 |
| Target genes of
downregulated DEMs |
|
|
|
|
| GO:0035567 | BP | Non-canonical Wnt
signaling pathway | 5 | −4.074 |
| GO:0031072 | MF | Heat shock protein
binding | 5 | −3.724 |
| GO:0062012 | BP | Regulation of small
Molecule metabolic process | 7 | −3.187 |
| GO:0001738 | BP | Morphogenesis of a
polarized epithelium | 4 | −3.151 |
| GO:0016055 | BP | Wnt signaling
pathway | 8 | −3.042 |
| GO:0198738 | BP | cell-cell signaling
by wnt | 8 | −3.030 |
| GO:0019904 | MF | Protein domain
specific binding | 10 | −3.026 |
| GO:0005793 | CC | Endoplasmic
reticulum-Golgi intermediate compartment | 4 | −2.684 |
| GO:0060071 | BP | Wnt signaling
pathway, planar cell polarity pathway | 3 | −2.599 |
| GO:0090175 | BP | Regulation of
establishment of planar polarity | 3 | −2.560 |
| hsa03013 | KEGG | RNA transport | 5 | −3.037 |
| hsa04142 | KEGG | Lysosome | 4 | −2.684 |
| hsa03015 | KEGG | mRNA surveillance
pathway | 3 | −2.132 |
PPI network construction and analysis
of modules
The PPI network was constructed and visualized by
NetworkAnalyst. The minimum network was used to keep seed proteins,
as well as essential non-seed proteins, that were suitable for
simplifying a dense network to study key associations. A zero-order
network was subsequently used to keep only seed proteins that
directly interact with each other and to visualize modules. Degree
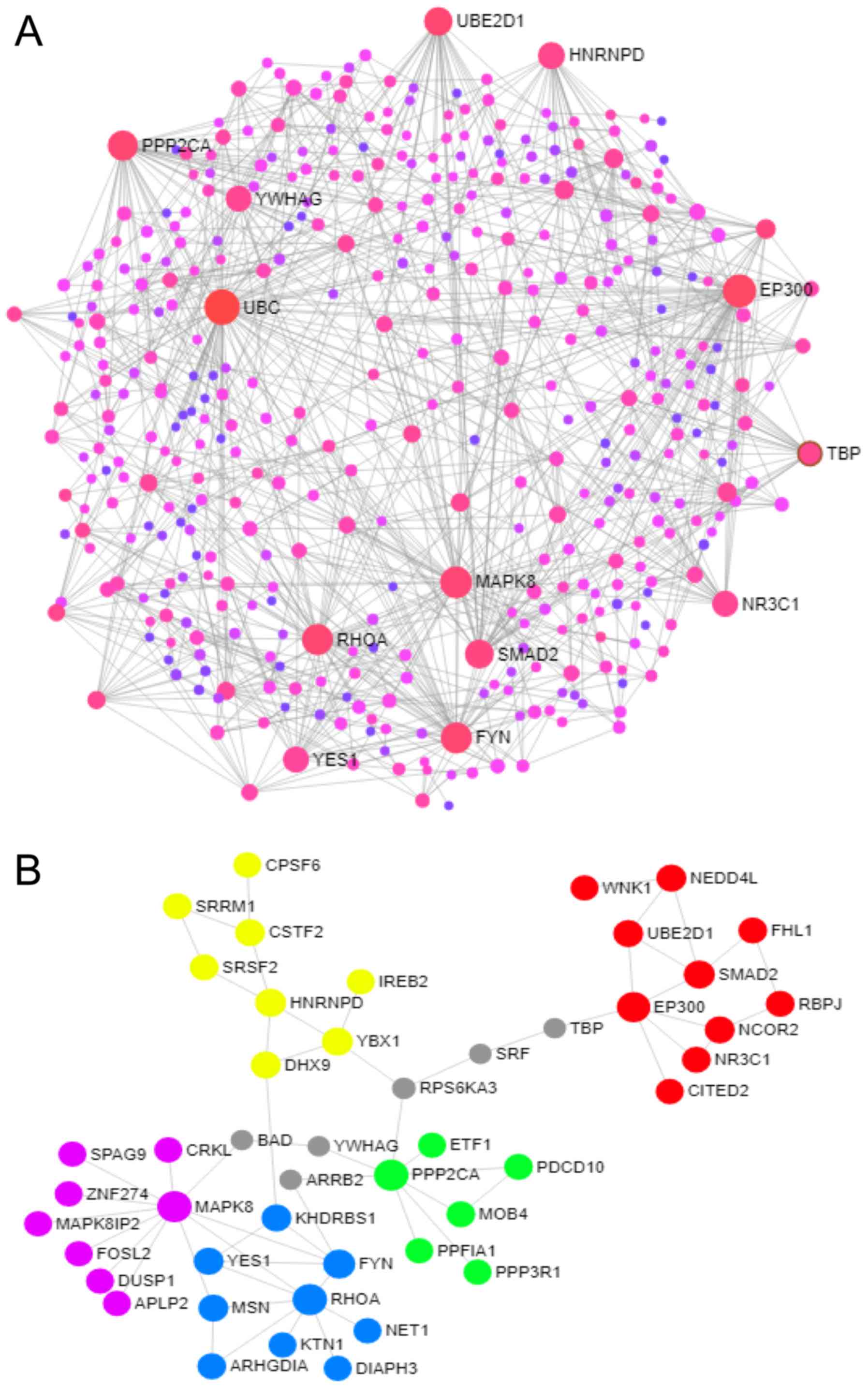
>20 was set as the cut-off criterion. A total of 13 genes were
chosen as hub genes, such as UBC, EP300, MAPK8, FYN, RHOA, PPP2CA,
SMAD2, UBE2D1, HNRNPD, YES1, YWHAG, NR3C1 and TBP. A total of 9
genes were target genes of hsa-miR-429 (EP300, FYN, RHOA, PPP2CA,
UBE2D1, HNRNPD, YWHAG, NR3C1 and TBP). In addition, the top 5
modules were selected, and the KEGG pathway enrichment analysis
revealed that target genes in these modules were mostly enriched in
the neurotrophin signaling pathway, the MAPK signaling pathway, the
pathways in cancer, the Wnt signaling pathway and the focal
adhesion (Fig. 5 and Table SIII).
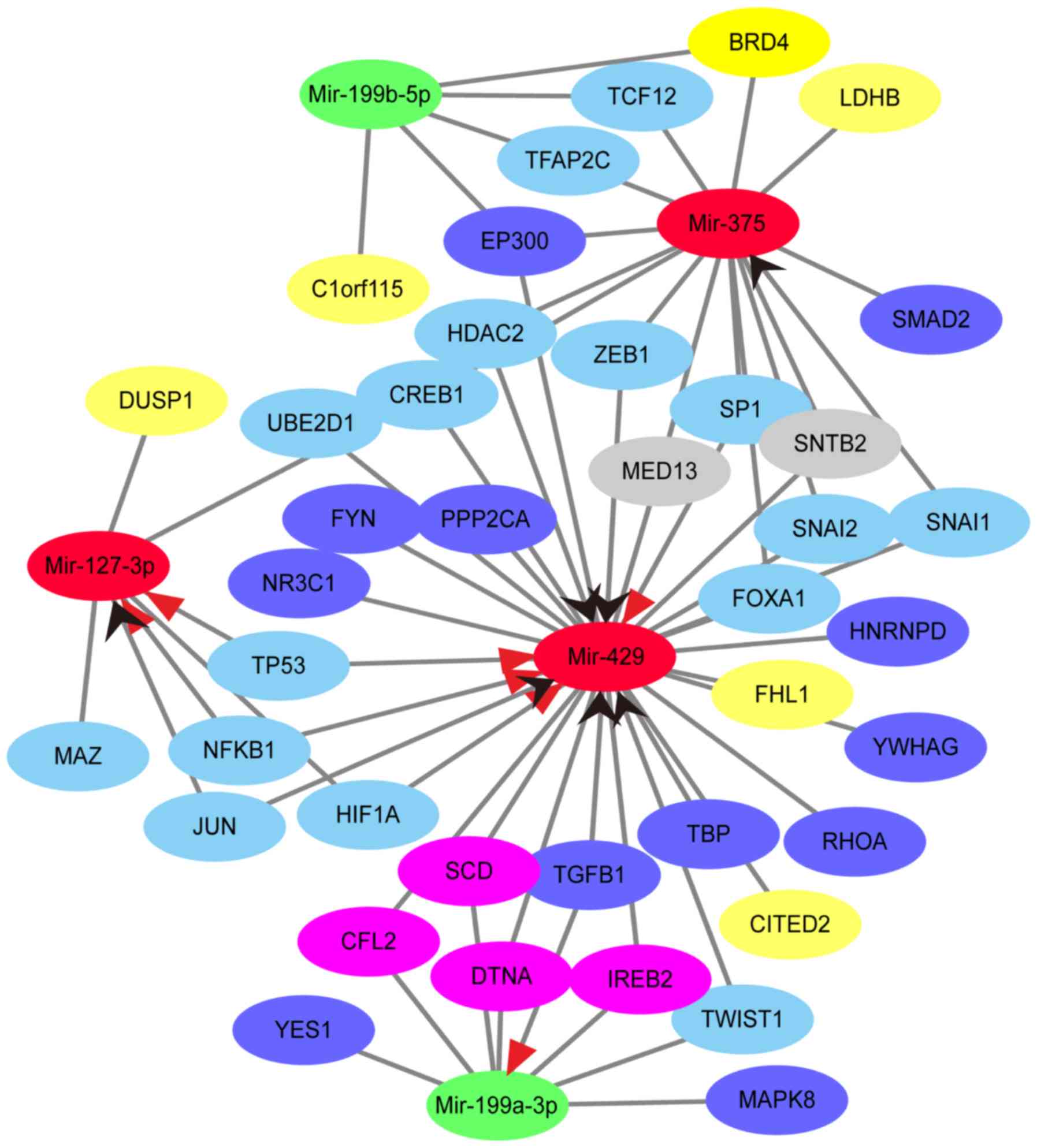
Analysis of TF-DEMs-target genes and
DEGs regulatory network
From the data of TransmiR, upregulated DEMs were
regulated by 51 TFs, and downregulated DEMs were regulated by 24
TFs. In addition, 17 TFs regulated 2 miRNAs, including upregulated
or downregulated DEMs, while CREB1 regulated all upregulated DEMs.
Furthermore, EP300 was detected as a TF of hsa-miR-375 and
hsa-miR-199b-5p, which was also a target gene of has-miR-429
(Table SIV). In summary, based on
the aforementioned results from the bioinformatics analysis, a
regulatory network was constructed to indicate the overlapped TFs,
DEMs, target genes and DEGs (Fig.
3).
Discussion
MTC is a rare malignancy with poor prognosis, as
lymph node metastases are found in 55% patients by the time of
diagnosis (29). Surgical
resection remains the most effective therapy of this disease,
however in advanced cases and patients with distant metastases,
this treatment method is not sufficient (30–33).
Therefore, it is important to study the molecular mechanisms of the
carcinogenesis and development of MTC.
In the present study, a bioinformatics approach was
used to identify candidate biomarker and therapeutic targets of
MTC. Following the analysis, 191 DEMs, including 99 upregulated
DEMs and 92 downregulated DEMs were identified. Among these,
hsa-miR-375, hsa-miR-127-3p and hsa-miR-429 were upregulated, and
hsa-miR-199a-3p and hsa-miR-199b-5p were downregulated in 2 miRNA
profiles, suggesting that they may function as carcinogens or tumor
suppressors in MTC. As shown by the OncomiR database,
hsa-miR-199a-3p and hsa-miR-199b-5p were upregulated in normal
tissues, and hsa-miR-375 was upregulated in thyroid carcinoma,
which is in accordance with the analysis of this study.
Furthermore, a number of researchers have reported that the
overexpression of hsa-miR-375 significantly contributes to the
pathophysiology and development of MTC (34–36).
GO and KEGG analysis results showed that target
genes of upregulated DEMs were significantly enriched in adherens
junction and focal adhesion, which might play important roles in
the tumor development and progression. Target genes of
downregulated DEMs were mainly involved in Wnt signaling pathway,
protein binding and RNA transport. The Wnt signaling pathway is a
group of signal transduction pathways, which begins with proteins
that pass signals into a cell through cell surface receptors. It is
an important signaling pathway in biological development and
tumorigenesis (37–39). In the comparison of
MTCM918T and MTC634, in addition to
MTCM918T and MTCWT, Maliszewska et al
reported that many biochemical pathways were involved in the
malignant behavior of MTC, including the Wnt pathway (40).
By constructing the PPI network, the present study
identified 13 hub genes, and 10 of these, EP300, MAPK8, FYN, RHOA,
PPP2CA, SMAD2, UBE2D1, HNRNPD, YES1 and NR3C1 were involved in the
top 5 modules. KEGG pathway enrichment analysis of modules showed
that Focal adhesion, TGF-β signaling pathway and Wnt signaling
pathway contained 4 hub genes respectively. The TGF-β signaling
pathway mediates intracellular signaling and participates in
embryonic development, tumorigenesis, and physiological processes
(41). Furthermore, TGF-β can
cause enhanced adhesion and motility of tumor cells (42). Santarpia et al observed the
effect of miRNAs in MTC tumorigenesis, migration, proliferation and
invasion. The cell lines were treated with miR-200 inhibitor and
analysis was performed in accordance to the array data, showing
that the members of the miR-200 family regulate the expression of
E-cadherin by directly targeting ZEB1,2 and through the enhanced
expression of tumor growth factor β-1,2 (43).
The hub genes MAPK8 and RHOA were involved in the 5
of the top 10 pathways, including the neurotrophin signaling
pathway, MAPK signaling pathway, colorectal cancer, adherens
junction, TGF-beta signaling pathway and Wnt signaling pathway.
RHOA encodes a member of the Rho family of small GTPases, which
cycle between inactive GDP-bound and active GTP-bound states, and
function as a molecular switch in signal transduction cascades. The
overexpression of this gene is associated with tumor cell
proliferation and metastasis. A number of studies have suggested
that RHOA can serve as a biomarker of colorectal cancer, with
regards to a therapeutic target (44,45).
Takahashi et al conducted an experiment on Rb1(+/-)Nras(+/-)
animals. The results of the aforementioned study revealed that
distant MTC metastases were associated with the loss of the
remaining wild-type Nras allele. In addition the loss of Nras in
Rb1-deficient C cells results in an elevated RHOA activity,
therefore leading to the malignant behavior of these cells
(46). MAPK8 is a member of the
MAP kinase family. The activation of this kinase by TNF-α is found
to be required for TNF-α induced apoptosis. The importance of the
MAPK pathway has been well established in the tumorigenesis of
papillary thyroid cancer (47,48).
For MTC, Chang et al conducted an exome-wide analysis of the
mutational spectrum and indicated that a number of pathways was
involved in the variant process, including MAPK pathway (49).
In the constructed TF-DEMs-target genes and DEGs
regulatory network, 5 downregulated DEGs (C1orf15, CITED2, DUSP1,
FHL1 and LDHB) were also the target genes of 3 upregulated DEMs,
namely hsa-miR-375, hsa-miR-127-3p and hsa-miR-429, and the
downregulated DEM, hsa-miR-199b-5p. In addition, DUSP1 and FHL1
were involved in the module of PPI network, and DUSP1 participated
in the MAPK pathway. The protein encoded by DUSP1 was involved in
several cellular processes and resulted in chemotherapy and
radiotherapy resistance, which indicated that DUSP1 can serve as a
target for cancer therapy. Despite that DUSP1 has been reported as
an oncogene, therapeutic target and a biomarker in many different
types of tumor (50–52), the study of DUSP1 expression and
function in thyroid carcinoma is limited and further investigations
are required.
As shown in the regulatory network, hsa-miR-429 and
hsa-miR-199a-3p were regulated by TGFB1, a TF which encodes a
secreted ligand of the TGF-β superfamily and regulates cell
proliferation, differentiation and growth. In addition, TGFB1 has
been recognized as an activator of hsa-miR-199a-3p and a repressor
of hsa-miR-429, based on the data from TransmiR by the evidence
level of literature. According to the results of target prediction,
4 genes, including CFL2, DTNA, IREB2 and SCD, were overlapped
between has-miR-429 and has-miR-199a-3p, whose effects have been
reported in tumors for their differential expression or potential
function in diagnosis and treatment (53–57).
However, to the best of our knowledge, CFL2, DTNA, IREB2 and SCD
has not been mentioned in MTC. In addition, 10 of the 13 hub genes
were the target genes of hsa-miR-429 and hsa-miR-199a-3p, including
MAPK8 and RHOA. Taken together, the regulatory network of TGFB1,
hsa-miR-429/hsa-miR-199a-3p and target genes may play important
roles in the development of MTC and warrant further
investigation.
Cancer is a complex disease caused by multiple
factors and integrated bioinformatics analysis can help in the
investigation and understanding of its molecular mechanism. The aim
of present study was to identify key DEMs and genes and to
determine potential biomarkers to predict the progression of MTC.
However, this study presents with a number of limitations. First,
the dataset sample size was limited, due to the difficulty to
obtain clinical samples. Second, there were only 5 DEMs that
overlapped in different GSE chips, suggesting that some valuable
miRNAs may be missing. Third, the incidence of MTC was low; thus,
studies of how those genes affect the prognosis of MTC were seldom
reported. Our team are collecting the clinical and pathological
data of MTC in order to carry out further investigations.
In conclusion, this study identified numerous DEMs
that may contribute to the initiation and development of MTC.
Furthermore, the present study also identified a series of
significant pathways and mechanisms for treatment. The regulatory
association between TGFB1, hsa-miR-429 and hsa-miR-199a-3p may
provide novel insight for the diagnosis and treatment of MTC. In
addition, we aim to perform further experiments to examine the
expression of the identified DEMS and genes in different sample
types, and subsequently confirm their utility in the diagnosis and
molecular therapy of MTC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QP, DL and MZ contributed to the design of the
study. LZ, ML and QP performed the bioinformatics analysis and
wrote the manuscript. LZ and DL were responsible for article
revision. LZ and ML contributed to language editing and the
revision of this manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohammadi M and Hedayati M: A brief review
on the molecular basis of medullary thyroid carcinoma. Cell J.
18:485–492. 2017.PubMed/NCBI
|
|
2
|
Figlioli G, Landi S, Romei C, Elisei R and
Gemignani F: Medullary thyroid carcinoma (MTC) and RET
proto-oncogene: Mutation spectrum in the familial cases and a
meta-analysis of studies on the sporadic form. Mutat Res.
752:36–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sippel RS, Kunnimalaiyaan M and Chen H:
Current management of medullary thyroid cancer. Oncologist.
13:539–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Milling RV, Grimm D, Krüger M, Grosse J,
Kopp S, Bauer J, Infanger M and Wehland M: Pazopanib, cabozantinib,
and vandetanib in the treatment of progressive medullary
thyroidcancer with a special focus on the adverse effects on
hypertension. Int J Mol Sci. 19(pii): E32582018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fagin JA and Wells SA Jr: Biologic and
Clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
American Thyroid Association Guidelines
Task Force, ; Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF,
Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M and Wells
SA Jr: Medullary thyroid cancer: Management guidelines of the
American Thyroid Association. Thyroid. 19:565–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agrawal N, Jiao Y, Sausen M, Leary R,
Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop J, et al:
Exomic sequencing of medullary thyroid cancer reveals dominant and
mutually exclusive oncogenic mutations in RET and RAS. J Clin
Endocrinol Metab. 98:E364–E369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
A T, F S, G P and M B: Genetic alterations
in medullary thyroid cancer: Diagnostic and prognostic markers.
Curr Genomics. 12:618–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elisei R, Cosci B, Romei C, Bottici V,
Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, et
al: Prognostic significance of somatic RET oncogene mutations in
sporadic medullary thyroid cancer: A 10-year follow-up study. J
Clin Endocrinol Metab. 93:682–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pishkari S, Paryan M, Hashemi M, Baldini E
and Mohammadi-Yeganeh S: The role of microRNAs in different types
of thyroid carcinoma: A comprehensive analysis to find new miRNA
supplementary therapies. J Endocrinol Invest. 41:269–283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shabani N, Razaviyan J, Paryan M, Tavangar
SM, Azizi F, Mohammadi-Yeganeh S and Hedayati M: Evaluation of
miRNAs expression in medullary thyroid carcinoma tissue samples:
miR-34a and miR-144 as promising overexpressed markers in MTC. Hum
Pathol. 79:212–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abraham D, Jackson N, Gundara JS, Zhao J,
Gill AJ, Delbridge L, Robinson BG and Sidhu SB: MicroRNA profiling
of sporadic and hereditary medullary thyroid cancer identifies
predictors of nodal metastasis, prognosis, and potential
therapeutic targets. Clin Cancer Res. 17:4772–4781. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romeo P, Colombo C, Granata R, Calareso G,
Gualeni AV, Dugo M, De Cecco L, Rizzetti MG, Zanframundo A, Aiello
A, et al: Circulating miR-375 as a novel prognostic marker for
metastatic medullary thyroid cancer patients. Endocr Relat Cancer.
25:217–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lassalle S, Zangari J, Popa A, Ilie M,
Hofman V, Long E, Patey M, Tissier F, Belléannée G, Trouette H, et
al: MicroRNA-375/SEC23A as biomarkers of the in vitro efficacy of
vandetanib. Oncotarget. 7:30461–30478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giordano TJ, Kuick R, Thomas DG, Misek DE,
Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al:
Molecular classification of papillary thyroid carcinoma: Distinct
BRAF, RAS, and RET/PTC mutation-specific gene expression profiles
discovered by DNA microarray analysis. Oncogene. 24:6646–6656.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giordano TJ, Au AY, Kuick R, Thomas DG,
Rhodes DR, Wilhelm KG Jr, Vinco M, Misek DE, Sanders D, Zhu Z, et
al: Delineation, functional validation, and bioinformatic
evaluation of gene expression in thyroid follicular carcinomas with
the PAX8-PPARG translocation. Clin Cancer Res. 12:1983–1993. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li D, Hao X and Song Y: Identification of
the key MicroRNAs and the miRNA-mRNA regulatory pathways in
prostate cancer by bioinformatics methods. Biomed Res Int.
2018:62041282018.PubMed/NCBI
|
|
20
|
Mou T, Zhu D, Wei X, Li T, Zheng D, Pu J,
Guo Z and Wu Z: Identification and interaction analysis of key
genes and microRNAs in hepatocellular carcinoma by bioinformatics
analysis. World J Surg Oncol. 15:632017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Qin Y and Zhang H: Identification of
key miRNA-gene pairs in chronic lymphocytic leukemia through
integrated analysis of mRNA and miRNA microarray. Oncol Lett.
15:361–367. 2018.PubMed/NCBI
|
|
22
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res 42 (Database Issue). D92–D97. 2014. View Article : Google Scholar
|
|
23
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44(D1): D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tripathi S, Pohl MO, Zhou Y,
Rodriguez-Frandsen A, Wang G, Stein DA, Moulton HM, DeJesus P, Che
J, Mulder LC, et al: Meta- and orthogonal integration of influenza
‘OMICs’ data defines a role for UBR4 in virus budding. Cell Host
Microbe. 18:723–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia J, Benner MJ and Hancock RE:
NetworkAnalyst-integrative approaches for protein-protein
interaction network analysis and visual exploration. Nucleic Acids
Res 42 (Web Server Issue). W167–W174. 2014. View Article : Google Scholar
|
|
26
|
Xia J, Gill E and Hancock RE:
NetworkAnalyst for statistical, visual and network-based
meta-analysis of gene expression data. Nat Protoc. 10:823–844.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Lu M, Qiu C and Cui Q: TransmiR: A
transcription factor-microRNA regulation database. Nucleic Acids
Res 38 (Database Issue). D119–D122. 2010. View Article : Google Scholar
|
|
28
|
Tong Z, Cui Q, Wang J and Zhou Y: TransmiR
v2.0: An updated transcription factor-microRNA regulation database.
Nucleic Acids Res. 47(D1): D253–D258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scollo C, Baudin E, Travagli JP, Caillou
B, Bellon N, Leboulleux S and Schlumberger M: Rationale for central
and bilateral lymph node dissection in sporadic and hereditary
medullary thyroid cancer. J Clin Endocrinol Metab. 88:2070–2075.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Griebeler ML, Gharib H and Thompson GB:
Medullary thyroid carcinoma. Endocr Pract. 19:703–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlumberger M, Carlomagno F, Baudin E,
Bidart JM and Santoro M: New therapeutic approaches to treat
medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab.
4:22–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conzo G, Polistena A, Calò PG, Bononi P,
Gambardella C, Mauriello C, Tartaglia E, Avenia S, Sanguinetti A,
Medas F, et al: Efficacy of combined treatment for anaplastic
thyroid carcinoma: Results of a multinstitutional retrospective
analysis. Int J Surg. 12 (Suppl 1):S178–S182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conzo G, Avenia N, Ansaldo GL, Calò P, De
Palma M, Dobrinja C, Docimo G, Gambardella C, Grasso M, Lombardi
CP, et al: Surgical treatment of thyroid follicular neoplasms:
Results of a retrospective analysis of a large clinical series.
Endocrine. 55:530–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi L, Zhao SM, Luo Y, Zhang AW, Wei LH,
Xie ZY, Li YY and Ma W: MiR-375: A prospective regulator in
medullary thyroid cancer based on microarray data and
bioinformatics analyses. Pathol Res Pract. 213:1344–1354. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galuppini F, Bertazza L, Barollo S,
Cavedon E, Rugge M, Guzzardo V, Sacchi D, Watutantrige-Fernando S,
Vianello F, Mian C and Pennelli G: MiR-375 and YAP1 expression
profiling in medullary thyroid carcinoma and their correlation with
clinical-pathological features and outcome. Virchows Arch.
47:651–658. 2017. View Article : Google Scholar
|
|
36
|
Hudson J, Duncavage E, Tamburrino A,
Salerno P, Xi L, Raffeld M, Moley J and Chernock RD: Overexpression
of miR-10a and miR-375 and downregulation of YAP1 in medullary
thyroid carcinoma. Exp Mol Pathol. 95:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tai D, Wells K, Arcaroli J, Vanderbilt C,
Aisner DL, Messersmith WA and Lieu CH: Targeting the WNT signaling
pathway in cancer therapeutics. Oncologist. 20:1189–1198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maliszewska A, Leandro-Garcia LJ,
Castelblanco E, Macià A, de Cubas A, Goméz-López G, Inglada-Pérez
L, Álvarez-Escolá C, De la Vega L, Letón R, et al: Differential
gene expression of medullary thyroid carcinoma reveals specific
markers associated with genetic conditions. Am J Pathol.
182:350–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santarpia L, Calin GA, Adam L, Ye L, Fusco
A, Giunti S, Thaller C, Paladini L, Zhang X, Jimenez C, et al: A
miRNA signature associated with human metastatic medullary thyroid
carcinoma. Endocr Relat Cancer. 20:809–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeong D, Park S, Kim H, Kim CJ, Ahn TS,
Bae SB, Kim HJ, Kim TH, Im J, Lee MS, et al: RhoA is associated
with invasion and poor prognosis in colorectal cancer. Int J Oncol.
48:714–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang GY, Yang WH and Chen Z: Upregulated
STAT3 and RhoA signaling in colorectal cancer (CRC) regulate the
invasion and migration of CRC cells. Eur Rev Med Pharmacol Sci.
20:2028–2037. 2016.PubMed/NCBI
|
|
46
|
Takahashi C, Contreras B, Iwanaga T,
Takegami Y, Bakker A, Bronson RT, Noda M, Loda M, Hunt JL and Ewen
ME: Nras loss induces metastatic conversion of Rb1-deficient
neuroendocrine thyroid tumor. Nat Genet. 38:118–123. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Zhang J, Gao J and Li Y:
MicroRNA-4728 mediated regulation of MAPK oncogenic signaling in
papillary thyroid carcinoma. Saudi J Biol Sci. 25:986–990. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan J, Zhang L, Xu S, Cheng X, Yu H, Bao J
and Lu R: Induction of apoptosis in human
papillary-thyroid-carcinoma BCPAP cells by diallyl trisulfide
through activation of the MAPK signaling pathway. J Agric Food
Chem. 66:5871–5878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chang YS, Chang CC, Huang HY, Lin CY, Yeh
KT and Chang JG: Detection of molecular alterations in taiwanese
patients with medullary thyroid cancer using whole-exome
sequencing. Endocr Pathol. 29:324–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Teng F, Xu Z, Chen J, Zheng G, Zheng G, Lv
H, Wang Y, Wang L and Cheng X: DUSP1 induces apatinib resistance by
activating the MAPK pathway in gastric cancer. Oncol Rep.
40:1203–1222. 2018.PubMed/NCBI
|
|
51
|
Fang J, Ye Z, Gu F, Yan M, Lin Q, Lin J,
Wang Z, Xu Y and Wang Y: DUSP1 enhances the chemoresistance of
gallbladder cancer via the modulation of the p38 pathway and DNA
damage/repair system. Oncol Lett. 16:1869–1875. 2018.PubMed/NCBI
|
|
52
|
Zhang Y, Zhang Y, Chen M, Liu C and Xiang
C: DUSP1 is involved in the progression of small cell carcinoma of
the prostate. Saudi J Biol Sci. 25:858–862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Kuramitsu Y, Ueno T, Suzuki N,
Yoshino S, Iizuka N, Zhang X, Oka M and Nakamura K: Differential
expression of up-regulated cofilin-1 and down-regulated cofilin-2
characteristic of pancreatic cancer tissues. Oncol Rep.
26:1595–1599. 2011.PubMed/NCBI
|
|
54
|
Yu BB, Lin GX, Li L, Qu S, Liang ZG, Chen
KH, Zhou L, Lu QT, Sun YC and Zhu XD: Cofilin-2 acts as a marker
for predicting radiotherapy response and is a potential therapeutic
target in nasopharyngeal carcinoma. Med Sci Monit. 24:2317–2329.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu J, Li H, Shen S, Sun L, Yuan Y and
Xing C: Alternative splicing events implicated in carcinogenesis
and prognosis of colorectal cancer. J Cancer. 9:1754–1764. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khiroya H, Moore JS, Ahmad N, Kay J,
Woolnough K, Langman G, Ismail I, Naidu B, Tselepis C and Turner
AM: IRP2 as a potential modulator of cell proliferation, apoptosis
and prognosis in nonsmall cell lung cancer. Eur Respir J. 49(pii):
16007112017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Siqingaow a, Sekar S, Gopalakrishnan V and
Taghibiglou C: Sterol regulatory element-binding protein 1
inhibitors decrease pancreatic cancer cell viability and
proliferation. Biochem Biophys Res Commun. 488:136–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|